Abstract
In mammalian systems, detoxification enzymes of the GST (glutathione S-transferase) family regulate JNK (c-Jun N-terminal kinase) signal transduction by interaction with JNK itself or other proteins upstream in the JNK pathway. In the present study, we have studied GSTs and their interaction with components of the JNK pathway from Diptera. We have evaluated the effects of four Delta class Anopheles dirus GSTs, GSTD1-1, GSTD2-2, GSTD3-3 and GSTD4-4, on the activity of full-length recombinant Drosophila HEP (mitogen-activated protein kinase kinase 7; where HEP stands for hemipterous) and the Drosophila JNK, as well as the reciprocal effect of these kinases on GST activity. Interestingly, even though these four GSTs are alternatively spliced products of the same gene and share >60% identity, they exerted different effects on JNK activity. GSTD1-1 inhibited JNK activity, whereas the other three GST isoforms activated JNK. GSTD2-2, GSTD3-3 and GSTD4-4 were inhibited 50–80% by HEP or JNK but GSTD1-1 was not inhibited by JNK. However, there were some similarities in the actions of HEP and JNK on these GSTs. For example, binding constants for HEP or JNK inhibiting a GST were similar (20–70 nM). Furthermore, after incubation of the GSTs with JNK, both JNK and the GSTs changed catalytic properties. The substrate specificities of both GSTs and JNK were also altered after their co-incubation. In addition, glutathione modulated the effects of JNK on GST activity. These results emphasize that different GST spliceforms possess different properties, both in their catalytic function and in their regulation of signalling through the JNK pathway.
Keywords: c-Jun N-terminal kinase (JNK), Drosophila, glutathione S-transferase, hemipterous (HEP; MKK7), JNK activity regulation, mosquito
Abbreviations: ASK1, apoptosis signal-regulating kinase 1; CDNB, 1-chloro-2,4-dinitrobenzene; DCNB, 1,2-dichloro-4-nitrobenzene; GST, glutathione S-transferase; HEP, hemipterous; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MKK, MAP kinase kinase; PNBC, p-nitrobenzyl chloride; PNPB, p-nitrophenyl bromide
INTRODUCTION
GSTs (glutathione S-transferases; EC 2.5.1.18) are a superfamily of multifunctional enzymes involved in normal cellular metabolism as well as in the detoxification of various hydrophobic endogenous and xenobiotic compounds [1]. The central importance of GSTs in detoxification lies in their unique capacity to conjugate glutathione with a wide variety of compounds [2]. This detoxification reaction is of critical importance in cell survival. As a consequence, GSTs have been found in virtually all organisms and are currently grouped into at least ten classes based on their primary sequence similarity, substrate specificity, immunological properties, and tertiary and quaternary structures [3]. Changes in glutathione levels have also been associated with the activation of a stress response [4] and GSTs can protect against electrophiles and oxidative stress by altering cellular glutathione levels [1]. GSTs also have glutathione peroxidase activity under conditions of oxidative stress and they have been further implicated in a range of physiological roles such as signal transduction, cell proliferation, differentiation and apoptosis [5].
Various classes of GSTs have been shown to be involved in these broader physiological roles. For example, GST Omega protected cells from apoptosis induced by Ca2+ mobilization from intracellular stores through its ability to regulate the Ca2+ channel activity of the ryanodine receptor [6]. Since the Bcl-2 family member Bax regulates programmed cell death by promoting apoptosis [7], a role for GST Theta in regulation of apoptotic cell death has also been suggested following its identification as a Bax-interacting protein [8].
Other interactions of GSTs to alter intracellular signal-transduction events have been reported. Most often these implicate GST in the regulation of the JNK (c-Jun N-terminal kinase) signal-transduction pathway. JNK is a member of the MAPK (mitogen-activated protein kinase) family, which is conserved across all eukaryotes ranging from yeast and insects to mammals [9]. JNK has been implicated in a variety of biological functions in response to stress, and the transmission of signals through the JNK pathway is achieved by sequential phosphorylation and activation of the pathway kinase components, the MKKKs (MAP kinase kinase kinase), the MKKs and the MAPKs. The activation of JNK relays extracellular cues to transcription factors such as c-Jun [10], activating transcription factor 2 [11] and Elk1 [12], thereby regulating gene expression, cellular homoeostasis, differentiation, apoptosis and cell death. Not only is the induction of c-jun and c-fos transcription dependent on JNK, but Jun and Fos can also induce the transcription of xenobiotic-metabolizing enzymes such as GST [13]. Thus c-Jun is directly involved in GST Pi expression in vivo [14]. This suggests a role of JNK in the induction of a cellular defence programme against cytotoxic xenobiotics.
JNK pathway components and GSTs are evolutionally conserved across mammals and insects. Different mammalian GST classes such as GST Pi and GST Mu have been reported to interact with different stress kinase proteins in the JNK pathway. For example, GST Pi is a JNK regulatory protein, and its association with JNK maintains a low basal level of JNK activity in the non-stressed cell [15]. The lack of GST Pi increased constitutive JNK activity in vivo and, therefore, regulated the expression of genes that were specific downstream targets of the JNK pathway [16]. Moreover, GST Pi co-ordinates ERK/p38/IKK activation as part of the mechanism underlying its ability to elicit protection against H2O2-induced cell death [17]. In contrast, GST Mu interacts with ASK1 (apoptosis signal-regulating kinase 1), an upstream activating kinase of JNK that participates in cell death [18].
In the present study, we evaluate the interaction of GST and kinase proteins in a Dipteran system using four different spliceforms of Anopheles dirus Delta class GSTs and two different Drosophila kinase proteins, Drosophila HEP7 (where HEP stands for hemipterous) and Drosophila JNK (JNK). The Drosophila JNK pathway, viewed as a linear cascade [19], comprises the Hemipterous (HEP or DMKK7) [20], basket (JNK) [21] and D-Jun [22], which are homologous proteins with mammalian MKK7, JNK and c-Jun respectively. The four Anopheles GSTs used in the present study are alternatively spliced products from a single gene [23]. To elucidate the mechanism by which GSTs modulate the JNK signalling pathway, we assessed both GST and kinase activities to provide evidence for direct protein–protein interactions and to measure the binding affinity. Our results show that the GSTs interact with protein kinases, the different GST isoforms appear to possess different regulatory mechanisms in the JNK pathway and JNK interaction also affects GST activities. This is the first report of the reciprocal regulation of GST and JNK pathway activities.
EXPERIMENTAL
Preparation of DNA constructs
The alternatively spliced products, GSTD1-1, GSTD2-2, GSTD3-3 and GSTD4-4, were cloned into a pET3a vector [24]. The recombinant proteins in the Drosophila JNK pathway consisting of Drosophila HEP7 (HEP; GenBank® accession number AAB63449.1), Drosophila JNK (JNK and also known as basket; GenBank® accession number AAB97094.1) and the transactivation domain of Drosophila Jun (amino acids 1–104; Jun 1–104; GenBank® accession number P18289) were obtained by reverse transcriptase–PCR from adult Drosophila melanogaster. The PCR products were then cloned into a pET28b vector (Stratagene). The HEP recombinant plasmid was also used as the template for a site-directed mutagenesis method to construct a constitutively active HEP mutant (HEP3E). The mammalian MKK7-β1 isoform mutant (MKK73E) has constitutive kinase activity after the substitutions of Ser271, Thr275 and Ser277 to Glu [25]; we therefore altered the three homologous residues (Ser348, Thr352 and Ser354) of Drosophila HEP7 to Glu by two-step PCR using a Quik Change™ site-directed mutagenesis kit (Stratagene). All recombinant clones were identified by restriction-digest of the plasmids and confirmed by full-length sequencing in both directions using a BigDye™ Terminator Cycle Sequencing kit (PerkinElmer).
Preparation of recombinant proteins
GST proteins were expressed and purified using either GSTrap or S-hexyl-glutathione affinity chromatography [24]. The four recombinant proteins, HEP, HEP3E, JNK and Jun 1–104, were expressed as histidine fusion proteins. The JNK and Jun 1–104 recombinant proteins were expressed as soluble proteins and purified using a standard Ni2+-nitrilotriacetate column method (Amersham Biosciences). In contrast, HEP and HEP3E recombinant proteins were expressed mainly in inclusion bodies. Therefore these HEP and HEP3E were purified using Ni2+-nitrilotriacetate column chromatography under denaturing conditions and renatured by slow dialysis using the Roti®-Fold reagent (Carl Roth GmbH+, Karlsruhe, Germany). Protein concentrations were determined using the BioRad protein reagent with BSA as the standard [26].
GST activity assays
GST activity was measured by the conjugation of GSH with the hydrophobic substrates CDNB (1-chloro-2,4-dinitrobenzene; Aldrich), DCNB (1,2-dichloro-4-nitrobenzene; Fluka, Buchs, Switzerland), PNBC (p-nitrobenzyl chloride; Aldrich) and PNPB (p-nitrophenyl bromide; Aldrich) [24]. We used the CDNB conjugation as our standard assay because GST-specific activity for this substrate was highest [24].
Effects of protein kinases HEP and JNK on GST activity
The effects of HEP and JNK on GST activity were examined by incubating GSTs and kinase proteins in a 1:1 molar ratio at room temperature (27–30 °C) for 5 min. GST activity was measured in the presence and absence of kinase proteins. The effects on GST activity were evaluated by the following methods.
(i) The percentage inhibition of GST activity. The percentage inhibition was determined by measuring GST activity in the presence of HEP or JNK as well as without kinase proteins as the control.
(ii) The type of inhibition and affinity binding (Ki). GST and kinase protein interactions were performed by varying the concentration of CDNB from 0.05 to 3.0 mM and measuring the kinetic parameters for CDNB and GSH conjugation [24]. The kinetic parameters and Ki were determined by both linear and non-linear regression analysis using GraphPad Prism 2.01 software.
(iii) The change of GST substrate specificity by JNK. GST and JNK were incubated in a 1:1 molar ratio and the effects of JNK on GST activity were determined using the hydrophobic substrates for GST, as mentioned above. A positive or negative change of GST activity towards a substrate, when compared with activity in the absence of JNK, indicated a substrate selectivity change of GST.
(iv) The effect of GSH on GST activity in the presence of JNK. A 1:1 molar ratio of GST/JNK was incubated at room temperature for 5 min in the presence and absence of 2 mM GSH. The GST activity was determined towards its hydrophobic substrates as described above.
In vitro protein kinase assays
Constitutively active HEP3E was used to activate JNK and then both HEP3E and Jun 1–104 were assessed as JNK substrates [21,22]. HEP3E/JNK/Jun 1–104 in 1:2:10 molar ratio were incubated in 20 mM Hepes, 20 mM MgCl2, 20 mM β-glycerophosphate (pH 7.6), containing 500 μM dithiothreitol, 100 μM sodium orthovanadate, supplemented with 20 μM ATP and 3 μCi of [γ-32P]ATP. The phosphorylation reactions were performed for 25 min at room temperature and separated by SDS/PAGE. Phosphorylated proteins were visualized by autoradiography and quantified by Cerenkov counting.
Effects of GST on protein kinase activity
The recombinant GSTs were incubated with kinase proteins in a 10:1 molar ratio for 10 min at room temperature. Kinase activity was then measured as described above.
RESULTS
JNK and HEP can inhibit the activity of Delta GST spliceforms
Previous studies have shown that GSTs of the mammalian Pi and Mu classes are capable of modulating the JNK pathway through their inhibition of JNK and interaction with ASK respectively [18]. In the present study, we have evaluated whether GSTs of the Dipteran Delta class could interact with two kinases from Drosophila, either JNK or its upstream kinase HEP, by measuring both the effects of the kinases on GST activity and the effects of the GSTs on kinase activity.
Previously, a comprehensive study of six GST isoforms yielded estimates of total GST concentrations in eukaryotic cells, depending on tissue type, ranging from 0.1 to 1.4 nmol of GST/mg of total soluble protein [27]. From our laboratory data, we estimate Drosophila SL2 cells to contain 66 pg of soluble protein/cell. Assuming a tissue culture cell volume of 1 pl [28], a total GST cell concentration of 7–90 μM can be estimated. A single MAPK protein has been estimated to have cellular concentrations of 1–3 μM [28]. In Anopheles gambiae, it has been reported that, probably, there are 32 different soluble GST encoding transcripts with 31 being found in the adult mosquito [29]. This is similar to the 37 putative GST genes identified in Drosophila [29]. It was also shown that, depending on the specific tissue/cell type, a single GST isoform can vary between 0.15 and 45 μM [27]. However, these concentration determinations do not address the issue of compartmental localization known to be important in controlling message propagation down signal-transduction pathways. We therefore chose an intermediate concentration range and used the ratio 1:1 for the GST/JNK experiments.
We began with an evaluation of the effects of JNK and HEP on the activity of four Delta GST spliceforms. Alternative splicing is a major mechanism of generating protein diversity in higher eukaryotes [30]. These four GSTs possess 61–77% amino acid identity and are products of the Adgst1AS1 gene [23,24]. They share an untranslated exon 1 and a translated exon 2 that code for 45 amino acids at the N-terminus. These two exons are spliced to one of four alternative exons 3, namely 3A, 3B, 3C or 3D. This generates four different mature transcripts coding for proteins containing 209–219 amino acids that have been called D4-4, D3-3, D2-2 and D1-1 respectively. These spliceforms therefore differ in their C-terminal amino acids only.
As shown in Table 1, GST activities, as assessed using the standard CDNB assay, were decreased in the presence of a 1:1 molar concentration of HEP. The activities of the GST spliceforms GSTD1-1 and GSTD2-2 were the greatest affected, but even the inhibition of GSTD3-3 and GSTD4-4 was >20%. A parallel series of experiments in which JNK was incubated with each Delta GST spliceform also showed that JNK could inhibit the activity of GSTD2-2, GSTD3-3 and GSTD4-4 towards CDNB. However, under these assay conditions, no inhibition of GSTD1-1 activity could be observed (Table 1). The differences in the effects of the HEP and JNK on the activities of the GST spliceforms would appear to be the result of different interactions with the different amino acids in the C-terminus of each GST spliceform.
Table 1. Effect of HEP and JNK proteins on GST activity.
The recombinant proteins of HEP and JNK were incubated with the different GST splice forms in 1:1 molar ratio for 5 min at room temperature. The percentage inhibition of GST activity was calculated using a reaction that contained no kinase proteins as control. No inhibition of GST activity of GSTD1-1 by JNK protein was detected. Results are means±S.D. for at least four independent experiments.
| GST | Inhibition of GSTs by HEP (%) | Inhibition of GSTs by JNK (%) |
|---|---|---|
| D1-1 | 54.64±3.83 | No inhibition detected |
| D2-2 | 53.29±7.71 | 85.35±6.72 |
| D3-3 | 23.71±1.88 | 44.64±6.92 |
| D4-4 | 29.27±2.99 | 68.45±4.00 |
Mechanism of GST inhibition by HEP and JNK
A more detailed kinetic study of inhibition was undertaken to yield data on the affinity of binding (Ki) and the mechanism of interaction for these four GST spliceforms and the kinases HEP and JNK (Table 2). With the exception of the JNK and GSTD1-1, which showed no inhibition of GST activity, the inhibition of the GST spliceforms by JNK or by HEP was non-competitive with respect to its substrate CDNB. This indicated that the interaction with each kinase did not block the GST active site despite inhibiting its transferase activity. This interaction was also of high affinity, with estimates of Ki in the range of 20–70 nM for GSTD1-1, GSTD2-2 and GSTD3-3 (Table 2). For GSTD4-4, Ki values were higher, being in the range of 100–200 nM; however, all of these interactions exhibited approx. 3–4 orders of magnitude greater affinity than the interactions of GSTs with glutathione [24]. Furthermore, these results show that HEP interacts with all the GST Delta spliceforms tested. JNK also interacts with GSTD2-2, GSTD3-3 and GSTD4-4, but it was not possible to observe an interaction of JNK with GSTD1-1 in this experiment.
Table 2. Mechanism of GST and kinase protein interaction.
The type of inhibition and affinity binding (Ki) of GST and kinase protein interaction were studied by varying the concentration of CDNB from 0.05 to 3.0 and measuring kinetic parameters under the standard conditions for each GST enzyme. Results are means±S.E.M. for at least four independent experiments.
| GST | Type of inhibition | Ki HEP-GST (nM) | Ki JNK-GST (nM) |
|---|---|---|---|
| D1-1 | Non-competitive | 50.0±2.84 | Not determined |
| D2-2 | Non-competitive | 21.3±5.18 | 73.9±20.1 |
| D3-3 | Non-competitive | 43.6±7.19 | 76.8±15.4 |
| D4-4 | Non-competitive | 125.0±45.2 | 218±86.3 |
Modulation of protein kinase activity in the presence of GST Delta spliceforms
We next tested the reciprocal regulation of GST Delta spliceforms on protein kinase activity. We began by assessing JNK activity towards its physiological substrate Jun. As shown in Figure 1(A), GSTD1-1 inhibited the ability of JNK to phosphorylate Jun by approx. 50%. In contrast, the inclusion of GSTD2-2, GSTD3-3 or GSTD4-4 increased JNK activity by up to 170%. Thus despite JNK not inhibiting GSTD1-1 activity towards CDNB (Table 1), GSTD1-1 was capable of inhibiting JNK activity towards Jun (Figure 1A).
Figure 1. GST spliceforms are both positive and negative regulators of JNK activity.
(A) The experiment was performed using GST/HEP3E/JNK/Jun 1–104 in 10:1:2:10 molar ratio. GST was incubated with constitutively active HEP3E for 10 min at room temperature before adding JNK and Jun with 3 μCi of [γ-32P]ATP for 25 min of phosphorylation time at room temperature. Proteins were then separated by SDS/PAGE, and JNK activity was visualized by autoradiography of the 32P-labelled Jun. The upper panel is an autoradiograph showing Jun phosphorylation in the presence and absence of different GST isoforms. The lower panel depicts SDS/polyacrylamide gel showing the sample loading for Jun. The histogram quantifies the effects of GSTs on Jun phosphorylation when compared with the reaction in the absence of these GSTs. Figures shown are representative of at least three independent experiments. (B) The experiment was performed using GST/HEP3E/JNK in 10:1:2 molar ratio. GST was incubated with constitutively active HEP3E for 10 min at room temperature before adding JNK with 3 μCi of [γ-32P]ATP for 25 min of phosphorylation time at room temperature. Proteins were then separated by SDS/PAGE, and kinase activity was visualized by autoradiography of the 32P-labelled substrates. The JNK protein was a substrate for HEP as well as HEP being a substrate for JNK [21]. The autoradiograph in the upper panel shows HEP and JNK phosphorylation in the presence and absence of different GST isoforms. The lower panel is an SDS/polyacrylamide gel showing the sample loading for HEP and JNK. The histogram quantifies the effects of the GSTs on HEP and JNK phosphorylation. Figures shown are representative of at least three independent experiments.
We next repeated these protein kinase assays, but without the inclusion of the Jun substrate protein. Thus we could assess the actions of the GST Delta spliceforms on both HEPEE and JNK activity using the ability of HEPEE to phosphorylate JNK as well as the ability of JNK to phosphorylate HEPEE [21]. As shown in Figure 1(B), the incubation of JNK alone did not result in its significant autophosphorylation. Similarly, prolonged incubation of HEPEE alone did not result in its significant autophosphorylation (results not shown). The inclusion of HEPEE with JNK resulted in weak phosphorylation of both HEPEE and JNK proteins. When the GST Delta spliceforms were also included, the most striking differences were noted with GSTD2-2 and GSTD3-3, which increased the phosphorylation of both HEPEE and JNK proteins up to 6-fold. Furthermore, GSTD1-1 and GSTD4-4 seemed to inhibit the phosphorylation of HEPEE, without inhibiting the phosphorylation of JNK. These results therefore show that GSTs can affect the activities of both JNK towards HEPEE and HEPEE towards JNK. They also show that GSTs were not JNK substrates because no phosphorylated GST protein was observed even after prolonged exposure of the autoradiographs. This is consistent with these GST proteins lacking a consensus phosphorylation site for MAPKs, namely S/TP or PXS/TP [31].
JNK affected GST substrate specificity
We next evaluated whether JNK changed the substrate specificity of the Delta GST spliceforms. First, the specific activity of each Delta GST spliceform was determined for the substrates CDNB, DCNB, PNBC and PNPB in the absence of any JNK protein. The results are presented in Table 3. The results illustrate the striking differences in the enzymic properties of these four GSTs. For example, a 10-fold difference in specific activity was noted for the CDNB substrate. Similarly, 8-fold differences for DCNB, 17-fold differences for PNBC substrate and >20-fold differences for PNPB are noted. These differences must arise from the differences in amino acids at the C-terminus. These C-terminal residues contribute to the H-site and have been shown to determine substrate specificity for different compounds within each class and amongst different classes [32–34].
Table 3. Specific activity of GST splice forms using various hydrophobic substrates.
Results are means±S.E.M. for at least five separate assays. The substrate concentrations used were: CDNB, 1 mM; DCNB, 1 mM; PNBC, 1.2 mM and PNPB, 0.1 mM.
| Specific activity [μmol·min−1·(mg of protein)−1] | ||||
|---|---|---|---|---|
| GST | CDNB | DCNB | PNBC | PNPB |
| D1-1 | 6.54±0.53 | 0.070±0.002 | 1.05±0.191 | 0.002±0.002 |
| D2-2 | 45.1±3.41 | 0.177±0.006 | 17.1±1.09 | 0.047±0.010 |
| D3-3 | 67.5±1.97 | 0.312±0.023 | 2.96±0.292 | 0.002±0.007 |
| D4-4 | 41.8±1.40 | 0.042±0.011 | 2.73±0.105 | 0.023±0.002 |
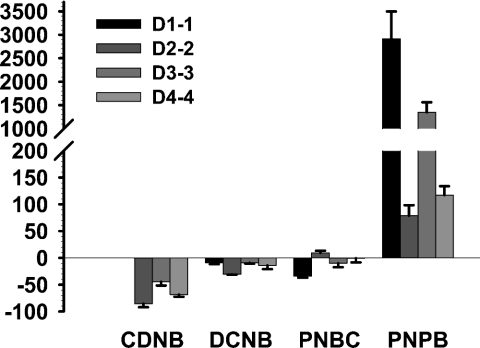
The changes in GST substrate specificity after incubation with JNK are shown in Figure 2. Some striking differences were observed. For example, GSTD1-1 activity towards CDNB was not influenced when incubated in the presence of JNK; nonetheless, it was significantly increased (approx. 2500-fold) towards PNPB. Similarly, incubation with JNK increased GSTD2-2 activity using PNBC and PNPB, whereas it was decreased on using other substrates. Both D3-3 and D4-4 displayed an increase in activity towards PNPB; however, the activities were decreased towards other substrates. These results suggest that the JNK interaction provokes a set of different conformational changes in each GST, thus affecting the active-site topologies, for instance, changes in hydrophobicity and size through residue movement [35], in a dissimilar manner.
Figure 2. JNK changes the substrate specificity of GST spliceforms.
GST and JNK were incubated in 1:1 molar ratio for 5 min at room temperature. GST activity was measured using various hydrophobic co-substrates. The percentage change in GST activity (y-axis) was determined by comparing the reactions in the presence and absence of JNK. The specific activity of each GST for the substrates in the absence of JNK is shown in Table 3. There was no activity change in GSTD1-1 activity using CDNB as the co-substrate due to the lack of a JNK effect (Table 1). Results shown are representative of at least four independent experiments with similar results.
Effect of glutathione on GST–JNK interaction
Under normal cellular conditions, intracellular GSH concentrations are supposed to be in the range 1–10 mM. At these concentrations, GSH would usually be bound to the GST active site [36] and, therefore, we evaluated whether the presence of GSH would alter the effects of GSTs on JNK activities. In the presence of 2 mM GSH, JNK had different effects on the Delta GST spliceforms for the GST substrates, CDNB, DCNB, PNBC and PNPB (Figure 3). Specifically, when activities of the GSTD1-1 and GSTD2-2 spliceforms were assessed after preincubation in the absence (Figure 3A) and presence (Figure 3B) of glutathione, the most striking differences were noted for activities towards the CDNB substrate. GSTD1-1 was now activated by JNK after preincubation in the presence of GSH, and GSTD2-2 was also activated rather than inhibited. We suggest that the known changes of GSH binding that result in GST-induced-fit conformational changes [37] could contribute to the changes noted in the GST–JNK interaction. These changes will be observed differently depending on the GST-specific isoforms and substrate employed.
Figure 3. Presence of glutathione changed the JNK effects on GST activity.
Effects of JNK on GST were determined in the absence and presence of GSH. GST and JNK were incubated in 1:1 molar ratio in the presence and absence of 2 mM GSH for 5 min at room temperature. GST activity was measured using various hydrophobic co-substrates. The percentage change in GST activity was then calculated: (A) specific activity of GSTD1-1 and GSTD2-2 affected by JNK, and it was employed for comparison with the reaction containing GSH, as shown in (B).
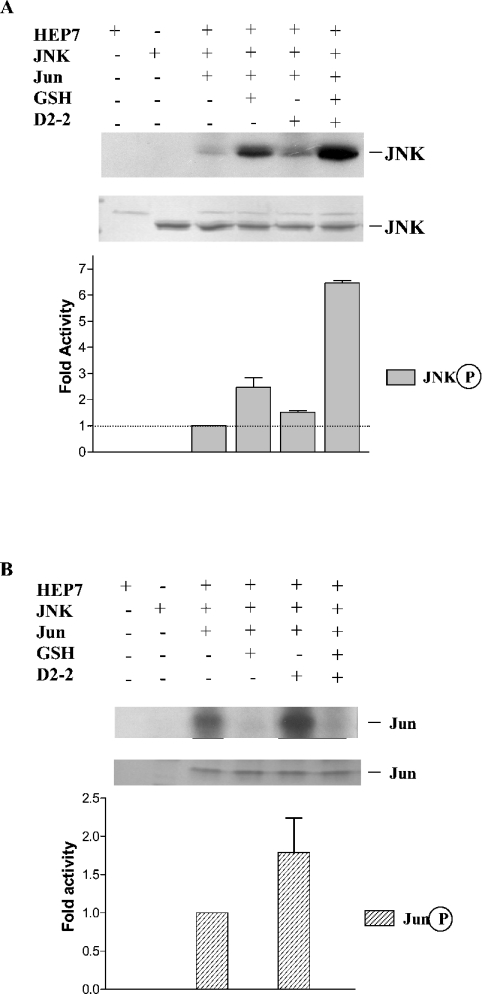
GSH also affected JNK activity by inducing JNK autophosphorylation (Figure 4A), but attenuated Jun phosphorylation (Figure 4B). As shown in the presence of Jun, the autophosphorylation of JNK was decreased; however, the GST and GSH-induced-fit conformational change induced a 3.5-fold increase in JNK autophosphorylation. The results also identify glutathione as a critical molecule directly involved in JNK regulation by controlling JNK substrate selectivity and specificity of interaction. Direct interaction of GSH and kinases has been reported previously for several isoforms of protein kinase C that were shown to be inhibited by GSH [38]. High GSH concentrations have been shown to inhibit the sphingomyelin/ceramide cycle where generation of ceramide leads to cell-cycle arrest and apoptosis [39]. In addition, the depletion of GSH in the cell during oxidative stress exerted negative regulation over protein kinase C isoenzymes [38] as well as the JNK/p38 pathway [4]. GSH has been shown to be a potent inhibitor of JNK activation, suggesting the existence of specific cellular components involved in JNK activation in response to different forms of cellular stress [4]. We suggest GSTs may be one of these components.
Figure 4. Presence of glutathione changed the GST effects on JNK activity.
GST/HEP/JNK/Jun 1–104 in 10:1:2:10 molar ratio were incubated in the presence and absence of 10 mM GSH with 3 μCi of [γ-32P]ATP for 25 min of phosphorylation time at room temperature. (A) The autoradiograph in the upper panel shows JNK phosphorylation in the presence and absence of GSH. The lower panel depicts an SDS/polyacrylamide gel showing the sample loading for JNK. The histogram quantifies in fold activity the GST's effect on JNK phosphorylation. (B) The autoradiograph in the upper panel shows Jun phosphorylation in the presence and absence of GSH. The lower panel depicts an SDS/polyacrylamide gel showing the sample loading for Jun. The histogram quantifies the GST's effects on Jun phosphorylation. Figures shown are representative of at least three independent experiments.
DISCUSSION
In the present study, we characterized the non-enzymic function of GST in the regulation of stress-activated kinase proteins of the JNK pathway as well as the reciprocal regulation of GST by proteins of the JNK pathway. In insects, HEP is a homologue of mammalian MKK7, whereas the single Drosophila JNK or basket protein corresponds to the ten human JNK isoforms with a 61–75% amino acid identity [21]. Furthermore, GSTs in insects currently fall into six classes, with the Delta class being the best studied [29,40,41]. Here, we have concentrated on the study of four spliceforms of a single Delta class GST, GSTD1-1, GSTD2-2, GSTD3-3 and GSTD4-4. Interestingly, the splicing of these GSTs generates products that share the same N-terminal 45 amino acids of the conserved glutathione-binding region [24]. Any differences in the actions of these spliceforms must therefore arise from the C-terminal regions of these proteins and we have previously shown that their substrate specificities, steady-state kinetics with respect to both GSH and CDNB and inhibition kinetics to the pyrethroid insecticide permethrin are very different [24].
Results of the present study have extended our previous studies by demonstrating an interaction between the Dipteran GSTD1-1, GSTD2-2, GSTD3-3 and GSTD4-4 and the JNK pathway components, JNK and HEP. Our inhibition study showed different interactions of these kinases with the GST splice products, particularly as seen with the inhibition of several of the GST spliceforms by JNK (Table 1). We observed, using the standard CDNB assay, that GSTD1-1 showed no inhibition, whereas the remaining GSTs were inhibited by their preincubation with JNK protein. We suggest that the interaction with the kinase could change the GST conformation and this results in different GST enzymic activity.
This is also the first report of the activation of JNK activity by their preincubation with GST. Previously, GSTs have been reported to serve as negative regulators in the JNK pathway, acting either on JNK or ASK1 [15,18]. In the present study, we report that although some alternatively spliced GSTs inhibit JNK activity, other spliceforms may also function as JNK activator proteins. These results again highlight the functional diversity of the GST spliceforms. Moreover, our study shows that the GSTs can associate with the immediate JNK upstream activator kinase in Drosophila, HEP. Hence, GST may play additional roles in the regulation of kinase proteins in the JNK pathway through an, as yet, unknown mechanism.
As a consequence of the different effects of GSTD4-4 on JNK activity, as shown by an increased phosphorylation of c-Jun (Figure 1A) and a decreased phosphorylation of HEP (Figure 1B), we suggest that the GSTs such as GSTD4-4 may contribute to a change in JNK substrate selectivity. Intriguingly, GST could be a pivotal molecule to switch the JNK downstream cascade direction by changing activation of transcriptional machinery components and thereby gene expression. One critical determinant of MAPK specificity and efficacy is the docking motif on the kinase surface, which interacts with the substrate target site. A structural change of JNK after interaction with GST may impact on this region critical for specificity determination [42,43]. Nonetheless, JNK has many functions in controlling cell stress responses, and it is possible that the GST–JNK interaction changes substrate specificities of JNK for other JNK substrates such as activating transcription factor 2 [11], Elk-1 [12] or p53 [44]. The GST–JNK interaction would therefore function as a switch or modulator for the various JNK processes on stimulation by cellular stresses (Figure 5). Owing to the nature of the signalling process, there must be other molecules participating in the regulation of the GST–JNK interaction. Since there are various classes and isoforms of GSTs [1,2] and JNKs [9] that are widely distributed in different tissues, the signalling specificity of GST–JNK interactions may also be controlled by the specific classes and isoforms present in any particular cell type. Previously, it was shown that distinct classes of GSTs played particular roles by interacting with different kinase proteins [15,18]. In the present study, we report that different isoforms of the same class of GST interact with JNK and modulate JNK activity in different ways. The variety of GST isoforms may be one of the keys determining signal specificity and controlling a particular cells biological response.
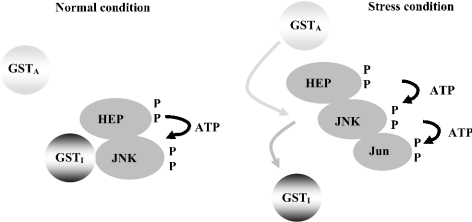
Figure 5. A proposed mechanism of GST regulation of stress kinase proteins through a dissociation/association process.
The different isoforms of GSTs possess different JNK effector properties. GSTI refers to a JNK inhibitor and GSTA represents a JNK activator. The JNK regulation occurs on stress. Left panel: under normal conditions, GSTI inhibits JNK and maintains JNK at basal level activity [15]. Right panel: once stress occurs, a mechanism to dissociate GSTI from JNK comes into effect. GSTA may now associate with JNK or an upstream activating kinase and increase or modulate the kinase cascade response.
The GST–JNK interaction may occur through the C-terminus of JNK, which has been reported as being important for providing direct protein–protein interaction with GSTP1-1 [45]. JNK has also been shown to interact electrostatically with other signalling molecules through a conserved docking motif called the common docking domain [46]. The conserved polar residues within the common docking domain may serve as energetic hot spots, which increase the specific protein–protein interaction [47,48]. In addition, hydrogen bonding and molecular surface shape complementarity are vital criteria that determine protein docking [49]. Thus the variation in surface residues of a GST interacting with a particular JNK may induce distinct conformational changes, yielding functional changes in both proteins. A shift in substrate specificity arising from the association of GST and JNK, in addition to the presence of different GST isoforms generated in the course of natural molecular evolution [50], would be a useful feature for a detoxification mechanism already well known for recognizing diverse substrate compounds.
In summary, the results show that distinct isoforms of GSTs specifically interact with JNK with different effects. Intriguingly, even though the studied GST isoforms are alternatively spliced products sharing >60% amino acid identity, the GST proteins displayed contrary roles in the regulation of JNK. It suggests that the specific isoforms of GSTs present may be important in controlling the final cellular response. The present study provides new insight into the mechanism of GSTs in regulating and conferring specificity of stress kinase proteins in the JNK pathway.
Acknowledgments
We are particularly grateful to Associate Professor Dr W. Chulalakasananukul (Genetics Program, Department of Botany, Chulalongkorn University, Bangkok, Thailand) for donating the D. melanogaster adults used in cloning of the Drosophila HEP, JNK and Jun. This work was supported by the Thailand Research Fund (TRF), a Royal Golden Jubilee Scholarship (R.U.) and Royal Golden Jubilee Grant (R.U.).
References
- 1.Hayes J. D., Pulford D. J. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. CRC Crit. Rev. Biochem. Mol. Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong R. N. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem. Res. Toxicol. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- 3.Sheehan D., Meade G., Foley V. M., Dowd C. A. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilhelm D., Bender K., Knebel A., Angel P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol. Cell. Biol. 1997;17:4792–4800. doi: 10.1128/mcb.17.8.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ketterer B. A bird's eye view of the glutathione transferase field. Chem. Biol. Interact. 2001;138:27–42. doi: 10.1016/s0009-2797(01)00277-0. [DOI] [PubMed] [Google Scholar]
- 6.Dulhunty A., Gage P., Curtis S., Chelvanayagam G., Board P. The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J. Biol. Chem. 2001;276:3319–3323. doi: 10.1074/jbc.M007874200. [DOI] [PubMed] [Google Scholar]
- 7.Fesik S. W. Insights into programmed cell death through structural biology. Cell (Cambridge, Mass.) 2001;103:273–282. doi: 10.1016/s0092-8674(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 8.Kampranis S. C., Damianova R., Atallah M., Toby G., Kondi G., Tsichlis P. N., Makris A. M. A novel plant glutathione S-transferase/peroxidase suppresses Bax lethality in yeast. J. Biol. Chem. 2000;275:29207–29216. doi: 10.1074/jbc.M002359200. [DOI] [PubMed] [Google Scholar]
- 9.Barr R. K., Bogoyevitch M. A. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs) Int. J. Biochem. Cell Biol. 2001;33:1047–1063. doi: 10.1016/s1357-2725(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 10.Hibi M., Lin A., Smeal T., Minden A., Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S., Campbell D., Dérijard B., Davis R. J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 12.Whitmarsh A. J., Shore P., Sharrocks A. D., Davis R. J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 13.Xia C. L., Cowell I. G., Dixon K. H., Pemble S. E., Ketterer B., Taylor J. B. Glutathione transferase π its minimal promoter and downstream cis-acting element. Biochem. Biophys. Res. Commun. 1991;176:233–240. doi: 10.1016/0006-291x(91)90914-s. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama Y., Sagara M., Sato S., Saito Y. Value of glutathione S-transferase p and the oncogene products c-Jun, c-Fos, c-H-Ras, and c-Myc as a prognostic indicator in endometrial carcinomas. Gynecol. Oncol. 1998;68:280–287. doi: 10.1006/gyno.1998.4936. [DOI] [PubMed] [Google Scholar]
- 15.Adler V., Yin Z., Fuchs S. Y., Benezra M., Rosario L., Tew K. D., Pincus M. R., Sardana M., Henderson C. J., Wolf C. R., et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsby R., Kitteringham N. R., Goldring C. E., Lovatt C. A., Chamberlain M., Henderson C. J., Wolf C. R., Park B. K. Increased constitute c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J. Biol. Chem. 2003;278:22243–22249. doi: 10.1074/jbc.M301211200. [DOI] [PubMed] [Google Scholar]
- 17.Yin Z., Ivanov V. N., Habelhah H., Tew K., Ronai Z. Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res. 2000;60:4053–4057. [PubMed] [Google Scholar]
- 18.Cho S.-G., Lee Y. H., Park H.-S., Ryoo K., Kang K. W., Park J., Eom S.-J., Kim M. J., Chang T.-S., Choi S.-Y., et al. Glutathione S-transferase Mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J. Biol. Chem. 2001;276:12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- 19.Stronach B. E., Perrimon N. Stress signaling in Drosophila. Oncogene. 1999;18:6172–6182. doi: 10.1038/sj.onc.1203125. [DOI] [PubMed] [Google Scholar]
- 20.Holland P. M., Suzanne M., Campbell J. S., Noselli S., Cooper J. A. MKK7 is a stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J. Biol. Chem. 1997;272:24994–24998. doi: 10.1074/jbc.272.40.24994. [DOI] [PubMed] [Google Scholar]
- 21.Sluss H. K., Han Z., Barrett T., Davis R. J., Ip Y. T. A JNK signal transduction pathway that mediates morphogenesis and immune response in Drosophila. Genes Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- 22.Zhang K., Chaillet J. R., Perkins L. A., Halazonetis T. D. Drosophila homolog of the mammalian jun oncogene is expressed during embryonic development and activates transcription in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 1990;87:6281–6285. doi: 10.1073/pnas.87.16.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pongjaroenkit S., Jirajaroenrat K., Boonchauy C., Chanama U., Leetachewa S., Prapanthadara L., Ketterman A. J. Genomic organization and putative promotors of highly conserved glutathione S-transferases originating by alternative splicing in Anopheles dirus. Insect Biochem. Mol. Biol. 2001;31:75–85. doi: 10.1016/s0965-1748(00)00107-7. [DOI] [PubMed] [Google Scholar]
- 24.Jirajaroenrat K., Pongjaroenkit S., Krittanai C., Prapanthadara L., Ketterman A. J. Heterologous expression and characterization of alternatively spliced glutathione S-transferases from a single Anopheles gene. Insect Biochem. Mol. Biol. 2001;31:867–875. doi: 10.1016/s0965-1748(01)00032-7. [DOI] [PubMed] [Google Scholar]
- 25.Wolter S., Mushinski J. F., Saboori A. M., Resch K., Kracht M. Inducible expression of a constitutively active mutant of mitogen activated protein kinase kinase 7 specifically activates c-JUN NH2-terminal protein kinase, alters expression of at least nine genes, and inhibits cell proliferation. J. Biol. Chem. 2002;277:3576–3584. doi: 10.1074/jbc.M105800200. [DOI] [PubMed] [Google Scholar]
- 26.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Rowe J. D., Nieves E., Listowsky I. Subunit diversity and tissue distribution of human glutathione S-transferase: interpretations based on electrospray ionization-MS and peptide sequence-specific antisera. Biochem. J. 1997;325:481–486. doi: 10.1042/bj3250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrell J. E., Jr Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. TIBS. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y., Ortelli F., Rossiter L. C., Hemingway J., Ranson H. The Anopheles gambiae glutathione transferase supergene family: annotation, phylogeny and expression profiles. BMC Genomics. 2003;4:35–50. doi: 10.1186/1471-2164-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurtdinov R. N., Artamonova I. I., Mironov A. A., Gelfand M. S. Low conservation of alternative splicing patterns in the human and mouse genomes. Hum. Mol. Genet. 2003;12:1313–1320. doi: 10.1093/hmg/ddg137. [DOI] [PubMed] [Google Scholar]
- 31.Pearson G., Robinson F., Gibson T. B., Xu B., Karandikar M., Berman K., Cobb M. H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 32.Allardyce C. S., McDonagh P. D., Lian L.-Y., Wolf C. R., Roberts G. C. K. The role of tyrosine-9 and the C-terminal helix in the catalytic mechanism of Alpha-class glutathione S-transferases. Biochem. J. 1999;343:525–531. [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsson L. O., Gustafsson A., Mannervik B. Redesign of substrate-selectivity determining modules of glutathione transferase A1-1 installs high catalytic efficiency with toxic alkenal products of lipid peroxidation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9408–9412. doi: 10.1073/pnas.150084897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal A., Gu Y., Pan S.-S., Ji X., Singh S. V. C-terminal region amino acid substitutions contribute to catalytic differences between murine class alpha glutathione transferases mGSTA1-1 and mGSTA2-2 toward anti-diol epoxide isomers of benzo[c]phenanthrene. Biochemistry. 2001;40:7047–7053. doi: 10.1021/bi010363r. [DOI] [PubMed] [Google Scholar]
- 35.Nuccetelli M., Mazzetti A. P., Rossjohn J., Parker M. W., Board P., Caccuri A. M., Federici G., Ricci G., Lo Bello M. Shifting substrate specificity of human glutathione transferase (from class pi to class alpha) by a single point mutation. Biochem. Biophys. Res. Commun. 1998;252:184–189. doi: 10.1006/bbrc.1998.9575. [DOI] [PubMed] [Google Scholar]
- 36.Oakley A. J., Rossjohn J., Lo Bello M., Caccuri A. M., Federici G., Parker M. W. The three-dimensional structure of the human pi class glutathione S-transferase P1-1 in complex with the inhibitor ethacrynic acid and its glutathione conjugate. Biochemistry. 1997;36:576–585. doi: 10.1021/bi962316i. [DOI] [PubMed] [Google Scholar]
- 37.Oakley A. J., Harnnoi T., Udomsinprasert R., Jirajaroenrat K., Ketterman A. J., Wilce M. C. J. The crystal structures of glutathione S-transferases isozymes 1-3 and 1-4 from Anopheles dirus species B. Protein Sci. 2001;10:2176–2185. doi: 10.1110/ps.ps.21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward N. E., Pierce D. S., Chung S. E., Gravitt K. R., O'Brian C. A. Irreversible inactivation of protein kinase C by glutathione. J. Biol. Chem. 1998;273:12558–12566. doi: 10.1074/jbc.273.20.12558. [DOI] [PubMed] [Google Scholar]
- 39.Liu B., Hannun Y. A. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J. Biol. Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- 40.Fournier D., Bride J.-M., Poirié M., Bergé J.-B., Plapp F. W. Insect glutathione S-transferases. Biochemical characteristics of the major forms from houseflies susceptible and resistant to insecticides. J. Biol. Chem. 1992;267:1840–1845. [PubMed] [Google Scholar]
- 41.Ranson H., Claudianos C., Ortelli F., Abgrall C., Hemingway J., Sharakhova M. V., Unger M., Collins F. H., Feyereisen R. Evolution of supergene families associated with insecticide resistance. Science. 2002;298:179–181. doi: 10.1126/science.1076781. [DOI] [PubMed] [Google Scholar]
- 42.Sharrocks A. D., Yang S.-H., Galanis A. Docking domains and substrate specificity determination for MAP kinases. TIBS. 2000;25:448–453. doi: 10.1016/s0968-0004(00)01627-3. [DOI] [PubMed] [Google Scholar]
- 43.Tanoue T., Maeda R., Adachi M., Nishida E. Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. EMBO J. 2001;20:466–479. doi: 10.1093/emboj/20.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adler V., Pincus M. R., Minamoto T., Fuchs S. Y., Bluth M. J., Brandt-Rauf P. W., Friedman F. K., Robinson R. C., Chen J. M., Wang X. W., et al. Conformation-dependent phosphorylation of p53. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1686–1691. doi: 10.1073/pnas.94.5.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang T., Arifoglu P., Ronai Z., Tew K. D. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J. Biol. Chem. 2001;276:20999–21003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- 46.Tanoue T., Adachi M., Moriguchi T., Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 47.Ma B., Elkayam T., Wolfson H., Nussinov R. Protein-protein interactions: structurally conserved residues distinguish between binding sites and exposed protein surfaces. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5772–5777. doi: 10.1073/pnas.1030237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Z., Ma B., Wolfson H., Nussinov R. Conservation of polar residues as hot spots at protein interfaces. Proteins. 2000;39:331–342. [PubMed] [Google Scholar]
- 49.Meyer M., Wilson P., Schomburg D. Hydrogen bonding and molecular surface shape complementarity as a basis for protein docking. J. Mol. Biol. 1996;264:199–210. doi: 10.1006/jmbi.1996.0634. [DOI] [PubMed] [Google Scholar]
- 50.Hansson L. O., Bolton-Grob R., Massoud T., Mannervik B. Evolution of differential substrate specificities in mu class glutathione transferases probed by DNA shuffling. J. Mol. Biol. 1999;287:265–276. doi: 10.1006/jmbi.1999.2607. [DOI] [PubMed] [Google Scholar]