Abstract
Taking into account a previous report of an unidentified enzyme from macrophages acting as a kininase, the ability of cysteine proteases to degrade kinins has been investigated. Wild-type fibroblast lysates from mice, by contrast with cathepsin K-deficient lysates, hydrolysed BK (bradykinin), and released two metabolites, BK-(1–4) and BK-(5–9). Cathepsin K, but not cathepsins B, H, L and S, cleaved kinins at the Gly4–Phe5 bond and the bradykinin-mimicking substrate Abz (o-aminobenzoic acid)-RPPGFSPFR-3-NO2-Tyr (3-nitrotyrosine) more efficiently (pH 6.0: kcat/Km=12500 mM−1·s−1; pH 7.4: kcat/Km=6930 mM−1·s−1) than angiotensin-converting enzyme hydrolysed BK. Conversely Abz-RPPGFSPFR-3-NO2-Tyr was not cleaved by the Y67L (Tyr67→Leu)/L205A (Leu205→Ala) cathepsin K mutant, indicating that kinin degradation mostly depends on the S2 substrate specificity. Kininase activity was further evaluated on bronchial smooth muscles. BK, but not its metabolites BK(1-4) and BK(5-9), induced a dose-dependent contraction, which was abolished by Hoe140, a B2-type receptor antagonist. Cathepsin K impaired BK-dependent contraction of normal and chronic hypoxic rats, whereas cathepsins B and L did not. Taking together vasoactive properties of kinins and the potency of cathepsin K to modulate BK-dependent contraction of smooth muscles, the present data support the notion that cathepsin K may act as a kininase, a unique property among mammalian cysteine proteases.
Keywords: cathepsin, cysteine protease, kinin, kininase, lung inflammation
Abbreviations: Ach, acetylcholine; AHR, airway hyper-responsiveness; Abz, o-aminobenzoic acid; ACE, angiotensin-converting enzyme; AMC, 7-amino-4-methylcoumarin hydrochloride; BAL, bronchoalveolar lavage; BK, bradykinin; p-CMPSA, p-chloromercuriphenylsulphonic acid; CP, cysteine protease; DTT, DL-dithiothreitol; E-64, trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane; EDDnp, N-(2,4-dinitrophenyl)ethylenediamine; ESI-Q-TOF, electrospray ionization–quadrupole–time-of-flight; Fmoc, fluoren-9-ylmethoxycarbonyl; hK1, human tissue kallikrein; Lys-BK, kallidin; MGTA, DL-2-mercaptomethyl-3-guanidinoethylthiopropanoic acid; 3-NO2-Tyr, 3-nitrotyrosine; PSS, physiological saline solution; TFA, trifluoroacetic acid; Z, benzyloxycarbonyl
INTRODUCTION
Owing to the strong proinflammatory properties of kinins resulting from plasma extravasation, activation of mast cells, fibroblasts and macrophages, and the release of inflammatory mediators (NO, prostaglandins, leukotrienes and cytokines), kinins are believed to play an important role in a variety of lung inflammatory diseases [1]. Asthma, which is characterized by repeated episodes of airflow obstruction [2], and chronic obstructive pulmonary disease display many similarities, including bronchoconstriction and AHR (airway hyper-responsiveness) [3]. Kinins [e.g. BK (bradykinin), and related kinins such as lysyl-bradykinin, also called kallidin], whose pharmacological effects are mediated by BK receptors, are peptide hormones that participate in the maintenance of cardiovascular homoeostasis (for a review, see [4]). It has been shown that, after an inflammatory challenge, lung kinin-releasing activity is increased in nasal washings and bronchoalveolar fluids [5]. Furthermore BK, which induces bronchoconstriction and AHR in asthmatic patients, has been proposed as a mediator of asthma [6]. Conversely, blockade of BK receptors by specific antagonists significantly reduced bronchoconstriction and AHR of patients suffering from perennial rhinitis and asthma [1].
In inflamed lung tissues, enhanced protein breakdown occurs as a result of protease/antiprotease imbalance, and may cause severe damage [7,8]. This process is accompanied by the release, from macrophages at the site of injury, of thiol-dependent cathepsins that participate in the spread of inflammation [9,10]. Furthermore, the activity of CPs (cysteine proteases) is favoured by the acidification of the pericellular environment of macrophages, via increased expression of an H+-ATPase pump [11,12]. Besides their housekeeping tasks (intracellular protein degradation and turnover) within the endosomal/lysosomal system, mammalian cathepsins B, K, L and S fulfil more specific physiological functions in MHC-II antigen presentation or in prohormone processing [13]. They are also involved in pathological processes such as Alzheimer's disease, tumour invasion, muscular dystrophy, osteoporosis or rheumatoid arthritis (for a review, see [14]). Among mammalian CPs, cathepsin K, which is predominantly expressed in osteoclasts, exhibits a unique chondroitin sulphate-dependent collagenolytic activity [15,16]. Cathepsin K has been also found in the serum of Gaucher patients, in lung epithelial cells, in cancer prostate cells, in macrophages, and has been proposed as a marker of inflammation [17–20]. During our preliminary efforts to characterize proteolytic activities of CPs from human inflammatory bronchoalveolar lavage fluids (C. Serveau-Avesque, M. Ferrer-Di Martino, and G. Lalmanach, unpublished work), we have observed that cathepsin K may hydrolyse BK at acidic pH [21]. On the other hand, p-CMPSA (p-chloromercuriphenylsulphonic acid), a thiol-group-blocking reagent, significantly delayed the breakdown of BK by macrophages, by inactivation of an unidentified kininase [22], that is not related to well-documented kininases such as neutral endopeptidase, carboxypeptidase N or ACE (angiotensin-converting enzyme) (for reviews, see [23,24]). In addition, evidence has been provided for the presence of potentially new enzymes and mechanisms of BK degradation [25]. Taken together, these data have led us to investigate the capacity of cathepsin K to act as a kininase. According to this hypothesis, kinetics studies using kinins and/or synthetic kinin-mimicking peptides were performed in order to characterize the kinin-degrading properties of cathepsin K. Moreover, the ability of cathepsin K to modulate the vasoactive properties of BK and its subsequent effects on BK receptors of isolated rat bronchial smooth muscles was investigated. The present report supports our hypothesis that cathepsin K, by impairing the BK-B2-receptor-mediated contraction phase, may be a new kininase of physiological relevance.
EXPERIMENTAL
Materials
E-64 [trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane], PMSF, pepstatin, chondroitin 4-sulphate, Hoe140 (a B2-type receptor antagonist) and des-Arg10-Hoe140 were obtained from Sigma–Aldrich (St Quentin le Fallavier, France). Captopril and Mergepta [MGTA (DL-2-mercaptomethyl-3-guanidinoethylthiopropanoic acid)] were supplied by Calbiochem (VWR International, Pessac, France). Z (benzyloxycarbonyl)-Phe-Arg-AMC (7-amino-4-methylcoumarin hydrochloride) and DTT (dithiothreitol) were purchased from Bachem (Weil am Rhein, Germany). All other reagents were of analytical grade.
Enzymes
Human cathepsins B, L and S were supplied by Calbiochem. Wild-type human cathepsin K and the Y67L (Tyr67→Leu)/L205A (Leu205→Ala) cathepsin K mutant were expressed in Pichia pastoris as described elsewhere [26]. Cathepsins were activated in 0.1 M phosphate buffer, pH 6.0, containing 2 mM DTT and 1 mM EDTA, for 5 min at 37 °C prior to kinetic measurements with a Kontron SFM 25 spectrofluorimeter. Their active sites were titrated with E-64 [27], using Z-Phe-Arg-AMC as substrate (excitation wavelength 350 nm; emission wavelength 460 nm). Human tissue kallikrein was obtained from Sigma–Aldrich, whereas bovine pancreatic trypsin was purchased from Boehringer (Roche Molecular Biochemicals, Mannheim, Germany). The buffer for trypsin was 0.1 M Tris/HCl. pH 8.5, containing 0.15 M NaCl, and that for hK1 (human tissue kallikrein) was 50 mM Tris/HCl, pH 8.3, containing 1 mM EDTA. Trypsin and hK1 were titrated as reported elsewhere [28].
Peptides
All Fmoc (fluoren-9-ylmethoxycarbonyl)-protected amino acids were of the L configuration, and were purchased from Neosystem (Strasbourg, France), Novabiochem (VWR international) and Advanced Chemtech (Cambridge, U.K.). The intramolecularly quenched fluorogenic substrate Abz (o-aminobenzoic acid)-RPPGFSPFR-(3-NO2-Tyr), also called Abz-BK-(3-NO2-Tyr), was prepared by Fmoc chemistry on an automated solid-phase peptide synthesizer (The Pioneer; Applied Biosystems, Warrington, U.K.), using a PAL-PEG-PS resin (Applied Biosystems). After removal of the sidechain protecting groups and cleavage from the resin, the peptidyl amide Abz-RPPGFSPFR-3-NO2-Tyr was purified by semipreparative reverse-phase chromatography (Vydac C18 218TPS1 column), using a 35 min linear (0–60%) gradient of acetonitrile in 0.1% TFA (trifluoroacetic acid). Finally, Abz-RPPGFSPFR-3-NO2-Tyr was checked for homogeneity by analytical reverse-phase HPLC (Brownlee C18 OD 300 column), using the elution conditions indicated above, and their molecular masses checked by MALDI-TOF (matrix-assisted laser-desorption ionization–time-of-flight) (Biflex III; Brüker, Wissembourg, France) or ESI-Q-TOF (electrospray ionization–quadrupole–time-of-flight) MS (Micromass, Birmingham, U.K.). BK and des-Arg9-BK were obtained from Sigma–Aldrich. Kallidin (Lys-BK) was from Advanced ChemTech (Louisville, KY, U.S.A.). The BK-derived pentapeptidylamide BK-(5–9) (Phe-Ser-Pro-Phe-Arg) was prepared as decribed in [21]. BK-(1–4) (Arg-Pro-Pro-Gly) was made by a similar procedure, using tetramethylfluoroformamidium hexafluorophosphate as activator and a Novasyn TGR resin (Novabiochem).
Kininase activity of cathepsin K
BK, Lys-BK and des-Arg9-BK were incubated with cathepsin K (enzyme/substrate molar ratio=1:2500) for 0–120 min at 37 °C in 0.1 M phosphate buffer, pH 6.0, containing 2 mM DTT and 1 mM EDTA and the products analysed by reverse-phase chromatography [C18 Brownlee ODS-032 column; 45-min linear (0–60%) gradient of acetonitrile in 0.1% trifluoroacetic acid]. Kinin metabolites were quantified by running the ChromQuest Chromatography Workstation (ThermoFinnigan, les Ulis, France), and were identified by N-terminal peptide sequencing (Procise sequencer; Applied Biosystems). Similar experiments were performed with cathepsins B, H, L, S and hK1. The same procedure was repeated in 0.1 M phosphate buffer, pH 7.4, containing 2 mM DTT and 1 mM EDTA.
The second-order rate constants for the hydrolysis of 0.5 μM Abz-RPPGFSPFR-3-NO2-Tyr by cathepsins B, L, S and K by the Y67L/L205A mutant, by trypsin and by hK1, were determined under pseudo-first-order conditions (excitation wavelength 320 nm; emission wavelength 420 nm). Calibration was performed as described elsewhere [29]. Triplicate assays (except for cathepsin K, n=10) were performed in the presence of cathepsins K (1–4 nM), B (30 nM), S (20 nM), L (20 nM), Y67L/L205A cathepsin K (20 nM), trypsin (20 nM) or hK1 (20 nM) in their respective activity buffer at 37 °C. Kinetic data were determined using the Enzfitter software (Biosoft, Cambridge, U.K.) and are reported as means±S.D. Michaelis (Km) and catalytic (kcat) constants for the hydrolysis of Abz-BK-3-NO2-Tyr (0.05–2 μM) by cathepsin K were determined by non-linear regression analysis.
Cathepsin K (3 nM) was incubated with Abz-BK-3-NO2-Tyr (8 μM) for 3 min at 37 °C in its assay buffer (final volume 200 μl), and the reaction stopped by adding 800 μl of ethanol. After removal of the precipitate, the supernatant containing the native peptide and/or its proteolytic fragments was evaporated to dryness and re-dissolved in 0.1% TFA. An aliquot of each sample was fractionated by reverse-phase HPLC on a C18 OD 300 column (Brownlee) using a 30 min linear (0–60%) gradient of acetonitrile in 0.1% TFA at a flow rate of 0.3 ml/min. Proteolysis products were analysed by running the ChromQuest Chromatography Workstation, and cleavage sites were located by N-terminal sequencing. The same protocol was repeated for hK1(70 nM), trypsin (100 nM) and cathepsin L (70 nM).
Cathepsin K activity assays on cultured fibroblasts
Cathepsin K knockout (cat K−/−) mice (C57BL/6J background) were generated by targeted disruption of the cathepsin K gene [30]. Skin fibroblasts from wild-type and cathepsin K-deficient mice were cultured as previously described [31]. Finally, cell lysates were obtained in 100 mM sodium acetate buffer, pH 5.5, 2.5 mM DTT, 2.5 mM EDTA, 10 μM PMSF and 10 μM pepstatin A. After centrifugation (13000 g for 10 min at 4 °C), the protein concentrations in the supernatants of wild-type and knockout cells were determined using the Bradford kit (Bio-Rad, Hercules, CA, U.S.A.). Western-blot analysis was performed using a rabbit polyclonal antibody directed against mouse cathepsin K [31].
After activation for 2 min in 0.1 M phosphate buffer, pH 6.0, containing 2 mM DTT, 1 mM EDTA, 0.01% Brij 35, 0.04 mM pepstatin A and 0.4 mM PMSF, supernatants (5 μg of total protein/assay) were incubated with BK (16.6 μM) for 6 h at 30 °C. After removal of proteins by ethanol, peptidyl fractions containing native BK and/or its hydrolysis products were separated by reverse-phase chromatography [C18 Merck LiChroCart Cartridge; 20 min linear (0–60%) gradient of acetonitrile in 0.1% TFA], and identified by N-terminal sequencing (Procise sequencer) and ESI-Q-TOF MS. The same procedure was repeated in the presence of 10 μM E-64.
Tissue preparation from normal and hypoxic rats
All animal experiments were conducted according to the ethical standards of the Ministère Français de l'Agriculture. On the day of experiments, Wistar rats were killed with sodium pentobarbital (60 mg/kg intraperitoneally). The thoraxes were opened and lungs quickly removed and immerged in cold (4 °C) physiological saline solution (PSS) containing 138.6 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 0.33 mM NaH2PO4, 10 mM Hepes and 11 mM glucose (the pH was adjusted to 7.4 using NaOH). The bronchi were then rapidly isolated and gently cleaned of parenchyma and adhering connective tissue under a dissection microscope. The bronchi were cut in rings 3 mm long and the epithelium was kept intact. Conversely a chronically hypoxic rat model was developed as previously described [32]. To summarize, the rats were set under chronic-hypoxia conditions by decreasing the atmospheric pressure to 50.5 kPa (pO2=10 kPa) for 21 days. Bronchi were isolated and prepared for isometric measurements as described above.
Recording of mechanical activity
Mechanical responses were measured as previously described [33]. Briefly, isolated rings were attached between a rigid support and the arm of a force transducer (Pioden Controls Ltd., Canterbury, U.K.) by slipping the ring over two stainless-steel wires. One of the wires was anchored to the rigid support and the other was connected to an isometric transducer. The rings were placed in an organ chamber and bubbled at a constant flow rate with a O2/N2 (21:79) gas mixture, then were set at optimal length by equilibration against a passive load. After undergoing a stress relaxation equilibration period of 60 min (to reach the residual passive tension) the isometric contractions were recorded. The rings were precontracted with Ach (acetylcholine) at 10−5 M (concentration inducing the maximal contraction) and when the contractile response reached a stable tension, kinin solutions, that is BK, BK-(1–4) and BK-(5–9), were applied (0.001 nM–0.01 mM). For some experiments, a cumulative concentration-response for BK was determined by increasing concentration from 10−12 to 10−5 M. Contraction amplitudes were expressed in milligrams and were measured from the resting tension. All the data were collected with a computerized data-acquisition system using Genie software (Advantech, Sunnyvale, CA, U.S.A.).
Analysis and statistics
Results are expressed as means±S.E.M. The contractions are expressed as percentages of Ach-induced contraction. All the analysis was done by using Origin 6 software (Microcal Software, Northampton, MA, U.S.A.). Statistical analysis was done using one-way factor ANOVA followed by Student's t test. Differences were considered significant when P<0.05. Statistical analysis was done using Minitab software (Minitab Inc., State College, PA, U.S.A.). The number of experiments (n) refers to the number of rings.
RESULTS AND DISCUSSION
Specific cleavage of BK by cathepsin K
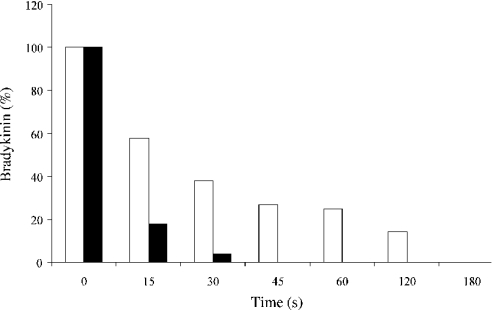
In our efforts to characterize proteolytic activities of CPs from human inflammatory BAL (bronchoalveolar lavage) fluids, we have explored the ability of cathepsin L-like enzymes to participate in the production of kinins [21,34], and observed that cathepsin K may hydrolyse BK. In the present study, in-depth analysis of the putative kininase activity of cathepsin K was performed. BK, Lys-BK and des-Arg9-BK were rapidly degraded by cathepsin K under mildly acidic conditions (pH 6.0) and at pH 7.4 (Figure 1), leading to the release of two stable products: BK-(1–4) (Arg1-Pro-Pro-Gly4) and BK-(5–9) (Phe5-Ser-Pro-Phe-Arg9) (enzyme/substrate molar ratio of 1:2500), in agreement with its pH-dependent activity profile. Interestingly neutral endopeptidase degrades BK at the same cleavage site [24]. By contrast, BK, Lys-BK and des-Arg9-BK remained uncleaved and stable upon incubation with cathepsins B, L, S and H when subjected to reverse-phase-chromatography analysis (results not shown).
Figure 1. Hydrolysis of BK by cathepsin K.
Human cathepsin K was incubated with BK (enzyme/substrate molar ratio 1:2500) at 37 °C, in 0.1 M phosphate buffer, pH 6.0, containing 2 mM DTT and 1 mM EDTA for periods of 0–5 min (black bar). The same procedure was repeated in 0.1 M phosphate buffer, pH 7.4, containing 2 mM DTT and 1 mM EDTA (white bar). Hydrolysis products were further fractionated by reverse-phase HPLC (C18 Brownlee ODS-032 column) (see the Experimental section for details). For the sake of clarity, the residual amount of BK was normalized.
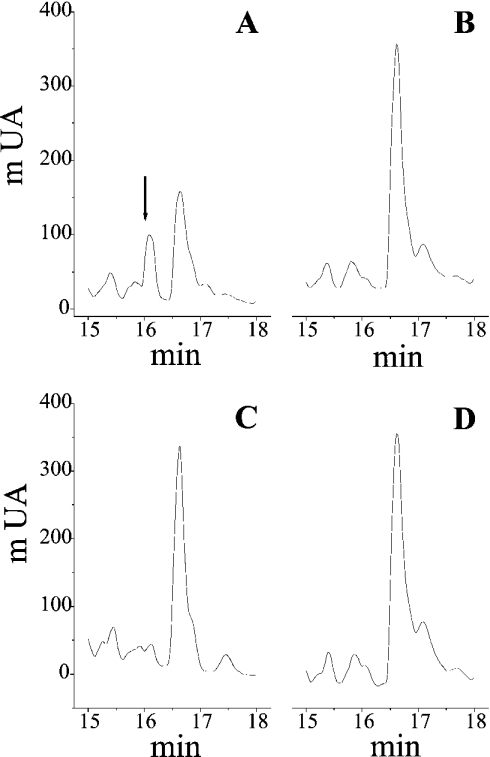
Beside its major well-documented expression in osteoclasts [13], the presence of both procathepsin K and its mature active form has been reported in synovial and skin fibroblasts, as shown by Western-blot analysis of cell lysates from wild-type and cathepsin K-deficient (cat K−/−) mice [31]. After 6 h incubation of cell extracts from skin fibroblasts with exogenous BK (16.6 μM) in the presence of a thiol-reducing reagent (2 mM DTT) and protease inhibitors (1 mM EDTA/400 μM PMSF/40 μM pepstatin A), peptidyl fractions were separated by reverse-phase HPLC and analysed by N-terminal sequencing and ESI-Q-TOF MS (Figure 2). Wild-type fibroblast lysates, but not cathepsin K-deficient lysates, hydrolysed BK and released a major proteolysis product whose retention time and molecular mass (experimental molecular mass 653.33 Da) corresponded to BK-(5–9) (theoretical molecular mass 653.34 Da). By contrast, addition of E-64 to wild-type lysates totally abolished the degradation of BK, suggesting that the kininase activity is indeed catalysed by cathepsin K.
Figure 2. Selective hydrolysis of BK by cathepsin K from skin fibroblasts.
Fibroblast lysates from wild-type and cathepsin K-deficient (cat K−/−) mice (C57BL/6J background) were prepared as described elsewhere [31]. After activation for 2 min in 0.1 M phosphate buffer, pH 6.0, containing 2 mM DTT and 0.01% Brij 35, and in presence of 1 mM EDTA, 0.04 mM pepstatin A and 0.4 mM PMSF, supernatants from fibroblast lysates (5 μg total protein/assay) were incubated with BK (0.16 μM) for 6 h at 30 °C. Native BK and/or its hydrolysis products were separated by reverse-phase HPLC, prior identification by N-terminal sequencing and ESI-Q-TOF MS (see the Experimental section). The major hydrolysis product, BK-(5–9), is identified by an arrow. (A) Wild-type lysates; (B) wild-type lysates+E-64 (10 μM); (C) cat K−/− lysates; (D) cat K−/− lysates+E-64 (10 μM). Abbreviation: mUA, milli-units of absorbance at 220 nm.
Enzymic activity on a fluorescent BK-mimicking peptide
We further analysed and quantified BK metabolism by cathepsin K using the intramolecularly quenched fluorogenic BK-derived peptidylamide Abz-BK-3-NO2-Tyr, consisting of the nonamer BK moiety flanked by a fluorescent N-terminal donor group (Abz) and a C-terminal quencher (3-NO2-Tyr). The second-order rate constants were measured under pseudo-first-order conditions for all enzymes assayed. In addition, Km and kcat values were determined by non-linear regression analysis, and the specificity constant (kcat/Km) deduced from individual Km and kcat values for cathepsin K. Abz-BK-3-NO2-Tyr was very efficiently hydrolysed by recombinant human cathepsin K with a second-order rate of hydrolysis of 12500 mM−1·s−1 (Km=0.4 μM, kcat=5 s−1). Similar rate constants were obtained under pseudo-first-order reaction conditions (kcat/Km=10370 mM−1·s−1; Table 1). Surprisingly, a significantly lower turnover rate by cathepsin K for a similar BK-derived peptide [Abz-RPPGFSPFR-EDDnp, where EDDnp is N-(2,4-dinitrophenyl)ethylenediamine] was recently reported (Km=1.7 μM, kcat=0.1 s−1 and kcat/Km=62.8 mM−1·s−1) [35]. Both substrates are only distinguished by their C-terminal quenching groups, 3-NO2-Tyr versus EDDnp. Presently it can only be speculated whether the observed differences in the rate constants are related to the quenching groups or variation in the assay conditions. Kinetics data support the notion that cathepsin K specificity for BK is comparable with that of angiotensin I-converting enzyme, which inactivates BK by the sequential removal of two C-terminal dipeptides [36]. Furthermore, cathepsin K was still highly efficient at pH 7.4 (kcat/Km=6930 mM−1·s−1). Similar second-order rate constants (pH 6.0: kcat/Km=10600 mM−1·s−1; pH 7.4: kcat/Km=6530 mM−1·s−1) were obtained in the presence of chondroitin 4-sulphate, a major glycosaminoglycan of the extracellular matrix. While chondroitin 4-sulphate is required for the collagenolytic activity of cathepsin K, present data indicate that its presence is not essential for BK hydrolysis, as previously reported for hydrolysis of small peptide substrates [37]. According to N-terminal sequencing results, cleavage occurred at the Gly4–Phe5 bond, as observed with native BK. This is in agreement with the substrate specificity of cathepsin K, since proline and glycine are favourable residues at the P2 and P1 positions respectively [31,35]. On the other hand, cathepsins B, L and S, as well as hK1, only weakly hydrolysed Abz-BK-3-NO2-Tyr, with approximately three orders of magnitude lower efficiency (Table 1). Accordingly, native BK is not a physiological target for cathepsins B, L and S, and hK1 [4]. Analysis of the hydrolysis pattern of Abz-BK-3-NO2-Tyr by hK1 and cathepsin L confirmed the absence of kininase activity, since the cleavage site was located at the amide bond between the C-terminal BK residue and the additional C-terminal fluorescence quencher, i.e. between Arg9 and (3-NO2-Tyr). This non-physiological cleavage site after Phe8-Arg9 is not surprising, since it corresponds to one of the preferred pairs of residues accommodated at the S2 and S1 subsites in trypsin-like enzymes as well as papain-like enzymes [38]. The Y67L/L205A cathepsin K mutant, which displays a S2 cathepsin L-like specificity [26], did not cleave at the Gly4–Phe5 bond but poorly hydrolysed Abz-BK-3-NO2-Tyr (kcat/Km=5 mM−1·s−1) at the Arg9–3-NO2-Tyr bond, indicating that the kininase activity of cathepsin K critically depends on P2–S2 interactions. Furthermore, cathepsin K hydrolysed Abz-BK-3-NO2-Tyr (kcat/Km=742±50 mM−1·s−1) with efficiency in PSS, a reducing-agent-free isotonic medium used to record isometric responses of isolated bronchial rings (see the next paragraph), supporting the notion that cathepsin K has the potential to degrade kinins extracellularly.
Table 1. Kinetic parameters for the hydrolysis of Abz-RPPGFSPFR-3-NO2-Tyr.
Experiments were carried out as described in the Experimental section. Kinetic data were determined using Enzfitter software (Biosoft, Cambridge, U.K.) and are reported as means±S.D. (triplicate assays; except for cathepsin K, n=10). The Michaelis constant (Km) and the catalytic constant (kcat) values were determined by non-linear regression analysis.
Effects of cathepsin K on BK-mediated contraction of bronchial smooth muscles
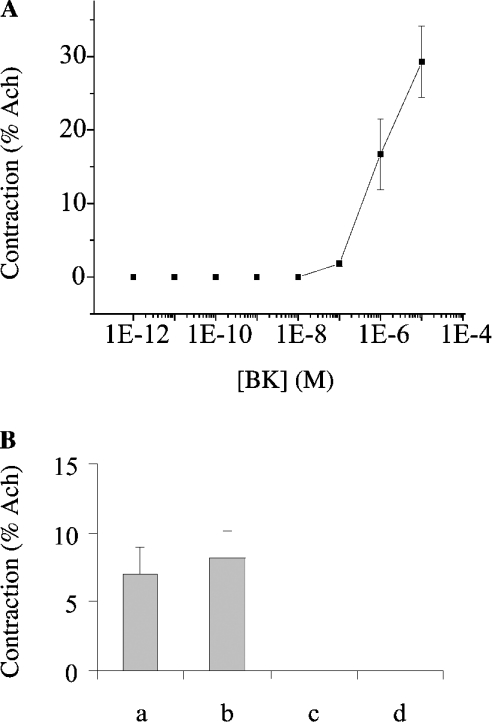
Briefly, contractions of freshly isolated bronchial smooth-muscle rings, placed in an organ chamber filled with PSS and bubbled at a constant flow rate with an O2/N2 (21:79) gas mixture, were measured by using an isometric transducer. After precontraction with ACh (10−5 M) and stabilization of the contractile response, BK, BK-(1–4) or BK-(5–9) was added (0.001 nM–0.01 mM). In contrast with its metabolites [BK-(1–4) and BK-(5–9)], BK (≥10 nM) induced a dose-dependent contractile response (Figure 3A). A similar BK-dependent contraction was observed in the presence of MGTA and captopril (inhibitors of carboxypeptidase M and ACE respectively), indicating that endogenous kininases [39] did not affect the contractile response of the bronchial smooth muscles under our experimental conditions. Moreover, the BK-dependent response was fully abolished in the presence of Hoe140 (1 μM), but not des-Arg10-Hoe140 (1 μM) (Figure 3B), indicating that bronchial smooth-muscle contraction is mediated by constitutive BK B2-type receptors.
Figure 3. Effect of BK on bronchial smooth muscles from normal rat.
(A) The dose–response curve was obtained by increasing concentrations of BK. After the initial administration of Ach (10−5 M), BK (10−12 M–10−5 M) was added as described in the Experimental section. (B) Effect of antagonists on BK-mediated contraction. Rings of bronchial smooth muscles were pretreated in the presence of des-Arg10-Hoe140 (1 μM), a BK B1 receptor antagonist (b, n=8), Hoe140 (1 μM), a BK B2 receptor antagonist (c, n=4) or with both antagonists (d, n=7), prior to the addition of 0.5 μM BK. The responses were compared with the control condition (a, n=19). Antagonists were applied 20 min prior to the addition of BK. Results are means±S.E.M. Contractions are expressed as percentage of the Ach response.
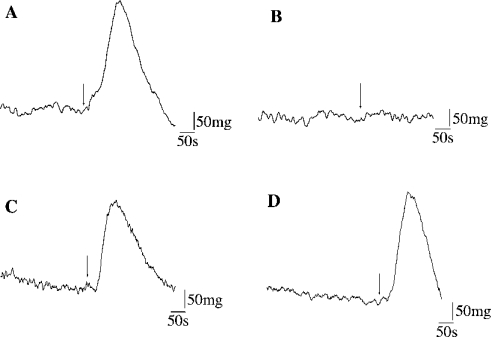
Cathepsin K (1 nM) was added to BK (0.5 μM), and the reaction mixture (40 μl) was immediately applied to the PSS bath solution of the organ chamber (4 ml). Cathepsin K totally abolished contraction of Ach-precontracted rings (Figure 4A), while treatment with E-64 fully restored the BK-induced contractile response (Figure 4B). On the other hand, cathepsins B and L did not impair BK-dependent contraction (Figures 4C and 4D). Removal of an aliquot and analysis of the corresponding peptide supernatant by reverse-phase HPLC confirmed that the loss of contraction was associated with BK hydrolysis and subsequent release of BK-(1–4) and BK-(5–9). This confirms that the ability of cathepsin K to modulate the activity of B2-receptors depends on its proteolytic activity. The same response was observed in presence of MGTA and captopril, indicating that the kininase activity was not related to endogenous lung carboxypeptidase M and ACE respectively [39]. Along with the kinetics data showing that cathepsin K hydrolysed efficiently Abz-BK-3-NO2-Tyr in the absence of reducing reagent (kcat/Km=742±50 mM−1·s−1), the present results confirmed the unique potency of cathepsin K among mammalian CPs to act as a physiological kininase.
Figure 4. Effect of cathepsins K, B, and L on BK-mediated transient contraction on normal bronchial smooth-muscle rings.
Original representative traces showing the effect of cathepsins on BK-dependent contractions are reported: (A) E-64-treated cathepsin K (1 nM); (B) cathepsin K (1 nM); (C) cathepsin B (1 nM); and (D) cathepsin L (1 nM). BK (0.5 μM) was mixed with cathepsins (enzyme/substrate molar ratio 1:500) and immediately added (arrow) to the organ bath solution containing PSS.
Cathepsin K-impaired BK-mediated contraction of hypoxic rats
Asthma and chronic obstructive pulmonary disease are associated with an inflammation, a hyper-responsiveness and a remodelling of the airways. Taking into account that BK induces bronchoconstriction and AHR in asthmatic patients [6], while BK antagonists reduce them significantly [1], it could be hypothesized that cathepsin K may exert a positive effect during asthma by inactivating proinflammatory kinins. We examined bronchial smooth muscles of chronically hypoxic rats [that is, 3 weeks in hypoxia; pO2=75 mmHg (1 mmHg≈133.3 Pa)], in which it was demonstrated that hypoxia, a condition often associated with chronic obstructive pulmonary disease, increased airway sensitivity [40,41]. As observed with normoxic rats, BK induced a contraction that was specifically impaired by a B2-type receptor antagonist (Hoe140, 1 μM). In the presence of cathepsin K, but not cathepsins B, L or E-64-inactivated cathepsin K, contraction was rapidly impaired (results not shown), confirming that blockade of the contractile response depends on the kininase activity of cathepsin K. It was noteworthy that cathepsin K prevented contraction of smooth muscles, despite hyper-responsiveness of hypoxic rats.
In summary, the presence of an unidentified kininase from macrophages has been reported and the involvement of new enzymes in BK catabolism was proposed [22,25]. Moreover, it has been shown that thiol-dependent cathepsins are released from macrophages during lung inflammation [9], and their active forms, including cathepsin K, from human inflammatory BAL fluids have been characterized (C. Serveau-Avesque, M. Ferrer-Di Martino and G. Lalmanach, unpublished work). Taken together with the potency of cathepsin K to impair BK-dependent contraction of smooth-muscle cells from normoxic and hypoxic rats, the present results support the notion that cathepsin K fulfils the criteria to be a kininase of physiological interest. The ability of cathepsin K to degrade pro-inflammatory kinins, and subsequently to impair activation of BK receptors, may attenuate bronchoconstriction during airway obstructive diseases.
Acknowledgments
We thank Ms Michèle Brillard-Bourdet for N-terminal peptide sequencing and Mr Laurent Galineau for BK-(5–9) synthesis. The technical assistance of Mr Norbert Bromet and Ms Colette Bounaix (Biotec SA, Orléans, France) and of Maya Belghazi (INRA de Tours-Nouzilly, Service de spectrométrie de masse pour la protéomique, Tours, France) for mass analysis are acknowledged. This work was partly supported by grants to G.L. from Vaincre les Maladies Lysosomales (Evry, France) and Biotechnocentre (Seillac, France) and by a grant to D.B. (AR46182) from the National Institutes of Health (Bethesda, MD, U.S.A.). E.G. holds a doctoral fellowship from MENRT (Ministère de l'Education Nationale de la Recherche et de la Technologie, France).
References
- 1.Heitsch H. Bradykinin B2 receptor as a potential therapeutic target. Drug News Perspect. 2000;13:213–225. [PubMed] [Google Scholar]
- 2.Wright A. L. Epidemiology of asthma and recurrent wheeze in childhood. Clin. Rev. Allergy Immunol. 2002;22:33–44. doi: 10.1007/s12016-002-0004-z. [DOI] [PubMed] [Google Scholar]
- 3.Coulson F. R., Fryer A. D. Muscarinic acetylcholine receptors and airway diseases. Pharmacol. Ther. 2003;98:59–69. doi: 10.1016/s0163-7258(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 4.Bhoola K. D., Figueroa C. D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol. Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 5.Scuri M., Forteza R., Lauredo I., Sabater J. R., Botvinnikova Y., Allegra L., Abraham W. M. Inhaled porcine pancreatic elastase causes bronchoconstriction via a bradykinin-mediated mechanism. J. Appl. Physiol. 2000;89:1397–1402. doi: 10.1152/jappl.2000.89.4.1397. [DOI] [PubMed] [Google Scholar]
- 6.Proud D. The kinin system in rhinitis and asthma. Clin. Rev. Allergy. Immunol. 1998;16:351–364. doi: 10.1007/BF02737656. [DOI] [PubMed] [Google Scholar]
- 7.Owen C. A., Campbell E. J. The cell biology of leukocyte-mediated proteolysis. J. Leukocyte Biol. 1999;65:137–150.. doi: 10.1002/jlb.65.2.137. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery P. K. Differences and similarities between chronic obstructive pulmonary disease and asthma. Clin. Exp. Allergy. 1999;29(Suppl. 2):14–26. [PubMed] [Google Scholar]
- 9.Wolters P. J., Chapman H. A. Importance of lysosomal cysteine proteases in lung disease. Respir. Res. 2000;1:170–177. doi: 10.1186/rr29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taggart C. C., Lowe G. J., Greene C. M., Mulgrew A. T., O'Neill S. J., Levine R. L., McElvaney N. G. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J. Biol. Chem. 2001;276:33345–33352. doi: 10.1074/jbc.M103220200. [DOI] [PubMed] [Google Scholar]
- 11.Reddy V. Y., Zhang Q. Y., Weiss S. J. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Punturieri A., Filippov S., Allen E., Caras I., Murray R., Reddy V., Weiss S. J. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J. Exp. Med. 2000;192:789–799. doi: 10.1084/jem.192.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecaille F., Kaleta J., Bromme D. Human and parasitic papain-like cysteine proteases: their role in physiology and pathology and recent developments in inhibitor design. Chem. Rev. 2002;102:4459–4488. doi: 10.1021/cr0101656. [DOI] [PubMed] [Google Scholar]
- 14.Turk V., Turk B., Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnero P., Borel O., Byrjalsen I., Ferreras M., Drake F. H., McQueney M. S., Foged N. T., Delmas P. D., Delaisse J. M. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J. Biol. Chem. 1998;273:32347–32352. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 16.Li Z., Hou W. S., Escalante-Torres C. R., Gelb B. D., Bromme D. Collagenase activity of cathepsin K depends on complex formation with chondroitin sulfate. J. Biol. Chem. 2002;277:28669–28676. doi: 10.1074/jbc.M204004200. [DOI] [PubMed] [Google Scholar]
- 17.Moran M. T., Schofield J. P., Hayman A. R., Shi G. P., Young E., Cox T. M. Pathologic gene expression in Gaucher disease: up-regulation of cysteine proteinases including osteoclastic cathepsin K. Blood. 2000;96:1969–1978. [PubMed] [Google Scholar]
- 18.Buhling F., Reisenauer A., Gerber A., Kruger S., Weber E., Bromme D., Roessner A., Ansorge S., Welte T., Rocken C. Cathepsin K – a marker of macrophage differentiation? J. Pathol. 2001;195:375–382. doi: 10.1002/path.959. [DOI] [PubMed] [Google Scholar]
- 19.Buhling F., Waldburg N., Kruger S., Rocken C., Wiesner O., Weber E., Welte T. Expression of cathepsins B, H, K, L, and S during human fetal lung development. Dev. Dyn. 2002;225:14–21. doi: 10.1002/dvdy.10134. [DOI] [PubMed] [Google Scholar]
- 20.Brubaker K. D., Vessella R. L., True L. D., Thomas R., Corey E. Cathepsin K mRNA and protein expression in prostate cancer progression. J. Bone Miner. Res. 2003;18:222–230. doi: 10.1359/jbmr.2003.18.2.222. [DOI] [PubMed] [Google Scholar]
- 21.Desmazes C., Galineau L., Gauthier F., Bromme D., Lalmanach G. Kininogen-derived peptides for investigating the putative vasoactive properties of human cathepsins K and L. Eur. J. Biochem. 2003;270:171–178. doi: 10.1046/j.1432-1033.2003.03382.x. [DOI] [PubMed] [Google Scholar]
- 22.Vietinghoff G., Paegelow I. Degradation of bradykinin by peritoneal and alveolar macrophages of the guinea pig. Peptides. 2000;21:1249–1255. doi: 10.1016/s0196-9781(00)00266-7. [DOI] [PubMed] [Google Scholar]
- 23.Blais C., Jr, Marceau F., Rouleau J. L., Adam A. The kallikrein–kininogen–kinin system: lessons from the quantification of endogenous kinins. Peptides. 2000;21:1903–1940. doi: 10.1016/s0196-9781(00)00348-x. [DOI] [PubMed] [Google Scholar]
- 24.Campbell D. J. The renin–angiotensin and the kallikrein–kinin systems. Int. J. Biochem. Cell. Biol. 2003;35:784–791. doi: 10.1016/s1357-2725(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 25.Marshall P., Heudi O., McKeown S., Amour A., Abou-Shakra F. Study of bradykinin metabolism in human and rat plasma by liquid chromatography with inductively coupled plasma mass spectrometry and orthogonal acceleration time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:220–228. doi: 10.1002/rcm.565. [DOI] [PubMed] [Google Scholar]
- 26.Lecaille F., Choe Y., Brandt W., Li Z., Craik C. S., Bromme D. Selective inhibition of the collagenolytic activity of human cathepsin K by altering its S2 subsite specificity. Biochemistry. 2002;41:8447–8454. doi: 10.1021/bi025638x. [DOI] [PubMed] [Google Scholar]
- 27.Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem. J. 1982;201:189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brillard-Bourdet M., Moreau T., Gauthier F. Substrate specificity of tissue kallikreins: importance of an extended interaction site. Biochim. Biophys. Acta. 1995;1246:47–52. doi: 10.1016/0167-4838(94)00179-k. [DOI] [PubMed] [Google Scholar]
- 29.Chagas J. R., Authie E., Serveau C., Lalmanach G., Juliano L., Gauthier F. A comparison of the enzymatic properties of the major cysteine proteinases from Trypanosoma congolense and Trypanosoma cruzi. Mol. Biochem. Parasitol. 1997;88:85–94. doi: 10.1016/s0166-6851(97)00085-6. [DOI] [PubMed] [Google Scholar]
- 30.Saftig P., Hunziker E., Wehmeyer O., Jones S., Boyde A., Rommerskirch W., Moritz J. D., Schu P., von Figura K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecaille F., Weidauer E., Juliano M. A., Bromme D., Lalmanach G. Probing cathepsin K activity with a selective substrate spanning its active site. Biochem. J. 2003;375:307–312. doi: 10.1042/BJ20030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Crescenzo V., Dubuis E., Constantin S., Rebocho M., Girardin C., Bonnet P., Vandier C. Halothane differentially decreases 5-hydroxytryptamine-induced contractions in normal and chronic hypoxic rat pulmonary arteries. Acta Physiol. Scand. 2001;173:247–255. doi: 10.1046/j.1365-201X.2001.00909.x. [DOI] [PubMed] [Google Scholar]
- 33.Vandier C., Delpech M., Rebocho M., Bonnet P. Hypoxia enhances agonist-induced pulmonary arterial contraction by increasing calcium sequestration. Am. J. Physiol. 1997;273:H1075–H1081. doi: 10.1152/ajpheart.1997.273.3.H1075. [DOI] [PubMed] [Google Scholar]
- 34.Desmazes C., Gauthier F., Lalmanach G. Cathepsin L, but not cathepsin B, is a potential kininogenase. Biol. Chem. 2001;382:811–815. doi: 10.1515/BC.2001.098. [DOI] [PubMed] [Google Scholar]
- 35.Alves M. F., Puzer L., Cotrin S. S., Juliano M. A., Juliano L., Bromme D., Carmona A. K. S3 to S3′ subsite specificity of recombinant human cathepsin K and development of selective internally quenched fluorescent substrates. Biochem. J. 2003;373:981–986. doi: 10.1042/BJ20030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corvol P., Williams T. A. Handbook of Proteolytic Enzymes. In: Barrett A. J., Rawlings N. D., Woessner F. J., editors. San Diego: Academic Press; 1998. pp. 1066–1076. [Google Scholar]
- 37.Li Z., Hou W. S., Bromme D. Collagenolytic activity of cathepsin K is specifically modulated by cartilage-resident chondroitin sulfates. Biochemistry. 2000;39:529–536. doi: 10.1021/bi992251u. [DOI] [PubMed] [Google Scholar]
- 38.Barrett A. J., Kirschke H. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Part C):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- 39.Skidgel R. A., Erdos E. G. Cellular carboxypeptidases. Immunol. Rev. 1998;161:129–141. doi: 10.1111/j.1600-065x.1998.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 40.Belouchi N. E., Roux E., Savineau J. P., Marthan R. Effect of chronic hypoxia on calcium signalling in airway smooth muscle cells. Eur. Respir. J. 1999;14:74–79. doi: 10.1034/j.1399-3003.1999.14a13.x. [DOI] [PubMed] [Google Scholar]
- 41.Roux E., Duvert M., Marthan R. Combined effect of chronic hypoxia and in vitro exposure to gas pollutants on airway reactivity. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2002;283:L628–L635. doi: 10.1152/ajplung.00387.2001. [DOI] [PubMed] [Google Scholar]