Abstract
ERp29 is a recently characterized resident of the ER (endoplasmic reticulum) lumen that has broad biological significance, being expressed ubiquitously and abundantly in animal cells. As an apparent housekeeper, ERp29 is thought to be a general folding assistant for secretory proteins and to probably function as a PDI (protein disulphide isomerase)-like molecular chaperone. In the present paper, we report the first purification to homogeneity and direct functional analysis of native ERp29, which has led to the unexpected finding that ERp29 lacks PDI-like folding activities. ERp29 was purified 4800-fold in non-denaturing conditions exploiting an unusual affinity for heparin. Two additional biochemical hallmarks that will assist the classification of ERp29 homologues were identified, namely the idiosyncratic behaviours of ERp29 on size-exclusion chromatography (Mr<globular homodimer) and SDS/PAGE (Mr>monomeric mass). In contrast with PDI and parallel-purified co-residents (calreticulin, ERp60), native ERp29 lacked classical chaperone, disulphide reductase and isomerase, and calcium-binding activities. In the chaperone assays, ERp29 neither protected substrate proteins against thermal aggregation nor interacted stably with chemically denatured proteins as detected by cross-linking. ERp29 also did not exhibit helper activity toward calreticulin (chaperone) or PDI and ERp60 (disulphide reductase). By refuting long-standing predictions about chaperone activity, these results expose ERp29 as a functionally distinct member of the ER machinery and prompt a revised hypothesis that ERp29 acts as a non-classical folding assistant. The native preparation and biochemical hallmarks established here provide a useful foundation for ongoing efforts to resolve the functional orphan status of ERp29.
Keywords: chaperone, endoplasmic reticulum, ERp29, orphan protein, protein purification, protein disulphide isomerase
Abbreviations: BiP, immunoglobulin heavy-chain binding protein; BS3, bis(sulphosuccinimidyl)suberate; ER, endoplasmic reticulum; MALDI–TOF, matrix-assisted laser-desorption ionization–time-of-flight; PDI, protein disulphide isomerase
INTRODUCTION
The ER (endoplasmic reticulum) holds key importance in biology, medicine and biotechnology. Embodying the start of the secretory pathway, the ER plays vital roles producing secretory proteins and providing safe storage of calcium. Malfunctions in the ER underlie serious disorders like cystic fibrosis and neurodegeneration [1,2], and industrial production of engineered proteins can benefit from customized tuning of ER functions [3]. Proteins residing in the ER lumen (reticuloplasmins) work co-operatively to ensure that secretory proteins are produced with high quality. The most abundant reticuloplasmins, expressed at millimolar levels in specialist secretory cells, include four ‘housekeeping’ proteins recognized primarily as catalysts of disulphidebond formation (PDI; protein disulphide isomerase) and chaperones of protein folding [BiP (immunoglobulin heavy-chain binding protein), calreticulin, endoplasmin/GRP94]. These classical protein-folding assistants all feature additional overlapping activities, as exemplified by their common ability to bind calcium and the chaperone activity of PDI [4–7]. Despite recent strides, understanding of the ER protein-folding machinery will be lacking until more is learned about a growing list of novel reticuloplasmins with less certain functions [8].
ERp29 is a prominent new player in the ER of animal cells that merits urgent functional investigation [9]. First characterized as a novel reticuloplasmin in the secretory cells that produce tooth enamel, ERp29 is now recognized as a ubiquitous and abundantly expressed ‘housekeeper’ [10–13]. ERp29 is traceable through evolution from insects and is highly conserved in mammals, and has been encountered in many different settings including phagocytosis, milk production, antibody secretion and neural pharmacology [9,14,15]. While endorsing a broad significance, such functional associations are limited by uncertainties over ERp29's specific role. Bioinformatics has failed to predict a function for ERp29 and so, as the founding member of a new protein class [16], ERp29 remains a ‘functional orphan’ despite much accumulated information, including a molecular structure and extensive expression profiling [9,12,17,18].
ERp29 is widely thought to be a folding assistant for secretory proteins and to probably function as a PDI-like molecular chaperone. We found that ERp29 is most abundant in the rough ER of actively secreting epithelial cells and complex neurons where its stoichiometric equivalence to BiP and PDI implies a protein-folding role [12,17,19]. ERp29 was also found to be co-ordinately up-regulated with other major reticuloplasmins during the onset of antibody production in lymphocytes [14]. Attention has latched on to a chaperone role, because ERp29 exhibits chaperone-like interactions with itself and other ER proteins [18,20–23], and a PDI-like chaperone from fruitfly, named Windbeutel, shares strong structural similarities with ERp29 [24,25]. With PDI as its closest structural relative, ERp29 has been classified as a PDI-family member that lacks the dithiol motif classically associated with thiol–disulphide oxidoreductase activities [10,21]. While these features are consistent with a protein-folding role, several lines of evidence suggest that ERp29 is functionally distinct from other major reticuloplasmins [12,19,25].
To advance functional understanding of ERp29, a pressing need exists for benchmark studies of pure native protein. Such a preparation will offer the best opportunity for preserving natural activities and allow the results to be assigned directly to ERp29. To date, evidence of interaction with other proteins has come from analysis of complex protein mixtures, leaving the identity of ERp29's immediate partners uncertain [20,22,23,26]. Since previous isolations of ERp29 from animal tissue involved denaturing steps and low yields [22,26], an effective procedure for purifying native ERp29 to homogeneity remained to be developed.
The present study has developed a robust procedure for purifying ERp29 to homogeneity from rat liver under non-denaturing conditions. Structural analyses were undertaken to validate the preparation and establish a characteristic set of properties for defining authentic ERp29. Native ERp29 was then used to evaluate the prime candidate activities as a chaperone or PDI analogue. Together, our findings justify a focal shift towards candidate activities for ERp29 that differ from those of its classically defined peers.
EXPERIMENTAL
Materials
Monospecific anti-ERp29-N-terminus antibodies originally used to develop the purification were raised conventionally in rabbit against a peptide (LHTKGALPLDTC) coupled to keyhole-limpet haemocyanin using NHS (N-hydroxysuccinimide)-maleimide, and affinity-purified on the same peptide conjugated to Sulfolink beads (from Pierce). Antiserum against whole ERp29 subsequently used for immunoblotting was raised in rabbit [16]. The following proteins (bovine unless specified) were from Sigma: PDI, insulin, glutathione peroxidase, carbonic anhydrase, serum albumin, scrambled and native RNaseA type 1-A, glutathione S-transferase, porcine malate dehydrogenase and citrate synthase, chicken lysozyme and ovalbumin. Heparin–Sepharose HiTrap cartridges were obtained from Amersham Biosciences.
Tissue isolation
Wistar-derived rats were given unlimited access to a standard pellet chow and kept as described in [27]. Livers were isolated from juveniles (6–8-weeks old, killed by CO2-induced asphyxiation and decapitation) then immediately frozen on solid CO2 and stored at −80 °C. All animal procedures were undertaken in accordance with national ethical requirements.
Purification of ERp29 from rat liver
Since no assayable activity existed for ERp29, immunoblotting was used to monitor the preparation during its development and refinement. Once robust, the procedure was followed more efficiently using chromatographic peak profiles and SDS/PAGE (Steps 4 and 5 below). All procedures were carried out at 4 °C unless stated otherwise.
Step 1: isolation of microsomes from rat liver
Based on conventional procedures, four rat livers were homogenized in 1.5 vol. of Buffer A (20 mM Tris/HCl, pH 8.0, 150 mM NaCl, 250 mM sucrose, 1 mM dithiothreitol and 0.5 mM PMSF) using a motorized glass/Teflon tissue disperser. Gross particulates were removed by centrifugation (20000 g for 30 min) and the supernatant was re-centrifuged. Microsomes were sedimented by ultracentrifugation (125000 g for 1 h) and washed by two cycles of sedimentation and resuspension. The microsomal pellet was suspended to 20 mg of protein/ml in Buffer B (2 mM Tris/HCl, pH 8.0, 0.1 mM dithiothreitol, 1 mM benzamidine and 0.1 mM PMSF) and stored at −80 °C.
Step 2: solubilization of microsomes
Thawed microsomes were diluted 8-fold with Buffer B containing 0.5% (v/v) Triton X-100 and incubated for 30 min with intermittent mixing. After addition of fresh PMSF (0.1 mM), solubilized microsomes were ultracentrifuged (125000 g for 1 h) to remove particulates.
Step 3: anion-exchange chromatography
The Triton-soluble fraction was applied to a DEAE–Sepharose column (10 cm×2.5 cm, 30 ml bed) equilibrated with Buffer C (20 mM Tris/HCl, pH 8.0, 0.1 mM dithiothreitol and 1 mM benzamidine). After washing with 5 bed-volumes of Buffer C, weakly acidic proteins were step-eluted with 4 bed-volumes of Buffer C containing 80 mM NaCl plus 0.1 mM PMSF.
Step 4: heparin-affinity chromatography
The eluate from Step 3 was immediately applied at 0.75 ml/min to a 1 ml Heparin–Sepharose HiTrap cartridge equilibrated in Buffer D (20 mM Tris/HCl, pH 8.0, 80 mM NaCl and 0.1 mM dithiothreitol). Heparin-bound proteins were eluted with a linear salt gradient (0–500 mM NaCl in Buffer D over 30 min) and 1 ml fractions were collected. Protease inhibitors (0.1 mM PMSF, 1 mM benzamidine, 1 μg/ml leupeptin and 1 μg/ml pepstatin) were added, and samples were rapidly subjected to SDS/PAGE. Fractions enriched with ERp29 and depleted of ERp60 were pooled, supplemented with non-ionic detergent (0.02% Thesit), and concentrated to 0.8 ml by centrifugal ultrafiltration (5000 g, Centricon C-10 device from Millipore).
Step 5: size-exclusion chromatography
ERp29 was polished by chromatography on a Sephacryl S-200HR column (120 cm×1 cm, 88 ml bed) running at 7 ml/h in Buffer E (20 mM Tris/HCl, pH 7.2, 160 mM NaCl and 1 mM dithiothreitol). Fractions (1.1 ml) embracing the A280 peak (Ve/Vo=1.5) were analysed by SDS/PAGE as in Step 4. Fractions of pure ERp29 (typically eight) were pooled, supplemented with Thesit (0.005%), concentrated 10-fold (Centricon C-10) and stored in aliquots at −80 °C. For maximizing recovery from centrifugal ultrafiltration, it was found important to not concentrate ERp29 excessively and so the retentate was always maintained at >0.5 ml.
Parallel purification of calreticulin and ERp60
Calreticulin and ERp60 were eluted from DEAE-Sepharose after Step 3 with 250 mM NaCl and purified to homogeneity using additional non-denaturing steps on heparin–Sepharose and a 1 ml UnoQ column (0–500 mM NaCl gradient, 20 °C).
Gel electrophoresis procedures
SDS/PAGE was carried out using reducing conditions with glycine- and Tricine-based discontinuous buffer systems, Coomassie Blue and silver staining, and recombinant 10-kDa ladder molecular-mass standards as before [16,19]. Immunoblotting, densitometric quantification and 45Ca-overlay analysis were done with established procedures [19,28]. For the latter, semi-pure reticuloplasmins were obtained by elution from DEAE with 250 mM NaCl (after Step 3) and the blot was probed with 50 μM 45Ca to maximize detection of low-affinity calcium-binding proteins [28].
Size-exclusion chromatography and sucrose-gradient analysis
Analytical size-exclusion chromatography on Sephacryl S-200HR was as described above (Step 5), except that the column was prepared in a 5 ml glass pipette (282 mm×6 mm, 8 ml bed, 1 ml/h) and Buffer E was modified by varying the NaCl concentration or adding 0.002% Thesit. Duplicate gels were run to enable parallel detection of ERp29 (20 μg loaded in 0.1 ml) and the internal standards (BSA, ovalbumin, carbonic anhydrase, cytochrome c; 50 μg each) using immunoblotting and Coomassie Blue staining respectively (see Figure 3). Control experiments verified that the Ve of ERp29 was unaffected by the internal standards and that a weak dimer (β-lactoglobulin) dissociated fully under these chromatography conditions. Linear sucrose gradients (1.1 ml, 5–30% sucrose in Buffer E) were ultracentrifuged (150000 g for 18 h at 4 °C) and fractionated manually. Sample loadings and detection were the same as for size-exclusion chromatography.
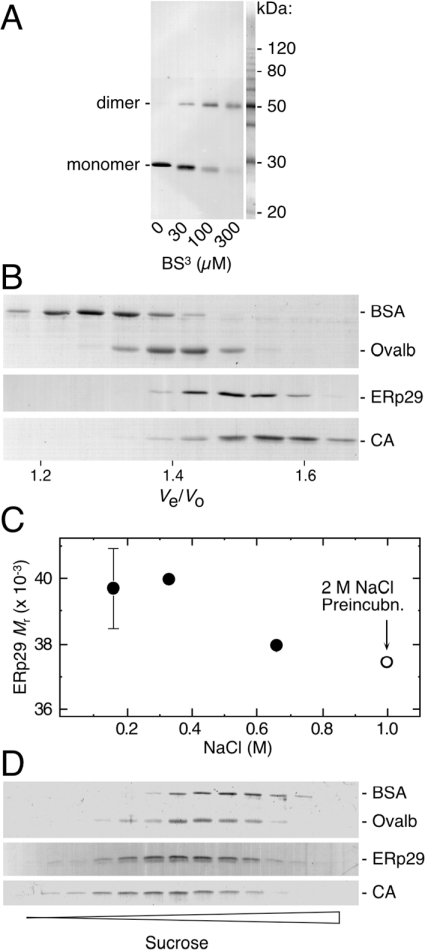
Figure 3. Native ERp29 behaves as a compact homodimer in solution.
(A) Cross-linking with BS3 followed by SDS/PAGE and ERp29 immunoblotting. ERp29 (2.5 μM monomer) was trapped as a homodimer almost quantitatively at higher BS3 exposures. No higher-order oligomers were observed here or in a parallel Coomassie-Blue-stained gel. The Amido-Black-stained mass markers were from an adjacent lane of the same blot. (B) Analytical size-exclusion chromatography of ERp29 and three internal globular-protein standards. ERp29 was eluted as a monodisperse peak between ovalbumin (Ovalb) and carbonic anhydrase (CA) (44.5 kDa and 29 kDa respectively). (C) Relative mass values derived for ERp29 from the experiment in (B) and other runs performed at this and higher ionic strengths (•; ±S.E.M. for 160 mM NaCl) showing that dimeric ERp29 consistently behaved as if smaller than expected for a 51 kDa protein. Where indicated (○), ERp29 was pre-incubated in 2 M NaCl for 16 h, then chromatographed in 1 M NaCl. (D) Analytical sucrose-gradient analysis showing that ERp29 again migrated between the 44.5 kDa and 29 kDa internal standards.
Protein microchemistry
Reverse-phase HPLC of ERp29 on a C18 column (1 mm×100 mm) was as described in [27], except that the starting solvent was 10% acetonitrile in 0.1% trifluoroacetic acid. MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) MS was performed in linear positive-ion mode with ubiquitin as internal calibrant as described [28]. For electrospray ionization MS, dynamic microspray and static nanospray sources were used on triple quadrupole (Sciex API 300) and quadrupole ion trap (Finnigan LCQ Deca) instruments respectively. Edman micro-sequencing and amino acid composition analysis followed established procedures [19,27].
Enzyme and molecular chaperone assays
For these activity assays, freezing was avoided during ERp29 purification (Steps 2 and 5), the final step was modified to exclude Thesit and ultrafiltration, and ERp29 was stored on ice until assay. All assays compared ERp29 (1 μM monomer) with equimolar or lower concentrations of pure-protein controls, except that a crude microsomal extract (from Step 2) was used for NADH oxidoreductase activity. Spectrophotometric and light-scattering data were recorded at 25 °C unless indicated otherwise. Enzyme assay conditions and substrates were as follows: disulphide reductase, insulin [29]; disulphide isomerase, scrambled RNase [30], dehydroascorbate reductase, bis(dehydroascorbic acid) [31]; glutathione peroxidase, dehydroascorbate and hydrogen peroxide [32]; glutathione S-transferase, 1-chloro-2,4-dinitrobenzene [33]; NADH oxidoreductase, lucigenin [34]. Chaperone activity towards two thermally labile substrates, malate dehydrogenase and citrate synthase, was assayed at 45 °C in the presence and absence of 0.5 mM MgATP as described [35]. For Figure 6(B), reticuloplasmins (2 μM) were incubated with equimolar substrate for 1 h, centrifuged at 20000 g for 5 min at 4 °C, then analysed by SDS/PAGE. Chaperone-like interactions with chemically denatured substrates were assayed by combining ERp29 (2 μM) with a 10-fold molar excess of RNase or lysozyme (both carboxyamidomethylated essentially as described in [36]) in the presence of BS3 [bis(sulphosuccinimidyl)suberate (Sigma); see cross-linking conditions below] followed by SDS/PAGE and immunoblotting.
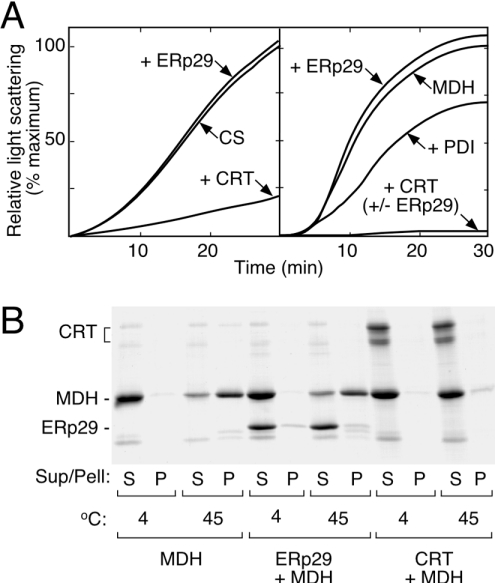
Figure 6. ERp29 lacks classical molecular chaperone activities.
(A) Thermal aggregation assay showing (left-hand panel) that the time-dependent denaturation of citrate synthase (CS) at 45 °C was unaffected by ERp29 (+ERp29), but was strongly suppressed by parallel-purified calreticulin (+CRT). Using malate dehydrogenase (MDH) as an alternative substrate (right-hand panel), ERp29 again lacked the chaperone properties of calreticulin and PDI. ERp29 also did not influence the chaperone activity of calreticulin when assayed together (+CRT+ERp29). The minor anti-chaperone effect of ERp29 on malate dehydrogenase (i.e. enhanced aggregation) was not observed consistently. (B) Coomassie-Blue-stained SDS/PAGE of supernatant (S) and pellet (P) fractions isolated from an equivalent experiment to that in (A), confirming that ERp29 did not prevent the heat-induced precipitation of malate dehydrogenase. Additionally, it is apparent that ERp29 remained soluble under these conditions and did not associate substantially with the precipitated substrate. As expected, calreticulin was heat-stable and prevented the thermal precipitation of malate dehydrogenase almost completely. Equivalent results were obtained using citrate synthase as substrate and with PDI as positive control (not shown).
Other procedures
For cross-linking analysis, samples in amine-free buffer (25 mM Hepes, pH 8.0, 100 mM NaCl and 0.1 mM dithiothreitol) were exposed to BS3 for 30 min at 20 °C, then quenched with 50 mM Tris/HCl, pH 8.0, and analysed by SDS/PAGE. Purified ERp29 was quantified by Coomassie-Blue-stained SDS/PAGE with carbonic anhydrase as standard. This procedure was effective because ERp29 and carbonic anhydrase samples equated by amino acid composition gave equal colour yields with Coomassie Blue. Quantification of ERp29 in complex mixtures by immunoblotting, and of protein extracts by dye-binding micro-assay, was as described previously [19].
RESULTS
Purification of ERp29 from rat liver
Since ERp29 had no known activity, our main design goal was to use non-denaturing purification conditions where the classical reticuloplasmins remained active. Rat liver was adopted as source tissue because we had previously established that ERp29-enriched microsomes could readily be prepared in sufficient bulk, a feature not shared by some other tissues that express ERp29 more highly [19]. As described in the Experimental section, the successful five-step purification procedure involved sequential chromatographies of Triton-solubilized microsomes on anion-exchange, heparin-affinity and size-exclusion columns (Figure 1A). With refinements made to expedite progress between the chromatography steps, it was practicable to complete the preparation in only 3 days.
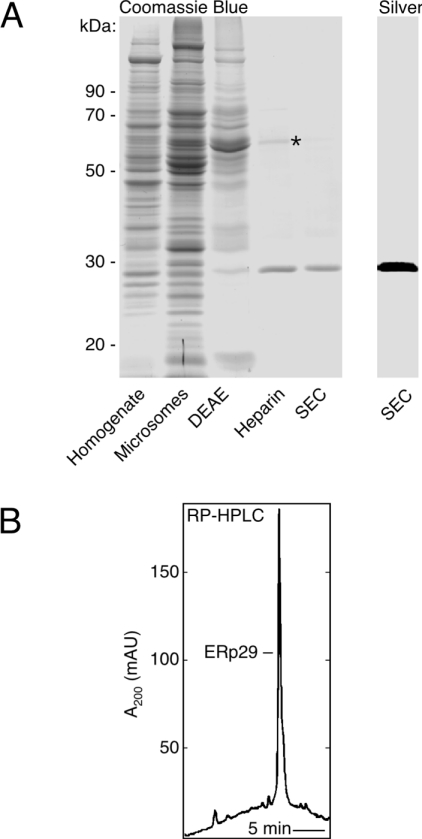
Figure 1. Purification of native ERp29 to homogeneity.
(A) SDS/PAGE representing the five preparative steps. A single major 29 kDa band was observed after the final size-exclusion chromatography (SEC), with both Coomassie Blue and silver staining as indicated. During heparin-affinity chromatography, ERp29 was largely resolved from a major 60 kDa protein (asterisk) identified as ERp60 by its N-terminal sequence (SDVLEL…). (B) Reverse-phase (RP) HPLC of material obtained from the final size-exclusion step, showing a single major peak corresponding to ERp29 (>95% pure in all four preparations analysed). mAU, milli-absorbance units.
ERp29 was obtained with high purity, being observed as a single major band at 29 kDa on SDS/PAGE after staining with either Coomassie Blue or silver (Figure 1A), and after immunoblotting (see Figure 3A). Using reverse-phase HPLC to check for small contaminants invisible to SDS/PAGE (Figure 1B), ERp29 was again the only major species detected. Edman analysis gave a single sequence (LHTKGALPLD…) that corresponded to the mature N-terminus of ERp29, as expected. Overall, ERp29 underwent a 4800-fold purification with 13% recovery, and a yield of 120–140 μg was obtained from the standard preparation that employed four rat livers. The heparin-affinity step was critical to the success of this procedure, providing a 40-fold enrichment (Figure 1 and Table 1).
Table 1. Purification of ERp29 from rat liver.
Data are from a typical preparation with four livers.
| Step | Volume (ml) | Protein (mg)* | ERp29 (mg)† | Purification (fold) | Recovery (%) |
|---|---|---|---|---|---|
| 1. Tissue extract | 100 | 4863 | 0.97 | 1 | 100 |
| 2. Microsomes | 160 | 120 | 0.80 | 33 | 82 |
| 3. DEAE | 120 | 37 | 0.53 | 72 | 55 |
| 4. Heparin | 3 | 0.285 | 0.18 | 3160 | 19 |
| 5. SEC‡ | 1 | 0.135 | 0.13 | 4815 | 13 |
* Determined by dye-binding assay and SDS/PAGE with Coomassie Blue staining (Steps 1–3 and 4–5 respectively, as defined in the Experimental section).
† Quantified by immunoblotting and Coomassie-Blue-stained SDS/PAGE (Steps 1–3 and 4–5 respectively).
‡ Size-exclusion chromatography.
Two practical issues concerning contamination and stability are noteworthy because of their potential to influence functional analysis of ERp29. ERp29 preparations were prone to contamination by a major heparin-binding protein that was identified as ERp60 (Figure 1A). However, as crucial for the PDI assays described below, ERp60 could be removed completely by appropriate selection of fractions or by repetition of the heparin-affinity step. ERp29 remained intact and soluble for >2 weeks when stored on ice after the size-exclusion step (assessed by SDS/PAGE after centrifugation at 20000 g), but major losses were observed from samples concentrated above 1 mg/ml or freeze–thawed repeatedly. These solubility problems were overcome by including trace amounts of a non-ionic detergent in the storage solution.
Structural characterization of native ERp29
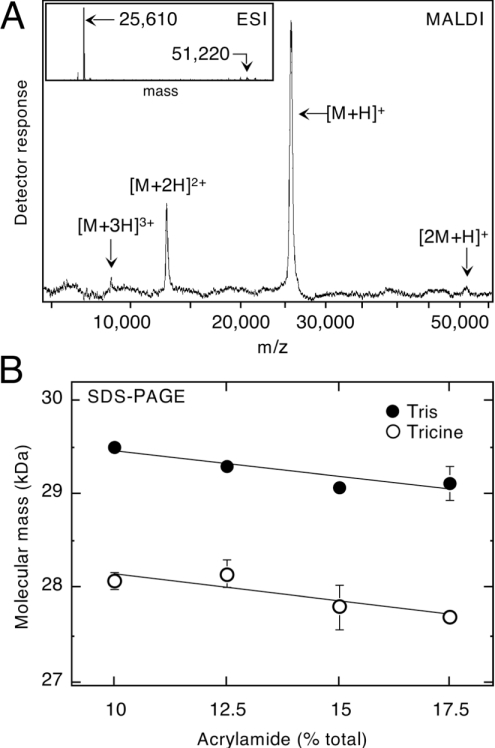
MS was applied to investigate an unexplained 12% mass discrepancy between the 29 kDa band observed on SDS/PAGE and that predicted from ERp29's cDNA sequence (25609.2 Da after removal of the signal sequence; [16]). With MALDI–TOF analysis, the two or three observable peaks all corresponded to charge variants of a 25.61 kDa species (Figure 2A). Higher resolution analyses using electrospray ionization gave a mass of 25609.9±0.5 Da (±S.E.M.), closely matching the predicted value for monomeric ERp29. Only trace amounts of a possible dimeric ERp29 adduct were observed in fresh preparations (Figure 2), and no other significant peaks were detected in the mass range investigated (0.8–100 kDa). These results indicated that ERp29 lacked additive post-translational modifications, and endorsed the high level of purity evident above (Figure 1).
Figure 2. Anomalous Mr of ERp29 on SDS/PAGE is an intrinsic property of the undecorated polypeptide.
(A) MALDI–TOF MS, showing that all detectable peaks corresponded to ERp29 (i.e. singly, doubly and tertiary charged forms of a 25.61 kDa species as indicated). Dimeric ERp29 ([2M+H]+) was barely detectable both with MALDI–TOF and (inset) electrospray ionization (ESI) analysis. (B) Ferguson analysis of SDS/PAGE buffered with glycine and Tricine as indicated. Gel density (percentage of acrylamide) had little effect on the relative mobility of ERp29, as reflected by the near-horizontal lines for both buffer systems. Although Tricine gave significantly faster migration, ERp29 still did not migrate as expected for a 25.6 kDa protein.
Ferguson analysis was used to characterize the behaviour of ERp29 on SDS/PAGE, which now seemed anomalous given the absence of post-translational additions. When relative electrophoretic mobility was plotted as a function of gel density, ERp29 was found to migrate near 29 kDa in gels buffered conventionally with glycine, whereas an alternative Tricine buffer resulted in distinctly different mobilities near 28 kDa (Figure 2B). However, even under the most favourable condition (Tricine, 17.5% acrylamide), the mobility of ERp29 remained 8% lower than expected for a 25.6 kDa protein.
Cross-linking analysis was undertaken to verify that native ERp29 existed as a homodimer, as reported previously for recombinant ERp29 [18,20]. Concordantly, when purified ERp29 was exposed to optimal amounts of the homobifunctional cross-linker BS3, the dimer was preserved almost quantitatively after SDS/PAGE (Figure 3A). No substantial cross-link products were observed at higher masses in contrast with the previous reports [18,20]. It was also apparent that BS3 treatment suppressed the characteristic slow migration of ERp29, to the extent that ERp29 dimer migrated near the mass (51 kDa) predicted by primary structure.
Size-exclusion analysis was pursued in some detail since the wide variation of previous reports (Mr values of 66000 [22], 55000 [20] and 40000 [19]) precluded a consistent view of the native oligomerization status and hydrodynamic shape of ERp29. To maximize accuracy, ERp29 was chromatographed together with a variety of globular proteins as internal standards. As illustrated (Figures 3B and 3C), ERp29 was eluted as a monodisperse peak between ovalbumin and carbonic anhydrase, corresponding to an Mr of 40000±1000 (±S.E.M.) at physiological ionic strength. Since this apparent mass was substantially less than expected for a globular dimer, potential causes of aberrant behaviour were investigated. Increasing salt concentrations up to 1 M NaCl resulted in a small reduction to an Mr of 37500, and this was unaltered after overnight pre-incubation of ERp29 in 2 M NaCl (Figure 3C). Non-ionic detergent did not affect Mr, and so the atypical behaviour could not be ascribed simply to retarding interactions with the column matrix or partial dissociation of ERp29 dimer during the chromatography.
Finally, sucrose-gradient analysis was used to test the ensuing proposal that ERp29 exists as a compact dimer in solution. When co-centrifuged with the same globular protein standards as above, ERp29 again migrated between ovalbumin and carbonic anhydrase (Figure 3D), consistent with an Mr value near 40000. Overall, this structural characterization suggested that native ERp29 exists exclusively as a tightly associated homodimer whose hydrodynamic volume is less than expected for a pair of 25.6 kDa monomers.
Functional analysis of native ERp29
Calcium-binding activity is a common property of all the major reticuloplasmins except ERp60, serving key roles in calcium storage and regulation of the ER machinery [6]. We used 45Ca-overlay analysis to test directly whether ERp29 lacks calcium-binding properties, as suggested by sequence analysis [10]. No calcium-binding activity was detected for ERp29 under conditions where equimolar amounts of endoplasmin, BiP, calreticulin and PDI were strongly labelled (Figure 4).
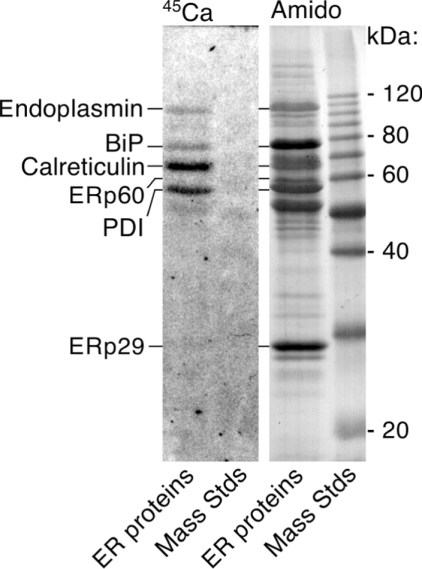
Figure 4. ERp29 distinctively lacks calcium-binding properties.
Purified ERp29 was mixed with other reticuloplasmins and subjected to 45Ca-overlay analysis. Autoradiography (left-hand panel) showed that ERp29 did not bind significant amounts of calcium, in contrast with endoplasmin, BiP, calreticulin and PDI. Post-staining with Amido Black (right-hand panel) confirmed a 2-fold molar excess of ERp29 over the other reticuloplasmins and that ERp60 did not bind 45Ca. Mass Stds, molecular-mass standards (sizes in kDa given on the right).
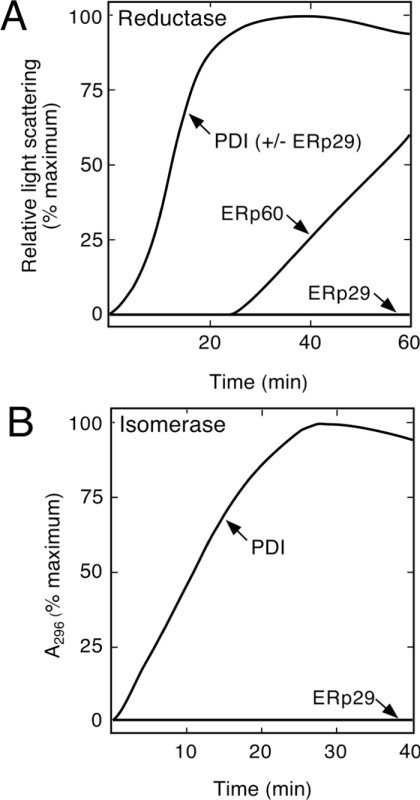
Disulphide isomerase activities were considered because ERp29 shares with PDI a structure based on the thioredoxin fold [18]. PDI functions primarily in the formation of disulphide bonds by acting as a dithiol oxidase. Since this oxidase activity depends on a dithiol catalytic element, it was theorized previously that with only a single cysteine residue, ERp29 would lack such properties [10]. This view was reinforced by confirmation that ERp29's monothiol lies outside the thioredoxin-like N-terminal domain [18]. However, the alternative activities of PDI in disulphide-bond editing (i.e. reductase, isomerase) still remained as possibilities for ERp29, because they can operate with a monothiol catalytic site and in other structural environments besides the thioredoxin fold [10,37,38]. To test for disulphide-reductase activity, we used a classical assay whereby PDI catalysed the glutathione-induced reduction of insulin disulphides, leading to aggregates of misfolded insulin detectable by light scattering (Figure 5A). In contrast with PDI, native ERp29 gave no detectable activity even at a 20-fold higher concentration (detection limit, <3% PDI) and with either dithiothreitol or glutathione as proton donor. Verifying this result, parallel-purified ERp60 was also highly active, albeit after a characteristic activation delay [29], as shown in Figure 5(A). Secondly, we questioned whether ERp29 might function as a helper of PDI during disulphide reduction, but no effect was found when ERp29 was assayed together with either PDI or ERp60 (Figure 5A, and results not shown). Thirdly, to evaluate disulphide isomerase activity, we used a classical assay in which an inactivated form of RNase with ‘scrambled’ disulphides was reactivated through the ability of PDI to edit out non-native disulphide bonds (Figure 5B). Again ERp29 gave no detectable activity (<5% compared with PDI). Fourthly, to evaluate the possibility of a specificity issue with protein substrates, we assayed for the reduction of dehydroascorbate, a small-molecule substrate of PDI and other thioredoxin-related proteins, including glutathione peroxidase and glutaredoxin [32]. ERp29 exhibited no detectable activity toward dehydroascorbate or a related substrate, hydrogen peroxide (<5% compared with glutathione peroxidase; results not shown). Fifthly, recognizing gross structural similarities between ERp29 and an emerging class of atypical, monothiol glutaredoxins/glutathione transferases [39], we examined glutathione S-transferase activity using a broad-specificity substrate, but again no activity was detected (<1% compared with control). Finally, we assayed more generally for NADH-oxido-reductase activity using lucigenin as substrate [34], but to no avail (ERp29<1% compared with liver microsomes).
Figure 5. ERp29 lacks classical disulphide-isomerase activities.
(A) Disulphide-reductase assay showing that ERp29 did not promote the aggregation of insulin, unlike PDI and parallel-purified ERp60. ERp29 also did not influence the activity of PDI when assayed together (PDI+ERp29). Assays were monitored for 1.5 h. (B) Disulphide-isomerase assay showing that, unlike PDI, ERp29 did not augment the renaturation of ‘disulphide-scrambled’ RNase detectably.
The possibility of ERp29 having molecular chaperone activity has been supported by several observations [12,18–23,25], but direct testing with pure protein remained to be done. We assayed for the ability to suppress aggregation of thermally labile protein substrates, a property shared by all classes of classical molecular chaperone [40]. As a positive control, the ability of parallel-purified calreticulin to act as a chaperone for citrate synthase [35] was tested and, as expected, the aggregation of citrate synthase at 45 °C was suppressed strongly. In contrast, equimolar ERp29 had no observable effect when added in place of calreticulin (Figure 6A, left-hand panel). The same behaviour pattern was observed with another substrate, malate dehydrogenase. Here, ERp29 failed to mimic the chaperone activities of calreticulin and PDI (Figure 6A, right-hand panel). Since some chaperones are ATPases, the effect of MgATP addition was checked, but no differences were found, consistent with the reported lack of ATP-binding properties of ERp29 [22]. We also found that calreticulin's chaperone activity was unaffected by the addition of equimolar ERp29 (Figure 6A), suggesting that ERp29 did not behave as a co-chaperone in this case. Although no aggregation of ERp29 was evident in controls heated up to 60 °C, the possibility that ERp29 had been thermally inactivated during these assays was explored further. SDS/PAGE analysis showed that, like calreticulin and PDI, ERp29 was soluble and intact under conditions where malate dehydrogenase was largely sedimented (Figure 6B). Interestingly, ERp60 did sediment under these conditions in both the presence and absence of substrate, providing an unforeseen control for the experiment (results not shown). These results suggested that not only was ERp29 stable in the assays at 45 °C, but also ERp29 did not form substantial associations with the denatured protein substrates. Because stoichiometric interaction with denatured proteins is a hallmark of chaperones, we investigated this aspect further using another approach that involved different substrates and no heating. Cross-linking analysis was used to assay for stable interactions between ERp29 and carboxyamidomethylated RNase, a commonly used model of denatured protein. No additional bands indicative of ERp29/RNase adducts were detected under conditions where the ERp29 homodimer was cross-linked effectively, and equivalent results were obtained using carboxyamidomethylated lysozyme as substrate (not shown).
DISCUSSION
To our knowledge, this is the first reported purification to homogeneity and direct functional analysis of ERp29, a major resident of the ER with seemingly general importance in animal cells. Using this native preparation, we have found that ERp29 lacks classical chaperone, PDI and calcium-binding activities that typify other major reticuloplasmins. A characteristic set of biochemical hallmarks that can be used to classify ERp29 homologues in the absence of a known activity has also been established. Contradicting repeated predictions about chaperone activity, these findings expose ERp29 as a functionally distinct member of the ER machinery and prompt a revised hypothesis that ERp29 acts as a non-conventional folding assistant.
The preparation of ERp29 isolated here from rat liver appears well suited for use in structural and functional investigations, being made in gentle conditions and exhibiting high purity. No harsh steps such as heating or precipitation were used, and the procedure was readily completed within 4 days, largely expedited by the striking enrichment achieved on heparin. High purity was evident at the molecular (SDS/PAGE, HPLC, Edman, MS) and activity levels, and integrity of the full-length sequence was verified by Edman and mass spectrometric analysis. Although less than typically offered by recombinant approaches, the yield was sufficient to make a variety of biochemical analyses practicable.
The present study has identified three biochemical hallmarks of ERp29 that reflect both its native folded structure in solution and its primary structure during denaturing PAGE. Distinguishing ERp29 from the classical reticuloplasmins, these features will be particularly useful for characterizing orthologues and recombinant analogues of ERp29. First, the finding that ERp29 binds heparin is invaluable since this property is shared by a minority of proteins and depends on the folded protein structure. Heparin binding usually involves surface patches of basic residues, several of which are apparent in ERp29 [18]. Affinity for heparin appears to be a conserved feature because we have successfully isolated ERp29 orthologues from human, possum and chicken using the same chromatographic conditions as detailed here for rat ([16]; and M. J. Hubbard, unpublished work). Interestingly, a sulphotransferase involved in heparin production was escorted to the Golgi apparatus by a distant relative of ERp29, Windbeutel, whereas ERp29 lacked this activity [25]. Any physiological relevance of the binding of ERp29 to heparin remains to be determined, but the apparent exclusion of ERp29 from the Golgi, where most heparin-related activities take place, is noteworthy [12,17,22]. Secondly, we have found that native ERp29 behaves as a compact homodimer in solution, or, in other words, does not behave as a typical 51 kDa protein hydrodynamically. This idiosyncratic behaviour was seen consistently during the size-exclusion and sucrose-gradient analyses, and parallels our previous results from size-exclusion analysis of microsomal lysates [19]. Non-globularity can also be inferred from the modelled structures of ERp29 and Windbeutel [18,25], suggesting that the compact shape of ERp29 is also conserved like its affinity for heparin. It is unclear whether contradictory reports that ERp29 behaves as a globular dimer during size-exclusion reflect procedural differences or the use of recombinant instead of native ERp29 [20,22]. Thirdly, extending earlier evidence [16,22], we have established by MS that the anomalously high Mr of ERp29 on SDS/PAGE is an intrinsic property of the undecorated polypeptide, not a result of post-translational additions. It appears from the corrective effect of lysine modification with BS3 (Figure 3A) that this unusual mobility could be due to the exceptionally high content of lysine (11% of residues) in ERp29. Precedents exist for this with other lysine-rich proteins [41]. Moreover, by showing that the Mr of ERp29 varies depending on the separation system used, the Ferguson analysis might partly explain the diversity of literature values (26–32 kDa; [9]). These three characteristics collectively delineate ERp29 from its peers PDI, ERp60, calreticulin, endoplasmin and BiP. That is, ERp29 exists uniquely as a tight homodimer, only calreticulin shares an anomalous Mr on SDS/PAGE, and only ERp60 and BiP bind to heparin like ERp29.
Undermining previous supposition, consistent direct evidence that ERp29 lacks classical protein-folding activities emerged here using different assay approaches and with parallel-purified reticuloplasmins as positive controls. However, we cannot dismiss the possibility that particular requirements of ERp29 were unmet during these analyses, perhaps relating to essential co-factors or substrate specificity, for example. Most surprisingly, ERp29 exhibited no chaperone activity when tested with a thermal-protection approach applied successfully to all classical chaperone classes [40] and with the cross-linking approach that traps ER chaperones bound to their substrates [42]. These results disfavour the idea that ERp29 has conventional chaperone roles akin to those established for BiP, endoplasmin, calreticulin and PDI. Notably, however, the contentious absence of BiP-like stress-response elements in ERp29 [10,11,22,26] and the difficulty of demonstrating stable interactions with other proteins in the ER [23,42] can now be viewed harmoniously. Although our analyses embraced four different substrates and two denaturation modes, potential still remains to uncover favoured substrates for ERp29 and helper roles with other chaperones besides calreticulin. Our results also did not support the possibilities that ERp29 has helper activity towards PDI or ERp60, or disulphide-editing activities like those retained in monothiol variants of PDI. The observed lack of ascorbate reductase, glutathione peroxidase and glutathione S-transferase activities suggests further that ERp29 is not a functional analogue of other thioredoxin-superfamily members, including the atypical glutaredoxins that bear gross structural resemblance to ERp29 [39]. In the absence of these activities, more-focused questions can now be asked about the strict conservation of the monothiol of ERp29 at its unusual location outside of the thioredoxin-fold domain. Indeed, using site-directed mutagenesis, we have recently obtained evidence suggesting that this residue might affect the structural behaviour of ERp29 (V. M. Hermann, J. F. Cutfield and M. J. Hubbard, unpublished work). Finally, because not all calcium-binding proteins are immediately recognizable at the sequence level, it was appropriate to assay ERp29 for this property directly. The resulting implication that ERp29 is neither modulated directly by calcium nor serves a calcium-storage role is consistent with previous evidence from sequence analysis, subcellular localization and expression profiling [10,17,19]. It follows from the lack of calcium binding and its relatively small size that ERp29 will probably have a narrower functional repertoire than the other major reticuloplasmins, which are all multifunctional.
In conclusion, we reflect that functional orphans are a burgeoning and difficult problem, and that housekeeping proteins such as ERp29 are among those most worthy of immediate attention. Pure native protein is a valuable resource for de novo functional analysis, and this goal has now been accomplished for ERp29. Using this preparation, we have ruled out the chaperone and PDI activities raised as prime candidates by our current understanding. While surprising in several regards, this outcome does fit the long-standing view that ERp29 is likely to be a functionally distinct entity [10]. Evidence that ERp29 lacks classical protein-folding activities is an important advance that, by underscoring the biochemical uniqueness of ERp29, should help the field to sculpt meaningful enquiry in future studies of structure–function relationships, interaction partners, gene silencing and the like. As conveyed by the revised hypothesis arising from the present study, exploration of non-classical folding activities is now strongly justified for ERp29. Candidate activities can be proposed by analogy with the chaperone-partner roles of ERp60 and the ERdj proteins [43,44], and recognizing that hypothetical roles remain vacant in the ER (e.g. modulators of Ero1 and thiol retention [45,46], and analogues of TPR (tetratricopeptide)-clamp/Bag domain co-chaperones and glutaredoxin [47]). The continued identification of novel reticuloplasmins [48–50] also necessitates ongoing appraisal of classical roles, as imposed on PDI by Ero1 [45]. Hence, for physiological relevance, it will be important to address ERp29 not only in splendid isolation as in the present study, but also in broader proteomic contexts like those that spawned its discovery [9].
Acknowledgments
We thank Patricia Flawn, Corey Moir and Diana Carne for their skilled technical assistance, and Dr Gillian Norris for helping with MS on the triple quadrupole instrument. We are also grateful to our other colleagues for their helpful suggestions and support. This work was primarily funded by New Zealand Lottery Health Research and the Health Research Council of New Zealand [HRC(NZ)]. M. J. H. was a Senior Research Fellow of the HRC(NZ), and acknowledges recent support from the Melbourne Research Unit for Facial Disorders and Melbourne University.
References
- 1.Kim P. S., Arvan P. Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: disorders of protein trafficking and the role of ER molecular chaperones. Endocr. Rev. 1998;19:173–202. doi: 10.1210/edrv.19.2.0327. [DOI] [PubMed] [Google Scholar]
- 2.Mattson M. P., LaFerla F. M., Chan S. L., Leissring M. A., Shepel P. N., Geiger J. D. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000;23:222–229. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- 3.Shusta E. V., Raines R. T., Pluckthun A., Wittrup K. D. Increasing the secretory capacity of Saccharomyces cerevisiae for production of single-chain antibody fragments. Nat. Biotechnol. 1998;16:773–777. doi: 10.1038/nbt0898-773. [DOI] [PubMed] [Google Scholar]
- 4.Freedman R. B., Klappa P., Ruddock L. W. Protein disulfide isomerases exploit synergy between catalytic and specific binding domains. EMBO Rep. 2002;3:136–140. doi: 10.1093/embo-reports/kvf035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gething M. J. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- 6.Michalak M., Robert Parker J. M., Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 7.Argon Y., Simen B. B. GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol. 1999;10:495–505. doi: 10.1006/scdb.1999.0320. [DOI] [PubMed] [Google Scholar]
- 8.Ellgaard L., Molinari M., Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 9.Hubbard M. J. Functional proteomics: the goalposts are moving. Proteomics. 2002;2:1069–1078. doi: 10.1002/1615-9861(200209)2:9<1069::AID-PROT1069>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Demmer J., Zhou C., Hubbard M. J. Molecular cloning of ERp29, a novel and widely expressed resident of the endoplasmic reticulum. FEBS Lett. 1997;402:145–150. doi: 10.1016/s0014-5793(96)01513-x. [DOI] [PubMed] [Google Scholar]
- 11.Sargsyan E., Baryshev M., Backlund M., Sharipo A., Mkrtchian S. Genomic organization and promoter characterization of the gene encoding a putative endoplasmic reticulum chaperone, ERp29. Gene. 2002;285:127–139. doi: 10.1016/s0378-1119(02)00417-1. [DOI] [PubMed] [Google Scholar]
- 12.Shnyder S. D., Hubbard M. J. ERp29 is a ubiquitous resident of the endoplasmic reticulum with a distinct role in secretory protein production. J. Histochem. Cytochem. 2002;50:557–566. doi: 10.1177/002215540205000413. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg E., Levanon E. Y. Human housekeeping genes are compact. Trends Genet. 2003;19:362–365. doi: 10.1016/S0168-9525(03)00140-9. [DOI] [PubMed] [Google Scholar]
- 14.van Anken E., Romijn E. P., Maggioni C., Mezghrani A., Sitia R., Braakman I., Heck A. J. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity. 2003;18:243–253. doi: 10.1016/s1074-7613(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 15.Toronen P., Storvik M., Linden A. M., Kontkane O., Marvanova M., Lakso M., Castren E., Wong G. Expression profiling to understand actions of NMDA/glutamate receptor antagonists in rat brain. Neurochem. Res. 2002;27:1209–1220. doi: 10.1023/a:1020985611667. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard M. J., McHugh N. J. Human ERp29: isolation, primary structural characterisation and two-dimensional gel mapping. Electrophoresis. 2000;21:3785–3796. doi: 10.1002/1522-2683(200011)21:17<3785::AID-ELPS3785>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Macleod J. C., Sayer R. J., Lucocq J. M., Hubbard M. J. ERp29, a general endoplasmic reticulum marker, is highly expressed throughout the brain. J. Comp. Neurol. 2004;477:29–42. doi: 10.1002/cne.20222. [DOI] [PubMed] [Google Scholar]
- 18.Liepinsh E., Baryshev M., Sharipo A., Ingelman-Sundberg M., Otting G., Mkrtchian S. Thioredoxin fold as homodimerization module in the putative chaperone ERp29: NMR structures of the domains and experimental model of the 51 kDa dimer. Structure. 2001;9:457–471. doi: 10.1016/s0969-2126(01)00607-4. [DOI] [PubMed] [Google Scholar]
- 19.Hubbard M. J., McHugh N. J., Carne D. L. Isolation of ERp29, a novel endoplasmic reticulum protein, from rat enamel cells evidence for a unique role in secretory-protein synthesis. Eur. J. Biochem. 2000;267:1945–1957. doi: 10.1046/j.1432-1327.2000.01193.x. [DOI] [PubMed] [Google Scholar]
- 20.Mkrtchian S., Baryshev M., Matvijenko O., Sharipo A., Sandalova T., Schneider G., Ingelman-Sundberg M., Mkrtchiana S. Oligomerization properties of ERp29, an endoplasmic reticulum stress protein. FEBS Lett. 1998;431:322–326. doi: 10.1016/s0014-5793(98)00786-8. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari D. M., Soling H. D. The protein disulphide-isomerase family: unravelling a string of folds. Biochem. J. 1999;339:1–10. [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari D. M., Nguyen Van P., Kratzin H. D., Soling H. D. ERp28, a human endoplasmic-reticulum-lumenal protein, is a member of the protein disulfide isomerase family but lacks a CXXC thioredoxin-box motif. Eur. J. Biochem. 1998;255:570–579. doi: 10.1046/j.1432-1327.1998.2550570.x. [DOI] [PubMed] [Google Scholar]
- 23.Sargsyan E., Baryshev M., Szekely L., Sharipo A., Mkrtchian S. Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex. J. Biol. Chem. 2002;277:17009–17015. doi: 10.1074/jbc.M200539200. [DOI] [PubMed] [Google Scholar]
- 24.Konsolaki M., Schüpbach T. windbeutel, a gene required for dorsoventral patterning in Drosophila, encodes a protein that has homologies to vertebrate proteins of the endoplasmic reticulum. Genes Dev. 1998;12:120–131. doi: 10.1101/gad.12.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Q., Guo C., Barnewitz K., Sheldrick G. M., Soling H. D., Uson I., Ferrari D. M. Crystal structure and functional analysis of Drosophila Wind, a protein-disulfide isomerase-related protein. J. Biol. Chem. 2003;278:44600–44607. doi: 10.1074/jbc.M307966200. [DOI] [PubMed] [Google Scholar]
- 26.Mkrtchian S., Fang C., Hellman U., Ingelman-Sundberg M. A stress-inducible rat liver endoplasmic reticulum protein, ERp29. Eur. J. Biochem. 1998;251:304–313. doi: 10.1046/j.1432-1327.1998.2510304.x. [DOI] [PubMed] [Google Scholar]
- 27.Hubbard M. J. Calbindin 28 kDa and calmodulin are hyperabundant in rat dental enamel cells. Identification of the protein phosphatase calcineurin as a principal calmodulin target and of a secretion-related role for calbindin 28 kDa. Eur. J. Biochem. 1995;230:68–79. doi: 10.1111/j.1432-1033.1995.tb20535.x. [DOI] [PubMed] [Google Scholar]
- 28.Hubbard M. J., McHugh N. J. Mitochondrial ATP synthase F1-β-subunit is a calcium-binding protein. FEBS Lett. 1996;391:323–329. doi: 10.1016/0014-5793(96)00767-3. [DOI] [PubMed] [Google Scholar]
- 29.Iida K. I., Miyaishi O., Iwata Y., Kozaki K. I., Matsuyama M., Saga S. Distinct distribution of protein disulfide isomerase family proteins in rat tissues. J. Histochem. Cytochem. 1996;44:751–759. doi: 10.1177/44.7.8675996. [DOI] [PubMed] [Google Scholar]
- 30.Hillson D. A., Lambert N., Freedman R. B. Formation and isomerization of disulfide bonds in proteins: protein disulfide-isomerase. Methods Enzymol. 1984;107:281–294. doi: 10.1016/0076-6879(84)07018-x. [DOI] [PubMed] [Google Scholar]
- 31.Stahl R. L., Liebes L. F., Farber C. M., Silber R. A spectrophotometric assay for dehydroascorbate reductase. Anal. Biochem. 1983;131:341–344. doi: 10.1016/0003-2697(83)90180-x. [DOI] [PubMed] [Google Scholar]
- 32.Xu D. P., Washburn M. P., Sun G. P., Wells W. W. Purification and characterization of a glutathione dependent dehydroascorbate reductase from human erythrocytes. Biochem. Biophys. Res. Commun. 1996;221:117–121. doi: 10.1006/bbrc.1996.0555. [DOI] [PubMed] [Google Scholar]
- 33.Mannervik B., Guthenberg C. Glutathione transferase (human placenta) Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- 34.Schepetkin I. A. Lucigenin as a substrate of microsomal NAD(P)H-oxidoreductases. Biochemistry (Moscow) 1999;64:25–32. [PubMed] [Google Scholar]
- 35.Saito Y., Ihara Y., Leach M. R., Cohen-Doyle M. F., Williams D. B. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999;18:6718–6729. doi: 10.1093/emboj/18.23.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirs C. H. Reduction and S-carboxymethylation of proteins. Methods Enzymol. 1967;11:199–203. [Google Scholar]
- 37.Walker K. W., Lyles M. M., Gilbert H. F. Catalysis of oxidative protein folding by mutants of protein disulfide isomerase with a single active-site cysteine. Biochemistry (Moscow) 1996;35:1972–1980. doi: 10.1021/bi952157n. [DOI] [PubMed] [Google Scholar]
- 38.O'Neill S., Robinson A., Deering A., Ryan M., Fitzgerald D. J., Moran N. The platelet integrin αIIbβ3 has an endogenous thiol isomerase activity. J. Biol. Chem. 2000;275:36984–36990. doi: 10.1074/jbc.M003279200. [DOI] [PubMed] [Google Scholar]
- 39.Board P. G., Coggan M., Chelvanayagam G., Easteal S., Jermiin L. S., Schulte G. K., Danley D. E., Hoth L. R., Griffor M. C., Kamath A. V., et al. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J. Biol. Chem. 2000;275:24798–24806. doi: 10.1074/jbc.M001706200. [DOI] [PubMed] [Google Scholar]
- 40.Buchner J., Grallert H., Jakob U. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Methods Enzymol. 1998;290:323–338. doi: 10.1016/s0076-6879(98)90029-5. [DOI] [PubMed] [Google Scholar]
- 41.Georgieva E. I., Sendra R. Mobility of acetylated histones in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 1999;269:399–402. doi: 10.1006/abio.1999.4050. [DOI] [PubMed] [Google Scholar]
- 42.Meunier L., Usherwood Y. K., Chung K. T., Hendershot L. M. A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol. Biol. Cell. 2002;13:4456–4469. doi: 10.1091/mbc.E02-05-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellgaard L., Frickel E. M. Calnexin, calreticulin, and ERp57: teammates in glycoprotein folding. Cell Biochem. Biophys. 2003;39:223–247. doi: 10.1385/CBB:39:3:223. [DOI] [PubMed] [Google Scholar]
- 44.Shen Y., Meunier L., Hendershot L. M. Identification and characterization of a novel endoplasmic reticulum (ER) DnaJ homologue, which stimulates ATPase activity of BiP in vitro and is induced by ER stress. J. Biol. Chem. 2002;277:15947–15956. doi: 10.1074/jbc.M112214200. [DOI] [PubMed] [Google Scholar]
- 45.Tu B. P., Weissman J. S. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tagliavacca L., Anelli T., Fagioli C., Mezghrani A., Ruffato E., Sitia R. The making of a professional secretory cell: architectural and functional changes in the ER during B lymphocyte plasma cell differentiation. Biol. Chem. 2003;384:1273–1277. doi: 10.1515/BC.2003.141. [DOI] [PubMed] [Google Scholar]
- 47.Young J. C., Barral J. M., Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem. Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Alanen H. I., Williamson R. A., Howard M. J., Lappi A. K., Jantti H. P., Rautio S. M., Kellokumpu S., Ruddock L. W. Functional characterization of ERp18, a new endoplasmic reticulum-located thioredoxin superfamily member. J. Biol. Chem. 2003;278:28912–28920. doi: 10.1074/jbc.M304598200. [DOI] [PubMed] [Google Scholar]
- 49.Anelli T., Alessio M., Bachi A., Bergamelli L., Bertoli G., Camerini S., Mezghrani A., Ruffato E., Simmen T., Sitia R. Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J. 2003;22:5015–5022. doi: 10.1093/emboj/cdg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knoblach B., Keller B. O., Groenendyk J., Aldred S., Zheng J., Lemire B. D., Li L., Michalak M. ERp19 and ERp46, new members of the thioredoxin family of endoplasmic reticulum proteins. Mol. Cell. Proteomics. 2003;2:1104–1119. doi: 10.1074/mcp.M300053-MCP200. [DOI] [PubMed] [Google Scholar]