Abstract
Background
Neighborhood socioeconomic deprivation has been linked to adverse health outcomes, yet it is unclear whether neighborhood-level social determinants of health (SDOH) measures affect overall survival in adolescent and young adult patients with cancer.
Methods
This study used a diverse cohort of adolescent and young adult patients with cancer (N = 10 261) seen at MD Anderson Cancer Center. Zip codes were linked to Area Deprivation Index (ADI) values, a validated neighborhood-level SDOH measure, with higher ADI values representing worse SDOH.
Results
ADI was statistically significantly worse (P < .050) for Black (61.7) and Hispanic (65.3) patients than for White patients (51.2). Analysis of ADI by cancer type showed statistically significant differences, mainly driven by worse ADI in patients with cervical cancer (62.3) than with other cancers. In multivariable models including sex, age at diagnosis, cancer diagnosis, and race and ethnicity, risk of shorter survival for people residing in neighborhoods with the least favorable ADI quartile was greater than for individuals in the most favorable ADI quartile (hazard ratio = 1.09, 95% confidence interval = 1.00 to 1.19, P = .043).
Conclusion
Adolescent and young adult patients with cancer and the worst ADI values experienced a nearly 10% increase in risk of dying than patients with more favorable ADI values. This effect was strongest among White adolescent and young adult survivors. Although the magnitude of the effect of ADI on survival was moderate, the presence of a relationship between neighborhood-level SDOH and survival among patients who received care at a tertiary cancer center suggests that ADI is a meaningful predictor of survival. These findings provide intriguing evidence for potential interventions aimed at supporting adolescent and young adult patients with cancer from disadvantaged neighborhoods.
The adolescent and young adult cancer population continues to grow and is expected to surpass 85 000 new cases in 2023 (1). Previous studies have identified survival disparities among adolescent and young adult cancer survivors, specifically noting poorer survival in Hispanic and Black adolescent and young adult patients with cancer than in White patients (2). Other studies have observed disparities in survival when comparing neighborhood type, insurance status, and poverty level (3-6). Though past research has highlighted these disparities in survival within the adolescent and young adult cancer population (7), the underlying sources for these disparities remain unclear.
Social determinants of health (SDOH) are the nonmedical factors that affect health outcomes (8). The adolescent and young adult community faces a unique set of challenges when it comes to SDOH, including difficulty with access to health care, finances, employment, social support, and housing. Thus, nonmedical factors can greatly affect the survival of this population and implicate SDOH as an important variable to study in the context of cancer outcomes in adolescent and young adult patients. Socioeconomic status and health insurance status are among the factors that have been shown to negatively affect overall survival and other outcomes in adolescent and young adult populations with cancer (9,10). The Area Deprivation Index (ADI) is a validated composite score and neighborhood measure of SDOH that incorporates 17 factors that reflect neighborhood housing quality, household characteristics, education quality, income, and employment (8). Recent analyses of ADI as an indicator of SDOH have underscored an independent impact of the local environment on cancer outcomes in diverse populations, even when accounting for the individual SDOH factors (11-13). Though there has been research assessing the impact of individual SDOH components in the setting of adolescent and young adult disparities, studies have yet to use a composite SDOH measure that focuses on the neighborhood environment to investigate cancer outcomes among adolescent and young adult patients.
In this study, we investigated the role of overall neighborhood-level SDOH via the ADI measure in observed survival disparities using a large, racially and ethnically diverse cohort of adolescent and young adult patients with cancer from a single institution. The research conducted in this study was designed to address 3 objectives: 1) to understand neighborhood-level socioeconomic disparities in the adolescent and young adult population, 2) to study the relationship between ADI and overall survival, and 3) to investigate the relationship between ADI and overall survival for different racial and ethnic groups. This project was established to help alleviate the significant knowledge gap regarding the association between neighborhood-level SDOH and disparities in overall survival in the adolescent and young adult patient population.
Methods
Study population
Study participants (N = 10 261) included adolescent and young adult patients with cancer diagnosed between the ages of 15 and 39 years and who received treatment at MD Anderson Cancer Center between 2000 and 2016. Patient and tumor characteristics (race and ethnicity, sex, age at diagnosis, date of diagnosis, cancer diagnosis, vital status, date of last follow-up, and zip code at presentation) were obtained from our institutional tumor registry. Race and ethnicity information was collected as Black, White, Hispanic, Asian, American Indian/Alaskan Native, other, or unknown. Due to the limited numbers of individuals in the Asian (n = 33), American Indian/Alaskan Native (n = 16), other (n = 601), or unknown (n = 5) categories, these patients were grouped as “Other”. Individuals with a prior diagnosis of childhood cancer were excluded from this study. This study was approved by the Institutional Review Board of MD Anderson Cancer Center.
Area Deprivation Index
The ADI from the Public Health Neighborhood Atlas is a composite of 17 SDOH measures (14). Patient zip codes at presentation to MD Anderson Cancer Center were linked to the ADI national ranking values and used for analyses in this study. The ADI is represented as a percentile ranging from 0% to 100%, with the 50% point denoting the “national midpoint.” A low ADI score suggests affluence or prosperity, while a high ADI score signifies elevated levels of deprivation. International patients and patients without zip code information were excluded from analysis.
Statistical analyses
Student t tests or analysis of variance were used to compare ADI values by patient characteristic. Survival was defined as the duration between date of diagnosis and death from any cause or last follow-up, as recorded by the institution’s tumor registry. Cox proportional hazards ratios (HRs) and 95% confidence intervals (CIs) were calculated for survival statistics by ADI mean, median, and quartile. Multivariate analyses were adjusted for sex, age at diagnosis (continuous variable), cancer diagnosis, and race and ethnicity, unless the variable was a stratification factor. Biological (additive) interaction analyses for race and ethnicity and ADI on overall survival were conducted using the method of Andersson et al. (15). Interactions were evaluated through interpretation of the synergy index [SI = (HR11 ‒1) / (HR10 ‒1) + (HR01 ‒1)], with values less than 1 indicative of negative (antagonistic) effects and values greater than 1 indicative of positive (synergistic) effects (15). All other analyses were conducted using Stata, version 17, software (StataCorp, College Station, TX), with a 2-sided P = .050 set as the threshold of statistical significance.
Results
Patient population
The study population of 10 261 adolescent and young adult patients with cancer was diverse in terms of race and ethnicity, with nearly 40% of the population identifying as non-White race or ethnicity—10.8% Black, 20.8% Hispanic, and 6.4% other races and ethnicities (Table 1). Breast cancer was the most common diagnosis, at 17.6%. The diagnoses with at least 400 patients in the population are shown in Table 1. There were slightly more female patients (55.6%) than male patients (44.4%), and a majority of individuals were diagnosed in the Young Adult age category, defined as individuals 26 to 39 years of age (73.3%). Over a median follow-up of 7.3 years, 4415 deaths were recorded, with more than 80% of the population surviving more than 2 years after diagnosis. Of note, the 5-year survival rate for this population was 65.8%, which is lower than the 85% reported for the adolescent and young adult cancer population nationally, likely because of the high number of patients with relapsed-refractory disease seen at MD Anderson Cancer Center.
Table 1.
Population characteristics
| Area Deprivation Index quartile |
|||||
|---|---|---|---|---|---|
| Total, No. (%) | Q1 (0-36.8) | Q2 (36.9-56.1) | Q3 (56.2-73.5) | Q4 (73.6-100) | |
| Self-identified race or ethnicity | 10 261 | ||||
| Black | 1112 (10.8) | 152 (5.9) | 256 (10.0) | 344 (13.3) | 360 (14.1) |
| Hispanic | 2133 (20.9) | 261 (10.2) | 428 (16.7) | 503 (20.0) | 941 (36.9) |
| White | 6361 (62.0) | 1850 (72.1) | 1713 (66.8) | 1615 (62.6) | 1183 (46.4) |
| Other | 655 (6.4) | 303 (11.8) | 168 (6.6) | 117 (4.5) | 67 (2.6) |
| Cancer type | |||||
| Breast | 1810 (17.6) | 506 (19.7) | 438 (17.1) | 429 (16.6) | 437 (17.1) |
| Cervical | 401 (3.9) | 56 (2.2) | 85 (3.3) | 112 (4.3) | 148 (5.8) |
| Central nervous system | 408 (4.0) | 114 (4.4) | 97 (3.8) | 105 (4.1) | 92 (3.6) |
| Colorectal | 648 (6.3) | 172 (6.7) | 168 (6.6) | 161 (6.2) | 147 (5.8) |
| Germ-cell tumor | 703 (6.9) | 138 (5.4) | 181 (7.1) | 188 (7.30 | 196 (7.7) |
| Hodgkin lymphoma | 964 (9.4) | 243 (9.5) | 254 (9.9) | 269 (10.4) | 198 (7.8) |
| Leukemia | 1370 (13.4) | 314 (12.2) | 323 (12.6) | 344 (13.3) | 389 (15.3) |
| Non-Hodgkin lymphoma | 843 (8.2) | 212 (8.3) | 241 (9.4) | 199 (7.7) | 191 (7.5) |
| Sarcoma | 1179 (11.5) | 314 (12.2) | 307 (12.0) | 294 (11.4) | 264 (10.4) |
| Other | 1935 (18.9) | 497 (19.4) | 471 (18.4) | 478 (18.5) | 489 (19.2) |
| Sex | |||||
| Female | 5701 (55.6) | 1440 (56.1) | 1422 (55.4) | 1417 (54.9) | 1422 (55.7) |
| Male | 4560 (44.4) | 1126 (43.9) | 1143 (44.6) | 1162 (45.1) | 1129 (44.3) |
| Age at diagnosis, median, y | 31.5 | 32 | 32 | 31 | 31 |
| Diagnosis, by age group | |||||
| Adolescent: 15-18 y | 752 (7.3) | 172 (6.7) | 191 (7.5) | 192 (7.4) | 197 (7.7) |
| Emerging adult: 19-25 y | 1984 (19.3) | 444 (17.3) | 479 (18.7) | 544 (21.1) | 517 (20.3) |
| Young adult: 26-39 y | 7525 (73.3) | 1950 (76.0) | 1895 (73.9) | 1843 (71.5) | 1837 (72.0) |
| 5-y survival rate, % | 65.8 | 67.4 | 67.3 | 64.4 | 64.2 |
| Follow-up time, median (IQR), ya | 7.3 (2.9-12.2) | 7.3 (3.0-11.9) | 7.4 (3.1-12.5) | 7.0 (2.7-11.9) | 7.2 (2.8-12.6) |
| Vital status | |||||
| Alive | 5801 (56.5) | 1495 (58.4) | 1470 (57.5) | 1431 (55.8) | 1405 (55.4) |
| Dead | 4415 (43.0) | 1063 (41.6) | 1087 (42.5) | 1133 (44.2) | 1132 (44.6) |
IQR = intra-quartile range.
ADI, by patient characteristics
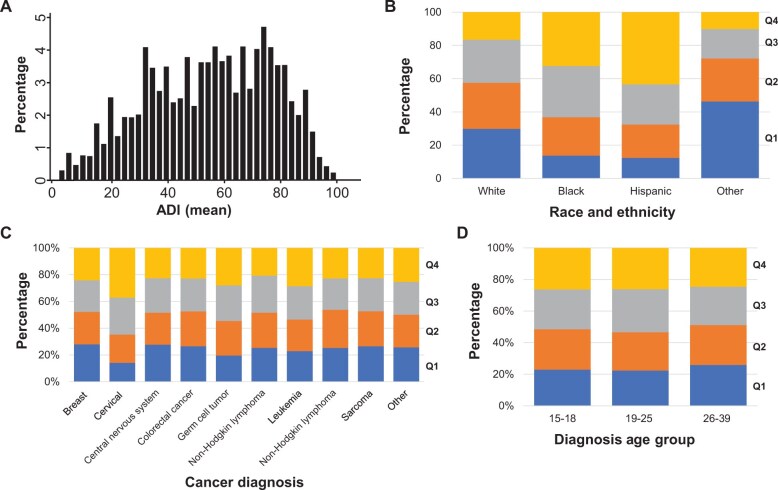
Overall, the mean (SD) ADI value for the adolescent and young adult cancer population was 54.7 (22.4) (Figure 1, A). Mean ADI was statistically significantly different by race and ethnicity (P < .050), with Hispanic (65.3) and Black (61.7) adolescent and young adult patients living in neighborhoods with worse area deprivation. When assessing the ADI distribution by quartiles (Figure 1, B), Hispanic and Black adolescent and young adult patients with cancer had a higher proportion of patients residing in neighborhoods with the highest levels of deprivation (quartile [Q] 4: 44% and 32%, respectively) compared with White patients and patients of other races and ethnicities (19% and 10%, respectively). Statistically significant differences in mean ADI were observed by cancer diagnosis. Notably, patients diagnosed with cervical cancer had a statistically significantly worse ADI value (62.3) than patients with other cancer diagnoses (pair-wise P < .050). Of note, the largest proportion of cancer diagnoses for patients within the Q4 ADI were for cervical cancer (Figure 1, C). ADI was statistically significantly different between diagnosis age groups (P < .0100), with the highest mean ADI among patients in the emerging adult category, defined as those diagnosed between 19 and 25 years of age. The distribution of ADI quartiles, however, did not differ between age groups (Figure 1, D). There were no statistically significant differences in ADI by sex or follow-up time category, defined as less than or more than 2 years.
Figure 1.
ADI, by patient characteristics. A) overall distribution of ADI values in the population. B) distribution of ADI quartiles, by race and ethnicity. C) distribution of ADI quartiles, by cancer diagnosis. D) distribution of ADI quartiles, by diagnosis age group. ADI = Area Deprivation Index; Q = quartile.
Survival analysis
Hispanic patients experienced favorable survival, and Black patients had worse outcomes compared to other groups (Figure 2, A). When accounting for sex, age at diagnosis, cancer diagnosis, and race and ethnicity, adolescent and young adult patients with cancer residing in the neighborhoods with the highest levels of area deprivation (Q4) had poorer survival than individuals living in areas with the lowest levels (Q1 HR = 1.09, 95% CI = 1.00 to 1.19, P = .043) (Table 2 and Figure 2, B). This statistically significant effect is evident in unadjusted and adjusted models for survival when analyzed by ADI as a continuous variable and median (Table 2). The effect sizes for Q3 and Q4 of the ADI were similar to each other, while Q2 did not show a statistically significant increase in hazard ratio compared with Q1. Therefore, we used median ADI categories for subsequent analyses (Figure 2, C).
Figure 2.
Overall survival in adolescent and young adult cancer survivors. A) race and ethnicity. B) ADI quartiles. C) ADI medians. ADI = Area Deprivation Index.
Table 2.
Overall survival, by ADI value
| 5-y survival rate, % | Log-rank P | Hazard ratio (95% confidence interval) a | P | |
|---|---|---|---|---|
| ADI continuous | 65.80 | 1.002 (1.000 to 1.003) | .011 | |
| ADI quartile | .082 | |||
| 0-25 | 64.46 | (Referent) | ||
| 26-49 | 64.44 | 1.03 (0.94 to 1.12) | .53 | |
| 50-74 | 61.97 | 1.10 (1.01 to 1.20) | .025 | |
| 75-100 | 61.58 | 1.09 (1.00 to 1.19) | .043 | |
| ADI value, median | < .0100 | |||
| Favorable | 64.45 | (Referent) | ||
| Unfavorable | 61.77 | 1.08 (1.02 to 1.15) | .012 |
Adjusted for sex, diagnosis age group, cancer type diagnosis, and race and ethnicity. ADI = Area Deprivation Index.
To investigate the potential relationships between ADI and race and ethnicity, stratified analyses were performed. Neighborhood deprivation was a statistically significant factor affecting survival only in White adolescent and young adult patients, with individuals residing in neighborhoods with high deprivation having an 11% increase in risk (HR = 1.11, 95% CI = 1.03 to 1.20, P < .0100) compared with those in low deprivation neighborhoods (Table 3). In contrast, ADI was not associated with overall survival in Hispanic patients (HR = 0.97, 95% CI = 0.84 to 1.13, P = .73). Among Black patients, the effect size of worse ADI vs favorable ADI (HR = 1.11, 95% CI = 0.93 to 1.33) with regard to survival was similar to that among White patients, but the result was not statistically significant (P = .24). This lack of association may be the result of a limited sample size from the Black and Hispanic populations when stratified by ADI categories. In analysis of survival by race and ethnicity, Black race was statistically significantly associated with poor survival in both high and low deprivation neighborhoods, conferring approximately 20% increased risk in both ADI categories (Table 3). No statistically significant interactions between ADI and race and ethnicity affecting overall survival were identified (data not shown), but a suggestive antagonistic interaction between race and ADI for the Hispanic patient population was observed, with a synergy index of 0.20 (95% CI = 0.012 to 3.20) nearing a value below 1.
Table 3.
Overall survival, by race and ethnicity and ADI category
| 5-y survival rate, % | Log-rank P | Hazard ratio (95% confidence interval) a | P | |
|---|---|---|---|---|
| Black only (n = 1103) | .64 | |||
| ADI favorable (n = 404) | 59.03 | (Referent) | ||
| ADI unfavorable (n = 699) | 57.02 | 1.11 (0.93 to 1.33) | .24 | |
| Hispanic only (n = 2122) | .81 | |||
| ADI favorable (n = 685) | 64.63 | (Referent) | ||
| ADI unfavorable (n = 1437) | 64.96 | 0.97 (0.84 to 1.13) | .73 | |
| White only (n = 6331) | < .0100 | |||
| ADI favorable (n = 3553) | 65.01 | (Referent) | ||
| ADI unfavorable (n = 2778) | 61.09 | 1.11 (1.03 to 1.20) | < .0100 | |
| ADI favorable (n = 4642) | .038 | |||
| Black (n = 404) | 59.03 | 1.23 (1.05 to 1.43) | < .0100 | |
| Hispanic (n = 685) | 64.63 | 1.04 (0.91 to 1.19) | .55 | |
| White (n = 3553) | 65.01 | (Referent) | ||
| ADI unfavorable (n = 4914) | < .00100 | |||
| Black (n = 699) | 57.02 | 1.19 (1.06 to 1.35) | < .0100 | |
| Hispanic (n = 1437) | 64.96 | 0.94 (0.85 to 1.04) | .23 | |
| White (n = 2778) | 61.09 | (Referent) |
Adjusted for sex, diagnosis age group, and cancer type diagnosis. ADI = Area Deprivation Index.
Discussion
In this study, we evaluated a large (N = 10 261) and diverse cohort of adolescent and young adults with cancer from a single institution to explore the association between neighborhood-level SDOH and overall survival. Adolescent and young adult patients with cancer residing in areas with higher neighborhood-level deprivation experienced a nearly 10% increased risk of dying than those in lower-deprivation areas, implicating SDOH as valuable prognostic factor affecting long-term survival. The potential implications of this finding are concerning because this effect was observed in adolescent and young adult patients with cancer at a single comprehensive cancer center that is biased towards individuals who are insured.
Past studies that have used ADI as a measure of SDOH have found inverse relationships between deprivation levels and survival that are aligned with our findings (16,17). A study focusing on disparities among patients diagnosed with primary central nervous system lymphoma made the same conclusion concerning the impact of worse ADI on overall survival. Primary central nervous system lymphoma is different from the cancers included in this analysis in that it is typically a disease of older adults and has a poor prognosis, yet the similarity of the relationship between ADI and survival is interesting. Another study in adult breast, prostate, lung, and colorectal cancers described similar relationships between SDOH and overall survival, even when accounting for individual-level socioeconomic status (18). This study used Surveillance, Epidemiology, and End Results data to gather patient information, indicating that the patients obtained their medical care across the country rather than at a single institution, as in this study. In addition, their population consisted of older adult patients, which differs from the population of the current study (18,19). Although past study populations have different features from the current adolescent and young adult cancer population, it provides compelling evidence that research using ADI is needed within other populations—particularly among individuals that are underinsured or uninsured. Thus, our analysis is the first to extend the effect of SDOH to adolescent and young adult patients with cancer treated in a single institution and further supports the importance of neighborhood-level deprivation as a potential driver of the persistent survival disparities observed in this community.
Racial disparities in overall survival among adolescent and young adult patients with cancer have been reported previously (20,21). In an analysis of more than 80 000 adolescent and young adult patients with cancer from Texas, Black men and women had poorer 5-year survival rates than White individuals diagnosed with the same cancer (7). It is thought that structural racism within health care may contribute to these disparities (22-26). Studies have linked structural racism to poor physical and mental health outcomes, with studies also identifying differences in time to treatment and treatments offered to Black patients that would potentially contribute to differences in survival (27-32). Black adolescent and young adult patients with cancer in our cohort also experienced worse survival than White and Hispanic patients. This effect was consistent when stratified by ADI, suggesting that the survival disadvantage among Black adolescent and young adults with cancer is independent of their neighborhood environment. It is worth noting, however, that the current study participants were all treated at a single academic cancer center, which could limit generalizability to other cancer care settings. Furthermore, the effects of structural racism that affect access and receipt of care are often difficult to measure. More investigation is needed in this area that incorporates detailed individual-level data, such as time to treatment, disease stage at treatment, and frequency of follow-up care, to fully explore this relationship.
Conversely, the Hispanic adolescent and young adult cancer population had a more favorable survival rate than other adolescent and young adult patients with cancer, and this effect was not affected by ADI. The Hispanic paradox, or what some call the “barrio-advantage,” may contribute to the improved survival observed in our study, regardless of local neighborhood disadvantage (33,34). This paradox describes improved outcomes among Hispanic vs non-Hispanic communities thought to be a result of strong social networks and support. Although not significant, we did observe a suggestively, slightly antagonistic interaction between race and ethnicity and ADI in the analysis of our Hispanic patients compared with White adolescent and young adult patients with cancer. This finding is in line with the Hispanic paradox hypothesis, where strong social support and other potentially beneficial cultural factors associated with Hispanic communities attenuate the adverse effect of neighborhood socioeconomic deprivation. More research on the impact of intricate social networks, possibly through assessment of neighborhood social capital and other measures of network support, in Hispanic adolescent and young adult cancer populations may provide more context for our results with respect to this phenomenon.
Worse ADI representing poorer neighborhood environment was associated with a survival disadvantage among White adolescent and young adults with cancer, with an 11% increase in death among individuals living in neighborhoods with a worse ADI. Although White people make up the majority of the total number of people living in poverty within the United States, disparities between high-income and low-income Whites patients are far less well studied than those disparities for racial and ethnic groups (35). Our results provide evidence that ADI may contribute to disparities within the White community, though more research is needed in this setting. This finding also suggests a multifaceted relationship among ADI, race and ethnicity, and overall survival.
Adolescent and young adult patients diagnosed with cervical cancer resided in neighborhoods with statistically significantly worse neighborhood-level deprivation compared with other cancer diagnoses. Underlying disparities in cervical cancer screening and prevention may be the driver of this result. Research evaluating human papillomavirus (HPV) vaccination by geographic measures in the adolescent and young adult population has concluded that people’s local area can affect vaccination rates, suggesting an increase in cervical cancer incidence and reinforcing our findings in the present cervical cancer population (36,37). Despite the presence of HPV vaccinations as a method of cervical cancer prevention that became available to the public in 2006 (38,39), disparities in vaccination uptake continue (40,41). Furthermore, current vaccine recommendations are for the nonavalent formulation of the HPV vaccine, which was not approved until 2014 (42). At the end of the cohort diagnosis period (2000-2016), the HPV vaccination rate in the United States was only about 43% for the completed HPV series and 60% for greater than or equal to 1 vaccine dose (43). In addition, cervical cancer screening rates are affected by county-level vulnerability and area deprivation level, either because of socioeconomic issues or geographical inaccessibility (44,45). Another study using ADI found higher deprivation levels associated with decreased cervical cancer screening, lending support to our own conclusions (45). Differences in health literacy may also be a contributing factor. In Texas, health literacy has been shown to be a barrier in the Hispanic and Black community (46,47). Interventions directed at disadvantaged groups have supported the importance of group-specific education materials and their ability to increase health-care knowledge and perceptions (48). The timeline of HPV vaccine approval, vaccination rates, general vaccine hesitancy, and health literacy may have all influenced the results we saw in this cohort (49-51).
This study has several strengths, including long follow-up to enable survival assessment for a population that is characterized by favorable prognosis overall. The large sample size and the diversity of the population in terms of race and ethnicity, ADI, and cancer diagnoses enabled us to conduct robust stratified and interaction analyses. Information regarding disease stage at presentation would be beneficial for exploring whether ADI is linked to delays in treatment and higher disease stage at presentation, both factors that would directly affect survival. Detailed treatment information would also enhance the robustness of this analysis, and efforts to collect these data are ongoing to enable cancer type–specific analyses. The single-center cohort of patients treated at MD Anderson Cancer Center is a strength in that it minimizes concerns regarding the effect of access to care: All participants included in this analysis received best practices–driven care, reducing heterogeneity in the treatments received. The preexisting barriers patients face to gain access to care at MD Anderson Cancer Center, a tertiary cancer center, such as lack of health-care access, financial means, transportation, and geographical location, may introduce a sampling bias and reduce the transferability of our findings to other adolescent and young adult cancer populations. Based on these barriers, our cohort would be expected to have worse ADI values than the general population or individuals receiving care at community hospitals. For patients unable to obtain care at MD Anderson Cancer Center, they may have less access to highly specialized cancer care and novel clinical trials. These barriers may have attenuated some of our results because the prognostic features of ADI were identified among a population with relatively high SDOH. Future studies in adolescent and young adult cancer populations with lower socioeconomic status and patients seen in other care-delivery settings would be beneficial to fully establish the prognostic ability of ADI. We would expect a larger magnitude of effect of the inverse relationship between ADI and survival when studied in the broader adolescent and young adult population.
The composite ADI values used in the analysis were generated by patient zip codes. The exact addresses would have allowed for census block–based ADI by geocoding and enable investigation of other SDOH measures beyond that captured by ADI. Future studies with robust longitudinal residential information would be of interest to assess geographical changes between the time of diagnosis and time of censoring study participants. This consideration is important for the adolescent and young adult population because of their increased mobility during young adulthood and the potential impact of these transitions on ADI values.
In conclusion, this study demonstrated the effect of ADI on overall survival in the context of a racially and ethnic diverse cohort to address survival disparities within the adolescent and young adult cancer population. Overall, our findings implicate ADI as an important prognostic factor in adolescent and young adult cancer survival. This information warrants continued investigation to better explain the impact of ADI on the adolescent and young adult population. With more investigation, ADI may guide individualized social interventions if it proves useful as a screening tool. Overall, the intriguing findings underscore the need for continued support in disadvantaged populations.
Acknowledgements
This work was presented as an abstract at the American Society for Clinical Oncology annual meeting in 2023 (E.R.R.). The study sponsors had no role in the design, collection, analysis, or interpretation of the data; writing of the manuscript; or decision to submit the manuscript for publication.
Contributor Information
Elizabeth R Rodriguez, Department of Lymphoma/Myeloma, University of Texas MD Anderson Cancer Center, Houston, TX, USA; UTHealth Houston McGovern Medical School, Houston, TX, USA.
Tori Tonn, Department of Lymphoma/Myeloma, University of Texas MD Anderson Cancer Center, Houston, TX, USA; San Juan Bautista School of Medicine, Caguas, PR, USA.
Midhat Jafry, Department of Lymphoma/Myeloma, University of Texas MD Anderson Cancer Center, Houston, TX, USA; UTHealth Houston McGovern Medical School, Houston, TX, USA.
Sairah Ahmed, Department of Lymphoma/Myeloma, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Branko Cuglievan, Division of Pediatrics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
J Andrew Livingston, Department of Sarcoma Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Christopher R Flowers, Department of Lymphoma/Myeloma, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Gregory J Aune, UTHealth San Antonio Greehey Children’s Cancer Research Institute, San Antonio, TX, USA.
Karen H Albritton, Cook Children’s Hospital, Fort Worth, TX, USA.
Michael E Roth, Division of Pediatrics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Qian Xiao, Department of Epidemiology, UTHealth School of Public Health, Houston, TX, USA.
Michelle A T Hildebrandt, Department of Lymphoma/Myeloma, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Data availability
Deidentified data may be made available upon reasonable request to the corresponding author.
Author contributions
Elizabeth R. Rodriguez, BS (Conceptualization; Formal analysis; Investigation; Visualization; Writing—original draft; Writing—review & editing); Tori Tonn, BS (Data curation; Formal analysis; Resources; Writing—review & editing); Midhat Jafry, MS (Data curation; Formal analysis; Resources; Writing—review & editing); Sairah Ahmed, MD (Investigation; Resources; Writing—review & editing); Branko Cuglievan, MD (Investigation; Resources; Writing—review & editing); J. Andrew Livingston, MD (Investigation; Resources; Writing—review & editing); Christopher Flowers, MD (Investigation; Methodology; Resources; Writing—review & editing); Gregory Aune, MD (Funding acquisition; Investigation; Resources; Writing—review & editing); Karen Albritton, MD (Funding acquisition; Investigation; Resources; Writing—review & editing); Michael Roth, MD (Conceptualization; Funding acquisition; Investigation; Writing—original draft; Writing—review & editing); Qian Xiao, PhD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing); Michelle A. T. Hildebrandt, PhD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Project administration; Supervision; Writing—original draft; Writing—review & editing).
Funding
This work was supported by the National Institutes of Health/National Cancer Institute through University of Texas MD Anderson Cancer Center’s Cancer Center Support Grant (P30 CA016672), Harry S. Moss Heart Trust Fund, and MD Anderson Cancer Survivorship Research Seed Fund. Christopher R. Flowers received support from the Cancer Prevention and Research Institute of Texas (RR190079), where he is a Cancer Prevention and Research Institute of Texas Scholar in Cancer Research.
Conflicts of interest
The authors declare no conflict of interest with regard to this work.
References
- 1. Surveillance, Epidemiology, and End Results (SEER) Program. https://seer.cancer.gov/statfacts/html/aya.html. Accessed June 26, 2023.
- 2. Keegan TH, DeRouen MC, Parsons HM, et al. Impact of treatment and insurance on socioeconomic disparities in survival after adolescent and young adult Hodgkin lymphoma: a population-based study. Cancer Epidemiol Biomarkers Prev. 2016;25(2):264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berkman AM, Andersen CR, Hildebrandt MAT, et al. Risk of early death in adolescents and young adults with cancer: a population-based study. J Natl Cancer Inst. 2023;115(4):447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berkman AM, Andersen CR, Roth ME, et al. Cardiovascular disease in adolescent and young adult cancer survivors: Impact of sociodemographic and modifiable risk factors. Cancer. 2023;129(3):450-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berkman AM, Mittal N, Roth ME. Adolescent and young adult cancers: unmet needs and closing the gaps. Current Opinion in Pediatrics. 2023;35(1):84-90. [DOI] [PubMed] [Google Scholar]
- 6. Kahn JM, Beauchemin M. Improving health equity and reducing disparities in pediatric and adolescent/young adult oncology: in support of clinical practice guidelines. J Natl Compr Cancer Netw. 2021;19(6):765-769. [DOI] [PubMed] [Google Scholar]
- 7. Murphy CC, Lupo PJ, Roth ME, et al. Disparities in cancer survival among adolescents and young adults: a population-based study of 88 000 patients. JNCI-J Natl Cancer Inst. 2021;113(8):1074-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hacker K, Auerbach J, Ikeda R, et al. Social determinants of health-an approach taken at CDC. J Public Health Manage Pract. 2022;28(6):589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ryder-Burbidge C, Diaz RL, Barr RD, et al. The burden of late effects and related risk factors in adolescent and young adult cancer survivors: a scoping review. Cancers. 2021;13(19):4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Semrad TJ, Li Q, Goldfarb M, et al. Influence of socioeconomic status on incident medical conditions in two-year survivors of adolescent or young adult differentiated thyroid cancer. J Adolesc Young Adult Oncol. 2021;10(5):521-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen JF, Bernard F, Sheppard VB, et al. Neighborhood disadvantage and biological aging biomarkers among breast cancer patients. Sci Rep. 2022;12(1):11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohlsen TJD, Doody DR, Mueller BA, et al. Population-based impact of rurality and neighborhood-level socioeconomic disadvantage on pediatric cancer mortality in Washington State. Cancer Epidemiol Biomarkers Prev. 2023;32(1):141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luningham JM, Seth G, Saini G, et al. Association of Race and Area Deprivation With Breast Cancer Survival Among Black and White Women in the State of Georgia. Jama Network Open. 2022;5(10):e2238183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. New Engl J Med. 2018;378(26):2456-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersson T, Alfredsson L, Kallberg H, et al. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20(7):575-579. [DOI] [PubMed] [Google Scholar]
- 16. Deng XY, Yang XG, Yang CL, et al. Socioeconomic deprivation and survival outcomes in primary central nervous system lymphomas. Front Oncol. 2022;12:929585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim T. Relationship of neighborhood and individual socioeconomic status on mortality among older adults: evidence from cross-level interaction analyses. PLoS One. 2022;17(5):e0267542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng E, Soulos PR, Irwin ML, et al. Neighborhood and individual socioeconomic disadvantage and survival among patients with nonmetastatic common cancers. JAMA Network Open. 2021;4(12):e2139593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akinyemiju TF, Soliman AS, Johnson NJ, et al. Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. J Cancer Epidemiol. 2013;2013:490472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berkman AM, Andersen CR, Puthenpura V, et al. Impact of race, ethnicity, and socioeconomic status over time on the long-term survival of adolescent and young adult Hodgkin lymphoma survivors. Cancer Epidemiol Biomarkers Prev. 2021;30(9):1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeRouen MC, Parsons HM, Kent EE, et al. Sociodemographic disparities in survival for adolescents and young adults with cancer differ by health insurance status. Cancer Causes Control. 2017;28(8):841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stiles-Shields C, Cummings C, Montague E, et al. A call to action: using and extending human-centered design methodologies to improve mental and behavioral health equity. Front Digit Health. 2022;4:848052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moke DJ, Tsai K, Hamilton AS, et al. Emerging cancer survival trends, disparities, and priorities in adolescents and young adults: a California cancer registry-based study. JNCI Cancer Spectr. 2019;3(2):pkz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avila JC, Livingston JA, Rodriguez AM, et al. Disparities in adolescent and young adult sarcoma survival: analyses of the Texas cancer registry and the national SEER data. J Adolesc Young Adult Oncol. 2018;7(6):681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keegan THM, Grogan RH, Parsons HM, et al. Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid. 2015;25(6):635-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nogueira LM, Sineshaw HM, Jemal A, et al. Association of race with receipt of proton beam therapy for patients with newly diagnosed cancer in the US, 2004-2018. JAMA Netw Open. 2022;5(4):e228970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schlottmann F, Gaber C, Strassle PD, et al. Disparities in esophageal cancer: less treatment, less surgical resection, and poorer survival in disadvantaged patients. Dis Esophagus. 2020;33(2):doz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blom EF, ten Haaf K, Arenberg DA, et al. Disparities in receiving guideline-concordant treatment for lung cancer in the United States. Ann Am Thorac Soc. 2020;17(2):186-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sukniam K, Kasbi AA, Ali M, et al. Disparities in time to treatment for breast cancer. Anticancer Res. 2022;42(12):5813-5818. [DOI] [PubMed] [Google Scholar]
- 31. Cox SR, Daniel CL. Racial and ethnic disparities in laryngeal cancer care. J Racial Ethn Health Disparities. 2022;9(3):800-811. [DOI] [PubMed] [Google Scholar]
- 32. Lillard JW, Moses KA, Mahal BA, et al. Racial disparities in Black men with prostate cancer: a literature review. Cancer. 2022;128(21):3787-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keegan THM, Quach T, Shema S, et al. The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC Cancer. 2010;10:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boen CE, Hummer RA. Longer-but harder-lives?: the Hispanic health paradox and the social determinants of racial, ethnic, and immigrant-native health disparities from midlife through late life. J Health Soc Behav. 2019;60(4):434-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bowie JV, Juon HS, Dubay LC, et al. Cancer prevention behaviors in low-income urban whites: an understudied problem. J Urban Health-Bull NY Acad Med. 2009;86(6):861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Do EK, Rossi B, Miller CA, et al. Area-level variation and human papillomavirus vaccination among adolescents and young adults in the united states: a systematic review. Cancer Epidemiol Biomarkers Prev. 2021;30(1):13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harper DM, Franco EL, Wheeler CM, et al. ; HPV Vaccine Study Group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet. 2006;367(9518):1247-1255. [DOI] [PubMed] [Google Scholar]
- 39. Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271-278. [DOI] [PubMed] [Google Scholar]
- 40. Hirth J. Disparities in HPV vaccination rates and HPV prevalence in the United States: a review of the literature. Hum Vaccin Immunother. 2019;15(1):146-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hirth J, McGrath CJ, Kuo YF, et al. Impact of human papillomavirus vaccination on racial/ethnic disparities in vaccine-type human papillomavirus prevalence among 14-26 year old females in the U.S. Vaccine. 2018;36(50):7682-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Markowitz LE, Dunne EE, Saraiya M, et al. ; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30. [PubMed] [Google Scholar]
- 43. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2016. MMWR-Morb Mort Weekly Rep. 2017;66(33):874-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bauer C, Zhang KH, Xiao Q, et al. County-level social vulnerability and breast, cervical, and colorectal cancer screening rates in the US, 2018. JAMA Netw Open. 2022;5(9):e2233429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kurani SS, McCoy RG, Lampman MA, et al. Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Network Open. 2020;3(3):e200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akinlotan M, Bolin JN, Helduser J, et al. Cervical cancer screening barriers and risk factor knowledge among uninsured women. J Commun Health. 2017;42(4):770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernandez ME, Savas LS, Lipizzi E, et al. Cervical cancer control for Hispanic women in Texas: strategies from research and practice. Gynecol Oncol 2014;132(suppl 1(01)):S26-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Casillas JN, Schwartz LF, Gildner JL, et al. Engaging Latino Adolescent and Young Adult (AYA) cancer survivors in their care: piloting a photonovela intervention. J Cancer Educ. 2021;36(5):971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morales-Campos DY, Zimet GD, Kahn JA. Human papillomavirus vaccine hesitancy in the United States. Pediatric Clin North Am. 2023;70(2):211-226. [DOI] [PubMed] [Google Scholar]
- 50. Victory M, Do TQN, Kuo YF, et al. Parental knowledge gaps and barriers for children receiving human papillomavirus vaccine in the Rio Grande Valley of Texas. Hum Vaccin Immunother. 2019;15(7-8):1678-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim S, Zhou K, Parker S, et al. Perceived barriers and use of evidence-based practices for adolescent HPV vaccination among east Texas providers. Vaccines. 2023;11(4):728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data may be made available upon reasonable request to the corresponding author.