Abstract
Background
Does incorporating Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors into endocrine therapy (ET) effectively enhance survival outcomes, notably overall survival (OS), among individuals with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer? This remains a clinical controversy. We compared the antitumor efficacy and adverse effects (AEs) between CDK4/6 inhibitors + ET (CET) and placebo + ET (PET) by conducting a phase III randomized controlled trials (RCTs) based meta-analysis.
Methods
Seven databases were searched to identify eligible studies, comprising Phase III RCTs comparing CET to PET. The primary endpoints were OS and progression-free survival (PFS), with secondary endpoints including responses and adverse events (AEs).
Results
Seven RCTs (DAWNA-2, MONALEESA-2, MONALEESA-3, MONALEESA-7, MONARCH-3, PALOMA-2, and PALOMA-4) were included. The CET group exhibited significantly improved OS (HR: 0.81 [0.74, 0.88]), PFS (HR: 0.57 [0.52, 0.63]), objective response rate (RR: 1.31 [1.20, 1.43]), and clinical benefit rate (RR: 1.11 [1.07, 1.15]). These benefits were consistent across almost all subgroups. Additionally, the CET group showed better overall survival rates (OSR) from 24 to 60 months (OSR 24–60 m) and progression-free survival rates (PFSR) from 6 to 60 months (PFSR 6–60 m). However, more total AEs, grade 3–5 AEs, and serious AEs were found in CET group. The top 5 grade 3–5 AEs in the CET group were neutropenia (59.39%), leukopenia (24.11%), decreased white blood cell count (12.99%), hypertension (7.03%), and increased alanine aminotransferase (5.91%).
Conclusions
The superiority of CET over PET in HR+/HER2- advanced breast cancer is evident, showing improved survival and responses. Nonetheless, the higher incidence of AEs, specifically hematologic AEs, requires cautious attention.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-12782-w.
Keywords: CDK4/6 inhibitors, Endocrine therapy, Breast Cancer, Meta-analysis, Randomized controlled trials
Introduction
For decades, breast cancer has been the most prevalent cancer worldwide, with approximately 2.26 million new cases diagnosed annually [1]. However, the five-year survival rate for hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) breast cancer patients still remains below 30% [2, 3]. These tumors predominantly rely on hormonal signaling for cell survival and proliferation, representing over 70% of breast cancer cases in clinical settings. This underscores the importance of endocrine therapy (ET) for these patients [4]. However, research indicates that approximately one-third of HR + patients experience a relapse within 15 years following ET. Additionally, initial ET fails to benefit 50% of HR + breast cancer patients [5]. Consequently, the exploration of new therapeutic strategies in conjunction with ET is imperative.
Recently, Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors have become a focal point for breast cancer [6]. CDKs are essential regulators of the tumor cell cycle, controlling cell proliferation through a series of enzymatic reactions [7]. One key mechanism underlying resistance to ET in HR + patients is the cyclin D-CDK4/6 signaling pathway activated by the estrogen pathway [8]. Consequently, the combination of CDK4/6 inhibitors with ET has emerged as a new treatment method for patients with HR+/HER2- breast cancer. Commonly used CDK4/6 inhibitors in clinical practice (Dalpiciclib, Palbociclib, Ribociclib, and Abemaciclib) have been proven effective, although results (survival, and adverse events [AEs]) vary across different studies [9–15].
Therefore, this study (a phase III randomized controlled trial [RCT]-based meta-analysis) aimed to assess and compare the efficacy of CDK4/6 inhibitors + ET versus placebo + ET for HR+/HER2- breast cancer patients.
Materials and methods
Search strategy
We searched PubMed, Scopus, EMBASE, ScienceDirect, Ovid MEDLINE, the Cochrane Library, and Web of Science from their inception until April 1, 2024, as detailed in Table S1. The search utilized keywords such as ‘CDK4/6 Inhibitors’ (including Palbociclib, Ribociclib, Abemaciclib, and Dalpiciclib), ‘Randomized’ (Randomized OR Randomly OR Randomised), and ‘Breast Cancer’. Furthermore, we reviewed the references of the selected RCTs to identify additional pertinent research.
Selection criteria
The studies selected for inclusion were published in English and adhered to the PICOS criteria:
(1) Participants: patients diagnosed with HR+/HER2- advanced breast cancer.
(2) Intervention: CDK4/6 inhibitors + ET, defined as the CET group.
(3) Control: placebo + ET, defined as the PET group.
(4) Outcomes: survival, responses, and AEs.
(5) Study design: phase III RCTs.
Studies lacking original data, such as meta-analyses, conference presentations, and case studies, were omitted. Various articles stemming from a single RCT that reported different results were evaluated. For outcomes that were the same, only the latest findings were incorporated.
Data extraction
The extracted data include: study characteristics (including registration number and study duration), patient particulars (menopausal status, ECOG PS, etc.), treatment details (CDK4/6 inhibitor utilized, ET utilized, etc.), cancer attributes (hormone receptor status, etc.), antitumor efficacy (overall survival [OS], objective response rate [ORR], etc.), and adverse event frequencies (total AEs, etc.). Two researchers independently gathered the data, and any inconsistencies were settled by deliberation.
Outcome assessments
The main endpoints examined in this investigation were OS and PFS. Additionally, we assessed the overall survival rate (OSR) and progression-free survival rate (PFSR) at intervals of 6, 12, 18, 24, 30, 36, 42, 48, 54, and 60 months. We also examined OS and PFS within specific subgroups. These subgroups included variables such as age, race category, menopausal status, hormone receptor status, ECOG PS, disease-free interval, number of metastatic sites, presence of liver or lung metastases, presence of bone-only disease, choice of CDK4/6 inhibitor therapy partner, choice of endocrine therapy partner, history of previous adjuvant or neoadjuvant ET, type of previous ET, and history of previous neoadjuvant or adjuvant chemotherapy.
Quality assessment
The quality of RCTs was evaluated using the Jadad scale, assessing randomization, blinding, and inclusion of patients based on a scale of five points. Trials achieving a score of three or higher were deemed of high quality [16]. Additionally, the Cochrane Risk of Bias Assessment Tool was employed to evaluate biases related to selection, performance, detection, attrition, and reporting. Risks were classified as low, unclear, or high, and depicted in a bias graph [17].
Moreover, the GRADE approach was applied to analyze result quality, examining bias, indirectness, imprecision, and publication bias. This method ranks certainty across four levels: very low, low, moderate, and high [18].
Statistical analysis
This meta-analysis was performed according to PRISMA guidelines (Table S2). Data was analyzed via Review Manager 5.3. Data on survival employed hazard ratios (HRs), which indicated an advantage for the CET group when HR was less than 1. Risk ratios (RRs) were used for dichotomous variables, RR > 1 favored the PET group in the analysis of AEs. Conversely, in analyzing OSR and PFSR, RR > 1 favored the CET group. To assess heterogeneity, the I2 statistic and χ2 test were utilized. Low heterogeneity, indicated by I2 being less than 50% or p-value greater than 0.1, led to the employment of a fixed-effects model. In contrast, a random-effects model was implemented. The determination of statistical significance was based on p-values less than 0.05. Publication bias was assessed visually using funnel plots. (Registered in PROSPERO: CRD42024539851)
Results
Search results
The analysis included twenty-six studies from seven RCTs (DAWNA-2, MONALEESA-2, MONALEESA-3, MONALEESA-7, MONARCH-3, PALOMA-2, and PALOMA-4) (Fig. 1) [9–15, 19–37]. Table 1 presents baseline information of the seven RCTs. The CET group comprised 2,103 patients, while the PET group comprised 1,463 patients. Of the RCTs, five (MONALEESA-2, MONALEESA-3, MONALEESA-7, MONARCH-3, and PALOMA-2) were global multicenter, one (PALOMA-4) was Asia multicenter, and one (DAWNA-2) was China multicenter. All seven phase III RCTs were deemed of high quality (Figure S1 and Table S3). According to the GRADE approach, the outcomes were assessed as medium to high quality (Table S4).
Fig. 1.
Study selection flow
Table 1.
Characteristics of the included studies
| Study | Register number | Phase | Period | Groups | Patients | Age (Mean, year) | CDK4/6 inhibitor | Endocrine therapy | Menopausal status | ECOG PS | Hormone receptor status | Follow up (months) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-M | Pre-M | 0 | 1 | 2 | ER+ | PR+ | ||||||||||
| DAWNA-2 [9] | NCT03966898 | III | 2019.07-2020.12 | CET group | 303 | 55 | Dalpiciclib | Letrozole or Anastrozole | 183 | 120 | 141 | 161 | 0 | 302 | 258 | 21.6 |
| PET group | 153 | 55 | 99 | 54 | 69 | 84 | 0 | 153 | 134 | |||||||
| MONALEESA-2 [10,19–23] | NCT01958021 | III | 2014.01-2015.03 | CET group | 334 | 62 | Ribociclib | Letrozole | 334 | 0 | 205 | 129 | 0 | 332 | 271 | 80.0 |
| PET group | 334 | 63 | 334 | 0 | 202 | 132 | 0 | 333 | 278 | |||||||
| MONALEESA-3 [11,24–26] | NCT02422615 | III | 2015.06-2016.06 | CET group | 237 | 63 | Ribociclib | Fulvestrant | 237 | 0 | 152 | 92 | 0 | 236 | 173 | 70.8 |
| PET group | 128 | 63 | 128 | 0 | 77 | 44 | 0 | 127 | 88 | |||||||
| MONALEESA-7 [12,27,28] | NCT02278120 | III | 2014.12-2016.08 | CET group | 288 | 43 | Ribociclib | Tamoxifen or Letrozole or Anastrozole | 0 | 288 | 211 | 75 | 0 | 285 | 249 | 53.5 |
| PET group | 290 | 45 | 0 | 290 | 219 | 67 | 1 | 288 | 248 | |||||||
| MONARCH-3 [13,29–32] | NCT02246621 | III | 2014.11-2015.11 | CET group | 328 | 63 | Abemaciclib | Letrozole or Anastrozole | 328 | 0 | 192 | 136 | 0 | 328 | 255 | 70.2 |
| PET group | 165 | 63 | 165 | 0 | 104 | 61 | 0 | 165 | 127 | |||||||
| PALOMA-2 [14,33–37] | NCT02246621 | III | 2013.02-2014.07 | CET group | 444 | 62 | Palbociclib | Letrozole | 444 | 0 | 257 | 178 | 9 | 444 | - | 90.1 |
| PET group | 222 | 61 | 222 | 0 | 102 | 117 | 3 | 222 | - | |||||||
| PALOMA-4 [15] | NCT02297438 | III | 2015.03-2020.08 | CET group | 169 | 54 | Palbociclib | Letrozole | 169 | 0 | 88 | 85 | 0 | 169 | - | 52.8 |
| PET group | 171 | 54 | 171 | 0 | 81 | 90 | 0 | 171 | - | |||||||
Abbreviations CET: CDK4/6 inhibitors plus endocrine therapy; CDK4/6: Cyclin-dependent kinase 4/6; ECOG PS: Eastern Cooperative Oncology Group Performance Status; M: Menopausal; PET: Placebo plus endocrine therapy
Survival (OS and PFS)
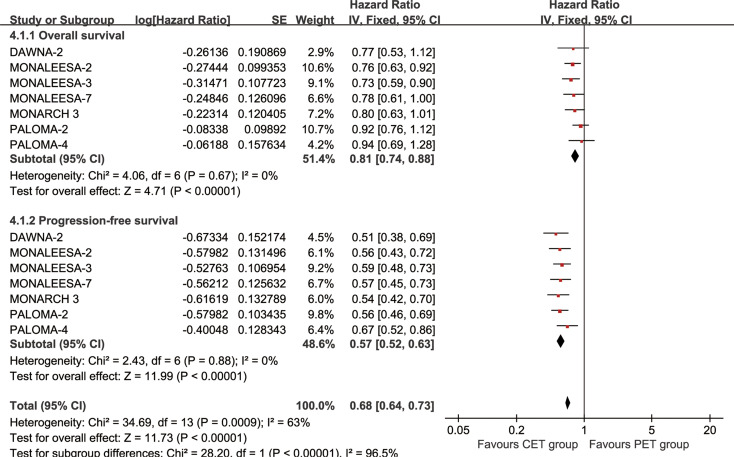
The CET group achieved better OS (HR: 0.81 [0.74, 0.88], p < 0.00001) and PFS (HR: 0.57 [0.52, 0.63], p < 0.00001) (Fig. 2). OSR 24–60 m significantly favored the CET group (Figure S2). Meanwhile, PFSR 6–60 m significantly favored the CET group (Figure S3). As survival prolonged, CET also exhibited a growing OS and PFS advantage over PET (Fig. 3).
Fig. 2.
Forest plots of overall survival and progression-free survival associated with CET group versus PET group
Fig. 3.
Comparisons of overall survival rate (6–60 months, A: trend of overall survival rate; C: trend of risk ratios) and progression-free survival rate (6–42 months, B: trend of progression-free survival rate; D: trend of risk ratios) associated with CET group versus PET group
Subgroup analysis of survival (OS and PFS)
We subgroup analyzed survival (OS and PFS) according to Age, Race category, Menopausal status, Hormone receptor status, ECOG PS, Disease-free interval, Metastatic status, CDK4/6 inhibitor therapy partner, ET partner, Previous adjuvant or neoadjuvant ET, Previous ET type, and Previous neoadjuvant or adjuvant chemotherapy. OS and PFS generally favored the CET group in all subgroups (Table 2).
Table 2.
Subgroup analysis of overall survival and progression-free survival
| Subgroups | No. of studies | Overall Survival | No. of studies | Progression-free survival | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Total | 7 | 0.81 [0.74, 0.88] | < 0.00001 | 7 | 0.57 [0.52, 0.63] | < 0.00001 |
| Age | ||||||
| < 65 years | 4 | 0.79 [0.69, 0.91] | 0.001 | 6 | 0.55 [0.49, 0.62] | < 0.00001 |
| > 65 years | 4 | 0.80 [0.69, 0.94] | 0.007 | 6 | 0.55 [0.49, 0.62] | < 0.00001 |
| Race category | ||||||
| Asian | 7 | 0.78 [0.66, 0.93] | 0.005 | 6 | 0.51 [0.38, 0.70] | < 0.0001 |
| Others | 5 | 0.81 [0.73, 0.91] | 0.0004 | 4 | 0.61 [0.53, 0.70] | < 0.00001 |
| ECOG PS | ||||||
| 0 | 5 | 0.80 [0.67, 0.96] | 0.02 | 7 | 0.56 [0.50, 0.64] | < 0.00001 |
| 1 | 5 | 0.78 [0.67, 0.90] | 0.0009 | 7 | 0.57 [0.50, 0.66] | < 0.00001 |
| Menopausal status | ||||||
| Postmenopausal | 4 | 0.78 [0.70, 0.88] | < 0.00001 | 6 | 0.58 [0.52, 0.64] | < 0.00001 |
| Premenopausal or perimenopausal | 1 | 0.78 [0.61, 1.00] | 0.05 | 2 | 0.56 [0.45, 0.70] | < 0.00001 |
| Hormone receptor status | ||||||
| ER positive + PR positive | 4 | 0.80 [0.70, 0.90] | 0.0004 | 4 | 0.58 [0.50, 0.67] | < 0.00001 |
| Others | 4 | 0.63 [0.51, 0.79] | < 0.0001 | 4 | 0.39 [0.29, 0.52] | < 0.00001 |
| Disease-free interval | ||||||
| De-novo metastatic disease | 4 | 0.69 [0.48, 1.01] | 0.06 | 6 | 0.50 [0.42, 0.60] | < 0.00001 |
| Existing disease | 3 | 0.89 [0.76, 1.03] | 0.12 | 4 | 0.58 [0.49, 0.68] | < 0.00001 |
| Number of metastatic sites | ||||||
| < 3 | 3 | 0.77 [0.66, 0.90] | 0.001 | 3 | 0.59 [0.49, 0.71] | < 0.00001 |
| > 3 | 5 | 0.81 [0.70, 0.94] | 0.004 | 5 | 0.55 [0.47, 0.64] | < 0.00001 |
| Visceral metastases at study entry | ||||||
| Yes | 2 | 0.81 [0.66, 0.99] | 0.04 | 4 | 0.61 [0.52, 0.72] | < 0.00001 |
| No | 1 | 0.98 [0.74, 1.30] | 0.89 | 3 | 0.53 [0.43, 0.65] | < 0.00001 |
| Presence of liver or lung metastases | ||||||
| Yes | 3 | 0.39 [0.10, 1.58] | 0.19 | 3 | 0.57 [0.48, 0.67] | < 0.00001 |
| No | 3 | 0.72 [0.60, 0.86] | 0.0003 | 3 | 0.58 [0.48, 0.71] | < 0.00001 |
| Bone-only disease | ||||||
| Yes | 5 | 0.72 [0.58, 0.88] | 0.002 | 5 | 0.50 [0.40, 0.64] | < 0.00001 |
| No | 4 | 0.81 [0.72, 0.91] | 0.0004 | 4 | 0.59 [0.52, 0.67] | < 0.00001 |
| CDK4/6 inhibitor therapy partner | ||||||
| Dalpiciclib | 1 | 0.77 [0.53, 1.12] | 0.17 | 1 | 0.51 [0.38, 0.69] | < 0.00001 |
| Ribociclib | 3 | 0.75 [0.67, 0.85] | < 0.00001 | 3 | 0.58 [0.50, 0.66] | < 0.00001 |
| Abemaciclib | 1 | 0.80 [0.63, 1.01] | 0.06 | 1 | 0.54 [0.42, 0.70] | < 0.00001 |
| Palbociclib | 2 | 0.93 [0.79, 1.09] | 0.36 | 2 | 0.60 [0.51, 0.70] | < 0.00001 |
| Endocrine therapy partner | ||||||
| Letrozole | 3 | 0.85 [0.75, 0.97] | 0.01 | 5 | 0.58 [0.51, 0.65] | < 0.00001 |
| Anastrozole | - | - | - | 2 | 0.51 [0.37, 0.71] | < 0.0001 |
| Fulvestrant | 1 | 0.73 [0.59, 0.90] | 0.003 | 1 | 0.59 [0.48, 0.73] | < 0.00001 |
| Tamoxifen | 1 | 0.70 [0.47, 1.04] | 0.08 | 1 | 0.59 [0.39, 0.89] | 0.01 |
| Previous adjuvant or neoadjuvant endocrine therapy | ||||||
| Yes | 4 | 0.81 [0.70, 0.94] | 0.005 | 5 | 0.57 [0.49, 0.66] | < 0.00001 |
| No | 5 | 0.79 [0.65, 0.96] | 0.02 | 6 | 0.56 [0.49, 0.65] | < 0.00001 |
| Previous endocrine therapy type | ||||||
| Selective oestrogenreceptor modulator | 2 | 0.88 [0.68, 1.14] | 0.32 | 5 | 0.61 [0.51, 0.74] | < 0.00001 |
| Aromatase inhibitors | 2 | 0.58 [0.40, 0.83] | 0.003 | 5 | 0.56 [0.47, 0.68] | < 0.00001 |
| Previous neoadjuvant or adjuvant chemotherapy | ||||||
| Yes | 3 | 0.83 [0.70, 0.98] | 0.03 | 3 | 0.58 [0.48, 0.70] | < 0.00001 |
| No | 3 | 0.79 [0.59, 1.06] | 0.11 | 3 | 0.53 [0.43, 0.64] | < 0.00001 |
Abbreviations CET: CDK4/6 inhibitors plus endocrine therapy; CDK4/6: Cyclin-dependent kinase 4/6; CI: Confidence interval; ECOG PS: Participants, Intervention, Control, Outcome and Study design Performance Status; HR: Hazard ratio; PET: Placebo plus endocrine therapy
Responses
In all patients, the ORR (RR: 1.31 [1.20, 1.43]) and clinical benefit rate (CBR, RR: 1.11 [1.07, 1.15]), along with partial response (PR, RR: 1.30 [1.19, 1.43]), were better in the CET group. Although the complete response (CR, RR: 1.53 [0.90, 2.59]) tended to favor the CET group, it lacked statistical significance. Conversely, the rate of stable disease (SD, RR: 0.90 [0.82, 0.98]) was higher in the PET group (Fig. 4).
Fig. 4.
Forest plots of responses associated with CET group versus PET group in all patients
Among patients with measurable disease, the ORR (RR: 1.31 [1.21, 1.42]), CBR (RR: 1.13 [1.08, 1.18]), and PR (RR: 1.29 [1.19, 1.41]) were better in the CET group. The CR (RR: 1.64 [0.93, 2.87]) tended to favor the CET group without statistical significance. The rate of SD (RR: 0.85 [0.77, 0.94]) was lower in the CET group (Figure S4).
Toxicity
In summary, the CET group resulted in more total AEs (RR: 1.05 [1.03, 1.06]), grade 3–5 AEs (RR: 2.96 [2.30, 3.81]), serious AEs (RR: 1.67 [1.37, 2.03]), AEs leading to treatment discontinuation (RR: 2.61 [1.88, 3.62]), AEs leading to dose reduction (RR: 11.70 [3.76, 36.36]), and AEs leading to dose interruption (RR: 5.67 [2.59, 12.43]). However, AEs leading to death (RR: 1.31 [1.21, 1.42]) were similar between the two groups (Fig. 5).
Fig. 5.
Forest plots of adverse events summary associated with CET group versus PET group
In the assessment of any grade AEs, more cases of neutropenia, decreased white blood cell count, leukopenia, nausea, fatigue, diarrhea, anemia, hyponatremia, thrombocytopenia, alopecia, vomiting, hypokalemia, cough, constipation, decreased appetite, abdominal pain, increased blood creatinine, rash, pruritus, urinary tract infection, hypokalaemia, stomatitis, pyrexia, prolonged electrocardiogram QT, dry skin, hypophosphataemia, dysgeusia, and oropharyngeal pain were observed in the CET group (Table S5). The top 5 any grade AEs were neutropenia (74.66%), decreased white blood cell count (49.55%), leukopenia (42.80%), nausea (37.54%), and fatigue (33.56%) (Table 3). Incidence rate of any grade interstitial lung diseases (ILDs) tended to be higher in the CET group without statistical significance (Figure S5).
Table 3.
Any grade adverse events (> 20% in the CET group)
| Adverse events | Studies involved | CET group | PET group | Risk ratio [95% CI] | P | ||
|---|---|---|---|---|---|---|---|
| Event/total | % | Event/total | % | ||||
| Neutropenia | 7 | 1570/2103 | 74.66% | 106/1463 | 7.25% | 10.92 [7.62, 15.65] | < 0.00001 |
| White blood cell count decreased | 2 | 328/662 | 49.55% | 50/499 | 10.02% | 4.93 [1.74, 13.99] | 0.003 |
| Leukopenia | 7 | 900/2103 | 42.80% | 79/1463 | 5.40% | 7.41 [5.69, 9.66] | < 0.00001 |
| Nausea | 6 | 726/1934 | 37.54% | 301/1292 | 23.30% | 1.67 [1.47, 1.90] | < 0.00001 |
| Fatigue | 6 | 604/1800 | 33.56% | 359/1310 | 27.40% | 1.18 [1.05, 1.32] | 0.003 |
| Diarrhea | 7 | 697/2103 | 33.14% | 288/1463 | 19.69% | 1.51 [1.16, 1.96] | 0.002 |
| Anemia | 7 | 670/2103 | 31.86% | 122/1463 | 8.34% | 3.64 [2.57, 5.14] | < 0.00001 |
| Hypercalcemia | 1 | 96/328 | 29.27% | 50/165 | 30.30% | 0.97 [0.73, 1.29] | 0.81 |
| Hyponatremia | 1 | 90/328 | 27.44% | 37/165 | 22.42% | 1.22 [0.88, 1.71] | 0.24 |
| Thrombocytopenia | 4 | 328/1204 | 27.24% | 27/836 | 3.23% | 7.59 [4.86, 11.86] | < 0.00001 |
| Alopecia | 6 | 472/1800 | 26.22% | 159/1310 | 12.14% | 2.10 [1.78, 2.48] | < 0.00001 |
| Arthralgia | 7 | 550/2103 | 26.15% | 409/1463 | 27.96% | 0.98 [0.88, 1.09] | 0.61 |
| Hot flush | 4 | 305/1303 | 23.41% | 275/974 | 28.23% | 0.86 [0.71, 1.05] | 0.05 |
| Headache | 5 | 375/1631 | 22.99% | 257/1139 | 22.56% | 1.05 [0.87, 1.26] | 0.50 |
| Vomiting | 6 | 436/1934 | 22.54% | 192/1292 | 14.86% | 1.63 [1.18, 2.25] | 0.003 |
| Hypokalemia | 2 | 110/497 | 22.13% | 29/336 | 8.63% | 2.25 [1.51, 3.35] | < 0.0001 |
| Hypocalcemia | 1 | 72/328 | 21.95% | 28/165 | 16.97% | 1.29 [0.87, 1.92] | 0.20 |
| Cough | 5 | 320/1472 | 21.74% | 201/1145 | 17.55% | 1.21 [1.03, 1.42] | 0.02 |
| Aspartate aminotransferase increased | 5 | 308/1422 | 21.66% | 167/1113 | 15.00% | 1.50 [0.94, 2.41] | 0.0002 |
| Constipation | 5 | 349/1631 | 21.40% | 182/1139 | 15.98% | 1.37 [1.16, 1.62] | 0.0001 |
| Alanine aminotransferase increased | 5 | 301/1422 | 21.17% | 165/1113 | 14.82% | 1.55 [0.90, 2.66] | 0.11 |
| Back pain | 5 | 331/1631 | 20.29% | 229/1139 | 20.11% | 1.04 [0.89, 1.21] | 0.64 |
Abbreviations CET: CDK4/6 inhibitors plus endocrine therapy; CI: Confidence interval; PET: Placebo plus endocrine therapy; RR: Risk ratio
In the assessment of grade 3–5 AEs, more cases of neutropenia, leukopenia, decreased white blood cell count, increased alanine aminotransferase, anemia, hyponatremia, increased aspartate aminotransferase, thrombocytopenia, diarrhea, fatigue, and decreased appetite were found in the CET group (Table S6). The top 5 grade 3–5 AEs were neutropenia (59.39%), leukopenia (24.11%), decreased white blood cell count (12.99%), hypertension (7.03%), and increased alanine aminotransferase (5.91%) (Table 4). Incidence rate of grade 3–5 ILDs also tended to be higher in the CET group without statistical significance (Figure S5).
Table 4.
Grade 3–5 adverse events (> 1% in the CET group)
| Adverse events | Studies involved | CET group | PET group | Risk ratio [95% CI] | P | ||
|---|---|---|---|---|---|---|---|
| Event/total | % | Event/total | % | ||||
| Neutropenia | 7 | 1249/2103 | 59.39% | 20/1463 | 1.37% | 42.16 [20.45, 86.90] | < 0.00001 |
| Leukopenia | 7 | 507/2103 | 24.11% | 7/1463 | 0.48% | 27.95 [12.00, 65.11] | < 0.00001 |
| White blood cell count decreased | 2 | 86/662 | 12.99% | 3/499 | 0.60% | 21.99 [6.99, 69.19] | < 0.00001 |
| Hypertension | 3 | 65/925 | 7.03% | 57/777 | 7.34% | 1.11 [0.79, 1.54] | 0.55 |
| Alanine aminotransferase increased | 5 | 84/1422 | 5.91% | 15/1113 | 1.35% | 3.51 [1.31, 9.44] | 0.01 |
| Hypokalemia | 2 | 25/497 | 5.03% | 4/336 | 1.19% | 3.06 [0.04, 240.13] | 0.62 |
| Anemia | 7 | 105/2103 | 4.99% | 25/1463 | 1.71% | 2.45 [1.54, 3.89] | < 0.00001 |
| Hyponatremia | 1 | 16/328 | 4.88% | 0/165 | 0.00% | 16.65 [1.01, 275.82] | 0.05 |
| Aspartate aminotransferase increased | 5 | 54/1422 | 3.80% | 13/1113 | 1.17% | 3.38 [1.84, 6.21] | < 0.0001 |
| Thrombocytopenia | 4 | 33/1204 | 2.74% | 4/836 | 0.48% | 4.45 [1.41, 14.02] | 0.001 |
| Diarrhea | 7 | 51/2103 | 2.43% | 10/1463 | 0.68% | 2.53 [1.16, 5.53] | 0.0008 |
| Electrocardiogram QT prolonged | 3 | 18/760 | 2.37% | 3/614 | 0.49% | 2.85 [0.81, 9.95] | 0.03 |
| Pneumonia | 1 | 6/303 | 1.98% | 1/153 | 0.65% | 3.03 [0.37, 24.94] | 0.30 |
| Fatigue | 6 | 33/1800 | 1.83% | 5/1310 | 0.38% | 3.76 [1.62, 8.76] | 0.002 |
| Dyspnea | 2 | 13/778 | 1.67% | 5/556 | 0.90% | 1.77 [0.38, 8.34] | 0.20 |
| Back pain | 5 | 27/1631 | 1.66% | 9/1139 | 0.79% | 2.10 [0.97, 4.52] | 0.03 |
| γ-Glutamyltransferase increased | 3 | 12/760 | 1.58% | 13/614 | 2.12% | 0.72 [0.33, 1.61] | 0.52 |
| Vomiting | 6 | 27/1934 | 1.40% | 12/1292 | 0.93% | 1.39 [0.49, 3.91] | 0.14 |
| Hypokalaemia | 1 | 4/303 | 1.32% | 0/153 | 0.00% | 4.56 [0.25, 84.14] | 0.31 |
| Asthenia | 3 | 13/1035 | 1.26% | 0/665 | 0.00% | 4.66 [0.82, 26.49] | 0.04 |
| Abdominal pain | 3 | 13/1060 | 1.23% | 3/677 | 0.44% | 2.19 [0.67, 7.18] | 0.16 |
| Blood creatinine increased | 2 | 7/631 | 1.11% | 0/318 | 0.00% | 7.57 [0.43, 131.71] | 0.16 |
| Dyspnoea | 1 | 3/288 | 1.04% | 1/290 | 0.34% | 3.02 [0.32, 28.87] | 0.34 |
Abbreviations: CET: CDK4/6 inhibitors plus endocrine therapy; CI: Confidence interval; PET: Placebo plus endocrine therapy; RR: Risk ratio
Sensitivity analysis
Analysis of OSR-6 m, PFS (Asian race category), and Grade 3–5 AEs revealed notable heterogeneity. The stability and reliability of the results remained unaffected by the exclusion of any study, as demonstrated by sensitivity analysis (Figure S6).
Publication bias
Funnel plots were observed for survival (OS and PFS), OSR, responses, and AEs summary, suggesting acceptable publication bias (Fig. 6).
Fig. 6.
Funnel plots of survival summary (A), OSR (B), responses (C), and AEs summary (D) associated with CET group versus PET group
Discussion
Although ET has established itself as the standard treatment for HR + breast cancer patients, its widespread application has gradually unveiled issues of drug resistance, diminishing its benefits and prompting clinicians to explore more effective and rational treatment strategies. Recent studies have highlighted the link between HR+/HER2- breast cancer and abnormal activation of the cyclin D1-CDK4/6 pathway [38]. Notably, CDK4/6 inhibitors such as Palbociclib have garnered FDA approval for breast cancer treatment [39, 40]. The use of CDK4/6 inhibitors in combination with other agents has emerged as a significant focus of recent research. The Phase III trial PALOMA-2 demonstrated Palbociclib’s ability to extend the median PFS of patients with advanced HR + breast cancer by over two years, underscoring its substantial efficacy in improving PFS outcomes [14]. Similarly, a study by Slamon et al. provided compelling evidence that Palbociclib in combination with letrozole significantly enhances PFS [37]. Furthermore, investigations have indicated that compared to the fulvestrant monotherapy group, the Ribociclib + fulvestrant combination therapy group exhibits significantly prolonged PFS [12].
This study further confirms the potential benefits of CET for HR+/HER2- breast cancer patients in terms of OS and PFS. However, previous clinical trials have yielded inconsistent OS data, leading to controversy regarding whether OS benefits are achieved. Notably, OS outcomes in combined drug treatment groups in the PALOMA-1 and PALOMA-3 studies did not demonstrate statistically significant differences. Nevertheless, recent updates from the MONALEESA-7 and MONALEESA-3 studies suggest significantly better OS for HR+/HER2- breast cancer in the CET group [11, 12, 41, 42]. In exploratory analyses of other secondary factors, this study found that CET significantly improved the ORR in the Intent-to-Treat (ITT) population. Incorporating data from MONALEESA-7 and MONALEESA-3 did not alter the overall conclusions of the study, and the consistency between these study results increased the reliability of the research. Furthermore, the analysis also confirmed that combined medication can increase the clinical benefit rate, with statistically significant differences observed [26, 32]. This contrasts with another meta-analysis, which indicated no significant differences, suggesting that the variation between the two study outcomes may be related to more comprehensive data updates. As of now, the MONALEESA-3 study, which has enrolled the most patients, indicates that Ribociclib combined with fulvestrant, compared to fulvestrant alone, offers a higher ORR and a clear PFS benefit advantage, consistent with the results of this study [26]. These findings underscore the significant impact of CET on the efficacy for patients with HR+/HER2- breast cancer.
The subgroup analysis indicate consistent improvement in OS and PFS prognosis across the included subgroups, even though some subgroups did not show statistically significant differences in OS. A systematic review conducted by Piezzo et al. demonstrated that CDK4/6 inhibitors improved PFS prognosis regardless of the presence of metastases, including bone metastases [43]. This result aligns with our findings, suggesting that CET is particularly effective for patients with visceral metastases. However, when considering OS outcomes, the study by Lin et al. suggested OS benefits for patients with visceral metastases [44]. In contrast, our study’s results indicated that patients with bone metastases also experienced improved OS prognosis. Nonetheless, it’s essential to acknowledge that the number of studies and total patient numbers varied across subgroups, suggesting potential bias. Therefore, further expansion of the study population is warranted to validate these findings. Importantly, our study found that patients who had previously received ET exhibited similar OS and PFS benefits compared to those who had not undergone adjuvant or neoadjuvant ET. Preclinical research suggested cyclin D-CDK4/6-retinoblastoma pathway changes link to breast cancer’s endocrine resistance. Thus, the CET could potentially offer clinical efficacy to the patients with HR+/HER2- breast cancer experiencing progression after ET [38].
In terms of safety, our results suggested that CET increased the incidence rates of neutropenia, decreased white blood cell count, leukopenia, and anemia in both any grade and grade 3–5 AEs. This suggests that CDK4/6 inhibitors may contribute to a higher frequency of hematotoxic AEs, with neutropenia being the most common, occurring at rates of 74.66% in any grade and 59.39% in grade 3–5, significantly higher than other adverse event rates. However, unlike chemotherapy drugs that induce DNA damage and cell apoptosis, CDK4/6 inhibitors primarily inhibit progenitor cells of neutrophils, thereby arresting the cell cycle and causing neutropenia, which often resolves quickly upon discontinuation of the CDK4/6 inhibitors [45]. This highlights the controllability of Neutropenia risk associated with CDK4/6 inhibitors, with no severe death events reported due to Neutropenia. Nonetheless, close monitoring of patients’ blood counts is still essential during CDK4/6 inhibitor use to promptly prevent or manage serious AEs [46]. The incidence of Grade 3–5 hypertension is higher in the CET group, highlighting the importance of monitoring blood pressure, managing thromboembolic risks, and addressing cardiovascular health in patients undergoing CDK4/6 inhibitor therapy. Meanwhile, in CDK4/6 inhibitor therapy, interstitial lung disease (ILD) is a rare but serious adverse event that requires close monitoring. In our analysis, the incidence of ILD is higher in the CET group (without statistical significance). To effectively manage ILD, it is recommended to conduct baseline lung function assessments and high-resolution chest CT scans before treatment, and regularly monitor respiratory symptoms and lung function changes during treatment [47]. If suspected ILD symptoms occur, treatment should be immediately paused, and further lung evaluations and appropriate medical interventions should be carried out [48].
Limitations of this study: (1) Including only English articles may introduce language bias; (2) The research includes fewer than 10 studies, and although a funnel plot test did not show significant publication bias, accurately determining publication bias results remains challenging. (3) No individual patient data hindered patient-level meta-analysis, possibly reducing clinical value. (4) Differences in follow-up times among RCTs may increase data heterogeneity.
Conclusion
CET appears to outperform PET in HR+/HER2- advanced breast cancer, demonstrating improved survival (OS and PFS) and responses. Survival benefits were consistent across most subgroups. However, the increased incidence of AEs, particularly hematologic AEs, requires careful consideration. Due to the limited number and quality of included studies, these conclusions require further validation through high-quality research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank professor Wenxiong Zhang, MD (Department of Thoracic Surgery, The second affiliated hospital of Nanchang University) for his data collection and statistical advice.
Abbreviations
- AEs
Adverse effects
- CBR
Clinical benefit rate
- CET
CDK4/6 inhibitors plus endocrine therapy
- CDK4/6
Cyclin-dependent kinase 4/6
- CI
Confidence interval
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- ER+
Estrogen receptor-positive
- ET
Endocrine therapy
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluation
- HER2-
Human epidermal growth factor receptor 2-negative
- HR
Hazard ratio
- HR+
Hormone receptor-positive
- ITT
Intent-to-Treat
- ORR
Objective response rate
- OS
Overall survival
- OSR
Overall survival rate
- PFS
Progression-free survival
- PFSR
Progression-free survival rate
- PET
Placebo plus endocrine therapy
- PICOS
Participants, Intervention, Control, Outcome and Study design
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PR
Partial response
- PR+
Progesterone receptors-positive
- SD
Stable disease
- RCT
randomized controlled trial
- RR
Risk ratio
Author contributions
Zhongkui Jin had full access to all of the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Cailu Luo, Kunlin Yu, Xiaodan Luo, Tao Lian, Xuejuan Liu, Wang Xu, and Zhongkui Jin. Acquisition, analysis, or interpretation of data: Cailu Luo, Kunlin Yu, Xiaodan Luo, Tao Lian, Xuejuan Liu, Wang Xu, and Zhongkui Jin. Drafting of the manuscript: Cailu Luo, Kunlin Yu and Zhongkui Jin. Critical revision of the manuscript for important intellectual content: Cailu Luo, Kunlin Yu, and Zhongkui Jin. Statistical analysis: Cailu Luo, Kunlin Yu, and Xiaodan Luo. Supervision: Cailu Luo, Kunlin Yu, and Zhongkui Jin.
Funding
This study was supported by Natural Science Foundation of Jiangxi Province (Grant number: 20212BAB206050). Role of the Funding: The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Declarations
Consent for publication
Not Applicable.
Ethical approval
Due to the nature of this study no ethical approval was required.
Informed consent
For this type of study formal consent is not required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. [DOI] [PubMed] [Google Scholar]
- 2.García-Sáenz JA, Marmé F, Untch M, Bonnefoi H, Kim SB, Bear H, et al. Patient-reported outcomes in high-risk HR+ /HER2- early breast cancer patients treated with endocrine therapy with or without palbociclib within the randomized PENELOPEB study. Eur J Cancer. 2024;196:113420. [DOI] [PubMed] [Google Scholar]
- 3.Wang QL, Zhang Y, Zeng E, Grassmann F, He W, Czene K. Risk of estrogen receptor-specific breast cancer by family history of estrogen receptor subtypes and other cancers. J Natl Cancer Inst. 2023;115(9):1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolpert KM, Schmidt JA, Ahern TP, Hjorth CF, Farkas DK, Ejlertsen B, et al. Clinical factors associated with patterns of endocrine therapy adherence in premenopausal breast cancer patients. Breast Cancer Res. 2024;26(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein HJ, DeMichele A, Fallowfield L, Somerfield MR, Henry NL. Endocrine and targeted therapy for hormone Receptor-Positive, human epidermal growth factor receptor 2-Negative metastatic breast Cancer-Capivasertib-Fulvestrant: ASCO Rapid Recommendation Update. J Clin Oncol. 2024;42(12):1450–3. [DOI] [PubMed] [Google Scholar]
- 6.Wekking D, Leoni VP, Lambertini M, Dessì M, Pretta A, Cadoni A, et al. CDK4/6 inhibition in hormone receptor-positive/HER2-negative breast cancer: Biological and clinical aspects. Cytokine Growth Factor Rev. 2024;75:57–64. [DOI] [PubMed] [Google Scholar]
- 7.Roberts EL, Greenwood J, Kapadia N, Auchynnikava T, Basu S, Nurse P. CDK activity at the centrosome regulates the cell cycle. Cell Rep. 2024;43(4):114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison L, Loibl S, Turner NC. The CDK4/6 inhibitor revolution-a game-changing era for breast cancer treatment. Nat Rev Clin Oncol. 2024;21(2):89–105. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Zhang Q, Tong Z, Sun T, Li W, Ouyang Q, et al. Dalpiciclib plus letrozole or anastrozole versus placebo plus letrozole or anastrozole as first-line treatment in patients with hormone receptor-positive, HER2-negative advanced breast cancer (DAWNA-2): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2023;24(6):646–57. [DOI] [PubMed] [Google Scholar]
- 10.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as First-Line therapy for HR-Positive, advanced breast Cancer. N Engl J Med. 2016;375(18):1738–48. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of Ribociclib and Fulvestrant in hormone Receptor-Positive, human epidermal growth factor receptor 2-Negative advanced breast Cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–72. [DOI] [PubMed] [Google Scholar]
- 12.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–15. [DOI] [PubMed] [Google Scholar]
- 13.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast Cancer. J Clin Oncol. 2017;35(32):3638–46. [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in Advanced breast Cancer. N Engl J Med. 2016;375(20):1925–36. [DOI] [PubMed] [Google Scholar]
- 15.Xu B, Hu X, Li W, Sun T, Shen K, Wang S, et al. Palbociclib plus Letrozole versus placebo plus letrozole in Asian postmenopausal women with oestrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: primary results from PALOMA-4. Eur J Cancer. 2022;175:236–45. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
- 19.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–7. [DOI] [PubMed] [Google Scholar]
- 20.O’Shaughnessy J, Petrakova K, Sonke GS, Conte P, Arteaga CL, Cameron DA, et al. Ribociclib plus Letrozole versus letrozole alone in patients with de novo HR+, HER2- advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat. 2018;168(1):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonke GS, Hart LL, Campone M, Erdkamp F, Janni W, Verma S, et al. Ribociclib with letrozole vs letrozole alone in elderly patients with hormone receptor-positive, HER2-negative breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat. 2018;167(3):659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yardley DA, Hart L, Favret A, Blau S, Diab S, Richards D, et al. Efficacy and safety of Ribociclib with Letrozole in US patients enrolled in the MONALEESA-2 study. Clin Breast Cancer. 2019;19(4):268–e2771. [DOI] [PubMed] [Google Scholar]
- 23.Slamon D, Lipatov O, Nowecki Z, McAndrew N, Kukielka-Budny B, Stroyakovskiy D, et al. Ribociclib plus Endocrine Therapy in early breast Cancer. N Engl J Med. 2024;390(12):1080–91. [DOI] [PubMed] [Google Scholar]
- 24.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with Ribociclib plus Fulvestrant in Advanced breast Cancer. N Engl J Med. 2020;382(6):514–24. [DOI] [PubMed] [Google Scholar]
- 25.Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32(8):1015–24. [DOI] [PubMed] [Google Scholar]
- 26.Neven P, Fasching PA, Chia S, Jerusalem G, De Laurentiis M, Im SA, et al. Updated overall survival from the MONALEESA-3 trial in postmenopausal women with HR+/HER2- advanced breast cancer receiving first-line ribociclib plus fulvestrant. Breast Cancer Res. 2023;25(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with Ribociclib plus endocrine therapy in breast Cancer. N Engl J Med. 2019;381(4):307–16. [DOI] [PubMed] [Google Scholar]
- 28.Lu YS, Im SA, Colleoni M, Franke F, Bardia A, Cardoso F, et al. Updated overall survival of Ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast Cancer in MONALEESA-7: a phase III Randomized Clinical Trial. Clin Cancer Res. 2022;28(5):851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toi M, Inoue K, Masuda N, Iwata H, Sohn J, Hae Park I, et al. Abemaciclib in combination with endocrine therapy for east Asian patients with HR+, HER2- advanced breast cancer: MONARCH 2 & 3 trials. Cancer Sci. 2021;112(6):2381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetz1 MP, Toi M, Huober J, Sohn J, Tredan O, Park IH et al. LBA15 MONARCH 3: interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC). Ann Oncol 33:S1384. [DOI] [PubMed]
- 32.Takahashi M, Tokunaga E, Mori J, Tanizawa Y, van der Walt JS, Kawaguchi T, et al. Japanese subgroup analysis of the phase 3 MONARCH 3 study of abemaciclib as initial therapy for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Breast Cancer. 2022;29(1):174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukai H, Shimizu C, Masuda N, Ohtani S, Ohno S, Takahashi M, et al. Palbociclib in combination with letrozole in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-2 subgroup analysis of Japanese patients. Int J Clin Oncol. 2019;24(3):274–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rugo HS, Finn RS, Gelmon K, Joy AA, Harbeck N, Castrellon A, et al. Progression-free survival outcome is Independent of Objective response in patients with estrogen Receptor-positive, human epidermal growth factor receptor 2-negative advanced breast Cancer treated with Palbociclib Plus Letrozole compared with letrozole: analysis from PALOMA-2. Clin Breast Cancer. 2020;20(2):e173–80. [DOI] [PubMed] [Google Scholar]
- 35.Im SA, Mukai H, Park IH, Masuda N, Shimizu C, Kim SB, et al. Palbociclib Plus Letrozole as First-Line therapy in postmenopausal Asian women with metastatic breast Cancer: results from the Phase III, Randomized PALOMA-2 study. J Glob Oncol. 2019;5:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelmon K, Walshe JM, Mahtani R, Joy AA, Karuturi M, Neven P, et al. Efficacy and safety of palbociclib in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with preexisting conditions: a post hoc analysis of PALOMA-2. Breast. 2021;59:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slamon DJ, Diéras V, Rugo HS, Harbeck N, Im SA, Gelmon KA, et al. Overall survival with Palbociclib Plus Letrozole in Advanced breast Cancer. J Clin Oncol. 2024;42(9):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peurala E, Koivunen P, Haapasaari KM, Bloigu R, Jukkola-Vuorinen A. The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res. 2013;15(1):R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA approval: Palbociclib for the Treatment of Postmenopausal Patients with estrogen Receptor-Positive, HER2-Negative metastatic breast Cancer. Clin Cancer Res. 2015;21(21):4760–6. [DOI] [PubMed] [Google Scholar]
- 40.Shah A, Bloomquist E, Tang S, Fu W, Bi Y, Liu Q, et al. FDA approval: Ribociclib for the Treatment of Postmenopausal Women with hormone Receptor-Positive, HER2-Negative Advanced or metastatic breast Cancer. Clin Cancer Res. 2018;24(13):2999–3004. [DOI] [PubMed] [Google Scholar]
- 41.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. [DOI] [PubMed] [Google Scholar]
- 42.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39. [DOI] [PubMed] [Google Scholar]
- 43.Piezzo M, Chiodini P, Riemma M, Cocco S, Caputo R, Cianniello D, et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast Cancer: a systematic review and Meta-analysis. Int J Mol Sci. 2020;21(17):6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin M, Chen Y, Jin Y, Hu X, Zhang J. Comparative overall survival of CDK4/6 inhibitors plus endocrine therapy vs. endocrine therapy alone for hormone receptor-positive, HER2-negative metastatic breast cancer. J Cancer. 2020;11(24):7127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai JI, Kuo TH, Huang KJ, Chai LMX, Lee MH, Liu CY, et al. Clinical and genotypic insights into higher prevalence of Palbociclib Associated Neutropenia in Asian patients. Oncologist. 2024;29(4):e455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavery L, DiSogra K, Lea J, Trufan SJ, Symanowski JT, Roberts A, et al. Risk factors associated with palbociclib-induced neutropenia in patients with metastatic breast cancer. Support Care Cancer. 2022;30(12):9803–9. [DOI] [PubMed] [Google Scholar]
- 47.Birnhuber A, Egemnazarov B, Biasin V, Bonyadi Rad E, Wygrecka M, Olschewski H, Kwapiszewska G, Marsh LM. CDK4/6 inhibition enhances pulmonary inflammatory infiltration in bleomycin-induced lung fibrosis. Respir Res. 2020;21(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlam I, Giordano A, Tolaney SM. Interstitial lung disease and CDK4/6 inhibitors in the treatment of breast cancer. Expert Opin Drug Saf. 2023;22(12):1149–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.