Abstract
Background
Antigen removal is a cornerstone of treatment of hypersensitivity pneumonitis (HP), but its association with transplant-free survival remains unclear. Further, HP guidelines conflict as to whether antigen removal is a recommended diagnostic test in patients with suspected HP.
Objective
The purpose of this study is to (1) evaluate the impact of antigen removal on transplant-free survival and (2) to describe the impact of antigen removal on pulmonary function testing and imaging in a retrospective cohort of patients with HP.
Methods
We retrospectively identified HP patients evaluated between 2011 and 2020. Demographic, physiologic, radiographic, and pathologic data were recorded.
Results
212 patients were included in the cohort. Patients who identified and removed antigen had a better transplant-free survival than patients who did not identify antigen and patients who identified but did not remove antigen. Antigen removal was associated with improvement in FVC by 10% predicted in 16.9% of patients with fibrotic HP and 56.7% of patients with nonfibrotic HP.
Discussion
Our results suggest that over 50% of nonfibrotic HP patients and 16.9% of fibrotic HP patients improve with exposure removal. In addition, antigen removal, rather than antigen identification, is associated with transplant-free survival in HP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-024-03098-3.
Introduction
Hypersensitivity pneumonitis (HP) is an interstitial lung disease (ILD) caused by repeated exposure to a sensitizing antigen [1, 2]. Prior studies have demonstrated that HP patients in whom a sensitizing antigen is identified have a better survival than those for whom a sensitizing antigen has not been identified [3–6]. As a result, antigen removal has become a cornerstone of HP treatment [7]. However, to date, the literature has been inconsistent in whether removal of antigen yields an improvement in pulmonary function testing (PFT) and has not shown a survival benefit for antigen removal [1, 6, 8–11]. Antigen removal, particularly mold remediation in the home, can be very costly and time-consuming for patients; [12, 13] thus, more data is needed on the predicted response to antigen removal to assist in a patient-centered discussion of home remediation.
Further, improvement in pulmonary function testing following removal of antigen has been suggested to support a diagnosis of HP [1]. However, there are no studies to determine the proportion of patients who have a beneficial response to exposure removal, particularly in those with fibrotic HP. Additional data is needed to determine what percentage of patients with HP have improvement with antigen removal to determine the utility of this intervention in the evaluation of patients with potential HP [7].
The purpose of this study is to (1) evaluate the impact of antigen removal on transplant-free survival and (2) to describe the impact of antigen removal on pulmonary function testing and imaging in a retrospective cohort of patients with HP.
Methods.
We retrospectively identified HP patients evaluated between 2011 and 2020 from the University of Texas Southwestern Medical Center (UTSW) in Dallas, TX. All patient seen in the ILD clinic at UTSW are automatically input into a registry in Epic. Our ILD clinic is a tertiary referral center designated as a Center of Excellence by the Pulmonary Fibrosis Foundation and our referral base includes our own institution and both community and academic pulmonologists in the surrounding region. We manually reviewed the diagnosis for each patient in the registry via multidisciplinary discussion. Patients who had a multidisciplinary diagnosis of moderate, high, or definite confidence of HP by current guidelines were included [2]. Patient charts were re-reviewed and diagnosis confirmed for the purposes of this study. This study was conducted in accordance with the amended Declaration of Helsinki and was approved by the UTSW Institutional Review Board (STU-2019-0913).
Clinical data extracted from the medical record included age, gender, smoking history, potential fibrogenic antigen exposure, removal of exposure, PFTs, GAP score, hypersensitivity pneumonitis panel where available, bronchoalveolar lavage (BAL) cell count and differential, histopathologic interpretation of the transbronchial biopsy (TBBx) and surgical lung biopsy (SLB), and date of death or lung transplant. GAP score was used because it correlates with survival in HP with a c-index of 0.79 and because it incorporates collinear variables including forced vital capacity (FVC) % predicted and diffusing capacity for carbon monoxide (DLCO) % predicted, both of which are important prognostic markers in HP [14]. Transplant-free survival was selected as an endpoint consistent with prior studies of HP [5, 15, 16]. Characteristics of exposure recorded included the location of the exposure, duration of the exposure, date of exposure removal and remediation efforts. HP was considered fibrotic if HRCT demonstrated reticulations, traction bronchiectasis, or honeycombing and nonfibrotic if all of those HRCT features were absent [1]. Fibrosis was severe if > 50% of the parenchyma was fibrotic, moderate if 10–50% was fibrotic, and mild if < 10% was fibrotic [17].
Antigen identification in our cohort was done during clinic visits using a template of questions consistent with recently published guidelines; [18] these exposure questions were asked by the provider during the initial clinic visit for all patients in our ILD cohort (supplementary data 1). Mold antigen in the home was considered to be a clinically significant if mold was visible and persistent and the mold exposure preceded the development of HP [1, 18]. Formal home inspections were noted in the chart; the actual reports were not uploaded into the medical record for review but results were briefly summarized in clinic notes. Information regarding specific mold species or concentrations are not available. Mold antigen outside the home was considered to be clinically significant if it occurred regularly (such as in the workplace) and predated the development of HP [2, 18]. Avian antigen was considered to be clinically significant if antigen exposure preceded the development of HP and the patient had a bird in the home, extensive bird exposure outside of the home (such as regular work in a chicken coop), or significant and persistent contact with feathers (such as sleeping on a feather pillow) [2, 8, 18]. Each exposure was reviewed by an occupational lung disease specialist (CSG) to ensure that the antigen was clinically significant [1].
Following identification of antigen in patients with HP, patients in our clinic were uniformly recommended to remove the antigen, which is standard of care for HP [12]. Mold was considered to be removed if any porous material with mold was fully removed from the home and the cause of the water damage was addressed with no residual leak or if the patient moved from the affected home to one without known water damage or mold [13]. Avian exposure was considered to be removed if the bird was removed followed by a professional house cleaning, if the patient stopped having contact with birds outside the home (for example, discontinuation of tending to the chicken coop), or if the feather product was removed. Patients were considered to have improved following exposure removal if their forced vital capacity (FVC) increased by at least 10% predicted within 3–6 months of exposure removal without a change in their medications, as 10% improvement in FVC is correlated with improved survival in HP [9].
Statistical analysis
Continuous variables were expressed with means and standard deviations, and categorical variables were expressed with counts and percentages. Cox proportional hazards regression was used to evaluate transplant-free survival in HP patients. A left truncated model was first created with explanatory variable of antigen removal with 3 categories: antigen identified and removed, antigen identified but not removed, and antigen not identified. Then left truncated model was created with explanatory variables GAP score, fibrosis status in conjunction with “treatment groups” of antigen removed or not removed. These variables were chosen due to their association with survival in HP in prior studies [2, 3, 12, 14]. In the latter model, antigen identified and not removed and antigen not identified were combined into the antigen not removed group due to limited sample size. The method used, left truncation, was defined as those patients diagnosed prior to study natural time origin (date of first clinic visit), due to the assumption that those that did not present to clinic for many years were healthier than similar patients with comparable dates of diagnoses, who may have experienced event prior to study initiation. We also utilized Direct-Adjusted Survival Curves to represent the survival experience of an average patient in the population by averaging the predicted survival functions for the combinations of categorical explanatory variables for in each treatment group (antigen removed vs. antigen not removed).
Results
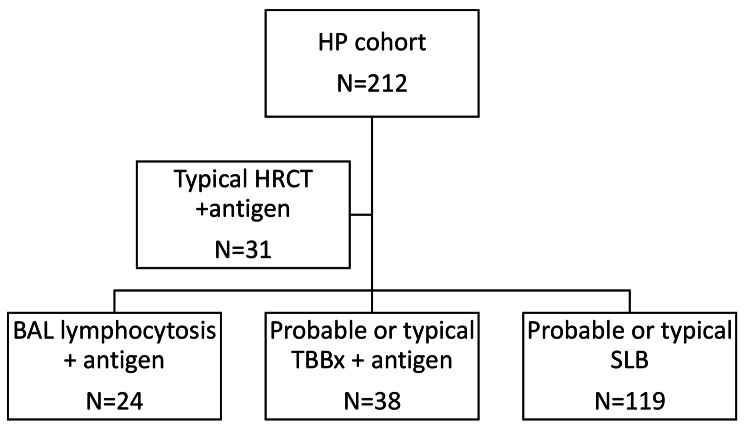
Two hundred twelve patients had a diagnosis of HP and were included in the analysis. Demographic characteristics of the cohort are listed in Table 1. Diagnostic evaluation that led to inclusion in the cohort is summarized in Fig. 1 [2]. Mean age was 62.4 ± 11.4 years and 102 (48.1%) were male. A total of 166 (78.3%) patients removed antigen, 20 (9.4) identified but did not remove the antigen, and 26 (12.3%) did not identify antigen. When stratified for antigen removal, there were no significant differences between groups in age, gender, smoking status, GAP score, baseline FVC, or baseline DLCO (data not shown).
Table 1.
Demographic characteristics of the cohort (N = 212)
| HP cohort (N = 212) | |
|---|---|
| Mean age at ILD diagnosis (SD) | 62.4 (11.4) |
| Male, No. (%) | 102 (48.1) |
| Ethnicity, No. (%) | |
|
Non-Hispanic White Black Hispanic or Latino Asian Unknown |
177 (83.5) 9 (4.2) 11 (5.2) 8 (3.8) 7 (3.3) |
| Ever Smoker, N (%) | 100 (47.2) |
|
Antigen identified Mold antigen Avian antigen Other antigen |
186 (87.8) 104 (49.1) 64 (30.2) 18 (8.5) |
| Professional mold inspection | 20 (9.4) |
| Antigen removal status | |
|
Antigen identified and removed Antigen identified and not removed Antigen not identified |
166 (78.3) 20 (9.4) 26 (12.3) |
| Baseline Lung Function, mean (SD), N | |
| FVC % predicted | 68.6 (18.5), 212 |
| DLCO % predicted | 51.4 (17.7), 212 |
|
HRCT available for scoring Typical HP Compatible HP Indeterminate HP |
212 (100) 136 (64.1) 24 (11.3) 52 (24.5) |
| Fibrotic HP | 190 (89.6) |
| Invasive procedure Performed | 187 (88.2) |
| Surgical Biopsy | 139 (65.6) |
|
TBBx BAL |
92 (43.4) 80 (37.8) |
| GAP score, N (%) | |
|
0–1 2–3 4–5 >5 |
34 (16.0) 92 (43.4) 75 (35.4) 11 (5.2) |
| Confidence HP diagnosis by American Thoracic Society Criteria, N (%) | |
|
Moderate High Definite |
85 (40.1) 33 (15.6) 94 (44.3) |
|
Outcomes Death Transplant Alive at censor date |
81 (38.2) 40 (18.9) 41 (19.3) 131 (61.8) |
| Median transplant-free survival in months | 53.3 |
Fig. 1.
Flow chart for HP diagnosis in the cohort
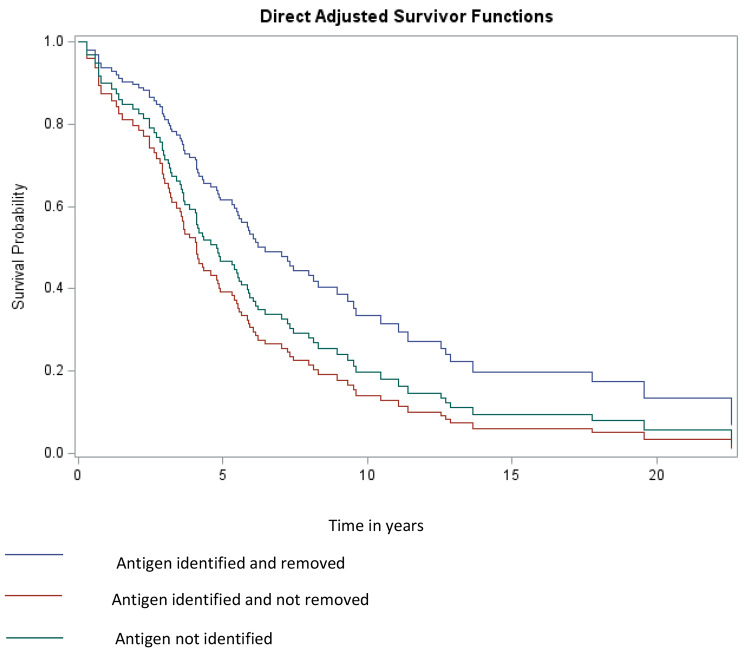
Of the 212 HP patients in the cohort, 207 were included in the left truncated Cox proportional hazard regression model. Of the 5 records that were removed, 4 had censor dates that were the same as the clinic date, and 1 had a censor date that was prior to clinic date. Missing data, and negative or zero time periods are not supported in this model. The follow up period for these patients varied due to available records, time from clinic visit to outcome or censor (mean ± sd: 3.57 ± 2.93 years). Of 207 observations, 127 were censored. Using direct adjusted survivor functions, there was no difference in the TFS of patients who identified and did not remove antigen and those for whom antigen was not identified; both of these groups had a worse TFS than those in whom antigen was identified and removed (Fig. 2).
Fig. 2.
Direct adjusted survivor functions comparing groups (1) antigen identified and removed; (2) antigen identified but not removed; and (3) antigen not identified
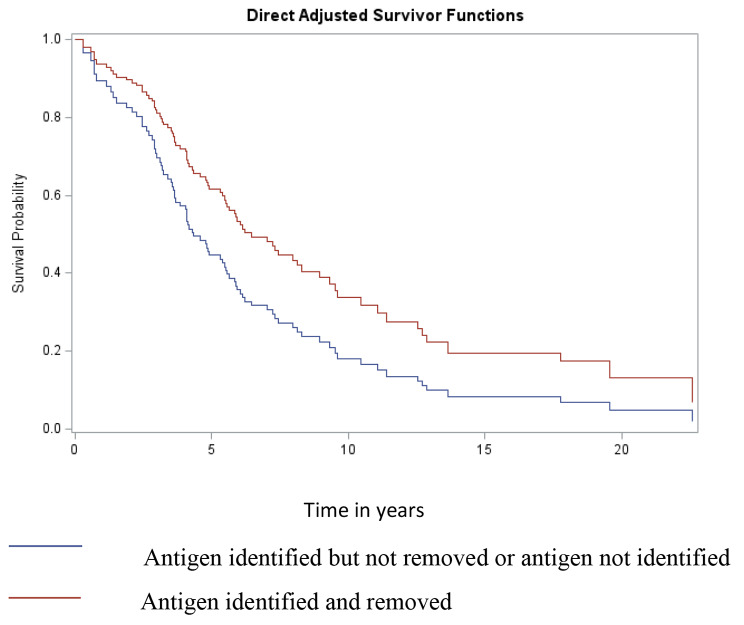
Due to the skewed distribution, with 78% of patients having identified and removed antigen, and the lack of a difference between the antigen identified but not removed and the antigen not identifiedgroups, the groups with antigen identified and not removed and not identified were combined for the Cox proportional hazard model and compared to patients for whom antigen was identified and removed. In the Cox proportional hazard model, patients in whom antigen was either not identified or not removed had a lower TFS than those in whom antigen was identified and not removed (HR 1.79, p = 0.02) (Table 2; Fig. 3). The presence of fibrosis by HRCT (HR 3.1, p = 0.05) and higher GAP score (HR 1.49, p < 0.001) were also associated with lower TFS.
Table 2.
Cox proportional hazard model for transplant-free survival (N = 207)
| Analysis of Maximum Likelihood Estimates | ||||
|---|---|---|---|---|
| Parameter | Parameter Estimate |
95% CI | Pr > ChiSq | Hazard Ratio |
| Antigen_removed | 0.58394 | 0.09–1.07 | 0.0201 | 1.793 |
| GAP_Score | 0.39921 | 0.23–0.57 | < 0.0001 | 1.491 |
| Fibrosis | 1.14731 | -0.02-2.31 | 0.0536 | 3.150 |
Fig. 3.
Direct adjusted survivor functions comparing groups based on known antigen removal: antigen identified but not removed or antigen not identified vs. antigen identified and removed
Of 166 patients in the HP cohort who identified and removed antigen, 30 (18.1%) were nonfibrotic HP and 136 (81.9%) were fibrotic HP. Seventeen (56.7%) patients with nonfibrotic HP who identified and removed antigen had an improvement in FVC by 10% predicted (Table 3). Of the patients who had improved FVC with exposure removal, all had at least one of the following: improvement in CT after exposure removal (N = 13), improvement in DLCO by 15% (N = 12), and improvement in symptoms after exposure removal (N = 14).
Table 3.
Characteristics of the response to exposure removal in the subset of patients with 10% improvement in FVC with exposure removal
| Nonfibrotic (N = 17 with FVC improvement by 10% with exposure removal) | Fibrotic (N = 23 with FVC improvement by 10% with exposure removal) | |
|---|---|---|
| DLCO improved by 15% within 3–6 months of exposure removal | 12 (70.6) | 18 (78.2) |
| % change in DLCO with exposure removal, Median (IQR) | 12 (7,20.5) | 16 (9.25,23) |
| Symptom improvement documented after exposure removal | 14 (82.3) | 19 (82.6) |
| CT available after exposure removal | 14 (82.3) | 19 (82.6) |
|
CT improvement after exposure removal Improvement in ground glass Resolution of ground glass Improvement in centrilobular nodules Resolution of centrilobular nodules Improvement in fibrosis |
13 (76.4) 6 (35.3) 5 (29.4) 2 (11.8) 0 (0) N/A |
7 (30.4) 6 (26.1) 0 (0) 1 (4.3) 0 (0) 0 (0) |
| FVC available > 1 year post exposure removal | 12 (70.6) | 22 (95.7) |
| FVC improvement maintained at 1 year post exposure removal | 11 (64.7) | 17 (73.9) |
Twenty-three (16.9%) patients with fibrotic HP who identified and removed antigen had an improvement in FVC by 10% predicted. The severity of fibrosis on HRCT was inversely correlated with the proportion of patients who had > 10% improvement of FVC with antigen removal (Fig. 4). Of those who had an FVC improvement by 10% with exposure removal, all but one had at least one of the following: improvement in CT after exposure removal (N = 7), improvement in DLCO by 15% (N = 18), and improvement in symptoms after exposure removal (N = 19).
Fig. 4.
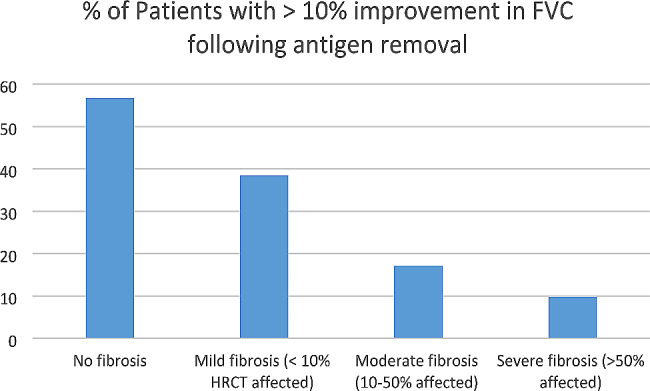
Proportion of patients with > 10% improvement in FVC following antigen removal based on degree of fibrosis on HRCT
Discussion
In this retrospective cohort of HP patients, we describe 212 patients with a moderate, high, or definite confidence of HP by current guidelines [2]. Our results suggest that patients who identified and removed antigen had a better transplant-free survival than patients who did not identify antigen and patients who identified but did not remove antigen. Antigen removal is associated with improvement in FVC by 10% predicted in 16.9% of patients with fibrotic HP and 56.7% of patients with nonfibrotic HP. Patients with a severe degree of fibrosis by HRCT were less likely to have FVC improvement following exposure removal compared to patients with a lesser degree of fibrosis.
Several studies have evaluated the relationship between antigen exposure and outcomes in hypersensitivity pneumonitis. The first landmark study published in Chest in 2013 evaluated 142 subjects with HP and antigen exposure and determined that the median survival time of patients with identified antigen was 18.2 years, compared to 9.3 years in patients without antigen. However, the major limitation of this study is that it did not assess for a change in survival with antigen removal, though antigen abatement was recommended for patients with identified antigen [3]. A Belgian cohort evaluated 202 patients with HP before and after exposure removal and found that antigen identification was associated with improved survival, but removal of antigen did not impact survival [6]. Antigen removal did, however, improve FVC compared to non-removal in this cohort [6]. In another study of 112 patients with fibrotic HP, antigen removal was not associated with FVC decline, but subjective improvement after antigen removal was associated with lower mortality [9]. A study of 17 HP patients revealed complete recovery based on symptoms, PFTs, and HRCT in 53% of patients following antigen avoidance but did not reveal a difference in TFS [19]. Finally, a study of 21 patients with hot tub lung revealed resolution of respiratory symptoms and radiographic abnormalities in 52% following exposure avoidance but with a median 5 month follow-up association with TFS could not be determined [11]. In our cohort, antigen removal was associated with transplant-free survival in univariable and multivariable analysis. Our results suggest that the removal, rather than the identification of antigen, is associated with improved TFS. These results demonstrate the importance of a thorough exposure history to identify potential HP antigens and provide support for the removal of those antigens as treatment for both fibrotic and non-fibrotic HP.
Our results also support the use of exposure removal as a diagnostic test for HP. Both the American Thoracic Society (ATS) and the American College of Chest Physicians (ACCP) have published guidelines on the diagnosis of HP in adults [1, 2]. However, these guidelines conflict as to whether improvement with antigen removal is supportive of a diagnosis of HP, with the ACCP guidelines suggesting that improvement exposure removal supports a diagnosis of HP and ATS guidelines not commenting on an exposure removal challenge in the diagnostic evaluation [1, 2]. Our study demonstrates that antigen removal is associated with improvement in FVC by 10% predicted in 16.9% of patients with fibrotic HP and 56.7% of patients with nonfibrotic HP. As expected, the probability of improvement with exposure removal is inversely proportional to the degree of fibrosis, with patients with lesser degrees of fibrosis having a higher probability of response. We therefore suggest that a trial of exposure removal when feasible could prevent over 50% of patients with nonfibrotic HP from undergoing invasive procedures for diagnosis. Diagnostic exposure removal challenge can be considered for patients with mild or moderate degrees of fibrosis but has a lower likelihood of a positive result in patients with a severe degree of fibrosis.
Strengths of our study include ascertainment of HP diagnosis based on the gold standard of multidisciplinary diagnosis based on current guidelines, and we had an occupational medicine specialist (CSG) assess each exposure to determine causality.
There are several limitations to our study. This is a retrospective study, and at our center serologic testing to document individual patient sensitization is not performed. IgG methods to establish the presence of exposure, while valuable in certain scenarios, are often insufficient and have at best modest sensitivity and specificity due to the lack of standardization of methodology and quality [1, 2, 18]. Further, our center does not require evaluation by an industrial hygienist for antigen identification or removal. While this limits our ability to definitively determine antigen removal, it increases the generalizability of the study, as industrial hygienists can be costly and not available in most centers. Finally, we were unable to control for the use of immunosuppressive medications in this study. Due to the retrospective nature of the study, we cannot confirm medication adherence and cannot adjust for selection bias with initiation of medication, as patients with more severe disease are more likely to be treated than patients with more mild disease. Further, immunosuppressive medications have not been associated with survival in HP and are confounded by the interaction with leukocyte telomere length, which we could not measure in our retrospective study [16]. Thus treatment data was excluded.
In conclusion, our results suggest that patient-reported antigen removal impacts TFS in patients with HP and that a diagnostic trial of antigen removal can reduce the number of patients who require an invasive procedure for diagnosis by about 50% in nonfibrotic HP.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
MR, CG, and TA collected data. AG performed the statistical analysis and prepared all figures. All authors wrote the main manuscript text and reviewed the manuscript.
Funding sources
none.
Data availability
We have included our deidentified data set in the supplementary files of this submission.
Declarations
Ethical approval
This study was conducted in accordance with the amended Declaration of Helsinki and was approved by the UTSW Institutional Review Board (STU-2019-0913). Informed consent was waived for this by the UTSW Institutional Review Board (STU-2019-0913) retrospective chart review. Written informed consent is not applicable to the present study or to a prior study published under the same IRB Impact of antigenidentification on transplant free survival in interstitial lung disease.
PubMed (nih.gov).
Consent for publication
Not applicable.
Conflict of interest
The authors have no conflicts of interest to report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fernandez Perez ER, Travis WD, Lynch DA, et al. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST Guideline and Expert Panel Report. Chest. 2021;160(2):e97–156. 10.1016/j.chest.2021.03.066 [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Remy-Jardin M, Ryerson CJ, et al. Diagnosis of hypersensitivity pneumonitis in adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(3):e36–69. 10.1164/rccm.202005-2032ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez Perez ER, Swigris JJ, Forssen AV, et al. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. 2013;144(5):1644–51. 10.1378/chest.12-2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kypreos M, Batra K, Glazer CS, Adams TN. Impact of number and type of identified antigen on transplant-free survival in hypersensitivity pneumonitis. PLoS ONE. 2022;17(9):e0273544. 10.1371/journal.pone.0273544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams TN, Newton CA, Glazer CS. Role of Antigen Type in Survival in Chronic Hypersensitivity Pneumonitis. Lung. 2019;197(1):113–4. 10.1007/s00408-018-0187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Sadeleer LJ, Hermans F, De Dycker E et al. Effects of Corticosteroid Treatment and Antigen Avoidance in a large hypersensitivity pneumonitis cohort: a single-centre cohort study. J Clin Med 2018;8(1). [DOI] [PMC free article] [PubMed]
- 7.Johannson KA, Barnes H, Bellanger AP, et al. Exposure Assessment Tools for Hypersensitivity Pneumonitis. An official American thoracic Society Workshop Report. Ann Am Thorac Soc. 2020;17(12):1501–9. 10.1513/AnnalsATS.202008-942ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano-Jimenez E, Rubal D, Perez de Llano LA, et al. Farmer’s lung disease: analysis of 75 cases. Med Clin (Barc). 2017;149(10):429–35. [DOI] [PubMed] [Google Scholar]
- 9.Gimenez A, Storrer K, Kuranishi L, Soares MR, Ferreira RG, Pereira CAC. Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax. 2018;73(4):391–2. 10.1136/thoraxjnl-2017-210035 [DOI] [PubMed] [Google Scholar]
- 10.Gu JP, Tsai CL, Wysham NG, Huang YT. Chronic hypersensitivity pneumonitis in the southeastern United States: an assessment of how clinicians reached the diagnosis. BMC Pulm Med. 2020;20(1):32. 10.1186/s12890-020-1072-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanak V, Kalra S, Aksamit TR, Hartman TE, Tazelaar HD, Ryu JH. Hot tub lung: presenting features and clinical course of 21 patients. Respir Med. 2006;100(4):610–5. 10.1016/j.rmed.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 12.Janssen B, Epstein S, Gomez-Manjarres D, Patel DC. Treatment of Hypersensitivity Pneumonitis (HP). Am J Respir Crit Care Med. 2022;205(10):P20–1. 10.1164/rccm.20510P20 [DOI] [PubMed] [Google Scholar]
- 13.Agency USEP. Mold Cleanup in Your Home. 2023; https://www.epa.gov/mold/mold-cleanup-your-home. Accessed January 17, 2024.
- 14.Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145(4):723–8. 10.1378/chest.13-1474 [DOI] [PubMed] [Google Scholar]
- 15.Adegunsoye A, Oldham JM, Fernandez Perez ER et al. Outcomes of immunosuppressive therapy in chronic hypersensitivity pneumonitis. ERJ Open Res 2017;3(3). [DOI] [PMC free article] [PubMed]
- 16.Zhang D, Adegunsoye A, Oldham JM et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J 2023;62(5). [DOI] [PMC free article] [PubMed]
- 17.Hansell DM, Goldin JG, King TE Jr., Lynch DA, Richeldi L, Wells AU. CT staging and monitoring of fibrotic interstitial lung diseases in clinical practice and treatment trials: a position paper from the Fleischner Society. Lancet Respir Med. 2015;3(6):483–96. 10.1016/S2213-2600(15)00096-X [DOI] [PubMed] [Google Scholar]
- 18.Barnes H, Morisset J, Molyneaux P, et al. A systematically derived exposure Assessment Instrument for Chronic Hypersensitivity Pneumonitis. Chest. 2020;157(6):1506–12. 10.1016/j.chest.2019.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TH, Wraith DG, Bennett CO, Bentley AP. Budgerigar fancier’s lung. The persistence of budgerigar precipitins and the recovery of lung function after cessation of avian exposure. Clin Allergy. 1983;13(3):197–202. 10.1111/j.1365-2222.1983.tb02588.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have included our deidentified data set in the supplementary files of this submission.