Abstract
Thiol-reactive agents such as thimerosal have been shown to modulate the Ca2+-flux properties of IP3 (inositol 1,4,5-trisphosphate) receptor (IP3R) via an as yet unidentified mechanism [Parys, Missiaen, De Smedt, Droogmans and Casteels (1993) Pflügers Arch. 424, 516–522; Kaplin, Ferris, Voglmaier and Snyder (1994) J. Biol. Chem. 269, 28972–28978; Missiaen, Taylor and Berridge (1992) J. Physiol. (Cambridge, U.K.) 455, 623–640; Missiaen, Parys, Sienaert, Maes, Kunzelmann, Takahashi, Tanzawa and De Smedt (1998) J. Biol. Chem. 273, 8983–8986]. In the present study, we show that thimerosal potentiated IICR (IP3-induced Ca2+ release) and IP3-binding activity of IP3R1, expressed in triple IP3R-knockout R23-11 cells derived from DT40 chicken B lymphoma cells, but not of IP3R3 or [Δ1–225]-IP3R1, which lacks the N-terminal suppressor domain. Using a 45Ca2+-flux technique in permeabilized A7r5 smooth-muscle cells, we have shown that Ca2+ shifted the stimulatory effect of thimerosal on IICR to lower concentrations of thimerosal and thereby increased the extent of Ca2+ release. This suggests that Ca2+ and thimerosal synergetically regulate IP3R1. Glutathione S-transferase pull-down experiments elucidated an interaction between amino acids 1–225 (suppressor domain) and amino acids 226–604 (IP3-binding core) of IP3R1, and this interaction was strengthened by both Ca2+ and thimerosal. In contrast, calmodulin and sCaBP-1 (short Ca2+-binding protein-1), both having binding sites in the 1–225 region, weakened the interaction. This interaction was not found for IP3R3, in agreement with the lack of functional stimulation of this isoform by thimerosal. The interaction between the IP3-binding and transmembrane domains (amino acids 1–604 and 2170–2749 respectively) was not affected by thimerosal and Ca2+, but it was significantly inhibited by IP3 and adenophostin A. Our results demonstrate that thimerosal and Ca2+ induce isoform-specific conformational changes in the N-terminal part of IP3R1, leading to the formation of a highly IP3-sensitive Ca2+-release channel.
Keywords: Ca2+ signalling; intramolecular interaction; isoformspecific conformational change; myo-inositol 1,4,5-trisphosphate receptor; thiol-reactive agent; thimerosal
Abbreviations: CaM, calmodulin; GST, glutathione S-transferase; IP3, myo-inositol 1,4,5-trisphosphate; IICR, IP3-induced Ca2+ release; IP3R, IP3 receptor; NEM, N-ethylmaleimide; sCaBP-1, short Ca2+-binding protein-1
INTRODUCTION
IP3 (inositol 1,4,5-trisphosphate) receptors (IP3Rs) are tetrameric intracellular Ca2+-release channels, located in the endoplasmic reticulum of many mammalian cell types [1] and encoded by three different genes [2]. Each monomer can be divided into three regions: an N-terminal IP3-binding domain, which is separated from the transmembrane C-terminal channel domain by a large internal coupling domain [2,3]. IP3 binding to the N-terminal part of an IP3R induces as yet unidentified intramolecular conformational changes in it and mediates subsequent opening of the C-terminal channel domain, followed by the release of Ca2+ from intracellular stores. Yoshikawa et al. [4] showed that IP3Rs are composed of five stable trypsinolytic fragments and these fragments could still form a functional channel, indicating that they were kept together by intramolecular interactions. Very recently, Uchida et al. [5] described different IP3R regions that are critical for the gating of the channel and pinpointed two essential, conserved cysteine residues in the C-terminal tail (Cys-2610 and Cys-2613) that are critical for IICR (IP3-induced Ca2+ release).
Ca2+ exerts a bell-shaped activation of the IICR [6–9]. Low concentrations stimulate channel activity, whereas high concentrations have an inhibitory effect. Multiple Ca2+-binding sites and a Ca2+-sensor region have already been identified in the primary sequence of IP3R1 [10–13]. Recently, it has been shown that Ca2+ significantly changes the conformation and molecular structure of purified IP3R1. Ca2+ reversibly promotes transition from a square-shaped to a windmill-like structure, with relocation of the four peripheral IP3-binding domains [14,15]. It was proposed that amino acids 651–1130, containing three possible Ca2+-binding sites [11], may fulfil the function of a hinge region that drastically changes the conformation of the receptor after Ca2+ binding [5].
Thimerosal also has a bell-shaped effect on Ca2+ flux through IP3R1 [16,17]. It increases the affinity of IP3R2 for IP3 in hepatocytes [18], whereas it has an inhibitory effect on Ca2+ flux through IP3R3 [19]. However, effects of thimerosal on IICR are not always mimicked by identical effects on IP3 binding [16,20]. The stimulatory effect of thimerosal on IP3 binding to IP3R1 from cerebellar microsomes [20] was also observed for the N-terminal IP3-binding domain of the receptor (amino acids 1–581) [21], suggesting that thimerosal has critical interaction sites in this part of the protein. However, the exact molecular mechanism by which thimerosal specifically stimulates IP3R1, but not IP3R3 functioning, has not been elucidated.
In the present study, we have compared the functional and molecular effects of thimerosal on IP3R1 and IP3R3. We have assessed IICR in permeabilized A7r5 cells, where IP3R1 is mainly expressed, and in R23-11 triple-knockout cells, which were originally derived from DT40 chicken B lymphoma cells, after the stable expression of one of these two isoforms. We have also analysed IP3 binding to wild-type IP3Rs and to a truncated IP3R1 lacking its N-terminal 225 amino acids ([Δ1–225]-IP3R1). We observed a co-ordinated effect of thimerosal and Ca2+ in stimulating IICR by IP3R1, but not by IP3R3. We found that the first 225 amino acids domain of IP3R1, but not that of IP3R3, directly interacted with the IP3-binding core and this interaction was enhanced by both thimerosal and Ca2+. In contrast, the interaction between the N- and C-terminal parts of IP3R1 was not affected by either thimerosal or Ca2+, but was significantly inhibited by IP3 and adenophostin A. It is conceivable that the C-terminal channel domain via an IP3-sensitive link can sense the Ca2+- and thimerosal-evoked conformational changes in the N-terminal part of IP3R1.
EXPERIMENTAL
Materials
Thimerosal and NEM (N-ethylmaleimide) were obtained from Sigma (St. Louis, MO, U.S.A.), [3H]IP3 from PerkinElmer (Boston, MA, U.S.A.), IP3 from Roche (Penzberg, Germany), 45Ca2+ from Amersham Biosciences (Uppsala, Sweden) and SIN-1 (3-morpholinosydnonimine/HCl) from EMD Biosciences (Darmstadt, Germany).
Cell culture
A7r5 embryonic rat aorta cells, obtained from A.T.C.C. (Bethesda, MD, U.S.A.) CRL 1444, and COS-1 SV40 African monkey kidney cells, obtained from A.T.C.C. CRL 1650, were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% (v/v) foetal calf serum, 3.8 mM L-glutamine, 0.9% (v/v) non-essential amino acids, 85 i.u./ml penicillin, 85 μg/ml streptomycin and 20 mM Hepes (pH 7.4). All cell culture media and supplements were purchased from Invitrogen (Paisley, Renfrewshire, U.K.). A7r5 cells were seeded in 12-well plates (Costar, Corning, NY, U.S.A.) at a density of approx. 4×104 cells/well. DT40 and R23-11 cell suspensions were cultured as described in [22]. R23-11 cells are IP3R-knockout cells derived from DT40 chicken B lymphoma cells by homologous recombination and were kindly provided by Dr T. Kurosaki (Department of Molecular Genetics, Institute for Liver Research, Kansai Medical University, Moriguchi, Japan).
Plasmid vector constructs
For the construction of the vector encoding [Δ1–225]-IP3R1, [pcDNA3.1(+) +mouse IP3R1] was used as a template for the PCR, with forward primer 5′-TATGTCGCTAGCCTTAAGGCCGCCATGTGGAGTGATAACAAAGACGAC-3′ and reverse primer 5′-GGGAGAGGTACCAATTTTC-3′, and the amplified cDNA was cut with NheI and KpnI (the respective restriction sites are underlined) and was ligated back into [pcDNA3.1(+) +mouse IP3R1], which was also cut with NheI and KpnI restriction enzymes.
For the construction of pGEX6p2 vectors (Amersham Biosciences) encoding amino acids 1–225, 226–604 and 1–604 of mouse IP3R1, all coding regions were amplified by PCR from [pcDNA3.1(+)+mouse IP3R1] used as a template. For the construction of pGEX6p2 vectors encoding the corresponding regions of rat IP3R3, [pcDNA3.1(+)+rat IP3R3] was used as a template. The sequences of the forward and reverse primers are listed in Table 1. The PCR products of IP3R1 were digested with BamHI and EcoRI (the respective restriction sites are underlined) and cloned into the BamHI–EcoRI-treated pGEX6p2 vector. The PCR products of IP3R3 were digested with EcoRI and XhoI (the respective restriction sites are underlined) and ligated into EcoRI–XhoI-treated pGEX6p2 vector.
Table 1. PCR primer sequences for the construction of pGEX6p2 vectors containing amino acids 1–225, 226–604 and 1–604 of mouse IP3R1 and rat IP3R3.
F, forward primer; R, reverse primer. See the text for details of the underlined regions.
| Sequence | ||
|---|---|---|
| Amino acids | Mouse IP3R1 | Rat IP3R3 |
| 1–225 | F: TAACCGGATCCATGTCTGACAAAATGTCGAG | F: GCTATGAATTCCCATGAATGAAATGTCCAGCTTTCTTC |
| R: CTCCCGAATTCTCATTTCATGAAAAGCACTATCTTC | R: GCCGTCACTCGAGTCACATGAATAAGTTGATCTTCCAGC | |
| 226–604 | F: TAACCGGATCCTGGAGTGATAACAAAGACGAC | F: GCTATGAATTCCCCAGTTCCGGGACCATCTGGAG |
| R: TTCCGAATTCTCATTTCCGGTTGTTGTGGAGCAGG | R: GCCGTCACTCGAGTCACTTCCGGTTGTTGTGCAGCAGG | |
| 1–604 | F: TAACCGGATCCATGTCTGACAAAATGTCGAG | |
| R: TTCCGAATTCTCATTTCCGGTTGTTGTGGAGCAGG | ||
For the construction of p3xFLAG-myc-CMV®-24 expression vector (Sigma) encoding amino acids 2170–2749 [FLAG(2170–2749)] of mouse IP3R1, [pcDNA3.1(+)+mouse IP3R1] was used as a template for the PCR, with forward primer 5′-CAACACAAGCTTCAAACCATGCTGAAACCTGGAGG-3′ and reverse primer 5′-TACTGGATCCTTGGGCCGGCTGCTGTGGGTTGACATTC-3′. The PCR product of IP3R1 was digested with HindIII and BamHI (the respective restriction sites are underlined) and cloned into the HindIII–BamHI-treated p3xFLAG-myc-CMV®-24 expression vector.
All constructs were sequenced using the Automated Fluorescent® sequencing system (Amersham Biosciences).
Transfections
COS-1 cells were plated in 175 cm2 dishes at 2.5×106 cells/dish, cultured for 24 h and transfected with 15–30 μg of DNA of FLAG(2170–2749) and 50 μl of FuGENE6® transfection reagent (Roche). Cells were harvested and/or analysed 72 h after transfection. R23-11 triple-knockout cells were transfected by electroporation using a Gene Pulser apparatus (Bio-Rad). Briefly, approx. 10×106 cells in 0.5 ml of serum-free medium were transferred to a 4 mm electroporation cuvette (Eurogentec, Seraing, Belgium) and pulsed at 550 V and 25 μF in the presence of 100 μg of plasmids. The electroporated cells were incubated in 30 ml of normal culture medium for 24 h before starting the selection with 1.5 mg/ml G418 to generate stable cell lines.
Preparation of microsomes and determination of protein concentration
Total microsomes from R23-11 cells and from COS-1 cells transfected with FLAG(2170–2749) DNA construct were prepared as described previously [23] and resuspended in 20 mM Tris/HCl (pH 7.4), 300 mM sucrose, 0.8 mM benzamidine and 0.2 mM PMSF. The microsomal preparations were stored at −80 °C. Protein concentration was determined by the method of Lowry et al. [24] after precipitation with 10% (w/v) ice-cold trichloroacetic acid and using BSA as a standard. Protein concentration of the recombinant proteins was determined using the BCA (bicinchoninic acid) Protein Assay kit (Pierce Biotechnology, Rockford, IL, U.S.A.) according to the manufacturer's instructions.
[3H]IP3-binding assay
Binding studies were performed as described previously [25]. [3H]IP3 binding was performed at 0 °C by incubating samples in 160 μl of binding buffer containing 50 mM Tris/HCl (pH 8.3), 1 mM EDTA, 10 mM 2-mercaptoethanol and 10 nM [3H]IP3 for 30 min. In the experiments with NEM and thimerosal, protein samples were preincubated with the respective agent for 10 min at 0 °C before starting the binding assay, and the reducing agent 2-mercaptoethanol was omitted from the binding buffer. After incubation, the samples were rapidly passed through glass-fibre filters using a Combi cell harvester (Skatron, Lier, Norway). Non-specific binding was determined in the presence of 12.5 μM unlabelled IP3. The amount of microsomes used was 200 μg.
SDS/PAGE and Western-blot analysis
Microsomes from different types of R23-11 cells were analysed by NuPAGE® 3–8% (v/v) Tris/acetate SDS/polyacrylamide gels (Invitrogen); microsomes from COS-1 cells transfected with FLAG(2170–2749) by NuPAGE® 4–12% (v/v) Bis-Tris SDS/polyacrylamide gels using Mops buffer (Invitrogen); GST (glutathione S-transferase) fusion proteins and amino acids 1–225 domains by NuPAGE® 4–12% Bis-Tris SDS/polyacrylamide gels using Mes buffer (Invitrogen) according to the manufacturer's instructions. After semi-dry electrophoretic transfer on to a PVDF membrane (Immobilon-P; Millipore, Bedford, MA, U.S.A.) and blocking with PBS containing 1% (v/v) Tween 20 and 5% (w/v) non-fat dry milk powder, the blots were incubated first with the primary antibody and then with the alkaline phosphatase-conjugated secondary antibody. The immunoreactive bands were visualized after dephosphorylation of the Vistra® enhanced chemifluorescence substrate (Amersham Biosciences) and quantified using a Storm840 FluorImager equipped with ImageQuant 5.2 software (Molecular Dynamics, Sunnyvale, CA, U.S.A.). Statistical comparisons were performed by Student's t test. For immunodetection of the N-terminal regions of the IP3R isoforms, we used the recently developed Rbt475 antibody (1:2500 to 1:3000). The epitope corresponds to amino acids 127–141 of the human IP3R1, which are conserved in all the isoforms and across species; consequently, the antibody recognizes the various IP3Rs having similar affinity ([26]; J. B. Parys, unpublished work). For the specific detection of IP3R1, a polyclonal antibody Rbt03 (1:2500), whose epitope lies within residues 2735–2749, was used [27]. For immunodetection of FLAG(2170–2749) fusion protein, ANTI-FLAG® M2 monoclonal antibody (1:1500; Sigma) was used.
Purification of GST fusion proteins
For the preparation of GST fusion proteins, pGEX6p2 vectors containing the coding sequences for amino acids 1–225, 226–604 and 1–604 of mouse IP3R1 and rat IP3R3 were transformed into BL21(DE3) Escherichia coli. For GST–226–604 and GST–1–604, colonies were grown overnight in 50 ml of Luria–Bertani medium at 30 °C. Luria–Bertani medium (400 ml) was added to this pre-culture and bacteria were grown at 22 °C until the A600 reached 1.5. Addition of 0.1 mM isopropyl β-D-thiogalactoside to the bacterial culture, which was further grown at 14 °C for another 20 h, induced protein expression. Expression of 1–225 recombinant proteins was performed as described previously [11]. All proteins were further purified as described previously [11]. For amino acids 1–225, after washing the beads, the immobilized GST fusion proteins were treated for 2 h at 4 °C with PreScission Protease (40 units; Amersham Biosciences) in cleavage buffer (50 mM Tris/HCl, pH 7.0/150 mM NaCl/1 mM EDTA/1 mM dithiothreitol). All the purified proteins were dialysed overnight against PBS using Slide-A-Lyzer with a cut-off of 10 kDa (Pierce Biotechnology) and stored at −80 °C.
GST-pull-down assay
A GST-pull-down assay was performed by using the Profound Pull-Down GST protein–protein interaction kit (Pierce Biotechnology). Dialysed, purified GST–226–604 fusion proteins or parental GST (control) were immobilized on 50 μl of glutathione–Sepharose 4B (50%) by incubation for 2 h at 4 °C in incubation buffer, i.e. 1 part TBS [20 mM Tris/HCl (pH 7.2)/150 mM NaCl] mixed with 1 part of ProFound® bacterial lysis buffer (Pierce Biotechnology) and supplemented with 1 mM 2-mercaptoethanol. After the GST fusion proteins were bound, the beads were washed twice with 500 μl of incubation buffer supplemented with 2-mercaptoethanol (1 mM) and twice with 500 μl of incubation buffer supplemented with either 2-mercaptoethanol (1 mM), thimerosal (100 μM) or NEM (300 μM) depending on the conditions. Purified 1–225 proteins were incubated with immobilized GST–226–604 for 2 h at 4 °C in incubation buffer, supplemented with 1 mM 2-mercaptoethanol, 100 μM thimerosal or 300 μM NEM. Unbound 1–225 protein was removed by washing the beads four times with 500 μl of the respective incubation buffer. Bound 1–225 proteins were eluted by incubating the beads with LDS® (Invitrogen) for 10 min at 70 °C and collected by centrifuging at 20000 g for 1 min at room temperature (25 °C).
Pull-down assay of FLAG(2170–2749) with GST–1–604
A GST-pull-down assay was performed by using the Profound Pull-Down GST protein–protein interaction kit (Pierce Biotechnology). Dialysed, purified GST–1–604 type 1 fusion proteins or parental GST (control) were immobilized on 50 μl of glutathione–Sepharose 4B (50%) by incubation for 2 h at 4 °C in incubation buffer, i.e. 1 part of PBS without Ca2+ and Mg2+ buffer containing 0.8 mM benzamidine, 0.2 mM PMSF and 1 μg/ml leupeptin mixed with 1 part of bacterial lysis buffer® (Pierce Biotechnology) and supplemented with 1 mM 2-mercaptoethanol. After the GST fusion proteins were bound, the beads were washed twice with 500 μl of incubation buffer supplemented with 2-mercaptoethanol (1 mM) and twice with 500 μl of incubation buffer supplemented with either 2-mercaptoethanol (1 mM) or thimerosal (100 μM) and EGTA (1 mM), Ca2+ (25 μM), IP3 (20 μM) or adenophostin A (20 μM) depending on the conditions. COS-1 microsomes, originating from cells transfected with FLAG(2170–2749), were first solubilized by incubation for 2 h at 4 °C in 400 μl of PBS without Ca2+ and Mg2+ buffer containing 1% Triton X-100, 0.8 mM benzamidine, 0.2 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatin A and 0.5 μM NaCl and centrifuged at 125000 g for 25 min. Solubilized FLAG(2170–2749) protein was incubated with immobilized GST–1–604 for 2 h at 4 °C in the corresponding incubation buffer. Unbound FLAG(2170–2749) was removed by washing the beads four times with 500 μl of PBS without Ca2+ and Mg2+ buffer containing 0.25% Triton X-100, 0.8 mM benzamidine, 0.2 mM PMSF, 1 μg/ml leupeptin, supplemented with either 2-mercaptoethanol (1 mM) or thimerosal (100 μM) and EGTA (1 mM), Ca2+ (25 μM), IP3 (20 μM) or adenophostin A (20 μM) depending on the conditions. Bound FLAG(2170–2749) proteins were eluted by incubating the beads with LDS® (Invitrogen) for 10 min at 70 °C and collected after centrifuging at 20000 g for 1 min.
Ca2+-release studies in permeabilized R23-11 and A7r5 cells
R23-11 cell pellets were resuspended in intracellular medium (120 mM KCl/30 mM Hepes, pH 7.4/1 mM MgCl2) in the presence of 1 mM ATP, 25 mM phosphocreatine, 50 i.u. of creatine kinase and 5 μM fluo-3 (Molecular Probes, Eugene, OR, U.S.A.) and transferred to a 4 ml fluorescence quartz cuvette thermostatically maintained at 37 °C. Cell density was 5×107 cells/ml. Mild treatment of the cells with digitonin (50 μM) disrupted the plasma membrane. The fluo-3 fluorescence (λex=503 nm and λem=530 nm) was measured with an Aminco–Bowman® Series 2 spectrometer (Spectronic Unicam, Rochester, NY, U.S.A.). A23187 (8 μM; Sigma) was added at the end of each experiment to measure the total releasable Ca2+. This value varied slightly but not significantly in different cell batches due to the time of storage of the cells on ice after pelleting. The fluorescence signal F was calibrated by first adding 0.5 mM Ca2+ (Fmax) and then adding 5 mM EGTA (Fmin). The free [Ca2+] was calculated using the equation [Ca2+]=Kd·(F−Fmin)/(Fmax−F) [28], with a dissociation constant Kd of 864 nM, as determined in the cytosol-like medium at 37 °C [29]. 45Ca2+ fluxes on monolayers of saponin-permeabilized A7r5 cells at 25 °C were measured exactly as described previously [30].
RESULTS
Expression and characterization of wild-type IP3R1, wild-type IP3R3 and [Δ1–225]-IP3R1 in IP3R-knockout R23-11 cells
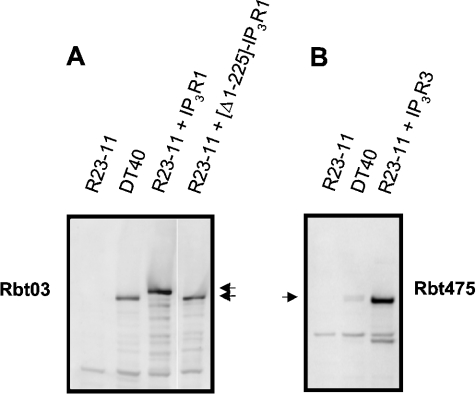
We stably expressed wild-type mouse IP3R1, wild-type rat IP3R3 and the deletion mutant of the mouse IP3R1 lacking its first 225 amino acids ([Δ1–225]-IP3R1) in R23-11 triple-knockout cells originating from DT40 chicken B lymphoma cells. Figure 1 shows a Western-blot analysis of the microsomal preparations from these stable cell lines together with the original DT40 cells using the polyclonal anti-IP3R1 Rbt03 antibody (Figure 1A) and anti-IP3R Rbt475 (Figure 1B). No IP3R expression was detected in R23-11 cells. The immunoreactive band in the DT40 cells corresponds to the endogenous peripheral IP3R1, which migrates faster than the heterologously expressed neuronal IP3R1 (Figure 1A, second and third lanes). The broad band detected in DT40 cells with Rbt475 (Figure 1B) corresponds to the sum of the endogenous IP3Rs.
Figure 1. Expression of IP3Rs in R23-11 cells.
Microsomes (10 μg) from different R23-11 cell lines were separated on SDS/polyacrylamide gels and analysed by immunoblotting. The blot in (A) was probed with the anti-IP3R1 polyclonal antibody Rbt03, whose epitope lies within residues 2735–2749, and the blot in (B) was probed with the anti-IP3R polyclonal antibody Rbt475, whose epitope corresponds to the conserved stretch of amino acids in the N-terminus of all IP3R isoforms. The arrows indicate the positions of the full-size IP3Rs.
IP3-binding activities of the wild-type IP3R1 and [Δ1–225]-IP3R1 were measured by equilibrium [3H]IP3-binding analysis with 10 nM [3H]IP3. After subtraction of background [3H]IP3 binding to the membranes of the R23-11 cells, we found that [Δ1–225]-IP3R1 bound approx. 7-fold more IP3 when compared with the wild-type IP3R1 (results not shown). This is compatible with previous studies reporting that a mutant IP3R1 lacking the first 223 amino acids exhibited a 10-fold higher affinity for IP3 when compared with the wild-type channel [5].
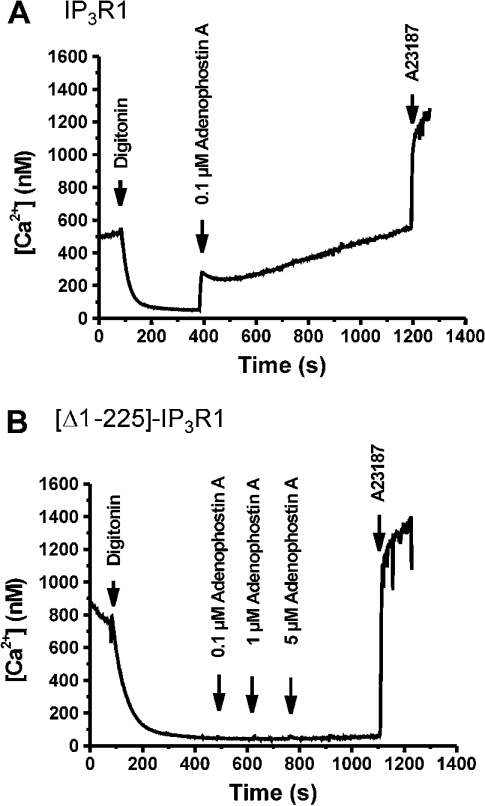
We then analysed the Ca2+-release activity of the truncated channel in digitonin-permeabilized R23-11 cells. Wild-type IP3R1 exhibited a strong Ca2+ release after the addition of IP3. In contrast, [Δ1–225]-IP3R1 did not respond to (up to 25 μM) IP3 (results not shown). Similarly, adenophostin A, a more potent and stable activator of IP3R, induced a long-lasting increase in the free [Ca2+] in cells heterologously expressing wild-type IP3R1 (Figure 2A), but had no effect on [Δ1–225]-IP3R1-expressing cells (Figure 2B). These findings are compatible with the other studies showing that the N-terminal 225 amino acids fragment is critical for the functional activation of IP3R [5].
Figure 2. Ca2+-release properties in permeabilized R23-11 cells heterologously expressing IP3R1 (A) and [Δ1–225]-IP3R1 (B).
Free [Ca2+] was measured using fluo-3 fluorescence. After permeabilization of the plasma membrane with 50 μM digitonin, the cells accumulated Ca2+ in their intracellular stores, resulting in a decrease in medium-free [Ca2+]. After stabilization of the signal, adenophostin A (concentrations are shown in the Figures) was added. At the end of each experiment, 8 μM A23187 was added to estimate the total releasable amount of Ca2+. Results are typical for four independent experiments.
Effect of thiol-reactive agents on IP3 binding and IICR
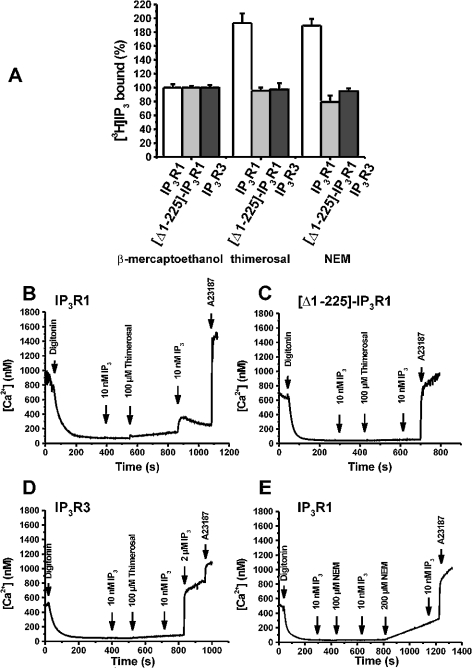
We next examined the effect of thiol-reactive agents on IP3-binding and Ca2+-release properties of the wild-type IP3R1 and [Δ1–225]-IP3R1. In addition, we also examined the effect on wild-type IP3R3, which was not stimulated by thimerosal [19–21]. We found that both thimerosal (100 μM), which catalyses the oxidation of thiol groups leading to the formation of S-S bridges, and NEM (300 μM), a thiol-alkylating agent, stimulated IP3 binding to the wild-type IP3R1 by approx. 2-fold (Figure 3A). Interestingly, the stimulatory effect of thimerosal and NEM on IP3 binding was completely abolished in the cell line heterologously expressing [Δ1–225]-IP3R1, indicating that the stimulatory effects of thimerosal and NEM on IP3 binding may involve cysteine residues in the first 225 amino acids. In agreement with previous studies [20,21], IP3 binding to the full-size IP3R3 was stimulated neither by incubation with thimerosal nor by incubation with NEM (Figure 3A).
Figure 3. Effects of thimerosal and NEM on IP3 binding and IICR in R23-11 cells heterologously expressing IP3R1, [Δ1–225]-IP3R1 and IP3R3.
(A) [3H]IP3-binding assay performed on 200 μg of microsomes in a binding buffer at pH 8.3 using 10 nM [3H]IP3 in the presence of 100 μM thimerosal or 300 μM NEM. Binding in the presence of 1 mM 2-mercaptoethanol was used as the control value (100%). Results are expressed as the means±S.E.M. for three independent experiments each performed in triplicate. (B–D) The effect of 100 μM thimerosal on IICR in permeabilized R23-11 cells heterologously expressing IP3R1 (B), [Δ1–225]-IP3R1 (C) and IP3R3 (D). The experimental procedure was the same as that described in Figures 2(A) and 2(B). In these experiments, a subthreshold concentration of IP3 (10 nM), which cannot provoke Ca2+ release, was used. After incubation with 100 μM thimerosal for 5 min, the same concentration of IP3 (10 nM) was added. (E) The effect of 100 and 200 μM NEM on IICR in permeabilized R23-11 cells heterologously expressing IP3R1. Results are typical for four independent experiments.
We observed that the preincubation with thimerosal enhanced IICR and rendered the subthreshold IP3 concentration of 10 nM effective to release Ca2+ through the wild-type IP3R1. This is demonstrated in Figure 3(B), where the free [Ca2+] is shown as a function of time in permeabilized R23-11 cells heterologously expressing the wild-type IP3R1. After preincubation with 100 μM thimerosal for approx. 5 min, the same concentration of IP3 provoked significant Ca2+ release. When water was added instead of thimerosal, 10 nM IP3 was still unable to induce Ca2+ mobilization (results not shown), indicating that the release induced by the second addition of IP3 (10 nM) was due to thimerosal-mediated sensitization of IP3R1. Furthermore, when a reducing agent such as 2-mercaptoethanol (1 mM) was used instead of thimerosal, 10 nM IP3 did not provoke any measurable Ca2+-release activity and preincubation with 2-mercaptoethanol completely abolished the potentiating effect of thimerosal on IICR (results not shown). NEM, which augmented IP3 binding to the same extent as thimerosal, did not enhance Ca2+ flux through IP3R1 (Figure 3E). At a total concentration of 300 μM, NEM inhibits the Ca2+-uptake activity of the SERCA (sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase) pumps, explaining the increase in the basal Ca2+ levels. This suggests that the oxidizing effect of thimerosal is essential for the stimulatory action and that alkylation of cysteine residues by NEM is unable to mimic the effect of thimerosal. We propose that thimerosal catalyses the formation of S–S bridges between different parts of IP3R1 and thereby influences the conformation.
The same experiment was performed for [Δ1–225]-IP3R1 and IP3R3. Thimerosal could neither render the truncated receptor active nor sensitize it to IP3 (Figure 3C). A similar observation was obtained for IP3R3, since up to 100 μM thimerosal could not potentiate IICR mediated by this isoform (Figure 3D). Moreover, at higher IP3 concentrations, there was no potentiation of IICR by 100 μM thimerosal (results not shown; [18]). The finding that thimerosal specifically exerts its activatory function on IP3R1, but not on IP3R3, suggests that thimerosal affects the cysteine residues that are not conserved between IP3R1 and IP3R3.
Effect of thimerosal and Ca2+ on IICR in permeabilized A7r5 cells
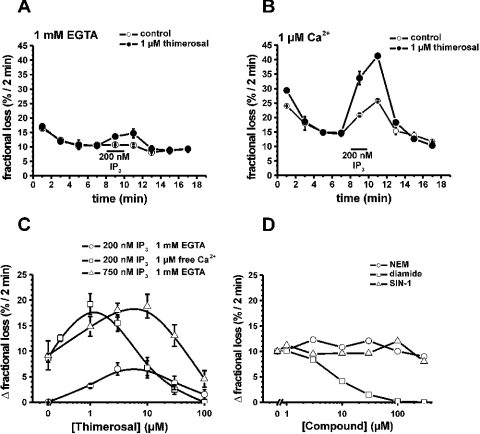
In A7r5 smooth-muscle cells, which mainly express IP3R1, a biphasic effect of thimerosal on IICR was found [16]. We now found that Ca2+ modulated this biphasic effect of thimerosal on IICR. Figures 4(A) and 4(B) illustrate the fractional loss (the amount of Ca2+ leaving the stores within 2 min divided by the total store Ca2+ content at that time) as a function of time. The non-mitochondrial Ca2+ stores of permeabilized A7r5 cells, loaded to steady state with 45Ca2+, slowly lost their accumulated Ca2+. Thimerosal (1 μM) strongly potentiated IICR and this was additive to the potentiation by Ca2+ (Figures 4A and 4B). A bell-shaped dependence on the thimerosal concentration was observed, but the maximum depended on the presence of Ca2+ (Figure 4C). In the absence of Ca2+, the maximum stimulation was reached with between 3 and 10 μM thimerosal, whereas in the presence of 1 μM free Ca2+, the maximum was obtained at 1 μM thimerosal. The shift to lower concentrations of thimerosal was not due to the higher Ca2+-release activity in the presence of Ca2+, since in the absence of Ca2+, but using 750 nM IP3 to evoke Ca2+ release, maximum stimulation was still reached with between 3 and 10 μM thimerosal. The additive effect of thimerosal and Ca2+ on the activation of IICR may indicate that both agents co-operate to induce a conformation that is much more sensitive towards activation by IP3.
Figure 4. Effect of thimerosal on IICR in the presence or absence of 1 μM free Ca2+ in permeabilized A7r5 cells.
Data are plotted as fractional loss (the amount of 45Ca2+ leaving the stores in 2 min divided by the total store Ca2+ content at that time) as a function of time in (A, B). (A) Effect of the presence (•) or absence (○) of 1 μM thimerosal on Ca2+ release provoked by 200 nM IP3 in the absence of Ca2+. (B) Effect of the presence (•) or absence (○) of 1 μM thimerosal on Ca2+ release provoked by 200 nM IP3 in the presence of 1 μM free Ca2+. (C) Bell-shaped dependence of the effect of thimerosal on IICR. Data are plotted as the difference between the fractional loss after the addition of IP3 and the fractional loss before the addition of IP3 as a function of the thimerosal concentration: ○, 200 nM IP3, 1 mM EGTA; □, 200 nM IP3, 1 μM free Ca2+; Δ, 750 nM IP3, 1 mM EGTA. (D) Effect of NEM (○), diamide (□) and SIN-1 (Δ) on IICR. Data are plotted as the difference between the fractional loss after the addition of IP3 and the fractional loss before the addition of IP3 as a function of the NEM, diamide or SIN-1 concentration respectively. The experiment was performed using 750 nM IP3 and in the presence of 1 mM EGTA. Each curve represents the means±S.E.M for three wells.
Other agents that interact with cysteine residues could not mimic the effect of thimerosal. NEM did not affect IICR, indicating that alkylation of cysteine residues is insufficient (Figure 4D). The NO donor SIN-1, capable of nitrosylation of cysteine residues, also had no effect on IICR, whereas diamide, which is known to cross-link disulphide bridges of cysteine residues, had an inhibitory effect on IICR.
Interaction of the N-terminal 225 amino acids with the IP3-binding core (amino acids 226–604)
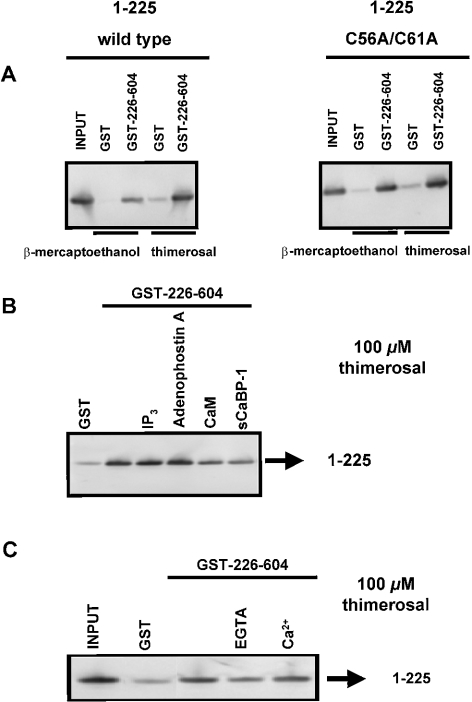
Since the N-terminal 225 amino acids seemed to be critical for the effect of thimerosal on IP3 binding (Figure 3A), we investigated the role of this domain in thimerosal-dependent intramolecular interactions. IP3-binding cores (amino acids 226–604) of both IP3R1 and IP3R3 were immobilized as GST fusion proteins on glutathione–Sepharose 4B. The suppressor domains (amino acids 1–225) of IP3R1 and IP3R3 were obtained from GST fusion proteins by proteolytic cleavage. Western-blot analysis using the Rbt475 antibody revealed that these 1–225 fragments were expressed as stable proteins and migrated in gels as single bands (results not shown). Under reducing conditions (1 mM 2-mercaptoethanol), we found that the suppressor domain of IP3R1 specifically interacted with the IP3-binding core of IP3R1 (Figure 5A, left panel). The binding was relatively weak, since only 5±2% (n=4 independent experiments) of the 1–225 fragments was retained by immobilized GST–226–604 (Table 2). In the presence of 100 μM thimerosal, the binding efficiency was increased approx. 6-fold, and up to 33±6% (n=4) of the input was retained on the column (Figure 5A, left panel). In the presence of 300 μM NEM, we could not detect any significant increase in the binding efficiency (Table 2).
Figure 5. Interaction between amino acids 1–225 and 226–604 of IP3R1.
(A) Pull-down experiment applied for GST–226–604 with 1–225 wild-type and with 1–225 C56A/C61A in the presence of 1 mM 2-mercaptoethanol or 100 μM thimerosal. C56 and C61, two conserved cysteine residues in the suppressor domain, were mutated into alanine residues. The recombinant suppressor domains were incubated with GST or GST–226–604 immobilized on glutathione–Sepharose 4B. After washing, the retained proteins were eluted. After SDS/PAGE, the proteins were analysed by immunoblotting with the anti-IP3R polyclonal antibody Rbt475. Input (1–225 protein) was always 50 ng, whereas the lanes representing samples obtained after GST-pull down contained 2.5-fold more material. (B) Pull-down experiment applied for GST–226–604 with 1–225 in the presence of 100 μM thimerosal. The experimental procedure was the same as that described in (A). During the incubation reaction, 25 μM IP3, 25 μM adenophostin A, 10 μM CaM or 10 μM sCaBP-1 was added. CaM and sCaBP-1 significantly inhibited the interaction to 44±13 and 37±12% of the control respectively. (C) Pull-down experiment applied for GST–226–604 with 1–225 in the presence or absence of Ca2+. Thimerosal (100 μM) was added during the incubation step. The experimental procedure was the same as that described in (A). The absence of Ca2+ inhibited the interaction to 26±18% of the control. Results are the means±S.D. for at least three independent experiments.
Table 2. Specific binding of 1–225 type 1 or 3 to immobilized GST–226–604 type 1 or 3 in the presence of 2-mercaptoethanol, NEM or thimerosal.
Purified 1–225 proteins were incubated with immobilized GST–226–604 fusion proteins in their respective interaction buffer, containing 2-mercaptoethanol (1 mM), NEM (300 μM) or thimerosal (100 μM). After washing and elution, the proteins (the equivalent of 125 or 200 ng) were separated on NuPAGE® 4–12% Bis-Tris gels and analysed by Western blotting using Rbt475 (1:3000). Immunoreactive bands were quantified by using ImageQuant 5.2 software and the binding efficiency was calculated against 50 ng of input protein (%). Specific binding was determined by subtracting the binding to immobilized GST in the presence of 2-mercaptoethanol, NEM or thimerosal. Results are the means±S.D. for at least four independent experiments. Abbreviation: NS, not significant.
| Specific binding (%) (means±S.D.) | ||
|---|---|---|
| GST–226–604 IP3R1 | GST–226–604 IP3R3 | |
| 1–225 protein IP3R1 | ||
| 2-Mercaptoethanol | 5±2 | NS |
| NEM | 7±2 | NS |
| Thimerosal | 33±6 | 6±2 |
| 1–225 protein IP3R3 | ||
| 2-Mercaptoethanol | NS | NS |
| NEM | NS | NS |
| Thimerosal | 4±2% | NS |
The effect of thimerosal on the intramolecular interaction appeared to be specific for IP3R1. The 1–225 fragment of IP3R3 did not interact with immobilized GST–226–604 protein of IP3R3 in the presence of 2-mercaptoethanol. Neither NEM nor thimerosal could induce a significant interaction (Table 2).
We also investigated the cross-interaction between types 1 and 3 by incubating the 1–225 fragment of IP3R1 with immobilized GST–226–604 of IP3R3 and vice versa. No significant interactions were observed and neither NEM nor thimerosal could stimulate domain interactions (Table 2). When we mutated the two cysteine residues conserved in all isoforms, i.e. at positions 56 and 61 of mouse IP3R1, into alanine residues, we found that thimerosal was still capable of stimulating this interaction to the same extent as the wild-type (Figure 5A, right panel). In addition, the full-size IP3R1 carrying those mutations was stimulated by thimerosal to the same extent as the wild-type IP3R1 (results not shown). This result suggests that thimerosal interacts with IP3R1 via non-conserved cysteine residues.
IP3, adenophostin A, CaM (calmodulin) and sCaBP-1 (short Ca2+-binding protein-1) interact within the first 604 amino acids of mouse IP3R1. In the presence of 2-mercaptoethanol, none of these compounds and none of these proteins modulated the interaction between the suppressor and IP3-binding core of IP3R1 (results not shown). However, the thimerosal-dependent interaction was significantly inhibited by CaM and sCaBP-1 to approx. 44±13 and 37±12% of the control conditions respectively (n=4 and P<0.01; P value obtained by paired Student's t test of the significantly different conditions; Figure 5B), whereas IP3 and adenophostin A did not have any effect on the binding efficiency (Figure 5B).
Since the effect of thimerosal on IICR via IP3R1 appeared to depend on Ca2+, we also investigated whether Ca2+ modulated the effect of thimerosal on the interaction between the suppressor and IP3-binding core of IP3R1. When Ca2+ was removed from the incubation buffer by the addition of 1 mM EGTA, the binding efficiency was indeed reduced to approx. 26±18% (n=3 and P<0.01) of the control conditions, which was done at nominal [Ca2+] (Figure 5C). However, addition of 25 μM Ca2+ did not significantly alter the binding efficiency.
Effects of thimerosal and IP3 on the interaction of the IP3-binding and transmembrane domains
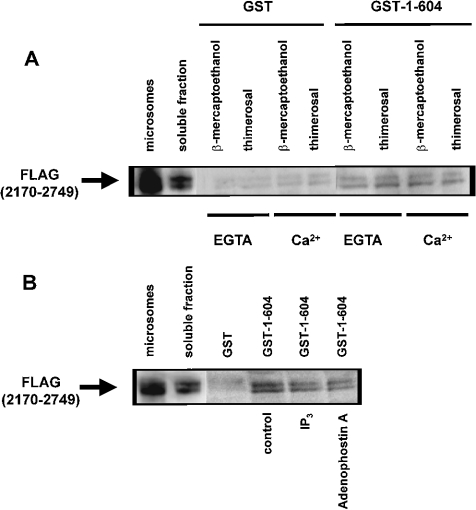
A weak interaction between ligand-binding and transmembrane domains has been described in homo- and hetero-tetrameric IP3Rs [31]. In the C-terminal tail, there are two highly conserved cysteine residues (Cys-2610 and Cys-2613) that appear to be essential for channel opening [5]. In addition, a C-terminal monoclonal antibody was shown to inhibit Ca2+ oscillations and thimerosal-induced sensitization of IICR [32]. Therefore we decided to characterize the effects of thimerosal and Ca2+ on the interaction between the IP3-binding and transmembrane domains. The IP3-binding domain of IP3R1 (amino acids 1–604) was expressed as a GST fusion protein (GST–1–604) and a pull-down assay was applied for the C-terminal transmembrane domain of IP3R1 (amino acids 2170–2749) tagged with three adjacent FLAG epitopes [FLAG(2170–2749)]. FLAG(2170–2749) was expressed in COS-1 cells and the microsomes were lysed in a Triton X-100-containing buffer. The N- and C-terminal association was confirmed as shown in Figure 6(A). Thimerosal (100 μM) or Ca2+ (25 μM) alone or a combination of both the compounds could not alter this interaction between the N- and C-terminal domains. The double band of the FLAG(2170–2749) is due to partial glycosylation of this protein. In contrast with previous findings [31], we found that 20 μM IP3 or adenophostin A significantly weakened the interaction (Figure 6B). For IP3, 45±5% (n=3 and P<0.05) of the control (without IP3) FLAG(2170–2749) was retained on the column. Adenophostin A had even a stronger effect, since only 32±8% (n=3 and P<0.05) compared with the control condition was retained by GST–1–604. This discrepancy between our results and results of previous studies is probably due to different experimental approaches.
Figure 6. Effect of thimerosal and IP3 on the interaction between the IP3-binding and transmembrane domains of IP3R1.
(A) Pull-down experiment applied for GST–1–604 with FLAG(2170–2749) in the presence of 1 mM 2-mercaptoethanol or 100 μM thimerosal. EGTA (1 mM) or 25 μM Ca2+ was added as indicated in the Figure. The recombinant FLAG(2170–2749) was incubated with GST or GST–1–604 immobilized on glutathione–Sepharose 4B. After washing, the retained proteins were eluted. After SDS/PAGE, the proteins were analysed by immunoblotting with ANTI-FLAG® M2 monoclonal antibody. Lane ‘microsomes’ contains 0.5 μg of protein and lane ‘soluble fraction’ contains 2 μg of protein, whereas the lanes representing samples obtained after GST-pull down contain 15-fold more material than the soluble fraction. (B) Pull-down experiment applied for GST–1–604 with FLAG(2170–2749) in the presence of 1 mM 2-mercaptoethanol. During the incubation reaction, 20 μM IP3 or adenophostin A was added. The experimental procedure was the same as that described in (A). IP3 and adenophostin A significantly inhibited the interaction to 45±5 and 32±8% of the control respectively. Results are the means±S.D. for at least three independent experiments.
DISCUSSION
In the present study, we have analysed the effects of the thiol-reactive agent thimerosal, which is a well-known activator of IP3R capable of inducing Ca2+ oscillations in a number of cells [32,33]. The effect of thimerosal has been linked to an increase in the affinity of IP3Rs for IP3 [17,18,34] and an increase in channel conductance and mean opening time [35]. Effects of thimerosal appear to be dependent on the redox state of the receptor, implying a direct action of thimerosal on one or more critical thiol groups [17]. However, the molecular mechanism and identity of these critical cysteine residues on IP3R are still not known. Thimerosal has been shown to lower significantly the threshold for IP3 action [36], whereas it only moderately enhances IP3 binding. Therefore it is conceivable that thimerosal interferes with conformational changes of IP3R that are crucial for activation.
The IP3-binding domain of IP3R1 is composed of two different functional and structural parts: an N-terminal 225-amino acid fragment and a succeeding 351-residue-fragment (amino acids 226–576), which forms the IP3-binding core [37,38]. The affinity for IP3 of this IP3-binding core is more than 10-fold higher compared with that of the full IP3-binding domain [37]. In addition, it was shown that the first 225-amino-acid fragment is critical for IP3R gating [5]. This domain was therefore referred to as a coupling/suppressor domain. In the present study, we describe a novel association between the coupling/suppressor domain and the IP3-binding core is described that was enhanced by both thimerosal and Ca2+. Accordingly, we observed that the previously demonstrated bell-shaped dependence of IICR on thimerosal in A7r5 cells [16] shifted to lower thimerosal concentrations in the presence of Ca2+. This analogy between the molecular data on the one hand and the functional experiments in A7r5 cells on the other allows us to conclude that Ca2+ and thimerosal co-operate to render IP3R1 more sensitive to IP3 and the interaction between the coupling/suppressor domain and the IP3-binding core is probably the molecular basis for this sensitization. This is compatible with experiments in hamster eggs, where it was also shown that both thimerosal and Ca2+ synergetically sensitize IICR [32]. This effect was isoform-specific. In agreement with the lack of effect of thimerosal on IP3R3 [19–21], we found that thimerosal did not enhance the interaction between the coupling/suppressor domain and the IP3-binding core of IP3R3. Also, in R23-11 cells stably expressing IP3R3, thimerosal could not affect IICR or IP3 binding.
The main question remains as to how changes in intramolecular interactions of the N-terminal part of the receptor are translated into altered Ca2+-flux properties of the C-terminal part of the receptor. Since it was suggested that the two conserved residues in the C-terminus (Cys-2610 and Cys-2613) could be the targets of thiol reagents [32], we investigated whether thimerosal might alter the interaction between the N- and C-terminal domains. However, we did not detect any thimerosal-dependent interaction between the N- and C-termini either in the presence or absence of Ca2+. Therefore the stimulatory action of thimerosal appears to be related solely to conformational changes in the N-terminal region of the receptor. These changes may be sensed by the C-terminal channel domain via the N-terminal coupling/suppressor domain [5], thereby enhancing the opening of the channel pore. Support for this view comes from the observation that, for IP3R3, the N-terminal intramolecular interaction and its modulation by thimerosal are completely absent, in agreement with the lack of stimulation of IP3R3 by thimerosal. The thimerosal-dependent N-terminal interaction in IP3R1 was inhibited by CaM and by CaM-like sCaBP-1. Both proteins interact specifically within the N-terminal domain of IP3Rs [39–41]. CaM inhibited IICR and IP3 binding [42,43], whereas sCaBP-1 was found to stimulate [40] or to inhibit [41,44] Ca2+ release. It is conceivable that CaM and sCaBP-1 exert their effects by disrupting the intramolecular interaction in the N-terminal region, which is required for activation of the channel and the increase in function by thimerosal. A similar situation is found for ryanodine receptor 1, where the activation by NO is dependent on endogenous CaM and antagonizes the inhibition by CaM without displacing it from the channel. Mutation of conserved Cys-3635, which is located in or near the CaM-binding domain of ryanodine receptor 1, resulted in the loss of CaM-dependent NO modulation and reduced S-nitrosylation by NO [45,46].
Although neither thimerosal nor Ca2+ modulated the interaction between the N-terminal IP3-binding and C-terminal transmembrane domains of IP3R1, we observed that IP3 and adenophostin A clearly weakened it. This may indicate that the N-terminal IP3-binding domain prevents unco-ordinated opening and activation of the channel in the absence of the ligand. When occupied by IP3 or adenophostin A, the affinity of the IP3-binding domain for the C-terminus decreases and this may allow proper opening of the channel pore. This IP3-sensitive link between the N- and C-terminal parts of the receptor may be a part of the mechanism for IP3-induced activation of the channel.
Although we cannot exclude the possibility that thimerosal may also affect other intramolecular interactions in the structure of IP3R1 that participate in coupling IP3 binding to channel domain, our results clearly show that the interaction of thimerosal with the N-terminal domain of IP3R1 is functionally important and enhances Ca2+ flux via conformational changes involving the N-terminal coupling domain. It is tempting to suggest that the windmill-like structure of the receptor that is achieved in the presence of Ca2+ [14,15] is more accessible for thimerosal. This would explain the synergetic effect of Ca2+ and thimerosal on IP3R1.
Our results also show that the oxidizing effect of thimerosal is specific and cannot be mimicked by the addition of NEM or diamide. We cannot exclude that these thiol-modifying agents act on cysteine residues other than that in thimerosal. Thimerosal exerts a biphasic effect: the stimulatory effect of thimerosal is only observed at lower concentrations, whereas higher concentrations have inhibitory effects on Ca2+ release and IP3 binding. This suggests that thimerosal may exert a specific action on a limited number of cysteine residues in the N-terminal part of IP3R1 at lower thimerosal concentrations, whereas it may affect other cysteine residues at high concentrations and thereby abolish the Ca2+-flux properties of the channel. Poirier et al. [47] have pointed out that the conformational state adopted by IP3R after a drastic treatment with a thiol-reactive agent is irreversible and corresponds to a nonbinding state. This may explain the bell-shaped dependence of IICR on thimerosal and the finding that diamide inhibits Ca2+ flux in a dose-dependent manner. Mutational analysis of the different cysteine residues in the IP3R1 constructs may reveal the critical component responsible for the thimerosal- and Ca2+-induced ‘gain of function’ process. At a later stage it will be necessary to evaluate different physiological agents that could mimic the effects of thimerosal on the intramolecular interaction and on the Ca2+-flux properties of IP3R1. The reactive oxygen species that are produced during different physiological processes may be good candidates.
Taken together, our results revealed a thimerosal-dependent intramolecular interaction within the N-terminal domain of IP3R1. This interaction is Ca2+-dependent and isoform-specific. These properties are entirely compatible with functional observations on IICR showing that thimerosal stimulation was potentiated in the presence of Ca2+ and the stimulatory effect of thimerosal was isoform-specific. This intramolecular interaction may therefore be responsible for at least a part of the conformational changes occurring during the sensitization of IP3R1 by thimerosal. In addition, IP3 may induce the opening of the channel pore by modifying the interaction of the C-terminal channel domain with the N-terminal IP3-binding domain.
Acknowledgments
We thank L. Bauwens, M. Crabbé, S. de Swaef and A. Florizoone for their technical assistance. We are also grateful to Dr T. Kurosaki for R23-11 triple-knockout cells, Dr K. Mikoshiba (Division of Molecular Neurobiology, Department of Basic Medical Sciences, Institute of Medical Science, University of Tokyo, Tokyo, Japan) for the p400C1 plasmid containing the cDNA of mouse IP3R1, Dr T.C. Südhof (Center for Basic Neuroscience, UT Southwestern Medical Center, Dallas, TX, U.S.A.) for the pCMV1-102 plasmid, and Dr G.I. Bell (Department of Biochemistry, University of Chicago, Chicago, IL, U.S.A.) for the pCB6 plasmid containing the cDNA of rat IP3R3. This work was supported by grant no. G.0210.03 (to H.D.S. and J.B.P.) from the Fund for Scientific Research Flanders (Belgium) and grant no. 99/08 from the Concerted Actions of the K.U. Leuven (to L.M., H.D.S., G.C. and J.B.P.). G.B. is a recipient of a postdoctoral fellowship from the Fund for Scientific Research Flanders.
References
- 1.Berridge M. J. Inositol trisphosphate and calcium signalling. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Furuichi T., Mikoshiba K. Inositol 1,4,5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J. Neurochem. 1995;64:953–960. doi: 10.1046/j.1471-4159.1995.64030953.x. [DOI] [PubMed] [Google Scholar]
- 3.Südhof T. C., Newton C. L., Archer B. T. D., Ushkaryov Y. A., Mignery G. A. Structure of a novel InsP3 receptor. EMBO J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshikawa F., Iwasaki H., Michikawa T., Furuichi T., Mikoshiba K. Trypsinized cerebellar inositol 1,4,5-trisphosphate receptor. Structural and functional coupling of cleaved ligand binding and channel domains. J. Biol. Chem. 1999;274:316–327. doi: 10.1074/jbc.274.1.316. [DOI] [PubMed] [Google Scholar]
- 5.Uchida K., Miyauchi H., Furuichi T., Michikawa T., Mikoshiba K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2003;278:16551–16560. doi: 10.1074/jbc.M300646200. [DOI] [PubMed] [Google Scholar]
- 6.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in smooth muscle cells of the guinea pig Taenia caeci. J. Gen. Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature (London) 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 8.Parys J. B., Sernett S. W., DeLisle S., Snyder P. M., Welsh M. J., Campbell K. P. Isolation, characterization, and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. J. Biol. Chem. 1992;267:18776–18782. [PubMed] [Google Scholar]
- 9.Marshall I. C., Taylor C. W. Biphasic effects of cytosolic Ca2+ on Ins(1,4,5)P3-stimulated Ca2+ mobilization in hepatocytes. J. Biol. Chem. 1993;268:13214–13220. [PubMed] [Google Scholar]
- 10.Sienaert I., De Smedt H., Parys J. B., Missiaen L., Vanlingen S., Sipma H., Casteels R. Characterization of a cytosolic and a luminal Ca2+ binding site in the type I inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1996;271:27005–27012. doi: 10.1074/jbc.271.43.27005. [DOI] [PubMed] [Google Scholar]
- 11.Sienaert I., Missiaen L., De Smedt H., Parys J. B., Sipma H., Casteels R. Molecular and functional evidence for multiple Ca2+-binding domains in the type 1 inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1997;272:25899–25906. doi: 10.1074/jbc.272.41.25899. [DOI] [PubMed] [Google Scholar]
- 12.Miyakawa T., Mizushima A., Hirose K., Yamazawa T., Bezprozvanny I., Kurosaki T., Iino M. Ca2+-sensor region of IP3 receptor controls intracellular Ca2+ signaling. EMBO J. 2001;20:1674–1680. doi: 10.1093/emboj/20.7.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu H., Nosyreva E., Miyakawa T., Wang Z., Mizushima A., Iino M., Bezprozvanny I. Functional and biochemical analysis of the type 1 inositol (1,4,5)-trisphosphate receptor calcium sensor. Biophys. J. 2003;85:290–299. doi: 10.1016/S0006-3495(03)74474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamada K., Miyata T., Mayanagi K., Hirota J., Mikoshiba K. Two-state conformational changes in inositol 1,4,5-trisphosphate receptor regulated by calcium. J. Biol. Chem. 2002;277:21115–21118. doi: 10.1074/jbc.C200244200. [DOI] [PubMed] [Google Scholar]
- 15.Hamada K., Terauchi A., Mikoshiba K. Three-dimensional rearrangements within inositol 1,4,5-trisphosphate receptor by calcium. J. Biol. Chem. 2003;278:52881–52889. doi: 10.1074/jbc.M309743200. [DOI] [PubMed] [Google Scholar]
- 16.Parys J. B., Missiaen L., De Smedt H., Droogmans G., Casteels R. Bell-shaped activation of inositol-1,4,5-trisphosphate-induced Ca2+ release by thimerosal in permeabilized A7r5 smooth-muscle cells. Pflügers Arch. 1993;424:516–522. doi: 10.1007/BF00374916. [DOI] [PubMed] [Google Scholar]
- 17.Kaplin A. I., Ferris C. D., Voglmaier S. M., Snyder S. H. Purified reconstituted inositol 1,4,5-trisphosphate receptors. Thiol reagents act directly on receptor protein. J. Biol. Chem. 1994;269:28972–28978. [PubMed] [Google Scholar]
- 18.Missiaen L., Taylor C. W., Berridge M. J. Luminal Ca2+ promoting spontaneous Ca2+ release from inositol trisphosphate-sensitive stores in rat hepatocytes. J. Physiol. (Cambridge, U.K.) 1992;455:623–640. doi: 10.1113/jphysiol.1992.sp019319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Missiaen L., Parys J. B., Sienaert I., Maes K., Kunzelmann K., Takahashi M., Tanzawa K., De Smedt H. Functional properties of the type-3 InsP3 receptor in 16HBE14o-bronchial mucosal cells. J. Biol. Chem. 1998;273:8983–8986. doi: 10.1074/jbc.273.15.8983. [DOI] [PubMed] [Google Scholar]
- 20.Vanlingen S., Sipma H., Missiaen L., De Smedt H., De Smet P., Casteels R., Parys J. B. Modulation of type 1, 2 and 3 inositol 1,4,5-trisphosphate receptors by cyclic ADP-ribose and thimerosal. Cell Calcium. 1999;25:107–114. doi: 10.1054/ceca.1998.0010. [DOI] [PubMed] [Google Scholar]
- 21.Vanlingen S., Sipma H., De Smet P., Callewaert G., Missiaen L., De Smedt H., Parys J. B. Modulation of inositol 1,4,5-trisphosphate binding to the various inositol 1,4,5-trisphosphate receptor isoforms by thimerosal and cyclic ADP-ribose. Biochem. Pharmacol. 2001;61:803–809. doi: 10.1016/s0006-2952(01)00540-8. [DOI] [PubMed] [Google Scholar]
- 22.Miyakawa T., Maeda A., Yamazawa T., Hirose K., Kurosaki T., Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parys J. B., De Smedt H., Borghgraef R. Calcium transport systems in the LLC-PK1 renal epithelial established cell line. Biochim. Biophys. Acta. 1986;888:70–81. doi: 10.1016/0167-4889(86)90072-8. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. F. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Sipma H., De Smet P., Sienaert I., Vanlingen S., Missiaen L., Parys J. B., De Smedt H. Modulation of inositol 1,4,5-trisphosphate binding to the recombinant ligand-binding site of the type-1 inositol 1,4,5-trisphosphate receptor by Ca2+ and calmodulin. J. Biol. Chem. 1999;274:12157–12162. doi: 10.1074/jbc.274.17.12157. [DOI] [PubMed] [Google Scholar]
- 26.Ma H. T., Venkatachalam K., Parys J. B., Gill D. L. Modification of store-operated channel coupling and inositol trisphosphate receptor function by 2-aminoethoxydiphenyl borate in DT40 lymphocytes. J. Biol. Chem. 2002;277:6915–6922. doi: 10.1074/jbc.M107755200. [DOI] [PubMed] [Google Scholar]
- 27.Parys J. B., De Smedt H., Missiaen L., Bootman M. D., Sienaert I., Casteels R. Rat basophilic leukemia cells as model system for inositol 1,4,5-trisphosphate receptor IV, a receptor of the type II family: functional comparison and immunological detection. Cell Calcium. 1995;17:239–249. doi: 10.1016/0143-4160(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 28.Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 29.Missiaen L., Taylor C. W., Berridge M. J. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature (London) 1991;352:241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- 30.Nadif Kasri N., Sienaert I., Parys J. B., Callewaert G., Missiaen L., Jeromin A., De Smedt H. A novel Ca2+-induced Ca2+ release mechanism in A7r5 cells regulated by calmodulin-like proteins. J. Biol. Chem. 2003;278:27548–27555. doi: 10.1074/jbc.M302026200. [DOI] [PubMed] [Google Scholar]
- 31.Boehning D., Joseph S. K. Direct association of ligand-binding and pore domains in homo- and heterotetrameric inositol 1,4,5-trisphosphate receptors. EMBO J. 2000;19:5450–5459. doi: 10.1093/emboj/19.20.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazaki S., Shirakawa H., Nakada K., Honda Y., Yuzaki M., Nakade S., Mikoshiba K. Antibody to the inositol trisphosphate receptor blocks thimerosal-enhanced Ca2+-induced Ca2+ release and Ca2+ oscillations in hamster eggs. FEBS Lett. 1992;309:180–184. doi: 10.1016/0014-5793(92)81090-9. [DOI] [PubMed] [Google Scholar]
- 33.Bootman M. D., Taylor C. W., Berridge M. J. The thiol reagent, thimerosal, evokes Ca2+ spikes in HeLa cells by sensitizing the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1992;267:25113–25119. [PubMed] [Google Scholar]
- 34.Hilly M., Pietri Rouxel F., Coquil J. F., Guy M., Mauger J. P. Thiol reagents increase the affinity of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1993;268:16488–16494. [PubMed] [Google Scholar]
- 35.Thrower E. C., Duclohier H., Lea E. J., Molle G., Dawson A. P. The inositol 1,4,5-trisphosphate-gated Ca2+ channel: effect of the protein thiol reagent thimerosal on channel activity. Biochem. J. 1996;318:61–66. doi: 10.1042/bj3180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Missiaen L., De Smedt H., Parys J. B., Sienaert I., Vanlingen S., Casteels R. Threshold for inositol 1,4,5-trisphosphate action. J. Biol. Chem. 1996;271:12287–12293. doi: 10.1074/jbc.271.21.12287. [DOI] [PubMed] [Google Scholar]
- 37.Yoshikawa F., Morita M., Monkawa T., Michikawa T., Furuichi T., Mikoshiba K. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1996;271:18277–18284. doi: 10.1074/jbc.271.30.18277. [DOI] [PubMed] [Google Scholar]
- 38.Bosanac I., Alattia J. R., Mal T. K., Chan J., Talarico S., Tong F. K., Tong K. I., Yoshikawa F., Furuichi T., Iwai M., et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature (London) 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 39.Sienaert I., Nadif Kasri N., Vanlingen S., Parys J. B., Callewaert G., Missiaen L., De Smedt H. Localization and function of a calmodulin-apocalmodulin-binding domain in the N-terminal part of the type 1 inositol 1,4,5-trisphosphate receptor. Biochem. J. 2002;365:269–277. doi: 10.1042/BJ20020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J., McBride S., Mak D. O., Vardi N., Palczewski K., Haeseleer F., Foskett J. K. Identification of a family of calcium sensors as protein ligands of inositol trisphosphate receptor Ca2+ release channels. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7711–7716. doi: 10.1073/pnas.102006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nadif Kasri N., Holmes A. M., Bultynck G., Parys J. B., Bootman M. D., Rietdorf K., Missiaen L., McDonald F., De Smedt H., Conway S. J., et al. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 2004;23:312–321. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadif Kasri N., Bultynck G., Sienaert I., Callewaert G., Erneux C., Missiaen L., Parys J. B., De Smedt H. The role of calmodulin for inositol 1,4,5-trisphosphate receptor function. Biochim. Biophys. Acta. 2002;1600:19–31. doi: 10.1016/s1570-9639(02)00440-5. [DOI] [PubMed] [Google Scholar]
- 43.Taylor C. W., Laude A. J. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32:321–334. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- 44.Haynes L. P., Tepikin A. V., Burgoyne R. D. Calcium binding protein 1 is an inhibitor of agonist-evoked, inositol 1,4,5-trisphosphate-mediated calcium signalling. J. Biol. Chem. 2003;279:547–555. doi: 10.1074/jbc.M309617200. [DOI] [PubMed] [Google Scholar]
- 45.Eu J. P., Sun J., Xu L., Stamler J. S., Meissner G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell (Cambridge, Mass.) 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 46.Sun J., Xin C., Eu J. P., Stamler J. S., Meissner G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poirier S. N., Poitras M., Laflamme K., Guillemette G. Thiol-reactive agents biphasically regulate inositol 1,4,5-trisphosphate binding and Ca2+ release activities in bovine adrenal cortex microsomes. Endocrinology. 2001;142:2614–2621. doi: 10.1210/endo.142.6.8195. [DOI] [PubMed] [Google Scholar]