Abstract
α-Sarcoglycan is a glycoprotein associated with the dystrophin complex at sarcolemma of skeletal and cardiac muscles. Gene defects in α-sarcoglycan lead to a severe muscular dystrophy whose molecular mechanisms are not yet clear. A first insight into the function of α-sarcoglycan was obtained by finding that it is an ATP-binding protein and that it probably confers ability to hydrolyse ATP to the purified dystrophin complex [Betto, Senter, Ceoldo, Tarricone, Biral and Salviati (1999) J. Biol. Chem. 274, 7907–7912]. In the present study, we present definitive evidence showing that α-sarcoglycan is an ATP-hydrolysing enzyme. The appearance of α-sarcoglycan protein expression was correlated with the increase in ecto-nucleotidase activity during differentiation of C2C12 cells. Approx. 25% of ecto-nucleotidase activity displayed by the C2C12 myotubes was inhibited by preincubating cells with an antibody specific for the ATP-binding motif of α-sarcoglycan. This demonstrates that α-sarcoglycan substantially contributes to total ecto-nucleotidase activity of C2C12 myotubes. To characterize further this activity, human embryonic kidney 293 cells were transfected with expression plasmids containing α-sarcoglycan cDNA. Transfected cells exhibited a significant increase in the ATP-hydrolysing activity that was abolished by the anti-α-sarcoglycan antibody. The enzyme had a substrate specificity for ATP and ADP, did not hydrolyse other triphosphonucleosides, and the affinity for ATP was in the low mM range. The ATPase activity strictly required the presence of both Mg2+ and Ca2+ and was completely inhibited by suramin and reactive blue-2. These results show that α-sarcoglycan is a Ca2+, Mg2+-ecto-ATPDase. The possible consequences of the absence of α-sarcoglycan activity in the pathogenesis of muscular dystrophy are discussed.
Keywords: α-sarcoglycan, ecto-nucleotidase, extracellular ATP signalling, limb-girdle muscular dystrophy
Abbreviations: DMEM, Dulbecco's modified Eagle's medium; E-NTPDase, ecto-nucleoside triphosphate diphosphohydrolase; FCS, foetal calf serum; HEK-293, human embryonic kidney 293 cells; HS, horse serum; LGMD, limb girdle muscular dystrophy; NCAM, neural cell-adhesion molecule; SCAN-1, soluble calcium-activated nucleotidase 1
INTRODUCTION
α-Sarcoglycan is part of a four-component subcomplex of single-pass transmembrane glycoproteins, the sarcoglycans [1]. Sarcoglycans are normally associated with the dystrophin complex of skeletal and cardiac muscles [1,2]. The functional role of the dystrophin complex is to form a membrane cytoskeleton devoted to the mechanical protection of muscle sarcolemma from tension produced by contractile activity [3]. The absence of dystrophin results in sarcolemmal instability and contraction-induced muscle fibre damage, events responsible for the severe clinical presentation of patients with Duchenne muscular dystrophy [2,4]. The contribution of sarcoglycans to dystrophin complex function is not well understood. However, it is worth noting that gene defects in α-sarcoglycan also lead to a severe muscular dystrophy, type 2D LGMD (limb-girdle muscular dystrophy) [5]. Since a primary deficiency of any of the sarcoglycans leads to the reduction or the absence of all other sarcoglycans without causing major alterations to the mechanical function of the dystrophin complex [2,6], it appears unlikely that sarcoglycans could play only a structural role [1,7]. Probably, sarcoglycans have additional functional roles in muscles. A first insight for a possible functional role of α-sarcoglycan was the identification of an ATP-binding site on the extracellular portion of the protein [8]. This finding gave rise to the hypothesis that α-sarcoglycan could be an enzyme involved in the control of the extracellular ATP concentration. This idea was reinforced by the demonstration that the ATPase activity of isolated dystrophin complexes was partly inhibited by an antibody specific for the extracellular portion of α-sarcoglycan [8].
The hydrolysis of ATP is of fundamental importance to the signalling cascades initiated by the presence of extracellular nucleotides. Extracellular nucleotides signal various physiological responses, including platelet activation and aggregation, immune responses, vascular tone, neurotransmission, nociception, cardiac function, tumour growth, renal transport, smooth-muscle contraction and apoptosis [9,10]. Nucleotides are released from cells into the extracellular fluids as a result of cell lysis, exocytosis or efflux from transport/channel proteins [11]. These signalling actions of nucleotides are exerted through their binding to specific cell-surface receptors, which are distinguished into two classes: P1 receptors, activated by adenosine and P2 receptors, activated by nucleotides [12]. P2 receptors are further subclassified into P2X, ionotropic ligand-gated channels [13] and P2Y, G-protein-coupled metabotropic receptors [14].
A central role in modulating extracellular nucleotide signalling is ascribed to a family of ecto-enzymes (ecto-nucleotidases) that sequentially degrade ATP to ADP and AMP, and eventually to adenosine. Therefore the action of nucleotide-hydrolysing enzymes is essential to terminate the signalling, to generate new signalling molecules and to salvage purines [15,16]. Ecto-nucleotidases include the following enzymes: E-NTPDases (ecto-nucleoside triphosphate diphosphohydrolase), ecto-nucleotide pyrophosphatase/phosphodiesterases, alkaline phosphatases and ecto-5′-nucleotidase [16,17]. So far, three E-NTPDases have been described, all containing conserved ‘apyrase’ regions [18] but differing in catalytic activity. Three additional structurally related NTPDases, containing the apyrase domains but with an intracellular localization, have also been described [16]. Recently, a novel soluble nucleotidase, called SCAN-1 (soluble calcium-activated nucleotidase 1), has been identified. This nucleotidase, however, does not possess apyrase domains and the preferred substrates are diphosphonucleosides [19,20]. An additional ecto-ATPase protein that lacks the apyrase motif, but possessing an ATP-binding site, has been identified as the neural cell-adhesion molecule (NCAM) [21].
In the present study, we present results showing that α-sarcoglycan is an ecto-ATPDase with distinctive enzymic properties. α-Sarcoglycan hydrolyses exclusively ATP and ADP and its activity strictly depends on the presence of bivalent cations. Thus α-sarcoglycan is an extracellular Ca2+, Mg2+-ATPDase, which is significantly inhibited by suramin and reactive blue-2.
EXPERIMENTAL
Materials
AMP, ADP, ATP, UTP, GTP, DMEM (Dulbecco's modified Eagle's medium), sodium orthovanadate, suramin and azide were from Sigma. FCS (foetal calf serum), HS (horse serum), penicillin and streptomycin were from Gibco. Reactive blue-2 and ouabain were from Research Biochemicals International (Natick, MA, U.S.A.). Thapsigargin and G418 sulphate were from Calbiochem. All other chemicals were of analytical grade.
Cell culture
Mouse C2C12 cells were cultured in DMEM proliferating medium supplemented with 10% (v/v) FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin on tissue flasks at the density of approx. 10000 cells/cm2 in a humidified atmosphere of 5% CO2 at 37 °C. After 3 days in the proliferating medium, myoblasts reached 80–90% confluence. To induce the formation of myotubes, FCS was substituted with 2% (v/v) HS. Myotubes were examined after 4, 6 and 8 days culture. HEK-293 cells (human embryonic kidney 293 cells) were seeded in tissue flasks at a density of approx. 25000 cells/cm2 and grown to approx. 70% confluence in DMEM containing 10% FCS, 100 units/ml penicillin and 100 μg/ml streptomycin. The cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C.
Preparation of α-sarcoglycan transformation vector
The full-length cDNA encoding human α-sarcoglycan (gift from M.L. Kunkel, Harvard University) was cloned into the EcoRI site of the pcDNA3 mammalian expression vector (Invitrogen), under the control of the cytomegalovirus promoter. This construct was slightly modified by the addition in the 3′-end of a His6 tag. Briefly, the stop codon of α-sarcoglycan was modified by PCR and a KpnI restriction endonuclease site was created. Within this site, a DNA fragment encoding the His6 tag plus three stop-codons was inserted. The recombinant plasmid was sequenced and then used in the transfection of HEK-293 cells.
Transient and stable transfection of HEK-293 cells
HEK-293 cells were transfected by calcium phosphate-DNA precipitation method, as described previously [22]. Approx. 400000 HEK-293 cells were transiently transfected in 6 cm plates with the α-sarcoglycan-HISTOP construct or with the empty vector. To select stable clones, cells were grown in culture for 8 h after transfection, and then washed five times with Ca2+- and Mg2+-free PBS, and finally harvested and plated in 10 cm plates. The cells were allowed to attach overnight after which the antibiotic, G418, was added (1.2 mg/ml). After 15 days, the established G418-resistant clones were subcloned and propagated in DMEM supplemented with 0.6 mg/ml G418.
Ecto-ATPases activity of intact cells
The extracellular nucleotide-hydrolysing capacity of cells was determined by measuring the amount of Pi released. Approx. 30000 C2C12 cells were plated in a 24-well plate and used after 2 days of culture to measure the activity of myoblasts. Alternatively, cells were cultured in DMEM supplemented with 2% (v/v) HS for an additional 4, 6 or 8 days to produce myotubes. Approx. 30000 HEK-293 cells, either stably or transiently transfected with the α-sarcoglycan construct or the pcDNA3 plasmid, were plated in a 24-well plate and cultured for 48 h. Cells were washed twice with the Activity Buffer, a phosphate-free physiological saline (140 mM NaCl/4 mM KCl/4 mM MgCl2/2 mM CaCl2/20 mM Hepes, pH 7.4). The substrate (either ATP, ADP, AMP, GTP or UTP) concentration was 4 mM, except in experiments for the determination of Km values for ATP hydrolysis, where ATP concentrations ranged from 0.1 to 8 mM. The following potential inhibitors were tested: suramin (100 μM), reactive blue-2 (200 μM), sodium azide (10 mM), thapsigargin (5 μM), sodium orthovanadate (100 μM) and ouabain (100 nM).
Enzyme activity was started by adding 0.3 ml/well of the selected nucleotide in the Activity Buffer, either in the absence or in the presence of selected inhibitors. After 30 min of incubation in a humidified atmosphere of 5% CO2 at 37 °C, the solution was removed from the cells and centrifuged at 500 g for 5 min. Then, two 100 μl aliquots of the supernatant were used to determine the Pi, using the Malachite Green method [23]. Cells were then lysed with 300 μl of 5% (w/v) deoxycholic acid with protease inhibitors (Complete; Roche, Mannheim, Germany) and a 100 μl aliquot was used to determine the protein concentration by the Lowry method using BSA as standard. The possible liberation of phosphate by activation of alkaline phosphatase was excluded by pilot experiments performed in the presence of the specific inhibitor, 2 mM levamisole. Cell-membrane integrity was evaluated by measuring the presence of lactate dehydrogenase activity in the supernatant of cells subjected to the nucleotidase assay.
Ecto-ATPases activity in the presence of α-sarcoglycan antibodies
Seven-day-old C2C12 myotubes or stably transfected HEK-293 cells grown in a 24-well plate for 2 days were washed twice with the Activity Buffer (see above) and then incubated for 30 min at 4 °C in Activity Buffer either in the absence or in the presence of a monoclonal antibody specific for the extracellular domain (1:50) (NCL-a-SARC; Novocastra, Newcastle upon Tyne, U.K.) or a polyclonal antibody specific for the C-terminal domain of α-sarcoglycan (1:200) [8]. Both the antibodies were previously dialysed in the Activity Buffer. Then, the incubation medium was replaced with the Activity Buffer, either containing the antibodies and 4 mM ATP or 4 mM ATP alone. The nucleotide-hydrolysing activity was measured, as described above.
Protein deglycosylation
Proteins of HEK-293 cells, either transfected with the α-sarcoglycan construct or with the empty vector, were solubilized with a PBS lysis buffer containing 1% Nonidet P40, 0.5% sodium deoxycholate, 0.1% SDS, 12 μg/ml PMSF, 30 μl/ml aprotinin and 1 mM leupeptin. Protein concentration was determined by the Lowry method, using BSA as standard. Proteins were deglycosylated by using the N-glycosidase F Deglycosylation kit (Roche) according to the manufacturer's instructions. Briefly, either 50 μg of HEK-293 cell protein or 5 μg of rabbit sarcolemma membrane protein [8] was denatured for 5 min at 95 °C. Denatured proteins were incubated with N-glycosidase F for 90 min; the reaction was stopped with SDS/PAGE solubilization buffer and the proteins were subjected to SDS/PAGE and Western-blot analysis.
Western-blot analysis
The proteins (40–50 μg/lane) were resolved by SDS/PAGE, blotted on to a nitrocellulose membrane and examined with the following antibodies: mouse monoclonal anti-α-sarcoglycan (NCL-a-SARC, 1:500), anti-dystrophin (NCL-DYS2, 1:100), and anti-β-dystroglycan (NCL-b-DG, 1:100) from Novocastra and rabbit polyclonal anti-β-actin (AC-15, 1:4000) from Sigma. The secondary antibody was either an alkaline phosphatase-labelled goat anti-mouse IgG (1:10000; Sigma) or a horseradish peroxidase-labelled goat anti-rabbit IgG (1:20000; Sigma). Blots were then developed either with p-nitrophenyl phosphate (Sigma) or with ECL® Plus (Amersham Biosciences).
Immunofluorescence
HEK-293 cells, transfected with either α-sarcoglycan construct or the empty vector, fixed with 4% (w/v) paraformaldehyde, were incubated overnight at 4 °C with the monoclonal antibody against α-sarcoglycan (1:500) in PBS with 0.5% BSA. After washing four times with PBS for 10 min, cells were incubated for 1 h at 37 °C with anti-mouse rhodamine-conjugated antibody (1:1000; DakoCytomation, Glostrup, Denmark). After washing four times with PBS for 10 min, cells were mounted in Elvanol and analysed on a Bio-Rad MRC 1024ES confocal laser-scanning microscope based on a Nikon Eclipse E600. Controls were performed by omitting the primary antibody.
RESULTS
Contribution of α-sarcoglycan to ecto-nucleotidase activity of C2C12 cells
The C2C12 cell line is a suitable model for the investigation of the initial steps of skeletal-muscle differentiation. The proliferating myoblast cells respond to serum deprivation by the cessation of proliferation and fuse to form myotubes. These multinucleated cells express a complete contractile apparatus assembled into sarcomeres as well as expressing cytoskeletal proteins, such as those of the dystrophin complex [24,25]. During differentiation, the expression of α-sarcoglycan progressively rises to be expressed fully in 8-day-old mature myotubes (Figure 1A).
Figure 1. Western-blot analysis and total ecto-nucleotidase activity of C2C12 cells.
(A) Western-blot analysis of cell lysates obtained from proliferating (P), confluent (C) and myotubes at different days of culture. Lysate proteins (50 μg) were separated on 10% SDS/polyacrylamide slab gels, blotted on nitrocellulose filters and probed with specific antibodies, as indicated. (B) ATP-hydrolysing activity of C2C12 myoblasts (Mb) and 4- and 8-day-old myotubes (4d-Mt and 8d-Mt respectively) was determined by measuring Pi generation as described by Lanzetta et al. [23]. Cells were incubated for 15 min in a medium with the following composition: 20 mM Hepes, 140 mM NaCl, 4 mM MgCl2 and 2 mM CaCl2. The reaction was started by adding 4 mM ATP and terminated by withdrawing a supernatant aliquot. Results are from four experiments performed in triplicate. (C) Effect of polyclonal (+pAb) and monoclonal (+mAb) antibodies specific for α-sarcoglycan on total ecto-ATPase activity of 7-day-old C2C12 myotubes incubated 30 min as above. Results are from three experiments performed in triplicate. *P<0.01; **P<0.0001.
C2C12 cells showed a low level of ecto-nucleotidase activity both during the proliferative phases and at subconfluence. In contrast, the activity progressively increased during differentiation, apparently in parallel with the rise of α-sarcoglycan expression (Figure 1B). However, since the presence of other ecto-nucleotidases has been reported in adult skeletal-muscle cells [26,27], other ecto-nucleotidases (in addition to α-sarcoglycan) may contribute to total extracellular ATP-hydrolysing activity seen in C2C12 cells. Thus to determine the contribution of α-sarcoglycan to over all ecto-ATPase activity of C2C12 cells, we preincubated myotubes with a commercial monoclonal antibody specific for the extracellular portion of α-sarcoglycan encompassing the putative ATP-binding site [8]. Total ecto-nucleotidase activity was reduced by approx. 25% in C2C12 myotubes treated with the monoclonal antibody (Figure 1C), whereas no effect was observed when cells were incubated with a polyclonal antibody specific for the C-terminal portion of α-sarcoglycan [8].
Expression of α-sarcoglycan in HEK-293 cells
To characterize further the ecto-ATPase activity of α-sarcoglycan, we expressed the protein in HEK-293 cells, a cell type that does not express α-sarcoglycan and possesses a low level of extracellular ATP-hydrolysing activity. Subsequent transient transfection experiments demonstrated that α-sarcoglycan was successfully expressed in HEK-293 cells (Figure 2A). The transfected protein has an apparent molecular mass (50 kDa) similar to that present in a purified sarcolemma fraction from rabbit leg muscles [8]. The molecular mass of 50 kDa is that expected for the glycosylated form of the protein, as α-sarcoglycan possesses two N-linked glycosylation sites [28]. The treatment with N-glycosidase F caused a molecular-mass reduction from 50 to 45 kDa of α-sarcoglycan whether present in sarcolemma membranes or in transfected HEK-293 cells (Figure 2B). Immunofluorescence analysis indicated that approx. 50–60% of cells were successfully transfected and, important for its putative activity, α-sarcoglycan was correctly expressed at the cell surfaces (Figure 2C). Most importantly, HEK-293 cells transiently transfected with α-sarcoglycan showed a 2–3-fold rise of ecto-nucleotidase activity with respect to both wild-type and empty vector-transfected cells (Figure 2D).
Figure 2. Transient transfection of HEK-293 cells with α-sarcoglycan.
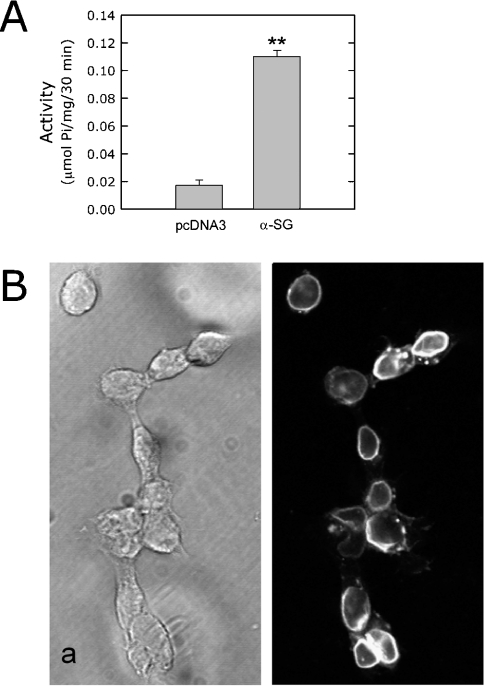
(A) Western-blot analysis of rabbit skeletal-muscle sarcolemma (rabbit SL), and HEK-293 cell lysates from α-sarcoglycan-transiently transfected cells (α-SG), empty vector (pcDNA3) transfected cells and untransfected cells (wild-type) by using an antibody specific to α-sarcoglycan. (B) Western-blot analysis of rabbit skeletal-muscle sarcolemma (rabbit SL), and HEK-293 cell lysates from α-sarcoglycan-transiently transfected cells (HEK α-SG) either untreated (left two lanes) or treated with N-glycosidase F (right two lanes). Amounts of protein from HEK-293 cell lysates were estimated (A, B) by using an antibody against β-actin. (C) Phase-contrast image (left panel) and immunofluorescence staining of non-permeabilized α-sarcoglycan transiently transfected HEK-293 cells with the antibody directed against the extracellular domain of α-sarcoglycan (right panel). (D) Extracellular ATP hydrolysis of wild-type HEK-293 cells (wt), or cells transiently transfected with the empty vector (pcDNA3) or with α-sarcoglycan (α-SG). Results are from three experiments performed in triplicate; **P < 0.0001.
Next, we produced HEK-293 clones stably expressing α-sarcoglycan. The time course of ATP hydrolysis of stably transfected cells was linear for more than 30 min (results not shown). The activity of the clones was 4–5-fold higher than that of control cells (Figure 3A), i.e. about double that of transiently transfected cells. The immunofluorescence analysis of α-sarcoglycan HEK-293 clones confirmed the membrane localization of the protein and that all cells expressed the protein (Figure 3B).
Figure 3. Ecto-nucleotidase activity of HEK-293 cells stably transfected with α-sarcoglycan.
(A) ATP-hydrolysing activity, measured as indicated in the Experimental section, was performed in HEK-293 cells transfected with the empty vector (pcDNA3) or with α-sarcoglycan (α-SG). Results are from four experiments performed in triplicate; **P<0.000005. (B) Phase-contrast image (a) and immunofluorescence staining of non-permeabilized α-sarcoglycan-expressing cells with the antibody directed against the extracellular domain of the protein (b).
An antibody against α-sarcoglycan inhibits the ATPase activity
To demonstrate further that the de novo ecto-ATPase activity of transfected HEK-293 cells could be ascribed to α-sarcoglycan expression, we tested the effects of antibodies specific for the protein (Figure 4). Accordingly, the stable transfected cells were preincubated for 30 min at 4 °C in the presence of either the monoclonal antibody specific for the extracellular portion of α-sarcoglycan, encompassing the putative ATP-binding site or the polyclonal antibody specific for the C-terminal portion of the protein [8]. The monoclonal antibody against α-sarcoglycan completely inhibited the ATP-hydrolysing activity of HEK-293 cells expressing the protein, whereas the polyclonal antibody was ineffective in blocking the activity (Figure 4).
Figure 4. Effects of antibodies against α-sarcoglycan on the ecto-nucleotidase activity of HEK-293 cells stably expressing the protein.
ATP-hydrolysing activity of cells incubated in the absence or in the presence of a monoclonal antibody against the extracellular domain of α-sarcoglycan encompassing the ATP-binding site (+mAb) or a polyclonal antibody specific for the C-terminal domain of the protein (+pAb). HEK-293 cells were preincubated for 30 min at 4 °C with or without antibodies. Results are from four experiments performed in triplicate; **P<0.000005.
Ca2+ and Mg2+ dependence
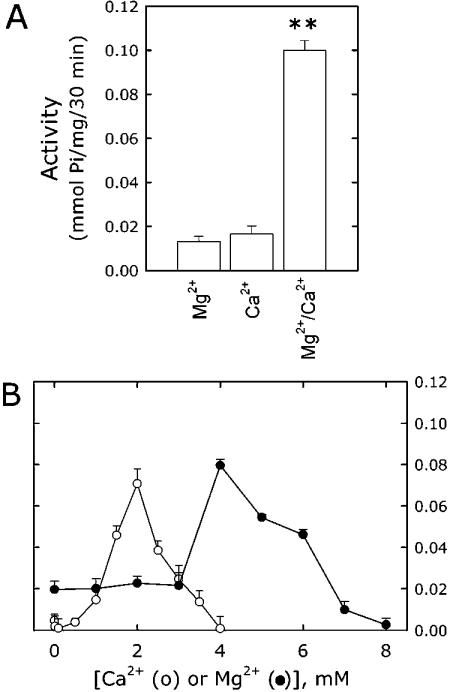
Typically, E-NTPDase activities are dependent on bivalent cations, either Ca2+ or Mg2+ [15,16]. The activity of the newly discovered soluble extracellular nucleotidase SCAN-1 is instead solely dependent on Ca2+ [19,20]. On the other hand, the ATP-hydrolysing activity of α-sarcoglycan-transfected HEK-293 clones required the presence of both Ca2+ and Mg2+ (Figure 5A). Figure 5(B) shows the concentration dependence of the enzyme for these two cations. In the presence of 4 mM ATP and 4 mM Mg2+, the hydrolysis became measurable at 1 mM Ca2+, was maximally stimulated at 2 mM Ca2+ and progressively inhibited at higher Ca2+ concentrations (Figure 5B). In contrast, in the presence of 4 mM ATP and 2 mM Ca2+, a very low level of activity was measured when Mg2+ was in the range 1–3 mM. At 4 mM Mg2+, a concentration equimolar to that of ATP, the protein was fully active, whereas at higher Mg2+ concentrations, the activity was progressively inhibited (Figure 5B). In addition, we tested whether other bivalent cations were capable of stimulating the activity in the presence of Mg2+-ATP. The tested bivalent cations activated the Mg2+-ATP hydrolysis of α-sarcoglycan in the following order: Sr2+≫Ca2+≫Co2+, whereas Mn2+ and Ba2+ were ineffective (results not shown).
Figure 5. Dependence of ecto-nucleotidase activity of α-sarcoglycan-expressing HEK-293 cells on the bivalent cation concentrations.
(A) ATP-hydrolysing activity was measured in stably transfected cells in the presence of 4 mM Mg2+ or 2 mM Ca2+ or both (4 mM Mg2+ and 2 mM Ca2+). (B) ATP-hydrolysing activity of α-sarcoglycan stably transfected HEK-293 cells was measured either in the presence of 4 mM Mg2+ and the indicated concentrations of Ca2+ (○) or in the presence of 2 mM Ca2+ and the indicated concentrations of Mg2+ (•). The plot shows the values after the subtraction of the empty vector-transfected activity. Results are from four experiments performed in triplicate. **P<0.000005.
Kinetic properties
The relationship between the substrate concentration and the enzymic activity was evaluated using ATP concentrations ranging between 0.1 and 8 mM, with calcium and magnesium fixed at 2 and 4 mM respectively (Figure 6). The ecto-ATPase activity of α-sarcoglycan-expressing HEK-293 cells exhibits Michaelis–Menten kinetics [Km=3.71±0.95 mM and Vmax=0.16±0.03 μmol·mg−1·(30 min)−1, n=3] that denotes a moderate affinity of the enzyme for the ATP. In Figure 6, it is also possible to note that data points are fairly scattered, a result that could be due to the expected difficulty in measuring kinetic properties of an enzyme in the cell membrane of living cells.
Figure 6. Kinetic properties of ecto-nucleotidase activity of HEK-293 cells stably transfected with α-sarcoglycan.
(A) The enzymic activity, as a function of ATP concentration, was measured in mock-transfected cells (○) and in stably expressing α-sarcoglycan cells (•), as described in Figure 1. Concentrations of Ca2+ and Mg2+ were 2 and 4 mM respectively. Results shown in the plot are the means±S.E.M. for three sets of experiments performed in triplicate on different cell clones. (B) The same enzymic activity of α-sarcoglycan expressing cells was plotted after subtracting the activity of the empty vector-transfected cells. Michaelis–Menten kinetics was calculated from the results of individual experiments. Inset: Lineweaver–Burk transformation of the data.
Substrate specificity
Ecto-nucleotidases normally display a low substrate specificity since they are capable of hydrolysing numerous nucleoside 5′-triphosphates and nucleoside 5′-diphosphates [16]. On the other hand, HEK-293 cells expressing α-sarcoglycan hydrolysed ATP and ADP only (an apyrase-like activity), but not AMP, UTP and GTP (Figure 7A). When the catalytic activity of wild-type cells was subtracted from the total activity of transfected cells, it became evident that the transfected protein was capable of hydrolysing ATP with respect to ADP with a 3-fold higher rate (Figure 7B). It is worth noting that the ecto-nucleotidase activity of mock-transfected HEK-293 cells displayed preference for UTP and GTP (Figure 7A).
Figure 7. Substrate specificity of and effects of selected inhibitors on ecto-nucleotidase activity of HEK-293 cells stably transfected with α-sarcoglycan.
(A) The enzymic activity was measured in mock-transfected HEK-293 cells (empty bars) and in stably expressing α-sarcoglycan cells (grey bars), as described in the legend to Figure 1. Substrates were added at a concentration of 4 mM in the presence of 4 mM Mg2+ and 2 mM Ca2+. (B) Specific activity of α-sarcoglycan stably transfected HEK-293 cells obtained by subtracting the activity of mock-transfected cells from the total activity, both reported in (A). Results are from four experiments performed in triplicate. (C) The ATP-hydrolysing activity was measured in the presence of ATP alone or ATP with either 100 μM suramin, 200 μM reactive blue-2 (RB-2), 10 mM azide, 100 nM ouabain, 100 μM orthovanadate or 5 μM thapsigargin. Results are mean values for at least three independent measurements. Values are indicated as percentage activity.
Effects of enzyme inhibitors
Next, we tested the effects of substances previously described as inhibitors of either ecto- or endo-ATPases on the activity of α-sarcoglycan-expressing cells (Figure 7C). Suramin (100 μM), reported as an antagonist of P2X receptors as well as ecto-ATPases [29–31], caused 94% inhibition of the specific α-sarcoglycan activity. Reactive blue (200 μM), a potent inhibitor of ecto-ATPases [29,31], was the most efficacious, causing the almost complete abrogation of the specific α-sarcoglycan activity. Azide (10 mM), an additional antagonist of ecto-ATPases [31,32], caused 49% reduction of α-sarcoglycan activity. Orthovanadate and thapsigargin, inhibitors of ion-pumping P-ATPases [15,33], decreased the activity by approx. 52 and 47% respectively. Ouabain, which at 100 nM concentration is a potent blocker of human Na+, K+-ATPase [34], had minimal effects on the activity of α-sarcoglycan-expressing HEK-293 cells.
DISCUSSION
Our previous work suggested that α-sarcoglycan was an ATP-binding protein and could account for the ATPase activity of the isolated dystrophin complex of skeletal muscles [8]. In the present study, we studied the catalytic activity of α-sarcoglycan, demonstrating for the first time that it is a Ca2+, Mg2+-dependent ecto-ATPDase. We first measured the contribution of α-sarcoglycan to the extracellular ATP-hydrolysing activity of differentiated C2C12 myotubes. Then, we showed that α-sarcoglycan conferred a novel extracellular enzymic activity to transfected HEK-293 cells, which was completely abolished by an antibody specific for the extracellular portion of α-sarcoglycan encompassing the ATP-binding site.
α-Sarcoglycan is a transmembrane glycoprotein crucial for the function/survival of skeletal-muscle fibres, as gene defects are responsible for the severe type 2D form of LGMD [5]. α-Sarcoglycan is one of the four sarcoglycans associated with the major dystrophin membrane complex [1,7]. The dystrophin complex is a cytoskeletal structure regularly distributed over the entire muscle fibre surface that provides mechanical protection to sarcolemma from intense/lateral tensions produced by contractile activity [2,3]. In addition to serving a purely structural role, the dystrophin complex also serves as a scaffold to locate specific proteins at the cell surface [1,2,35]. Thus it is not yet clear whether the sarcoglycans directly participate in the mechanical/structural function of the dystrophin complex or if they are associated with the complex to locate the ecto-ATPase activity of α-sarcoglycan at the cell surface.
During differentiation, the ecto-ATPase activity of C2C12 skeletal-muscle cells progressively increased. We showed that α-sarcoglycan substantially contributed to this activity, as an antibody against the protein reduced the activity by approx. 25%. These results suggest that ATP and, in particular, the regulation of its concentration, could be of great relevance during muscle differentiation. On the other hand, the importance of ATP signalling during this process is also suggested by the variable sensitivity to nucleotides of C2C12 and embryonic muscle cells [36–39].
The expression of α-sarcoglycan in HEK-293 cells allowed the analysis of its nucleotide-hydrolysing activity. HEK-293 cells transfected with α-sarcoglycan presented a de novo apyrase-like activity, as demonstrated by the fact that both ATP and ADP were good substrates. At variance with other ecto-enzymes, which have a wide range of substrate specificity, α-sarcoglycan does not hydrolyse other triphosphonucleosides, but only ATP. On the other hand, the preference for specific tri-diphosphonucleosides is known for other nucleotidases. For example, NTPDase 4 is reported to hydrolyse preferentially UTP and UDP, whereas SCAN-1, NTPDases 5 and 6 hydrolyse diphosphonucleosides [16,19,40]. Similar to other ecto-nucleotidases, α-sarcoglycan ecto-ATPase activity is completely inhibited by reactive blue-2 and suramin [29–31].
Our results also show that α-sarcoglycan activity possesses an additional distinctive property compared with other NTPDases. Whereas the activity of known ecto-nucleotidases is dependent on either Ca2+ or Mg2+ [15], the activity of α-sarcoglycan requires both bivalent cations. However, other nucleotidases have a peculiar bivalent cation dependence. For example, SCAN-1, the soluble nucleotidase, can only utilize Ca2+, but not Mg2+ [19,20]. The need for Ca2+ of α-sarcoglycan activity is also supported by the presence of a putative Ca2+-binding site in the extracellular portion of the protein. Sequence analysis of α-sarcoglycan identified the presence of a cadherin-like domain, between amino acids 28 and 133, which conserves the amino acid residues of the Ca2+-binding pocket [41].
Taken together, these results indicate α-sarcoglycan as a new component of the family of ecto-nucleotidases, possessing peculiar features of substrate specificity and ion dependence. The distinctive enzymic properties of α-sarcoglycan could be ascribed to the different molecular structure compared with that of cloned ecto-nucleotidases until now. α-Sarcoglycan is a single pass type 1 transmembrane protein and does not possess the conserved apyrase regions that probably are of major relevance for the enzymic activity of NTPDases [18]. The absence of apyrase motifs is, however, found also in other non-conventional ecto-ATPases, such as NCAM [21] and SCAN-1 [19]. On the other hand, similar to NCAM, α-sarcoglycan possesses in the extracellular domain a consensus site for the binding of ATP [8].
α-Sarcoglycan activity shows a low affinity for ATP, in the low-mM range. Diversely, other E-NTPDases display Km values for ATP in the micromolar range [15,16]. The only nucleotidase with high Km values for ADP (approx. 5 mM) is SCAN-1, the soluble nucleotidase discovered recently [20]. Thus, similar to SCAN-1, it is conceivable that α-sarcoglycan could be active only in the presence of high concentrations of the nucleotide [20]. High levels of extracellular ATP can occur when cells are disrupted and lose their cytoplasm. In this respect, α-sarcoglycan activity could represent a protection for muscle fibres after the traumatic injury of the tissue, after unaccustomed intense exercise [42] or during the degenerative processes of muscular dystrophy [2,7]. An alternative explanation for the low affinity for the substrate of α-sarcoglycan may derive from the molecular organization of sarcoglycans. α-Sarcoglycan in skeletal-muscle sarcolemma is part of a hetero-tetrameric complex that comprises β-, γ- and δ-sarcoglycans, and several biochemical results indicate that the complex behaves as a single unit [25,43,44]. Since HEK-293 cells expressing the sole α-sarcoglycan were characterized by a low affinity for ATP, we speculate that for α-sarcoglycan to express its optimal catalytic activity, it may require the presence of the other sarcoglycans. On the basis of this hypothesis, α-sarcoglycan may represent the catalytic subunit of the ATP-hydrolysing activity of the complex and the other regulatory elements. Consistent with this hypothesis, treatments that favour the oligomerization of E-NTPDases results in the stimulation of their activity [45,46].
A convincing evidence for the important role of α-sarcoglycan ecto-ATPase activity is the fact that 30 out of 31 of the mutations that produce the type 2D LGMD phenotype map to the extracellular domain of the protein. In particular, several of them affect residues crucial for the activity of α-sarcoglycan. Among these, it appears particularly relevant that the P228Q (Pro228→Gln) substitution maps to the ATP-binding site of α-sarcoglycan and that other residue substitutions (R34C/H, D97G and R98C/H) map to the putative Ca2+-binding site of the protein [41]. Therefore mutations of the α-sarcoglycan gene causing the loss of its enzymic function could represent a key to explain the pathogenetic mechanisms leading to the severe dystrophy.
In conclusion, our results imply α-sarcoglycan as a novel component of the ecto-nucleotidase family that could exert an important protective function of muscle fibres and/or in the control of extracellular ATP signalling. This finding also advances a novel possible explanation for the pathogenetic mechanisms of several severe muscular dystrophies.
Acknowledgments
We thank Professor R.A. Sabbadini (San Diego State University, San Diego, CA, U.S.A.) and Professor F. Di Virgilio (University of Ferrara, Ferrara, Italy) for the critical reading of the paper and Professor T. Pozzan (University of Padova, Padova, Italy) for helpful discussion. The financial support of TELETHON ITALY (grant no. 1286) is gratefully acknowledged. The study was also supported by institutional funds from Consiglio Nazionale delle Ricerche, Italy.
References
- 1.Hack A. A., Groh M. E., McNally E. M. Sarcoglycans in muscular dystrophy. Microsc. Res. Tech. 2000;48:167–180. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<167::AID-JEMT5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Straub V., Campbell K. P. Muscular dystrophies and the dystrophin–glycoprotein complex. Curr. Opin. Neurol. 1997;10:168–175. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Pasternak C., Wong S., Elson E. L. Mechanical function of dystrophin in muscle cells. J. Cell Biol. 1995;128:355–361. doi: 10.1083/jcb.128.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman E. P., Brown R. H., Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell (Cambridge, Mass.) 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 5.Roberds S. L., Leturcq F., Allamand V., Piccolo F., Jeanpierre M., Anderson R. D., Lim L. E., Lee J. C., Tome F. M., Romero N. B., et al. Missense mutations in the adhalin gene linked to autosomal recessive muscular dystrophy. Cell (Cambridge, Mass.) 1994;78:625–633. doi: 10.1016/0092-8674(94)90527-4. [DOI] [PubMed] [Google Scholar]
- 6.Lim L. E., Campbell K. P. The sarcoglycan complex in limb-girdle muscular dystrophy. Curr. Opin. Neurol. 1998;11:443–452. doi: 10.1097/00019052-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Bushby K. M. D. The limb-girdle muscular dystrophies – multiple genes, multiple mechanisms. Hum. Mol. Gen. 1999;8:1875–1882. doi: 10.1093/hmg/8.10.1875. [DOI] [PubMed] [Google Scholar]
- 8.Betto R., Senter L., Ceoldo S., Tarricone E., Biral D., Salviati G. Ecto-ATPase activity of α-sarcoglycan. J. Biol. Chem. 1999;274:7907–7912. doi: 10.1074/jbc.274.12.7907. [DOI] [PubMed] [Google Scholar]
- 9.Brake A. J., Julius D. Signaling by extracellular nucleotides. Annu. Rev. Cell Dev. Biol. 1996;12:519–541. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G., Willliams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J. Pharmacol. Exp. Ther. 2000;295:862–869. [PubMed] [Google Scholar]
- 11.Bodin P., Burnstock G. Purinergic signalling: ATP release. Neurochem. Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 12.Ralevic V., Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 13.North R. A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 14.von Kügelgen I., Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 15.Plesner L. Ecto-ATPases: identities and functions. Int. Rev. Cytol. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 17.Bollen M., Gijsbers R., Ceulemans H., Stalmans W., Stefan C. Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit. Rev. Biochem. Mol. Biol. 2000;35:393–432. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 18.Handa M., Guidotti G. Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum) Biochem. Biophys. Res. Commun. 1996;218:916–923. doi: 10.1006/bbrc.1996.0162. [DOI] [PubMed] [Google Scholar]
- 19.Smith T. M., Hicks-Berger C. A., Kim S., Kirley T. L. Cloning, expression, and characterization of a soluble calcium-activated nucleotidase, a human enzyme belonging to a new family of extracellular nucleotidases. Arch. Biochem. Biophys. 2002;406:105–115. doi: 10.1016/s0003-9861(02)00420-4. [DOI] [PubMed] [Google Scholar]
- 20.Murphy D. M., Ivanenkov V. V., Kirley T. L. Bacterial expression and characterization of a novel, soluble, calcium-binding, and calcium-activated human nucleotidase. Biochemistry. 2003;42:2412–2421. doi: 10.1021/bi026763b. [DOI] [PubMed] [Google Scholar]
- 21.Dzhandzhugazyan K., Bock E. Demonstration of an extracellular ATP-binding site in NCAM: functional implications of nucleotide binding. Biochemistry. 1997;36:15381–15395. doi: 10.1021/bi9709351. [DOI] [PubMed] [Google Scholar]
- 22.Rizzuto R., Brini M., Pozzan T. Targeting recombinant aequorin to specific intracellular organelles. Methods Cell Biol. 1994;40:339–358. doi: 10.1016/s0091-679x(08)61121-8. [DOI] [PubMed] [Google Scholar]
- 23.Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 1979;100:95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- 24.Liu L., Vachon P. H., Kuang W., Xu H., Wewer U. M., Kylsten P., Engvall E. Mouse adhalin: primary structure and expression during late stages of muscle differentiation in vitro. Biochem. Biophys. Res. Commun. 1997;235:227–235. doi: 10.1006/bbrc.1997.6757. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi S., Wakabayashi E., Imamura M., Yoshida M., Ozawa E. Formation of sarcoglycan complex with differentiation in cultured myocytes. Eur. J. Biochem. 2000;267:640–648. doi: 10.1046/j.1432-1327.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- 26.Delgado J., Moro G., Saborido A., Megias A. T-tubule membranes from chicken skeletal muscle possess an enzymatic cascade for degradation of extracellular ATP. Biochem. J. 1997;327:899–907. doi: 10.1042/bj3270899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kegel B., Braun N., Heine P., Maliszewski C. R., Zimmermann H. An ecto-ATPase and an ecto-ATP-diphosphohydrolase are expressed in rat brain. Neuropharmacology. 1997;36:1189–1200. doi: 10.1016/s0028-3908(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 28.Roberds S. L., Anderson R. D., Ibraghimov-Berskrovnaya O., Campbell K. P. Primary structure and muscle-specific expression of the 50-kDa dystrophin-associated glycoprotein (adhalin) J. Biol. Chem. 1993;268:23739–23742. [PubMed] [Google Scholar]
- 29.Stout J. G., Kirley T. L. Inhibition of purified chicken gizzard smooth muscle ecto-ATPAse by P2 purinoceptor antagonists. Biochem. Mol. Biol. Int. 1995;36:927–934. [PubMed] [Google Scholar]
- 30.Heine P., Braun N., Heilbronn A., Zimmermann H. Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after heterologous expression in CHO cells. Eur. J. Biochem. 1999;262:102–107. doi: 10.1046/j.1432-1327.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 31.Yegutkin G. G., Burnstock G. Inhibitory effects of some purinergic agents on ecto-ATPase activity and pattern of stepwise ATP hydrolysis in rat liver plasma membranes. Biochim. Biophys. Acta. 2000;1466:234–244. doi: 10.1016/s0005-2736(00)00165-6. [DOI] [PubMed] [Google Scholar]
- 32.Knowles A. F., Nagy A. K. Inhibition of an ecto-ATP-diphosphohydrolase by azide. Eur. J. Biochem. 1999;262:349–357. doi: 10.1046/j.1432-1327.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 33.Sagara Y., Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J. Biol. Chem. 1991;266:13503–13506. [PubMed] [Google Scholar]
- 34.Clausen T. The Na+, K+ pump in skeletal muscle: quantification, regulation and functional significance. Acta Physiol. Scand. 1996;156:227–235. doi: 10.1046/j.1365-201X.1996.209000.x. [DOI] [PubMed] [Google Scholar]
- 35.Betto R., Biral D., Sandonà D. Functional roles of dystrophin and of associated proteins. New insights for the sarcoglycans. Neurol. Sci. 1999;20:371–379. doi: 10.1007/s100720050054. [DOI] [PubMed] [Google Scholar]
- 36.Henning R. H. Purinoceptors in neuromuscular transmission. Pharmacol Ther. 1997;74:115–128. doi: 10.1016/s0163-7258(97)00015-6. [DOI] [PubMed] [Google Scholar]
- 37.Ryten M., Hoebertz A., Burnstock G. Sequential expression of three receptor subtypes for extracellular ATP in developing rat skeletal muscle. Dev. Dyn. 2001;221:331–341. doi: 10.1002/dvdy.1147. [DOI] [PubMed] [Google Scholar]
- 38.Wells D. G., Zawisa M. J., Hume R. I. Changes in responsiveness to extracellular ATP in chick skeletal muscle during development and upon denervation. Dev. Biol. 1995;172:585–590. doi: 10.1006/dbio.1995.8062. [DOI] [PubMed] [Google Scholar]
- 39.Ryten M., Dunn P. M., Neary J. T., Burnstock G. ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2×5 receptor on satellite cells. J. Cell Biol. 2002;158:345–355. doi: 10.1083/jcb.200202025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hicks-Berger C. A., Chadwick B. P., Frischauf A. M., Kirley T. L. Expression and characterization of soluble and membrane-bound human nucleoside triphosphate diphosphohydrolase 6 (CD39L2) J. Biol. Chem. 2000;275:34041–34045. doi: 10.1074/jbc.M004723200. [DOI] [PubMed] [Google Scholar]
- 41.Dickens N. J., Beatson S., Ponting C. P. Cadherin-like domains in α-dystroglycan, α/ε-sarcoglycan and yeast and bacterial proteins. Curr. Biol. 2002;12:R197–R199. doi: 10.1016/s0960-9822(02)00748-0. [DOI] [PubMed] [Google Scholar]
- 42.Allen D. G. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol. Scand. 2001;171:311–319. doi: 10.1046/j.1365-201x.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 43.Holt K. H., Campbell K. P. Assembly of the sarcoglycan complex. Insights for muscular dystrophy. J. Biol. Chem. 1998;273:34667–34670. doi: 10.1074/jbc.273.52.34667. [DOI] [PubMed] [Google Scholar]
- 44.Jung D., Leturcq F., Sunada Y., Duclos F., Tomé F. M. S., Moomaw C., Merlini L., Azibi K., Chaouch M., Slaughter C., et al. Absence of γ-sarcoglycan (35 DAG) in autosomal recessive muscular dystrophy linked to chromosome 13ql2. FEBS Lett. 1996;381:15–20. doi: 10.1016/0014-5793(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 45.Caldwell C. C., Hornyak S. C., Pendleton E., Campbell D., Knowles A. F. Regulation of chicken gizzard ecto-ATPase activity by modulators that affect its oligomerization status. Arch. Biochem. Biophys. 2001;387:107–116. doi: 10.1006/abbi.2000.2216. [DOI] [PubMed] [Google Scholar]
- 46.Stout J. G., Kirley T. L. Control of cell membrane ecto-ATPase by oligomerization state: intermolecular cross-linking modulates ATPase activity. Biochemistry. 1996;35:8289–8298. doi: 10.1021/bi960563g. [DOI] [PubMed] [Google Scholar]