Abstract
Objectives
To compare the sensitivity of 2019 European Alliance of Associations for Rheumatology/American College of Rheumatology (EULAR/ACR) classification criteria against 1997 ACR criteria for systemic lupus erythematosus (SLE), for incident SLE cases in the presumably complete population-based Nor-SLE cohort from Southeast Norway (2.9 million inhabitants).
Methods
All cases International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) coded as SLE during 2000–2017 were individually reviewed. Those with a confirmed SLE diagnosis by expert clinical assessment were included in the Nor-SLE cohort. Core clinical data were recorded, and the cases were classified according to 2019 EULAR/ACR and 1997 ACR criteria. Juvenile SLE was defined as <16 years at diagnosis and adult SLE was defined as ≥16 years at diagnosis.
Results
We included 737 incident SLE cases (701 adults, 36 juveniles). At diagnosis, 2019 EULAR/ACR criteria were more sensitive than 1997 ACR criteria for adults (91.6% vs 77.3%; p<0.001), but not for juveniles (97.2% vs 88.9%). The 2019 EULAR/ACR counts at diagnosis differed by age group and ethnicity, being higher in young cases and those originating from Asia. From time of diagnosis to study end the fulfilment rate of 2019 EULAR/ACR criteria for the adult cohort increased from 92.5% and 86.5% to 94.6% and 91.0%, respectively, for females and males (mean disease duration of 7.5 years).
Conclusion
Showing 92% criteria fulfilment already at time of SLE diagnosis by 2019 EULAR/ACR criteria versus 77% by 1997 ACR criteria, the results from this population-based study suggest that the 2019 EULAR/ACR criteria will achieve its goal of capturing more early-SLE cases for clinical trials.
Keywords: lupus erythematosus, systemic; classification; epidemiology; sensitivity and specificity
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
In a presumably complete population-based prospective cohort following 737 SLE cases from time of diagnosis, we found that the 2019 EULAR/ACR classification criteria were more sensitive than the 1997 ACR criteria. A key, novel finding was that more than 90% of our incident SLE cases met the 2019 criteria already at time of diagnosis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The high sensitivity at time of diagnosis observed for the 2019 EULAR/ACR classification criteria suggests the feasibility of capturing highly representative, new-onset SLE cohorts for clinical trials, providing unprecedented opportunities to study the effects of early treatment.
Introduction
Systemic lupus erythematosus (SLE) is a complex, heterogeneous autoimmune disease, with partly overlapping phenotypes that evolve from disease onset and undergo multiple changes across the course of the disease.1,3 Immune complexes containing autoantibodies is a hallmark of the disease and compiled data from the recent international SLE classification project (detailed below) showed that 98% of patients scored positive for antinuclear antibodies (ANA) by standardised indirect immunofluorescence testing.4 While the presence of additional, more specific autoantibodies binding double-stranded DNA (anti-dsDNA), Smith ribonucleoprotein (anti-Sm) and membrane phospholipids (anti-PL) makes the diagnosis more likely, they are never more than diagnostic aids.5
In SLE, the immune-mediated inflammation has destructive potential.1 2 Thus, if the disease is left untreated, targeted organs are at high risk of structural damage and loss of function.6 This implies that diagnostic delay, with the thwarted start-up of immune-suppressive therapies, is critical to avoid, as it may result in a situation where the patient already at time of diagnosis has severe, and sometimes irreversible, damage in one or more organ systems. Moreover, there is evidence that delayed diagnosis may increase the likeliness of persistent disease activity and flares, which has an impact on disease burden, damage accrual and mortality.6,9
In the absence of diagnostic criteria, the current ‘gold-standard’ case definition for SLE and the basic eligibility criterion for all types of SLE research (including studies of early SLE), is ‘diagnosis confirmed by expert clinical assessment’. However, this case definition is liable to bias, thus the case definition for research must be accompanied by classification criteria to secure homogeneity and comparability. Requiring high specificity, classification, in general, comes with the risk of low sensitivity. Accordingly, classification may exclude a significant proportion of patients, for example, from testing of new treatments in clinical trials.
Reflecting the need for better representation of SLE patients in clinical trials, it was an expressed ambition of the 2019 European Alliance of Associations for Rheumatology/American College of Rheumatology (EULAR/ACR) classification criteria to increase sensitivity, especially for patients with early disease, compared with the 1997 ACR criteria.10,13 This consequently accommodates inclusion earlier in the disease course to trials on newer treatments.14 Moreover, the new criteria were constructed as an additive scoring system, and as proposed when the criteria were launched, recent studies have linked higher scores to a more severe disease progression and higher risk of organ damage.10 11 15 16
Complementing and extending data from the original derivation and validation cohorts applied in the primary criteria studies, the sensitivity of the 2019 EULAR/ACR criteria has been estimated in many established SLE cohorts around the world.1011 17,19 In a recent systematic literature review from Aringer et al, which included 22 studies, the aggregated sensitivity was 93%.17 These studies provide some information on criteria sensitivity in SLE cases with relatively short disease duration. However, information about criteria sensitivity at time of clinical diagnosis is lacking. Moreover, and most notably, there are no data on sensitivity in complete population-based incident SLE cohorts. Hence, there is a need to assess whether the highly promising sensitivity improvement holds up in an incident, population-based SLE cohort assessed at time of diagnosis
We have established a large and complete, population-based, incident SLE cohort from Norway.20 From this cohort, we have comprehensive data on all the items included in the 2019 EULAR/ACR classification criteria from time of diagnosis and the corresponding item scores. Here, we estimate the sensitivity for the 2019 EULAR/ACR vs the 1997 ACR criteria at time of diagnosis (inception), at 2-year follow-up, and at last visit in study period. We assessed sensitivity in the total cohort and in subsets defined by age, sex and ethnic background.
Methods
Study area, study period and study cohort
For this study, we used data from the established population-based Nor-SLE cohort, which has been described in detail elsewhere.9 20 Briefly, the Nor-SLE cohort was designed to include every incident and prevalent SLE case living in the Southeast Norway region, an area populated by 2.9 million people and equivalent to 56% of the total population in Norway, during 1999–2017.
As incident SLE cases, we defined cases who were diagnosed with SLE within the study period and resided in the study area at time of diagnosis or settled in the area within 1 year of diagnosis. Cases were defined by a two-step process. First, we identified all cases in the study area International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) coded as SLE (M32.1/M32.8/M32.9) during the study period. Next, we set up a research network that performed individual-level chart review to confirm or reject SLE diagnosis in the cases identified with SLE-specific ICD-10 codes.20 As guidance for this expert chart review, and determination of time of diagnosis in the incident cases, the network applied the principles of Fries and Holman, which requires the presence of multisystemic disease, manifestations typical of SLE in at least two organ systems and immunological disturbance compatible with SLE.21
As we, for technical reasons, in the Nor-SLE cohort lacked complete follow-up data for 29 cases diagnosed in 1999, we excluded incident cases diagnosed in 1999 from the current study. Hence, this study includes all incident Nor-SLE cases diagnosed during 2000–2017. Juvenile-onset SLE was defined as <16 years at diagnosis and adult SLE was defined as ≥16 years at diagnosis. Follow-up lasted to end of study period, death or migration out of study area. The study was defined as an observational, population-based cohort study with prospective follow-up. Geographical origin of cases was defined by Statistics Norway: By parents’ country of birth (with offspring being Norwegian if one parent was born in Norway), and by the four geographical origin groups Europe (including Russia), Asia (including Turkey), Africa and South and Central America.
Application of SLE classification criteria
As part of the individual-level chart review process, we collected and recorded data in predefined data-extraction study forms. The dataset for the incident SLE cases included demographics and presence or absence of core SLE parameters at three different time points: (1) time of diagnosis, (2) after 2 years disease duration (or specifically, at a visit 24±6 months after diagnosis) and (3) at the visit closest to study end in 2017. The core SLE parameters included all the 2019 EULAR/ACR and 1997 ACR classification criteria items.
Performance of the sensitivity analysis and the criteria item analysis
We estimated the sensitivity of the 2019 EULAR/ACR and the 1997 ACR criteria by first calculating the number of cases fulfilling the two sets of classification criteria and then using the confirmed SLE diagnosis by individual-level chart review as ‘gold standard’ and for verification. The sensitivity estimate at time of diagnosis and at study end was based on all incident cases in the Nor-SLE cohort during 2000–2017, whereas the estimates after 2 years disease duration included only incident cases during 2000–2015 to allow for 2-year follow-up for all cases. We stratified all cases fulfilling the 2019 EULAR/ACR criteria at time of diagnosis by a score less than 20 points, or 20 or more points, to analysed possible group-level characteristics.
Statistical analysis
Analyses were performed by using IBM SPSS Statistics V.28 (IBM SPSS) or STATA V.17 (StataCorp). The sensitivity of the 2019 EULAR/ACR and 1997 ACR classification criteria was calculated by using the clinical SLE diagnosis as ‘gold standard’. We calculated 95% CIs for rates using Poisson distribution. Population characteristics were summarised as means with SD, median values with IQR or proportions. χ2 test or two-sample z-test of proportions was used to detect differences in categorical variables. All tests were two sided with a 5% significance level.
Results
Study cohort characteristics
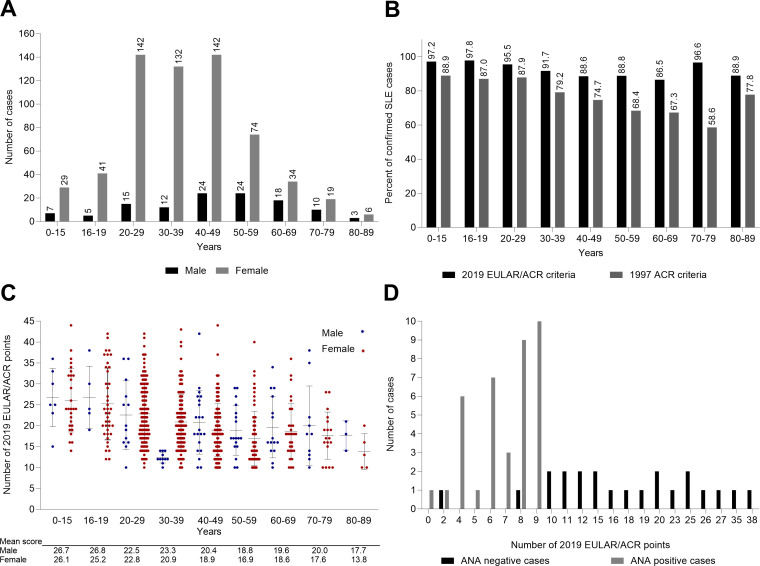
To assess the performance of the 2019 EULAR/ACR criteria at time of diagnosis, we included in the analyses all the incident cases from the Nor-SLE cohort who had complete registrations of classification criteria items. Among the 744 incident cases diagnosed with SLE in the years 2000–2017, we found that 737 (99.1 %) had complete registrations of the classification criteria items (figure 1). Among these 737 cases, 95.1% had adult-onset disease, the female to male ratio was 5.3 and 715 cases (97.0%) had positive ANA (table 1 and table 2). The median time from onset of first SLE symptom to confirmed diagnosis was 1 year (IQR 0–4 year). Mean age at diagnosis was 39.5 (SD 16.5) years. The age distribution of the cases at time of diagnosis is shown in figure 2A.
Figure 1. Flow chart for the incident Southeast Norway SLE cohort during 2000–2017 at time of diagnosis, showing SLE case definitions and distribution of cases meeting the 1997 ACR classification criteria for SLE (1997 ACR criteria) and the 2019 EULAR/ACR classification criteria for SLE (2019 EULAR/ACR criteria), respectively. ACR, American College of Rheumatology; EULAR, European Alliance of Associations for Rheumatology; SLE, systemic lupus erythematosus.
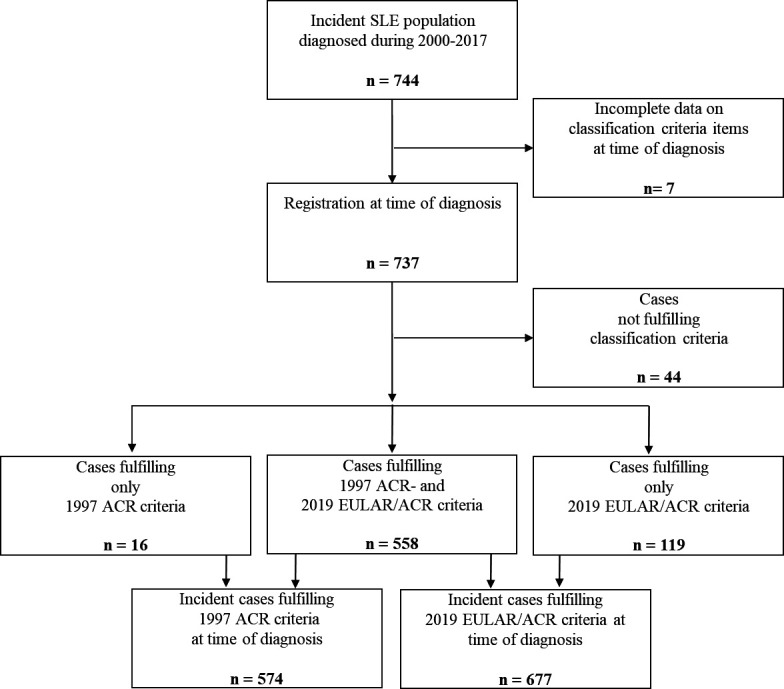
Table 1. Demographics of the incident Southeast Norway SLE cohort during 2000–2017 for cases with confirmed SLE diagnosis by chart review.
| TotalN=737 | FemaleN=619 | MaleN=118 | |
| Adult onset, n (%) | 701 (95.1) | 590 (95.3) | 111 (94.1) |
| Juvenile onset, n (%) | 36 (4.9) | 29 (4.7) | 7 (5.9) |
| Mean age at diagnosis, years (SD) | 39.5 (16.5) | 38.1 (15.7) | 46.7 (18.7) |
| Mean follow-up time from time of diagnosis to study end in 2017, years (SD) | 7.9 (5.2) | 7.8 (5.1) | 8.5 (5.2) |
| Median time from first SLE symptom to diagnosis*, years (IQR) | 1.0 (0–4) | 1.0 (0–4) | 1.0 (0–3.3) |
| Mean time from first SLE symptom to diagnosis*, years (SD) | 3.5 (5.5) | 3.6 (5.6) | 2.9 (4.7) |
| European descent, n (%) | 609 (82.6) | 503 (81.3) | 106 (89.8) |
| Asian descent, n (%) | 97 (13.2) | 87 (14.1) | 10 (8.5) |
| African descent, n (%) | 21 (2.8) | 19 (3.1) | 2 (1.7) |
| Other descent, n (%) | 10 (1.4) | 10 (1.6) | 0 (0.0) |
| Number of cases fulfilling the 1997 ACR criteria at diagnosis, n (%) | 574 (77.9) | 492 (79.5) | 82 (69.5) |
| Number of cases fulfilling the 2019 EULAR/ACR criteria at diagnosis, n (%) | 677 (91.9) | 574 (92.7) | 103 (87.3) |
| Deceased by end of 2017, n (%) | 57 (7.7) | 36 (5.8) | 21 (17.7) |
1997 ACR criteria, 1997 ACR classification criteria for SLE; 2019 EULAR/ACR criteria, 2019 EULAR/ACR classification criteria for SLE.
Missing information for 42 of 737 cases.
#, missing information for 42 of 737 cases2019criteria, the 2019 European Alliance of Associations for Rheumatology/American College of Rheumatology classification criteria for Systemic Lupus Erythematous; 1997criteria, the 1997 American College of Rheumatology classification criteria for Systemic Lupus Erythematous; EULAR/ACR, European Alliance of Associations for Rheumatology/American College of Rheumatology; N, number of cases of the incident SLE population; SD, standard deviation; SLE, systemic lupus erythematosus
Figure 2. For the incident Southeast Norway SLE cohort during 2000–2017, at time of diagnosis: (A) Distribution of age and sex for cases with a confirmed diagnosis. (B) Estimated percentage of cases with a confirmed SLE diagnosis fulfilling the 2019 EULAR/ACR classification criteria for SLE and the 1997 ACR classification criteria for SLE. (C) Mean and actual number of classification points for all cases fulfilling the 2019 EULAR/ACR classification criteria. (D) Calculated number of classification points for the ANA-positive and ANA-negative subsets of SLE cases not classifiable via the 2019 EULAR/ACR criteria. ACR, American College of Rheumatology; ANA, antinuclear antibodies; EULAR, European Alliance of Associations for Rheumatology; SLE, systemic lupus erythematosus.
Fulfilment of SLE classification criteria in incident cases assessed at time of diagnosis
Already at the time point of the clinical SLE diagnosis, 677 of the 737 incident cases (91.9%) fulfilled the 2019 EULAR/ACR criteria while 574/737 (77.9%) fulfilled the 1997 ACR criteria (figure 1). Obviously, all the 677 cases fulfilling the 2019 EULAR/ACR criteria were ANA positive at time of diagnosis. Among the 60 incident cases who did not fulfil the 2019 EULAR/ACR criteria at time of diagnosis, only 38 (63.3 %) were ANA positive (table 2). At diagnosis, 18 (2.4%) out of the 737 incident cases were ANA negative. This percentage decreased over time. At the visit 2 years after diagnosis, 2.3% of the patients were ANA negative vs 1.8% at study end. The immunological findings for the 13 cases never positive for ANA are summarised in table 3. Stratification by age and sex indicated that, in both females and males, the proportion of cases meeting the 2019 criteria at time of diagnosis was highest in the age groups <30 years and lower in the older age groups (figure 2B).
Table 2. Case characteristics and fulfilment of the 2019 EULAR/ACR classification criteria items for SLE at time of diagnosis for the incident Southeast Norway SLE cohort during 2000–2017 (N=737).
| Cohort fulfilling2019 EULAR/ACR criteria | Cohort not fulfilling2019 EULAR/ACR criteria | ||||||
| TotalN=677 | Female N=574 | MaleN=103 | TotalN=60 | FemaleN=45 | MaleN=15 | ||
| Case characteristics | |||||||
| Adults, n (%) | 642 (94.8) | 546 (95.1) | 96 (93.2) | 59 (98.3) | 44 (97.8) | 15 (100) | |
| Mean age at diagnosis, years (SD) | 39.1 (16.6) | 37.8 (15.7) | 46.3 (19.3) | 44.2 (14.6) | 42.6 (14.6) | 49.1 (13.7) | |
| European descent, n (%) | 553 (81.7) | 460 (80.1) | 93 (90.3) | 56 (93.3) | 43 (95.6) | 13 (86.7) | |
| Cases meeting 1997 ACR criteria, n (%) | 558 (82.4) | 479 (83.4) | 79 (76.7) | 16 (26.7) | 13 (28.9) | 3 (20.0) | |
| 2019 EULAR/ACR criteria-items at time of diagnosis | P value† | ||||||
| ANA positive, n (%) | 677 (100) | 574 (100) | 103 (100) | 38 (63.3) | 30 (66.7) | 8 (53.3) | |
| Mean 2019 EULAR/ACR criteria points, n (SD) | 20.8 (7.4) | 20.7 (7.3) | 21.2 (7.7) | 10.9 (7.8) | 10.5 (8.0) | 12.2 (7.5) | |
| Constitutional, n (%) | 267 (39.4) | 225 (39.2) | 42 (40.8) | 9 (15.0) | 5 (11.1) | 4 (26.7) | Total: **Female: ** |
| Haematologic, n (%) | 328 (48.4) | 279 (48.6) | 49 (47.6) | 18 (30.0) | 13 (28.9) | 5 (33.3) | Total: *Female: * |
| Neuropsychiatric, n (%) | 16 (2.4) | 15 (2.6) | 1 (1.0) | 4 (6.7) | 3 (6.7) | 1 (6.7) | Total: * |
| Mucocutaneous, n (%) | 480 (70.9) | 426 (74.2) | 54 (52.4) | 38 (63.3) | 32 (71.1) | 6 (40.0) | |
| Serositis, n (%) | 139 (20.5) | 110 (19.2) | 29 (28.2) | 4 (6.7) | 2 (4.4) | 2 (13.3) | Total: *Female: * |
| Musculoskeletal, n (%) | 534 (78.9) | 459 (80.0) | 75 (72.8) | 26 (43.3) | 21 (46.7) | 5 (33.3) | Total: **Female: **Male: * |
| Renal, n (%) | 191 (28.2) | 144 (25.1) | 47 (45.6) | 11 (18.3) | 6 (13.3) | 5 (33.3) | |
| Anti-phospholipid antibodies, n (%) | 231 (34.1) | 203 (35.4) | 28 (272) | 13 (21.7) | 7 (15.6) | 6 (40.0) | Total: *Female: * |
| Complementary proteins, n (%) | 307 (45.3) | 258 (44.9) | 49 (47.6) | 12 (20) | 9 (20.0) | 3 (20.0) | Total: **Female: *Male: * |
| SLE-specific antibodies, n (%) | 513 (75.8) | 437 (76.1) | 76 (73.8) | 8 (13.3) | 6 (13.3) | 2 (13.3) | Total: **Female: **Male: ** |
1997 ACR criteria, 1997 American College of RheumatologyACR classification criteria for Systemic Lupus Erythematous; 2019 EULAR/ACR criteria, 2019 European Alliance of Associations for Rheumatology/American College of RheumatologyEULAR/ACR classification criteria for Systemic Lupus Erythematous; ANA, antinuclear antibodies; N, number of cases of the incident SLE population; n, number of cases; , standard deviation; § P-value for 2019 EULAR/ACR criteria fulfilment not fulfilment; *; **SLE.
p<0.05, **p<0.001.
P value for 2019 EULAR/ACR criteria fulfilment versus not fulfilment.
ANAantinuclear antibodiesEULAR/ACREuropean Alliance of Associations for Rheumatology/American College of Rheumatologynnumber of casesSDstandard deviationSLE, systemic lupus erythematosus
Table 3. Autoantibodies and complement factors in the 13 cases never positive for ANA in the incident Southeast Norway SLE cohort during 2000–2017 (N=737).
| Anti-dsDNA | Case | Anti-ENA | Anti-PL | Low complement | |||||
| Anti-SSA | Anti-SSB | Anti-Sm | Anti-CL | LA | Anti-ß2 GPI | C3 | C4 | ||
| 1 | – | –* | – | – | – | – | – | – | – |
| 2 | + | + | + | – | + | – | – | + | + |
| 3 | – | – | – | – | – | + | – | – | – |
| 4 | + | – | – | + | + | + | + | + | + |
| 5 | – | + | – | – | – | – | – | – | – |
| 6 | – | – | – | – | – | – | – | + | – |
| 7 | – | – | – | – | + | + | + | – | – |
| 8 | – | + | – | – | – | – | – | – | – |
| 9 | – | – | – | – | – | + | – | + | – |
| 10 | – | – | – | – | – | – | – | – | – |
| 11 | – | – | – | – | – | – | – | – | – |
| 12 | – | – | – | – | – | – | – | – | – |
| 13 | – | – | – | – | – | – | – | – | – |
cCase 1 has positive anti-ENA, but negative anti-SSA/SSB.
aCL, anti-cardiolipin; aCL, anti-cardiolipin; ANA, antinuclear antibodies; anti-ß2 GPIanti-beta2glycoprotein Ianti-PL, anti-phospholipid; C3, C3 proteins; C4, C4 proteinsdsDNA, double-stranded DNA; ENA, extractable nuclear antigen; LA, lupus anticoagulantPLphospholipidSSA, Sjögren’s-syndrome-related antigen ASSB, Sjögren’s-syndrome-related antigen B
Assessment of the individual 2019 EULAR/ACR criteria items at time of diagnosis
Individual classification items that were more frequently present at time of diagnosis in the cases that met the 2019 criteria compared with those that did not fulfil the criteria included SLE-specific autoantibodies, antiphospholipid antibodies, reduced levels of complement protein C3 and/or C4, haematology parameters, musculoskeletal symptoms and serositis (table 2).
As for sex-related differences, renal involvement was significantly more common in males versus females in the cohort fulfilling the criteria at 45.6% vs 25.1% (p<0.001), and mucocutaneous symptoms were significantly more common in females versus males at 74.2% vs 52.4% (p<0.001).
Of the 18 ANA-negative incident SLE cases at time of diagnosis, 3 (16.7%) had SLE-specific antibodies, 6 (33.3%) had complementary proteins, 6 (33.3%) had antiphospholipid antibodies, and 12 and 13, respectively, had mucocutaneous and musculoskeletal symptoms. Only five (27.8%) had haematological findings.
For the 11 cases with renal involvement not fulfilling the 2019 EULAR/ACR criteria, ANA was negative in 10 cases, for the last case, the status was unknown. Out of the 10 ANA-negative cases, 2 were dsDNA positive at time of diagnosis while 2 more cases were positive at last visit. Nine of the 10 cases had chart information on biopsy findings. For the 10th case, we have no biopsy information. Of the nine biopsy-proven lupus nephritis cases according to the Society of Nephrology/Renal Pathology Society classification, one case was in class II, two cases were in class III, five cases were in class IV and the last case was in class V.
Assessment of the total 2019 EULAR/ACR criteria point scores for classified cases at time of diagnosis
Both in males and females, the mean total item scores were higher in the younger age groups (figure 2C). Highest overall mean score (26.8 SD 7.4) was noted in males who were <20 years at time of diagnosis. From 40 years of age, the mean 2019 EULAR/ACR point scores were consistently <20, except in males aged 40–49 and 70–79 years at time of diagnosis (n=24 and n=10, respectively), where it was 20.4 and 20.0, respectively (figure 2C). Among cases originating from Europe, 46.1% had an item score of 20 or more (mean 20.2, SD 7.0), whereas in cases originating from Asia 67% had item scores of 20 or above (mean 23.5, SD 8.2).
When comparing cases with items scores ≥20 to those with <20 at time of diagnosis, mean age was lower (34.5 (SD 15.7) vs 43.6 (SD 16.3) years). Cases with scores ≥20 more often had fever (53.0% vs 26.2%), haematological (64.1% vs 33.2%), mucocutaneous (78.4% vs 63.6%), musculoskeletal (84.4% vs 73.5%), serosal (28.7% vs 12.5%) and renal (44.3% vs 12.5%) involvement, as well as antiphospholipid antibodies (46.1% vs 22.4%), low complement factors (69.5% vs 21.9 %) and SLE-specific antibodies (93.1% vs 58.9%), all items p<0.05.
For the ANA positive and ANA-negative subsets of SLE patients not classifiable via the 2019 EULAR/ACR criteria, the total item scores are shown in figure 2D.
Sensitivity of the 2019 EULAR/ACR vs 1997 ACR criteria
The sensitivity analysis of the 2019 EULAR/ACR criteria at time of diagnosis was performed for the incident cases during 2000–2017 and included 701 adult and 36 juvenile cases (table 4). For the adult incident SLE cases, the 2019 EULAR/ACR criteria showed higher sensitivity at diagnosis compared with the 1997 ACR criteria (91.6% vs 77.3 %, p<0.001), also evident in both the female and male population (table 4, upper panel).
Table 4. Estimated sensitivity of the 1997 ACR classification criteria for SLE and the 2019 EULAR/ACR classification criteria for SLE in the incident Southeast Norway SLE cohort during 2000–2017, at time of diagnosis, 2 years after diagnosis and at last visit in study period.
| Classification criteria | Proportion of cases meeting criteria, % (95% CI) n1/n2, | |||
| At time of diagnosis | 2 years after diagnosis* | At last visit in study period | ||
| Adult-onset SLE | ||||
| Total | 2019 EULAR/ACR | 91.6 (89.5–93.6)642/701 | 92.7 (90.6–94.7)568/613 | 94.0 (92.3–95.8)659/701 |
| 1997 ACR | 77.3 (74.2–80.4)542/701 | 82.5 (79.5–85.5)506/613 | 84.3 (81.6–87.0)591/701 | |
| P<0.001 | P<0.001 | P<0.001 | ||
| Female | 2019 EULAR/ACR | 92.5 (90.4–94.7)546/590 | 93.6 (91.4–95.7)480/513 | 94.6 (92.7–96.4)558/590 |
| 1997 ACR | 79.0 (75.7–82.3)466/590 | 83.6 (80.4–86.8)429/513 | 85.6 (82.8–88.4)505/590 | |
| P<0.001 | P<0.001 | P<0.001 | ||
| Male | 2019 EULAR/ACR | 86.5 (80.1–92.8)96/111 | 88.0 (81.6–94.4)88/100 | 91.0 (85.7–96.3)101/111 |
| 1997 ACR | 68.5 (59.8–77.1)76/111 | 77.0 (68.6–85.2)77/100 | 77.5 (69.7–85.2)86/111 | |
| P<0.001 | P<0.05 | P<0.05 | ||
| Juvenile-onset SLE‡ | ||||
| Total | 2019 EULAR/ACR | 97.2 (91.9–102.6)35/36 | 100 (100–100)25/25 | 97.2 (91.9–102.6)35/36 |
| 1997 ACR | 88.9 (78.6–99.2)32/36 | 92.0 (81.4–102.6)23/25 | 91.7 (82.6–100.7)33/36 | |
| Female | 2019 EULAR/ACR | 96.6 (89.9–103.1)28/29 | 100 (100–100)21/21 | 96.6 (89.9–103.2)28/29 |
| 1997 ACR | 89.7 (78.5–100.7)26/29 | 90.5 (77.9–102.6)19/21 | 89.7 (78.6–100.7)26/29 | |
| Male | 2019 EULAR/ACR | 100†7/7 | 100†4/4 | 100†7/7 |
| 1997 ACR | 85.7†6/7 | 100†4/4 | 100†7/7 | |
The 1997 ACR criteria, the 1997 American College of RheumatologyACR classification criteria for Systemic Lupus ErythematousSLE; the 2019 EULAR/ACR criteria, the 2019 European Alliance of Associations for Rheumatology/American College of RheumatologyEULAR/ACR classification criteria for Systemic Lupus Erythematous; n, number of cases fulfilling the criteria; n total number of cases at visit,SLE.
n1, number of cases fulfilling the criteria; n2,, total number of cases at visit
eEstimated sensitivity two2 years after diagnosis includes only the 64238 incident cases during 2000–2015 to allow for two2-year follow upfollow-up for all cases.
lLess than 10 cases cases, and to scarce for statistical analysis.
2019 EULAR/ACR criteria fulfilment vs 1997 ACR criteria fulfilment did not differ significantly.
EULAR/ACREuropean Alliance of Associations for Rheumatology/American College of RheumatologySLEsystemic lupus erythematosus
In the juvenile SLE population, the sensitivity at time of diagnosis for the 2019 EULAR/ACR vs the 1997 ACR criteria did not differ significantly at 97.2% vs 88.9% for the total population, and similarly for the female cases at 96.6 vs 89.7% (see table 4, lower panel, for details). For male subjects, the numbers were too small for statistical analysis (table 4, lower panel).
To assess whether sensitivity of the 2019 ACR/EULAR and the 1997 ACR classification criteria rose with increasing disease duration, we assessed cumulative fulfilment of the classification criteria items after 2 years disease duration and at study end. For the assessment after 2 years disease duration, we focused on the 613 adult-onset cases and 25 juvenile cases diagnosed in the period from 2000 to 2015 (to allow for 2 years follow-up data from diagnosis in all cases). In the adult incident cases diagnosed from 2000 to 2015, the sensitivity of the 2019 EULAR/ACR criteria was superior to the 1997 ACR criteria after 2 years disease duration, for both female and male cases (table 4).
Finally, we also assessed cumulative fulfilment of the classification criteria items at the visit closest to study end in the total cohort. At this assessment, the median disease duration was 7 years (range 0–17 years). We found that the superiority of the 2019 EULAR/ACR criteria was maintained (table 4).
Proportion of cases meeting 2019 EULAR/ACR criteria already at time of diagnosis
Among the 558 (of 590) adult female subjects meeting the 2019 EULAR/ACR criteria at study end (when mean disease duration was 7.5 years (SD 5.1), 546 (97.8%) met the criteria already at time of diagnosis. Correspondingly, among the 101 male subjects who met the criteria at study end, 96 (95.0%) did so already at time of diagnosis. All the 28 female and seven male juvenile-onset cases who met the 2019 EULAR/ACR criteria did so already at time of diagnosis.
Discussion
A major aim of the recent 2019 EULAR/ACR classification criteria was to enhance the representation of SLE patients in clinical trials by increasing the sensitivity of classification criteria, particularly for early-onset SLE.10 11
In this study, we assessed sensitivities of the 2019 EULAR/ACR criteria and compared them against the 1997 ACR criteria12 13 in the total Nor-SLE cohort, a large, presumably complete, population-based, incident SLE cohort from Southeast Norway, and in the juvenile and adult subsets therein.20
A key and novel finding was the significantly higher sensitivity for incident SLE in a population-based setting by the 2019 EULAR/ACR criteria compared with the 1997 ACR criteria already at time of diagnosis, in the male and female subsets, and across the adult age groups. In fact, in the juvenile subset, the 2019 EULAR/ACR criteria were met by as much as 97% of SLE cases at time of diagnosis. Overall, the results indicated the excellent performance of the 2019 EULAR/ACR criteria across the whole spectre of new-onset disease phenotypes and at all ages in a population-based setting.
To our knowledge, the current study provides the first external validation of the sensitivity of the 2019 EULAR/ACR criteria at time of inception, and in a population-based SLE cohort, for both juvenile and adult cases. Our results extend and complement the international validation cohort data presented in the original EULAR/ACR criteria study and in a later subset of the study on early disease by Johnson et al.10 11 22 In the latter study, only 3% of the total cohort (34/1266) had early SLE when defined as less than 1-year disease duration. In these 34 early adult-onset SLE cases, a sensitivity of 89% was noted for the 2019 EULAR/ACR criteria vs 56% with the 1997 ACR criteria.22 For comparison, in our cohort the sensitivity of the 2019 EULAR/ACR criteria for the 701 adult cases was estimated to be 91.6% at time of diagnosis (defined as no disease duration).
Interestingly, 98% of the adult female subjects fulfilling the 2019 EULAR/ACR criteria at study end also met the same criteria at time of diagnosis. Only 17 more cases with confirmed SLE were classified from time of diagnosis to study end by the 2019 EULAR/ACR criteria compared with 50 cases classified by the 1997 ACR criteria. This underscores the impact of the higher sensitivity of the newer criteria for SLE in the early stages.
For the 36 juvenile cases, the sensitivity by the 2019 EULAR/ACR criteria was high already at time of diagnosis (97.2%) and not significantly different from the 1997 ACR criteria. The two most likely explanations for this are higher sensitivity of the 1997 ACR criteria in juvenile SLE and low statistical power due to relatively low number of juvenile cases. Eight previous studies on sensitivity of the 2019 EULAR/ACR criteria in juvenile SLE were included in the 2022 systematic literature review by Aringer et al. While none of these studies were population based, and thus not directly comparable to our study, the overall sensitivity was 91% and the range was from 85% to 100%.17
The same Aringer review identified sensitivity data from 22 adult SLE studies, with a total of 6467 SLE cases. Three studies were from Europe. None of these was population based, but one, from a referral-centre cohort in Greece, assessed an early cohort with disease duration less than 3 years and reported a sensitivity of 87%.14
To our knowledge, three population-based studies, all from the USA, have assessed sensitivity of the the 2019 EULAR/ACR criteria. Two of these were included in the review by Aringer et al, while the last, from New York, was published in 2023.19 23 24 Of these three, the study from Rochester, Minnesota, was the only one to assess sensitivity in early disease. This study included 139 incident SLE cases diagnosed during 2000–2018. The Rochester study is, however, not directly comparable to our study as it did not estimate sensitivity of criteria at time of SLE diagnosis (inception), but rather at the time when the incident cases fulfilled at least one of three SLE classification criteria (1997 ACR, 2019 ACR/EULAR or 2012 Systemic Lupus International Collaborating Clinics criteria). Hence, their case definition for incident SLE was fulfilment of at least one set of classification criteria, while we, in our study, applied ‘SLE diagnosis confirmed by expert chart review guided by the diagnostic principles of Fries and Holman’ as incident case definition.20 Notwithstanding, the estimated sensitivity reported from Rochester (90.6% in adult cases) is in line with the results from our larger cohort at 91.6%. Notably, in the Rochester cohort the sensitivity of the 1997 ACR criteria was as low as 66.2% while it was 77.3% in our study. Time from first SLE manifestation to fulfilment of the 2019 ACR/EULAR criteria was 29.4 months in the Rochester study. In our cohort, the estimated time from symptom onset to diagnosis was 3.6 months (SD 8.6 months) in the juvenile cases and 28.8 months (SD 3.0) in the adult cases.
As far as we know, our study represents the largest external validation cohort published for the 2019 EULAR/ACR criteria. Both the above-mentioned Greek and German cohorts were sizeable at 690 and 606 cases, respectively, but they were not population based and did not assess the cases at time of diagnosis.14 18
The lack of extensive data on the sensitivity of the 2019 EULAR/ACR criteria in early SLE (inception cohorts), may reflect a quite common methodological practice in SLE clinical research where SLE cases are included at some random time point since diagnosis rather than from the time of diagnosis. For prospective studies on SLE, this may be due to the low incidence of SLE and, consequently, the long time horizon required to build a sufficiently large prospective incident cohort. For retrospective and observational prospective cohorts, it could depend on the present referral practice and organisation of the healthcare system. Complete registrations of classification criteria items from the time of diagnosis may not be available, at least not for studies originating from tertiary centres. Additionally, funding may be a limitation, as complete individual-level chart reviews and classification in retrospect require substantial efforts by trained researchers. This scarcity of robust data on the 2019 EULAR/ACR criteria from population-based cohorts from the time of diagnosis puzzled us and encouraged us in the pursuit for more sturdy data.
The clinical picture of SLE in the early course of SLE development is often characterised by non-specific signs and symptoms not easily recognisable as SLE.25 This corresponds with our results, as only 30.4% of cases were diagnosed with SLE within the same year as the first SLE related symptom occurred.
At time of diagnosis, for the 2019 EULAR/ACR classified cohort the musculoskeletal criteria item was the most frequent, followed by SLE-specific antibodies and mucocutaneous symptoms. These findings correspond with prior reports on arthralgia and arthritis as common clinical symptoms in early SLE, and also the most frequent symptoms of both the 1997 ACR and the 2019 EULAR/ACR criteria.14 26 Of all cases with a confirmed SLE diagnosis, 97% were ANA positive at time of diagnosis, which levels with the results from the meta-regression analysis performed for the development of the 2019 EULAR/ACR criteria at 97.8%.4
In accordance with previous suggestions for the use of the 2019 EULAR/ACR criteria items score as a proxy for disease severity10 11 and corresponding to work published by Whittall Garcia et al,15 we divided cases fulfilling the 2019 EULAR/ACR criteria at time of diagnosis into subsets of item scores of 20 points or more, or less than 20 points. We discovered that those carrying the higher scores were more likely to be of younger age. It was out of the scope of this article to link the item score to outcome, however, it is interesting that recently published data from the Nor-SLE cohort indicated higher mortality rates in juvenile-onset cases and young adults compared with cases with onset in the older adult ages.9
The current study has several strengths. In the Norwegian health system, complex diseases like SLE are assessed early and followed through the disease course by universal specialist healthcare, as early referral from primary care is encouraged. Date, ICD-10 code and a unique identity number are recorded at all visits, which make it feasible to identify almost every SLE case in the study area, and to characterise, classify and track clinical disease features from the time of diagnosis to study end. To our knowledge, the Nor-SLE cohort is among the largest medical-record-confirmed, population-based SLE cohorts and one of few that follow the cases across so many years, which further strengthens our results. The repeated recordings of classification items from diagnosis to study end also secures robustness of results.
The main limitation of this study is the retrospective case definition, by medical record confirmation of the diagnosis, as it depends on prior workup by other clinicians. Second, the resolution for time of diagnosis and assessment of classification criteria fulfilment was at the year level. With real-time assessment, data could have been recorded at monthly level and provided more accurate determination of disease onset and diagnostic delay. Finally, as the incidence of SLE varies with ethnicity, the dominance of cases with European ancestry limits generalisation.
In conclusion, this study provides novel data on the sensitivity of the 2019 EULAR/ACR criteria at time of diagnosis and shows that more than 90% of the incident cases fulfilled the criteria at time of diagnosis. Implicitly, the goal of the 2019 EULAR/ACR criteria to capture more early-SLE cases for clinical trials seems highly feasible. The outlook of earlier treatment for more patients brings hope for a better prognosis for future SLE patients.
Acknowledgements
Parts of the data have been presented as abstract at the ACR Convergence 2023.27
The funding sources have not been involved in the planning and execution of this study.
Footnotes
Funding: This study was supported by The Norwegian Women's Public Health Association, the DAM Foundation, Vivi Irene Hansen’s Foundation, Ragna and Egil Eiken’s Foundation and the Norwegian Rheumatism Association.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and the Regional Committee for Medical and Health Research Ethics approved this study and gave exemption from informed consent for identification of patients and chart review (REK 2009/2017).
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Hilde Haukeland, Email: hildehaukeland1@gmail.com.
Sigrid Reppe Moe, Email: sigmoe@ous-hf.no.
Cathrine Brunborg, Email: uxbruc@ous-hf.no.
Antonela Botea, Email: BXBOAN@betanienhospital.no.
Nenad Damjanic, Email: nenad.damjanic@mhh.no.
Gro Årthun Wivestad, Email: Gro.Arthun@sshf.no.
Heidi Kverneggen Øvreås, Email: RXOVH01@revmatismesykehuset.no.
Thea Bjerkestrand Bøe, Email: thea_bbhotmail.com.
Anniken Orre, Email: anniken_orre@hotmail.com.
Garen Torhild, Email: tgaren@ous-hf.no.
Helga Sanner, Email: helga.sanner@medisin.uio.no.
Karoline Lerang, Email: klerang@ous-hf.no.
Øyvind Molberg, Email: oyvind.molberg@medisin.uio.no.
Data availability statement
Data are available on reasonable request.
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 3.Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 4.Leuchten N, Hoyer A, Brinks R, et al. Performance of antinuclear antibodies for classifying systemic lupus erythematosus: a systematic literature review and meta-regression of diagnostic data. Arthritis Care Res (Hoboken) 2018;70:428–38. doi: 10.1002/acr.23292. [DOI] [PubMed] [Google Scholar]
- 5.Aringer M, Johnson SR. Classifying and diagnosing systemic lupus erythematosus in the 21st century. Rheumatol (Oxford) 2020;59:v4–11. doi: 10.1093/rheumatology/keaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kernder A, Richter JG, Fischer-Betz R, et al. Delayed diagnosis adversely affects outcome in systemic lupus erythematosus: Cross sectional analysis of the LuLa cohort. Lupus (Los Angel) 2021;30:431–8. doi: 10.1177/0961203320983445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce IN, O’Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74:1706–13. doi: 10.1136/annrheumdis-2013-205171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers SA, Allen E, Rahman A, et al. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford) 2009;48:673–5. doi: 10.1093/rheumatology/kep062. [DOI] [PubMed] [Google Scholar]
- 9.Moe SR, Haukeland H, Brunborg C, et al. Persisting mortality gap in systemic lupus erythematosus; a population-based study on juvenile- and adult-onset SLE in Norway 1999-2022. Rheumatol (Oxford) 2024;63:2109–17. doi: 10.1093/rheumatology/kead519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151–9. doi: 10.1136/annrheumdis-2018-214819. [DOI] [PubMed] [Google Scholar]
- 11.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71:1400–12. doi: 10.1002/art.40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 14.Adamichou C, Nikolopoulos D, Genitsaridi I, et al. In an early SLE cohort the ACR-1997, SLICC-2012 and EULAR/ACR-2019 criteria classify non-overlapping groups of patients: use of all three criteria ensures optimal capture for clinical studies while their modification earlier classification and treatment. Ann Rheum Dis. 2020;79:232–41. doi: 10.1136/annrheumdis-2019-216155. [DOI] [PubMed] [Google Scholar]
- 15.Whittall Garcia LP, Gladman DD, Urowitz M, et al. New EULAR/ACR 2019 SLE classification criteria: defining ominosity in SLE. Ann Rheum Dis. 2021;80:767–74. doi: 10.1136/annrheumdis-2020-218670. [DOI] [PubMed] [Google Scholar]
- 16.Carneiro AC, Ruiz MM, Freitas S, et al. Comparison of three classification criteria sets for systemic lupus erythematosus: a study looking at links to outcome and mortality. Arthritis Care Res (Hoboken) 2020;72:1611–4. doi: 10.1002/acr.24061. [DOI] [PubMed] [Google Scholar]
- 17.Aringer M, Costenbader K, Dörner T, et al. Advances in SLE classification criteria. J Autoimmun. 2022;132:S0896-8411(22)00053-1. doi: 10.1016/j.jaut.2022.102845. [DOI] [PubMed] [Google Scholar]
- 18.Schmidtmann I, Kraus D, Weinmann A, et al. Validation of the 2019 EULAR/ACR classification criteria for systemic lupus erythematosus in an academic tertiary care centre. RMD Open. 2023;9:e003037. doi: 10.1136/rmdopen-2023-003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guttmann A, Denvir B, Aringer M, et al. Evaluation of the EULAR/American college of rheumatology classification criteria for systemic lupus erythematosus in a population-based registry. Arthritis Care Res (Hoboken) 2023;75:1007–16. doi: 10.1002/acr.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haukeland H, Moe SR, Brunborg C, et al. Declining incidence of systemic lupus erythematosus in Norway 1999-2017: Data from a population cohort identified by ICD-10 code and verified by classification. Arthritis Rheumatol. 2023 doi: 10.1002/art.42775. [DOI] [PubMed] [Google Scholar]
- 21.Fries JF, Holman HR. Systemic lupus erythematosus: a clinical analysis. Maj Probl Intern Med. 1975;6:v–199. [PubMed] [Google Scholar]
- 22.Johnson SR, Brinks R, Costenbader KH, et al. Performance of the 2019 EULAR/ACR classification criteria for systemic lupus erythematosus in early disease, across sexes and ethnicities. Ann Rheum Dis. 2020;79:1333–9. doi: 10.1136/annrheumdis-2020-217162. [DOI] [PubMed] [Google Scholar]
- 23.Duarte-García A, Hocaoglu M, Osei-Onomah S-A, et al. Population-based incidence and time to classification of systemic lupus erythematosus by three different classification criteria: a Lupus Midwest Network (LUMEN) study. Rheumatology (Oxford) 2022;61:2424–31. doi: 10.1093/rheumatology/keab807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubio J, Krishfield S, Kyttaris VC. Application of the 2019 European league against rheumatism/American college of rheumatology systemic lupus erythematosus classification criteria in clinical practice: a single center experience. Lupus (Los Angel) 2020;29:421–5. doi: 10.1177/0961203320908939. [DOI] [PubMed] [Google Scholar]
- 25.Nossent J, Kiss E, Rozman B, et al. Disease activity and damage accrual during the early disease course in a multinational inception cohort of patients with systemic lupus erythematosus. Lupus (Los Angel) 2010;19:949–56. doi: 10.1177/0961203310366572. [DOI] [PubMed] [Google Scholar]
- 26.Rees F, Doherty M, Lanyon P, et al. Early clinical features in systemic lupus erythematosus: Can they be used to achieve earlier diagnosis? A risk prediction model. Arthritis Care Res (Hoboken) 2017;69:833–41. doi: 10.1002/acr.23021. [DOI] [PubMed] [Google Scholar]
- 27.Haukeland H, Moe SR, Brunborg C, et al. Sensitivity of the 2019 European alliance of associations for rheumatology /American college of rheumatology classification criteria for systemic lupus erythematosus in a population-based cohort; a study set in Norway 2000-2015 [abstract] Arthritis Rheumatol. 2023;75 https://acrabstracts.org/abstract/sensitivity-of-the-2019-european-alliance-of-associations-for-rheumatology-american-college-of-rheumatology-classification-criteria-for-systemic-lupus-erythematosus-in-a-population-based-cohort-a-st/ Available. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.