Abstract
The novel Ca2+-mobilizing second messengers cADPr (cyclic ADP-ribose) and NAADP (nicotinic acid–adenine dinucleotide phosphate) are both synthesized by ADP-ribosyl cyclases. Using HSR (heavy sarcoplasmic reticulum) fractions from rabbit skeletal muscle, NAADP-induced Ca2+ release was observed. In the present paper, we show in HSR membranes the formation of authentic cADPr, cGDPr (cyclic GDP-ribose) and NAADP. The cyclization reaction to form cADPr and cGDPr as well as the base-exchange reaction to form NAADP were strictly dependent on pH. Although the formation of cGDPr is optimized at pH 6, the synthesis of NAADP was most pronounced at a pH below 5. A novel regulation mechanism is provided for nicotinic acid, the co-substrate for NAADP synthesis. Nicotinic acid had virtually no influence on the cyclization reaction, but increased the affinity of NADP at an acidic pH and had the opposite effect at alkaline pH. Nicotinamide, the side product of cADPr synthesis, is an inhibitor of the cyclization reaction (IC50, 0.7±0.1 mM) and was 30-fold more potent at suppressing the base-exchange reaction. Although the synthesis of NAADP was highly sensitive to nicotinamide inhibition, this was not via a competition with the nicotinic-acid-binding site. In contrast with the ecto-ADP-ribosyl cyclase (CD38), the cyclization and base-exchange reaction of the skeletal muscle isoform was inhibited by Cu2+ and Zn2+, while other bivalent cations such as Ca2+, Mg2+ and Mn2+ had virtually no effect. These findings allow for the prediction of a novel ADP-ribosyl cyclase isoform in skeletal muscle HSR, other than CD38. Hence the enzymic prerequisite for cADPr- and NAADP-mediated Ca2+ signalling is present.
Keywords: ADP-ribosyl cyclase, CD38, cyclic ADP-ribose, nicotinic acid–adenine dinucleotide phosphate (NAADP), sarcoplasmic reticulum, skeletal muscle
Abbreviations: BST-1, bone marrow stromal cell antigen 1; cADPr, cyclic ADP-ribose; cGDPr, cyclic GDP-ribose; HSR, heavy sarcoplasmic reticulum; MALDI, matrix-assisted laser-desorption ionization; NAADP, nicotinic acid–adenine dinucleotide phosphate; NADP, nicotinamide–adenine dinucleotide phosphate; NEM, N-ethylmaleimide; NGD, nicotinamide–guanine dinucleotide
INTRODUCTION
The signalling cascades leading to Ins(1,4,5)P3-mediated activation of Ins(1,4,5)P3 receptors are well understood [1]. In contrast, little is known about the regulation of the novel second messengers cADPr (cyclic ADP-ribose) and NAADP (nicotinic acid–adenine dinucleotide phosphate). It is evident that cADPr- and NAADP-mediated Ca2+-release cascades are conserved from plants to mammalian cells [2,3]. Recently, we could show in rabbit skeletal muscle that NAADP activates Ca2+ release from sarcoplasmic reticulum and open probability of the ryanodine receptor in concentrations between 20 and 200 nM [4]. Under similar conditions, the related compound, cADPr, was ineffective to trigger ryanodine receptor opening.
Interestingly, the synthesis of the two signalling compounds cADPr and NAADP is managed by one enzyme family, the ADP-ribosyl cyclases [5]. These enzymes are highly conserved during evolution and are found in plants and sea urchin eggs [5]. Thus ADP-ribosyl cyclase activity has been identified in a variety of mammalian cell types and tissues [6–11]. Three members of the ADP-ribosyl cyclase family have been characterized in more detail and share overall sequence identity of approx. 30%: (i) the ADP-ribosyl cyclase from Aplysia californica (sea slug) ovotestis, (ii) the CD38 surface antigen, a marker for differentiation and activation of T- and B-lymphocytes, and (iii) CD157/BST-1 (bone marrow stromal cell antigen 1) [2,5,12–15]. Unlike the ADP-ribosyl cyclase from Aplysia californica, CD38 is an integral membrane protein. CD38 is not only found in lymphocytes and other haematopoetic cells, but is also widely distributed in tissues such as brain, smooth muscle and cardiac muscle [9,11,16,17]. Most of the CD38-specific activity found in various tissues was not retained in CD38-knockout mice, implicating that this is the major enzyme for cADPr and NAADP synthesis [11]. Nevertheless, small amounts of ADP-ribosyl cyclase activity were found in developing brain, with, interestingly, intracellular localization in CD38-knockout mice [16]. Experimental evidence accumulates to indicate that CD38-like activity is not restricted to the plasma membrane, but is also found in several intracellular organelles, including mitochondria, nuclei and other microsomal fractions [11,16,18–20].
At present, limited information is available on the regulation of ADP-ribosyl cyclase activity with intracellular localization. The fact that an ADP-ribosyl cyclase is co-enriched in the HSR (heavy sarcoplasmic reticulum) together with the ryanodine receptor, the target of NAADP action, highlights the question of whether or not this enzyme is related to the well characterized CD38 [4]. It is unclear whether the features identified for CD38, such as strict pH-dependence and substrate promiscuity, also fully account for the ADP-ribosyl cyclase enriched in the HSR.
In the present study, we show that the ADP-ribosyl cyclase from the skeletal muscle HSR is capable to form authentic cGDPr (cyclic GDP-ribose), cADPr and NAADP. We demonstrate that the co-substrate nicotinic acid channels the ADP-ribosyl cyclase affinity for NADP in a novel pH-dependent manner. The behaviour of the skeletal muscle ADP-ribosyl cyclase towards the bivalent cations and reducing agents is different from CD38 and CD157/BST-1, and highlights functional relevant cysteine residues in the catalytic site of the enzyme.
MATERIALS AND METHODS
Materials
NAD, NADP (nicotinamide–adenine dinucleotide phosphate), NAADP, NGD (nicotinamide–guanine dinucleotide), cADPr, cGDPr, nicotinic acid, nicotinamide and leupeptin were purchased from Sigma (St. Louis, MO, U.S.A.). Alternatively, NAD and NADP, as well as protease inhibitors were obtained from Roche Diagnostics (Vienna, Austria). All other reagents were of analytical grade.
Protein preparations and cell culture
HSR membranes were prepared from rabbit white skeletal muscle as described previously [4,21].
Jurkat T-lymphocytes were cultured in RPMI 1640 medium supplemented with newborn bovine calf serum (10%, v/v), Hepes (25 mM, pH 7.4), penicillin (100 units/ml) and streptomycin (50 μg/ml).
ADP-ribosyl cyclase activity
ADP-ribosyl cyclase activity was determined by an assay that relies on the fluorescence increase caused by the cyclization of NGD to form cGDPr [22]. cGDPr is resistant to hydrolysis, unlike cADPr. The reaction (final volume of 250–500 μl) was carried out in buffer A containing 20 mM Hepes/HCl (pH 7.35), 0.5 mM MgCl2, 0.45 mM CaCl2, 0.5 mM EGTA, 15 mM NaCl and 125 mM KCl. HSR (50–100 μg) was allowed to equilibrate for 2 min before the reaction was started with NGD at a concentration given in the Figure legends. The fluorescence intensity was monitored continuously at an excitation wavelength of 300 nm and an emission wavelength of 410 nm (using a F-4500 Hitachi fluorescence photometer). The fluorescence signal was calibrated with cGDPr. Drugs and compounds were added to give the indicated concentrations.
Synthesis of [32P]cADPr
The formation of [32P]cADPr from 1 mM [32P]NAD (6000 c.p.m./pmol) was carried out similarly to a procedure described previously [23,24]. Briefly, HSR (0.35–0.5 mg/ml) was incubated in buffer A. At the corresponding time points, 2 μl aliquots were spotted on to a poly(ethyleneimine)-cellulose membrane (Roche, Mannheim, Germany). The nucleotides were separated by TLC using a solvent containing 0.2 M NaCl and 30% ethanol. Under similar conditions, [32P]cADPr was synthesized by the purified ADP-ribosyl cyclase (5 μg/ml) from Aplysia californica (Sigma) and used as a standard. In order to ascertain the identity of [32P]cADPr, the nucleotide was exposed to heat or acidic pH which resulted in an almost complete destruction of the nucleotide. The dried TLC plates were exposed for autoradiography, and the corresponding radioactive spots were excised, counted and expressed as nmol of [32P]cADPr per mg.
Synthesis of NAADP
The base-exchange reaction was carried out similarly to the method previously described [4]. Briefly, buffer A was mixed with 0.5 mM NADP and 10 mM nicotinic acid. The pH of the mixture was adjusted to the corresponding value given in the respective Figure legends. The synthesis of NAADP was initiated by the addition of HSR membranes (0.25–0.5 mg/ml) and carried out for 30–90 min at 37 °C. Aliquots of the reaction were immediately loaded on to the HPLC system. Alternatively, the base-exchange reaction was carried out with the fluorescent nucleotide etheno-NADP in order to give etheno-NAADP.
HPLC analysis
Nucleotides from the base-exchange reaction were separated on an AG MP-1 column (4.6 mm×26 mm; Bio-Rad) using a Hitachi HPLC system similar to the method used in our previous work [4]. In brief, the column was equilibrated in 100% buffer A (water). The nucleotides were detected at 257 nm and eluted by a non-linear gradient of buffer B (150 mM trifluoroacetic acid) at a flow rate of 3 ml/min: 0–1 min, 2% buffer B; 1–2.5 min, 4% buffer B; 2.5–4.5 min, 8% buffer B; 4.5–6.5 min, 16% buffer B; 6.5–10 min, 32% buffer B; 10–11.5 min, 100% buffer B; and 11.5–14.5 min, 0% buffer B. The specific activity of NAADP formation was determined from the area under the curve of the NAADP peak (see Figure 3A). In the case of etheno-NAADP synthesis, a fluorescence detector (excitation, 300 nm, emission, 410 nm) was used to visualize the nucleotide. The identity of the NAADP peak was confirmed by comparison with the retention time of a commercial available NAADP standard, etheno-NAADP synthesized by Aplysia californica ADP-ribosyl cyclase, and by NMR and MS analysis.
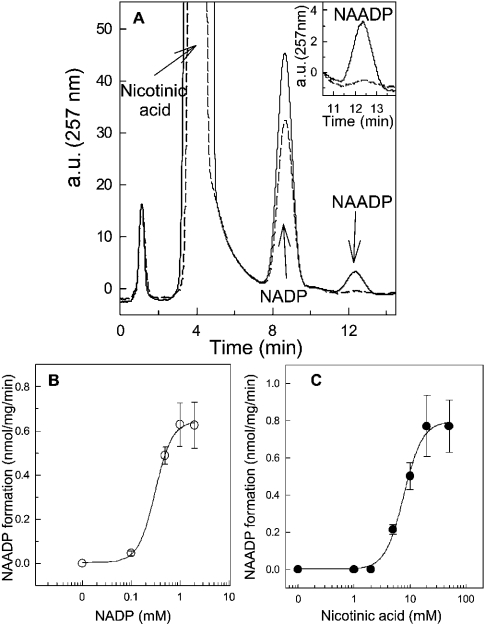
Figure 3. Synthesis of NAADP in HSR membranes.
(A) HPLC chromatograms of a typical synthesis of NAADP in the absence (broken line) and presence of HSR membranes (solid line; 0.5 mg/ml). The HSR was incubated with 0.5 mM NADP and 10 mM nicotinic acid for 45 min at 37 °C. The pH of the mixture was adjusted to 4.3 with ethanoic acid. The peaks for nicotinic acid, NADP and NAADP were indicated by arrows. The insert depicts a magnification of the elution profile of NAADP in the absence (broken line) and presence of HSR (solid line). a.u., arbitrary units. (B) Substrate-dependence of the base-exchange reaction. The reaction was carried out under similar conditions to those in (A) with increasing concentrations of NADP. (C) Formation of NAADP was measured in the presence of increasing concentrations of nicotinic acid and 0.5 mM NADP. The pH was kept constant at 4.3. Results are means±S.E.M. of a representative experiment carried out in duplicate.
1H NMR and MS
For NMR analysis, the presumptive NAADP peak from HPLC separation was collected, freeze-dried and re-dissolved in 2H2O. 1H NMR spectra were recorded on a Bruker Avance DPx200 at 200 MHz. MS was performed on a PE Sciex API QSTAR™ (applying electrospray ionization; samples were dissolved in acetonitrile) and on a Kratos Kompact SEQ [using the MALDI (matrix-assisted laser-desorption ionization) technique]. Samples for MALDI–TOF (time-of-flight) were dissolved in water and mixed with triethanolamine as a matrix for negative ion spectra. The masses of the presumptive NAADP and etheno-NAADP peak obtained from HPLC separation were identical with the respective mass of the nucleotides. The proton spectra of NAADP were identical with the spectra of the commercially available NAADP standard.
Western blot analysis
Whole-cell suspensions from Jurkat T-lymphocytes (3 μg) and HSR membranes (10 μg) were resolved on an SDS/12% polyacrylamide gel, and transferred on to a nitrocellulose membrane (pore size, 0.45 μm; Schleicher & Schuell, Dassel, Germany). Quantitative transfer of the proteins was controlled with Ponceau S staining of the nitrocellulose membrane and Coomassie Blue staining of the gel. For immunodetection, the membrane was blocked with 4% (w/v) BSA and exposed to antibodies against CD38 [AT-1, N-17 and M-19 (Santa Cruz), and Ab-3 (Neomarkers, Union City, CA, U.S.A.)] at a dilution of 1:500. The first antibodies were incubated overnight at 4 °C, and secondary antibodies (1:2500 or 1:6000) were incubated for 1 h at room temperature (23 °C). Appropriate secondary antibodies conjugated to horseradish peroxidase enabled detection by the SuperSignal enhanced chemiluminescence detection system (Pierce, Rockford, IL, U.S.A.).
Miscellaneous procedures
Curve-fitting was carried out using the standard Maquardt–Levenberg algorithm provided by Sigma plot (Jandel, San Rafael, CA, U.S.A.). Statistical analysis was carried out using Student's t test, and for multiple comparisons by ANOVA and post hoc Scheffe's test. Experiments were carried out at least three times in duplicate and results are presented as means±S.D., unless otherwise stated.
RESULTS
Cyclization and base-exchange reaction of the ADP-ribosyl cyclase from sarcoplasmic reticulum
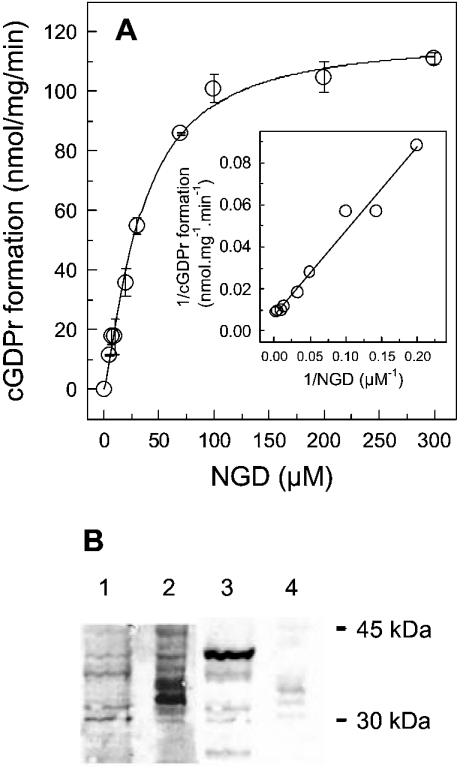
A reliable assay to monitor the cyclization of ADP-ribosyl cyclase is to use NGD as a substrate. Upon cyclization, the fluorescent product cGDPr is generated (Figure 1). Since neither NGD nor GDP-ribose share this fluorescent behaviour, this experimental approach is ideal to continuously monitor ADP-ribosyl cyclase activity, and to discriminate from unrelated NADases [5]. The dependence of the cyclization reaction on NGD is shown in Figure 1. The Vmax calculated from the saturation isotherm gives a value of 118.1±7.4 nmol·mg−1·min−1 with a Km of 33.4±5.3 μM. These values are broadly in line with previously determined parameters with different HSR preparations [4]. This ADP-ribosyl cyclase activity is not due to CD38 contamination found in the HSR preparation (Figure 1B). As CD38 from Jurkat T-lymphocytes is clearly responsive to anti-CD38 antibodies in the HSR, almost no signal was detectable, as exemplified for anti-CD38 antibody N-17 in Figure 1(B). Similar results were obtained with other anti-CD38 antibodies (results not shown). In the HSR, no bands were visualized at the molecular mass of interest; however, faint bands are visible in the 35 kDa region (lane 4 in Figure 1B). At higher dilutions of the secondary antibody, these bands were not seen, while CD38 in Jurkat T-lymphocytes was still reactive. The hardly detectable bands in the HSR were therefore attributed as unspecific. Overall, from these results, the conclusion is drawn that the similarity between CD38 and the HSR ADP-ribosyl cyclase is low.
Figure 1. Saturation isotherm of cGDPr formation in HSR membranes and Western blot analysis.
(A) HSR (60 μg) was equilibrated in buffer A for 2 min at room temperature. Subsequently, NGD was added at the concentrations indicated to initiate the formation of cGDPr. The slope of the increment in fluorescence intensity was calculated by linear regression from time intervals of 5–10 min. The insert depicts the Lineweaver–Burk transformation of the saturation isotherm. Results are means±S.E.M. of duplicates. The experiment was repeated twice with different HSR preparations and gave similar results. (B) Whole-cell lysates from Jurkat T-lymphocytes (3 μg) (lanes 1 and 3) and HSR membranes (10 μg) (lanes 2 and 4) were resolved by SDS/12% PAGE and transferred on to a nitrocellulose membrane. The proteins were stained with Ponceau S (lanes 1 and 2), and Western blot analysis was carried out as described under the Materials and methods section with a specific anti-CD38 antiserum (N-17) (lanes 3 and 4). The molecular mass standards (kDa) are indicated at the right.
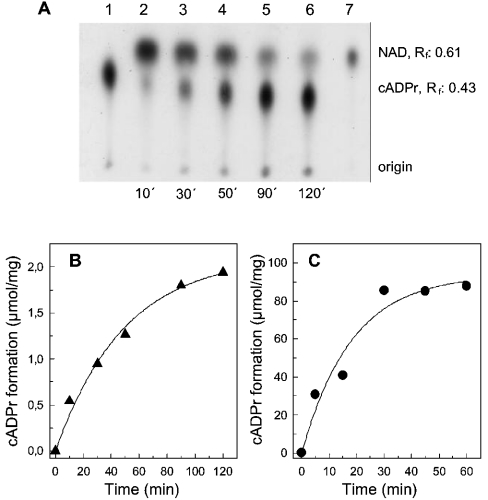
The ADP-ribosyl cyclase from sarcoplasmic reticulum is also capable of forming cADPr (Figure 2). Using a TLC assay similar to that described by Graeff et al. [24], we resolved the substrate [32P]NAD from the product [32P]cADPr, which were visualized by autoradiography (Figure 2A). The formation of authentic [32P]cADPr was confirmed by the following controls. First, the relative motility obtained in our system was comparable with that given in the literature under similar conditions [24]. Secondly, the product was highly sensitive to acidic treatment and heating (15 min exposure to 95 °C or 1 M HCl). Under both conditions, the [32P]cADPr spot on the autoradiograph was hardly detectable, while degradation products occurred at the origin and running front of the TLC (results not shown). Thirdly, for comparison, the cyclization reaction was also carried out with the purified ADP-ribosyl cyclase from Aplysia californica (Figure 2C). This approach was also used to obtain a standard for [32P]cADPr (Figure 2A, lane 1).
Figure 2. Synthesis of [32P]cADPr by the ADP-ribosyl cyclase from Aplysia californica and HSR.
(A) HSR membranes (0.35 mg/ml) were incubated with [32P]NAD in buffer A. At the indicated time points, aliquots of the reaction (3 μl) were transferred to a poly(ethyleneimine)-coated plastic sheet (lanes 2–6). The [32P]cADPr standard was generated by incubation with the purified ADP-ribosyl cyclase (5 μg/ml) from Aplysia californica (lane 1). An aliquot (1 μl) of the assay mix without protein is depicted in lane 7 and represents [32P]NAD. The autoradiography of the radioactive TLC is shown. The relative motility (Rf) is indicated for [32P]NAD and [32P]cADPr. (B and C) Enzyme kinetics in the presence of HSR (0.35 mg/ml) and the purified ADP-ribosyl cyclase (5 μg/ml) from Aplysia californica respectively. The amount of [32P]cADPr synthesis was determined by liquid-scintillation counting of the radioactive [32P]cADPr spots identified by autoradiography similar to that given in (A). Results are means of duplicates of an experiment that was repeated.
Fitting the data from Figures 2(B) and 2(C) to a mono-exponential raise gave a half-life of 34.5±6.9 and 12.6±4.8 min for the ADP-ribosyl cyclase from the HSR and the purified enzyme from Aplysia respectively. In comparison, the catalytic activity of the cyclization reaction with NGD (Vmax, 118.1±7.4 nmol of cGDPr·mg−1·min−1; Figure 1) is approx. 4-fold higher than that obtained from the initial linear part of the kinetics given for [32P]cADPr in Figure 2(B) (33.8±6.1 nmol of [32P]cADPr·mg−1·min−1). Interestingly, the later time points in the kinetic provided in Figure 2(A) depicted an increment in radioactivity of the origin, which may be indicative for the hydrolytic product, [32P]ADPr [24]. For this reason, the following cyclization experiments were carried out with NGD to form cGDPr, as this nucleotide is resistant to enzymic hydrolysis [22].
Beside the cyclization reaction, ADP-ribosyl cyclases have been described to catalyse a base-exchange reaction [2,3,5,10]. Similar to our previous observations, authentic NAADP can be detected when HSR membranes were incubated with NADP and nicotinic acid (Figure 3). We can exclude the possibility that NAADP was synthesized by a chemical mechanism, since in the absence of HSR, an NAADP peak was not detectable (Figure 3A, inset). The production of NAADP was strictly dependent on the concentration of NADP (Figure 3B) and nicotinic acid (Figure 3C). Synthesis of NAADP was hardly detected in some tissues or cell lines. In the literature, variations in the detection of the base-exchange reactions may be due to our observation that the commercially available NADP contains an inhibitory component [11]. Above 4–5 mM NADP, a further increment in the substrate concentration results in successively decreased NAADP synthesis. This inhibitory component could be successfully removed by re-chromatography of NADP (results not shown). Using 10 mM re-chromatographed NADP under the conditions given in Figure 3(B), the base-exchange reaction proceeded with 0.72±0.1 nmol of NAADP·mg−1·min−1, which is virtually identical with the plateau reached with commercially available NADP. The apparent Km for commercially available NADP determined from Figure 3(B) was 0.31±0.04 mM with a Hill coefficient of 2.4±0.58. Nicotinic acid propagated the formation of NAADP with an EC50 of 7.7±0.5 mM (Figure 3C). This concentration–response curve is also very steep, which is reflected in a Hill coefficient of 2.6±0.3. Thus this positive co-operativity, documented by the steep concentration–response curves may be indicative of enzyme dimerization (Figures 3B and 3C) [5].
Dependence of ADP-ribosyl cyclase activity on pH
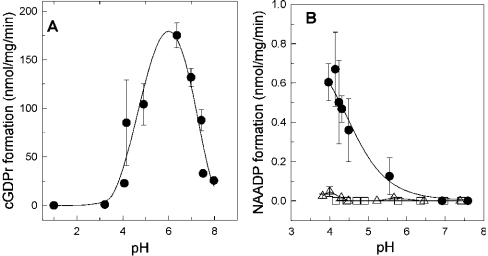
The ADP-ribosyl cyclase from sea urchin eggs and CD38 synthesize cADPr and NAADP in a pH-dependent manner [11,24]. The ADP-ribosyl cyclase in skeletal muscle described in the present paper gave a bell-shaped curve with an optimum at a pH of 6.2 for the cyclization reaction with NGD (Figure 4A). This is surprising, since the cyclization reaction has been shown to reach a plateau at a pH between 7–8 [10,24]. The base-exchange reaction is also sensitive to pH, but promoted at acidic conditions below a pH of 5 (Figure 4B). As a control, in the absence of nicotinic acid, the nucleotide NAADP was not detectable and therefore a chemical mechanism of NAADP production under the depicted pH conditions can be excluded (Figure 4B, open squares). Even sub-threshold concentrations of 1 mM nicotinic acid were not sufficient to stimulate the enzyme significantly (compare with Figure 3C and Figure 4B, open triangles). Increasing the substrate concentration from 0.5 mM NADP to 5 mM NADP (both in the presence of 10 mM nicotinic acid) had no influence on the half maximum pH threshold for NAADP synthesis (pH 4.7±0.35 compared with 4.3±0.12 respectively; results not shown).
Figure 4. pH-dependence of the ADP-ribosyl cyclase activity.
(A) HSR (60 μg) was incubated at the indicated pH. The cyclization reaction was initiated by the addition of 100 μM NGD. (B) Measuring the pH-dependence of the base-exchange reaction was carried out with HSR membranes (100 μg) supplemented with 0.5 mM NADP (□) or plus 1 mM (▵) or 10 mM (•) nicotinic acid. Results are means±S.D. for three experiments.
Substrate specificity of the skeletal muscle ADP-ribosyl cyclase
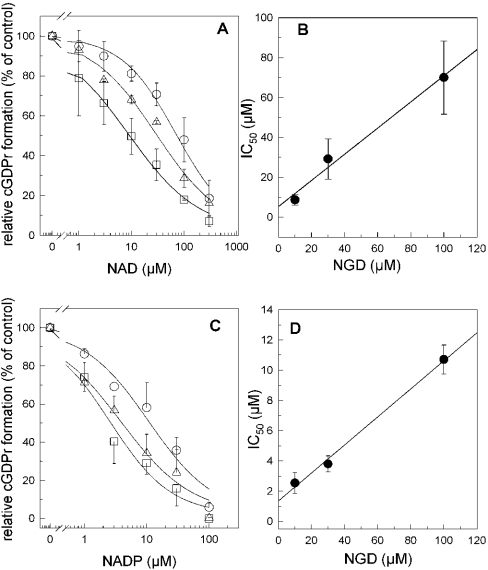
In order to determine the substrate specificity of the skeletal muscle ADP-ribosyl cyclase we have measured cGDPr formation in the presence of increasing concentrations of alternative substrates such as NAD and NADP (Figure 5). From the linear regressions of the IC50 values obtained with increasing concentrations of NGD, we could estimate the Ki values of 5.4 μM (r2=0.98) and 1.3 μM (r2=0.99) for NAD and NADP respectively (Figures 5B and 5D). For comparison, the reported Km value of CD38 is 14 μM NAD [22]. These data show a 4-fold higher potency of NADP to inhibit the enzyme compared with NAD. However, these competition experiments were carried out in the absence of nicotinic acid. We have therefore investigated the role of the co-substrate nicotinic acid as a possible regulator of the NADP affinity.
Figure 5. Substrate competition of the cyclization reaction.
(A) Increasing concentrations of NAD were allowed to compete with 100 μM (○), 30 μM (▵) or 10 μM (□) NGD under conditions as described in the Materials and methods section. The respective curves were fitted to the Hill equation. (B) IC50 values obtained from (A) were plotted against the corresponding NGD concentration, and the data were fitted to a linear regression. (C) similar to (A) but with increasing concentrations of NADP competed with 100 μM (○), 30 μM (▵) or 10 μM (□) NGD. (D) IC50 values obtained from (C) were plotted against the corresponding NGD concentration, analogous to (B). Results are means±S.D. for three experiments.
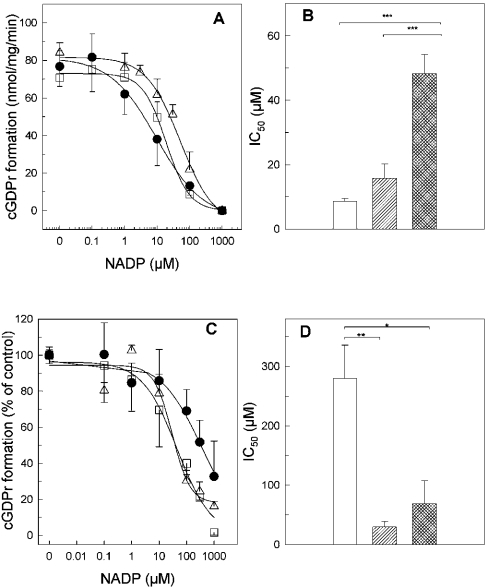
As depicted in Figure 6, at pH 7.35, the potency of NADP to compete with NGD is shifted to the right by increasing the concentration for nicotinic acid (Figures 6A and 6B). This shift in the IC50 values is not significant with 1 mM nicotinic acid (control, 8.6±0.85 μM; +1 mM nicotinic acid, 15.8±4.4 μM; n=3–4), but became highly significant with 10 mM nicotinic acid (48.2±5.9 μM, n=3; P<0.001, ANOVA and post hoc Scheffe's test). Under acidic buffer conditions, an IC50 shift was observed in the opposite direction (Figures 6C and 6D). At pH 5.5, nicotinic acid increased the affinity for NADP significantly (Figures 6C and 6D).
Figure 6. Nicotinic acid regulates the apparent affinity of NADP in a pH-dependent manner.
HSR membranes (100 μg) were incubated in buffer A at a constant pH of 7.35 (A and B) or 5.5 (C and D). The formation of cGDPr was measured with increasing concentrations of NADP in the absence (•), or presence of 1 mM (□) or 10 mM (▵) nicotinic acid (A and C). The IC50 values obtained from three experiments similar to that in (A and C) are summarized in (B and D), in the absence (white bar), or presence of 1 mM (hatched squares) or 10 mM (cross-hatched) nicotinic acid. Statistical significance between groups (B and D) was determined by ANOVA and post hoc Scheffe's test and indicated by brackets (***P<0.001; **P<0.01; *P<0.05).
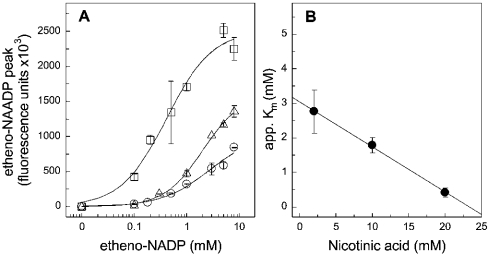
The same was true when the base-exchange reaction was measured directly at a fixed pH of 4.3 in the presence of increasing concentrations of nicotinic acid and etheno-NADP (Figure 7). Nicotinic acid gradually increased the affinity for etheno-NADP as depicted in Figure 7(B). However, the apparent Km for etheno-NADP in the absence of nicotinic acid extrapolates to 3 mM. Compared with the affinity for NADP determined indirectly by competition with NGD, this value is lower by one order of magnitude (compare with Figures 6C and 6D).
Figure 7. Nicotinic acid increases the affinity for etheno-NADP and augments the efficacy of the base-exchange reaction.
(A) Concentration–response curves for etheno-NAADP formation in the presence of 2 mM (○), 10 mM (▵) or 20 mM nicotinic acid (□). Similar to the conditions described in Figure 3, the formation of etheno-NAADP from nicotinic acid and etheno-NADP was determined with HPLC. The HSR (0.5 mg/ml) was incubated with etheno-NADP and nicotinic acid for 60 min at 37 °C. The pH of the mixture was adjusted to 4.3. In (B), the apparent (app.) Km calculated from the concentration–response curves given in (A) were plotted against the respective nicotinic acid concentration. The linear regression reveals an intercept with the y-axis at 3.05 mM (r2=0.99). Results are means±S.D. for a representative experiment carried out in duplicate.
Regulation of the ADP-ribosyl cyclase activity by nicotinic acid and nicotinamide
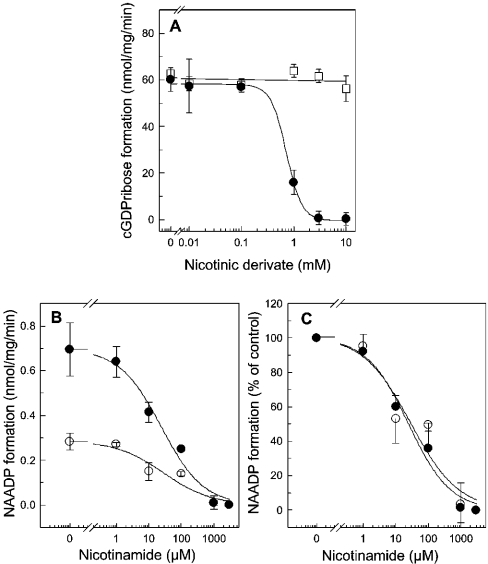
In order to draw the conclusion that nicotinic acid is an allosteric regulator of NADP binding, one has to exclude the possibility that nicotinic acid has no effect on the cyclization reaction. As expected, it is clear from Figure 8(A) that nicotinic acid up to 10 mM had no effect on the formation of cGDPr (Figure 8A, open squares). This confirms the conjecture that nicotinic acid indeed regulates the skeletal muscle ADP-ribosyl cyclase allosterically. In contrast, nicotinamide, the side product of the cyclization reaction inhibited the formation of cGDPr completely with an IC50 of 0.72±0.16 mM at pH 7.35 (Figure 8A). The base-exchange reaction was also inhibited by nicotinamide (Figure 8B). However, this inhibition was not due to a competition with the co-substrate nicotinic acid, as can be seen for the normalized concentration–response curves (Figure 8C, 5 and 15 mM nicotinic acid, open and filled circles respectively). These are superimposable with IC50 values of 24.7±1.6 and 29.5±5.0 μM. Apparently, the base-exchange reaction was 25–30-fold more sensitive towards inhibition by nicotinamide than the cyclization reaction (Figure 8B).
Figure 8. Inhibition of the ADP-ribosyl cyclase activity by nicotinamide.
(A) HSR membranes (100 μg) were incubated with buffer A, and the synthesis of cGDPr was initiated by the addition of 100 μM NGD. The cyclization reaction was measured as described in the Material and methods section in the presence of increasing concentrations of nicotinamide (•) or nicotinic acid (□). In all samples, the pH was adjusted to 7.35. (B) The base-exchange reaction was monitored in the presence of 0.5 mM NADP and 5 mM (○) or 15 mM (•) nicotinic acid. HSR membranes (100 μg) were incubated for 60 min at pH 4.3, and the production of NAADP was quantified by HPLC. (C) Normalized data from (B), expressed as means±S.D. for multiple determinations (n=3–7).
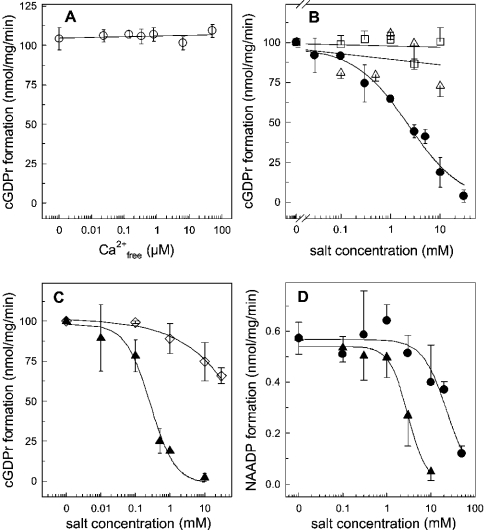
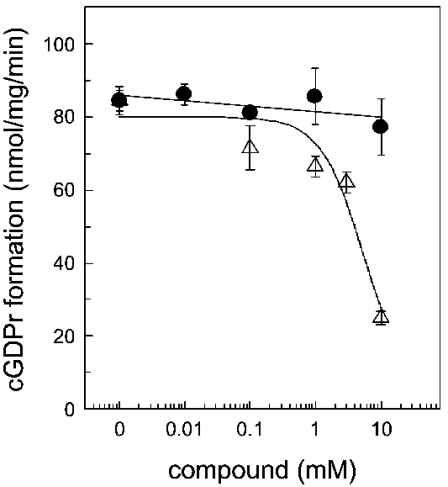
In order to identify ionic regulators of the skeletal muscle ADP-ribosyl cyclase, we have focused on various bivalent cations (Figure 9). Since Ca2+ plays an essential role in skeletal muscle contraction–relaxation cycles, the free Ca2+ concentration in the assay buffer may contribute to ADP-ribosyl cyclase regulation in the HSR. However, the cyclization reaction was insensitive to free Ca2+ concentration in the range 20 nM–100 μM (Figure 9A). As expected, the enzyme was also not regulated by Mg2+. It is worthy of mention that BST-1 is activated by Mn2+, while CD38 was insensitive to this metal ion [13]. In our system, we found that the HSR ADP-ribosyl cyclase is insensitive to Mn2+ (Figure 9B). The bivalent cations Zn2+ and Cu2+ were both shown to activate the CD38 and BST-1 ADP ribosyl cyclases [23]. This was clearly not the case for the skeletal muscle isoform. The formation of cGDPr in the HSR was inhibited with an IC50 of 2.3±1.1 μM Zn2+ and 0.27±0.07 μM Cu2+ (Figures 9B and 9C). Zn2+ and Cu2+ also inhibited the base-exchange reaction (Figure 9D). Similar to the cyclization reaction, the rank order of potency between Zn2+ and Cu2+ was retained; however, the IC50 values were shifted to the right, 23.3±9.5 μM and 3.03±0.3 μM respectively (Figure 9D). In order to control for the ionic strength under these experimental conditions, potassium phosphate was added, but had no significant effect at the relevant concentrations (Figure 9C). The CD38 and Aplysia californica ADP-ribosyl cyclases contain ten conserved cysteine residues. Deriving information from the crystal structure of the Aplysia ADP-ribosyl cyclase and site-directed mutagenesis of the catalytic centre of the enzyme highlights the importance of these residues to form disulphide bridges [25,26]. Treatment of CD38 with reducing agents has been described to inhibit ADP-ribosyl cyclase activity [26,27]. The ADP-ribosyl cyclase from skeletal muscle HSR was not sensitive to the sulphydryl-side chain-reducing agent, 1,4-dithiothreitol (Figure 10). Conversely, NEM (N-ethylmaleimide), a thiol-alkylating reagent, reduced the formation of cGDPr in a concentration-dependent manner (IC50 5.1±1.2 mM; Figure 10, open triangles). Thus a free cysteine residue is essentially participating in the catalytic activity of the skeletal muscle ADP-ribosyl cyclase.
Figure 9. ADP-ribosyl cyclase activity in the presence of various ions.
HSR membranes (100 μg) were incubated in buffer A (pH 7.35) and the cyclization reaction was initiated by 100 μM NGD. (A) Enzymic formation of cGDPr was determined at various free Ca2+ concentrations, which were adjusted by the ratio of EGTA/CaCl2, according to the algorithm provided by Schoenmakers and co-workers [36]. (B) Formation of cGDPr in buffer A (pH 7.35) supplemented with ZnCl2 (•), MgCl2 (▵) or MnCl2 (□). (C) Similar to (B), the formation of cGDPr was measured in the presence of CuSO4 (▴) or potassium phosphate adjusted to pH 7.35 (□). Two experiments were pooled and cGDPr formation under control conditions was set 100% (102.8 nmol of cGDPr·mg−1·min−1). (D) The base-exchange reaction was determined in the presence of 0.5 mM NADP, 10 mM nicotinic acid, 100 μg of HSR membranes in buffer A adjusted to pH 4.3. The synthesis of NAADP was inhibited by increasing concentrations of CuSO4 (▴) and ZnCl2 (•). Results are means±S.D. for duplicates of representative experiments which were repeated twice.
Figure 10. NEM inhibits the cyclization reaction of the ADP-ribosyl cyclase.
Under conditions described in the Materials and methods section, the formation of cGDPr was monitored in the presence of increasing concentrations of dithiothreitol (•) or NEM (▵). Before NGD, the HSR was pre-incubated with NEM on ice for 15 min. Results are means±S.E.M. for duplicates of a representative experiment.
DISCUSSION
ADP-ribosyl cyclase activity was first described in sea urchin eggs and thereafter in various mammalian tissues (reviewed in [3,5]). The prototypical mammalian representatives of this enzyme family are the type II transmembrane glycoprotein CD38 and CD157/BST-1, a surface antigen on bone marrow cells [13,15]. For the first instance, CD38 was described as a multifunctional ecto-enzyme in lymphocytes capable of forming the potent Ca2+-mobilizing products cADPr and NAADP [10,28]. Breaking with the initial observation that CD38-like ADP-ribosyl cyclase activity is targeted to the plasma membrane, experimental evidence accumulates that intracellular compartments such as sarcoplasmic reticulum, mitochondria, cytosol or nucleus may also contain the enzymic equipment for cADPr or NAADP synthesis [9,11,16,18–20,29]. In the present study, we demonstrate that the enzyme that generates these nucleotides is present as a membrane-bound ADP-ribosyl cyclase in the sarcoplasmic reticulum. Our results provide evidence that a novel ADP-ribosyl cyclase in skeletal muscle HSR is clearly distinguishable in many aspects from CD38 and CD157/BST-1 proteins. First, the ADP-ribosyl cyclase in skeletal muscle HSR is not detected by antibodies against CD38 (Figure 1B). Moreover, both reactions (the cyclization of cADPr and the base-exchange reaction to give NAADP) follow a tightly regulated pH-dependent switch mechanism. Authentic cADPr and NAADP are synthesized by the ADP-ribosyl cyclase from HSR (Figures 2 and 3). The cyclization reaction is described by a bell-shaped curve with an optimum at pH 6.2. At alkaline pH, the cyclization reaction is successively decreased. Such behaviour has recently been described for ADP-ribosyl cyclases in rat ventricular myocyte membranes and brain fractions from CD38 negative mice [16,30]. In contrast, CD38, and the ADP-ribosyl cyclase from Aplysia and sea urchin eggs support the cyclization reaction with a plateau in the pH range 7–8 [10,24]. Although the sensitivity of the cyclization reaction towards pH differs between the stated ADP-ribosyl cyclase isoforms, the base-exchange reaction works best at a pH below 5 in all ADP-ribosyl cyclases (Figure 4B) [3,5].
We could also elucidate a novel pH-dependent switch mechanism of the ADP-ribosyl cyclase by nicotinic acid (Figure 6). In a dose-dependent manner, nicotinic acid decreased the affinity for NADP at alkaline pH. Conversely, under acidic conditions, the affinity for NADP was significantly increased by nicotinic acid (Figures 6B and 7). The co-substrate nicotinic acid is therefore not only a prerequisite for the base-exchange reaction, but also an allosteric regulator of this catalytic pathway by enhancing or reducing the affinity for NADP, depending on the pH of the environment. From these observations, one may conjecture that binding of nicotinic acid channels the enzyme for the base-exchange reaction, which is corroborated by the fact that nicotinic acid had no direct effect on the cyclization reaction at alkaline pH (Figure 8A). Additionally, the binding site for nicotinic acid is distinct from the inhibitory binding site of nicotinamide. This assumption is confirmed by two observations. First, the potency of nicotinamide to inhibit the base-exchange reaction was not shifted in the presence of various concentrations of nicotinic acid (Figure 8). Secondly, although the base-exchange reaction was carried out in the presence of nicotinic acid, it was approx. 30-fold more sensitive to nicotinamide-induced inhibition compared with the cyclization reaction.
Interestingly, several features of the brain ADP-ribosyl cyclase found in CD38-negative mice fit to the enzyme characterization that we observed in the HSR: intracellular localization, optimal cyclization at pH 6.0 and inhibition by zinc [16]. The cyclization and the base-exchange reaction of the skeletal muscle ADP-ribosyl cyclase were potently inhibited by Zn2+ and Cu2+. In contrast, CD38 or CD157/BST-1 was activated by Zn2+ [9,23]. It is worthy of mention that Zn2+ is stored in neurotransmitter vesicles in the central nervous system and in motor neurons [31]. It has also been shown that Zn2+ permeates the skeletal muscle nicotinic acetylcholine receptor and is thus available for intracellular Zn2+-binding proteins [32]. It is unclear at the moment if the ADP-ribosyl cyclase of the HSR is a direct target for Zn2+ via a cation-binding site or indirectly via an accessory Zn2+-binding protein. Nevertheless, the primary sequence of CD38 does not provide similarity to a typical zinc finger, zinc cluster or zinc twist motif [33]. Possibly, Zn2+ may bind to cysteine residues. This conjecture is corroborated by our finding that at least one cysteine residue is accessible for NEM, which results in an inhibition of the enzyme (Figure 10). According to sequence homology, structural analogy between the Aplysia ADP-ribosyl cyclase and CD38 was obtained, and highlighted ten conserved cysteine residues that form disulphide bonds [25,26]. Such cysteine residues do not participate in the cyclization reaction of the ADP-ribosyl cyclase of the HSR, as the reducing agent dithiothreitol had no effect in the tested concentration range (Figure 10). Taken together, despite the overall similarities, such as the cyclization and the base-exchange reaction, the ADP-ribosyl cyclase from the HSR is divergent from CD38 and CD157/BST-1 as we have described in pharmacological terms.
The question remains if in skeletal muscle physiological conditions exist which support a decrease in pH so that the base-exchange reaction is favoured? In mammalians, predominantly, two skeletal muscle fibre types exist: slow twitch fibres (type I) and fast twitch fibres (type II). Under exercise conditions, the slow twitch fibres use aerobic energy sources, while the fast twitch fibres are better equipped for anaerobic glycolysis. The latter metabolic situation leads to the production of protons and consequently lowers the pH in these fibres [34]. In healthy volunteers already under conditions of 2–10 min of exercise, using 31P-NMR, significant falls in pH were detected in the tibial anterior muscle [35]. The pH values at the end of 30% and 60% of maximal voluntary contraction were 6.69 and 6.28 respectively. In individual measurements, a fall in pH to 6.0 was reached. The HSR we used within the present study was prepared from white rabbit skeletal muscle, which is consistent with fast twitch muscle fibres (type II). We therefore conclude that under conditions of physiological exercise, the pH may fall to acidic values that are sufficient to support a switch in ADP-ribosyl cyclase activity from the cyclization to the base-exchange reaction, which is supported by the co-substrate nicotinic acid.
Acknowledgments
We thank Anton Karel for technical assistance, and Dr Michael Freissmuth and Dr Lukas Weigl for helpful discussion. This work was supported by the Fonds zur Förderung der wissenschaftlichen Forschung (P-14940 to M.H.).
References
- 1.Berridge M. J. Inositol trisphosphate and calcium signaling. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Lee H. C. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 3.Guse A. H. Cyclic ADP-ribose: a novel Ca2+-mobilising second messenger. Cell. Signalling. 1999;11:309–316. doi: 10.1016/s0898-6568(99)00004-2. [DOI] [PubMed] [Google Scholar]
- 4.Hohenegger M., Suko J., Gscheidlinger R., Drobny H., Zidar A. Nicotinic acid–adenine dinucleotide phosphate activates the skeletal muscle ryanodine receptor. Biochem. J. 2002;367:423–431. doi: 10.1042/BJ20020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H. C. Enzymatic functions and structures of CD38 and homologs. Chem. Immunol. 2000;75:39–59. doi: 10.1159/000058774. [DOI] [PubMed] [Google Scholar]
- 6.Rusinko N., Lee H. C. Widespread occurrence in animal tissues of an enzyme catalyzing the conversion of NAD+ into a cyclic metabolite with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 1989;264:11725–11731. [PubMed] [Google Scholar]
- 7.Lee H. C., Graeff R., Walseth T. F. Cyclic ADP-ribose and its metabolic enzymes. Biochimie. 1995;77:345–355. doi: 10.1016/0300-9084(96)88145-4. [DOI] [PubMed] [Google Scholar]
- 8.Kim H., Jacobson E. L., Jacobson M. K. Synthesis and degradation of cyclic ADP-ribose by NAD glycohydrolases. Science. 1993;261:1330–1333. doi: 10.1126/science.8395705. [DOI] [PubMed] [Google Scholar]
- 9.de Toledo F. G., Cheng J., Liang M., Chini E. N., Dousa T. P. ADP-ribosyl cyclase in rat vascular smooth muscle cells: properties and regulation. Circ. Res. 2000;86:1153–1159. doi: 10.1161/01.res.86.11.1153. [DOI] [PubMed] [Google Scholar]
- 10.Aarhus R., Graeff R. M., Dickey D. M., Walseth T. F., Lee H. C. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J. Biol. Chem. 1995;270:30327–30333. doi: 10.1074/jbc.270.51.30327. [DOI] [PubMed] [Google Scholar]
- 11.Chini E. N., Chini C. C., Kato I., Takasawa S., Okamoto H. CD38 is the major enzyme responsible for synthesis of nicotinic acid–adenine dinucleotide phosphate in mammalian tissues. Biochem. J. 2002;362:125–130. doi: 10.1042/0264-6021:3620125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessio M., Roggero S., Funaro A., De Monte L. B., Peruzzi L., Geuna M., Malavasi F. CD38 molecule: structural and biochemical analysis on human T lymphocytes, thymocytes, and plasma cells. J. Immunol. 1990;145:878–884. [PubMed] [Google Scholar]
- 13.Itoh M., Ishihara K., Tomizawa H., Tanaka H., Kobune Y., Ishikawa J., Kaisho T., Hirano T. Molecular cloning of murine BST-1 having homology with CD38 and Aplysia ADP-ribosyl cyclase. Biochem. Biophys. Res. Commun. 1994;203:1309–1317. doi: 10.1006/bbrc.1994.2325. [DOI] [PubMed] [Google Scholar]
- 14.Dong C., Willerford D., Alt F. W., Cooper M. D. Genomic organization and chromosomal localization of the mouse Bp3 gene, a member of the CD38/ADP-ribosyl cyclase family. Immunogenetics. 1996;45:35–43. doi: 10.1007/s002510050164. [DOI] [PubMed] [Google Scholar]
- 15.States D. J., Walseth T. F., Lee H. C. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem. Sci. 1992;17:495. doi: 10.1016/0968-0004(92)90337-9. [DOI] [PubMed] [Google Scholar]
- 16.Ceni C., Muller-Steffner H., Lund F., Poochon N., Schweitzer A., De Waard M., Schuber F., Villaz M., Moutin M. J. Evidence for an intracellular ADP-ribosyl cyclase/NAD+-glycohydrolase in brain from CD38 deficient mice. J. Biol. Chem. 2003;278:40670–40678. doi: 10.1074/jbc.M301196200. [DOI] [PubMed] [Google Scholar]
- 17.Meszaros L. G., Wrenn R. W., Varadi G. Sarcoplasmic reticulum-associated and protein kinase C-regulated ADP-ribosyl cyclase in cardiac muscle. Biochem. Biophys. Res. Commun. 1997;234:252–256. doi: 10.1006/bbrc.1997.6620. [DOI] [PubMed] [Google Scholar]
- 18.Liang M., Chini E. N., Cheng J., Dousa T. P. Synthesis of NAADP and cADPR in mitochondria. Arch. Biochem. Biophys. 1999;371:317–325. doi: 10.1006/abbi.1999.1463. [DOI] [PubMed] [Google Scholar]
- 19.White T. A., Johnson S., Walseth T. F., Lee H. C., Graeff R. M., Munshi C. B., Prakash Y. S., Sieck G. C., Kannan M. S. Subcellular localization of cyclic ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities in porcine airway smooth muscle. Biochim. Biophys. Acta. 2000;1498:64–71. doi: 10.1016/s0167-4889(00)00077-x. [DOI] [PubMed] [Google Scholar]
- 20.Sternfeld L., Krause E., Guse A. H., Schulz I. Hormonal control of ADP-ribosyl cyclase activity in pancreatic acinar cells from rats. J. Biol. Chem. 2003;278:33629–33636. doi: 10.1074/jbc.M301043200. [DOI] [PubMed] [Google Scholar]
- 21.Klinger M., Freissmuth M., Nickel P., Stäbler-Schwarzbart M., Kassack M., Suko J., Hohenegger M. Suramin and suramin analogs activate skeletal muscle ryanodine receptor via a calmodulin binding site. Mol. Pharmacol. 1999;55:462–472. [PubMed] [Google Scholar]
- 22.Graeff R. M., Walseth T. F., Fryxell K., Branton W. D., Lee H. C. Enzymatic synthesis and characterizations of cyclic GDP-ribose: a procedure for distinguishing enzymes with ADP-ribosyl cyclase activity. J. Biol. Chem. 1994;269:30260–30267. [PubMed] [Google Scholar]
- 23.Kukimoto I., Hoshino S., Kontani K., Inageda K., Nishina H., Takahashi K., Katada T. Stimulation of ADP-ribosyl cyclase activity of the cell surface antigen CD38 by zinc ions resulting from inhibition of its NAD+ glycohydrolase activity. Eur. J. Biochem. 1996;239:177–182. doi: 10.1111/j.1432-1033.1996.0177u.x. [DOI] [PubMed] [Google Scholar]
- 24.Graeff R. M., Franco L., De Flora A., Lee H. C. Cyclic GMP-dependent and -independent effects on the synthesis of the calcium messengers cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate. J. Biol. Chem. 1998;273:118–125. doi: 10.1074/jbc.273.1.118. [DOI] [PubMed] [Google Scholar]
- 25.Munshi C., Aarhus R., Graeff R., Walseth T. F., Levitt D., Lee H. C. Identification of the enzymatic active site of CD38 by site-directed mutagenesis. J. Biol. Chem. 2000;275:21566–21571. doi: 10.1074/jbc.M909365199. [DOI] [PubMed] [Google Scholar]
- 26.Tohgo A., Takasawa S., Noguchi N., Koguma T., Nata K., Sugimoto T., Furuya Y., Yonekura H., Okamoto H. Essential cysteine residues for cyclic ADP-ribose synthesis and hydrolysis by CD38. J. Biol. Chem. 1994;269:28555–28557. [PubMed] [Google Scholar]
- 27.Guida L., Franco L., Zocchi E., De Flora A. Structural role of disulfide bridges in the cyclic ADP-ribose related bifunctional ectoenzyme CD38. FEBS Lett. 1995;368:481–484. doi: 10.1016/0014-5793(95)00715-l. [DOI] [PubMed] [Google Scholar]
- 28.Franco L., Zocchi E., Usai C., Guida L., Bruzzone S., Costa A., De Flora A. Paracrine roles of NAD+ and cyclic ADP-ribose in increasing intracellular calcium and enhancing cell proliferation of 3T3 fibroblasts. J. Biol. Chem. 2001;276:21642–21648. doi: 10.1074/jbc.M010536200. [DOI] [PubMed] [Google Scholar]
- 29.Adebanjo O. A., Anandatheerthavarada H. K., Koval A. P., Moonga B. S., Biswas G., Sun L., Sodam B. R., Bevis P. J., Huang C. L., Epstein S., et al. A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nat. Cell. Biol. 1999;1:409–414. doi: 10.1038/15640. [DOI] [PubMed] [Google Scholar]
- 30.Higashida H., Egorova A., Higashida C., Zhong Z. G., Yokoyama S., Noda M., Zhang J. S. Sympathetic potentiation of cyclic ADP-ribose formation in rat cardiac myocytes. J. Biol. Chem. 1999;274:33348–33354. doi: 10.1074/jbc.274.47.33348. [DOI] [PubMed] [Google Scholar]
- 31.Choi D. W., Koh J. Y. Zinc and brain injury. Annu. Rev. Neurosci. 1998;21:347–75. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 32.Ragozzino D., Giovannelli A., Degasperi V., Eusebi F., Grassi F. Zinc permeates mouse muscle ACh receptor channels expressed in BOSC 23 cells and affects channel function. J. Physiol. (London) 2000;529:83–91. doi: 10.1111/j.1469-7793.2000.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallee B. L., Coleman J. E., Auld D. S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc. Natl. Acad. Sci. U.S.A. 1991;88:999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Essen B., Jansson E., Henriksson J., Taylor A. W., Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol. Scand. 1975;95:153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- 35.Houtman C. J., Heerschap A., Zwarts M. J., Stegeman D. F. pH heterogeneity in tibial anterior muscle during isometric activity studied by 31P-NMR spectroscopy. J. Appl. Physiol. 2001;91:191–200. doi: 10.1152/jappl.2001.91.1.191. [DOI] [PubMed] [Google Scholar]
- 36.Schoenmakers J. M., Visser G. J., Flick G., Theuvene A. P. R. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. BioTechniques. 1992;12:870–879. [PubMed] [Google Scholar]