Summary
Acute liver failure is a rare and dynamic condition, with a broad aetiology and an incompletely understood pathophysiology. Management of this life-threatening disease requires critical care and organ support and frequently early liver transplantation. Proper identification, prevention and treatment of complications such as intracranial hypertension and sepsis are critical to optimising outcomes. The identification of the cause of acute liver failure and the prompt initiation of the aetiological treatment can also improve prognosis. Survival has progressively improved in parallel to advances in medical treatment. Intracranial hypertension complicating hepatic encephalopathy is less frequent than in the past and intracranial pressure monitoring now relies on non-invasive techniques. Current prognostic models have good accuracy to identify patients who will die without liver transplantation but are not able to identify those in whom transplantation is futile. New prognostic markers to select patients for transplantation are still in the pipeline. Therapeutic plasma exchange and, in some centers, early renal replacement therapy are well established treatments for the disease. The use of other artificial liver devices in clinical practice is not supported by evidence. This review is intended to provide a clinical update on the management of acute liver failure, incorporating the most recent advances in the field.
Keywords: prognosis, mortality, liver transplantation, liver support, intracranial hypertension
Keypoints.
-
•
Acute liver failure is a rare clinical condition with a broad aetiology that varies among geographical areas.
-
•
It is the most severe clinical condition in hepatology. This life-threatening disease requires critical care admission and frequently organ support and emergency liver transplantation.
-
•
The identification of the cause of acute liver failure and the prompt initiation of aetiological treatment can improve prognosis.
-
•
Proper management of complications such as intracranial hypertension and sepsis is critical to optimising outcomes.
-
•
Plasma exchange and renal replacement therapy are increasingly used in the treatment of the disease.
-
•
Survival has progressively improved in parallel to advances in medical treatment.
Introduction
Acute liver failure (ALF) is a rare life-threatening condition characterised by abnormal liver tests, elevated international normalised ratio (INR) >1.5 and hepatic encephalopathy (HE) without preexisting chronic liver disease.[1], [2], [3] Most cases are preceded by acute liver injury (ALI), a condition characterised by liver damage and impaired liver function with coagulopathy (INR >1.5) but without HE. An acute deterioration in liver function in patients with chronic liver disease or cirrhosis should be categorised as acute-on-chronic liver failure, a completely different entity which will not be considered further in this review.4 ALF usually affects young patients without comorbidities, yet it is associated with high mortality. This entity may progress to either spontaneous recovery or to extrahepatic complications, multiorgan failure and death. Emergency liver transplantation (ELT) is the most effective treatment for this condition given the limited effectiveness of the currently available liver support systems. ELT and extensive research in the last decades have transformed ALF from a poorly understood disease that was almost always fatal to a well-known entity with established diagnostic and treatment protocols and good prognosis in developed countries.5 Prompt diagnosis and treatment in expert centres can prevent ALI from progressing to ALF. Once ALF develops, rapid transfer to centres with expertise in ELT and admission to the intensive care unit (ICU) is pivotal for aetiologic diagnosis and treatment, organ support and expedited evaluation for ELT. This review will focus on practical clinical aspects and recent advances in the management of ALF.
Pathogenesis of hepatic and extrahepatic organ failures
ALF is preceded by ALI which manifests with varying degrees of hepatocyte cell death. The mechanisms involved in liver injury differ by aetiology: acute hepatitis B infection is associated with massive lysis of infected hepatocytes by the immune system, paracetamol (acetaminophen) overdose with toxic metabolites, Amanita phalloides intoxication with RNA synthesis blockage, shock with ischaemic necrosis, and acute fatty liver of pregnancy with mitochondrial dysfunction, among others. On the other hand, the host response to massive liver necrosis with the release of damage-associated molecular patterns from dying hepatocytes and endothelial cells6,7 underpins a significant immune reaction that may further cause a severe systemic inflammatory response with the release of both pro-inflammatory cytokines such as IL-6, TNF-α, HGF and anti-inflammatory cytokines. Liver failure and the deranged systemic inflammatory response may lead to the development of vasodilatory shock, acute kidney injury (AKI), metabolic abnormalities and vulnerability to infections. Moreover, the failing liver is unable to convert ammonia to urea causing an accumulation of this neurotoxin which may lead to astrocyte swelling and intracranial hypertension.8 Sepsis with multiorgan failure and intracranial hypertension complicated by cerebral herniation are the main causes of death in ALF. In a recent cohort of patients with ALF who were on the waitlist for ELT, 28% of the deaths were due to complications from cerebral oedema and the rest due to multiorgan failure.9
Epidemiological changes and current aetiology
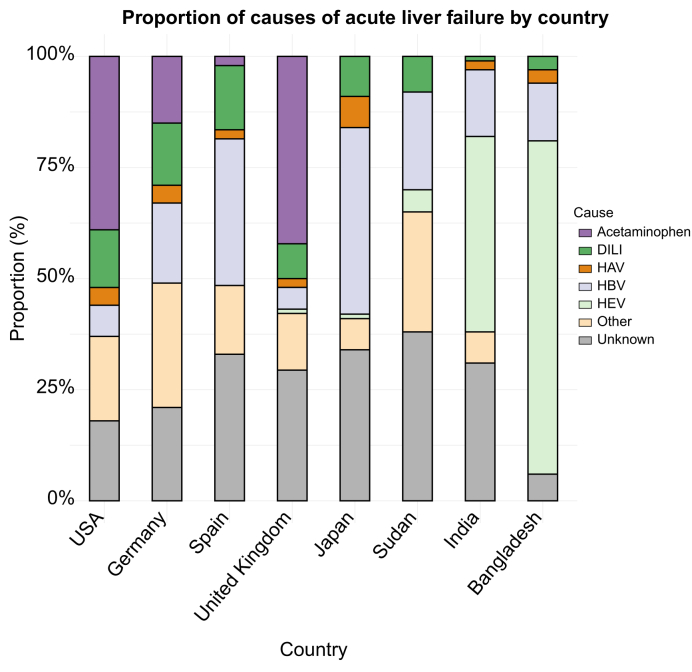
ALF is a rare disease with relevant differences in frequency between geographical areas (Fig. 1). A yearly incidence of 1–6 cases per million population has been reported in developed countries.9,10 In contrast, ALF is much more common in developing countries given the lack of extended vaccination programmes for hepatitis viruses. Prognosis is also worse in these geographical areas given the limited access to medical treatment.11 Drug-induced liver injury (DILI) is currently the main cause of ALF in Europe and the US. Paracetamol is the leading drug involved in DILI. Other causes that may lead to ALF are autoimmune hepatitis (AIH), shock (ischaemic hepatitis), Wilson’s disease, Budd-Chiari syndrome, toxic mushrooms, heatstroke, acute fatty liver of pregnancy, HELLP (haemolysis, elevated liver enzymes and low platelets) syndrome and malignancies.12 Box 1 describes the whole aetiological picture in ALF. Up to 35% of cases reported worldwide are of indeterminate origin,11,12 a feature that may be related to limitations in the aetiologic work-up, undiagnosed AIH or unrecognised viral pathogens or drugs. It is anticipated that this percentage of indeterminate causes of ALF may decrease when data from more recent cohorts are published.
Fig. 1.
Causes of acute liver failure by country.
DILI, drug-induced liver injury.
Box 1. Causes of acute liver failure.
Virus
Acute hepatitis due to HAV, HBV, HCV (exceptional), HDV (HBV and HDV coinfection; HDV superinfection in HBsAg carrier) and HEV Acute infections due to non-hepatotropic viruses: herpes simplex 1 and 2, varicella-zoster, cytomegalovirus, Epstein-Barr virus, herpesvirus type 6, parvovirus B19, adenovirus, haemorrhagic fever virus and Coxsackie B virus.
Drugs
Paracetamol (overdose; therapeutic doses in alcoholic, malnourished patients or under treatment with enzyme inducers)
Antibiotics: isoniazid, pyrazinamide, tetracyclines, amoxicillin-clavulanic acid
Halothane, sevoflurane and other derivatives
Antidepressants: MAO inhibitors, imidazopyridine derivatives, etc.
Non-steroidal anti-inflammatory drugs
Antithyroid drugs
Others: hydantoins, α-methyldopa, ketoconazole, amphetamines, sulfonamides, etc.
Toxics
Mushrooms of the Amanita group: A. phalloides, A. verna and Lepiota
Industrial solvents: carbon tetrachloride, trichlorethylene, white phosphorus
Illegal drugs: cocaine, ecstasy (3,4-methylenedioxymethamphetamine)
Others: traditional Chinese medicine, chaparral, other "natural" products
Vascular diseases
Ischaemic hepatitis: shock liver, heart failure
Ligation of the hepatic artery (especially if portal thrombosis coexists)
Acute Budd-Chiari syndrome
Others
Chronic autoimmune hepatitis (as presentation of the disease)
Wilson’s disease
Pregnancy (third trimester): acute fatty liver, HELLP syndrome
Hyperthermia, heat stroke
Massive tumour infiltration (due to solid neoplasms, lymphomas, leukaemia)
Reye syndrome (in children and adults)
Urea cycle disorders
Partial hepatectomy
Undetermined
HELLP, haemolysis, elevated liver enzymes and low platelets; MAO, monoamine oxidase.
Alt-text: Box 1
ALF may present as a hyperacute disease with an interval between jaundice and HE of a few days (≤1 week) or as acute or subacute forms with an interval that can last several weeks. Intracranial hypertension is mainly observed in hyperacute forms of ALF which are associated with a higher probability of spontaneous recovery. Paracetamol, viral hepatitis, heatstroke, and hypoxic hepatitis tend to follow a hyperacute course. Subacute aetiologies may develop features of chronic liver disease such as irregular liver borders on imaging, ascites and even hepatorenal syndrome. Development of those features is associated with low probability of spontaneous recovery. Examples include non-paracetamol DILI or some cases of AIH.1
Viral infection
Viruses account for most ALF cases worldwide. In Japan, 40% of cases of ALF are due to hepatitis B virus (HBV) infection, whereas hepatitis E virus (HEV) causes half of cases in India and Bangladesh.1,13 Even in developed countries with extended vaccination programmes and blood product monitoring, hepatitis viruses currently account for 12% of cases of ALF.1 ALF due to HBV is a severe condition leading to death or ELT in 80% of cases.12 Also, coinfection with hepatitis delta virus may lead to a more aggressive phenotype. In addition, there has been an increase in cases of reactivation of HBV in patients with subclinical chronic HBV infection treated with immunosuppressive therapy, especially steroids and rituximab. This reactivation may lead to acute or subacute ALF that can be fatal.14 Therefore, screening for past or chronic HBV infection is mandatory in all patients before initiating immunosuppression and specific recommendations regarding either prophylactic treatment or regular monitoring must be defined.14 Hepatitis A virus is the second most common viral cause of ALF globally. However, it is a decreasing entity in developed countries due to the extension of vaccination programmes and sanitization measures, and is currently a marginal cause of ELT in these countries.12 HEV-related ALF is relatively rare in developed countries since acute viral infection is usually self-limited, but it should always be suspected. In fact, there is data estimating nearly 2 million cases of locally acquired HEV infections in Europe every year.15 It should be suspected in patients with neurologic abnormalities such as encephalitis, Guillain-Barre syndrome, or neuralgic amyotrophy.15 Acute hepatitis C is rare and generally not associated with ALF, but some cases have been reported and specific treatment with direct-acting antiviral agents should be promptly initiated. In immunocompromised (but also in immunocompetent) patients, other viruses such as herpes simplex, herpes zoster, cytomegalovirus, Epstein-Barr virus, parvovirus, and adenovirus may cause a disseminated infection with ALF, multiorgan failure, and high mortality despite antiviral treatment. Disseminated herpes simplex infection must be suspected in relatively young patients or pregnant women with high fever, very high transaminase and LDH levels and rapid progression to multiorgan failure. ELT is usually contraindicated in the setting of disseminated herpes infection.
Drugs and herbal products
Paracetamol overdose, either intentional or accidental, is the most common cause of ALF in developed countries, especially in the UK (60% of cases) and the US (46%).12 Paracetamol-related ALF follows a hyperacute phase with very high transaminases and INR elevation and relatively low bilirubin levels. It usually self-limits with supportive therapy and N-acetylcysteine treatment. Of all ELT for ALF, only 8% were related to paracetamol overdose.12
Non-paracetamol DILI is a leading cause of ALF in developed countries (5% to 20% of all cases of ALF). DILI may have different phenotypes depending on the responsible drug. Most cases of DILI are idiosyncratic, i.e. unpredictable, variable from person to person and generally dose-independent drug reactions related to the exposure to antimicrobials, anticonvulsants, rheumatologic and antineoplastic drugs. Patterns of liver injury may be hepatocellular, cholestatic or mixed. However, a large variety of phenotypes exists. Some drugs may cause an immunoallergic reaction with eosinophilia and systemic symptoms (DRESS), others may induce an AIH, a secondary sclerosing cholangitis, a granulomatous hepatitis or even a ductopenic syndrome. There has been a recent increase in herbal medicines causing ALF. In fact, in the US, almost 20% of all DILI cases are caused by such products. Recreational drugs such as cocaine and ecstasy may cause severe cases of ALF and should be suspected in young patients with ALF of unknown origin with hyperthermia, rhabdomyolysis, disseminated intravascular coagulation and multiorgan failure.16 Exertional heatstroke is a severe condition characterised by the presence of hyperthermia above 40 °C, associated with neurological impairment that may lead to multiple organ failure. Liver injury may develop in this context, mainly manifesting as variable elevations of serum aminotransferases. While most patients will regain normal liver function with medical treatment, some may progress to ALF requiring ELT or even death.17 Rising global temperatures, more frequent heatwaves, and intense exercise routines make this condition increasingly important to monitor and treat.
Autoimmune and metabolic diseases
The onset of AIH can lead to the development of acute or subacute forms of liver failure. Both phenotypes are increasingly observed in routine clinical practice. AIH should be suspected in young women with high transaminase and bilirubin levels, and high IgG levels and autoantibodies (e.g. smooth muscle antibody, antinuclear antibody). Liver biopsy is often required for the diagnosis since autoantibodies are negative in up to half of AIH-related cases.18
The fulminant presentation of Wilson’s disease almost always follows a fatal clinical course without ELT. It must be suspected in young patients with a family history of liver disease and psychiatric conditions, mild increase in transaminases, a alkaline phosphatase/total bilirubin ratio <2 and Coombs negative haemolysis.
Other aetiologies
HELLP syndrome and acute fatty liver of pregnancy are complications of preeclampsia occurring in the third trimester of pregnancy. Despite immediate delivery some patients may progress to ALF and require ELT. Hypoxic hepatitis, also called shock liver or ischaemic hepatitis, is a rare cause of ALF in patients with decompensated congestive heart failure or acute cardiac failure. It follows a hyperacute clinical course and it usually resolves with the treatment of cardiovascular disease. HE is usually mild.
Diagnostic work-up
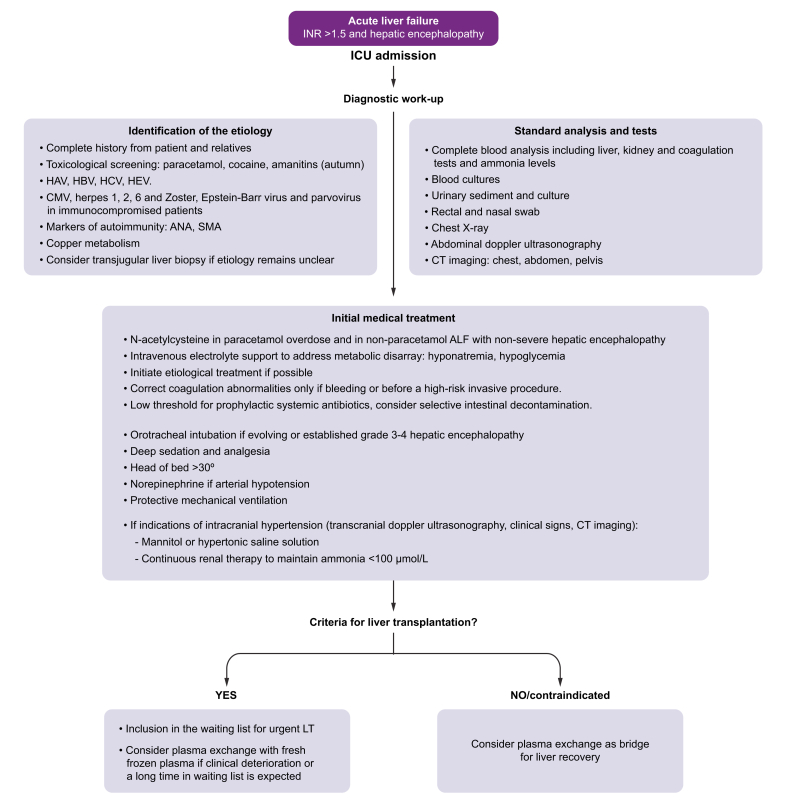
Once diagnosis of ALF has been established (abnormal liver test, severe coagulopathy, and HE), efforts should be made to rapidly determine its aetiology as it impacts prognosis, may delineate specific treatments, and can also unveil a contraindication for LT (i.e. malignant infiltration). Diagnostic evaluation of ALF requires a comprehensive approach (Fig. 2). Clinical assessment should be exhaustive at the time of admission, asking both the patient and their family members about recent consumption of medications or herbs, risky sexual behaviour, and recent travel. Consumption of such herbs should be actively investigated since many patients do not consider them as a potentially harmful drug. Comorbid conditions should also be investigated.1,3,19
Fig. 2.
Recommended initial management of acute liver failure.
ALF, acute liver failure; ANA, antinuclear antibody; CMV, cytomegalovirus; INR, international normalised ratio; LT, liver transplantation; SMA, smooth muscle antibody.
At admission, specific laboratory tests should be obtained to determine not only the aetiology, severity, and prognosis of ALF but also to identify potential complications (infection, AKI, among others) (See Box 2). Chest X-ray and abdominal ultrasonography are also required at baseline assessment. Abdominal ultrasonography should evaluate the patency of the hepatic veins (exclusion of acute Budd-Chiari syndrome), portal vein and hepatic artery, liver morphology and spleen size (data from chronic liver disease) and exclude potential infiltrative disorders. Echocardiography should always be performed in haemodynamically unstable patients or in candidates for ELT. Abdominal tomography is sometimes required to characterise vascular anatomy or upon suspicion of acute pancreatitis.1,3
Box 2. Suggested laboratory work-up in acute liver failure.
Disease severity and complications
Full coagulation screen including prothrombin time, INR, fibrinogen and factor V
Liver tests including LDH and conjugated and unconjugated bilirubin and creatinine kinase
Renal function tests including serum creatinine, urea* and urine output
Arterial blood gases and serum lactate
Arterial ammonia
Serum amylase and lipase
Blood and urine cultures
Aetiology
Toxicological screening in urine
Paracetamol serum levels in paracetamol overdose
Amanitin in urine in mushroom poisoning
Microbiological screen for virus infection:
-
•
HBsAg, anti-HBc IgM, HBV DNA, delta virus if HBV
-
•
Anti hepatitis A virus IgM
-
•
Anti hepatitis E virus IgM, HEV RNA
-
•
Anti hepatitis C virus, HCV RNA
-
•
Cytomegalovirus, herpes simplex, herpes zoster, Epstein-Barr virus, parvovirus PCR
Markers of autoimmunity: serum levels of immunoglobulins, ANA, SMA, anti-soluble liver antigen
ANA, antinuclear antibody; INR, international normalised ratio; LDH, lactate dehydrogenase; SMA, smooth muscle antibody.
*Low urea levels indicate severe liver dysfunction.
∧Immunocompromised patients.
Alt-text: Box 2
In all cases of suspected viral ALF, blood viral load determination by quantitative PCR techniques is recommended in addition to serologic screening (Box 2, Fig. 2).1,3,19
The diagnosis of other entities such as acute Wilson’s disease, HELLP or acute fatty liver of pregnancy is mainly based on clinical grounds. Copper metabolism should be evaluated if Wilson’s disease is suspected. The determination of copper excretion in urine (8-24 h) is the simplest and fastest diagnostic biomarker of Wilson’s disease, having an excellent positive predictive value. The usefulness of relative exchangeable copper in ALF remains to be elucidated.20,21
There are other aetiologies of ALF that may only be diagnosed by liver biopsy like malignant infiltration of the liver or granulomatous hepatitis.22 It has been demonstrated that transjugular liver biopsy is safe in patients with ALF and provides enough tissue sample to establish the diagnosis. Current guidelines recommend its use if clinically indicated.1,23 Table 1 shows the clinical, analytical and histological characteristics of the main causes of ALF.
Table 1.
Multiparametric diagnosis guide for acute liver failure.
| Aetiologic trigger | Clinical phenotype | Testing | Liver histology |
|---|---|---|---|
| HAV, HBV, HCV, HEV | Epidemiological background Viral prodromes (malaise, fever, diarrhoea) Hyperacute course High AST, ALT, and INR |
Anti-HAV IgM Anti-HB core IgM, HBV surface antigen, and HBV-DNA Anti-HCV Ig and HCV-RNA Anti-HEV IgM and HEV-RNA |
Massive or submassive hepatocellular necrosis Lymphocytic infiltrates Varying degrees of fibrosis in HBV reactivation Immunohistochemical staining |
| HSV, VZV, CMV, EBV, ADV, PB1924 | Immunocompromised (but also in immunocompetent) Hyperacute course High fever with or without skin/mucosal lesions in HSV and VZV Disseminated infection Multiorgan failure |
IgM and blood viral load | Massive or submassive hepatocellular necrosis Viral inclusions Immunohistochemical staining |
| Paracetamol overdose | Intentional (younger) or accidental overdose Hyperacute course Metabolic acidosis and acute kidney injury |
Drug serum concentration | Coagulative confluent hepatocellular necrosis in centrilobular areas |
| DILI | Epidemiological background Acute or subacute clinical course Varying patterns of liver injury |
Exclude other causes of ALF | Necroinflammatory pattern Massive or submassive hepatocellular necrosis Lymphocytic infiltrates |
| Autoimmune hepatitis25 | Young women History of other autoimmune disorders High transaminases and high bilirubin May follow a subacute course (ascites) |
High gamma globulin levels Autoantibodies (not in 50% of cases) |
Centrilobular and confluent necrosis Lymphoplasmacytic infiltration Interface hepatitis Varying degrees of fibrosis |
| Wilson’s disease | Young patients Neurologic or psychiatric background Acute intravascular non-immune haemolysis High bilirubin and low alkaline phosphatase Modest elevations of transaminases with high AST/ALT ratio Progression to AKI |
High 24h-cupruria High relative exchangeable copper Low serum copper and low ceruloplasmin |
Microvesicular steatosis Glycogenated nuclei Varying degrees of fibrosis |
| Hypoxic hepatitis | History of congestive heart failure or refractory shock Very sudden and high increase in transaminases and INR with abrupt recovery Transient elevation of bilirubin after recovery Other signs of end-organ damage |
Perform liver imaging to assess vascular permeability | Predominant centrilobular necrosis |
| Heatstroke | Hyperthermia > 40 °C History of physical exhaustion (exercise, heat wave) Concomitant recreational use of cocaine or MDMA Very high transaminases and INR Other signs of end-organ damage (AKI, rhabdomyolysis, ARDS) |
Exclude other causes of ALF | Predominant centrilobular necrosis |
| Mushroom poisoning | Epidemiologic background Severe gastroenteritis High transaminases |
Amatoxins in urine | Massive hepatocellular coagulative necrosis |
| Malignant infiltration | Lymphoma, leukaemia, breast cancer, colon cancer Toxic syndrome, B symptoms High bilirubin and cholestasis Hepatomegaly and lymphadenopathies |
Liver biopsy | Atypical cell infiltration in sinusoids |
| HELLP syndrome26 | Third trimester of pregnancy (frequent history of preeclampsia/eclampsia) Coombs negative haemolysis High transaminases and low platelets May progress to liver rupture |
Exclude other causes of ALF | Microvascular fibrin deposition, neutrophilic infiltrate, fatty infiltration, lobular necrosis, and periportal haemorrhage |
| Acute fatty liver of pregnancy26 | Third trimester of pregnancy (infrequent history of preeclampsia/eclampsia) Abdominal pain and vomiting Ascites Severe coagulopathy and encephalopathy Low transaminases Progression to AKI |
Swansea criteria | Microvesicular steatosis |
| Acute Budd-Chiari syndrome25 | Myeloproliferative neoplasm or other prothrombotic disease Abdominal pain Painful hepatomegaly and ascites |
Hepatic vein congestion on ultrasound Vein thrombosis identification on imaging |
Centrilobular vein dilation |
ADV, adenovirus; AKI, acute kidney injury; ALF, acute liver failure; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; CMV, cytomegalovirus; DILI, drug-induced liver injury; EBV, Epstein-Barr virus; HSV, herpes simplex virus; PB19, parvovirus B19; VZV, herpes zoster virus.
Criteria for referral to transplant centres
The progression of ALF is highly unpredictable, especially in its hyperacute clinical presentation. In all cases where there is significant acute liver damage, transfer of the patient to a liver transplant centre should be considered. Even patients who may not seem ideal candidates for ELT should be assessed for potential transfer, as management in specialised centres could enhance their chances of survival. Mental status changes can be subtle, and even mild HE may indicate a critical, life-threatening condition within a short timeframe. Therefore, it is highly recommended to transfer the patient upon observing any alteration in mental status, an INR >1.5, or hypoglycaemia or metabolic acidosis. Table 2 describes the suggested criteria for transferring a patient with ALI or ALF related or not related to paracetamol to a LT centre. This early and proactive policy can be crucial for decision-making and improving clinical outcomes in patients with ALF.1,2 The only general measure advisable during patient transfer is hypertonic glucose infusion (due to the risk of hypoglycaemia).
Table 2.
Suggested criteria for referral of patients with acute liver failure to specialised units.3
| Paracetamol and hyperacute aetiologies | Non-paracetamol |
|---|---|
| Arterial pH <7.30 or HCO3 <18 | pH <7.30 or HCO3 <18 |
| INR >3.0 day 2 or >4.0 thereafter | INR >1.8 |
| Oliguria and/or elevated creatinine | Oliguria/renal failure or Na <130 mmol/L |
| Altered level of consciousness | Encephalopathy, hypoglycaemia, or metabolic acidosis |
| Hypoglycaemia | Bilirubin >300 μmol/L (17.6 mg/dl) |
| Elevated lactate unresponsive to fluid resuscitation | Shrinking liver size |
INR, international normalised ratio.
General management and organ support
Critical care management of patients with ALF could resemble that of any other patient in the ICU but has specific aspects that deserve consideration. If ALF is suspected, all drugs that the patient was previously receiving should be suspended (except hormone replacement treatment if applicable). Additionally, manoeuvres that further worsen or could resemble HE (i.e. administering sedatives or metoclopramide) should be avoided. There is no evidence that treatment with disaccharides or rifaximin are effective in the treatment of HE in ALF. Lactulose enemas are contraindicated due to the risk of increasing intracranial pressure (ICP).27 The administration of intravenous N-acetylcysteine improves transplant-free survival in cases of non-paracetamol ALF with early stage HE (grade 1 and 2). This treatment is ineffective if HE worsens to grade 3 or 4.28,29
A close control of consciousness level is essential in the management of patients with ALF. It should be checked very frequently, usually every 6 or even 2 h. Patients with grade 3-4 HE require intubation for airway protection and prevention/management of intracranial hypertension. Once intubated, sedation and analgesia must be initiated.1,3,5 Recommended general management of patients with ALF is detailed in Fig. 2 and Table 3.
Table 3.
General management of acute liver failure.
| Organs and systems | General management | Treatment |
|---|---|---|
| Admission and patient monitoring in the ICU (risk of rapid clinical deterioration) Assessment of arterial ammonia |
N-acetylcysteine in all cases of paracetamol overdose and in non-paracetamol ALF with grades 1 or 2 hepatic encephalopathy | |
| Brain | Orotracheal intubation in case of grade 3-4 hepatic encephalopathy Non-invasive intracranial pressure monitoring:
|
Deep sedation, RASS -5 No nociceptive stimuli Raise head of bed >30º Core temperature <37 °C Avoid hyponatremia If data of intracranial hypertension:
|
| Cardiovascular | Invasive blood pressure monitoring in intubated patient Echocardiography |
Avoid arterial hypotension Volume resuscitation in hypotensive patients Norepinephrine as the vasopressor of choice Hydrocortisone at stress doses in refractory shock |
| Respiratory | Reasons for intubation:
|
Protective mechanical ventilation
|
| Renal | Close analytical and clinical monitoring (urinary output) | Avoid nephrotoxic drugs Early continuous renal replacement therapy |
| Metabolic and gastrointestinal | Tight glycaemic control (2 h) | Hypertonic glucose (10%-20%) Blood glucose levels: 110-180 mg/dl PPI: stress ulcer prophylaxis Early enteral nutrition (discontinue if worsening of hepatic encephalopathy or of arterial ammonium) |
| Coagulation | Close analytical monitoring Viscoelastic tests if bleeding or invasive procedures |
Correct coagulation abnormalities only if bleeding or before a high-risk invasive procedure. |
| Infections | Rule out infection: cultures (blood, urine, respiratory secretions), chest X-ray, and urine sediment at admission and whenever patient deteriorates | Potential prophylactic role of selective intestinal decontamination or systemic antibiotics Low threshold to initiate empirical antibiotic therapy |
ALF, acute liver failure; ICP, intracranial pressure; ICU, intensive care unit; PPI, proton pump inhibitor; RASS, Richmond agitation-sedation scale.
Aetiological treatment
Early initiation of treatment targeting the cause of ALF, when available, is of paramount clinical relevance. Administration of N-acetylcysteine in paracetamol overdose (150 mg/kg in 1 h, 50 mg/kg over 4 h, 100 mg/kg in 16 h and 150 mg/kg in 24 h for 3-4 days); glucocorticoids in AIH without severe HE (1 mg/kg); penicillin G in Amanita phalloides poisoning, intravenous acyclovir in herpes 1, 2 or zoster infection (10 mg/kg every 8 h), tenofovir or entecavir in HBV ALF and expedited delivery in acute fatty liver of pregnancy or HELLP are measures that should be initiated immediately after the diagnosis has been established.11,27 Ribavirin may also be considered in ALF caused by HEV since it may reduce morbidity, but there is no evidence of its efficacy.15 Therapeutic plasma exchange (TPE) is indicated to treat acute haemolysis in Wilson's disease.17 Once major liver damage is established (i.e. grade 3-4 HE) aetiological measures are much less effective and potentially harmful (i.e. steroids in AIH).30
Cardiovascular support
Cardiovascular alterations in ALF are characterised by hyperdynamic circulation (high cardiac output and peripheral vasodilation) that could compromise end-organ perfusion causing lactic acidosis and AKI.27 Hypotensive patients should be carefully resuscitated, preferably with normal saline, to avoid congestion and ensure organ perfusion. Fluid overload is especially deleterious in ALF since it increases the risk of intracranial hypertension due to worsening cerebral oedema. In a study involving patients with ALF, fluid responsiveness was observed in only 29%. Preload dependency parameters, based on pulse contour analysis, effectively predicted fluid responsiveness.31 In persistently hypotensive patients, vasopressors should be initiated, norepinephrine being the preferred drug. Vasopressin should be added to norepinephrine in cases of refractory hypotension. Stress dose hydrocortisone (50 mg intravenous q6h) should be considered in vasopressor-resistant hypotension given the high prevalence of relative adrenal insufficiency in ALF.32 Several studies show that molecular adsorbent recirculating system and high-volume plasma exchange improve haemodynamic stability in patients with ALF requiring vasopressor support.33,34
Respiratory support
The incidence of respiratory failure is low in ALF. However, ELT is contraindicated in patients developing refractory respiratory failure.35,36 Ventilatory settings should be protective using low tidal volumes (6 ml/kg/ideal body) and appropriate, usually low to moderate positive end expiratory pressure. Hypercarbia and hypocarbia should be avoided, with CO2 targets between 4.5 and 5.5 kPa (34–41 mmHg).3 Extracorporeal membrane oxygenation could be considered as a bridge to ELT in highly selected patients with reversible respiratory failure or as rescue therapy for refractory respiratory failure in post-transplant patients.37
Acute kidney injury and renal replacement therapy
AKI is highly prevalent in ALF and is associated with poor outcome.38,39 Up to 70% of patients in a US cohort had AKI with 30% requiring renal replacement therapy (RRT).40 Continuous renal replacement therapy (CRRT) is the preferred modality because it is associated with a lower risk of cardiovascular instability and cerebral oedema. CRRT effectively decreases ammonia level and, in the last decade, hyperammonaemia has become an increasingly common indication for RRT in ALF independently of the presence of AKI. Several studies suggest that early CRRT improves survival in this setting by preventing severe hyperammonaemia and its associated complications.[41], [42], [43]
Nutritional support and metabolic control
The role of nutritional therapy in ALF is to provide sufficient energy, vitamins, and trace elements, facilitate adequate protein synthesis, and avoid metabolic complications, such as hypoglycaemia and hyperammonaemia.44 In patients with ALF with high arterial ammonia levels who are at risk of cerebral oedema, nutritional protein support can be deferred for 24–48 h until hyperammonaemia is controlled. When protein administration is started, arterial ammonia should be closely monitored to ensure no pathological elevation occurs.45
Hypoglycaemia is a frequent manifestation in patients with ALF due to decreased hepatic glycogen stores and impaired gluconeogenesis and glycogenolysis. Hypoglycaemia can contribute to HE and has been associated with increased mortality; therefore, periodic monitoring of glycaemia is recommended every 6 h1.
Management of coagulopathy
Global assessment of haemostasis in ALF indicates a “rebalanced” state. Compensatory mechanisms have been identified for each phase of haemostasis. Pro-haemostatic drivers may overcompensate for deficient liver-derived coagulation factors and result in a relative hypercoagulable state.46 Therefore, prophylactic transfusion of fresh frozen plasma (FFP) or other blood products is unwarranted and may expose patients to harmful effects, such as volume overload, brain oedema and transfusion reactions, without any clinical benefit.47 A recent study by Stravitz et al., identified an association between coagulopathy assessed by rotational thromboelastometry, bleeding events, and mortality in patients with ALF, highlighting the clinical utility of rotational thromboelastometry in this setting.48
Prevention and treatment of infections
In patients with ALF, sepsis can manifest at any time in the course of the disease. Early infections are mainly attributed to immune dysfunction, while late episodes are also facilitated by the high degree of instrumentation required in the management of these patients (hospital-acquired infections occurring in the setting of disease progression).49 Bacteria implicated in early infections arise from the patient's normal flora while later infections involve exogenous bacteria and fungi. Most endogenous infections occur within the first week.50
The susceptibility to bacterial and fungal infections, sepsis, and septic shock is notably increased in patients with ALF, with infectious complications being a leading cause of death in this setting. Bacterial infections have been documented in 60–80% of patients, most commonly presenting as pneumonia (50%), urinary tract infections (22%), catheter-related bacteraemia (12%), and spontaneous bacteraemia (16%). Infections by multidrug-resistant organisms are associated with a higher incidence of grade 3-4 HE, multiorgan failure, longer hospital stay and a trend towards higher mortality.51 Fungal infections, largely caused by Candida species, occur in about one-third of patients requiring prolonged critical care support.52
The diagnosis of infection in patients with ALF is difficult; the clinical features are non-specific and tests such as C reactive protein and procalcitonin measurements are frequently unhelpful. A high level of clinical suspicion should be maintained. Empirical broad-spectrum antibiotics should be administered to patients with ALF who have signs of sepsis, persistent hypotension or unexplained progression to higher grades of HE. Antifungal therapy should be considered in patients with prolonged organ support. Use of biomarkers to guide treatment for invasive fungal infections is recommended (serum galactomannan antigen and 1.3-β-D glucan).3 Empiric antibiotics are recommended for patients listed for super urgent LT, since the development of infection and sepsis may prompt delisting.52
Cerebral oedema and intracranial hypertension
Intracranial hypertension is a complication mainly observed in hyperacute forms of ALF, having declined in incidence in recent years (50% in the past vs. 30% in more recent series).53 The two main pathogenic factors are: high ammonia levels leading to astrocyte swelling and systemic inflammation. Patients with more than one of the following: young patients with acute or hyperacute forms, persistent increase in ammonium >150-200 μmol/L, AKI and norepinephrine >0.1 μg/kg/min are at higher risk of intracranial hypertension.3,54 Clinical monitoring of this complication is essential. Insertion of an ICP sensor, mainly in the extradural space, was frequently used in the past for this purpose. This sensor allows for the continuous evaluation of ICP and the estimation of cerebral perfusion pressure; ICP should be maintained <20 mmHg and cerebral perfusion pressure (CPP) >50 mmHg. This invasive technique has been replaced by non-invasive methods, mainly transcranial Doppler ultrasound of cerebral arteries (cerebral flow, pulsatility index) and by the evaluation of the optic nerve sheath diameter (a measure >0.48 cm is suggestive of intracranial hypertension).55 The disadvantage of non-invasive methods is that the information given is discontinuous.
Several measures are applied to prevent cerebral oedema and intracranial hypertension in ALF including facilitating venous drainage (head elevation 30° or more, semiflexed head), avoidance of nociceptive stimuli; control of agitation (sedoanalgesia); avoidance of hyponatremia (serum sodium between 140 and 145 mEq/L), prevention of hypo- and hyperglycaemia and avoidance of fever and hyperhydration.3,54 Osmotic therapy with mannitol (0.5-1 g/kg) or hypertonic saline (10-30%) is the first line treatment for intracranial hypertension. CRRT can be used if there is no response to osmotic therapy in the presence of serum ammonia >150-200 mmol/L. Muscle relaxants and moderate hyperventilation (pCO2 30-35 mmHg) are other measures that can help to control intracranial hypertension. Second-level interventions, indicated in refractory cases (CPP <50 mmHg, ICP >20 mmHg), are moderate hypothermia (central temperature of 33-34 °C) and barbiturate coma.32 In desperate cases of uncontrolled intracranial hypertension, refractory shock and toxic liver due to massive necrosis, emergent hepatectomy is indicated in some centres, with the aim of achieving a clinical improvement while awaiting an organ.3,56
Liver support systems
Hepatic failure in ALF is reversible in many cases given the huge regenerative capacity of the liver. This explains why liver support systems could have great potential in ALF, as a bridge to liver regeneration or to ELT. These systems should support all liver functions including synthesis, excretion, and metabolism. Unfortunately, such a system does not exist. The greatest clinical experience is with artificial liver support systems (without liver cells). Albumin dialysis systems (MARS [molecular adsorbent recirculation system] and Prometheus) are based on the scavenger capacity of albumin that is capable of removing water-soluble and albumin-bound toxic substances, which accumulate and promote systemic and hepatic inflammation. Unfortunately, these systems have not been shown to improve survival in ALF and are not recommended in current guidelines.57 Bioartificial liver support systems contain hepatocytes in the bioreactor. Published studies performed in the past also failed to show a consistent reduction in mortality.58 Currently, bioartificial liver support systems are not available on the market.
TPE is the most effective liver support treatment currently available and the only one indicated according to current guidelines.,3,34 It is used as a bridge to LT or to spontaneous recovery in cases with good prognosis (paracetamol, hepatitis A virus) and when LT is contraindicated. It has detoxifying and immunomodulatory effects. High-volume plasma exchange (3 plasma volumes) improves transplant-free survival. Recent studies suggest the effectiveness of TPE using lower doses (1.5-2 volumes of plasma) with fewer adverse events. In recent years, TPE has become a routine therapy in patients with ALF in several centres given its effectiveness, safety, ease of use, and low cost compared to other therapies such as albumin dialysis.59 It is important to remark that the replacement solution used is FFP. Therefore, INR cannot be used as a prognostic marker after the initiation of TPE.
Liver transplantation for ALF
Selection of candidates for emergency liver transplantation in ALF
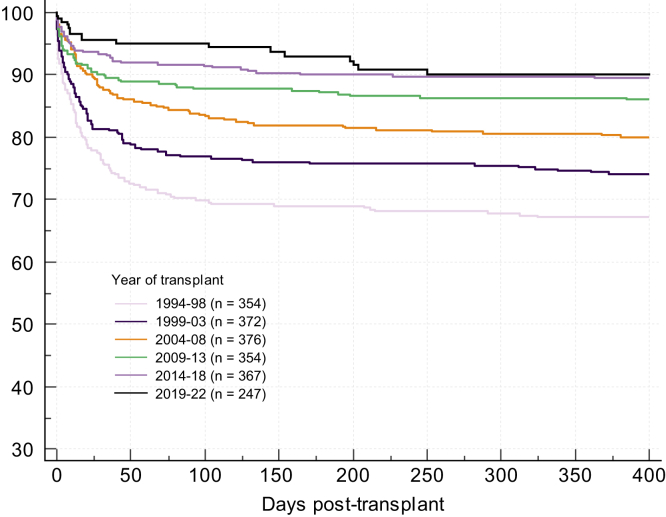
The standard clinical approach to the care of the patient with ALF is to deliver supportive critical care to the multiple organ systems involved and identify (and where possible treat) the specific agent responsible for liver injury. In doing so, conditions for native liver regeneration are optimised and with effective regeneration and return of liver function, multiorgan failure will resolve. Prioritised ELT is reserved for those patients in whom sufficient regeneration will not occur and death is inevitable. Since its first introduction, patient outcomes have incrementally improved over time (Fig. 3) reflecting better understanding of the matching of liver grafts with recipients and progressive improvement in pre-, intra-, and post-operative care.8,9,60
Fig. 3.
Patient survival after liver transplantation for ALF, United Kingdom 1994-2022.
ALF, acute liver failure.
Successful utilisation of ELT requires a reliable means of identifying patients who will benefit at an early enough stage (i.e. before the severity of their illness precludes surgery). Recipient selection criteria therefore require a rapid result with high accuracy, as the consequences of incorrect selection are huge. A false positive result from a selection test will result in unnecessary transplantation in a patient who would otherwise survive with medical management and loss of a graft from the donor pool. The consequences of a false negative selection test are even greater, resulting in a missed ELT candidate and a likely preventable death.
A number of prognostic systems are in current use and share common features. The presence of HE is generally a prerequisite for consideration for ELT, given that in its absence, transplant-free survival (TFS) is generally very good.61 Criteria then consider severity of liver injury, usually assessed through laboratory measures including bilirubin and coagulation disturbance, as reflected by INR or factor V levels.62,63 In some criteria the aetiology of liver injury is also considered, reflecting that for ‘favourable’ causes of liver injury such as paracetamol, TFS may be good despite apparently very severe liver injury, as capacity for native liver regeneration is retained.64 In ‘unfavourable’ aetiologies – exemplified by indolent presentations of ALF of indeterminant cause – equivalent measures of liver injury may be associated with a markedly worse TFS.65,66 Criterion thresholds triggering consideration for ELT may thus vary by aetiology.
The King’s College criteria (KCC) are amongst the most widely utilised prognostic criteria and illustrate this approach and its strengths and weaknesses (Box 3). They were derived and validated from two large retrospective cohorts of patients with ALF treated without ELT between 1973 and 1986.67 The KCC mandate the presence of HE, differentiate between patients with paracetamol-induced hepatotoxicity and other causes of ALF, and utilise basic clinical features and standard laboratory parameters. Initial validation analyses of criteria performance suggested a high specificity such that few cases fulfilling criteria would be unnecessarily transplanted. This performance and apparent simplicity of their clinical application led to their widespread adoption and use. Several meta-analyses have subsequently assessed criteria performance. One of the largest assessed 23 studies comprising 2,153 patients from 1983-2012 and demonstrated high pooled specificity (79%, 95% CI 77-81%) but lower pooled sensitivity (59%, 95% CI 56-62%).68 Subgroup analyses found these findings to be consistent when exploring key confounding factors including size and quality of studies, inclusion/exclusion of transplanted patients as ‘non-survivors’ and the era of the cases reported.68,69
Box 3. The original Kings College criteria.
Non-paracetamol aetiology
Consider transplantation if: encephalopathy and INR >6.7, or any three of: unfavourable aetiology (non-APAP DILI, indeterminate), acute/sub-acute presentation, bilirubin >300 μmol/L, INR >3.5
Paracetamol aetiology
Consider transplantation if: arterial pH <7.3 after adequate intravenous volume resuscitation or concurrent findings of: HE ≥ Grade 3, creatinine >300 μmol/L, INR >6.5
Source: 64.
Alt-text: Box 3
Issues in practical application
The widespread use of the KCC has made apparent some issues in their practical application. What constitutes ‘adequate intravenous fluid administration’ in paracetamol ALF is difficult to quantify and will have significant impact on both KCC parameters of arterial pH and blood lactate concentration. Both may also be modulated by very high concentrations of circulating paracetamol early after overdose, through its impact on mitochondrial function that may precede hepatocellular necrotic injury by many hours.70,71 Further, the clinical and laboratory features of an unintentional, staggered overdose may differ significantly from those of single timepoint consumption.72
Medical interventions may also act as confounders and affect the variables assessed. The administration of sedation may make assessment of HE severity difficult, and RRT will affect both arterial pH and blood creatinine concentration. Administration of blood products such as FFP or TPE will artefactually alter the INR and factor levels.34,73 Finally, ALF may be a dynamic and rapidly progressive illness and patient’s condition, and clinical and laboratory features, may change for better or worse over hours.74
Alternative and supplementary predictive scores and biomarkers
To address the shortfalls in the diagnostic performance of the KCC, proposals have included its replacement with alternate scoring systems and/or the supplemental inclusion of standard laboratory variables or non-standard biomarkers most often reflecting hepatic injury, and less commonly regeneration or severity of multiorgan failure.75,76 To date, most reports of their performance are in cohorts of limited size and are subject to the systematic limitations in assessment as outlined above.69 External validation has been limited for most, and many of the non-standard biomarkers are not measured in routine clinical laboratory settings. Examples of different approaches are presented in Table 4.
Table 4.
Illustrative alternative systems and biomarkers for mortality prediction in acute liver failure.
| Criteria | Era | Aetiology | Cases assessed | Diagnostic performance∗ | Comment |
|---|---|---|---|---|---|
| SOFA score | 1993-2010 | APAP | 125 | Sensitivity 0.67, specificity 0.80 | MOF scoring system77 |
| ALFSG model | 1998-2013 | All | 1,974 | Sensitivity 0.37, specificity 0.95 | Combined clinical criteria65 |
| Arterial lactate | 1998-2000 | APAP | 210 | Sensitivity 0.81, specificity 0.97 | Addition to original KCC78 |
| MELD | 2001-2015 | All | 2,153 | AUROC 0.78 | Meta-analysis68 |
| Dynamic | 2004-2014 | APAP | 762 | AUROC 0.91 | Dynamic clinical model74 |
| MicroRNA | 1998-2014 | APAP | 194 | AUROC 0.83 | Regeneration biomarker model79 |
| FABP1 | 1998-2014 | APAP | 198 | AUROC 0.82 | Cellular injury biomarker80 |
| Factor V | 2001-2017 | All | 90 | AUROC 0.86 | Coagulation factor81 |
| IL-10 | 2007-2009 | APAP | 51 | AUROC 0.92 | Immunoparesis biomarker82 |
| M-30 | 2010-2012 | APAP | 26 | Sensitivity 0.89, specificity 0.61 | Apoptosis biomarker83 |
| 13C-MBT | 2016-2019 | All | 62 | AUROC 0.88 | Hepatic metabolic function test84 |
13C-MBT, 13C-methacetin breath test; ALFSG, Acute Liver Failure Study Group; APAP, acetaminophen; FABP1, fatty acid binding protein 1; IL-10, interleukin 10; M-30, caspase cleaved cytokeratin-18; MELD, model for end-stage liver disease; MOF, multiorgan failure; SOFA, sequential organ failure assessment score.
diagnostic performance in discriminating survivors and non-survivors.
With the exception of arterial blood lactate measurement, few have been widely adopted. Lactate has the advantage of being routinely, rapidly and accurately measured in most critical care settings and persistent elevation is closely related to poor outcomes in many critical illnesses.70 In 2002, blood lactate was proposed for inclusion into the KCC to address their limited sensitivity.78
The UK revised criteria
In the UK, the ELT selection criteria have been revised and recalibrated, recognising marked improvements in TFS over time in some aetiologies of ALF, and increased confidence in the poor prognosis of others (Box 4).8,85,86 These UK Revised Criteria (UKRC), though based around the original KCC, now have seven categories, and include new thresholds for key variables. Recognising the issues in their application, specific guidance is now offered about their interpretation. By example, for the paracetamol criteria, arterial pH thresholds have been lowered and those for blood lactate increased, with specific guidance on their utilisation and interpretation. A further transplant criterion (selection category number 4) has been introduced to give more scope for clinical judgement, recognising that some patients may die without fulfilling the standard criteria. For non-paracetamol cases different laboratory marker thresholds are now provided for favourable and unfavourable causes, and waitlisting is now permitted for some specific cases in advance of the onset of HE (i.e. idiopathic aetiology or idiosyncratic drug reactions with gradual illness onset, deep jaundice and significant coagulopathy and shrinking liver volume). External validation of the UKRC has been limited, but to date the indications are of good performance, with maintained specificity and enhanced sensitivity.
Box 4. The United Kingdom revised ELT selection criteria for acute liver failure in adults.
Category 1
Paracetamol: pH <7.25 more than 24 hours after overdose and after fluid resuscitation.
Category 2
Paracetamol: co-existing INR >6.5, creatinine >300 μmol/L or anuria, and grade 3–4 HE
Category 3
Paracetamol: significant liver injury and coagulopathy following exclusion of other causes of hyperlactatemia (e.g. pancreatitis, intestinal ischaemia) after adequate fluid resuscitation: Arterial lactate >5 mmol/L on admission and >4 mmol/L 24 hours later in presence of HE.
Category 4
Paracetamol: two of the three criteria from category 2 with clinical evidence of deterioration (e.g. increased ICP, FiO2 >50%, increasing inotrope requirements) in the absence of clinical sepsis.
Category 5
Favourable non-paracetamol aetiologies such as acute viral hepatitis or ecstasy/cocaine-induced ALF: the presence of clinical hepatic encephalop-athy is mandatory and: prothrombin time >100 seconds, or INR >6.5, or any three from the following: age >40 or 50 seconds or INR >3.5; any grade of hepatic encephalopathy with jaundice to encephalopathy time >7 days; serum bilirubin >300 μmol/L.
Category 6
Unfavourable non-paracetamol aetiologies such as seronegative or idiosyncratic drug reactions: a) prothrombin time >100 seconds, or INR >6.5, or b) in the absence of clinical hepatic encephalopathy then INR >2 after vitamin K repletion is mandatory and any two from the following: age >40 or 50 seconds or INR >3.5; if hepatic encephalopathy is present then jaundice to encephalopathy time >7 days; serum bilirubin >300 μmol/L.
Category 7
Acute presentation of Wilson’s disease, or Budd-Chiari syndrome. A combination of coagulopathy, and any grade of encephalopathy.
ELT, emergency liver transplantation.
Alt-text: Box 4
Transplant selection criteria and cases transplanted
It is important to recognise that fulfilling ELT selection criteria may identify a patient with poor survival without ELT, but it does not identify those who will tolerate the procedure or have acceptable longer term post-transplant survival. The principal determinants of recipient survival after ELT are age – with an upper threshold of 40-50 years – the severity of multiorgan failure, and the use of a sub-optimal graft.[87], [88], [89] Impaired early graft function is poorly tolerated by critically ill recipients and predisposes to sepsis, the most common cause of early post-ELT death. Many potential recipients may have an underlying comorbidity or acute abdominal complications contraindicating surgery, meaning that ELT may never be an option for them. Other contra-indications include active sepsis or fungal infection, irreversible brain injury from intracranial hypertension, and overwhelming multiorgan failure. Thresholds for the latter must be individualised and take into account candidate age and underlying physical condition – though respiratory failure or high-level vasopressor dependence are a particular concern. Multiorgan failure is generally more severe in cases of paracetamol-related ALF who are more likely to be removed from the waitlist due to deterioration.9,64 A particular challenge is the patient who has been waitlisted for ELT after fulfilling poor prognosis criteria and is now showing signs of improvement: is it better to proceed with transplantation or to remove from the waitlist? Paradoxically, this is also a particular issue for paracetamol-induced ALF, wherein native liver recovery is still possible even in the setting of severe multiorgan failure, and decision-making must be individualised. In more unfavourable aetiologies decision-making is more straightforward and removal from the waitlist is seldom undertaken.
Abbreviations
AIH, autoimmune hepatitis; AKI, acute kidney injury; ALF, acute liver failure; ALI, acute liver injury; CRRT, continuous renal replacement therapy; DILI, drug-induced liver injury; ELT, emergency liver transplantation; FFP, fresh frozen plasma; HBV, hepatitis B virus; HE, hepatic encephalopathy; HELLP, haemolysis, elevated liver enzymes and low platelets; HEV, hepatitis E virus; ICP, intracranial pressure; ICU, intensive care unit; INR, international normalised ratio; KCC, King’s College criteria; RASS, Richmond agitation-sedation scale; RRT, renal replacement therapy; TFS, transplant-free survival; TPE, therapeutic plasma exchange; UKRC, UK Revised criteria.
Financial support
The authors did not receive any financial support to produce this manuscript.
Conflict of interest
The authors of this study declare that they do not have any conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
All authors contributed equally to this work by writing the assigned sections. Sections were suggested by JF and approved by DT, OB and WB.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101131.
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Shingina A., Mukhtar N., Wakim-Fleming J., et al. Acute liver failure guidelines. Am J Gastroenterol. 2023 Jul;118(7):1128–1153. doi: 10.14309/ajg.0000000000002340. [DOI] [PubMed] [Google Scholar]
- 2.Vincent J.L., Moore F.A., Bellomo R., et al., editors. Textbook of critical care. ed. Elsevier; Amsterdam: 2024. [Google Scholar]
- 3.Wendon J., Cordoba J., Dhawan A., et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017 May;66(5):1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Arroyo V., Moreau R., Jalan R. Acute-on-Chronic liver failure. N Engl J Med. 2020 May 28;382(22):2137–2145. doi: 10.1056/NEJMra1914900. [DOI] [PubMed] [Google Scholar]
- 5.Bernal W., Lee W.M., Wendon J., et al. Acute liver failure: a curable disease by 2024? J Hepatol. 2015 Apr 1;62(1, Supplement):S112–S120. doi: 10.1016/j.jhep.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Antoniades C.G., Berry P.A., Wendon J.A., et al. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008 Nov;49(5):845–861. doi: 10.1016/j.jhep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Rolando N. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000 Oct;32(4):734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 8.Bernal W., Hyyrylainen A., Gera A., et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013 Jul;59(1):74–80. doi: 10.1016/j.jhep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Karvellas C.J., Leventhal T.M., Rakela J.L., et al. Outcomes of patients with acute liver failure listed for liver transplantation: a multicenter prospective cohort analysis. Liver Transplant. 2023 Mar;29(3):318–330. doi: 10.1002/lt.26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bower W.A., Johns M., Margolis H.S., et al. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007 Nov;102(11):2459–2463. doi: 10.1111/j.1572-0241.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 11.Bernal W., Wendon J. Acute liver failure. N Engl J Med. 2013 Dec 26;369(26):2525–2534. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 12.Ichai P., Samuel D. Etiology and prognosis of fulminant hepatitis in adults. Liver Transpl. 2008 Sep 29;14(S2):S67–S79. doi: 10.1002/lt.21612. [DOI] [PubMed] [Google Scholar]
- 13.Oketani M., Ido A., Tsubouchi H. Changing etiologies and outcomes of acute liver failure: a perspective from Japan. J Gastro Hepatol. 2011 Jan;26(s1):65–71. doi: 10.1111/j.1440-1746.2010.06574.x. [DOI] [PubMed] [Google Scholar]
- 14.Hwang J.P., Lok A.S.F. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014 Apr;11(4):209–219. doi: 10.1038/nrgastro.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalton H.R., Kamar N., Baylis S.A., et al. EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol. 2018 Jun;68(6):1256–1271. doi: 10.1016/j.jhep.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Epstein Y., Yanovich R. Heatstroke. New Engl J Med. 2019;380(25):2449–2459. doi: 10.1056/NEJMra1810762. [DOI] [PubMed] [Google Scholar]
- 17.Ichai P., Laurent-Bellue A., Camus C., et al. Liver transplantation in patients with liver failure related to exertional heatstroke. J Hepatol. 2019 Mar 1;70(3):431–439. doi: 10.1016/j.jhep.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 18.EASL Clinical Practice Guidelines Autoimmune hepatitis. J Hepatol. 2015 Oct;63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Trovato F.M., Rabinowich L., McPhail M.J.W. Update on the management of acute liver failure. Curr Opin Crit Care. 2019 Apr;25(2):157–164. doi: 10.1097/MCC.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 20.Guillaud O., Brunet A.S., Mallet I., et al. Relative exchangeable copper: a valuable tool for the diagnosis of Wilson disease. Liver Int. 2018 Feb;38(2):350–357. doi: 10.1111/liv.13520. [DOI] [PubMed] [Google Scholar]
- 21.European Association for Study of Liver EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol. 2012 Mar;56(3):671–685. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Évole H., López Del Campo R., Bassegoda O. A worrisome liver doppelgänger. Gastroenterology. 2024 Jan;8(24):S0016–S5085. doi: 10.1053/j.gastro.2023.12.025. 00008-8. [DOI] [PubMed] [Google Scholar]
- 23.Stift J., Semmler G., Walzel C., et al. Transjugular aspiration liver biopsy performed by hepatologists trained in HVPG measurements is safe and provides important diagnostic information. Dig Liver Dis. 2019 Aug;51(8):1144–1151. doi: 10.1016/j.dld.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Ichai P., Roque Afonso A.M., Sebagh M., et al. Herpes simplex virus-associated acute liver failure: a difficult diagnosis with a poor prognosis. Liver Transpl. 2005 Dec;11(12):1550–1555. doi: 10.1002/lt.20545. [DOI] [PubMed] [Google Scholar]
- 25.Lefkowitch J.H., Scheuer P.J. Elsevier; Edinburgh: 2016. Scheuer’s liver biopsy interpretation. [Google Scholar]
- 26.Williamson C., Nana M., Poon L., et al. EASL Clinical Practice Guidelines on the management of liver diseases in pregnancy. J Hepatol. 2023 Sep 1;79(3):768–828. doi: 10.1016/j.jhep.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Tujios S., Stravitz R.T., Lee W.M. Management of acute liver failure: update 2022. Semin Liver Dis. 2022 Aug;42(3):362–378. doi: 10.1055/s-0042-1755274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee W.M., Hynan L.S., Rossaro L., et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009 Sep;137(3):856–864. doi: 10.1053/j.gastro.2009.06.006. 864.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amjad W., Thuluvath P., Mansoor M., et al. N-acetylcysteine in non-acetaminophen-induced acute liver failure: a systematic review and meta-analysis of prospective studies. Prz Gastroenterol. 2022;17(1):9–16. doi: 10.5114/pg.2021.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karkhanis J., Verna E.C., Chang M.S., et al. Steroid use in acute liver failure. Hepatology. 2014 Feb;59(2):612–621. doi: 10.1002/hep.26678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Audimoolam V.K., McPhail M.J.W., Willars C., et al. Predicting fluid responsiveness in acute liver failure: a prospective study. Anesth Analg. 2017 Feb;124(2):480–486. doi: 10.1213/ANE.0000000000001585. [DOI] [PubMed] [Google Scholar]
- 32.Stravitz R.T., Fontana R.J., Karvellas C., et al. Future directions in acute liver failure. Hepatology. 2023 Oct 1;78(4):1266–1289. doi: 10.1097/HEP.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald A.J., Subramanian R.M., Olson J.C., et al. Use of the molecular adsorbent recirculating system in acute liver failure: results of a multicenter propensity score-matched study. Crit Care Med. 2022 Feb 1;50(2):286–295. doi: 10.1097/CCM.0000000000005194. [DOI] [PubMed] [Google Scholar]
- 34.Larsen F.S., Schmidt L.E., Bernsmeier C., et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. 2016 Jan 1;64(1):69–78. doi: 10.1016/j.jhep.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Dong V., Sun K., Gottfried M., et al. Significant lung injury and its prognostic significance in acute liver failure: a cohort analysis. Liver Int. 2020 Mar;40(3):654–663. doi: 10.1111/liv.14268. [DOI] [PubMed] [Google Scholar]
- 36.Audimoolam V.K., McPhail M.J.W., Wendon J.A., et al. Lung injury and its prognostic significance in acute liver failure. Crit Care Med. 2014 Mar;42(3):592–600. doi: 10.1097/01.ccm.0000435666.15070.d5. [DOI] [PubMed] [Google Scholar]
- 37.Park J., Lin M.Y., Wray C.L., et al. Applications and outcomes of extracorporeal life support use in adult liver transplantation: a case series and review of literature. ASAIO J. 2022 May 1;68(5):683–690. doi: 10.1097/MAT.0000000000001562. [DOI] [PubMed] [Google Scholar]
- 38.Cardoso F.S., Fidalgo P., Bagshaw S.M., et al. Persistent but not transient acute kidney injury was associated with lower transplant-free survival in patients with acute liver failure: a multicenter cohort study. Crit Care Med. 2022 Sep 1;50(9):1329–1338. doi: 10.1097/CCM.0000000000005563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coelho S., Fonseca J.N., Gameiro J., et al. Transient and persistent acute kidney injury in acute liver failure. J Nephrol. 2019 Apr;32(2):289–296. doi: 10.1007/s40620-018-00568-w. [DOI] [PubMed] [Google Scholar]
- 40.Tujios S.R., Hynan L.S., Vazquez M.A., et al. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol. 2015 Feb;13(2):352–359. doi: 10.1016/j.cgh.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warrillow S., Fisher C., Tibballs H., et al. Continuous renal replacement therapy and its impact on hyperammonaemia in acute liver failure. Crit Care Resusc. 2020 Jun;22(2):158–165. doi: 10.51893/2020.2.oa6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong V., Robinson A.M., Dionne J.C., et al. Continuous renal replacement therapy and survival in acute liver failure: a systematic review and meta-analysis. J Crit Care. 2024 Jan 8;81 doi: 10.1016/j.jcrc.2023.154513. [DOI] [PubMed] [Google Scholar]
- 43.Cardoso F.S., Gottfried M., Tujios S., et al. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology. 2018 Feb;67(2):711–720. doi: 10.1002/hep.29488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasques F., Cavazza A., Bernal W. Acute liver failure. Curr Opin Crit Care. 2022 Apr;28(2):198. doi: 10.1097/MCC.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 45.Bischoff S.C., Bernal W., Dasarathy S., et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. 2020 Dec;39(12):3533–3562. doi: 10.1016/j.clnu.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Stravitz R.T., Ellerbe C., Durkalski V., et al. Bleeding complications in acute liver failure. Hepatology. 2018 May;67(5):1931–1942. doi: 10.1002/hep.29694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim A., Niu B., Woreta T., et al. Clinical considerations of coagulopathy in acute liver failure. J Clin Transl Hepatol. 2020 Dec 28;8(4):407–413. doi: 10.14218/JCTH.2020.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stravitz R.T., Fontana R.J., Meinzer C., et al. Coagulopathy, bleeding events, and outcome according to rotational thromboelastometry in patients with acute liver injury/failure. Hepatology. 2021 Aug;74(2):937–949. doi: 10.1002/hep.31767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karvellas C.J., Pink F., McPhail M., et al. Predictors of bacteraemia and mortality in patients with acute liver failure. Intensive Care Med. 2009 Aug;35(8):1390–1396. doi: 10.1007/s00134-009-1472-x. [DOI] [PubMed] [Google Scholar]
- 50.Rolando N., Philpott-Howard J., Williams R. Bacterial and fungal infection in acute liver failure. Semin Liver Dis. 1996 Nov;16(4):389–402. doi: 10.1055/s-2007-1007252. [DOI] [PubMed] [Google Scholar]
- 51.Kaur P., Verma N., Valsan A., et al. Prevalence, risk factors, and impact of bacterial or fungal infections in acute liver failure patients from India. Dig Dis Sci. 2023 Oct;68(10):4022–4038. doi: 10.1007/s10620-023-07971-9. [DOI] [PubMed] [Google Scholar]
- 52.Zider A.D., Zopey R., Garg R., et al. Prognostic significance of infections in critically ill adult patients with acute liver injury: a retrospective cohort study. Liver Int. 2016 Aug;36(8):1143–1150. doi: 10.1111/liv.13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacDonald A.J., Speiser J.L., Ganger D.R., et al. Clinical and neurologic outcomes in acetaminophen-induced acute liver failure: a 21-year multicenter cohort study. Clin Gastroenterol Hepatol. 2021 Dec;19(12):2615–2625.e3. doi: 10.1016/j.cgh.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escorsell À., Mas A., De La Mata M., et al. Acute liver failure in Spain: analysis of 267 cases. Liver Transpl. 2007 Oct;13(10):1389–1395. doi: 10.1002/lt.21119. [DOI] [PubMed] [Google Scholar]
- 55.Robba C., Santori G., Czosnyka M., et al. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2018 Aug;44(8):1284–1294. doi: 10.1007/s00134-018-5305-7. [DOI] [PubMed] [Google Scholar]
- 56.Jalan R., Pollok A., Shah S.H.A., et al. Liver derived pro-inflammatory cytokines may be important in producing intracranial hypertension in acute liver failure. J Hepatol. 2002 Oct;37(4):536–538. doi: 10.1016/s0168-8278(02)00240-4. [DOI] [PubMed] [Google Scholar]
- 57.Larsen F.S. Artificial liver support in acute and acute-on-chronic liver failure. Curr Opin Crit Care. 2019 Apr;25(2):187–191. doi: 10.1097/MCC.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 58.Demetriou A.A., Brown R.S., Busuttil R.W., et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004 May;239(5):660–667. doi: 10.1097/01.sla.0000124298.74199.e5. discussion 667-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maiwall R., Sarin S.K. Plasma exchange in acute and acute on chronic liver failure. Semin Liver Dis. 2021 Nov;41(4):476–494. doi: 10.1055/s-0041-1730971. [DOI] [PubMed] [Google Scholar]
- 60.O’Grady J.G., Williams R., Calne R. Transplantation in fulminant hepatic failure. Lancet. 1986 Nov 22;2(8517):1227. doi: 10.1016/s0140-6736(86)92247-6. [DOI] [PubMed] [Google Scholar]
- 61.Nakao M., Nakayama N., Uchida Y., et al. Nationwide survey for acute liver failure and late-onset hepatic failure in Japan. J Gastroenterol. 2018 Jun;53(6):752–769. doi: 10.1007/s00535-017-1394-2. [DOI] [PubMed] [Google Scholar]
- 62.Portmann B., Talbot I.C., Day D.W., et al. Histopathological changes in the liver following a paracetamol overdose: correlation with clinical and biochemical parameters. J Pathol. 1975 Nov;117(3):169–181. doi: 10.1002/path.1711170307. [DOI] [PubMed] [Google Scholar]
- 63.Harrison P.M., O’Grady J.G., Keays R.T., et al. Serial prothrombin time as prognostic indicator in paracetamol induced fulminant hepatic failure. BMJ. 1990 Oct 27;301(6758):964–966. doi: 10.1136/bmj.301.6758.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernal W. Acute liver failure on the transplant waiting list: acetaminophen both better and worse? Liver Transpl. 2022 Jan;28(1):11–12. doi: 10.1002/lt.26274. [DOI] [PubMed] [Google Scholar]
- 65.Koch D.G., Tillman H., Durkalski V., et al. Development of a model to predict transplant-free survival of patients with acute liver failure. Clin Gastroenterol Hepatol. 2016 Aug;14(8):1199–1206.e2. doi: 10.1016/j.cgh.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel P.V., Livingston S., Rakela J.L., et al. Indeterminate etiology of acute liver failure in North America: less common, still grave prognosis. Clin Transpl. 2023 Dec;37(12) doi: 10.1111/ctr.15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Grady J.G., Alexander G.J., Hayllar K.M., et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989 Aug;97(2):439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 68.McPhail M.J.W., Farne H., Senvar N., et al. Ability of King’s College criteria and model for end-stage liver disease scores to predict mortality of patients with acute liver failure: a meta-analysis. Clin Gastroenterol Hepatol. 2016 Apr;14(4):516–525. doi: 10.1016/j.cgh.2015.10.007. e5; quiz e43–5. [DOI] [PubMed] [Google Scholar]
- 69.Bernal W., Wendon J. Liver transplantation in adults with acute liver failure. J Hepatol. 2004 Feb;40(2):192–197. doi: 10.1016/j.jhep.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 70.Shah A.D., Wood D.M., Dargan P.I. Understanding lactic acidosis in paracetamol (acetaminophen) poisoning. Br J Clin Pharmacol. 2011 Jan;71(1):20–28. doi: 10.1111/j.1365-2125.2010.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esterline R.L., Ray S.D., Ji S. Reversible and irreversible inhibition of hepatic mitochondrial respiration by acetaminophen and its toxic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI) Biochem Pharmacol. 1989 Jul 15;38(14):2387–2390. doi: 10.1016/0006-2952(89)90481-4. [DOI] [PubMed] [Google Scholar]
- 72.Craig D.G.N., Bates C.M., Davidson J.S., et al. Staggered overdose pattern and delay to hospital presentation are associated with adverse outcomes following paracetamol-induced hepatotoxicity. Br J Clin Pharmacol. 2012 Feb;73(2):285–294. doi: 10.1111/j.1365-2125.2011.04067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maiwall R., Bajpai M., Singh A., et al. Standard-volume plasma exchange improves outcomes in patients with acute liver failure: a randomized controlled trial. Clin Gastroenterol Hepatol. 2022 Apr;20(4):e831–e854. doi: 10.1016/j.cgh.2021.01.036. [DOI] [PubMed] [Google Scholar]
- 74.Bernal W., Wang Y., Maggs J., et al. Development and validation of a dynamic outcome prediction model for paracetamol-induced acute liver failure: a cohort study. Lancet Gastroenterol Hepatol. 2016 Nov;1(3):217–225. doi: 10.1016/S2468-1253(16)30007-3. [DOI] [PubMed] [Google Scholar]
- 75.Bernal W., Williams R. Beyond KCH selection and options in acute liver failure. Hepatol Int. 2018 May;12(3):204–213. doi: 10.1007/s12072-018-9869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rakela J.L., Karvellas C.J., Koch D.G., et al. Acute liver failure: biomarkers evaluated by the acute liver failure study Group. Clin Transl Gastroenterol. 2023 Apr 1;14(4) doi: 10.14309/ctg.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cholongitas E., Theocharidou E., Vasianopoulou P., et al. Comparison of the sequential organ failure assessment score with the King’s College Hospital criteria and the model for end-stage liver disease score for the prognosis of acetaminophen-induced acute liver failure. Liver Transpl. 2012 Apr;18(4):405–412. doi: 10.1002/lt.23370. [DOI] [PubMed] [Google Scholar]
- 78.Bernal W., Donaldson N., Wyncoll D., et al. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002 Feb 16;359(9306):558–563. doi: 10.1016/S0140-6736(02)07743-7. [DOI] [PubMed] [Google Scholar]
- 79.Tavabie O.D., Karvellas C.J., Salehi S., et al. A novel microRNA-based prognostic model outperforms standard prognostic models in patients with acetaminophen-induced acute liver failure. J Hepatol. 2021 Aug;75(2):424–434. doi: 10.1016/j.jhep.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karvellas C.J., Speiser J.L., Tremblay M., et al. Elevated FABP1 serum levels are associated with poorer survival in acetaminophen-induced acute liver failure. Hepatology. 2017 Mar;65(3):938–949. doi: 10.1002/hep.28945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patidar K.R., Davis B.C., Slaven J.E., et al. Admission factor V predicts transplant-free survival in acute liver failure. Dig Dis Sci. 2021 Feb;66(2):619–627. doi: 10.1007/s10620-020-06197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berry P.A., Antoniades C.G., Hussain M.J., et al. Admission levels and early changes in serum interleukin-10 are predictive of poor outcome in acute liver failure and decompensated cirrhosis. Liver Int. 2010 May;30(5):733–740. doi: 10.1111/j.1478-3231.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- 83.Possamai L.A., McPhail M.J.W., Quaglia A., et al. Character and temporal evolution of apoptosis in acetaminophen-induced acute liver failure. Crit Care Med. 2013 Nov;41(11):2543–2550. doi: 10.1097/CCM.0b013e31829791a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fontana R.J., Stravitz R.T., Durkalski V., et al. Prognostic value of the 13 C-methacetin breath test in adults with acute liver failure and non-acetaminophen acute liver injury. Hepatology. 2021 Aug;74(2):961–972. doi: 10.1002/hep.31783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reuben A., Tillman H., Fontana R.J., et al. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann Intern Med. 2016 Jun 7;164(11):724–732. doi: 10.7326/M15-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donnelly M.C., Davidson J.S., Martin K., et al. Acute liver failure in Scotland: changes in aetiology and outcomes over time (the Scottish Look-Back Study) Aliment Pharmacol Ther. 2017 Mar;45(6):833–843. doi: 10.1111/apt.13943. [DOI] [PubMed] [Google Scholar]
- 87.Germani G., Theocharidou E., Adam R., et al. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol. 2012 Aug;57(2):288–296. doi: 10.1016/j.jhep.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 88.Bernal W., Cross T.J.S., Auzinger G., et al. Outcome after wait-listing for emergency liver transplantation in acute liver failure: a single centre experience. J Hepatol. 2009 Feb;50(2):306–313. doi: 10.1016/j.jhep.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 89.Porteous J., Cioccari L., Ancona P., et al. Outcome of acetaminophen-induced acute liver failure managed without intracranial pressure monitoring or transplantation. Liver Transpl. 2019 Jan;25(1):35–44. doi: 10.1002/lt.25377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.