Abstract
Background
The combination of immunotherapy and antiangiogenic therapy has shown potential in the treatment of numerous malignant tumors, but limited evidence was available for soft tissue sarcomas (STS). Therefore, the aim of the present study is to assess the efficacy and safety of immunotherapy in conjunction with antiangiogenic therapy in patients diagnosed with advanced STS (aSTS).
Methods
The study enrolled patients with aSTS from January 2014 to October 2022. Eligible participants had previously received anthracycline-based chemotherapy, presented with an anthracycline-resistant sarcoma subtype, or were ineligible for anthracycline treatment due to medical conditions. Following enrollment, these patients received a combination of immunotherapy and antiangiogenic therapy. The primary endpoints were the objective response rate (ORR) and progression-free survival (PFS), while the secondary endpoints included the disease control rate (DCR), overall survival (OS), and the incidence of adverse events.
Results
Fifty-one patients were included in this cohort study. The median duration of follow-up was 15.8 months. The ORR and DCR were 17.6%, and 76.5%, respectively. The median PFS (mPFS) was 5.8 months (95% CI: 4.8–6.8) for all patients, and the median OS had not been reached as of the date cutoff. Multivariate analysis indicated that Eastern Cooperative Oncology Group performance status of 0–1 and ≤ second-line treatment were positive predictors for both PFS and OS. Patients with alveolar soft part sarcoma or clear cell sarcoma had longer mPFS (16.2 months, 95% CI: 7.8–25.6) when compared to those with other subtypes of STS (4.4 months, 95% CI: 1.4–7.5, P < 0.001). Among the observed adverse events, hypertension (23.5%), diarrhea (17.6%), and proteinuria (17.6%) were the most common, with no treatment-related deaths reported.
Conclusion
The combination of immunotherapy and antiangiogenic agents showed promising efficacy and acceptable toxicity in patients with aSTS, especially those with alveolar soft part sarcoma or clear cell sarcoma.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-12810-9.
Keywords: Efficacy, Safety, Immunotherapy, Antiangiogenic therapy, Soft tissue sarcoma
Introduction
As a cohort of rare malignancies, soft tissue sarcomas (STS) constitute a mere 1% of all adult cancers, with a prevalence of less than 6 cases per 100,000 [1, 2]. For patients with localized STS, wide resection surgery with negative margins is the standard treatment, and both radiotherapy and chemotherapy are recommended for those with a high risk of local recurrence or metastasis [3]. Unfortunately, about one-third of patients with localized STS will encounter disease relapse or metastasis [3–5], resulting in unfavorable prognosis. For those with advanced STS (aSTS), anthracycline-based chemotherapy is commonly utilized as the initial treatment approach. However, the objective response rate (ORR) ranges only from 5 to 20%, with a poor median progression-free survival (mPFS) of 4.2 months [3]. Further lines of chemotherapy including eribulin, dacarbazine, trabectedin, and gemcitabine plus docetaxel also showed limited efficacy or poor tolerance for some patients [6–10]. Consequently, there is an urgent need for treatment innovations to improve the prognosis of patients with aSTS.
Recently, a few studies have explored the role of immunotherapy combined with antiangiogenic therapy in patients with aSTS. A previous study has shown that the ORR for STS receiving immunotherapy plus antiangiogenic agents was approximately 20%, with a mPFS of 7.0 months [11]. A recent meta-analysis revealed an ORR of 24% for this combination in patients with STS, whereas immune monotherapy alone yielded a lower pooled ORR of only 14% [12]. Notably, the study by You et al. demonstrated the ORR among STS treated with immune checkpoint inhibitors and antiangiogenic agents reached 30% [13], while another retrospective study conducted by Liu and his colleagues reported a remarkable ORR of 48.1% in patients with STS [14]. In a previous prospective phase II trial, the combination of axitinib and pembrolizumab demonstrated a 3-month PFS rate of 65.6%, indicating its preliminary effectiveness in patients with aSTS [15]. Furthermore, findings from Martin revealed that nearly half of the patients treated with nivolumab and sunitinib remained progression-free at the 6-month mark, underscoring the promising potential of this combination therapy for managing aSTS [16].
To date, several studies with limited samples have examined the efficacy and safety of combined immunotherapy and antiangiogenic therapy for aSTS in real-world settings. The results also varied from different studies. Of note, a few studies further identified the potential patients who may benefit from this treatment strategy. Herein, in this retrospective study, we aimed to evaluate the efficacy and safety of immunotherapy in conjunction with antiangiogenic therapy in aSTS, and further explored the prognostic factors associated with this combination strategy.
Methods and materials
This retrospective study enrolled hospitalized patients admitted to the Department of Medical Oncology at the Second Affiliated Hospital, Zhejiang University School of Medicine from January 2014 to October 2022. Due to the anonymous, retrospective, and nonintervention nature of this study, informed consent from all patients was waived and this study was approved by the Ethic Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine (approve number: 20230918).
Study subjects
With the assistance of the Electronic Case Record System, patients with a discharge diagnosis of aSTS and receiving immunotherapy combined with antiangiogenic therapy were screened. Patients who met the following criteria were included: (1) diagnosed with STS based on histology, with metastasis at the time of initial diagnosis or experiencing progression after previous treatment; (2) not qualified for surgery, and were anthracycline-resistant or not eligible for chemotherapy; (3) at least one measurable tumor lesion as confirmed by Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). Subjects were excluded if they presented (1) with a second primary tumor; (2) with a history of immune disease; (3) with only one cycle therapy; (4) no available data for baseline assessment and response assessment; and (5) no available follow-up data. For patients enrolled, the demographic and clinical profiles, including gender, age, the Eastern Cooperative Oncology Group (ECOG) performance status score, primary and metastasis site of the tumor, the subtype of STS, and previous treatment were collected.
Efficacy and safety assessment
The efficacy measures of this study included ORR, disease control rate (DCR), PFS (time from initial treatment to disease progression), and overall survival (OS, time from initial treatment to death). ORR is defined as the percentage of patients with complete response (CR) or partial response (PR) according to the RECIST 1.1, while DCR refers to the portion of patient with CR, PR or disease stable (SD) as per RECIST 1.1.
All patients were evaluated for treatment-related adverse events (TRAEs) according to the Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE 5.0). Patients were observed for adverse events which occurred during the treatment. The clinical manifestations, the occurrence and severity were recorded.
Statistical analysis
All the statistical analysis was performed with SPSS 25.0 software (IBM, SPSS, Chicago, IL, USA) and GraphPad Prism 9.2 (GraphPad Software, San Diego, CA, USA). The categorical variables were tested for normality by the Shapiro-Wilk test. When variables conformed to a normal distribution, they were presented as mean ± standard deviations and compared using a one-way analysis of variance and t-test. When data did not exhibit normal distribution, they were presented as median and interquartile range and analyzed using a non-parametric equivalent. Categorical variables were presented as numbers (frequency) and compared using the Chi-square test. The Kaplan-Meier method was used to estimate the median PFS and OS for each treatment group with 95% confidence intervals (CIs). Differences in Kaplan–Meier survival curves were assessed using the log-rank test. Univariate analysis and a multivariate Cox regression model were utilized to investigate the risk factors of PFS and OS, which was estimated by Hazard ratio (HR) and 95% CI. The statistical significance was set at P < 0.05, and all tests were 2-tailed.
Results
Demographic and clinical characteristics
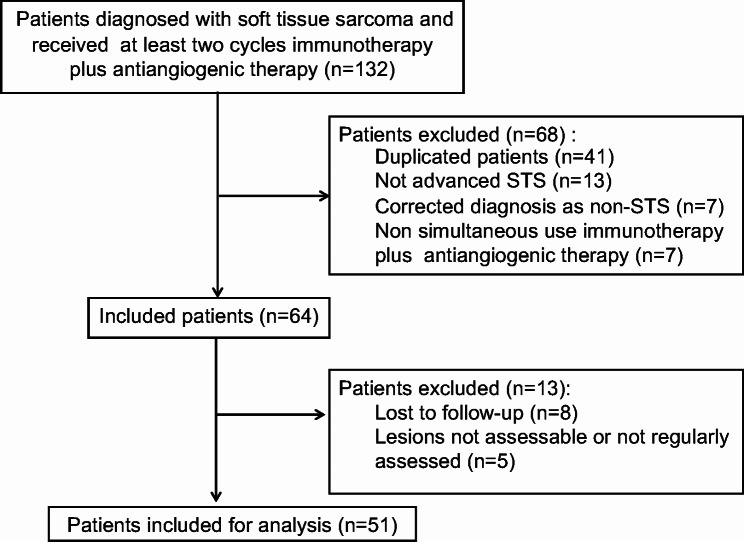
In total, 51 patients with aSTS met the criteria and were included in the final analysis (see Fig. 1). The combination strategy was conducted according to individual characteristics, previous treatment, patients’ willingness, and economic status. The basic characteristics of the included patients are presented in Table 1. Among the included patients, 26 patients (51.0%) were female, and the remaining 25 (49.0%) were male. The median age of all patients was 54.0 years (range: 15–80 years), and 28 patients (54.9%) had an ECOG performance status score of 0 or 1. Approximately half of the patients received this combination as second-line treatment (n = 24, 47.1%), 12 patients as first-line treatment, and the remaining 15 patients as ≥ third-line treatment. In this cohort, patients were given different combination strategies of immunotherapy and antiangiogenic drugs (see Supplementary Material Table S1). Anlotinib was the most commonly used antiangiogenic agent (n = 39, 76.5%), while tislelizumab was the most frequently used immunotherapy (n = 17, 33.3%), followed by camrelizumab (n = 11, 21.6%), sintilimab (n = 10, 19.6%), and pembrolizumab (n = 7, 13.7%).
Fig. 1.
The flow chart of population selection
Table 1.
Demographic and clinical profiles of all subjects
| Characteristics and previous therapeutic strategies | n (%) |
|---|---|
| Gender | |
| Male | 25 (49.0) |
| Female | 26 (51.0) |
| Age | |
| < 54 years old | 24 (45.1) |
| ≥ 54 years old | 27 (54.9) |
| ECOG performance status | |
| 0–1 | 28 (54.9) |
| 2–3 | 23 (45.1) |
| Subtype | |
| Leiomyosarcoma | 11 (21.6) |
| Alveolar soft part sarcoma | 6 (11.8) |
| Rhabdomyosarcoma | 6 (11.8) |
| Clear cell sarcoma | 5 (9.8) |
| Myxofibrosarcoma | 5 (9.8) |
| Synovial sarcoma | 3 (5.9) |
| Malignant peripheral nerve sheath tumor | 3 (5.9) |
| Undifferentiated pleomorphic sarcoma | 3 (5.9) |
| Others | 9 (17.6) |
| Primary site | |
| Limb | 28 (54.9) |
| Trunk | 17 (33.3) |
| Others | 6 (11.8) |
| Metastasis present | |
| Lung | 33 (64.7) |
| Bone | 18 (35.3) |
| Liver | 6 (11.8) |
| Pancreas | 5 (9.8) |
| Brain | 3 (5.9) |
| Treatment lines | |
| 1 | 12 (23.5) |
| 2 | 24 (47.1) |
| ≥ 3 | 15 (29.4) |
| Prior chemotherapy | |
| Yes | 32 (62.7) |
| No | 19 (37.3) |
| Prior radiotherapy | |
| Yes | 9 (17.6) |
| No | 42 (82.4) |
| Prior antiangiogenic therapy | |
| Yes | 21 (41.2) |
| No | 30 (58.8) |
| Prior immunotherapy | |
| Yes | 4 (7.8) |
| No | 47 (92.2) |
Abbreviation: ECOG: Eastern Cooperative Oncology Group
Efficacy assessment and prognostic predictors
The patients in this study underwent radiographic evaluation at an average of 7.2 ± 2.9 weeks after receiving the initial combination treatment. By the time of data lock (Feb 1st, 2023), the median duration of follow-up was 15.8 months (95% CI: 11.5–20.1). Out of the 51 patients, 1 patient (2.0%) achieved CR, 8 patients (15.7%) achieved PR, and 30 patients (58.8%) had SD. The ORR and DCR were 17.6% and 76.5%, respectively.
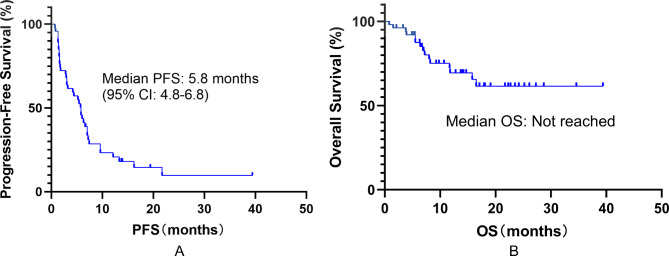
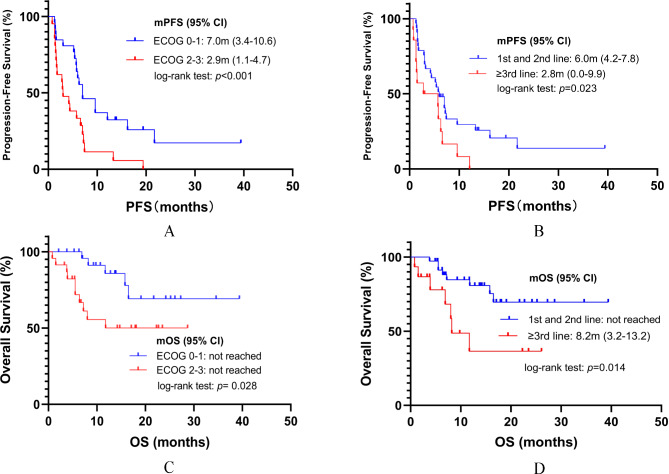
The mPFS for all patients was 5.8 months (95% CI: 4.8–6.8), and the median OS (mOS) had not been reached as of the date cut-off (see Fig. 2). We further explored the effect of different variables on survival outcomes using univariate and multivariate Cox regression analysis. All variables displaying significant correlations (P < 0.05) in univariate analysis were included in multivariate analysis. The results of multivariate Cox regression indicated ECOG performance status of 2–3 and ≥ third-line treatment were negative predictors for both PFS (ECOG performance status: HR: 3.26, 95% CI: 1.64–6.25, P = 0.001; treatment line: HR: 2.28, 95% CI: 1.13–4.57, P = 0.021) and OS (ECOG performance status: HR: 2.96, 95% CI: 1.00-8.70, P = 0.049; treatment line: HR: 3.12, 95% CI: 1.12–8.59, P = 0.030, see Tables 2 and 3). Kaplan–Meier survival curves were performed based on the significant prognostic factors in multivariate Cox regression analysis. Patients with ECOG performance status 2–3 (7.0 vs. 2.9 months P < 0.001) or ≥ third-line treatment (6.0 vs. 2.8 months P = 0.023) had significantly shorter mPFS when compared to those without it (Fig. 3A and B). In addition, ECOG performance status 2–3 (P = 0.028) and ≥ third-line treatment (P = 0.014) were also significantly associated with shorter mOS (Fig. 3C and D). Furthermore, in our cohort, patients with ASPS had the longest mPFS (not reached), followed by CCS (16.2 months, 95% CI: 0.0-35.9). while patients with myxofibrosarcoma had the shortest mPFS (0.8 months, 95% CI: 0.0-4.4), followed by LMS (2.8 months, 95%CI: 0.6-5.0, see Supplementary Material Table S2). The result of the log-rank test also showed that patients with ASPS or CCS had longer mPFS (16.2 months, 95% CI: 7.8–25.6) when compared to those with other subtypes of STS (4.4 months, 95% CI: 1.4–7.5, P < 0.001).
Fig. 2.
(A) Kaplan-Meier estimates for PFS in all 51 patients; (B) Kaplan-Meier estimates for OS in all 51 patients
Abbreviation: PFS: progression-free survival; OS: overall survival; CI: confidence interval
Table 2.
Univariate analyses of factors associated with PFS and OS
| Characteristic | Category | PFS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Gender | Male vs. female | 1.31 (0.69–2.50) | 0.412 | 0.74 (0.27–2.04) | 0.554 |
| Age | < 54 vs. ≥54 years | 0.53 (0.26–1.06) | 0.071 | 0.72 (0.26–2.04) | 0.541 |
| ECOG performance status | 0–1 vs. 2–3 | 0.32 (0.16–0.63) | 0.001 | 0.32 (0.11–0.94) | 0.038 |
| Primary site | Limbs vs. trunks vs. others | 0.59 (0.31–1.12) | 0.108 | 0.68 (0.25–1.89) | 0.464 |
| Metastasis organs | None/single vs. multiple organs | 0.54 (0.26–1.12) | 0.098 | 0.42 (0.12–1.48) | 0.176 |
| Lung metastasis | Yes vs. no | 0.85 (0.44–1.66) | 0.639 | 1.01 (0.34–2.95) | 0.991 |
| Liver metastasis | Yes vs. no | 2.00 (0.74–5.36) | 0.171 | 2.39 (0.67–8.52) | 0.179 |
| Bone metastasis | Yes vs. no | 1.00 (0.52–1.95) | 0.989 | 0.84 (0.30–2.38) | 0.748 |
| Treatment lines | 1–2 vs. ≥3 | 0.47 (0.23–0.92) | 0.028 | 0.30 (0.11–0.83) | 0.022 |
| Prior chemotherapy | Yes vs. no | 2.00 (0.99–4.04) | 0.055 | 4.42 (1.00-19.62) | 0.051 |
| Prior radiotherapy | Yes vs. no | 2.02 (0.94–4.35) | 0.074 | 2.24 (0.76–6.57) | 0.142 |
| Prior antiangiogenic therapy | Yes vs. no | 1.13 (0.59–2.17) | 0.713 | 0.84 (0.28–2.47) | 0.746 |
| Prior immunotherapy | Yes vs. no | 1.10 (0.50–2.42) | 0.806 | 0.36 (0.05–2.72) | 0.319 |
Abbreviation: PFS: progression-free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; ECOG: Eastern Cooperative Oncology Group
Table 3.
Multivariate analyses of factors associated with PFS and OS
| Characteristic | Category | PFS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| ECOG performance status | 0–1 vs. 2–3 | 0.31 (0.16–0.61) | 0.001 | 0.34 (0.12-1.00) | 0.049 |
| Treatment lines | 1–2 vs. ≥3 | 0.44 (0.22–0.88) | 0.021 | 0.32 (0.12–0.89) | 0.030 |
Abbreviation: PFS: progression-free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; ECOG: Eastern Cooperative Oncology Group
Fig. 3.
(A) Kaplan-Meier analyses for PFS based on the ECOG level; (B) Kaplan-Meier analyses for PFS based on the treatment line; (C) Kaplan-Meier analyses for OS based on the ECOG level; (D) Kaplan-Meier analyses for OS based on the treatment line
Abbreviation: mPFS: median progression-free survival; ECOG: Eastern Cooperative Oncology Group; CI: confidence interval; mOS: median overall survival
Safety assessment
Among the 51 patients included in this study, 39 patients (76.5%) experienced one or more TRAEs. The most frequently reported TRAEs of any grade were hypertension (n = 12, 23.5%), diarrhea (n = 9, 17.6%), proteinuria (n = 9, 17.6%), and anemia (n = 8, 15.7%). Grade 3–4 TRAEs were observed in 15.7% of patients, with anemia (n = 2, 3.9%), hand-foot syndrome (n = 2, 3.9%), and hemorrhage (n = 2, 3.9%) being the most common. Notably, no deaths related to the treatment occurred. The detailed TRAEs of included patients are shown in Table 4.
Table 4.
Treatment-related adverse events (TRADEs) of all patients
| TRADEs | Any grade n (%) |
3/4 grade n (%) |
|---|---|---|
| Hypertension | 12(23.5) | 0 |
| Diarrhea | 9(17.6) | 1(2.0) |
| Proteinuria | 9(17.6) | 1(2.0) |
| Anemia | 8(15.7) | 2(3.9) |
| Fatigue | 7(13.7) | 0 |
| Hand foot syndrome | 7(13.7) | 2(3.9) |
| Renal insufficiency | 7(13.7) | 0 |
| Thrombocytopenia | 6(11.8) | 0 |
| White blood cell count decreased | 6(11.8) | 0 |
| Hemorrhage | 5(9.8) | 2(3.9) |
| Liver dysfunction | 4(7.8) | 0 |
| Nausea / Vomiting | 3(5.9) | 0 |
| Interstitial pneumonia | 3(5.9) | 0 |
| Toothache | 2(3.9) | 0 |
| Oral mucositis | 2(3.9) | 0 |
| Interstitial pneumonia | 2(3.9) | 0 |
| Hypothyroidism | 2(3.9) | 0 |
| Elevated bilirubin | 2(3.9) | 0 |
| Musculoskeletal pain | 1(2.0) | 0 |
Discussion
In this study, we assessed the efficacy and safety of combining immunotherapy and antiangiogenic therapy in a real-world group of individuals with aSTS. The overall ORR was 17.6% and the mPFS was 5.8 months, with manageable toxicity observed in all patients. Consistent with the previous studies, our study supports the potential of immunotherapy plus antiangiogenic therapy for patients with advanced ASPS and CCS. In Liu’s study, the ORR was up to 48.1%, and one of the most possible explanations is that nearly one-third of the included patients were diagnosed with ASPS, which is more sensitive to immunotherapy. Until now a few studies have explored factors influencing prognosis in patients with STS being treated with immunotherapy, and our analysis revealed that for aSTS treated with immunotherapy plus antiangiogenic therapy, ECOG performance status of 0–1 and treatment-line of 1–2 were positive predictors for both PFS and OS. It is noteworthy that our study included four patients with brain metastases who received immunotherapy combined with antiangiogenic treatment. During the follow-up period, no severe adverse events such as intracranial hemorrhage or central nervous system changes were observed, suggesting the potential of this combination strategy for patients with brain metastases.
In our study, the response to combination treatment also differed among different subtypes, with patients diagnosed with ASPS or CCS exhibiting a more favorable prognosis compared to other subtypes. Several studies have indicated that traditional chemotherapy is not effective in treating patients with ASPS, whereas the combination of immunotherapy and antiangiogenic therapy significantly improves the prognosis for this subtype [12, 17]. In a previous phase II trial, ASPS patients treated with axitinib plus pembrolizumab achieved ORR of 54.5%, which was higher than the non-ASPS group (9.5%), and the mPFS for ASPS was 12.4 months [15]. Another study demonstrated that out of 7 patients with ASPS, 5 (71.4%) achieved PR, and 2 had SD [14]. Of note, Liu et al. reported an ORR of 80% and an mPFS of 19.3 months for ASPS patients receiving this combined therapy [11]. In our study, among 6 diagnosed ASPS patients, 1 achieved PR, and the remaining 5 patients achieved SD. The mPFS was not reached in this subgroup. These findings suggest that ASPS exhibits greater sensitivity to the combination of immunotherapy and antiangiogenic therapy, which warrants further verification. Similar to ASPS, CCS is not recommended for chemotherapy due to its low chemosensitivity. Previous studies have shown that immunotherapy improves the prognosis for patients with advanced CCS [18]. In addition, a phase II study revealed the promising efficacy of apatinib in patients with untreated or chemotherapy-refractory CCS, with an ORR of 33.3% [19]. Wang and his colleagues also demonstrated the potential of combining apatinib with camrelizumab for patients with advanced CCS [20]. Additionally, anlotinib, a multi-kinase angiogenesis inhibitor, has been reported to exhibit antitumor activity in CCS patients [21]. Among the 5 CCS cases in our cohort, 2 patients achieved PR, 2 had SD and one experienced progressive disease (PD), reporting an mPFS of 16.2 months. These results highlight the potential of this combination strategy for patients with CCS. In the cohort of six patients with rhabdomyosarcoma, the mPFS was 7.2 months. Among these patients, one exhibited PR, four demonstrated SD, and one experienced PD. Notably, the patient with PD was classified as exhibiting the embryonal subtype, in contrast to the pleomorphic subtype identified in the other five patients. These findings suggest that rhabdomyosarcoma subtypes may respond differentially to this combination treatment.
Previous studies have investigated the role of immunotherapy in patients with aSTS. A multicenter, phase II trial (SARC-028) demonstrated that pembrolizumab monotherapy maintained objective responses in 7 out of 40 (18%) patients with aSTS [22]. Another study conducted by Yang et al. reported an ORR of 25% among patients diagnosed with advanced or recurrent ASPS who received toripalimab treatment [23]. In a noteworthy single-arm phase II trial, treatment with atezolizumab yielded an ORR of 37% among 52 patients with unresectable or metastatic ASPS, predominantly consisting of PR. The PFS was recorded at 20.8 months, with a median duration of response lasting nearly 25 months [24]. However, a previous Phase II single-arm trial indicated a low ORR of only 4% in aSTS treated with nivolumab [25]. Additionally, the combination of nivolumab and ipilimumab also showed limited efficacy in aSTS [26]. These results suggest that immunotherapy alone does not appear to work well for aSTS, except in some specific subtypes such as ASPS. To address this issue, previous investigations have explored combination strategies to enhance the prognosis of aSTS. Livingston et al. showed that the combination of doxorubicin and pembrolizumab demonstrated an ORR of 36.7% [27], which is higher than another similar study conducted by Pollack et al. (ORR = 22%) [28]. Although immunotherapy plus chemotherapy has improved the efficacy to some extent, many patients still experience disease progression and further exploration is necessary.
With the rapid advancement in understanding the molecular mechanisms of tumor growth, antiangiogenic therapy has been widely used in various malignant tumors. Previous clinical trials have demonstrated that antiangiogenic agents, such as regorafenib, pazopanib, and anlotinib, exhibited favorable efficacy as second-line treatments for aSTS [29–31]. Several real-world studies have also suggested the potential benefits of axitinib, apatinib, sorafenib, and lenvatinib in patients with aSTS [32–35]. Moreover, antiangiogenic therapy has shown significant progress in subtypes that exhibit high resistance to chemotherapy, such as ASPS and CCS [36, 37]. Further studies have indeed presented evidence endorsing the substantial efficacy of antiangiogenic drugs, namely pazopanib, sunitinib, and anlotinib, as first-line treatments specifically for ASPS [21, 38, 39]. In a previous randomized phase II study, cediranib exhibited notable efficacy in patients with metastatic ASPS, thereby reaffirming the therapeutic potential of VEGFR inhibitors in this malignancy [40]. In the case of CCS, both anlotinib and pazopanib have exhibited considerable efficacy as first-line treatment options [21, 29]. In the past few years, cellular and zoological studies have demonstrated that immune checkpoint inhibitors can promote tumor vascular normalization, reduce metastasis, and exhibit a synergistic effect with antiangiogenic agents [41]. Antiangiogenic therapy has been proven to promote immune activation by reshaping the immune microenvironment, enhancing antigen presentation, and facilitating immune cell recruitment. These findings suggest a potential synergistic effect when combining immunotherapy with antiangiogenic agents [42, 43]. Of note, promising results have been reported for the combination of durvalumab and pazopanib in aSTS with an ORR of 30.4% and mPFS of 8.6 months [44]. These present studies have shown the potential of this combination strategy for patients with aSTS.
Several limitations of this study need to be addressed. First, it was conducted as a single-center retrospective research with a small sample size. Consequently, the patient’s characteristics were heterogeneous, encompassing variations in histopathological types, prior treatment history, combination strategies, and other factors. As a real-world study, the follow-up data included information derived from patients’ self-reported statements, which may introduce potential inaccuracies. To obtain a more comprehensive understanding of the anti-tumor effects of immunotherapy combined with antiangiogenic agents for aSTS, future prospective studies with larger sample sizes are warranted. Second, the mOS for this cohort and the mPFS for ASPS were not reached although the median follow-up time was nearly 16 months. Further follow-up is required, particularly for patients with favorable prognoses, such as ASPS and CCS. Third, the combinations of immunotherapy and antiangiogenic agents employed by patients vary significantly due to a multitude of factors, potentially impacting treatment efficacy in the real world. Given the unique properties of each immunotherapeutic and antiangiogenic agent, further research is imperative to elucidate and identify the optimal drug combinations for enhanced therapeutic outcomes. Fourth, several biomarkers such as tumor mutation burden, microsatellite instability, expression level of PD-L1, and VEGFR/PDGFR, may potentially impact the efficacy of this combination therapy in patients with aSTS. Nonetheless, we failed to analyze the correlation between them due to insufficient data, and further exploration of biomarkers to predict efficacy could potentially enhance the prognosis of patients with aSTS.
Conclusion
In conclusion, our study suggested that the immunotherapy plus antiangiogenic agents demonstrated potential activity and tolerable adverse events in patients with aSTS, especially in ASPS or CCS. Further prospective studies with larger sample sizes, fixed combination strategy, comparable patients’ characteristics, and biomarker detection are needed to verify our findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not Applicable.
Author contributions
YD. designed the research. SLL. QAS. and R.B wrote the protocol. SLL. QAS. HW. HFC. collected the data. SLL. QAS. RB. and YPW. analyzed the data. SLL. and QAS. wrote the manuscript. RB. YPW. and YD. revised the manuscript. All authors reviewed the manuscript.
Funding
Not Applicable.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
This study was approved by the Ethic Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine (approve number: 20230918). Due to the anonymous, retrospective, and nonintervention nature of this study, informed consent from all patients was waived.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaoli Li and Qunan Sun contributed equally to this work.
References
- 1.Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021;32(11):1348–65. 10.1016/j.annonc.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 2.Hui JYC. Epidemiology and etiology of sarcomas. Surg Clin North Am. 2016;96(5):901–14. 10.1016/j.suc.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 3.von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network: JNCCN. 2022;20(7):815–33. 10.6004/jnccn.2022.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vezeridis MP, Moore R, Karakousis CP. Metastatic patterns in soft-tissue sarcomas. Arch Surg. 1983;118(8):915–8. 10.1001/archsurg.1983.01390080023007 [DOI] [PubMed] [Google Scholar]
- 5.Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchère D, Sastre X, Vilain MO, Bonichon F, N’Guyen Bui B. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French federation of cancer centers sarcoma group. Cancer. 2001;91(10):1914–26. [DOI] [PubMed] [Google Scholar]
- 6.Schöffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, Grignani G, Camargo V, Bauer S, Rha SY, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet (London England). 2016;387(10028):1629–37. 10.1016/S0140-6736(15)01283-0 [DOI] [PubMed] [Google Scholar]
- 7.Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K, Tawbi H, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34(8):786–93. 10.1200/JCO.2015.62.4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai A, Araki N, Sugiura H, Ueda T, Yonemoto T, Takahashi M, Morioka H, Hiraga H, Hiruma T, Kunisada T, et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: a randomised, open-label, phase 2 study. Lancet Oncol. 2015;16(4):406–16. 10.1016/S1470-2045(15)70098-7 [DOI] [PubMed] [Google Scholar]
- 9.Seddon B, Strauss SJ, Whelan J, Leahy M, Woll PJ, Cowie F, Rothermundt C, Wood Z, Benson C, Ali N, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18(10):1397–410. 10.1016/S1470-2045(17)30622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Del-Muro X, López-Pousa A, Maurel J, Martín J, Martínez-Trufero J, Casado A, Gómez-España A, Fra J, Cruz J, Poveda A, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish group for research on sarcomas study. J Clin Oncol. 2011;29(18):2528–33. 10.1200/JCO.2010.33.6107 [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Wang X, Wang J, Zhang P, Li C, Wang B, Gao S, Liu O, Yao W. The efficacies and biomarker investigations of antiangiogenic agents and PD-1 inhibitors for metastatic soft tissue sarcoma: a multicenter retrospective study. Front Oncol. 2023;13:1124517. 10.3389/fonc.2023.1124517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saerens M, Brusselaers N, Rottey S, Decruyenaere A, Creytens D, Lapeire L. Immune checkpoint inhibitors in treatment of soft-tissue sarcoma: a systematic review and meta-analysis. Eur J Cancer. 2021;152:165–82. 10.1016/j.ejca.2021.04.034 [DOI] [PubMed] [Google Scholar]
- 13.You Y, Guo X, Zhuang R, Zhang C, Wang Z, Shen F, Wang Y, Liu W, Zhang Y, Lu W, et al. Activity of PD-1 inhibitor combined with anti-angiogenic therapy in advanced sarcoma: a single-center retrospective analysis. Front Mol Biosci. 2021;8:747650. 10.3389/fmolb.2021.747650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Xu J, Liu M, Hu W, Xu N, Zhu D. Efficacy and safety of angiogenesis inhibitors plus immune checkpoint inhibitors in advanced soft tissue sarcoma: a real-world, single-center study. Sci Rep. 2023;13(1):3385. 10.1038/s41598-023-30412-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilky BA, Trucco MM, Subhawong TK, Florou V, Park W, Kwon D, Wieder ED, Kolonias D, Rosenberg AE, Kerr DA, et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019;20(6):837–48. 10.1016/S1470-2045(19)30153-6 [DOI] [PubMed] [Google Scholar]
- 16.Martin-Broto J, Hindi N, Grignani G, Martinez-Trufero J, Redondo A, Valverde C, Stacchiotti S, Lopez-Pousa A, D’Ambrosio L, Gutierrez A et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: a multicenter, single-arm, phase Ib/II trial. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed]
- 17.Italiano A, Bellera C, D’Angelo S. PD1/PD-L1 targeting in advanced soft-tissue sarcomas: a pooled analysis of phase II trials. J Hematol Oncol. 2020;13(1):55. 10.1186/s13045-020-00891-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wetterwald L, Riggi N, Kyriazoglou A, Dei Tos G, Dei Tos A, Digklia A. Clear cell sarcoma: state-of-the art and perspectives. Expert Rev Anticancer Ther. 2023;23(3):235–42. 10.1080/14737140.2023.2183846 [DOI] [PubMed] [Google Scholar]
- 19.Yu W, Zhang H, Chen J, Zhang X, Chen Y, Qu G, Huang G, Zhou Y, Ye T, Fan Z, et al. Efficacy and safety of apatinib in patients with untreated or chemotherapy-refractory soft tissue sarcoma: a multicenter, phase 2 trial. Annals Translational Med. 2022;10(18):981. 10.21037/atm-22-4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Gao S, Yang Y, Liu X, Zhang P, Dong S, Wang X, Yao W. Clinical experience with apatinib and camrelizumab in advance clear cell sarcoma: a retrospective study. Cancer Manage Res. 2021;13:8999–9005. 10.2147/CMAR.S337253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, Du F, Sun Y, Wu Q, Qu G, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. 2018;24(21):5233–8. 10.1158/1078-0432.CCR-17-3766 [DOI] [PubMed] [Google Scholar]
- 22.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D’Angelo S, Attia S, Riedel RF, Priebat DA, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–501. 10.1016/S1470-2045(17)30624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Dong L, Yang S, Han X, Han Y, Jiang S, Yao J, Zhang Z, Zhang S, Liu P, et al. Safety and clinical efficacy of toripalimab, a PD-1 mAb, in patients with advanced or recurrent malignancies in a phase I study. Eur J Cancer. 2020;130:182–92. 10.1016/j.ejca.2020.01.028 [DOI] [PubMed] [Google Scholar]
- 24.Chen AP, Sharon E, O’Sullivan-Coyne G, Moore N, Foster JC, Hu JS, Van Tine BA, Conley AP, Read WL, Riedel RF, et al. Atezolizumab for advanced alveolar soft part sarcoma. N Engl J Med. 2023;389(10):911–21. 10.1056/NEJMoa2303383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai A, Okamura TNN, Shibata T, Tamaura K, Ueda G. Efficacy and safety of nivolumab monotherapy in patients with unresectable clear cell sarcoma and alveolar soft part sarcoma (OSCAR TRIAL, NCCH1510): a multicenter, phase 2 clinical trial. In: CTOS. 2020.
- 26.Keung EZ, Lazar AJ, Torres KE, Wang W-L, Cormier JN, Ashleigh Guadagnolo B, Bishop AJ, Lin H, Hunt KK, Bird J, et al. Phase II study of neoadjuvant checkpoint blockade in patients with surgically resectable undifferentiated pleomorphic sarcoma and dedifferentiated liposarcoma. BMC Cancer. 2018;18(1):913. 10.1186/s12885-018-4829-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livingston MB, Jagosky MH, Robinson MM, Ahrens WA, Benbow JH, Farhangfar CJ, Foureau DM, Maxwell DM, Baldrige EA, Begic X, et al. Phase II study of pembrolizumab in combination with doxorubicin in metastatic and unresectable soft-tissue sarcoma. Clin Cancer Res. 2021;27(23):6424–31. 10.1158/1078-0432.CCR-21-2001 [DOI] [PubMed] [Google Scholar]
- 28.Pollack SM, Redman MW, Baker KK, Wagner MJ, Schroeder BA, Loggers ET, Trieselmann K, Copeland VC, Zhang S, Black G, et al. Assessment of doxorubicin and pembrolizumab in patients with advanced anthracycline-naive sarcoma: a phase 1/2 nonrandomized clinical trial. JAMA Oncol. 2020;6(11):1778–82. 10.1001/jamaoncol.2020.3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Graaf WTA, Blay J-Y, Chawla SP, Kim D-W, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP, Beppu Y, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (London England). 2012;379(9829):1879–86. 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 30.Mir O, Brodowicz T, Italiano A, Wallet J, Blay J-Y, Bertucci F, Chevreau C, Piperno-Neumann S, Bompas E, Salas S, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(12):1732–42. 10.1016/S1470-2045(16)30507-1 [DOI] [PubMed] [Google Scholar]
- 31.Chi Y, Yao Y, Wang S, Huang G, Cai Q, Shang G, Wang G, Qu G, Wu Q, Jiang Y, et al. Anlotinib for metastasis soft tissue sarcoma: a randomized, double-blind, placebo-controlled and multi-centered clinical trial. J Clin Oncol. 2018;36(15suppl):11503–11503. 10.1200/JCO.2018.36.15_suppl.11503 [DOI] [Google Scholar]
- 32.Liu J, Deng Y-T, Wu X, Jiang Y. Rechallenge with multi-targeted tyrosine kinase inhibitors in patients with Advanced Soft tissue sarcoma: a single-center experience. Cancer Manage Res. 2021;13:2595–601. 10.2147/CMAR.S300430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray-Coquard I, Italiano A, Bompas E, Le Cesne A, Robin Y-M, Chevreau C, Bay J-O, Bousquet G, Piperno-Neumann S, Isambert N, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French sarcoma group (GSF/GETO). Oncologist. 2012;17(2):260–6. 10.1634/theoncologist.2011-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stacchiotti S, Simeone N, Lo Vullo S, Morosi C, Greco FG, Gronchi A, Barisella M, Collini P, Zaffaroni N, Dagrada GP, et al. Activity of axitinib in progressive advanced solitary fibrous tumour: results from an exploratory, investigator-driven phase 2 clinical study. Eur J Cancer. 2019;106:225–33. 10.1016/j.ejca.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Wang J, Sun B, Sun Y, Liu N, Niu X, Li C, Li L, Zhang Q, Hao J, et al. Efficacy and safety of apatinib in advanced refractory soft tissue sarcoma and association with histologic subtypes: a multicenter retrospective study. Annals Translational Med. 2022;10(18):961. 10.21037/atm-22-3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichardt P, Lindner T, Pink D, Thuss-Patience PC, Kretzschmar A, Dörken B. Chemotherapy in alveolar soft part sarcomas. What do we know? Eur J Cancer. 2003;39(11):1511–6. 10.1016/S0959-8049(03)00264-8 [DOI] [PubMed] [Google Scholar]
- 37.Jones RL, Constantinidou A, Thway K, Ashley S, Scurr M, Al-Muderis O, Fisher C, Antonescu CR, D’Adamo DR, Keohan ML, et al. Chemotherapy in clear cell sarcoma. Med Oncol. 2011;28(3):859–63. 10.1007/s12032-010-9502-7 [DOI] [PubMed] [Google Scholar]
- 38.Stacchiotti S, Negri T, Zaffaroni N, Palassini E, Morosi C, Brich S, Conca E, Bozzi F, Cassinelli G, Gronchi A, et al. Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol. 2011;22(7):1682–90. 10.1093/annonc/mdq644 [DOI] [PubMed] [Google Scholar]
- 39.Kim M, Kim TM, Keam B, Kim YJ, Paeng JC, Moon KC, Kim D-W, Heo DS. A phase II trial of Pazopanib in patients with metastatic alveolar soft part sarcoma. Oncologist. 2019;24(1). [DOI] [PMC free article] [PubMed]
- 40.Judson I, Morden JP, Kilburn L, Leahy M, Benson C, Bhadri V, Campbell-Hewson Q, Cubedo R, Dangoor A, Fox L, et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): a double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol. 2019;20(7):1023–34. 10.1016/S1470-2045(19)30215-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544(7649):250–4. 10.1038/nature21724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Datta M, Coussens LM, Nishikawa H, Hodi FS, Jain RK. Reprogramming the tumor microenvironment to improve immunotherapy: emerging strategies and combination therapies. Am Soc Clin Oncol Educational book Am Soc Clin Oncol Annual Meeting. 2019;39:165–74. 10.1200/EDBK_237987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–40. 10.1038/nrclinonc.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HS, Cho HJ, Yun KH, Lee YH, Kim SH, Baek W, Jeon MK. Durvalumab and pazopanib in patients with advanced soft tissue sarcoma: a single-center, singlearm, phase 2 trial. J Clin Oncol. 2021;39(15 SUPPL).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.