Abstract
Repair of the mature mammalian myocardium following injury is impaired by the inability of the majority of cardiomyocytes to undergo cell division. We show that overexpression of the cyclin B1–CDC2 (cell division cycle 2 kinase) complex re-initiates cell division in adult cardiomyocytes. Thus strategies targeting the cyclin B1–CDC2 complex might re-initiate cell division in mature cardiomyocytes in vivo and facilitate myocardial regeneration following injury.
Keywords: cardiomyocyte, CDC2, cell cycle, cell division, cyclin B
Abbreviations: CAK, cyclin-dependent-kinase-activating kinase; CDC2, cell division cycle 2 kinase; CDC2AF, constitutively active CDC2; CDK, cyclin-dependent kinase; E18, gestational day 18; EGFP, enhanced green fluorescent protein; FAM, 5-carboxyfluorescein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; P2, 2-days old; P3, 3-days old; P5, 5-days old; TAMRA, 6-carboxytetramethylrhodamine; tTA, tetracycline transactivator
INTRODUCTION
The ability of the myocardium to repair itself following a myocardial infarction is severely impaired by the limited capacity of adult ventricular cardiomyocytes to divide (for a review, see [1]). Although a very small proportion of adult cardiomyocytes (<0.1%) has been shown to undergo division in the border zone surrounding an infarct [2], this rate of division is not sufficient to repair damaged cardiac tissue. Consequently, the damaged region of the myocardium is replaced by scar tissue, and cardiac function is often irreversibly impaired.
The ability of the mammalian cardiomyocyte to divide decreases significantly during development, consistent with a down-regulation in the activities and expressions of cyclin and CDK (cyclin-dependent kinase) complexes [3,4]. Thus foetal and early neonatal cardiomyocytes can divide, whereas late neonatal and adult cells cannot; instead, these cells grow by the process of hypertrophy [3,4]. Cell-cycle withdrawal of cardiomyocytes occurs at different developmental ages in different species. For example, in humans, cell division in cardiomyocytes ceases by 7 months of age [5], whereas in the mouse, it occurs at or around birth [6]. In the rat, the vast majority of cardiomyocytes withdraw progressively from the cell cycle shortly after birth, such that late neonatal (>4 days old) and adult cardiomyocytes normally do not divide as a consequence of cell-cycle arrest in G0/G1 and G2/M [3,4]. However, these cells retain the ability to undergo partial cell-cycle reactivation following hypertrophic stimulation. Thus increases in the activities of the G1/S phase cyclin–CDK complexes occur, followed by progression of cardiomyocytes through the G1/S transition before accumulation in G2/M [7,8]. Interestingly, expression and activity of the G2/M phase cyclin–CDK complex, cyclin B1–CDC2 (cell division cycle 2 kinase), is not detected in normal adult cardiomyocytes nor in cardiomyocytes undergoing hypertrophy [3,7].
Manipulation of the expressions of specific cell-cycle regulators might permit re-initiation of cardiomyocyte cell division in the mature heart, which could have important therapeutic benefits for patients post-infarct. A number of studies have targeted the expressions of G1- and S-phase-acting molecules, including E2F1, cyclin D1 and CDK2 [9–13]. However, while cell-cycle progression was observed in these studies, no increase in cardiomyocyte number was demonstrated. Since these G1/S-phase-acting molecules are re-expressed in cardiomyocytes during hypertrophy [7,8], it is unlikely that they are limiting for the proliferative potential of cardiomyocytes; however, targeting specific G2/M-acting molecules might prove more beneficial. Accordingly, in the present study, we have examined the effects of overexpressing the mitotic cell-cycle-regulatory complex, cyclin B1–CDC2, on cardiomyocyte proliferation.
EXPERIMENTAL
Materials
Dual-labelled quantitative PCR probes were synthesized by Applied Biosystems (Warrington, U.K.). All other oligonucleotides were synthesized by Sigma-Genosys (Pampisford, U.K.). Universal PCR Master mix, rodent GAPDH (glyceraldehyde-3-phosphate dehydrogenase) control reagent kit and quantitative PCR consumables were supplied by Applied Biosystems. The High-Fidelity PCR system was supplied by Roche Diagnostics (Lewes, U.K.). Anti-(cyclin B1) (GNS1) and anti-(CDC2 p34) (17) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.) and the anti-tropomyosin (sarcomeric) antibody (clone CH1) was obtained from Sigma (Poole, U.K.). Horseradish-peroxidase-conjugated secondary antibodies were purchased from DakoCytomation (Ely, U.K.) and AlexaFluor® 568 anti-mouse IgG secondary antibody was purchased from Molecular Probes (Eugene, OR, U.S.A.). Vectashield mounting medium containing DAPI (4′,6′-diamidino-2-phenylindole) was purchased from Vector Laboratories (Orton Southgate, Peterborough, U.K.). Expression vector pIRES2-EGFP (where EGFP is enhanced green fluorescent protein) was obtained from BD Clontech UK (Basingstoke, U.K.). Quantity One® software package was from Bio-Rad (Hemel Hempstead, U.K.). BD Matrigel™ Matrix (growth factor reduced) was supplied by Fahrenheit Laboratories (Milton Keynes, U.K.). All other chemicals and biochemicals were supplied by Sigma.
Cardiomyocyte isolation
All experimental procedures using animals were performed in accordance with the U.K. Animals (Scientific Procedures) Act, 1986. Foetal [gestational day 18 (E18)] and neonatal [2- (P2), 3- (P3) and 5- (P5) days-old] ventricular cardiomyocytes were isolated from 30–70 excised ventricles of Wistar rats as described previously [8]. Cardiomyocyte-enriched cultures were at least 95% pure, as determined by anti-tropomyosin staining. Adult ventricular cardiomyocytes were isolated from the excised hearts of male Wistar rats (175–200 g) by Langendorff perfusion [3,8].
Real-time PCR and immunoblotting
Total RNA was extracted and reverse transcribed as described in [8]. Real-time PCR analysis was performed using an ABI Prism 7700 sequence detector. Rat cyclin B1 was amplified using sense (5′-TTATGGAAGTGAAACACTCTCCTGA-3′) and antisense (5′-CGGAGAAAGCCTGACACAGAT-3′) oligonucleotides and detected using a cyclin B1 probe (5′-FAM-TTCTTCGGCAGGTGCACATCCAGA-TAMRA-3′, where FAM is 5-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine). Rat CDC2 was amplified using sense (5′-GGCCAGAAGTAGAGTCCCTGC-3′) and antisense (5′-GACCAGCATTTTCGAGAGCAA-3′) oligonucleotides, and detected using a CDC2 probe (5′-FAM-TCCAAGCCGTTTTCATCCAGGTTCTTG-TAMRA-3′). Reactions were normalized using GAPDH mRNA expression.
Expression levels of cyclin B1 and CDC2 in 20 μg of total protein extract were determined as described previously [3,8].
Expression constructs and transfection studies
Rat cyclin B1 and rat CDC2 coding regions were amplified using the High-Fidelity PCR system and the following oligonucleotides: cyclin B1, sense (5′-TCGGCGAACCTCTGCTTCAGGAGGAG-3′), antisense (5′-GTACAGGCGCACATGGTGCCAACTGC-3′); CDC2, sense (5′-TTGAGTAACTATGGAGGA-3′) and antisense (5′-GTTACATCTTCTTGATCTGATTGTCC-3′). Products were cloned and sequenced before subcloning into pAG-IRES2-EGFP, generated by replacing the CMV (cytomegalovirus) promoter cassette of pIRES2-EGFP with the pAG promoter cassette from pCAGGS [14].
For neonatal cardiomyocyte transfection studies, cardiomyocytes were plated in 0.5 mM 5-bromo-2′-deoxyuridine for 24 h to inhibit proliferation of contaminating cells. Neonatal cardiomyocytes, at 50–70% confluency, were transfected in six-well plates using integrin-targeting peptide-mediated transfection as described in [15], and transfection efficiencies of 15–30% were achieved consistently.
Adenovirus-infection studies
Recombinant adenoviruses expressing tTA (tetracycline transactivator), constitutively active CDC2 (CDC2AF) or cyclin B1 were a gift from Professor David O. Morgan (University of California, San Francisco, CA, U.S.A.) [16]. For adult cardiomyocyte-infection studies, isolated adult myocytes were plated in serum-free modified M199 medium (M199 containing 2 mM creatine, 5 mM taurine and 2 mM carnitine) for 1 h on Matrigel™-coated six-well dishes. Adherent adult cardiomyocytes were washed and then infected with the tTA virus alone, tTA virus and CDC2AF and/or cyclin B1 at a MOI (multiplicity of infection) of 100 plaque-forming units of each virus per cell in modified M199 medium. When only one or two viruses were used, a recombinant adenovirus overexpressing EGFP or the tTA virus was used to adjust for differences in viral load. Infected cultures were maintained in modified M199 medium for 24 h before analysis.
Immunocytochemistry
Neonatal cardiomyocytes were cultured on laminin-coated glass coverslips for 24 h before transfection. Adult cardiomyocytes were plated on to Matrigel™-coated chamber slides for 1 h before infection with recombinant adenoviruses. Cell monolayers, 72 h post-transfection or 24 h post-infection, were fixed in 1% (v/v) formaldehyde in PBS for 10 min at room temperature (21 °C), permeabilized with 0.5% (v/v) Triton X-100 in PBS for 15 min and blocked in 10% (v/v) normal goat serum/2% (w/v) BSA in PBS for 30 min. Anti-tropomyosin (sarcomeric) monoclonal antibody (clone CH1) was used to distinguish cardiomyocytes from non-myocytes and antibody binding was detected using AlexaFluor® 568 anti-mouse IgG secondary antibody. Stained monolayers were mounted and fluorescence microscopy performed using a Nikon Eclipse TE200 inverted microscope.
In vitro kinase assays
Cyclin B1–CDC2 complexes were immunoprecipitated from 250 μg of protein extracted from transfected or infected cultures using anti-cyclin B1 antibody and kinase activity measured as described previously [17].
Statistics
All results are presented as means±S.E.M. Data were analysed by one-way ANOVA followed by Bonferroni t test, with P<0.05 considered to be statistically significant.
RESULTS
Neonatal and adult cardiomyocytes retain the capacity to transverse the G1/S transition during hypertrophic growth, although these cells subsequently become arrested in G2/M [7]. In the present study, we have shown that this G2/M arrest is, at least in part, mediated by the absence of the expressions and/or activities of cyclin B1 and its catalytic partner CDC2 in adult cardiomyocytes, as demonstrated by a combination of real-time PCR (mRNA) and immunoblotting (protein) (Figure 1) and in vitro CDC2-associated kinase assays (activity) [3]. Real-time quantitative PCR was performed on cDNAs obtained from P2, P5 and adult cardiomyocytes (Figure 1A) and results normalized to GAPDH expression. Cyclin B1 mRNA expression was down-regulated significantly in cell-cycle-arrested adult cardiomyocytes compared with P2 and P5 cardiomyocytes by 91.7±14.9-fold and 69.6±2.5-fold respectively. In addition, the expression of CDC2 mRNA was decreased significantly in adult cells compared with P2 and P5 cardiomyocytes by 43.9±4.5-fold and 31.2±6.5-fold respectively. A similar pattern of expression was observed for cyclin B1 and CDC2 proteins, such that levels decreased progressively during development with high expression in proliferative foetal E18 cardiomyocytes, lower levels in neonatal cells and no detectable protein in adult cardiomyocytes (Figure 1B). In support of these observations, we have demonstrated previously that CDC2-associated kinase activity decreases progressively during cardiomyocyte development [3]. Hence we hypothesized that loss of cyclin B1 and/or CDC2 expressions and activity might be responsible for cell-cycle arrest in maturing cardiomyocytes and that overexpression of cyclin B1 and/or CDC2 in these cells might influence their proliferative capacity.
Figure 1. CDC2 and cyclin B1 mRNA and protein expressions decrease during rat cardiomyocyte development.
(A) mRNA expressions of CDC2 and cyclin B1 were measured in neonatal (P2 and P5) and adult cardiomyocytes using real-time PCR. *P<0.05 compared with P2 values; (B) Protein expression levels of CDC2 and cyclin B1 in foetal (E18), neonatal (P2 and P5) and adult cardiomyocytes are presented.
In view of these observations, we have targeted the G2/M-regulatory complex, cyclin B1–CDC2, to investigate the effect that overexpression of cyclin B1 and/or CDC2 has upon the proliferative potential of cardiomyocytes at different stages of postnatal development. Initially, we transfected P3 cardiomyocytes (that retain a limited capacity for proliferation) with (i) cyclin B1, (ii) CDC2, (iii) cyclin B1 in combination with CDC2, or (iv) vector alone. Effects on proliferation were measured 72 h post-transfection by direct cell counting following immunocytochemistry. An increase in tropomyosin-positive cells in cyclin-B1-transfected cultures (177±20%) compared with vector controls (100±7%; n=3; P<0.05) indicated that increased cell numbers resulted from proliferation of transfected cardiomyocytes (Figure 2A). Tropomyosin-positive cardiomyocytes represented approx. 84±14% of cells present 96 h after plating, and the number of contaminating tropomyosin-negative cells was constant between transfected cultures. Transfection of cyclin B1 alone, CDC2 alone or a combination of cyclin B1 and CDC2 expression constructs resulted in increases in CDC2 protein expression of approx. 1.25±0.03-fold, 2.25±0.09-fold and 1.81±0.03-fold respectively, compared with vector controls, as estimated by densitometry (Figure 2B). Moreover, transfection of cyclin B1, CDC2 or a combination of cyclin B1 and CDC2 expression constructs resulted in increases in cyclin B1 protein expression of approx. 2.39±0.19-fold, 1.31±0.06-fold and 2.24±0.20-fold respectively (Figure 2B). Additionally, cyclin B1–CDC2 kinase activity was increased significantly in cyclin-B1-transfected cultures (158±3-fold) compared with vector-transfected controls (Figure 2C). Interestingly, overexpression of CDC2 alone (2 μg) did not alter the proliferative capacity of these cultures nor did co-transfection of cyclin B1 and CDC2 (1 μg of each), although a significant increase in cyclin B1–CDC2 kinase activity was observed in these latter transfectants compared with vector controls (Figure 2C). The increase in neonatal cardiomyocyte proliferation was specific to overexpression of cyclin B1, since an increase in the proliferative potential of neonatal cardiomyocytes was not observed when cyclin E was overexpressed (results not shown).
Figure 2. Overexpression of cyclin B1, but not CDC2, increases the proliferative capacity of P3 neonatal rat cardiomyocytes.
(A) Proliferation of tropomyosin-positive neonatal cardiomyocytes following transfection is shown (means±S.E.M.; n=3). (B) Protein expression levels of cyclin B1 and CDC2 in vector, cyclin B1 and/or CDC2 transfected neonatal cardiomyocytes. (C) A representative kinase assay showing cyclin B1–CDC2 kinase activities in transfected cultures is presented. Relative levels of histone H1 phosphorylation in transfected cardiomyocytes are represented graphically below (means±S.E.M.; n=3). *P<0.05 compared with vector controls.
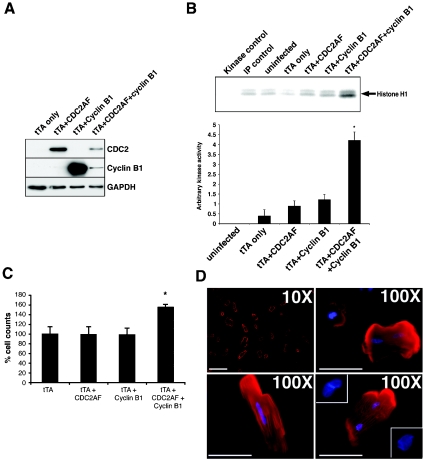
Our previous flow-cytometric analyses have demonstrated that P3 cardiomyocytes display some proliferative potential [3]. In order to determine whether or not cyclin B1 had a similar effect on the growth potential of cell-cycle-arrested cardiomyocytes, we used recombinant adenoviruses to re-express cyclin B1 and/or CDC2 in adult rat cardiomyocytes (Figure 3A). To overcome the requirement for activation of the re-expressed cyclin B1–CDC2 complex in adult cardiomyocytes, we used a constitutively active form of CDC2, CDC2AF, in these experiments. Re-introduction of cyclin B1 or CDC2AF alone did not result in an increase in cyclin B1–CDC2 kinase activity nor an increase in cellular proliferation in adult cardiomyocytes compared with control cultures (Figures 3B and 3C). However, following re-expression of both cyclin B1 and CDC2 in these cultures, cyclin B1–CDC2 kinase activity was increased 2.9±0.2-fold in cyclin B1 and CDC2AF co-infected cultures compared with controls (Figure 3B). Furthermore, re-expression of both components of the cyclin B1–CDC2 complex in adult cardiomyocyte cultures resulted in a significant increase in total cell number (155±3%) 24 h after infection compared with control cultures (100±8%; n=4; P<0.05) (Figure 3C). To confirm that this increase in cell number was due to an increase in the number of adult cardiomyocytes and not non-myocytes, we counted the number of tropomyosin-positive cells present following immunocytochemistry. Consistent with the increase in total cell counts, a significant increase in the number of tropomyosin-positive adult cardiomyocytes (144±16%) was observed compared with control cultures (100±8%; n=5; P<0.05). In parallel with the increase in cell number in cyclin B1 and CDC2AF co-infected cultures, an increased number of smaller mononuclear tropomyosin-positive cardiomyocytes was observed in cultures overexpressing cyclin B1 and CDC25AF (17.5%), compared with cultures infected with tTA virus alone (7.1%). Moreover, an increased number of tropomyosin-positive cells were observed to display abnormal nuclei (Figure 3D).
Figure 3. Adenovirus-mediated expression of constitutively active cyclin B1–CDC2 complex increases the proliferative capacity of adult rat cardiomyocytes.
(A) Protein expression levels of CDC2 and cyclin B1 in adult myocyte cultures infected with tTA virus alone, tTA and CDC2AF and/or cyclin B1 viruses. Protein expression of GAPDH was used as a loading control. (B) A representative kinase assay showing cyclin B1–CDC2 kinase activities in infected adult cardiomyocyte cultures is shown. Controls include no immunoprecipitated protein added to kinase reaction (kinase control), immunoprecipitation in absence of antibody (IP control) and protein immunoprecipitated from uninfected adult myocytes (uninfected). Relative levels of histone H1 phosphorylation in infected adult cardiomyocytes are represented graphically (means±S.E.M.; n=3). (C) Proliferation of adult cardiomyocytes in infected cultures is shown by direct cell counting (means±S.E.M.; n=4). (D) Immunocytochemistry shows DAPI (4′,6′-diamidino-2-phenylindole)-stained nuclei (blue) of representative cyclin B1- and CDC2AF-infected tropomyosin-positive (red) adult cardiomyocytes (×10 or ×100 magnification, as indicated). Insets show the effect of cyclin B1–CDC2AF expression on adult myocyte nuclei. Scale bar, 200 μm. *P<0.05 compared with tTA control.
DISCUSSION
The extent, and indeed occurrence, of cell division in mammalian adult cardiomyocytes is the subject of much debate at the present time [2,18–20]. It is most likely that mitosis does occur partially in some cardiomyocytes, leading to multinucleation (endoreduplication), but that the vast majority of these cells are unable to complete division and undergo cytokinesis. Indeed, it has been demonstrated that a small percentage of adult cardiomyocytes do undergo cell division in normal and infarcted hearts, although the majority of adult cardiomyocytes (99.92%) are terminally differentiated and do not divide [2]. Using flow cytometry, we have reported previously that 85% of adult cardiomyocytes are arrested in the G0/G1 phases with the remaining 15% of cardiomyocytes being blocked in G2/M of the cell cycle [7]. One of the limitations of flow-cytometric DNA analysis is that this technique is unable to distinguish between a G2-phase-arrested cell and an M-phase-arrested cell. However, since the mRNA and protein expressions and kinase activities of the cyclin B1–CDC2 complex are very low in adult cardiomyocytes, it is likely that these cells arrest before the G2/M transition. Interestingly, during pressure-overload-induced hypertrophy in adult rat cardiomyocytes, a partial reactivation of the cell-cycle machinery does occur, such that cardiomyocytes progress through the G1/S phase transition and accumulate in G2/M with no evidence of cell division [7,21]. This observation suggests that the ability of adult cardiomyocytes to progress through the G2 or M phases is limited.
The inability of cardiomyocytes to progress through the G2/M checkpoint has been highlighted in previous studies that overexpressed certain G1- and S-phase-acting molecules in these cells [9–13]. Thus, whereas overexpression of E2F1 in neonatal and adult cardiomyocytes promoted a partial reactivation of the cell cycle, with myocytes progressing through the G1/S phase transition and undergoing DNA synthesis, cells failed to complete division and accumulated in G2/M [9,10,12]. Moreover, overexpression of E2F1 induced apoptosis in cardiomyocytes; however, the presence of the adenoviral gene, E1B, or IGF-1 (insulin-like growth factor-1) rescued cardiomyocytes from E2F1-induced apoptosis [9,12]. Soonpaa et al. [11] showed that over-expression of cyclin D1 in adult cardiomyocytes promoted DNA synthesis and multinucleation, suggesting that endoreduplication, rather than cytokinesis, was the predominant effect. Recently, Tamamori-Adachi et al. [13] reported that exogenous expression of nuclear-localized cyclin D1 and CDK4 increased proliferation of P3 neonatal rat cardiomyocytes, with relative cell numbers increasing 3-fold by 120 h post-infection. However, it is not clear from this study [13] whether or not exogenous expression of cyclin D1 alone is sufficient to promote cardiomyocyte proliferation, since P3 cardiomyocytes retain a limited proliferative capacity and express other cell-cycle molecules that are required for cell-cycle progression, such as cyclin B1 and CDC2 ([3,4]; Figure 1). In addition, these authors reported that adenoviral-delivery of nuclear-localized cyclin D1 and CDK4 into rat hearts in vivo led to increased DNA synthesis in infected adult cardiomyocytes and expression of Ki-67, a marker of cell proliferation. However, no direct measure of adult cardiomyocyte cell number was reported in this study [13]. Although Ki-67 is expressed in all proliferating cells, it is not a definitive marker of cell division, since expression of the Ki-67 antigen decreases rapidly during the latter stages of mitosis (in anaphase and telophase) and Ki-67 expression is not down-regulated if a cell is arrested in its cell cycle [22–24]. Collectively, these previous studies, while demonstrating increased DNA synthesis in cardiomyocytes, do not demonstrate definitively that overexpression of G1/S-phase-acting molecules induce cell division in cell-cycle-arrested myocytes.
The formation and activation of the cyclin B1–CDC2 complex regulates entry into mitosis. The catalytic activities of the CDKs are regulated by a combination of the expression of their cyclin partners, cyclin–CDK complex formation and activation of the cyclin–CDK complex both by phosphorylation and dephosphorylation events (reviewed in [1]). Since late neonatal (>4-days-old in the rat) and adult cardiomyocytes are unable to undergo cell division [3,4], it is feasible that the decreased expression and activity of the cyclin B1–CDC2 complex in these cells limits their proliferative capacity. In the present study, we have shown that in P3 cardiomyocytes, which retain some proliferative capacity [4], overexpression of cyclin B1 alone, but not CDC2 alone, is sufficient to extend their proliferative capacity. Additionally, overexpression of cyclin B1 alone, but not CDC2 alone, resulted in an increase in CDC2-associated kinase activity. In these transfection studies, increased kinase activity and resultant cell-cycle activation, requires the overexpressed protein (either cyclin B1 or CDC2) to associate with its endogenously expressed partner to form an active cyclin–CDK complex and promote proliferation. Thus, in cultures transfected with CDC2 alone, overexpressed CDC2 must associate with endogenously expressed cyclin B1 to form an active cyclin B1–CDC2 complex. Although we have shown that both cyclin B1 and CDC2 are expressed endogenously in P3 neonatal cardiomyocytes, the fact that overexpression of CDC2 does not result in an increase in CDC2-associated kinase activity suggest that levels of endogenous cyclin B1 or the molecules that activate the cyclin B1–CDC2 complex are limiting. Indeed, since overexpression of cyclin B1 leads to increased CDC2-associated kinase activity, it is unlikely that activation of the cyclin B1–CDC2 complex in these cells is restrictive. Taken together, the data from these transfection studies suggest that cyclin B1 is a limiting factor for neonatal cardiomyocyte division, since the activity of CDC2 kinase is primarily dependent upon the availability of, and its binding to, cyclin B1.
Despite observing a significant increase in proliferation in neonatal cardiomyocytes transfected with cyclin B1 alone, we did not observe a significant increase in proliferation in cultures transfected with both cyclin B1 and CDC2. In the present study, we adjusted the total amount of plasmid DNA transfected to be consistent between cultures and thus, in cultures co-transfected with cyclin B1 and CDC2, only 1 μg of each plasmid construct was transfected, whereas cultures overexpressing cyclin B1 alone were transfected with 2 μg of plasmid DNA. We have observed that transfecting neonatal cardiomyocytes with increasing amounts of cyclin B1 plasmid DNA leads to a concentration-dependent increase in myocyte proliferation (K. A. Bicknell and G. Brooks, unpublished work). Consistent with the expression level of exogenous genes being affected by the amount of DNA transfected, lower levels of cyclin B1 protein expression and reduced kinase activities were observed in cultures transfected with both cyclin B1 and CDC2 compared with cultures expressing cyclin B1 alone. Moreover, since the level of cyclin B1 expression appears to modulate cardiomyocyte proliferation and endogenous CDC2 is not limiting, it is not surprising that cardiomyocytes co-transfected with 1 μg of cyclin B1 DNA and 1 μg of CDC2 DNA had a reduced effect on myocyte proliferation compared with cardiomyocytes expressing higher levels of cyclin B1 as a result of transfection with 2 μg of cyclin B1 plasmid. This observation substantiates further the key role that cyclin B1 expression levels play in modulating neonatal cardiomyocyte proliferation.
Since neonatal cardiomyocytes retain a limited proliferative potential, we also investigated the effect that cyclin B1 and CDC2 had upon cell division in cell-cycle-arrested adult cardiomyocytes. Consistent with a cell-cycle arrest, cyclin B1 and CDC2 mRNA and protein expressions are not detected in adult cardiomyocytes. Similarly, the expressions and/or activities of the molecules that regulate the activation of the cyclin B1–CDC2 complex, such as CDC25 dual-specificity phosphatases and CAK (CDK-activating kinase) are absent in adult cardiomyocytes [25]. In the absence of CAK and CDC25 activities in adult cardiomyocytes, we predicted that overexpression of wild-type CDC2 with cyclin B1 in adult cardiomyocytes might lead to increased expression of an inactive cyclin B1–CDC2 complex and, in the absence of cyclin B1–CDC2 kinase activity, re-initiation of cell-cycle progression in adult cardiomyocytes would not be observed. To overcome the requirement for activation of the re-expressed cyclin B1–CDC2 complex, we overexpressed a constitutively active form of CDC2, CDC2AF, in our infection studies. Although premature or prolonged activation of cyclin B1–CDC2 can result in mitotic catastrophe, premature chromatin condensation, centrosome over-duplication and/or apoptosis [16,26], results from our present study supports the observation that cell division is re-initiated in a population of adult cardiomyocytes expressing an active cyclin B1–CDC2 complex. Although an increase in the number of condensed nuclei was observed, the increase in adult cardiomyocyte cell numbers and an increased number of mononuclear myocytes compared with controls suggest that mitotic progression and cell division, not mitotic catastrophe, was occurring in cyclin B1–CDC2-expressing adult cardiomyocytes.
The present study has demonstrated for the first time that overexpression of the G2/M regulator, cyclin B1–CDC2 complex, is sufficient to re-initiate proliferation in adult cardiomyocytes that have exited the cell cycle. Thus it is possible that the cyclin B1–CDC2 complex could be targeted in post-mitotic cardiomyocytes in vivo as an approach for re-initiating controlled cell division in healthy cardiomyocytes that surround an infarct.
Acknowledgments
This work was funded by a British Heart Foundation (BHF) Project Grant (PG/99149), awarded to G.B. K.A.B. is funded by a BHF intermediate fellowship. pCAGGS was kindly supplied by Dr Miyazaki, University of Tokyo, Tokyo, Japan. Recombinant adenoviruses expressing tTA, CDC2AF or cyclin B1 were gifts from Professor David O. Morgan, University of California, San Francisco, CA, U.S.A.
References
- 1.Bicknell K. A., Surry E. L., Brooks G. Targeting the cell cycle machinery for the treatment of cardiovascular disease. J. Pharm. Pharmacol. 2003;55:571–591. doi: 10.1211/002235703765344487. [DOI] [PubMed] [Google Scholar]
- 2.Beltrami A. P., Urbanek K., Kajstura J., Yan S. M., Finato N., Bussani R., Nadal-Ginard B., Silvestri F., Leri A., Beltrami C. A., Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N. Engl. J. Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 3.Brooks G., Poolman R. A., McGill C. J., Li J. M. Expression and activities of cyclins and cyclin-dependent kinases in developing rat ventricular myocytes. J. Mol. Cell. Cardiol. 1997;29:2261–2271. doi: 10.1006/jmcc.1997.0471. [DOI] [PubMed] [Google Scholar]
- 4.Poolman R. A., Brooks G. Expressions and activities of cell cycle regulatory molecules during the transition from myocyte hyperplasia to hypertrophy. J. Mol. Cell. Cardiol. 1998;30:2121–2135. doi: 10.1006/jmcc.1998.0808. [DOI] [PubMed] [Google Scholar]
- 5.Huttenbach Y., Ostrowski M. L., Thaller D., Kim H. S. Cell proliferation in the growing heart: MIB-1 immunostaining in preterm and term infants at autopsy. Cardiovasc. Pathol. 2001;10:119–123. doi: 10.1016/s1054-8807(01)00065-5. [DOI] [PubMed] [Google Scholar]
- 6.Soonpaa M. H., Kim K. K., Pajak L., Franklin M., Field L. J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 1996;271:H2183–H2189. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- 7.Li J. M., Poolman R. A., Brooks G. Role of G1 phase cyclins and cyclin-dependent kinases during cardiomyocyte hypertrophic growth in rats. Am. J. Physiol. 1998;275:H814–H822. doi: 10.1152/ajpheart.1998.275.3.H814. [DOI] [PubMed] [Google Scholar]
- 8.Vara D., Bicknell K. A., Coxon C. H., Brooks G. Inhibition of E2F abrogates the development of cardiac myocyte hypertrophy. J. Biol. Chem. 2003;278:21388–21394. doi: 10.1074/jbc.M212612200. [DOI] [PubMed] [Google Scholar]
- 9.Kirshenbaum L. A., Abdellatif M., Chakraborty S., Schneider M. D. Human E2F-1 reactivates cell cycle progression in ventricular myocytes and represses cardiac gene transcription. Dev. Biol. 1996;179:402–411. doi: 10.1006/dbio.1996.0270. [DOI] [PubMed] [Google Scholar]
- 10.Agah R., Kirshenbaum L. A., Abdellatif M., Truong L. D., Chakraborty S., Michael L. H., Schneider M. D. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J. Clin. Invest. 1997;100:2722–2728. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soonpaa M. H., Koh G. Y., Pajak L., Jing S., Wang H., Franklin M. T., Kim K. K., Field L. J. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J. Clin. Invest. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Harsdorf R., Hauck L., Mehrhof F., Wegenka U., Cardoso M. C., Dietz R. E2F-1 overexpression in cardiomyocytes induces downregulation of p21CIP1 and p27KIP1 and release of active cyclin-dependent kinases in the presence of insulin-like growth factor 1. Circ. Res. 1999;85:128–136. doi: 10.1161/01.res.85.2.128. [DOI] [PubMed] [Google Scholar]
- 13.Tamamori-Adachi M., Ito H., Sumrejkanchanakij P., Adachi S., Hiroe M., Shimizu M., Kawauchi J., Sunamori M., Marumo F., Kitajima S., Ikeda M. A. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ. Res. 2003;92:e12–e19. doi: 10.1161/01.res.0000049105.15329.1c. [DOI] [PubMed] [Google Scholar]
- 14.Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 15.Saurin A. T., Martin J. L., Heads R. J., Foley C., Mockridge J. W., Wright M. J., Wang Y., Marber M. S. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J. 2000;14:2237–2246. doi: 10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- 16.Jin P., Hardy S., Morgan D. O. Nuclear Localization of cyclin B1 controls mitotic entry after DNA damage. J. Cell Biol. 1998;141:875–885. doi: 10.1083/jcb.141.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks G. Cyclin-dependent kinases and cyclin-dependent kinase inhibitors: detection methods and activity measurements. Methods Mol. Biol. 2001;124:161–170. doi: 10.1385/1-59259-059-4:161. [DOI] [PubMed] [Google Scholar]
- 18.Anversa P., Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ. Res. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Soonpaa M. H., Field L. J. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ. Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 20.Pasumarthi K. B. S., Field L. J. Cardiomyocyte cell cycle regulation. Circ. Res. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 21.Li J. M., Brooks G. Downregulation of cyclin-dependent kinase inhibitors p21 and p27 in pressure-overload hypertrophy. Am. J. Physiol. 1997;273:H1358–H1367. doi: 10.1152/ajpheart.1997.273.3.H1358. [DOI] [PubMed] [Google Scholar]
- 22.Braun N., Papadopoulos T., Muller-Hermelink H. K. Cell cycle dependent distribution of the proliferation-associated Ki-67 antigen in human embryonic lung cells. Virchows Arch. B. 1988;56:25–33. doi: 10.1007/BF02889998. [DOI] [PubMed] [Google Scholar]
- 23.van Oijen M. G., Medema R. H., Slootweg P. J., Rijksen G. Positivity of the proliferation marker Ki-67 in noncycling cells. Am. J. Clin. Pathol. 1998;110:24–31. doi: 10.1093/ajcp/110.1.24. [DOI] [PubMed] [Google Scholar]
- 24.Scholzen T., Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Coxon C. H., Bicknell K. A., Brooks G. Inhibition of CDC25 phosphatases blocks hypertrophic growth in rat cardiac myocytes. Heart. 2002;87(Suppl. II):70. [Google Scholar]
- 26.Castedo M., Perfettini J.-L., Roumier T., Andreau K., Medema R., Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]