Abstract
Objectives:
Laryngomalacia is an established cause of stridor and sleep-disordered breathing in children. However, the relationship between laryngomalacia and dysphagia has not been well characterized. The objectives of this study were to 1) describe the patient characteristics, symptoms, and prevalence of dysphagia in children with laryngomalacia and 2) examine the effectiveness of supraglottoplasty in improving feeding.
Methods:
This was a retrospective study of patients with laryngomalacia who underwent a modified barium swallow study (MBSS) at a tertiary academic pediatric medical center between 03/01/2014 and 03/01/2018. Patients were excluded if they did not undergo a MBSS. Comorbidities, airway and feedings symptoms, MBSS results, and surgical history were recorded from each patient’s electronic medical record.
Results:
Forty four children met inclusion/exclusion criteria. The median age at presentation was 96 days. There was a male predominance (66%). About one third had a genetic or neuromuscular comorbidity. Most children had stridor (93%) and feeding difficulty (86%), while 50% had parent-reported poor weight gain. 57% of patients had evidence of penetration or aspiration on MBSS. All patients with a positive MBSS had dysphagia symptoms. 57% of patients underwent supraglottoplasty. Post-operatively, 92% reported improvement in dysphagia symptoms.
Conclusion:
Dysphagia is prevalent among a subset of children with laryngomalacia. Symptomatic children may benefit from a swallow evaluation to help determine the need for further intervention.
Children who undergo supraglottoplasty for laryngomalacia have improved dysphagia at follow-up, though the amount of improvement directly attributable to surgery is unclear and warrants further investigation.
Introduction
Laryngomalacia is the most common cause of stridor in infants and a well-established cause of sleep disordered breathing. However, there is a paucity of data examining the relationship between laryngomalacia and swallowing dysfunction. The objectives of this study were the following:
1) Estimate the prevalence of dysphagia in children with laryngomalacia who underwent a modified barium swallow study (MBSS).
2) Identify predictors of abnormality on MBSS among children with laryngomalacia.
3) Determine impact of supraglottoplasty on dysphagia outcomes in children with laryngomalacia.
Methods
The medical records of all patients who were diagnosed with laryngomalacia and subsequently underwent a MBSS at a pediatric academic medical center between March 1, 2014 and March 1, 2018 were retrospectively reviewed and considered for inclusion in the study. Patients were excluded from the study if they did not have laryngomalacia or did not undergo a MBSS at our institution. Pertinent clinical information including medical comorbidities, swallow and airway symptoms, and weight at initial presentation and at follow-up was recorded from each patient’s medical record.
MBSS results and speech language pathology (SLP) diet treatment recommendations before and after treatment were recorded. MBSS findings were quantified using the SLP recommended diet liquid thickness (RLT) at baseline and follow-up time points as follows:
| SLP Diet Recommendation | Numerical Score |
|---|---|
| No Diet Restrictions/Thickeners | 0 |
| Nectar Thick Liquids | 1 |
| Nectar Plus or Thin Honey | 2 |
| Honey Thick | 3 |
| Pureed | 4 |
| Nothing By Mouth (NPO) | 5 |
Subgroups defined by treatment group (observation vs supraglottoplasty) were compared. Differences in categorical variables were tested using Fisher’s exact test and differences in continuous variables were tested using student’s t-test. In addition to these bivariate tests, the impact of supraglottoplasty vs observation on weight gain was also assessed using multivariate linear regression, adjusting for relevant covariates. Statistical analysis was performed using STATA v15.0 software with statistical significance set at p <0.05.
Results
65 children met initial inclusion criteria while 21 patients met exclusion criteria, leaving a final sample size of 44 patients. The median age at initial presentation was 96 days after birth. There were slightly more males than females (28/44, 64%). Almost one third (13/44, 30%) had an underlying genetic, craniofacial, or neuromuscular comorbidity (see Table 1).
Table 1:
Demographics of Study Population
| Total | Observation N = 19 (42%) | Supraglottoplasty N = 25 (57%) | P value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 29 | 14 (74%) | 14 (56%) | 0.23 |
| Female | 16 | 5 (26%) | 11 (44) | |
| Comorbidities | ||||
| Down syndrome | 2 | 0 (0%) | 2 (8%) | 0.50 |
| Genetic syndrome | 10 | 1 (5%) | 8 (32%) | 0.03* |
| Neuromuscular disorder | 9 | 3 (16%) | 5 (20%) | >0.99 |
| Prematurity (<36 wks) | 6 | 3 (16%) | 2 (8%) | 0.64 |
| Craniofacial anomaly | 5 | 1 (5%) | 4 (16%) | 0.37 |
| GERD | 6 (32%) | 12 (48%) | 0.36 | |
| Failure to thrive | 19 | 4 (21%) | 9 (36%) | 0.34 |
| Asthma/reactive airway disease | 14 | 0 (0%) | 1 (4%) | >0.99 |
| Congenital heart/lung anomaly | 1 | 3 (16%) | 5 (20%) | >0.99 |
| Additional upper airway anomaly | 8 | 0 (0%) | 6 (24%) | 0.03* |
| Surgical History | ||||
| Tracheostomy | 2 | 0 (0%) | 2 (8%) | 0.50 |
| Gastrostomy tube placement | 11 | 1 (5%) | 9 (36%) | 0.03* |
| Presenting Symptoms | ||||
| Stridor | 42 | 10 (53%) | 19 (76%) | 0.12 |
| Retractions | 27 | 9 (47%) | 18 (72%) | 0.13 |
| Feeding difficulty | 39 | 15 (79%) | 23 (92%) | 0.38 |
| Poor weight gain | 23 | 4 (21%) | 18 (72%) | 0.002* |
Unless otherwise indicated, (%) represent column percentages. Legend: SD = standard deviation, n = number, MBSS = modified barium swallow study, GERD = gastroesophageal reflux disease.
Statistically significant (p value <0.05)
Most children had symptoms of stridor (41/44, 93%) or feeding difficulty (38/44, 86%). Half had a history of poor weight gain (22/44, 50%) and the majority were found to have laryngeal penetration or aspiration on MBSS (25/44, 57%). All patients with a positive swallow study reported dysphagia or aspiration symptoms such as coughing or choking with feeds.
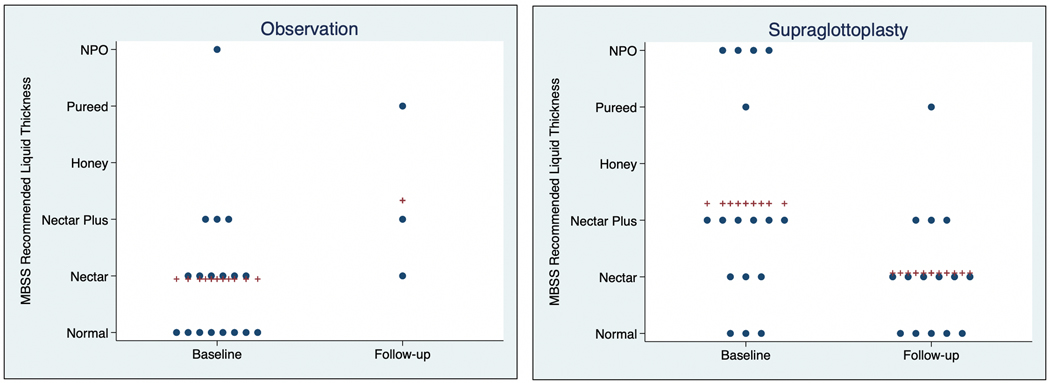
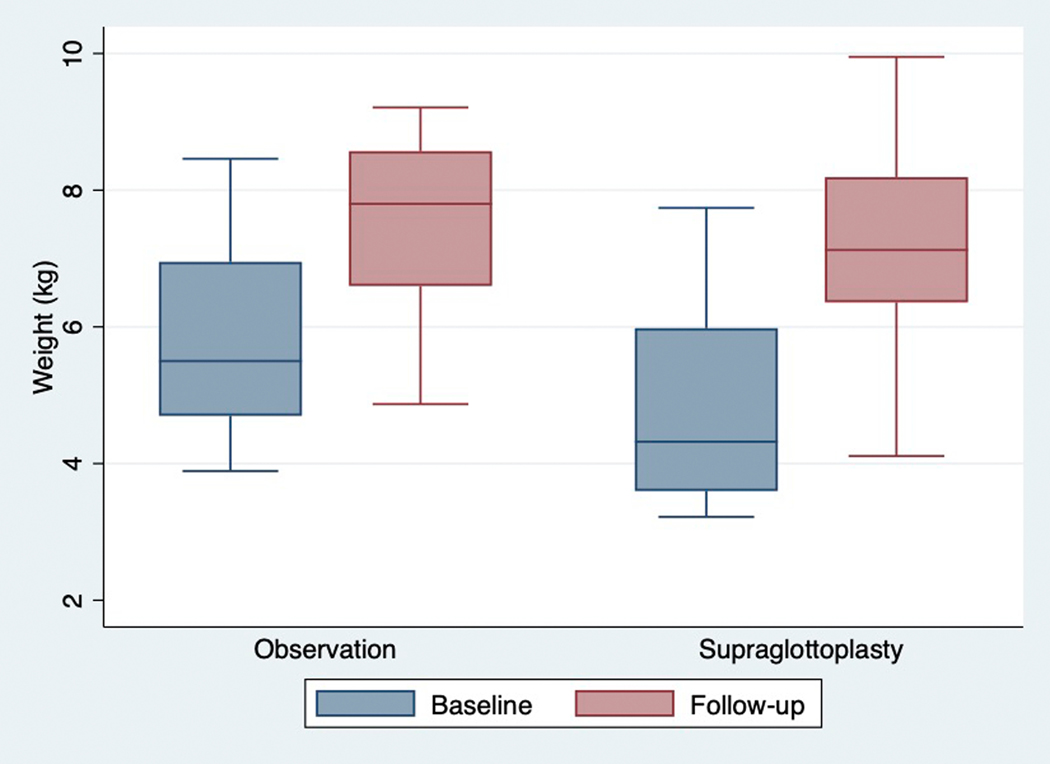
Just over half (25/44, 57%) of patients underwent supraglottoplasty. Compared to children who were observed, children who underwent supraglottoplasty were more likely to have a genetic syndrome (p=0.03), have additional airway abnormalities (p=0.03), and have a history of gastrostomy tube placement (p=0.03) (see Table 1). Most children (23/25 92%) who underwent supraglottoplasty experienced major improvement or resolution of dysphagia symptoms. The two patients who did not report improvement in dysphagia symptoms following supraglottoplasty had an underlying genetic syndrome. Pre-operatively, the mean diet score was 2.3 (nectar plus/thin honey) which improved to a mean of 1.1 (nectar thick), as demonstrated in Figure 1. There was no significant difference in final weight or weight gain between children treated with observation vs supraglottoplasty (1.6 vs 2.3 kg, p = 0.06, see Table 2 and Figure 2). In linear regression analysis, when adjusting for baseline age and length of follow-up, there was no significant association between treatment type (observation vs supraglottoplasty) and weight gain (beta = 0.21, p = 0.52).
Figure 1.
Speech Language Pathology Recommended Liquid Thickness at Baseline and Follow-up
Legend: Each dot represents an individual patient’s RLT. The crosses represent the mean RLT
Table 2.
Mean Weight and Modified Barium Swallow Study Outcomes
| Mean (SD) | N | Observation N = 19 (43%) | N | Supraglottoplasty N = 25 (57%) | P value |
|---|---|---|---|---|---|
| Baseline | 19 | 24 | |||
| Weight in kg | 5.8 (1.4) | 5.3 (2.2) | 0.43 | ||
| Age in days | 117 (82) | 127 (124) | 0.76 | ||
| Follow-Up | 17 | 24 | |||
| Weight in kg | 7.4 (1.4) | 7.6 (1.8) | 0.63 | ||
| Age in days | 202 (100) | 286 (175) | 0.08 | ||
| Weight change | 17 | 1.6 (0.8) | 24 | 2.3 (1.3) | 0.06 |
| Follow-up time | 82 (49) | 162 (103) | <0.01* | ||
| Recommended Liquid Thickness (RLT) | |||||
| Baseline | 18 | 0.9 (1.3) | 17 | 2.3 (1.8) | 0.02* |
| Follow-Up | 3 | 2.3 (1.5) | 15 | 1.1 (1.1) | 0.10 |
| Mean difference in RLT | + 1.39 | −1.27 | |||
| p = 0.10 | p = 0.03* | ||||
Unless otherwise indicated, (%) represent column percentages. Legend: SD = standard deviation, n = number, RLT = recommended liquid thickness.
Statistically significant (p value <0.05).
Figure 2.
Baseline and Follow-up Weight by Treatment Group
Legend: Box plot demonstrating baseline and follow-up weights. Horizontal bar represents the mean weight.
Discussion
Laryngomalacia is a common congenital anomaly which leads to supraglottic tissue laxity and collapse into the airway. Laryngomalacia is the most common cause of stridor in infants, with a symptom peak at around 6 months of age and gradual resolution in most children by age 24 months.1 In addition to stridor, laryngomalacia is a well-established and treatable cause of respiratory distress and sleep disordered breathing.1,2
In our study population, children who underwent supraglottoplasty had improvement in dysphagia with less stringent diet restrictions based on MBSS at follow up. However, we were not able to demonstrate a statistically significant difference in degree of MBSS improvement when comparing patients who underwent surgery and those who did not, likely due to insufficient follow-up MBSS data in the observation group.
The relationship between laryngomalacia and dysphagia has not been well characterized in the literature.1–2 Potential mechanisms which might link laryngomalacia with dysphagia include insufficient laryngeal inlet mechanical coverage during swallow, increased negative pressure in the subglottis due to inspiratory obstruction, and disruption of the highly coordinated normal suck-swallow-breathe mechanism.
Determining the true prevalence of dysphagia in children with laryngomalacia is difficult due to the low sensitivity (<50%) of the bedside swallow evaluation in identifying dysphagia in this population due to high rates of silent aspiration.3 Hence, many children with laryngomalacia likely go undiagnosed. In our study, 57% of the children with laryngomalacia who were tested with a MBSS showed evidence of aspiration or laryngeal penetration. However, patients at our institution only undergo a MBSS if there is clinical suspicion for aspiration, so this likely overestimates the true prevalence of aspiration in children with laryngomalacia.
With regards to the efficacy of supraglottoplasty in improving swallow, our findings are in agreement with prior studies on the topic. Richter et al. studied 50 patients with laryngomalacia who underwent functional endoscopic evaluation of swallow (FEES) and subsequent supraglottoplasty. Of this group, 44/50 (88%) had laryngeal penetration and 36/50 (72%) frank aspiration on FEES. After surgical repair of the laryngomalacia, they found an 82% resolution rate of aspiration and 86% resolution of penetration.4 Simmons et al. studied 324 patients with laryngomalacia who were evaluated in an academic medical center’s aerodigestive clinic. In that study, 140/185 (75%) patients who underwent a swallowing assessment had abnormal results.1 Our research is also in general agreement with the work by previous authors who have demonstrated improved oral intake and weight gain following supraglottoplasty.5–6
In contrast, Rastatter et al. examined 39 patients with laryngomalacia who underwent a surgical repair (supraglottoplasty) for respiratory distress. Of this cohort, 10/39 were found to aspirate preoperatively by clinical exam or videofluoroscopic swallow study (VFSS), and only 2/10 patients had resolution of aspiration post-operatively.7
The conclusions which can be drawn from this study are limited by a small sample size and the retrospective nature of the study design. Children in our study had high rates of genetic syndromes, medical comorbidities, and symptoms of dysphagia, so care should be taken in generalizing our results to the broader population of children with laryngomalacia who often lack these same attributes and tend to see resolution of symptoms with conservative management. In addition, our study is limited by a selection bias, as children who underwent supraglottoplasty generally had more severe dysphagia and more comorbid medical problems. The fact that children who were observed had less severe dysphagia at baseline, might explain why we did not find a relatively greater weight gain in children who underwent surgical repair. In addition, given the natural self-limited nature of laryngomalacia, we are unable to definitively determine how much of the improvement in swallowing outcomes can be attributed directly to supraglottoplasty.
Another limitation was lack of consistent patient follow-up in certain subsets of patients. In particular, few patients who did not undergo supraglottoplasty had repeat evaluations by speech language pathology, and it is likely that those who did continue to follow-up were those still experiencing swallowing or feeding difficulty. This may account for the greater degree of recommended thickening at follow-up among those children who were observed. As a result, it is difficult to make conclusions about the impact of supraglottoplasty on recommended liquid thickness relative to patients who did not undergo an operation.
Given the paucity of research on this topic, and the potential to impact clinical practice, randomized controlled trials are needed to further evaluate the relationship between laryngomalacia and dysphagia.
Conclusion
Children who undergo supraglottoplasty for laryngomalacia have improved dysphagia with less restrictive diets at follow-up. However, determining whether this improvement is directly attributable to surgery rather than the natural history of the disease warrants further investigation.
Footnotes
Conflicts of Interest: The authors declare that there are no conflicts of interest
References
- [1].Simons JP, Greenberg LL, Mehta DK, Fabio A, Maguire RC, Mandell DL, Laryngomalacia and Swallowing Function in Children, Laryngoscope. 126 (2016) 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Farhood Z, Ong AA, Nguyen SA, Gillespie MB, Discolo CM, White DR, Objective Outcomes of Supraglottoplasty for Children With Laryngomalacia and Obstructive Sleep Apnea: A Meta-analysis, JAMA Otolaryngol Head Neck Surg. 142 (2016) 665–71. [DOI] [PubMed] [Google Scholar]
- [3].Gasparin M, Schweiger C, Manica D, Maciel AC, Kuhl G, Levy DS, Marostica PJ, Accuracy of clinical swallowing evaluation for diagnosis of dysphagia in children with laryngomalacia or glossoptosis, Pediatr Pulmonol. 52 (2017) 41–47. [DOI] [PubMed] [Google Scholar]
- [4].Richter GT, Thompson DM, The surgical management of laryngomalacia, Otolaryngol Clin North Am. 41 (2008) 837–64. [DOI] [PubMed] [Google Scholar]
- [5].Eustaquio M, Lee EN, Digoy GP, Feeding outcomes in infants after supraglottoplasty, Otolaryngol Head Neck Surg. 145 (2011) 818–22. [DOI] [PubMed] [Google Scholar]
- [6].Czechowicz JA, Chang KW, Catch-up growth in infants with laryngomalacia after supraglottoplasty, Int J Pediatr Otorhinolaryngol. 79 (2015) 1333–6. [DOI] [PubMed] [Google Scholar]
- [7].Rastatter JC, Schroeder JW, Hoff SR, Holinger LD, Aspiration before and after Supraglottoplasty regardless of Technique, Int J Otolaryngol. 2010 (2010) 912814. [DOI] [PMC free article] [PubMed] [Google Scholar]