Abstract
OBJECTIVE:
To describe syphilis treatment status and prenatal care among people with syphilis during pregnancy to identify missed opportunities for preventing congenital syphilis.
METHODS:
Six jurisdictions that participated in SET-NET (Surveillance for Emerging Threats to Pregnant People and Infants Network) conducted enhanced surveillance among people with syphilis during pregnancy based on case investigations, medical records, and linkage of laboratory data with vital records. Unadjusted risk ratios (RRs) were used to compare demographic and clinical characteristics by syphilis stage (primary, secondary, or early latent vs late latent or unknown) and treatment status during pregnancy (adequate per the Centers for Disease Control and Prevention’s “Sexually Transmitted Infections Treatment Guidelines, 2021” vs inadequate or not treated) and by prenatal care (timely: at least 30 days before pregnancy outcome; nontimely: less than 30 days before pregnancy outcome; and no prenatal care).
RESULTS:
As of September 15, 2023, of 1,476 people with syphilis during pregnancy, 855 (57.9%) were adequately treated and 621 (42.1%) were inadequately treated or not treated. Eighty-two percent of the cohort received timely prenatal care. Although those with nontimely or no prenatal care were more likely to receive inadequate or no treatment (RR 2.50, 95% CI, 2.17–2.88 and RR 2.73, 95% CI, 2.47–3.02, respectively), 32.1% of those with timely prenatal care were inadequately or not treated. Those with reported substance use or a history of homelessness were nearly twice as likely to receive inadequate or no treatment (RR 2.04, 95% CI, 1.82–2.28 and RR 1.83, 95% CI, 1.58–2.13, respectively).
CONCLUSION:
In this surveillance cohort, people without timely prenatal care had the highest risk for syphilis treatment inadequacy; however, almost a third of people who received timely prenatal care were not adequately treated. These findings underscore gaps in syphilis screening and treatment for pregnant people, especially those experiencing substance use and homelessness, and the need for systems-based interventions, such as treatment outside of traditional prenatal care settings.
Syphilis is a sexually transmitted infection (STI) that, if left untreated, causes substantial morbidity and pregnancy-specific problems such as stillbirth, preterm birth, low birth weight, and fetal hydrops, anemia, and hepatosplenomegaly.1–4 Congenital syphilis cases reported to the Centers for Disease Control and Prevention (CDC) doubled from 1,325 in 2018 to 2,875 in 2021.5 This, in combination with rising rates of syphilis in all segments of the population, prompted the declaration of a syphilis epidemic.6
Congenital syphilis is preventable with timely identification and treatment before or early during pregnancy, which also prevents long-term health sequelae for the pregnant person with syphilis.3 Syphilis screening is universally recommended during the first prenatal care visit and is mandated in most states in the United States, and typically requires both non-treponemal and treponemal tests.7–9 Repeat screening in the third trimester and at delivery is additionally recommended where syphilis prevalence is high or with high individual risk for syphilis.8,9 For people without optimal prenatal care, syphilis screenings and treatments should be performed at the time of pregnancy testing if follow-up is uncertain.1
As of October 2023, benzathine penicillin G is the only medication recommended for treatment of syphilis during pregnancy given its demonstrated efficacy and safety in treating both the pregnant person and fetus.9 However, there is concern for lack of identification and treatment of syphilis during pregnancy, despite available therapy given the rise in cases nationally. A 2023 study of nationally reported congenital syphilis cases found that 51% were attributed to inadequate treatment despite a timely diagnosis during pregnancy, and 37% were attributed to a nontimely testing.10 The objective of this analysis is to examine missed opportunities for congenital syphilis prevention by describing characteristics of people with syphilis during pregnancy that are associated with the lack of complete treatment during pregnancy.
METHODS
SET-NET (Surveillance for Emerging Threats to Pregnant People and Infants Network), in collaboration with the CDC and state and local health departments, is a longitudinal, pregnant person–infant linked surveillance program designed to identify exposures to infections during pregnancy and monitor pregnant person and infant outcomes.11 As of September 2023, six participating jurisdictions (Arizona, Georgia, Michigan, New Jersey, New York State, and Washington) submitted enhanced surveillance data on syphilis during pregnancy to the CDC based on case investigations, medical records, and linkage of laboratory surveillance with vital records. Arizona’s surveillance included Maricopa, Pima, and Yuma Counties, representing 80% of the state’s births; surveillance in all other jurisdictions was statewide.
Pregnant people were included if they 1) met the Council of State and Territorial Epidemiologists’ case definition for syphilis (all stages) at any point during pregnancy or had a stillborn or liveborn neonate with syphilis or a child who met the 2018 Council of State and Territorial Epidemiologists’ case definition for probable or confirmed congenital syphilis,12 2) had a reported surveillance stage (primary, secondary, or early latent; late latent or unknown), and 3) had a reported pregnancy outcome and a pregnancy outcome date that occurred between January 1, 2018, and December 31, 2021. Arizona and New Jersey reported data from 2018–2021, Georgia from 2018–2019, Michigan from 2020–2021, New York State from 2018 and 2020–2021, and Washington from 2018–2020. Pregnant people reported as being in the “Other” surveillance stage were grouped with late latent or unknown stages, based on CDC surveillance guidance.13
Surveillance stages were grouped by treatment recommendations such that those diagnosed with primary, secondary, or early latent syphilis were grouped together and those diagnosed with late latent or unknown syphilis were similarly grouped together. Adequate treatment status was defined by the CDC’s “Sexually Transmitted Infections Treatment Guidelines, 2021” (2021 STI Treatment Guidelines) and syphilis stage: for people diagnosed with primary, secondary, or early latent syphilis, at least one dose (2.4 million units intramuscularly) of benzathine penicillin G initiated at least 30 days before the pregnancy outcome; for people diagnosed with late latent or unknown stages, at least three (2.4 million units intramuscularly) doses of benzathine penicillin G dosed 5 to 9 days apart, with the initial dose initiated at least 30 days before pregnancy outcome.8 Guidance in both the 2015 and 2021 CDC STI Treatment Guidelines indicates doses should be no more than 9 days apart for pregnant people, without a lower bound of days for the treatment interval.9,14 This analysis implemented a lower bound of 5 days between doses to be classified as adequate treatment for the multidose benzathine penicillin G regimen. For people with neurosyphilis, adequate treatment was defined as aqueous crystalline penicillin G (a form of benzathine penicillin G) administered as 3–4 million units intravenously every 4 hours or a continuous infusion for 10–14 days for a total of 18–24 million units.9
For people with multiple categories of inadequate treatment, a primary reason was assigned using a prioritization hierarchy: 1) treatment other than benzathine penicillin G, 2) treatment initiated less than 30 days before pregnancy outcome, 3) receipt of fewer than three doses (for late latent or unknown syphilis only), and 4) doses outside of the recommended dosing interval (for late latent or unknown syphilis only; Appendices 1 and 2, available online at http://links.lww.com/AOG/D643). People who received treatment after the pregnancy outcome and people with no treatment data reported were categorized as no treatment during pregnancy. Antibiotic doses were included if they were administered at any time from the date of the last menstrual period through one day before the pregnancy outcome; for people diagnosed with late latent or unknown syphilis, doses received up to 18 days before the last menstrual period were included, with the assumption that two doses occurred before pregnancy and dosing intervals were met.
Prenatal care was characterized by the number of prenatal care visits and the timing of the prenatal care relative to the pregnancy outcome. The timing of prenatal care was categorized as: timely (at least one prenatal care visit at least 30 days before pregnancy outcome); nontimely (prenatal care initiated less than 30 days before pregnancy outcome), and none (no prenatal care documented).15 Demographic characteristics, including social determinants of health, and clinical characteristics were described by treatment status (adequate vs inadequate or no treatment), syphilis stage, and timeliness of prenatal care. Race and ethnicity were included as a proxy for lived experiences that may include systemic racism and implicit bias influencing health care access and management and do not reflect physiologic differences in these heterogenous groups.16 The categories for inadequate treatment were reported, as well as the number of benzathine penicillin G doses received overall and by prenatal care status.
Unadjusted associations between clinical characteristics and treatment status were assessed using modified Poisson regression to obtain unadjusted risk ratios (RRs), 95% CIs, and P values. Models that assessed the association between demographics and treatment status (adequate vs inadequate vs no treatment) are shown in Appendix 3, available online at http://links.lww.com/AOG/D643. Given the current role of prenatal care visits as the main setting for administration of syphilis treatment5 and to assess additional opportunities for treatment, demographic and clinical characteristics were stratified by prenatal care and treatment status. Statistical significance was set a priori at P<.05. All analyses were conducted using SAS 9.4 and R 4.1.2 statistical software. This activity was deemed as public health surveillance and exempt from IRB review; it has been reviewed by the CDC and conducted consistent with applicable federal law and policy (45 C.F.R. part 46.102(l)(2), 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a).
RESULTS
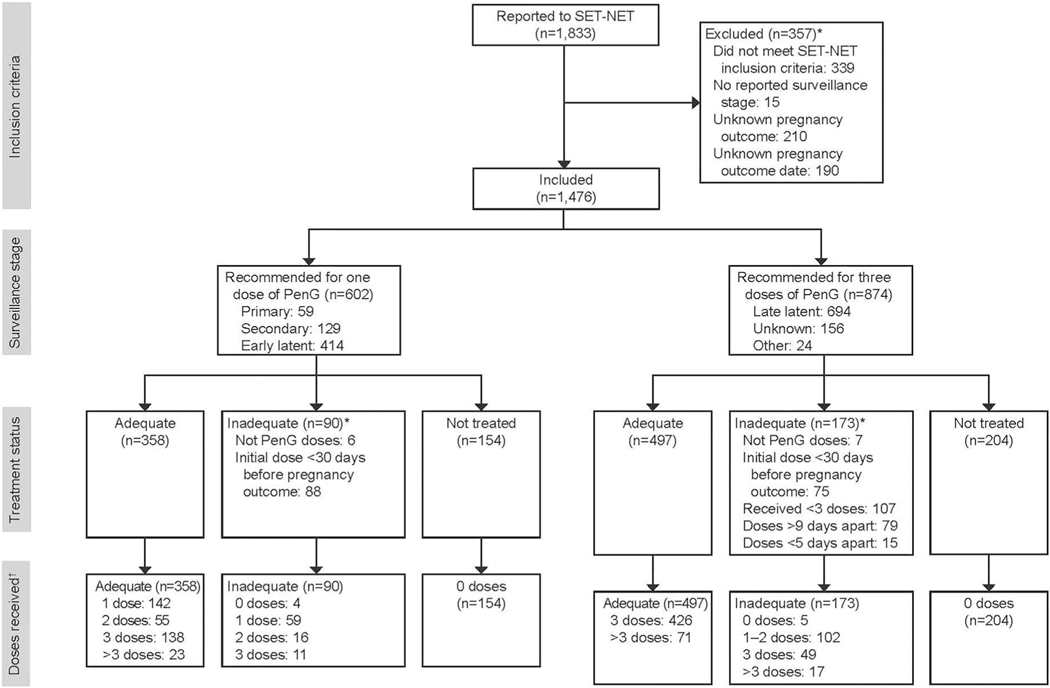
As of September 15, 2023, six jurisdictions reported 1,833 people with syphilis during pregnancy to SET-NET, and 1,476 (80.5%) of these met inclusion criteria (Fig. 1). The median age was 27 years, and 88.0% lived in medium–large metropolitan areas (Table 1). The majority (82.2%) received timely prenatal care, 5.1% had nontimely prenatal care, and 12.7% had no prenatal care. One in five pregnant people reported substance use during pregnancy. Additionally, 8.5% reported a history of incarceration, and 11.7% reported a history of homelessness. Overall, 40.8% of pregnant people in this cohort were diagnosed with primary, secondary, or early latent syphilis, and 59.2% were diagnosed with late latent or unknown syphilis.
Fig. 1.
Flowchart of people with syphilis during pregnancy reported to SET-NET (Surveillance for Emerging Threats to Pregnant People and Infants Network) as of September 15, 2023. Surveillance stage, treatment status, and number of benzathine penicillin G (PenG) doses received are reported. *Not mutually exclusive. †Doses are defined as doses of PenG or aqueous crystalline penicillin G (as treatment for neurosyphilis) received during pregnancy.
Table 1.
Distribution of Demographic and Clinical Characteristics by Syphilis Surveillance Stage and Treatment Status, SET-NET (Surveillance for Emerging Threats to Pregnant People and Infants Network), Six States, 2018–2021
| Not Treated or Inadequately Treated(n=621, 42.1%) |
||||
|---|---|---|---|---|
| Characteristic | Total (N=1,476) | P/S/E (n=244)* | LL/U (n=377)† | Total (n=621) |
|
| ||||
| Urbanicity# | ||||
| Medium–large metropolitan (250,000 or more) | 1,297 (88.0) | 222 (91.7) | 339 (89.9) | 561 (90.6) |
| Rural–small metropolitan (249,999 or fewer) | 177 (12.0) | 20 (8.3) | 38 (10.1) | 58 (9.4) |
| Missing | 2 (0.1) | 2 (0.8) | 0 (0.0) | 2 (0.3) |
| Age at first diagnosis (y) | 27 (23–31) | 27 (22–31) | 28 (24–33) | 28 (23–32) |
| 24 or younger | 574 (39.4) | 90 (37.8) | 114 (30.7) | 204 (33.5) |
| 25–29 | 434 (29.8) | 74 (31.1) | 111 (29.9) | 185 (30.4) |
| 30–34 | 289 (19.8) | 45 (18.9) | 85 (22.9) | 130 (21.3) |
| 35 or older | 160 (11.0) | 29 (12.2) | 61 (16.4) | 90 (14.8) |
| Missing | 19 (1.3) | 6 (2.5) | 6 (1.6) | 12 (1.9) |
| Race and ethnicity** | ||||
| American Indian, American Native, Native Hawaiian, Pacific Islander | 78 (5.4) | 14 (5.8) | 22 (5.9) | 36 (5.9) |
| Asian | 30 (2.1) | 5 (2.1) | 4 (1.1) | 9 (1.5) |
| Black, non-Hispanic | 486 (33.8) | 81 (33.8) | 102 (27.3) | 183 (29.8) |
| Hispanic or Latinx | 530 (36.9) | 83 (34.6) | 144 (38.5) | 227 (37.0) |
| Multiple or other unspecified | 14 (1.0) | 3 (1.3) | 6 (1.6) | 9 (1.5) |
| White, non-Hispanic | 298 (20.8) | 54 (22.5) | 96 (25.7) | 150 (24.4) |
| Missing | 40 (2.7) | 4 (1.6) | 3 (0.8) | 7 (1.1) |
| Health insurance at delivery | ||||
| Private | 174 (15.0) | 25 (14.3) | 25 (8.6) | 50 (10.8) |
| Public | 897 (77.2) | 133 (76.0) | 230 (79.3) | 363 (78.1) |
| Other, none, self-pay | 91 (7.8) | 17 (9.7) | 35 (12.1) | 52 (11.2) |
| Missing | 314 (21.3) | 69 (28.3) | 87 (23.1) | 156 (25.1) |
| History of incarceration†† | ||||
| Yes | 91 (8.5) | 15 (8.6) | 33 (13.0) | 48 (11.2) |
| No | 984 (91.5) | 159 (91.4) | 220 (87.0) | 379 (88.8) |
| Missing | 401 (27.2) | 70 (28.7) | 124 (32.9) | 194 (31.2) |
| History of homelessness†† | ||||
| Yes | 126 (11.7) | 31 (18.7) | 53 (20.2) | 84 (19.6) |
| No | 946 (88.2) | 135 (81.3) | 209 (79.8) | 344 (80.4) |
| Missing | 404 (27.4) | 78 (32.0) | 115 (30.5) | 193 (31.1) |
| No. of prenatal care visits | 8 (3–12) | 6 (2–10) | 2 (0–8) | 4 (0–9) |
| Receipt of prenatal care | ||||
| Timely (at least 30 d before pregnancy outcome) | 1,143 (82.2) | 169 (74.1) | 198 (56.4) | 367 (63.4) |
| Nontimely (less than 30 d before pregnancy outcome) | 71 (5.1) | 21 (9.2) | 36 (10.3) | 57 (9.8) |
| No prenatal care | 177 (12.7) | 38 (16.7) | 117 (33.3) | 155 (26.8) |
| Missing | 86 (5.8) | 16 (6.6) | 26 (6.9) | 42 (6.8) |
| MOUD receipt‡‡ | ||||
| Yes | 79 (5.5) | 17 (7.6) | 33 (9.3) | 50 (8.6) |
| No | 1,345 (94.5) | 208 (92.4) | 321 (90.7) | 529 (91.4) |
| Missing | 52 (3.5) | 19 (7.8) | 23 (6.1) | 42 (6.8) |
| Any substance use§§ | ||||
| Yes | 279 (19.6) | 55 (24.4) | 137 (38.7) | 192 (33.2) |
| No | 1,145 (80.4) | 170 (75.6) | 217 (61.3) | 387 (66.8) |
| Missing | 52 (3.5) | 19 (7.8) | 23 (6.1) | 42 (6.8) |
| Gestational age at pregnancy outcome (wk) | ||||
| Less than 35 | 222 (15.0) | 53 (21.7) | 114 (30.2) | 167 (26.9) |
| 35 or more | 1,254 (85.0) | 191 (78.3) | 263 (69.8) | 454 (73.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Adequately Treated (n=855, 57.9%) |
|||||
|---|---|---|---|---|---|
| Characteristic | P/S/E (n=358)‡ | LL/U (n=497)§ | Total (n=855) | RR (95% CI)║ | P ¶ |
|
| |||||
| Urbanicity# | .008 | ||||
| Medium–large metropolitan (250,000 or more) | 323 (90.2) | 413 (83.1) | 736 (86.1) | 1.00 (ref) | |
| Rural–small metropolitan (249,999 or fewer) | 35 (9.8) | 84 (16.9) | 119 (13.9) | 0.76 (0.61–0.94) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Age at first diagnosis (y) | 26 (22–30) | 26 (23–31) | 26 (23–31) | <.001 | |
| 24 or younger | 156 (43.8) | 214 (43.5) | 370 (43.6) | 1.00 (ref) | |
| 25–29 | 107 (30.1) | 142 (28.9) | 249 (29.4) | 1.20 (1.03–1.40) | |
| 30–34 | 65 (18.3) | 94 (19.1) | 159 (18.8) | 1.27 (1.07–1.50) | |
| 35 or older | 28 (7.9) | 42 (8.5) | 70 (8.3) | 1.58 (1.33–1.89) | |
| Missing | 2 (0.6) | 5 (1.0) | 7 (0.8) | ||
| Race and ethnicity** | .004 | ||||
| American Indian, American Native, Native Hawaiian, Pacific Islander | 18 (5.2) | 24 (5.0) | 42 (5.1) | 0.92 (0.70–1.20) | |
| Asian | 5 (1.4) | 16 (3.4) | 21 (2.6) | 0.60 (0.34–1.04) | |
| Black, non-Hispanic | 135 (39.1) | 168 (35.2) | 303 (36.9) | 0.75 (0.64–0.88) | |
| Hispanic or Latinx | 125 (36.2) | 178 (37.3) | 303 (36.9) | 0.85 (0.73–0.99) | |
| Multiple or other unspecified | 2 (0.6) | 3 (0.6) | 5 (0.6) | 1.28 (0.85–1.92) | |
| White, non-Hispanic | 60 (17.4) | 88 (18.4) | 148 (18.0) | 1.00 (ref) | |
| Missing | 13 (3.6) | 20 (4.0) | 33 (3.9) | ||
| Health insurance at delivery | <.001 | ||||
| Private | 45 (15.7) | 79 (19.3) | 124 (17.8) | 1.00 (ref) | |
| Public | 227 (79.1) | 307 (74.9) | 534 (76.6) | 1.41 (1.10–1.80) | |
| Other, none, self-pay | 15 (5.2) | 24 (5.9) | 39 (5.6) | 1.99 (1.48–2.67) | |
| Missing | 71 (19.8) | 87 (17.5) | 158 (18.5) | ||
| History of incarceration†† | .008 | ||||
| Yes | 13 (4.9) | 30 (7.8) | 43 (6.6) | 1.37 (1.11–1.69) | |
| No | 250 (95.1) | 355 (92.2) | 605 (93.4) | 1.00 (ref) | |
| Missing | 95 (26.5) | 112 (22.5) | 207 (24.2) | ||
| History of homelessness†† | <.001 | ||||
| Yes | 23 (8.7) | 19 (5.0) | 42 (6.5) | 1.83 (1.58–2.13) | |
| No | 241 (91.3) | 361 (95.0) | 602 (93.5) | 1.00 (ref) | |
| Missing | 94 (26.3) | 117 (23.5) | 211 (24.7) | ||
| No. of prenatal care visits | 9 (6–12) | 10 (7–12) | 10 (6–12) | ||
| Receipt of prenatal care | <.001 | ||||
| Timely (at least 30 d before pregnancy outcome) | 328 (95.3) | 448 (95.7) | 776 (95.6) | 1.00 (ref) | |
| Nontimely (less than 30 d before pregnancy outcome) | 5 (1.5) | 9 (1.9) | 14 (1.7) | 2.50 (2.17–2.88) | |
| No prenatal care | 11 (3.2) | 11 (2.4) | 22 (2.7) | 2.73 (2.47–3.02) | |
| Missing | 14 (3.9) | 29 (5.8) | 43 (5.0) | ||
| MOUD receipt‡‡ | <.001 | ||||
| Yes | 16 (4.5) | 13 (2.6) | 29 (3.4) | 1.61 (1.34–1.93) | |
| No | 338 (95.5) | 478 (97.4) | 816 (96.6) | 1.00 (ref) | |
| Missing | 4 (1.1) | 6 (1.2) | 10 (1.2) | ||
| Any substance use§§ | <.001 | ||||
| Yes | 42 (11.9) | 45 (9.2) | 87 (10.3) | 2.04 (1.82–2.28) | |
| No | 312 (88.1) | 446 (90.8) | 758 (89.7) | 1.00 (ref) | |
| Missing | 4 (1.1) | 6 (1.2) | 10 (1.2) | ||
| Gestational age at pregnancy outcome (wk) | <.001 | ||||
| Less than 35 | 32 (8.9) | 23 (4.6) | 55 (6.4) | 2.08 (1.87–2.31) | |
| 35 or more | 326 (91.1) | 474 (95.4) | 800 (93.6) | 1.00 (ref) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
P/S/E/, primary, secondary, or early latent; LL/U, late latent or unknown; RR, risk ratio; ref, reference; MOUD, medications for opioid use disorder.
Data are n (%) or median (interquartile range) unless otherwise specified.
Not treated or inadequately treated, inclusive of treatment other than penicillin or initial dose given less than 30 days before pregnancy outcome.
Not treated or inadequately treated, inclusive of treatment other than penicillin, initial dose given less than 30 days before pregnancy outcome, fewer than three doses given, or doses given outside of the recommended window (more than 9 days apart or less than 5 days apart).
Adequately treated with at least one dose of penicillin during pregnancy, with the dose administered at least 30 days before pregnancy outcome.
Adequately treated with at least three doses of penicillin, spaced 5–9 days apart, with the first dose administered at least 30 days before pregnancy outcome and the final dose occurring during pregnancy.
Combined across syphilis stages, excluding missing cases. Risk ratio describes the risk of inadequate or no syphilis treatment in each category vs the risk of inadequate syphilis treatment in the referent category.
P values from overall x2 tests of association or Fisher exact test of the null hypotheses that the distribution of the demographic or clinical characteristic are similar across the treatment categories (inadequate or no syphilis treatment vs adequate treatment).
The National Center for Health Statistics Urban-Rural Classification Scheme for Counties was used to categorize urbanicity as follows: medium–large metropolitan (population of 250,000 or greater) and rural–small metropolitan (population 249,000 or fewer).24
Race and ethnicity is top-coded in the following hierarchy: 1) Native Hawaiian/Pacific Islander, 2) American Indian/Alaska Native, 3) Asian, 4) Hispanic ethnicity, 5) multiple non-Hispanic races. “Other” race category was a response option in SET-NET (Surveillance for Emerging Threats to Pregnant People and Infants Network) for people whose race was not Native Hawaiian, Pacific Islander, American Indian, Alaska Native, Asian, Black or African American, or White.
Within the 12 months preceding case report or positive test results or during this pregnancy.
The pregnant person received MOUD during this pregnancy.
Defined as illicit use of opioids (eg, prescription opioids not taken as prescribed, fentanyl, or heroin) and other illicit, nonprescription substances (eg, cocaine, methamphetamines, inhalants, ecstasy, or hallucinogens, such as LSD or PCP) during this pregnancy.
Missing data were excluded from the denominators for calculating percentages.
Just more than half of the cohort was adequately treated for syphilis during pregnancy (Table 1). Of the characteristics analyzed, prenatal care was most strongly associated with treatment status; compared with pregnant people who received timely prenatal care, those with nontimely or no reported prenatal care were approximately three times more likely to be inadequately treated or not treated. Several social determinants of health were associated with treatment status. Inadequate treatment or no treatment was associated with a lack of health insurance, self-pay status, or reported other insurance type (RR 1.99, 95% CI, 1.48–2.67), compared with those with private health insurance. Inadequate treatment or no treatment was also associated with a history of incarceration (RR 1.37, 95% CI, 1.11–1.69), a history of homelessness (RR 1.83, 95% CI, 1.58–2.13), and reported substance use during pregnancy (RR 2.04, 95% CI, 1.82–2.28). The receipt of medications for opioid use disorder (MOUD) was also associated with inadequate treatment or no treatment (RR 1.61, 95% CI, 1.34–1.93). Additionally, a pregnancy outcome before 35 weeks of gestation was associated with inadequately treated or not treated syphilis (RR 2.08, 95% CI, 1.87–2.31). Differences between adequate treatment and inadequate treatment groups were similar to differences between adequate treatment and no treatment groups (Appendix 3, http://links.lww.com/AOG/D643).
The proportion of pregnant people who received adequate treatment for syphilis was similar across surveillance stage groups, despite differences in the number of doses: the percentage of people adequately treated was 59.5% among those diagnosed with primary, secondary, or early latent syphilis and was 56.9% among those diagnosed with late latent or unknown syphilis. The percentage of people inadequately treated (excluding untreated people) was 15.0% among those diagnosed with primary, secondary, or early latent syphilis (90/602) and was 19.8% among those diagnosed with late latent or unknown syphilis (173/874; Appendix 3, http://links.lww.com/AOG/D643). Among those with primary, secondary, or early latent syphilis who received inadequate treatment (n=90), the most common type of inadequate treatment was receiving an initial dose less than 30 days before the pregnancy outcome (97.8%; Fig. 1). Among those with late latent or unknown syphilis who received inadequate treatment (n=173), the most common type of inadequate treatment was receiving fewer than three doses (61.8%), followed by receiving doses more than 9 days apart (45.7%), and receiving an initial dose less than 30 days before the pregnancy outcome (43.4%; Fig. 1).
Prenatal care timing was available for 1,391 (94.2%) pregnant people (Table 2). Statistical testing was not performed for analyses stratified by prenatal care timing due to small cell sizes. Most pregnant people with reported timely prenatal care were diagnosed between 2017 and 2019 (56.6%); the majority of those with nontimely prenatal care (55.9%) and without prenatal care (74.8%) were diagnosed between 2020 and 2021. Among those without prenatal care, histories of incarceration (26.5%) and homelessness (47.5%) and reported substance use (63.4%) were common, and 11.6% received MOUD. In contrast, among those with timely prenatal care, histories of incarceration (6.8%) and homelessness (6.4%), reported substance use (12.8%), and MOUD receipt (4.6%) were less common. Diagnosis of syphilis during the first trimester decreased from 42.1% among those receiving timely prenatal care to 36.8% those receiving nontimely prenatal care and 9.5% among those with no prenatal care.
Table 2.
Demographics and Clinical Characteristics by Timing of Receipt of Prenatal Care* and Treatment Status, SET-NET (Surveillance for Emerging Threats to Pregnant People and Infants Network), Six States, 2018–2021
| Timely Prenatal Care† |
||||
|---|---|---|---|---|
| Treatment Status |
||||
| Characteristic | Total (n=1,143) | Adequate (n=776) | Inadequate (n=198) | Not Treated (n=169) |
|
| ||||
| Urbanicity§ Medium–large metropolitan (250,000 or greater) |
1,000 (87.5) | 670 (86.3) | 173 (87.4) | 157 (92.9) |
| Rural–small metropolitan (249,999 or fewer) | 143 (12.5) | 106 (13.7) | 25 (12.6) | 12 (7.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Age at first diagnosis (y) History of incarceration║ |
26 (23–31) | 26 (23–31) | 27 (22–31) | 27 (23–32) |
| Yes | 59 (6.8) | 36 (6.1) | 12 (7.3) | 11 (9.0) |
| No | 815 (93.2) | 551 (93.9) | 153 (92.7) | 111 (91.0) |
| Missing History of homelessness║ |
269 (23.5) | 189 (24.4) | 33 (16.7) | 47 (27.8) |
| Yes | 56 (6.4) | 31 (5.2) | 12 (7.8) | 13 (11.1) |
| No | 815 (93.6) | 569 (94.8) | 142 (92.2) | 104 (88.9) |
| Missing | 272 (23.8) | 176 (22.7) | 44 (22.2) | 52 (30.8) |
| MOUD receipt¶ Yes |
51 (4.6) | 27 (3.5) | 14 (7.4) | 10 (6.5) |
| No | 1,063 (95.4) | 744 (96.5) | 174 (92.6) | 145 (93.5) |
| Missing | 29 (2.5) | 5 (0.6) | 10 (5.1) | 14 (8.3) |
| Any substance use# Yes |
143 (12.8) | 71 (9.2) | 38 (20.2) | 34 (21.9) |
| No | 971 (87.2) | 700 (90.8) | 150 (79.8) | 121 (78.1) |
| Missing | 29 (2.5) | 5 (0.6) | 10 (5.1) | 14 (8.3) |
| No. of prenatal care visits | 10 (6–12) | 10 (7–12) | 8 (5–11) | 6 (3–10) |
| Surveillance stage P/S/E |
497 (43.5) | 328 (42.3) | 68 (34.3) | 101 (59.8) |
| LL/U | 646 (56.5) | 448 (57.7) | 130 (65.7) | 68 (40.2) |
| Year of diagnosis**
2017–2019 |
602 (56.6) | 452 (62.0) | 101 (53.2) | 49 (33.8) |
| 2020–2021 | 462 (43.4) | 277 (38.0) | 89 (46.8) | 96 (66.2) |
| Missing | 79 (6.9) | 47 (6.1) | 8 (4.0) | 24 (14.2) |
| Test-to-treat time (d)†† | 9 (5–19) | 10 (6–20) | 7 (2–18) | NA |
| Trimester of 1st positive syphilis test result 1st |
445 (42.1) | 377 (51.8) | 48 (25.3) | 20 (14.3) |
| 2nd | 319 (30.2) | 260 (35.7) | 44 (23.2) | 15 (10.7) |
| 3rd | 223 (21.1) | 88 (12.1) | 95 (50.0) | 40 (28.6) |
| At delivery | 71 (6.7) | 3 (0.4) | 3 (1.6) | 65 (46.4) |
| Missing Gestational age at pregnancy outcome (wk) |
85 (7.4) | 48 (6.2) | 8 (4.0) | 29 (17.2) |
| Less than 35 | 105 (9.2) | 47 (6.1) | 29 (14.6) | 29 (17.2) |
| 35 or more | 1,038 (90.8) | 729 (93.9) | 169 (85.4) | 140 (82.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nontimely Prenatal Care‡ |
No Prenatal Care |
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Status |
||||||||
| Characteristic | Total(n=71) | Adequate (n=14) | Inadequate (n=30) | Not Treated (n=27) | Total (n=177) | Adequate (n=22) | Inadequate (n=28) | Not Treated (n=127) |
|
| ||||||||
| Urbanicity§ | ||||||||
| Medium–large metropolitan (250,000 or greater) | 58 (81.7) | 7 (50.0) | 24 (80.0) | 27 (100) | 167(94.9) | 22 (100) | 28 (100) | 117 (92.9) |
| Rural–small metropolitan (249,999 or fewer) | 13 (18.3) | 7 (50.0) | 6 (20.0) | 0 (0.0) | 9 (5.1) | 0 (0.0) | 0 (0.0) | 9 (7.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Age at first diagnosis (y) History of incarceration║ |
29 (23–34) | 27 (23–33) | 31 (22–35) | 27 (25–32) | 29 (25–32) | 26 (24–29) | 29 (26–33) | 29 (25–33) |
| Yes | 4 (9.3) | 1 (9.1) | 1 (6.3) | 2 (12.5) | 26 (26.5) | 5 (31.3) | 3 (20.0) | 18 (26.9) |
| No | 39 (90.7) | 10 (90.9) | 15 (93.8) | 14 (87.5) | 72 (73.5) | 11 (68.8) | 12 (80.0) | 49 (73.1) |
| Missing History of homelessness║ |
28 (39.4) | 3 (21.4) | 14 (46.7) | 11 (40.7) | 79 (44.6) | 6 (27.3) | 13 (46.4) | 60 (47.2) |
| Yes | 9 (19.6) | 2 (18.2) | 4 (23.5) | 3 (16.7) | 56 (47.5) | 8 (47.1) | 14 (58.3) | 34 (44.2) |
| No | 37 (80.4) | 9 (81.8) | 13 (76.5) | 15 (83.3) | 62 (52.5) | 9 (52.9) | 10 (41.7) | 43 (55.8) |
| Missing | 25 (35.2) | 3 (21.4) | 13 (43.3) | 9 (33.3) | 59 (33.3) | 5 (22.7) | 4 (14.3) | 50 (39.4) |
| MOUD receipt¶ | ||||||||
| Yes | 3 (4.4) | 1 (7.7) | 0 (0.0) | 2 (7.4) | 19 (11.6) | 0 (0.0) | 6 (22.2) | 13 (11.3) |
| No | 65 (95.6) | 12 (92.3) | 28 (100) | 25 (92.6) | 145 (88.4) | 22 (100) | 21 (77.8) | 102 (88.7) |
| Missing | 3 (4.2) | 1 (7.1) | 2 (6.7) | 0 (0.0) | 13 (7.3) | 0 (0.0) | 1 (3.6) | 12 (9.4) |
| Any substance use# | ||||||||
| Yes | 18 (26.5) | 4 (30.8) | 3 (10.7) | 11 (40.7) | 104 (63.4) | 9 (40.9) | 21 (77.8) | 74 (64.3) |
| No | 50 (73.5) | 9 (69.2) | 25 (89.3) | 16 (59.3) | 60 (36.6) | 13 (59.1) | 6 (22.2) | 41 (35.7) |
| Missing | 3 (4.2) | 1 (7.1) | 2 (6.7) | 0 (0.0) | 13 (7.3) | 0 (0.0) | 1 (3.6) | 12 (9.4) |
| No. of prenatal care visits | 1 (1–3) | 3 (1–8) | 2 (1–3) | 1 (1–2) | NA | NA | NA | NA |
| Surveillance stage | ||||||||
| P/S/E | 26 (36.6) | 5 (35.7) | 8 (26.7) | 13 (48.1) | 49 (27.7) | 11 (50.0) | 13 (46.4) | 25 (19.7) |
| LL/U | 45 (63.4) | 9 (64.3) | 22 (73.3) | 14 (51.9) | 128 (72.3) | 11 (50.0) | 15 (53.6) | 102 (80.3) |
| Year of diagnosis** | ||||||||
| 2017–2019 | 30 (44.1) | 11 (84.6) | 11 (37.9) | 8 (30.8) | 41 (25.2) | 6 (33.3) | 5 (18.5) | 30 (25.4) |
| 2020–2021 | 38 (55.9) | 2 (15.4) | 18 (62.1) | 18 (69.2) | 122 (74.8) | 12 (66.7) | 22 (81.5) | 88 (74.6) |
| Missing | 3 (4.2) | 1 (7.1) | 1 (3.3) | 1 (3.7) | 14 (7.9) | 4 (18.2) | 1 (3.6) | 9 (7.1) |
| Test-to-treat time (d)†† | 9 (4–15) | 17 (6–37) | 7 (3–12) | NA | 3 (1–11) | 7 (2–22) | 3 (1–9) | NA |
| Trimester of 1st positive syphilis test result | ||||||||
| 1st | 25 (36.8) | 6 (46.2) | 9 (31.0) | 10 (38.5) | 15 (9.5) | 7 (38.9) | 3 (11.1) | 5 (4.4) |
| 2nd | 9 (13.2) | 4 (30.8) | 2 (6.9) | 3 (11.5) | 40 (25.3) | 7 (38.9) | 6 (22.2) | 27 (23.9) |
| 3rd | 30 (44.1) | 3 (23.1) | 18 (62.1) | 9 (34.6) | 50 (31.6) | 4 (22.2) | 18 (66.7) | 28 (24.8) |
| At delivery | 4 (5.9) | 0 (0.0) | 0 (0.0) | 4 (15.4) | 53 (33.5) | 0 (0.0) | 0 (0.0) | 53 (46.9) |
| Missing | 3 (4.2) | 1 (7.1) | 1 (3.3) | 1 (3.7) | 19 (10.7) | 4 (18.2) | 1 (3.6) | 14 (11.0) |
| Gestational age at pregnancy outcome (wk) | ||||||||
| Less than 35 | 23 (32.4) | 1 (7.1) | 7 (23.3) | 15 (55.6) | 78 (44.1) | 5 (22.7) | 11 (39.3) | 62 (48.8) |
| 35 or more | 48 (67.6) | 13 (92.9) | 23 (76.7) | 12 (44.4) | 99 (55.9) | 17 (77.3) | 17 (60.7) | 65 (51.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
MOUD, medications for opioid use disorder; NA, not applicable P/S/E/, primary, secondary, or early latent; LL/U, late latent or unknown. Data are n (%) or median interquartile range.
Individuals with missing information on receipt of prenatal care (n=85) were excluded from the denominator.
Received prenatal care at least 30 days before pregnancy outcome.
Received prenatal care less than 30 days before pregnancy outcome.
The National Center for Health Statistics Urban-Rural Classification Scheme for Counties was used to categorize urbanicity as follows: medium–large metropolitan (population of 250,000 or greater) and rural–small metropolitan (population of 249,000 or fewer).24
Within the 12 months preceding case report or positive test results or during pregnancy.
The pregnant person received MOUD during this pregnancy.
Defined as illicit use of opioids (eg, prescription opioids not taken as prescribed, fentanyl, or heroin) and other illicit, nonprescription substances (eg, cocaine, methamphetamines, inhalants, ecstasy, or hallucinogens, such as LSD or PCP) during this pregnancy.
Year of the first positive treponemal or nontreponemal test reported (whichever comes first). No single test or combination of tests can definitively diagnose and stage syphilis in the absence of a comprehensive history and physical examination.
Number of days between first positive treponemal or nontreponemal test during pregnancy and first treatment date during pregnancy. Missing data were excluded from the denominators for calculating percentages.
Among pregnant people who received timely prenatal care (n=1,143), 32.1% did not receive adequate treatment, 17.3% received inadequate treatment, and 14.8% did not receive treatment during pregnancy. The most common reasons for inadequate treatment among those with timely prenatal care were treatment initiation less than 30 days before pregnancy outcome (57.0%, despite receiving timely prenatal care), receiving fewer than three doses for those diagnosed with late latent or unknown syphilis (39.4%), and receiving doses more than 9 days apart (31.3%; data not shown). The median (interquartile range) time from testing to treatment was 9 days (5–19 days) overall and 7 days (2–18 days) for people who received inadequate treatment. Substance use was prevalent among people with inadequate treatment (20.2%) and no treatment (21.9%).
More than half of the people with timely prenatal care and no treatment (n=169) were diagnosed with primary, secondary, or early latent syphilis (59.8%). The timing of first syphilis testing was known for 82.8% of those not receiving syphilis treatment; of those, 25.0% were first tested for syphilis during the first or second trimester, and 46.4% were not tested for syphilis until delivery (ie, date of the pregnancy outcome). Among those with timely prenatal care, the pregnancy outcome occurred before 35 weeks of gestation for 6.1% of those with adequate treatment, 14.6% of those with inadequate treatment, and 17.2% of those with no treatment.
Among those with nontimely prenatal care (n=71), 19.7% were adequately treated, 42.3% had inadequate treatment (0% not benzathine penicillin G, 86.7% less than 30 days before pregnancy outcome, 23.2% outside recommended window of 5–9 days), and 38.0% were not treated during pregnancy. The pregnancy outcome occurred before 35 weeks of gestation for 32.4% of those with nontimely prenatal care. Among those without prenatal care (n=177), 12.4% received adequate treatment, 15.8% received inadequate treatment (14.3% not benzathine penicillin G, 78.6% less than 30 days before pregnancy outcome, 42.9% fewer than three doses), and 71.8% were not treated. Of those with known timing of positive testing, a third had their first positive syphilis test during the first or second trimester, and one-fourth of these people (14/55) received adequate treatment. Nineteen (11.6%) people who did not receive prenatal care received MOUD during their pregnancies; none of these people received adequate syphilis treatment. Forty-four percent of people without reported prenatal care had a pregnancy outcome before 35 weeks of gestation.
DISCUSSION
Despite recommendations for screening and treatment during pregnancy, 32.1% of people with timely prenatal care were not adequately treated for syphilis, including 14.8% who did not receive any treatment. Inadequate treatment was most frequently related to prenatal care initiation less than 30 days before pregnancy outcome. Findings from this analysis are consistent with previous literature on missed opportunities for congenital syphilis prevention and evaluating structural barriers to accessing prenatal care and preventing congenital syphilis.5,15,17–19
Treatment less than 30 days before pregnancy outcome might relate to patient-specific barriers and lack of awareness among clinicians about the need for immediate benzathine penicillin G treatment after diagnosis. Patient counseling at the time of testing or coordination with health departments might address these issues and create opportunities to educate and plan for future benzathine penicillin G doses, if indicated. Overall, 17.8% of pregnant people received prenatal care too late to receive adequate treatment or did not receive treatment at all, and recommendations for screening at first prenatal care visit would not reach these individuals. Furthermore, 15% experienced a pregnancy outcome before 35 weeks of gestation, shortening the window to commence adequate treatment.
These findings support recent CDC proposals to perform recommended syphilis screening during pregnancy outside of traditional prenatal care, including in emergency rooms, substance use treatment facilities, carceral settings, and homeless shelters.10 Given challenges with accessing care across the United States—especially among people experiencing homelessness, incarceration, or substance use disorder—broader availability of rapid testing (ie, point of care) with immediate treatment is critical. Additionally, systems-based solutions, such as the use of pregnancy laboratory panels in other encounters and employing electronic health record notifications when syphilis testing or treatment are needed.
The findings in this report highlight that syphilis testing and treatment is already likely occurring outside of traditional prenatal care. Among pregnant people with nontimely or no prenatal care, 35.9% had an initial positive syphilis test during the first or second trimester. Among those without prenatal care, more than a quarter still received some syphilis treatment, indicating that clinicians and public health professionals are already facilitating testing and treating outside of traditional prenatal care. More work is needed to bolster these efforts as testing and treating exclusively during prenatal care is no longer acceptable.20
Seventy-five percent of pregnant people without prenatal care and who were diagnosed between 2020 and 2021 (ie, the start of the coronavirus disease 2019 [COVID-19] pandemic) were not treated. The COVID-19 pandemic led to shifts to telemedicine models, changes in resource allocation, and patient hesitancy around face-to-face care, all of which likely contributed to less treatment adequacy.21,22
Clinical factors might also have contributed to inadequate treatment. These include syphilis testing and treatment recommendations that are difficult to interpret and implement, diagnostic algorithms including two-step testing, test turnaround time, the need for intramuscular injections, multidose regimens with defined dosing intervals for late latent or unknown syphilis, and inadequate partner treatment options. Additionally, clinicians often lack the time and administrative support for rapid communication of results, facilitation of expedited treatment, and communicating with health departments.17–19 The intermittent U.S. shortages of benzathine penicillin G also play a role in inadequate treatment23; moreover, these data suggest that multiple doses of benzathine penicillin G were given to those diagnosed with primary, secondary, or early latent syphilis (ie, more doses than the minimum recommended by the CDC’s 2021 STI Treatment Guidelines9), potentially exacerbating shortages.
This analysis has several limitations. First, the data are from six U.S. jurisdictions; observed patterns might differ from other areas. Furthermore, data are aggregated across four surveillance years (2018–2021), with jurisdictions reporting data from differing years. National STI treatment guidelines evolved during the surveillance period, and the optimal minimum dosing interval for pregnant people diagnosed with late latent or unknown syphilis remains unclear.14 Statutory mandates dictating screening during pregnancy vary across jurisdictions, and implementation might have led to increased timeliness of case finding in some jurisdictions.7,22 Data on the location of testing and treatment were not available and would help inform interventions. Possible syphilis reinfection could not be assessed, potentially biasing the results toward third trimester testing and treatment. Finally, certain characteristics (eg, histories of incarceration or homelessness, receipt of MOUD, and substance use) had low numbers and high levels of missingness, which might produce unstable estimates. These characteristics and other unmeasured social determinants of health might be stigmatizing and could reflect reporting bias, resulting in misclassification, and attenuating their observed associations with treatment status.
Although timely prenatal care remains an important opportunity for treating people with syphilis during pregnancy and preventing congenital syphilis, additional solutions are needed to address persistent missed opportunities. Research and development are needed around syphilis testing and treatment, especially highly sensitive and specific rapid tests, and oral treatment options during pregnancy. Effective interventions will require innovative partnerships between public health, patient advocacy groups, prenatal care clinicians, and clinicians outside of traditional prenatal care.
Supplementary Material
Acknowledgments
This study was performed as regular work of the Centers for Disease Control and Prevention. This work is supported by the Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases (ELC) Cooperative Agreement (ELC CK19-1904) and through contractual mechanisms, including the Local Health Department Initiative to Chickasaw Health Consulting (200-2021-F-12655). Staffing support for this work was funded by CDC to a contract to Eagle Global Scientific (200-2019-06754) and a contract to Lukos, LLC (200-2023F-17882).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The Surveillance for Emerging Threats to Pregnant People and Infants Network does not collect data on the sex or gender of the birthing person. For clarity in terminology, “maternal” is used to identify the person who is pregnant or postpartum throughout this manuscript; the authors are aware that pregnancy is not equated with the decision to parent nor do all parents who give birth identify as mothers. The following text makes use of concepts or descriptions that align with the traditional gender definitions by using concepts such as “maternal” however, the concepts described are translatable to all people who experience a pregnancy, regardless of their gender identity.
The authors thank the staff supporting the SET-NET work, including Genna Owens, Tia Falzarano, and Heather Marshall with the Michigan Department of Health and Human Services, Arizona Department of Health Services, Georgia Department of Public Health, New Jersey Department of Health, New York State Department of Health, and Washington State Department of Health for data collection, reporting, and partnership in this important work. Permission has been obtained from all named persons.
Footnotes
Each author has confirmed compliance with the journal’s requirements for authorship.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Eppes CS, Stafford I, Rac M. Syphilis in pregnancy: an ongoing public health threat. Am J Obstet Gynecol 2022;227:822–38. doi: 10.1016/j.ajog.2022.07.041 [DOI] [PubMed] [Google Scholar]
- 2.Hook EW. Syphilis. Lancet 2017;389:1550–7. doi: 10.1016/s0140-6736(16)32411-4 [DOI] [PubMed] [Google Scholar]
- 3.Cooper JM, Sánchez PJ. Congenital syphilis. Semin Perinatol 2018;42:176–84. doi: 10.1053/j.semperi.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 4.Tsimis ME, Sheffield JS. Update on syphilis and pregnancy. Birth Defects Research 2017;109:347–52. doi: 10.1002/bdra.23562 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Sexually transmitted infections surveillance, 2022. Accessed February 15, 2024. https://www.cdc.gov/std/statistics/2022/
- 6.Centers for Disease Control and Prevention. U.S. STI epidemic showed no signs of slowing in 2021 – cases continued to escalate. Accessed February 15, 2024. https://www.cdc.gov/media/releases/2023/s0411-sti.html
- 7.Centers for Disease Control and Prevention. State statutory and regulatory language regarding prenatal syphilis screenings in the United States. Accessed June 12, 2023. https://www.cdc.gov/std/treatment/syphilis-screenings.htm
- 8.US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for syphilis infection in pregnant women: US preventive services task force reaffirmation recommendation statement. JAMA 2018;320: 911–7. doi: 10.1001/jama.2018.11785 [DOI] [PubMed] [Google Scholar]
- 9.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021;70:1–187. doi: 10.15585/mmwr.rr7004a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald R, O’Callaghan K, Torrone E, Barbee L, Grey J, Jackson D, et al. Vital signs: missed opportunities for preventing congenital syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep 2023;72:1269–74. doi: 10.15585/mmwr.mm7246e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodworth KR, Reynolds MR, Burkel V, Gates C, Eckert V, McDermott C, et al. A preparedness model for mother-baby linked longitudinal surveillance for emerging threats. Matern Child Health J 2021;25:198–206. doi: 10.1007/s10995-020-03106-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Syphilis, congenital 2018 case definition. Accessed June 12, 2023. https://ndc.services.cdc.gov/case-definitions/syphilis-congenital-2018/
- 13.U.S. Department of Health & Human Services. Congenital syphilis case investigation and reporting form instructions. Accessed November 30, 2023. https://www.cdc.gov/std/program/ConSyphReporting-Instructions7-10-2014.pdf
- 14.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 15.Kimball A, Torrone E, Miele K, Bachmann L, Thorpe P, Weinstock H, et al. Missed opportunities for prevention of congenital syphilis - United States, 2018. MMWR Morb Mortal Wkly Rep 2020;69:661–5. doi: 10.15585/mmwr.mm6922a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin EG, Ansari B, Rosenberg ES, Hart-Malloy R, Smith D, Bernstein KT, et al. Variation in patterns of racial and ethnic disparities in primary and secondary syphilis diagnosis rates among heterosexually active women by region and age group in the United States. Sex Transm Dis 2022;49:330–7. doi: 10.1097/olq.0000000000001607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan EYL, Smullin C, Clavijo S, Papp-Green M, Park E, Nelson M, et al. A qualitative assessment of structural barriers to prenatal care and congenital syphilis prevention in Kern County, California. PLoS One 2021;16:e0249419. doi: 10.1371/journal.pone.0249419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harville EW, Giarratano GP, Buekens P, Lang E, Wagman J. Congenital syphilis in East Baton Rouge parish, Louisiana: providers’ and women’s perspectives. BMC Infect Dis 2021;21:64. doi: 10.1186/s12879-020-05753-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroeger KA, Sangaramoorthy T, Loosier PS, Schmidt R, Gruber D. Pathways to congenital syphilis prevention: a rapid qualitative assessment of barriers, and the public health response, in Caddo Parish, Louisiana. Sex Transm Dis 2018;45:442–6. doi: 10.1097/olq.0000000000000787 [DOI] [PubMed] [Google Scholar]
- 20.Barfield WD, Besera G, Cox S, Warner L, Azeez O, Riggs J, et al. From data to action: CDC’s public health surveillance for women, infants, and children. Accessed November 30, 2023. https://www.cdc.gov/reproductivehealth/productspubs/data-to-action-e-book/index.htm#:;:text5This%20updated%20monograph%20offers%20health,the%20health%20of%20these%20populations
- 21.Fryer K, Delgado A, Foti T, Reid CN, Marshall J. Implementation of obstetric telehealth during COVID-19 and beyond. Matern Child Health J 2020;24:1104–10. doi: 10.1007/s10995-020-02967-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury JE, Atkinson L, Bennett T, Jack SM, Gonzalez A. Prenatal distress, access to services, and birth outcomes during the COVID-19 pandemic: findings from a longitudinal study. Early Hum Dev 2022;170:105606. doi: 10.1016/j.earlhumdev.2022.105606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nurse-Findlay S, Taylor MM, Savage M, Mello MB, Saliyou S, Lavayen M, et al. Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: an evaluation from multi-country surveys and stakeholder interviews. PLoS Med 2017;14:e1002473. doi: doi: 10.1371/journal.pmed.1002473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingram DD, Franco SJ. 2013 NCHS Urban-Rural Classification Scheme for Counties. Vital Health Statistics Ser 2014;2:1–73. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.