Abstract
Background
Only a few reports have currently studied the efficacy of dutasteride in patients with small benign prostatic hyperplasia (BPH). We investigated the efficacy of dutasteride on reducing lower urinary tract symptoms among them.
Materials and methods
A total of 81 patients with BPH who completed 52weeks of 0.5?mg dutasteride treatment were enrolled. Each patient filled out the International Prostatic Symptom Score (IPSS) and overactive bladder symptom score (OABSS) at baseline and at the 6- and 12-month follow-up visits. Total testosterone, prostate-specific antigen, adenoma/prostate volume (PV), uroflowmetry analysis, and postvoid residual volume were evaluated at baseline and at the 12-month follow-up visit. The enrolled patients were divided into 2 groups according to PV at baseline. The groups were as follows: Group A (PV ≥ 30 mL) and Group B (PV < 30 mL).
Results
Groups A and B had mean PVs of 52.1 and 23.6 mL and mean IPSS scores of 16.7 and 14.4, respectively. Group A had significantly higher OABSS and prostate-specific antigen levels at baseline than Group B, while no significant differences in any other baseline characteristics was observed. After dutasteride treatment, adenoma volume and PV decreased significantly, while testosterone level showed a significant increase in both groups. Group A showed significant improvements in the total IPSS, voiding and storage subscore of the IPSS, OABSS, maximum flow rate, and postvoid residual volume. Group B, on the other hand, also showed significant improvements only in the total IPSS, voiding subscore of the IPSS, and maximum flow rate.
Conclusions
The present study suggests a possible beneficial effect of dutasteride treatment on the reduction of lower urinary tract symptoms in patients with small and large BPH. However, the effectiveness of dutasteride was limited compared to patients with large BPH (PV ≥ 30 mL).
Keywords: Benign prostatic hyperplasia, Dutasteride, Lower urinary tract symptoms, Testosterone, Voiding dysfunction
1. Introduction
Dutasteride is a dual 5-α-reductase inhibitor that suppresses the conversion of testosterone to dihydrotestosterone, an active form of testosterone, and potentially decreases the prostate volume (PV) by strongly suppressing dihydrotestosterone. Hence, dutasteride reduces bladder outlet obstruction (BOO) by decreasing the PV, which significantly improves lower urinary tract symptoms (LUTS) among patients with benign prostatic hyperplasia (BPH).[1,2] A randomized controlled study in Japan also demonstrated that dutasteride administration for 1 year resulted in the improvement of urinary symptoms and flow rate by reducing the PV.[3] Therefore, dutasteride has often been used to treat LUTS in patients with relatively large PVs. Nevertheless, the efficacy and safety of dutasteride have been demonstrated among patients with large PV (>30 mL) only.[1–3] On the other hand, only a few reports have currently studied the efficacy of dutasteride in patients with small PV (<30 mL).
In daily clinical practice, management of severe LUTS in those with small BPH with only α1-blocker and/or phosphodiesterase- 5 inhibitor occasionally results in treatment failure. Thus, exploration of other treatment strategies in this population is beneficial. Thus, in this study, we aimed to evaluate the effects of dutasteride on reducing LUTS according to the PV and to compare the drug's efficacy between patients with small and large BPH.
2. Materials and methods
2.1. Study subjects
The present study was a subanalysis of a previous prospective study (UMIN000005173) that examined the clinical effects of dutasteride on LUTS and on the systemic body.[4] Among the 93 patients with BPH enrolled in the previous study between June 2010 and March 2012, 81 who completed the 52-week 0.5-mg dutasteride treatment were included in the present analysis.
Participants aged ≥50years who were diagnosed with clinical BPH were included. Benign prostatic hyperplasia is defined as having an International Prostate Symptom Score (IPSS) of >7 and a PV of ≥20 mL, which is measured using ultrasonography.[5] The exclusion criteria were as follows: patients with definite neurogenic bladder; those with prostate cancer; and those receiving antiandrogenic agents, finasteride, or testosterone within 6 months prior to the start of the study. In addition, patients who required additional medication or drug modifications due to worsening urinary symptoms were excluded from the present analysis. All patients with prostate-specific antigen (PSA) levels >4.0 ng/mL underwent additional clinical or histological evaluation to rule out prostate cancer. The study was conducted in accordance with the Declaration of Helsinki and its amendments and was approved by our hospital ethics committee.
2.2. Study protocols
Upon screening, all patients with BPH underwent medical history evaluation, physical examination, ultrasonography, and blood analysis, including total testosterone (TT) and PSA, to determine their eligibility for dutasteride treatment. Their adenoma volume and PV were also determined by the attending physician via transrectal ultrasonography.
At baseline, all eligible patients answered 2 questionnaires: the IPSS and overactive bladder symptom score (OABSS). In addition, eligible patients underwent uroflowmetry (UFM) analysis and postvoid residual (PVR) volume measurement via abdominal ultrasonography.
Physical examination and blood analysis were performed at baseline and at 6 and 12 months after treatment. Total testosterone and PSA were determined at baseline and 12 months after treatment initiation. Questionnaire surveys were evaluated at baseline and 6 and 12 months after treatment initiation. Adenoma volume, PV, UFM analysis, and PVR volume were measured at baseline and 12 months after treatment initiation.
2.3. Analysis
The enrolled patients were divided into 2 groups according to their baseline PV: Group A (patients with large BPH [≥30 mL]) and Group B (those with small BPH [<30 mL]). Patient characteristics were compared using the Mann-Whitney U test, while categorical data were analyzed using the unpaired chisquare test. The Wilcoxon signed-rank test was used to compare changes in each parameter after 6 and 12months in both groups. All statistical analyses were performed using the Statistical Package for the Social Sciences version 22 (SPSS Inc., Chicago, IL). A p value of <0.05 indicated statistical significance in all analyses.
3. Results
Groups A and B had a mean ± standard deviation age of 72.9 ± 7.0 and 73.4 ± 7.0years, respectively (Table 1). The mean adenoma volume and PV were 30.1 ± 15.6 and 52.1 ± 19.2 mL in Group A and 9.8 ± 4.3 and 23.6 ± 4.6 mL in Group B, respectively. Groups A and B had a mean IPSS of 16.7 and 14.4, respectively, which suggests a severity of moderate LUTS in both groups. Group A had a significantly higher OABSS and PSA level at baseline than Group B, while no significant differences in any other baseline characteristics, including IPSS, UFM data, PVR volume, and TT levels were observed between both groups. Therefore, despite having a diagnosis of small BPH, patients in Group B had a similar LUTS severity to those in Group A.
Table 1.
Patients’characteristics in Groups A and B at baseline visit (mean ± SD).
| Characteristics | Group A (n = 59) | Group B (n = 22) | p |
|---|---|---|---|
| Age, yr | 72.9±7.0 | 73.4±7.0 | 0.401 |
| Body mass index, kg/m2 | 23.7±2.6 | 22.8±3.6 | 0.112 |
| Waist size, cm | 86.7±7.4 | 85.2±10.2 | 0.229 |
| Prostate adenoma, mL | 30.1±15.6 | 9.8±4.3 | <0.001 |
| Total volume, mL | 52.1±19.2 | 23.6±4.6 | <0.001 |
| IPSS total score | 16.7±7.6 | 14.4±7.2 | 0.107 |
| Voiding subdomain score | 7.8±4.1 | 6.7±5.3 | 0.152 |
| Storage subdomain score | 6.9±3.8 | 5.5±3.7 | 0.0631 |
| OABSS | 5.7±3.5 | 3.7±3.4 | 0.0134 |
| Uroflowmetry data Qmax, mL/s | 9.9±4.3 | 10.1±5.5 | 0.454 |
| Voided volume, mL | 172±119 | 140±76 | 0.124 |
| PVR volume, mL | 70.7±74.0 | 61.5±69.6 | 0.308 |
| Total testosterone, ng/mL | 4.66±1.30 | 4.20±1.82 | 0.117 |
| PSA, ng/mL | 6.74±5.68 | 2.57±2.41 | <0.001 |
| Concomitant medicine, n (%) | |||
| α1-blocker | 48 (81) | 17 (77) | 0.922 |
| Anti-OAB drugs | 3 (5.1) | 3 (13) | 0.336 |
| Other | 2 (3.4) | 1 (4.5) | 1.000 |
IPSS = international prostatic symptoms score; OAB = overactive bladder; OABSS = overactive bladder symptom score; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate.
After the 52-week dutasteride treatment, both groups showed a significant decrease in adenoma volume and PV, although no significant difference in PV reduction rate was observed between both groups (–29.5% and –27.0% in Groups A and B; Table 2). Group A exhibited a significant improvement in the total IPSS, voiding and storage subscores of the IPSS, and OABSS 6 and 12 months after dutasteride treatment, as shown in Figure 1. In addition, dutasteride promoted a significant improvement in the maximum flow rate (Qmax) and PVR volume in Group A. Group B, on the other hand, showed a significant improvement only in the total IPSS and voiding subscore of the IPSS 6 and 12months after treatment initiation. Furthermore, a significant improvement in the Qmax was observed 12months after treatment initiation. However, Group B showed no significant changes in the storage subscore of the IPSS, OABSS, and PVR volume. Thus, patients in Group B were only able to achieve a significant improvement in voiding symptoms. Moreover, both groups showed a significant increase in TT levels, with a mean increase of 20.3% in Group A and 22.1% in Group B (p = 0.399) and a significant decline in PSA levels.
Table 2.
Changes from baseline to 12-month visit in each parameter in Groups A and B (mean ± SD).
| Variables | Baseline | 12-mo visit | p |
|---|---|---|---|
| Group A | |||
| Prostate adenoma, mL | 30.1±15.6 | 23.8±12.2 | <0.001 |
| Total volume, mL | 52.1±19.2 | 36.7±16.2 | <0.001 |
| IPSS total score | 16.7±7.6 | 13.1±7.5 | <0.001 |
| Voiding subdomain score | 7.8±4.1 | 5.9±4.1 | <0.001 |
| Storage subdomain score | 6.9±3.8 | 5.7±3.6 | 0.001 |
| OABSS | 5.7±3.5 | 4.6±3.1 | 0.00217 |
| Uroflowmetry data Qmax, mL/s | 9.9±4.3 | 11.5±5.4 | 0.00771 |
| Voided volume, mL | 172±119 | 184±125 | 0.189 |
| PVR volume, mL | 70.7±74.0 | 50.7±60.6 | <0.001 |
| Total testosterone, ng/mL | 4.66±1.30 | 5.50±1.70 | <0.001 |
| PSA, ng/mL | 6.74±5.68 | 2.98±2.54 | <0.001 |
| Group B | |||
| Prostate adenoma, mL | 9.8±4.3 | 7.0±2.5 | <0.001 |
| Total volume, mL | 23.6±4.6 | 17.2±5.1 | <0.001 |
| IPSS total score | 14.4±7.2 | 12.3±6.5 | 0.0178 |
| Voiding subdomain score | 6.7±5.3 | 4.8±3.2 | 0.0278 |
| Storage subdomain score | 5.5±3.7 | 5.6±3.4 | 0.393 |
| OABSS | 3.7±3.4 | 3.8±2.4 | 0.498 |
| Uroflowmetry data Qmax, mL/s | 10.1±5.5 | 11.1±7.3 | 0.0229 |
| Voided volume, mL | 140±76 | 182±136 | 0.108 |
| PVR volume, mL | 61.5±69.6 | 57±64 | 0.0754 |
| Total testosterone, ng/mL | 4.20±1.82 | 4.82±1.84 | 0.00593 |
| PSA, ng/mL | 2.57±2.41 | 0.98±0.85 | <0.001 |
IPSS = international prostatic symptoms score; OABSS = overactive bladder symptom score; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate.
Figure 1.
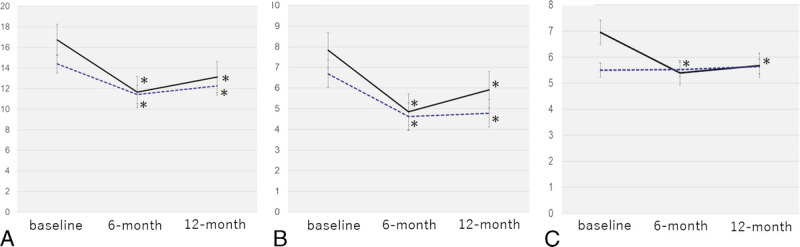
Changes in the total IPSS, voiding subscore of IPSS, and storage subscore of IPSS from baseline to 6 and 12 months after the initiation of dutasteride treatment. (A) IPSS, (B) voiding subscore of IPSS, (C) storage subscore of IPSS. Group A: solid line; Group B: dotted line. *significant difference (p < 0.05). IPSS = international prostatic symptoms score.
4. Discussion
The present study demonstrated that treatment with dutasteride for 1 year in patients with small BPH significantly improved their total IPSS score, voiding subscore, and Qmax through PV reduction. Their outcomes are similar to those with large BPH.
Moreover, subjective symptoms improved 6 months after treatment initiation, which continued until the 12-month follow-up visit. These findings suggest that dutasteride can improve voiding symptoms even among patients with small BPH. To our knowledge, this is the first study to demonstrate the efficacy of dutasteride administration among Asian patients with small BPH.
Given that the efficacy of 5-α reductase inhibitors generally depends on the PV size,[6] dutasteride is assumed to be more effective in patients with larger BPH. Therefore, many previous studies demonstrating the efficacy of dutasteride for LUTS included populations consisting of patients with large BPH (PV ≥30 mL) only.[1–3,7] These previous studies showed that dutas- teride could promote better IPSS and UFM data through continuous PV reduction compared to placebo, suggesting that the clinical efficacy of dutasteride could likely be due to the decrease in BOO via PV reduction. Indeed, a previous study investigating the urodynamic efficacy of dutasteride treatment for 1 year on LUTS among patients with large BPH (mean PV = 57.6 mL) revealed that dutasteride resulted in the improvement in LUTS and a reduction in the BOO index and PV 6 and 12months after treatment initiation.[8]
On the other hand, only a few studies investigated the efficacy of dutasteride on LUTS among patients with small BPH. A secondary analysis of the REDUCE trial that determined the effects of dutasteride on LUTS according to the baseline PV quantiles (<30, 30–40, 40–50, 50–60, and 60–80 mL) among 8122 patients with BPH showed that the dutasteride group had a significantly better IPSS after 6 months of treatment with dutasteride compared with those treated with placebo. In addition, the efficacy of those stabilized at 48 months had a PV of <30 mL.[9] However, statistically significant differences between dutasteride and placebo were lowest among patients with a PV of <30 mL and highest among those with a PV of 60–80 mL. In the aforementioned study, no significant difference in the Qmax was found between the 2 groups at baseline and after 48 months. In the present study, subjective symptoms were improved 6 months after treatment initiation. This finding is similar to that in the REDUCE trial. Together, these findings suggest a possible beneficial effect of dutasteride treatment on LUTS even among patients with small BPH. However, another previous prospective study in Japan compared the efficacy of adding dutasteride to α1-blocker therapy, which was administered to patients who were having a BPH that was intermediate between small and large. The results showed that dutasteride only temporarily improved the IPSS, voiding subscore of IPSS, and OABSS among 17 male participants with small BPH. Nevertheless, the efficacy of dutasteride was not sustained.[10] This difference in results may be attributed to differences in the types and duration of prior BPH therapies, LUTS severity, and form of BPH. In addition, an extremely small number of patients were included in this previous study. Further prospective studies that include a large number of subjects are required to establish a more definite conclusion.
The present study found the same PV reduction rates (–29.5% and –27.0%) in Groups A and B, which were similar to those reported previously in Japan.[3,11] Although patients in Group B had small BPH, no significant differences in the IPSS, Qmax, PV, and PVR had been found between Groups A and B at baseline. Our study population included patients with relatively moderate to severe LUTS, regardless of their PV, and those who were likely to have a similar degree of BOO in both groups. Therefore, dutasteride treatment for 1 year might have improved LUTS even among subjects with small BPH. This improvement may beattributed to the PV reduction. However, given the lack of urodynamic data in the present study, we were unable to clearly determine whether BOO was present among patients with small BPH. Hence, further studies, including urodynamic analyses, are warranted to confirm our hypothesis and findings. On the other hand, patients in Group B showed no improvement in OABSS and storage symptoms. This reason may have been caused by their milder OAB symptoms (mean OABSS = 3.7) compared with those in Group A.
One study showed that dutasteride suppressed serum dihydrotestosterone levels by more than 90%, together with an 18% increase in TT levels.[12] Similarly, a randomized controlled trial in Japan found that TT was increased by 18.8% after 24 weeks of dutasteride treatment.[3] On the other hand, patients included herein had similar increases in TT (Group B: 22.1%; Group A: 20.3%), with TT increases observed in Group B being relatively higher than those reported previously.[3,11] Recently, testosterone deficiency was reported to be significantly associated with BPH and LUTS in men.[13] Indeed, a few previous studies suggested some beneficial effects of increased TT levels on LUTS concurrent with dutasteride treatment.[14,15] Higher increases in TT levels might contribute to LUTS improvement in Group B, which might have resulted in the improvement of LUTS among the patients in Group B.
The significant decrease in the mean IPSS score shown in our results was only 2.2 points in Group B, which was undoubtedly less than those (from 4 to 6 points in terms of the mean IPSS) reported by many previous studies, in which the study subjects were comprised with patients with large BPH.[3,7,8,11] Therefore, the clinical significance of this minimal improvement remained to be considered. In a previous subanalysis of REDUCE trial, IPSS was significantly improved among patients with small BPH. In particular, the mean IPSS was reduced from 0.5 to 1.0 points 12–48 months after dutasteride treatment,[9] which was relatively less than our present results. However, dutasteride treatment was useful in reducing the risk for BPH progression even among patients with small BPH.[9] The slight decrease in the mean IPSS demonstrated in the present study might also have some merits among patients with small BPH, although its effect was limited compared with those with large BPH. Further studies including long-term observation should be conducted to validate our conclusions.
Limitations
There are some limitations in this study that warrants consideration. Firstly, the present study included a relatively small population, especially in Group B who only had 22 patients. Therefore, the results involving patients with small BPH may not be conclusive. Second, numerous patients also underwent α1-blocker therapy. Therefore, the present study aimed to investigate the add-on effects of dutasteride among patients taking α1-blocker therapy. Third, given that lack of urodynamic data, we had difficulty determining the exact mechanism through which dutasteride improved LUTS among patients with small BPH. In addition, dutasteride was administered based on the discretion of each patient's attending physician, which could have resulted in selection bias. Therefore, a large number of subjects are required in future studies to establish a more precise conclusion regarding the efficacy of dutasteride on LUTS among patients with small BPH.
5. Conclusions
The present study suggests that dutasteride treatment may have a possible beneficial effect on LUTS even among patients with small BPH (PV <30 mL), although its effectiveness is limited compared to patients with large BPH (PV≥30 mL).
Acknowledgments
None.
Statement of ethics
The study was conducted in accordance with the Declaration of Helsinki and its amendments and was approved by our hospital ethics committee. Written informed consent was obtained from all participants prior to the start of this study.
Funding source
The authors received no financial support for the research, authorship, and/or publication of this article.
Author contributions
All authors contributed equally in this study.
Data availability
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Footnotes
How to cite this article: Shigehara K, Kato Y, Kawaguchi S, Izumi K, Kadono Y, Mizokami A. A comparison of the efficacy of dutasteride on reducing lower urinary tract symptoms among patients with small versus large benign prostatic hyperplasia. Curr Urol 2024;18(3):199–202. doi: 10.1097/CU9.0000000000000103
Contributor Information
Yuki Kato, Email: kato1402@gmail.com.
Shohei Kawaguchi, Email: shohei_k2001@yahoo.co.jp.
Kouji Izumi, Email: azuizu2003@yahoo.co.jp.
Yoshifumi Kadono, Email: yskadono@yahoo.co.jp.
Atsushi Mizokami, Email: mizokami@staff.kanazawa-u.ac.jp.
Conflict of interest statement
No conflict of interest has been declared by the author.
References
- 1.Roehrborn CG Boyle P Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002;60 (3):434–441. [DOI] [PubMed] [Google Scholar]
- 2.Debruyne F, Barkin J, Van Erps P, Reis M, Tammela TLJ, Roehrborn C. Efficacy and safety of long-term treatment with the dual 5α-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol 2004;46 (4):488–495. [DOI] [PubMed] [Google Scholar]
- 3.Tsukamoto T, Endo Y, Narita M. Efficacy and safety of dutasteride in Japanese men with benign prostatic hyperplasia. Int J Urol 2009;16 (9):745–750. [DOI] [PubMed] [Google Scholar]
- 4.Shigehara K Koh E Sakamoto J, et al. Effects of dutasteride on lower urinary tract symptoms and general health in men with benign prostatic hypertroplasia and hypogonadism: A prospective study. Aging Male 2014;17 (1):51–56. [DOI] [PubMed] [Google Scholar]
- 5.Homma Y Kawabe K Tsukamoto T, et al. Estimate criteria for diagnosis and severity in benign prostatic hyperplasia. Int J Urol 1996;3 (4):261–266. [DOI] [PubMed] [Google Scholar]
- 6.Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: Meta-analysis of randomized clinical trials. Urology 1996;48 (3):398–405. [DOI] [PubMed] [Google Scholar]
- 7.Roehrborn CG Marks LS Fenter T, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology 2004;63 (4):709–715. [DOI] [PubMed] [Google Scholar]
- 8.Matsukawa Y, Gotoh M, Kato M, Funahashi Y, Narita M, Mitsui K. Effects of dutasteride on storage and voiding symptoms in male patients with lower urinary tract symptoms as a result of benign prostatic obstruction: The 1-year outcomes from a prospective urodynamic study. Int J Urol 2014;21 (8):826–830. [DOI] [PubMed] [Google Scholar]
- 9.Roehrborn CG Nickel JC Andriole GL, et al. Dutasteride improves outcomes of benign prostatic hyperplasia when evaluated for prostate cancer risk reduction: Secondary analysis of the reduction by dutasteride of prostate cancer events (REDUCE) trial’. Urology 2011;78 (3):641–646. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto M Shimizu N Sugimoto K, et al. Efficacy of adding dutasteride to (-blocker therapy treated benign prostatic hyperplasia patients with small volume prostate (<30 mL). Low Urin Tract Symptoms 2017;9 (3):157–160. [DOI] [PubMed] [Google Scholar]
- 11.Tsukamoto T, Endo Y, Narita M. Assessment of recommended dose of dutasteride on Japanese men with benign prostatic hyperplasia: Arandomized, double-blind, placebo-controlled, parallel-group, dose response study. Hinyokika Kiyo 2009;55 (4):209–214. [PubMed] [Google Scholar]
- 12.Rittmaster R, Hahn RG, Ray P, Shannon JB, Wurzel R. Effect of dutasteride on intraprostatic androgen levels in men with benign prostatic hyperplasia or prostate cancer. Urology 2008;72 (4):808–812. [DOI] [PubMed] [Google Scholar]
- 13.Shigehara K, Namiki M. Late-onset hypogonadism syndrome and lower urinary tract symptoms. Korean J Urol 2011;52 (10):657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada N Hashidume K Tamaki G, et al. Add-on effect of dutasteride in patients with benign prostatic hyperplasia treated with alpha blocker: Its effect on overactive bladder. Hinyokika Kiyo 2012;58 (9):475–480. [PubMed] [Google Scholar]
- 15.Shigehara K Miyagi T Nakashima T, et al. Effects of dutasteride on lower urinary tract symptoms: A prospective analysis based on changes in testosterone/dihydrotestosterone levels and total prostatic volume reduction. Aging Male 2016;19 (2):128–133. [DOI] [PubMed] [Google Scholar]


