Abstract
Prostate cancer (PC) is the most frequently diagnosed cancer and second leading cause of cancer-related deaths in men. It is heterogeneous, as is evident from the wide spectrum of therapeutic approaches. Most patients with PC are initially responsive to androgen deprivation therapy; however, the majority of cases are either hormone-sensitive PC or castration-resistant PC. Current therapeutic protocols follow the evolution of PC, a continuously progressive process involving a combination of widespread genomic alterations. These genomic alterations are either hereditary germline mutations, such as mutations in BRCA2, or specific only to tumor cells (somatic). Tumor-specific genomic spectra include genomic structural rearrangements, canonical androgen response genes, and many other specific genes such as TMPRSS2-ERG fusion, SPOP/FOXA1, TP53/RB1/PTEN, and BRCA2. New evidence indicates the involvement of signaling pathways including PI3K, WNT/β-catenin, SRC, and IL-6/STAT, which have been shown to promote epithelial-mesenchymal transition cancer stem cell–like features/stemness, and neuroendocrine differentiation in PC. Over the last decade, our understanding of the genotype-phenotype relationships has been enhanced considerably. The genetic background of PC related to canonical genetic alterations and signaling pathway activation genes has shed more insight into the molecular subtype and disease landscape, resulting in a more flexible role of individual therapies targeting diverse genotypes and phenotypes.
Keywords: Prostate cancer, Prostate cancer treatment, Prostate cancer genomics, Hereditary prostate cancer, Androgen deprivation therapy, Hormone sensitive prostate cancer, Kinase signaling
1. Introduction
Prostate cancer (PC) remains the most common cancer in males and the second most common cancer overall in the United Kingdom, accounting for 14% of all new cancer cases.[1] Worldwide, 1.4 million cases of PC are diagnosed each year.[2] Incidence and disease stage distribution patterns follow a combination of biological, genetic, and lifestyle factors but are also influenced by national and international screening and diagnosis recommendations.[3] The Swedish survey on PC provides data on the economic burden thereof.[4] This prevalence-based PC registry study provides data on the societal costs of existing PC testing, diagnosis, management, and treatment of PC and additionally provides reference values for future cost-effectiveness analyses of PC screening and treatment. The analysis methods and data are relevant for cost-effectiveness evaluation of PC screening and treatment.
The epidemiology of PC includes several closely interrelated determinants, namely, geographic location; ethnic origin; sociocultural environment; dietary habits; personal lifestyle; natural aging process; consumption of and/or exposure to toxins, including smoking and alcohol; occupational hazards such as working with hazardous chemicals; and possible risks associated with radiation (Fig. 1).[5] In addition, genetic and genomic factors play a crucial role in the risk and pathogenesis of PC.[6] These might include somatic mutations in genes regulating prostate structure and function or part of the inherited predisposition segregating in either the Mendelian manner or complex polygenic mechanisms.[7]
Figure 1.
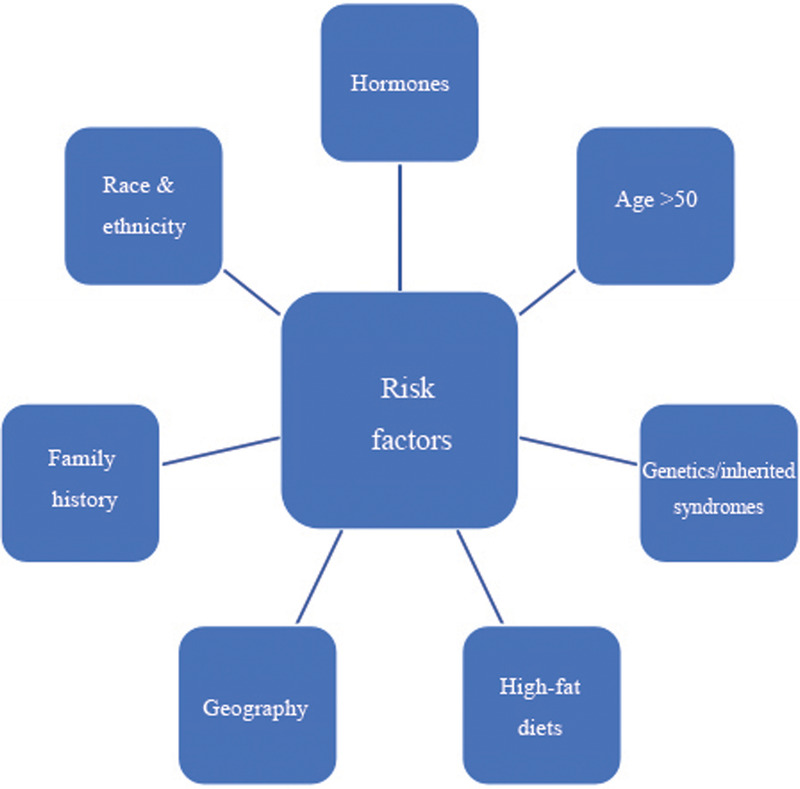
Risk factors for prostate cancer.
Prostate cancer diagnosis remains a significant area of focus in clinical and laboratory-based research, with tools constantly evolving. The current tools available to clinicians include prostate-specific antigen (PSA) testing, prostate magnetic resonance imaging (MRI), and prostate biopsies,[3] and PC guidelines are available from the European Consortium.[8] These tools have been subjected to constant refinement, and recently, the PRECISION trial has demonstrated the use of MRI-directed targeted biopsies.[9] Furthermore, use of prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (CT) scans has been suggested to offer further enhancement of imaging modalities.[10–12]
However, the diagnostic conundrum still facing clinicians is genetic counseling and testing in both men with strong family histories and those in the wider population. Specific germline mutations can lead to aggressive PC development, particularly in patients with a family history of PC.[13] Apart from autosomal loci, several Y chromosome–linked genes are relevant for PC predisposition and growth. Y-chromosome PC genotyping may reveal specific genes (BPY2, RPS4Y1, NLGN4Y, SMAD3, and others), DYZ region mutations (DYS458, allele 12 of DYS393, and others), tumor suppressor genes (KDM5D and MSY), and several loci.[14]
Evidence supports the implementation of germline testing with concomitant genetic counseling,[15,16] and several commercial genetic screening tools are now available to assess germline mutations in genes that have been identified to increase the risk of PC. These include BRCA2, ATM, CHEK2, BRCA1, and HOXB13 genes and carriers of MSH2 mutation in the DNA-MMR genes.[17,18] However, their overall impact is yet to be ascertained, as published data suggest that germline BRCA1 and BRCA2 mutations occur in approximately 0.2% to 0.3% of the general population.[19,20] It is argued that most genetic factors are nonspecific and cannot be used on their own to accurately stratify patients with indolent versus more aggressive PC tumors.[21] It is imperative to use more sensitive and specific biomarkers that can predict and allow for the proper selection of patients at high risk of developing life-threatening PC.[22–24] The ultimate aim would be to plan and institute personalized and precision therapy for better outcomes, which may also prevent overtreatment in patients with low-risk PC.
In this article, the role and current clinical status of genomic and molecular biomarkers in PC treatment are reviewed with the aim of developing precision treatment. Although the emphasis is on medical therapy, some elements of personalized surgical management are also included.
2. Prostate cancer diagnosis and treatment
Current PC diagnoses, treatment recommendations, and protocols are available from various sources. The National Institute for Health and Care Excellence (www.nice.org.uk; NICE) produced an updated set of PC guidelines in 2021 (https://www.nice.org.uk/guidance/ng131/chapter/Recommendations).[23] However, there is still insufficient evidence from formalized population screening programs.[25,26]
2.1. Clinical diagnosis of PC
All informed men requesting an early diagnosis are offered a PSA test and undergo digital rectal examination (DRE).[24] Upon fast-track referral to a secondary care facility, multiparametric MRI is the first-line investigation for patients with suspected clinically localized PC, and the results are reported using a 5-point Likert scale. All patients with a Likert score of ≥3 should be offered the MRI-guided prostate biopsy.[27] Those with a multiparametric MRI Likert score of 1 or 2 could consider omitting prostate biopsy after discussing the risks and benefits and reaching a shared decision.[28] Alternatively, a CT scan can be performed in patients with contraindications to MRI. Additional factors to be considered are as follows:
the patient’s PSA level
their DRE findings (including prostate size estimation)
any comorbidities, together with their risk factors
increasing age
Afro-Caribbean ethnicity
any history of a previous negative prostate biopsy
Additional tests, including an isotope bone scan, may be requested if aggressive PC is clinically suspected. However, sclerotic metastases can also be identified using plain radiography. All cases are discussed at a urological cancer multidisciplinary team meeting.[29] Even if a prostate biopsy is reported as negative, the NICE advises that a review of the risk factors of all people who have had a negative first prostate biopsy must be discussed. Factors that must be discussed include the following:
There remains a risk that PC is present.
-
The risk is slightly higher if any of the following risk factors are present:
-
▪
the biopsy showed high-grade prostatic intraepithelial neoplasia,
-
▪
the biopsy showed atypical small acinar proliferation, and
-
▪
an abnormal DRE.
-
▪
2.2. Risk stratification for localized or locally advanced PC
There are different options for risk stratification of local or advanced PC. One such approach is based on the current NICE (www.nice.org.uk) guidance in the context of all urological cancers. This is broadly similar to the National Comprehensive Cancer Network (NCCN)–recommended criteria based on serum PSA level and clinical tumor stage and grade, which define 3 major groups of localized disease based on the probability of biochemical recurrence after local therapy: low, intermediate, and high risk.[8,24] The NCCN risk classification for the high-risk category is further subdivided into favorable high-risk, standard high-risk, and very high-risk disease (Fig. 2).[30] In a later review, high-risk PC is further subdivided into 3 categories based on the Decipher genomic testing. This includes 2 additional categories: standard high risk and very high risk.[30,31]
Figure 2.
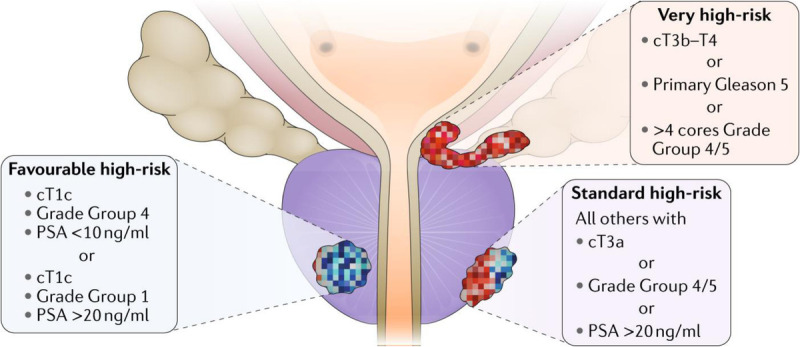
Heterogeneity of prostate cancer risk.[30] PSA = prostate-specific antigen.
The NICE guidelines[24] recommend that the urological cancer multidisciplinary teams assign a Cambridge Prognostic Group Criteria risk category to all people with newly diagnosed localized or locally advanced PC:
(1) Gleason score 6 (grade group 1) and PSA <10 μg/L and Stages T1–T2
(2) Gleason score 3 + 4 = 7 (grade group 2) or PSA 10–20 μg/L and Stages T1–T2
(3) Gleason score 3 + 4 = 7 (grade group 2) and PSA 10–20 μg/L and Stages T1–T2 or Gleason 4 + 3 = 7 (grade group 3) and Stages T1–T2
(4) One of Gleason score 8 (grade group 4), PSA >20 μg/L, or Stage T3
(5) Two or more of Gleason score 8 (grade group 4); PSA >20 μg/L; Stage T3 or Gleason score 9 to 10 (grade group 5) or Stage T4
2.3. Treatment options for localized or locally advanced PC
The NICE guidelines recommend evidence-based PC treatment according to the Cambridge Prognostic Group risk category (Table 1).
Table 1.
NICE guidelines for localized or locally advanced PC.[24]
| CPG 1 localized PC | Offer active surveillance |
| Consider radical prostatectomy or radiotherapy if active surveillance is not suitable or acceptable to the patient | |
| CPG 2 localized PC, offer a choice between | Active surveillance |
| Radical prostatectomy or radical radiotherapy, if suitable | |
| CPG 3 localized PC | Offer radical prostatectomy or radical radiotherapy |
| Consider active surveillance for patients who choose not to have immediate radical treatment |
CPG = Cambridge Prognostic Group; NICE = National Institute for Health and Care Excellence; PC = prostate cancer.
The current NICE recommendations for active surveillance protocols in Year 1 include PSA testing at 3- to 4-month intervals and a DRE at 12 months with an MRI. In the second year, the NICE guidance suggests every 6 months serum PSA measurements and yearly DRE assessment. If there is evidence of disease progression in the active surveillance protocols at any stage, patients should receive radical treatment. In all cases, it should be explained to patients and, if they wish, their partner that radical treatment for PC will result in an alteration of sexual experience and may result in loss of sexual function. In addition, patients must be warned that radical treatment for PC could affect their urinary continence.
Important consideration should be given to people with localized CPG 4 and 5 and any man with locally advanced PC; in these situations, active surveillance should not be offered. However, patients with CPG 4 and 5 localized and locally advanced PC can be offered radical prostatectomy or radical radiotherapy with androgen deprivation therapy (ADT) when it is likely that the cancer can be controlled in the long term. The NICE recommends the use of docetaxel chemotherapy in specially selected cases of T3/4 PC that are to be managed long-term with ADT.[32]
2.4. Treatment options for metastatic PC
Evidence-based guidelines are available from leading urology organizations, including the American Urology Association (www.auanet.org), UK National Institute of Clinical Excellence (www.NICE.org.uk), and European Association of Urology (www.uroweb.org). Most recommendations are broadly similar for nonmetastatic, metastatic, hormone-sensitive, and castration-resistant PC. According to all guidelines, all patients should consider commencing ADT. In addition, all guidelines suggest offering docetaxel chemotherapy to patients with newly diagnosed metastatic PC who do not have significant comorbidities:
start treatment within 12 weeks of starting ADT
use six thrice weekly cycles at a dose of 75 mg/m2 (with or without daily prednisolone)
Other options that may be offered include bilateral orchidectomy (as an alternative to continuous luteinizing hormone–releasing hormone agonist therapy). Patients with metastatic PC who are willing to accept the adverse impact on overall survival and gynecomastia, with the aim of retaining sexual function, are offered antiandrogen monotherapy with 150 mg of bicalutamide.
In cases with biochemical evidence of hormone-relapsed disease, an oncologist and/or specialist palliative care opinion is best sought where appropriate. In these situations, docetaxel is recommended if the Karnofsky Performance Status score is 60%.[33] After 10 cycles of docetaxel, if serious adverse effects occur, or if there is disease progression according to any criteria, docetaxel must be discontinued. The next step would be to offer a daily corticosteroid, such as dexamethasone (0.5 mg), as a third-line hormonal therapy after ADT and antiandrogen therapy in patients with hormone-relapsed PC. In men with hormone-relapsed metastatic PC, zoledronic acid should be considered to prevent or reduce skeletal-related events. These treatments can be combined with oral or intravenous bisphosphonates for pain relief in patients with hormone-relapsed metastatic PC when other treatments, including analgesics and palliative radiotherapy, do not provide satisfactory pain relief.[34,35]
3. Role of genetic and genomic factors in PC
Cancer is fundamentally a genetic condition involving 1 or more genetic factors that interfere with the molecular regulation of different stages of the cell cycle (Fig. 3).
Figure 3.
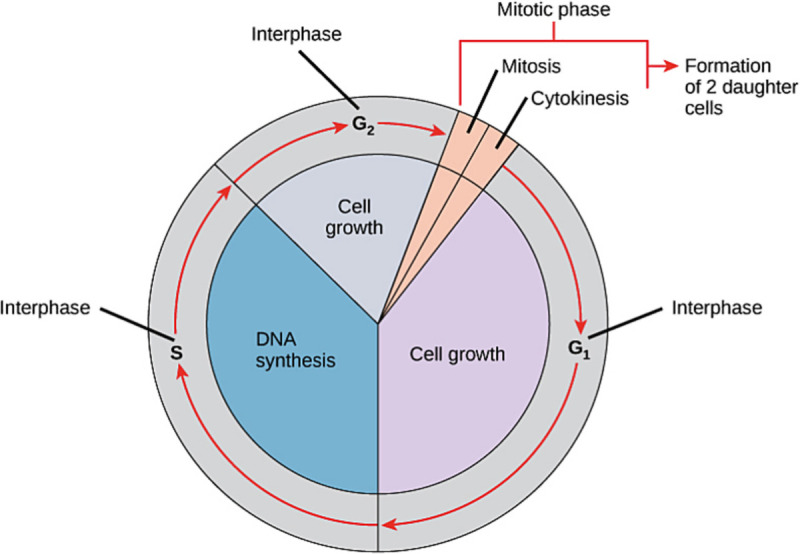
Schematic display of stages of the cell cycle. Source: OpenStax. https://cnx.org/contents/FPtK1zmh@8.25:fEI3C8Ot@10/Preface.
Genetic factors predominantly control DNA synthesis (S phase) and cell growth (G2, mitosis, and G1). In most cases, genetic factors are nonspecific and are similar in many eukaryotes. However, these can be cell- or tissue-specific, with a predilection for a particular organ, such as neural components or endocrine glands. Understanding these complex molecular and cellular changes is the key to cancer diagnosis, progression, metastasis, and treatment.[36,37]
Genetic factors involved in cancer are heterogeneous and may include gross imbalances in genome structure, such as aneuploidy or copy number variations ranging from gain (insertion) to loss (deletion), commonly referred to as indels. Specific DNA sequence changes within an exon may result in chain termination (nonsense mutations) or alter the selection of amino acids for peptide synthesis (missense mutations). Sequence changes at the exon-intron splice site may also interfere with peptide synthesis by blocking transcription. Moreover, there are several RNA molecules that may interfere with the cell cycle.
Morbid genetic factors, or diseases that cause genetic changes, are inherited from one or both parents and are referred to as germline mutations. In contrast, mutations that are confined to a particular tissue are called somatic mutations (Fig. 4). Conceptually, this distinction is important when dealing with a family history of cancer. Most tissue-specific neoplasms carry somatic mutations except for small subsets with de novo or inherited germline mutations. Thus, it is imperative to identify germline mutations in tumors with somatic mutations. Germline mutations in certain genes, such as DNA repair genes, may involve different tissues in multiple organs, including the breasts, ovaries, and prostate.[38,39] Further, presence or absence of germline mutations may influence risk for distant metastasis or poor prognosis.
Figure 4.
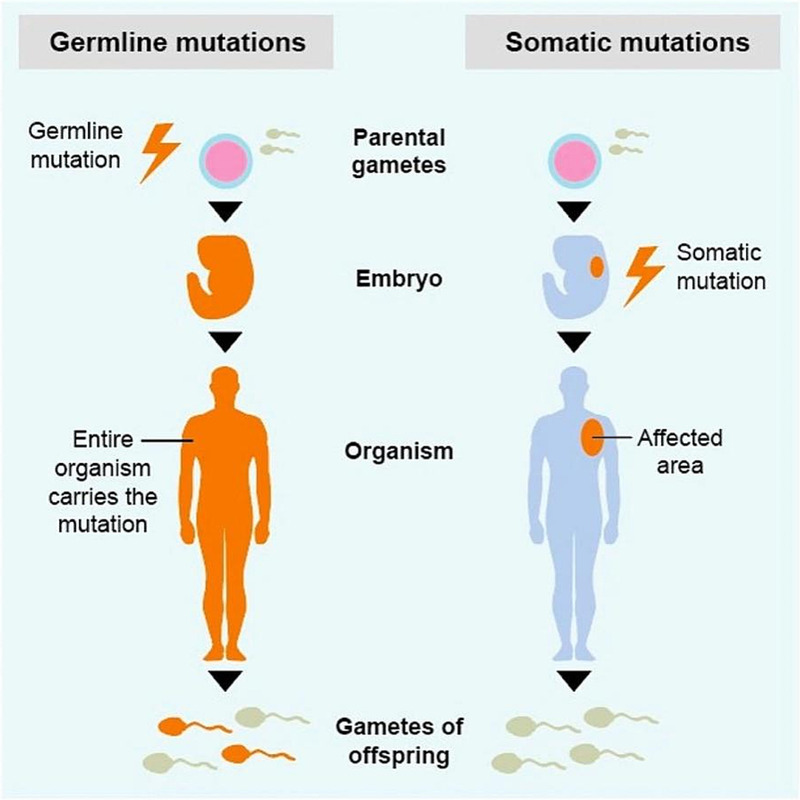
Fundamental difference between germline and somatic mutations (www.learn.colontown.org).
Several acquired and nongenetic factors are involved in cancer development and progression. These factors are diverse, including geographic location, dietary habits, adverse or extreme environmental habitats, occupational hazards, exposure or use of chemicals or drugs, hormonal changes, aging, and comorbidity (Fig. 1). Family history, although strongly influenced by inherited or genetic traits, may be limited to sharing nongenetic or acquired factors.
Although PC tumor genotyping provides insight into the molecular pathology, it cannot offer reliable predictions for very high-risk metastatic PC, particularly the risk of distant metastasis. Genome-wide studies, specifically genome-wide association studies, have led to the construction of morbid PC genome maps.[40] The karyogram (Fig. 5) provides a graphical display of the PC-associated gene density correlated with lifelong PC risk. Next-generation genome sequencing has enabled the identification of PC-specific genes, most of which are either involved in or regulated by complex molecular mechanisms in DNA repair.[41,42]
Figure 5.
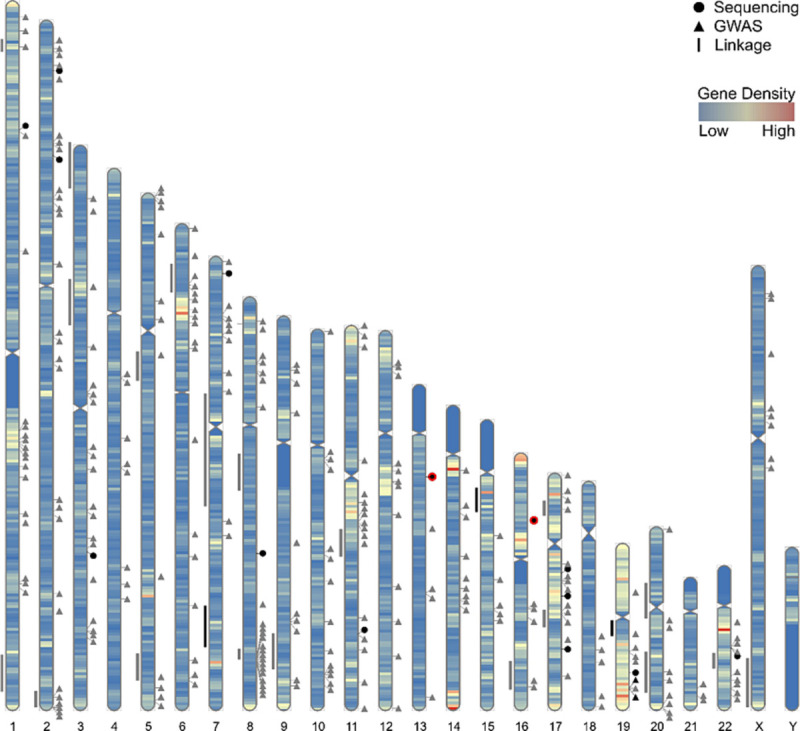
Karyogram showing the approximate genome mapping of candidate prostate cancer risk loci based on linkage, GWAS, and sequencing study analyses.[40] GWAS = genome-wide association studies.
There are several molecular targets of PC (Table 2). These include genes, proteins, and other molecules involved in genomic instability or diversity, androgen receptors (ARs), signaling pathways, molecular subtypes, biomarkers, and cancer evolution.[43,44] The International Consortium for Prostate Cancer Genetics has identified a list of genes from PC genome-wide association studies data, some of which confer susceptibility to PC and/or poor prognosis (Table 3).[40]
Table 2.
Different molecular targets and genes in prostate cancer.
| Genomic instability | Aberrations of AR, TP53/PTEN, BRCA |
| Androgen signaling | AR sensitive, AR mutations, AR independent, AR resistance, AR variants, AR loss |
| Signal pathways | CYP17, P13K, SRC, JAK/STAT/PGE2 |
| Molecular subtypes | ERG/SPOP/FOXA1PTEN/TP53/CHD1, AR-V7, DDR genes |
| Biomarkers | PSA, DNA methylation, UBE2C, CgA/Syn/CD56 |
| Cancer evolution | HSPC, AIPC, CRPC-Adeno, CRPC-NE |
AR = androgen receptor; PSA = prostate-specific antigen.
Table 3.
DNA repair genes associated with high-risk/poor-prognosis prostate cancer.
| Gene | Chromosome |
|---|---|
| ATM | 11 |
| ATR | 3 |
| BRCA1 | 17 |
| BRCA2 | 13 |
| BRIP1 | 17 |
| CHECK2 | 22 |
| ERCC2 | 19 |
| GEN1 | 2 |
| MSH2 | 2 |
| MUTYH | 1 |
| NBN | 8 |
| PALB2 | 16 |
| PMS2 | 7 |
| RAD51D | 17 |
4. Genotyping for PC risk stratification
Germline genotyping can reveal the level of lifetime PC risk (Table 4). The derived risk estimates thus derived are used for genetic counseling, particularly in the context of family history.[45] It is now recommended that men should be carefully selected for effective PC surveillance and prophylactic intervention.
Table 4.
The lifetime PC risk with selected genes associated with cancer family syndromes (www.prostatematters.co.uk).
| Mutated gene | Increased lifetime risk of PC |
|---|---|
| BRCA1 | 7%–25% |
| BRCA2 | 19%–60% |
| HOXB13 | 33%–60% |
| Lynch syndrome | 12%–52% |
| ATM and CHEK2 | Moderate risk |
PC = prostate cancer.
The majority of moderate to severe PC genetic susceptibilities are correlated with mutations interfering with either DNA damage repair or damage response. In contrast, mutations in the HOXB13 confer a higher risk of developing PC, probably through an AR repressor mechanism[46–48] (Fig. 6).
Figure 6.
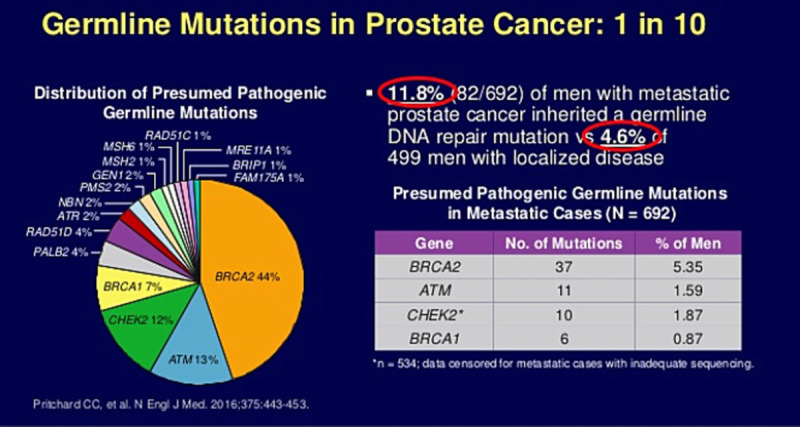
Genomic and molecular heterogeneity in prostate cancer. Reproduced from www.prostatematters.co.uk.[49,50]
Approximately 1 in 10 men with PC is likely to harbor mutations in one of the germline PC genes (Table 5; Fig. 7). The bulk of PC risk is associated with BRCA2 followed by ATM, both of which are predominantly involved in DNA damage repair.[52,53] Delineation of genomic and molecular heterogeneity in PC has led to the widespread use of PC germline genotyping, particularly in metastatic PC. Approximately 12% of men with metastatic PC have inherited one of the DNA repair gene mutations compared with less than 5% of men with localized disease. DNA repair genotyping is relevant in the selection of men treated with olaparib for castration-resistant PC.[54]
Table 5.
Summary of different gene mutations and the respective PC risk.
| Gene | PC risk | Mechanism |
|---|---|---|
| ATM | Elevated | DNA damage response |
| BRCA1 | ~20% | DNA damage repair |
| BRCA2 | ~20% | DNA damage repair |
| CHEK2 | Elevated | DNA repair through phosphorylation of BRCA2 |
| EPCAM | Up to 30% | Upregulate c-myc |
| HOXB13 | Up to 60% | AR repressor |
| MLH1 | Up to 30% | DNA repair |
| MSH2 | Up to 30% | DNA repair |
| MSH6 | Up to 30% | DNA repair |
| NBN | Elevated | DNA repair |
| PMS2 | Up to 30% | DNA mismatch repair |
| TP53 | Unknown | Tumor suppressor |
| PALB2 | Preliminary | Tumor suppressor |
| RAD51D | Preliminary | DNA repair |
PC = prostate cancer.
Courtesy: American Urological Association.
Figure 7.
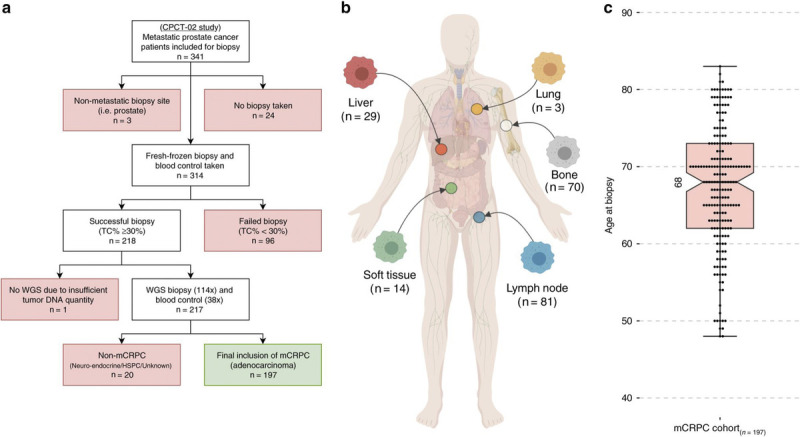
Genotyping in castration-resistant metastatic prostate cancer.[51]
Genome sequencing analysis of the biopsy and blood samples of men with castration-resistant PC generated data supporting genotyping as a reliable tool for predicting distant metastases (Fig. 8).[51,55] The NCCN guidelines explicitly recommend PC germline genotyping as opposed to molecular biopsy (Table 6).
Figure 8.
Schematic overview of liquid biopsy molecular profiling in prostate cancer.[5]
Table 6.
NCCN definition of both high-risk and very high-risk prostate cancer.
| Risk group | Clinical/pathological features | Imaging | Germline testing | Molecular/biochemical analysis of tumor |
|---|---|---|---|---|
| High | No very high features and 1 high-risk feature: T3a OR Gleason 4 or 5 OR PSA >20 ng/mL |
Bone scan Pelvic ± abdominal imaging |
Recommended | Consider if life expectancy >10 yr |
| Very high | At least one of the following: T3b–T4 Primary Gleason 5 2–3 high-risk features >4 cores with grade 4 or 5 |
Bone scan Pelvic ± abdominal imaging |
Recommended | Not routinely recommended |
NCCN = National Comprehensive Cancer Network; PSA = prostate-specific antigen.
Courtesy: Christopher J. D. Wallis, University of Toronto Twitter: @WallisCJD during the 2021 American Urological Association Annual Meeting; September 10–13, 2021.
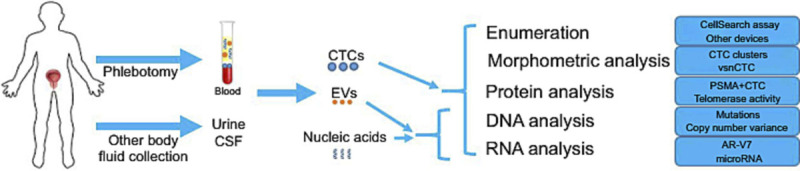
In the context of the genomic analysis of malignant PC, recent advances in molecular diagnosis of PC include the emerging role and clinical application of liquid biopsy.[56,57] This approach is considered most beneficial in the very early stages of PC, particularly in those with an aggressive course, and early diagnosis may allow for early treatment with favorable outcomes. This technique uses peripheral blood and other body fluids, such as urine and cerebrospinal fluid.[58] The peripheral blood analysis includes quantitative assays of circulating tumor cells and extracellular vesicles and nucleic acid sequence analysis (mutations or variants) of cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA) (Fig. 9).[59] In addition to the ctDNA or cfDNA analysis, circulating RNAs, particularly microRNAs, are emerging as potential molecular markers in the clinical setting.[60] The liquid biopsy is a complex process performed in a limited number of laboratories. Briefly, various analytical approaches include enumeration and morphometric, protein, DNA, and RNA analyses (Fig. 9).
Figure 9.
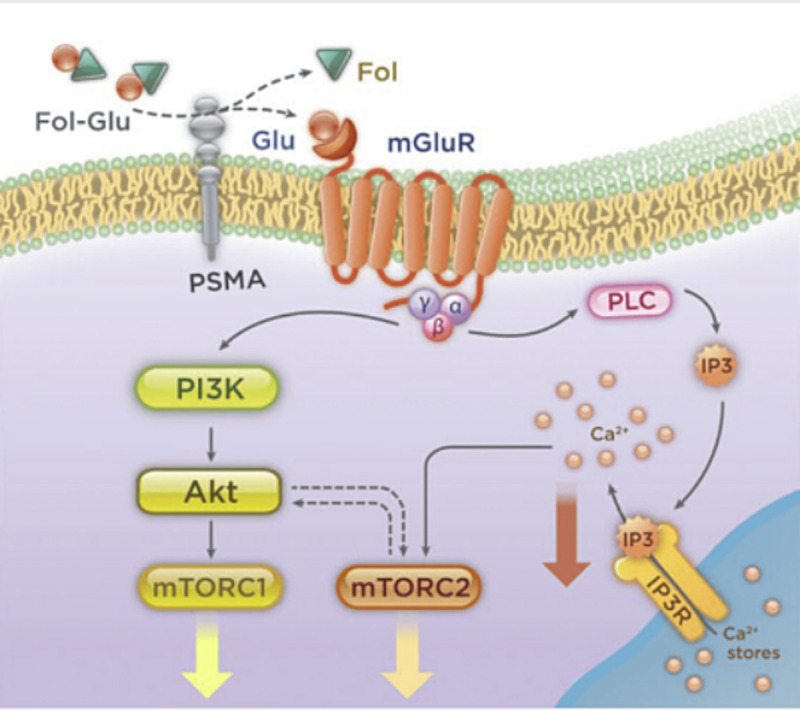
PSMA-P13K/Akt pathway. PSMA = prostate-specific membrane antigen.
5. Molecular basis for novel PC therapy
Among the many molecular targets for PC therapy, the PSMA is an excellent target for both PC imaging and therapy.[9] Prostate-specific membrane antigen is involved in the PI3K/Akt pathway (Fig. 10) and is a diagnostic biomarker and a potential therapeutic target, enabling a phenotypic precision medicine approach to guide patient selection for therapy in advanced PC.[12]
Figure 10.
PSMA and targeted prostate cancer therapy.[8] PSMA = prostate-specific membrane antigen.
Prostate cancer inhibitors targeting PSMA and other kinase molecules are effective in treating both localized and metastatic PC. The use of PSMA inhibitors along with positron emission tomography/CT imaging has been shown to be effective in tailored precision PC therapy (Fig. 9).[61]
Liquid biopsy–based molecular profiling has rapidly modified PC therapy. Quantitative analysis of circulating tumor cells, particularly AR-V7 phenotyping, is helpful for delivering AR surface inhibitor therapy.[12] The cfDNA and ctDNA analyses are powerful tools for detecting AR and DNA damage repair genes. These mutations determine the outcomes of PC therapy using taxanes or poly(ADP-ribose) polymerase inhibitors.[13]
6. Discussion
Diagnosis of PC has been the subject of significant clinical and laboratory-based research, leading to the constant evolution of clinical guidelines and evidence-based research. The ideal diagnostic pathway is a streamlined, individual patient-focused protocol that allows busy clinicians to use a validated, reliable, and reproducible method to diagnose and determine the risk of PC.
Over the past few decades, much of the evidence has evolved and stemmed from the use of clinicopathological data, such as those utilized by the PREDICT prostate trial. Cancer-specific and overall survival data from the PREDICT Prostate trial have been endorsed by the NICE, as they provide up to 15 years of data.[62] However, these data typically involve available clinicopathological data (age, PSA level, grade, stage, biopsy involvement, treatment types, and comorbidities).[63] Furthermore, PREDICT does not include the impact of a family history of PC or the role of genetic factors. The NICE recognizes the role of family history as a risk factor for PC.[24] Patients are at a higher risk if they have a close relative, such as a brother or father, with PC. It is estimated that approximately 5% to 10% of PC cases have a substantial inherited component.[14,15]
The NICE therefore acknowledged the significance of a meta-analysis reporting a pooled relative risk (RR) of 2.48 in men with 1 first-degree relative (brother or father) with PC compared with no first-degree family history. The risk was higher if the first-degree relative was a brother (RR, 3.14) rather than a father (RR, 2.35). An RR of 4.39 was reported in men with 2 or more first-degree relatives with a history of PC.[16,17] In addition, strong predisposing genes could be responsible for up to 40% of cases in men up to the age of 55 years.[18,19] For example, a recurrent HOXB13 G84E germline mutation has recently been shown to be significantly associated with an increased risk of PC and is significantly more common in men with early-onset familial disease.[20,21] It can therefore be hypothesized that the diagnostic conundrum in patients with a strong family history will not be fully evaluated unless genetic counseling and molecular testing are incorporated into future guidelines. In addition, previous reports have shown that patients with hereditary PC are diagnosed 6 to 7 years before spontaneous cases, indicating that younger age at diagnosis plays a role.[15] The underlying genetic mechanisms of hereditary PC have now been established as several PC-specific germline and somatic mutations, particularly in HOXB13 and BRCA2 (Table 7). A recurrent HOXB13 G84E germline mutation is significantly associated with an increased risk of PC and is significantly more common in men with early-onset familial disease.[47] Germline mutations associated with other cancers (such as those in PTEN and BRCA1) are also associated with an increased risk.[64] The link between PC and a family history of breast cancer, specifically due to the BRCA1 and BRCA2 gene mutations, has been established.[22,23] Clinicians need a low threshold to investigate PC risk in men with a family history of breast cancer.
Table 7.
Various commercial multigene prostate cancer genetic testing kits for risk stratification.
| Genetic testing kit (no. of genes tested) | Genes tested |
|---|---|
| Ambry Genetics “ProstateNext” (14) | ATM, BRCA1, BRCA2, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, PALB2, PMS2, RAD51D, TP53 |
| Fulgent “Prostate Cancer Panel” (12) | ATM, BRCA1, BRCA2, CHEK2, EPCAM, HOXB13, MLH1, MSH2, MSH6, NBN, PMS2, TP53 |
| GeneDx “Prostate Cancer Panel” (12) | ATM, BRCA1, BRCA2, CHEK2, EPCAM, HOXB13, MLH1, MSH2, MSH6, NBN, PMS2, TP53 |
| Invitae “Prostate Cancer Panel” (15) |
ATM, BRCA1, BRCA2, CHEK2, EPCAM, HOXB13, MLH1, MSH2, MSH6, NBN, PMS2, TP53; ADD ON FANCA, PALB2, RAD51D NB: HOXB13 Analysis limited to the NM_006361.5.c.251G > A, p.Gly84Glu variant |
| NeoGenomics “Hereditary DNA Repair Panel for Prostate Cancer” (20) | ATM, ATR, BAP1, BARD1, BRCA1, BRCA2, BRIP1, CHEK2, FAM175A, GEN1, MLH1, MRE11A, MSH2, MSH6, NBN, PALB2, PMS2, RAD51C, RAD51D, XRCC2 |
| Strand Diagnostics “UroSeq” (12) | BRCA1/2, ATM, CHEK2, RAD51D, HOXB13, PALB2, MLH1, MSH2, MSH6, PMS2, EPCAM |
Ultimately, this raises the question of whether there is a role for genetic testing, but how, when, and for whom have yet to be established. Although several commercial genetic screening tools are now available, further work is needed to ascertain which of the germline mutations in genes increasing the risk of PC should be tested. As discussed, germline BRCA1 and BRCA2 mutations occur in approximately 0.2% to 0.3% of the general population, and their impact on the diagnosis of blood relatives with PC needs further research. However, these factors are nonspecific and do not accurately distinguish patients with indolent PC tumors from those with more aggressive tumors. Thus, it is imperative to use more sensitive and specific biomarkers that can predict and allow the proper selection of patients at high risk of developing life-threatening PC.
The overriding aim should be to plan and implement personalized and precision therapies to achieve better outcomes. Similar strategies may also prevent overtreatment in patients with low-risk PC. This review suggests that comprehensive PC tumor and blood genotyping should be included in PC stratification for targeted treatment, prognostic prediction, long-term surveillance, and effective prophylaxis. Several commercial panel kits for PC genotyping are available (Table 7). In the future, the scope of whole-genome sequencing will likely increase with the development of annotated cancer genome databases. Prostate cancer remains on the priority list of the International Cancer Genome Consortium, and further work to translate specialized investigation pathways must be incorporated into daily practice.
Prostate cancer is a priority cancer on the agenda of the International Cancer Genetics Consortium (www.icgc.org). Multiple centers worldwide have contributed a large amount of epidemiological and genotyping data. This risk resource is extremely important for planning and delivering the most effective and efficient PC management, including that for males in communities with high PC risk, particularly those with a likely aggressive clinical course. Digital health technologies, specifically artificial intelligence (AI), are now being rapidly used to manage genome- and gene-specific databases. Appropriate and judicious use of AI might further strengthen genotyping-based PC risk assessment, clinical course prediction, selection of optimal therapeutic options, facilitating comprehensive and integrated multidisciplinary management of patients with PC, and risk reduction in close male members of the family. A consortium of leading academic institutions in England is engaged in a pioneering study to improve PC diagnoses using AI (www.transform.england.nhs.uk).
7. Conclusions
Comprehensive PC tumor and blood genotyping should be included in PC stratification for risk evaluation, planning and delivery of targeted molecular therapy, predicting prognosis, long-term surveillance, and effective prophylaxis. Currently, several commercial PC genotyping panel kits with enhanced accuracy and predictability are available. Technical advances in laboratory genomics and the refinement of highly sensitive methods, such as PC liquid biopsy, have greatly improved PC diagnosis. Increasingly, the specific prescription of expensive new therapeutic drugs such as taxanes and poly(ADP-ribose) polymerase inhibitors has been based on PC genotyping. In the future, the scope of whole-genome sequencing will likely increase with the development of annotated cancer genome databases. Prostate cancer remains a priority for the International Cancer Genome Consortium. In this context, the rapid expansion and healthcare applications of digital health or AI would further strengthen genotyping-based PC risk assessment, clinical course prediction, selection of optimal therapeutic options, facilitating comprehensive and integrated multidisciplinary management of patients with PC, and risk reduction in close male members of the family.
Acknowledgments
None.
Statement of ethics
Not applicable.
Funding source
None.
Author contributions
Author contributed to paper in entirety.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Footnotes
How to cite this article: Kumar AA. Prostate cancer genotyping for risk stratification and precision treatment. Curr Urol 2024;18(2):87–97. doi: 10.1097/CU9.0000000000000222
Conflict of interest statement
No conflict of interest has been declared by the author.
References
- 1.https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer.
- 2.Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol 2020;77(1):38–52. [DOI] [PubMed] [Google Scholar]
- 3.Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol 2017;14(1):26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao S Östensson E Eklund M, et al. The economic burden of prostate cancer—A Swedish prevalence-based register study. BMC Health Serv Res 2020;20(1):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker AR, Walker BF. Puzzles in the causation and epidemiology of prostate cancer—A sombre outlook. S Afr Med J 2003;93(10):773–774. [PubMed] [Google Scholar]
- 6.Dean M, Lou H. Genetics and genomics of prostate cancer. Asian J Androl 2013;15(3):309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallis CJ, Nam RK. Prostate cancer genetics: A review. EJIFCC 2015;26(2):79–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Mottet N van den Bergh RCN Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 Update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol 2021;79(2):243–262. [DOI] [PubMed] [Google Scholar]
- 9.Emmett L Buteau J Papa N, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): A prospective multicentre study. Eur Urol 2021;80(6):682–689. [DOI] [PubMed] [Google Scholar]
- 10.Kasivisvanathan V Stabile A Neves JB, et al. Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: A systematic review and meta-analysis. Eur Urol 2019;76(3):284–303. [DOI] [PubMed] [Google Scholar]
- 11.Kasivisvanathan V Rannikko AS Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378(19):1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ptasznik G Papa N Kelly BD, et al. High prostate-specific membrane antigen (PSMA) positron emission tomography (PET) maximum standardized uptake value in men with PI-RADS score 4 or 5 confers a high probability of significant prostate cancer. BJU Int 2022;130 suppl 3(suppl 3):5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szymaniak B, Ross AE, Morgans AK. Genetics in prostate cancer: Implications for clinical practice. Curr Opin Support Palliat Care 2021;15(4):241–246. [DOI] [PubMed] [Google Scholar]
- 14.Patel R, Khalifa AO, Isali I, Shukla S. Prostate cancer susceptibility and growth linked to Y chromosome genes. Front Biosci (Elite Ed) 2018;10(3):423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo J McDougall C Bowler N, et al. Pretest genetic education video versus genetic counseling for men considering prostate cancer germline testing: A patient-choice study to address urgent practice needs. JCO Precis Oncol 2021;5:PO.21.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomella LG, Giri VN. Prostate cancer genetics: Changing the paradigm of care. Urol Clin North Am 2021;48(3):xiii–xiv. [DOI] [PubMed] [Google Scholar]
- 17.Loeb S, Giri VN. Clinical implications of germline testing in newly diagnosed prostate cancer. Eur Urol Oncol 2021;4(1):1–9. [DOI] [PubMed] [Google Scholar]
- 18.Leyten GH Hessels D Smit FP, et al. Identification of a candidate gene panel for the early diagnosis of prostate cancer. Clin Cancer Res 2015;21(13):3061–3070. [DOI] [PubMed] [Google Scholar]
- 19.Eggener S Karsh LI Richardson T, et al. A 17-gene panel for prediction of adverse prostate cancer pathologic features: Prospective clinical validation and utility. Urology 2019;126:76–82. [DOI] [PubMed] [Google Scholar]
- 20.Barnes DR Silvestri V Leslie G, et al. Breast and prostate cancer risks for male BRCA1 and BRCA2 pathogenic variant carriers using polygenic risk scores. J Natl Cancer Inst 2022;114(1):109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John EM Miron A Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA 2007;298(24):2869–2876. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Acebo I Dierssen-Sotos T Fernandez-Navarro P, et al. Risk model for prostate cancer using environmental and genetic factors in the Spanish multi-case-control (MCC) study. Sci Rep 2017;7(1):8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Autier P. Risk factors and biomarkers of life-threatening cancers. Ecancermedicalscience 2015;9:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NICE Prostate Cancer Guidelines 2021. https://www.nice.org.uk/guidance/ng131/chapter/Recommendations. Accessed March 11, 2023.
- 25.Brawley OW, Thompson IM, Jr., Grönberg H. Evolving recommendations on prostate cancer screening. Am Soc Clin Oncol Educ Book 2016;35:e80–e87. [DOI] [PubMed] [Google Scholar]
- 26.Loeb S Gonzalez CM Roehl KA, et al. Pathological characteristics of prostate cancer detected through prostate specific antigen based screening. J Urol 2006;175(3 pt 1):902–906. [DOI] [PubMed] [Google Scholar]
- 27.Ahn H Hwang SI Kim TM, et al. Diagnostic value of multiparametric MRI in detecting residual or recurrent prostate cancer after high-intensity focused ultrasound. Prostate Cancer Prostatic Dis 2023;26(2):360–366. [DOI] [PubMed] [Google Scholar]
- 28.Caglic I, Sushentsev N, Shah N, Warren AY, Lamb BW, Barrett T. Integration of prostate biopsy results with pre-biopsy multiparametric magnetic resonance imaging findings improves local staging of prostate cancer. Can Assoc Radiol J 2022;73(3):515–523. [DOI] [PubMed] [Google Scholar]
- 29.Sciarra A, Gentile V, Panebianco V. Multidisciplinary management of prostate cancer: How and why. Am J Clin Exp Urol 2013;1(1):12–17. [PMC free article] [PubMed] [Google Scholar]
- 30.Muralidhar V Chen MH Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: Implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys 2015;93(4):828–835. [DOI] [PubMed] [Google Scholar]
- 31.Mohler JL Antonarakis ES Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17(5):479–505. [DOI] [PubMed] [Google Scholar]
- 32.Graham J, Kirkbride P, Cann K, Hasler E, Prettyjohns M. Prostate cancer: Summary of updated NICE guidance. BMJ 2014;348:f7524. [DOI] [PubMed] [Google Scholar]
- 33.Kearns B Pandor A Stevenson M, et al. Cabazitaxel for hormone-relapsed metastatic prostate cancer previously treated with a docetaxel-containing regimen: An evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics 2017;35(4):415–424. [DOI] [PubMed] [Google Scholar]
- 34.Beer TM Hotte SJ Saad F, et al. Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): A randomised, open-label, international, phase 3 trial. Lancet Oncol 2017;18(11):1532–1542. [DOI] [PubMed] [Google Scholar]
- 35.Boopathi E, Birbe R, Shoyele SA, Den RB, Thangavel C. Bone health management in the continuum of prostate cancer disease. Cancers (Basel) 2022;14(17):4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dräger DL. Bisphosphonates or RANK ligand inhibitors for men with prostate cancer and bone metastases: Comments on the network meta-analysis [in German]. Urologie 2022;61(8):855–859. [DOI] [PubMed] [Google Scholar]
- 37.Cheung AH Hui CH Wong KY, et al. Out of the cycle: Impact of cell cycle aberrations on cancer metabolism and metastasis. Int J Cancer 2023;152(8):1510–1525. [DOI] [PubMed] [Google Scholar]
- 38.Jamasbi E, Hamelian M, Hossain MA, Varmira K. The cell cycle, cancer development and therapy. Mol Biol Rep 2022;49(11):10875–10883. [DOI] [PubMed] [Google Scholar]
- 39.Holeckova K, Baluchova K, Hives M, Musak L, Kliment J, Sr., Skerenova M. Germline mutations in DNA repair genes in patients with metastatic castration-resistant prostate cancer. In Vivo 2020;34(4):1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirotsu Y Nakagomi H Sakamoto I, et al. Multigene panel analysis identified germline mutations of DNA repair genes in breast and ovarian cancer. Mol Genet Genomic Med 2015;3(5):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders EJ, Kote-Jarai Z, Eeles RA. Identification of germline genetic variants that increase prostate cancer risk and influence development of aggressive disease. Cancers (Basel) 2021;13(4):760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darst BF Dadaev T Saunders E, et al. Germline sequencing DNA repair genes in 5545 men with aggressive and nonaggressive prostate cancer. J Natl Cancer Inst 2021;113(5):616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunders EJ Dadaev T Leongamornlert DA, et al. Gene and pathway level analyses of germline DNA-repair gene variants and prostate cancer susceptibility using the iCOGS-genotyping array. Br J Cancer 2018;118(6):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng L, MacLennan GT, Lopez-Beltran A, Montironi R. Anatomic, morphologic and genetic heterogeneity of prostate cancer: Implications for clinical practice. Expert Rev Anticancer Ther 2012;12(11):1371–1374. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh CL Oakley-Girvan I Balise RR, et al. A genome screen of families with multiple cases of prostate cancer: Evidence of genetic heterogeneity. Am J Hum Genet 2001;69(1):148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo J, Giri VN. Germline testing and genetic counselling in prostate cancer. Nat Rev Urol 2022;19(6):331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ewing CM Ray AM Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med 2012;366(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beebe-Dimmer JL Hathcock M Yee C, et al. The HOXB13 G84E mutation is associated with an increased risk for prostate cancer and other malignancies. Cancer Epidemiol Biomarkers Prev 2015;24(9):1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang J, Beckta JM, Bindra RS. Re: Catherine H. Marshall, Alexandra O. Sokolova, Andrea L. McNatty, et al. Differential response to olaparib treatment among men with metastatic castration-resistant prostate cancer harboring BRCA1 or BRCA2 versus ATM mutations. Eur Urol 2019;76:452–458. Eur Urol 2019;76:e109–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abida W Cyrta J Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 2019;116(23):11428–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med 2016;375(18):1804–1805. [DOI] [PubMed] [Google Scholar]
- 52.Johng D Torga G Ewing CM, et al. HOXB13 interaction with MEIS1 modifies proliferation and gene expression in prostate cancer. Prostate 2019;79(4):414–424. [DOI] [PubMed] [Google Scholar]
- 53.Chi KN Barnicle A Sibilla C, et al. Detection of BRCA1, BRCA2, and ATM alterations in matched tumor tissue and circulating tumor DNA in patients with prostate cancer screened in PROfound. Clin Cancer Res 2023;29(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaur HB Vidotto T Mendes AA, et al. Association between pathogenic germline mutations in BRCA2 and ATM and tumor-infiltrating lymphocytes in primary prostate cancer. Cancer Immunol Immunother 2022;71(4):943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muralidhar V Zhang J Wang Q, et al. Genomic validation of 3-tiered clinical subclassification of high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2019;105(3):621–627. [DOI] [PubMed] [Google Scholar]
- 56.Lozano R Castro E Aragón IM, et al. Genetic aberrations in DNA repair pathways: A cornerstone of precision oncology in prostate cancer. Br J Cancer 2021;124(3):552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Dessel LF van Riet J Smits M, et al. The genomic landscape of metastatic castration-resistant prostate cancers reveals multiple distinct genotypes with potential clinical impact. Nat Commun 2019;10(1):5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao JV, Liu F, Schooling CM, Li J, Gu D, Lu X. Using genetics to assess the association of commonly used antihypertensive drugs with diabetes, glycaemic traits and lipids: A trans-ancestry Mendelian randomisation study. Diabetologia 2022;65(4):695–704. [DOI] [PubMed] [Google Scholar]
- 59.Lu YT, Delijani K, Mecum A, Goldkorn A. Current status of liquid biopsies for the detection and management of prostate cancer. Cancer Manag Res 2019;11:5271–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu J Pang J Chen Y, et al. Application of microfluidic chips in separation and analysis of extracellular vesicles in liquid biopsy for cancer. Micromachines (Basel) 2019;10(6):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Formosa A Markert EK Lena AM, et al. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene 2014;33(44):5173–5182. [DOI] [PubMed] [Google Scholar]
- 62.Thurtle DR, Jenkins V, Pharoah PD, Gnanapragasam VJ. Understanding of prognosis in non-metastatic prostate cancer: A randomised comparative study of clinician estimates measured against the PREDICT prostate prognostic model. Br J Cancer 2019;121(8):715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thurtle DR, Greenberg DC, Lee LS, Huang HH, Pharoah PD, Gnanapragasam VJ. Individual prognosis at diagnosis in nonmetastatic prostate cancer: Development and external validation of the PREDICT prostate multivariable model. PLoS Med 2019;16(3):e1002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gómez V Galazi M Weitsman G, et al. HER2 mediates PSMA/mGluR1-driven resistance to the DS-7423 dual PI3K/mTOR inhibitor in PTEN wild-type prostate cancer models. Mol Cancer Ther 2022;21(4):667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]


