Abstract
Background
Urinary incontinence (UI) is a debilitating and common condition that adversely affects quality of life. Prescriptive and surgical approaches for managing UI symptoms may result in undesirable risks and complications. This randomized, double-blind, placebo-controlled, parallel study investigated the efficacy of 2 nonsolvent flower pollen extracts on UI in healthy women.
Materials and methods
One-hundred and fourteen women aged 40–75 years who scored ≥5 on the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-SF) were randomized to receive either Graminex® RCT Fem™ UI, Graminex® PollenBerry®, or placebo for 24 weeks. The primary outcome was the change in the ICIQ-SF score between the trial and placebo groups after 24 weeks of supplementation. The secondary outcomes included changes in the frequency of nocturia (recorded in 3-day void diaries) and 24-hour leakage volume (assessed via pad weight) after 6, 12, 18, and 24 weeks of supplementation and changes in stress-induced urinary leakage volume (after completion of a provocative maneuver challenge) after 24 weeks of supplementation.
Results
All the groups demonstrated improvement in ICIQ-SF scores at week 24 (p < 0.001). The RCT Fem™ UI group had the greatest improvement in ICIQ-SF scores (−4.07 ± 3.4), followed by the PollenBerry® group (−3.34 ± 2.87) and placebo group (−2.61 ± 3.52). The RCT Fem™ UI group had corresponding improvements in 24-hour leakage volume (−17.68 ± 39.84 g) and frequency of nocturia (−0.52 ± 1.26) (p ≤ 0.05). PollenBerry® supplementation significantly improved stress-induced urinary leakage volume (−7.12 ± 15.64 g) at week 24. The study products demonstrated safe hematological and chemical profiles.
Conclusions
RCT Fem™ UI supplementation resulted in significant and clinically meaningful reductions in UI severity, with corresponding improvements in daily urinary leakage volume and frequency of nocturia. PollenBerry® significantly improved stress-induced urinary leakage volume, suggesting that it may be efficacious in women who are prone to stress UI. The study products were safe and well tolerated in this population.
Keywords: Urinary incontinence, Pollen extract, Healthy women, Dietary supplement, ICIQ-SF, Urinary leakage
1. Introduction
Urinary incontinence (UI) is defined as the involuntary loss of urine and is associated with adverse effects on overall quality of life (QoL).[1] Individuals with UI report a higher tendency to distance themselves from social and recreational activities because of feelings of distress, embarrassment, and fear of emitting odor.[2,3] Urinary incontinence is typically classified into 3 common forms: stress UI (leakage that results from an increase in intra-abdominal pressure, e.g., coughing or standing up), urgency UI, and mixed UI (a combination of stress and urgency).[4,5] Although the prevalence of UI is high, affecting approximately 51.1% of women in the United States, social stigma discourages the reporting of UI and a large proportion of those who suffer do not seek treatment.[6,7] Owing to the silence surrounding UI, its prevalence is underappreciated. This may further exacerbate feelings of isolation and lead women to mistakenly believe that successfully managing UI has little or no hope.
The management of UI generally follows a personalized approach based on the type, nature, frequency, and severity.[5]] Current approaches include behavioral therapy, physiotherapy, vaginal inserts, medication, Botox injections, laser therapy, and/or surgery. Varying degrees of success have been reported with monotherapy and combination approaches; however, these options have been associated with side effects or risk of complications.[8]] Health-conscious consumers are looking for noninvasive, nonprescription alternative therapies for UI treatment.
Pollen extracts may exert a positive effect on UI symptoms because of their action on smooth muscles in the bladder wall, resulting in better contractibility and relaxation of the urethra.[9,10] They contain amino acids, enzymes, carbohydrates, fats, vitamins, and minerals,[11,12] and have been explored as a treatment for male urinary conditions for decades.[13]] Previous research has suggested that pollen extracts help manage the clinical symptoms of prostatitis by reducing inflammation and oxidative stress,[11,14] including nocturia and the frequency of urination.[13] However, studies specifically investigating the effects of pollen extracts on women in the context of UI are limited. Therefore, this study aimed to investigate the efficacy of 2 nonsolvent flower pollen extracts on UI in healthy women.
2. Materials and methods
2.1. Ethical and regulatory approval
This randomized, double-blind, placebo-controlled, parallel study was conducted at KGK Science, Inc (London, Ontario, Canada) between June 2019 and October 2021. As the investigational products (IPs) utilized in the current study included nonsolvent flower pollen extract supplements, this study was reviewed by the Natural and Non-Prescription Health Products Directorate, Health Canada, Ottawa, Ontario, and was approved on March 28, 2019. Ethics approval was granted on April 26, 2019 by the Institutional Review Board Services, Aurora, Ontario (Pro00033511). The trial followed the CONSORT guidelines for randomized controlled trials[15] (Table S1, http://links.lww.com/CURRUROL/A58), and written informed consent was obtained from all participants prior to study initiation. This study was registered at ClinicalTrials.gov (NCT05510999).
2.2. Study design
This study implemented a 24-week supplementation period in which participants were randomized to receive 1 of 4 IPs or placebo (Fig. 1). Results are presented for the comparison of Graminex® RCT Fem™ UI, Graminex® PollenBerry®, and placebo only.
Figure 1.
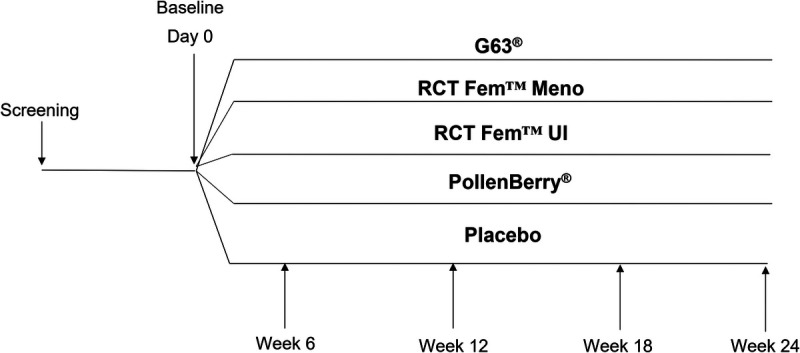
Study design.
2.3. Participants
Enrolled participants were women aged 40–75 years, with a body mass index of 18.5–34.9 kg/m2, and UI persisting for at least 1 month as assessed by a score ≥5 (slight to very severe UI)[16] on the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-SF). The participants were healthy as determined by laboratory results, medical history, and physical examination, and were assessed by the medical director (MD).
Participants were excluded if they were pregnant, breastfeeding, or planning to become pregnant; had an allergy, sensitivity, or intolerance to active or inactive ingredients of IPs; utilized a catheter for urination; were treated for overactive or neurogenic bladder or initiation of pelvic floor therapy 3 months prior to the study; had a urinary tract infection (UTI) or had history of recurrent UTIs; were treated for UI by a continence specialist within the past 5 years; had a sexually transmitted infection or self-reported infection within the last 3 months; used concomitantly prescribed (e.g., antimuscarinics/anticholinergics, β3-adrenoceptor agonists, and diuretics) or over-the-counter medications (e.g., pumpkin seed extract, soy germ extract, and horsetail), or supplements, food, and/or drinks that may have impacted the efficacy and/or safety of the IPs; had clinically significant abnormal laboratory results; or had a recent or active unstable medical condition that may have adversely affected ability to fully participate in the study or posed significant risk, as assessed by the MD.
2.4. Investigational products and placebo
Each capsule of Graminex® RCT Fem™ UI contained 9 mg of lipid-soluble pollen extract fraction, plus excipients, and each capsule of Graminex® PollenBerry® contained a combination of 42 mg of water-soluble pollen extract fraction and 125 mg of cranberry powder, plus excipients. Both IPs were nonsolvent flower pollen extract supplements/natural health products and were not medications or drugs intended to treat disease.
The placebo capsules contained microcrystalline cellulose, corn starch, maltodextrin, silicon dioxide, hydroxypropyl cellulose, and calcium stearate.
The participants were instructed to take 1 capsule twice daily for 24 weeks, beginning on day 1 (1 day after the baseline visit). When participants missed a dose, they were instructed to take it with their next scheduled dose.
2.5. Randomization and blinding
The participants were assigned a randomization number derived from a randomization list (www.randomization.com) by a blinded investigator. The investigators, study personnel, and participants were blinded to the study product. The placebo was similar in appearance to the IPs to ensure allocation concealment. The IPs and placebo were sealed in identical bottles and labeled by personnel not involved in the study assessments, according to the requirements of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Guidelines for Good Clinical Practice and applicable local regulatory guidelines.
2.6. Outcomes
The primary outcome was the difference in change in UI severity between the trial groups (RCT Fem™ UI and PollenBerry®) and placebo group after 24 weeks of supplementation.
The secondary outcomes were changes in the severity of UI between the trial groups and placebo group after 6, 12, and 18 weeks of supplementation; change in frequency of UI; daily urinary leakage volume; frequency of nocturia; frequency of daytime urination; incontinence-related QoL; degree of discomfort; sleep quality; lower urinary tract symptom score (LUTSS) between the trial groups and placebo group after 6, 12, 18, and 24 weeks of supplementation; and changes in bone density and stress-induced urinary leakage volume between the trial groups and placebo group after 24 weeks of supplementation.
2.6.1. UI severity
The primary outcome of UI severity was assessed using the ICIQ-SF. The ICIQ-SF is a brief and psychometrically robust patient-completed questionnaire used in research and clinical practice to evaluate the frequency, severity, and impact of UI on women’s QoL.[17] It is scored on a scale of 0 to 21, with higher scores indicating greater symptom severity.
2.6.2. Incontinence-related quality of life
The Incontinence Quality of Life (I-QoL) Questionnaire,[18] a condition-specific self-report measure, was used to assess the impact of UI on health-related QoL. Scores were transformed into a range of 0–100, with higher scores indicating better incontinence-related QoL.
2.6.3. Degree of bother
The degree of bother was assessed using the Overactive Bladder (short form) Questionnaire (OAB-q).[19] The OAB-q assesses how much the participant has been bothered by selected bladder symptoms during the past 4 weeks. It was scored from 0 to 100, with higher scores indicating a greater degree of bother.
2.6.4. Lower urinary tract symptoms
Lower urinary tract symptoms were assessed using the LUTSS. The LUTSS is a validated questionnaire that assesses the full range of lower urinary tract symptoms including storage, voiding, and bother.[20] The total scores are summed and range from 0 to 56, with higher scores indicating greater symptom severity.
2.6.5. Sleep quality
Sleep quality was assessed using the Healthy People Sleep Quality Index (HPSQI). The HPSQI has been previously used to assess sleep quality in populations with occasional sleeplessness[21] and measured the following aspects of sleep quality: sleep efficiency (>85% considered normal), subjective sleep quality, sleep duration, sleep latency, perceived sleep debt (sleep loss), sleep difficulty, and sleep-related QoL.
2.6.6. Frequency of UI, nocturia, and daytime urination
The frequency of UI, nocturnal urination (nocturia), and daytime urination were assessed using a 3-day void diary. Participants were instructed to record the time they woke up and went to bed, fluid intake, number of toilet urinations, leaks, and pad changes on each of the 3 days prior to scheduled clinic visits.
2.6.7. Daily urinary leakage volume
Daily urinary leakage volume was assessed by 24-hour pad weight. Participants were required to wear pads over a 24-hour period prior to clinic visits (the final day of 3-day void diary). The participants were provided with clean pads that were weighed by the study coordinators prior to use. After each use, participants placed each used pad and packaging into their original Ziploc bag and returned it to the clinic at each visit, at which time the study coordinators weighed the used pad and bag. To calculate the leakage volume, the equivalent of 1 mL of urine to 1 g of pad weight was used. A pad weight increase of <1 g was defined as the dry weight to account for perspiration.
2.6.8. Stress-induced urinary leakage volume
Stress-induced urinary leakage volume was assessed by participants performing a provocative maneuver challenge, adapted from the procedure described by Krhut et al.[22] After consuming 500 mL of water and inserting a preweighed, standardized pad into their undergarments, the participants performed the following: 1) walking up and down a flight of stairs (10-minute duration), 2) washing hands in running water (1 minute), 3) standing up and sitting down in succession (10 times), 4) coughing (10 times), 5) running on the spot (1 minute), and 6) squatting (10 times). The pad was then removed and weighed by the study coordinators (equivalent of 1 mL of urine to 1 g of pad weight was used).
2.6.9. Bone density
Bone density was measured using dual-energy X-ray absorption scans, which were conducted at KGK Science, Inc.
2.6.10. Safety
Safety outcomes included postemergent adverse events (AEs), vital signs, hematology, and clinical chemistry. Hematological parameters included white blood cell count with differential (neutrophils, lymphocytes, monocytes, eosinophils, and basophils), red blood cell count, hemoglobin, hematocrit, platelet count, and red blood cell indices (mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and red cell distribution width). Clinical chemistry parameters included liver function (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and total bilirubin), kidney function (creatinine, electrolytes [sodium, potassium, and chloride], and estimated glomerular filtration rate), and glucose. Hematological and clinical chemistry parameters were analyzed by Dynacare (London, Canada) using standardized procedures. Participants recorded AEs in their study diary, which were classified according to the description, duration, intensity, frequency, and outcome using MEDRA 22.1.
2.7. Study procedures
Anthropometrics and vitals were assessed at each clinic visit, and blood was extracted for clinical chemistry and hematology at screening and at week 24. During screening, the ICIQ-SF was administered to assess the severity of UI. Urine samples were collected to test for pregnancy in women with childbearing potential at screening, baseline (maximum 45 days after screening), and at the end of the study. Urine samples were also collected during screening to detect the presence of sexually transmitted infection and UTI and analyzed by a central laboratory (Dynacare, London, Canada). At baseline and weeks 6, 12, 18, and 24, the ICIQ-SF, I-QoL, OAB-q, LUTSS, and HPSQI questionnaires were administered, study and 3-day void diaries were reviewed, and 24-hour leakage volume was measured. Participants completed the provocative maneuver challenge at baseline and at week 24, with dual-energy X-ray absorption scans scheduled within 1 week of each clinic visit.
2.8. Statistical analyses
A sample size of 38 participants per group provided 92% power (2-sided effect size = 0.88, alpha = 0.05, 2-sided) to detect differences between groups and accounted for a 20% attrition rate. An effect size of 0.88 was considered large.[23]
Summary statistics, including means, medians, and standard deviations of primary and secondary outcomes, are presented at each study time point. All outcomes were evaluated for normality using the Shapiro-Wilk test. The difference in the change in the severity of UI scores between the study groups was evaluated using a 1-way analysis of variance followed by Tukey test for post hoc pairwise comparisons.
The secondary outcomes were evaluated using linear mixed-effects models, with time and group as fixed effects and subjects as random effects. Pairwise comparisons of the differences between baseline and each time point were performed using this model. All categorical secondary objective efficacy variables were evaluated using chi-square or Fisher exact tests.
For the AEs, a descriptive analysis of nature, incidence, severity, and causality was conducted. The significance of the differences in each safety objective within the study arms was evaluated using a paired t-test, and significant differences between groups were assessed using a 1-way analysis of variance. Categorical safety outcome variables were evaluated using the chi-square or Fisher exact tests.
Analyses are reported for the intention-to-treat population, comprising participants who received the study products and had available post-randomization efficacy data. All statistical analyses were completed using the R Statistical Software Package Version 3.6.3 (R Core Team) or newer for Microsoft Windows.[24] Statistical significance was set at p ≤ 0.05. Data are presented as means ± standard deviations unless otherwise stated.
3. Results
3.1. Study population
Three-hundred and seventy-one individuals consented to participate and were screened between June 2019 and October 2021, and 190 were enrolled in the study (Fig. 2). In the RCT Fem™ UI, PollenBerry®, and placebo groups, 114 participants (38 per group) were included in the intention-to-treat population. In total, 28 early terminations were recorded, including 9 in the PollenBerry® group, 9 in the RCT Fem™ UI group, and none in the placebo group. The reasons for early termination included auto-dropping (n = 5), inability to use a computer (n = 1), loss to follow-up (n = 2), schedule conflicts (n = 1), general practitioner recommendation (n = 1), fractured leg (n = 1), cramps in the right leg (n = 1), nausea and tiredness (n = 1), severe abdominal pain (n = 1), withdrawal of consent (n = 2), and participant request (n = 2).
Figure 2.
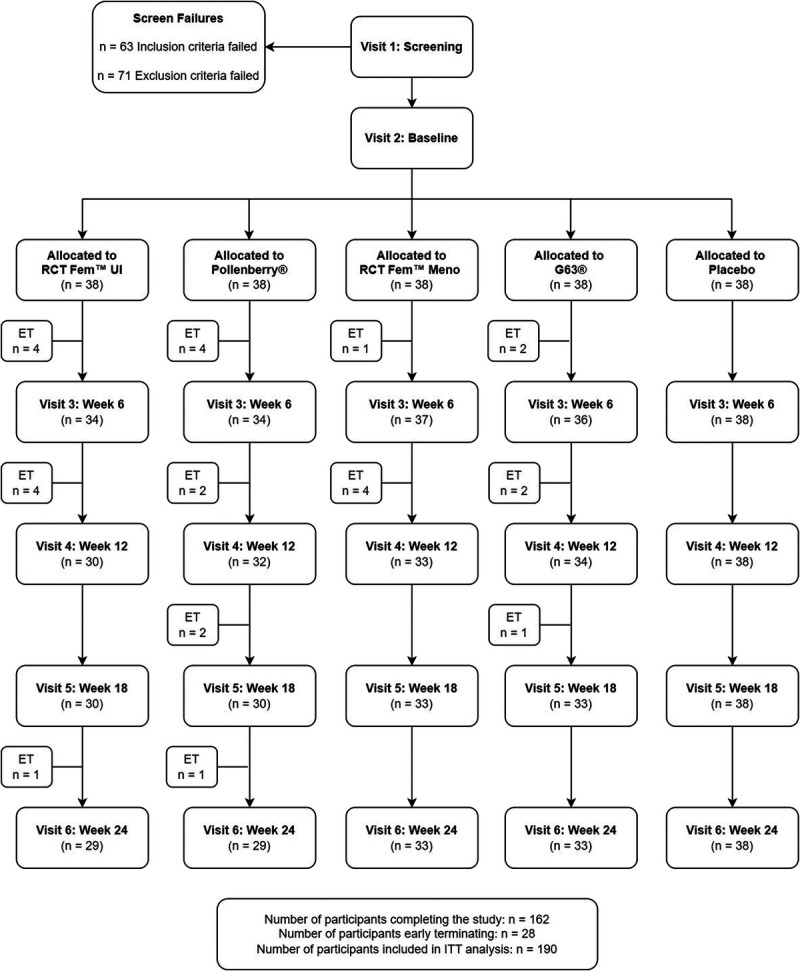
Disposition of study participants. ET = Early Termination; ITT = Intention-to-Treat.
The enrolled participants were predominantly Western European White (≥79%) between the ages of 40 and 73 years (Table 1). The participants in the PollenBerry® group had significantly lower body weight compared to those in the placebo group at baseline. All the participants were deemed healthy by the MD based on their medical history, anthropometric measurements, vital signs, and hematology and clinical chemistry parameters.
Table 1.
Participant demographics.
| Characteristics | Level | RCT Fem™ UI (n = 38) | PollenBerry® (n = 38) | Placebo (n = 38) |
|---|---|---|---|---|
| Age, yr, mean ± SD p |
Continuous | 56.45 ± 8.15 0.54* |
56.13 ± 8.5 0.65* |
55.18 ± 9.7 |
| Race, n (%) | East Asian | 0 | 1 (2.63) | 0 |
| Eastern European White | 3 (7.89) | 5 (13.16) | 4 (10.53) | |
| Hispanic or Latino | 1 (2.63) | 0 | 0 | |
| Middle Eastern | 0 | 0 | 2 (5.26) | |
| Native American | 1 (2.63) | 0 | 0 | |
| South American | 0 | 0 | 0 | |
| South Asian | 0 | 1 (2.63) | 1 (2.63) | |
| South East Asian | 1 (2.63) | 1 (2.63) | 0 | |
| Western European White | 32 (84.21) | 30 (78.95) | 31 (81.58) | |
| p | 0.61 | 0.42 | ||
| Mean weight, kg, mean ± SD p |
Continuous | 71.19 ± 12.09 0.1 |
70.66 ± 11 0.05† |
75.78 ± 12.65 |
| BMI, kg/m2, mean ± SD p |
Continuous | 27.67 ± 3.8 0.74 |
26.88 ± 3.6 0.22 |
27.97 ± 4.18 |
| Alcohol use, n (%) | None | 7 (18.42) | 5 (13.16) | 9 (23.68) |
| Daily | 1 (2.6) | 3 (7.9) | 3 (7.9) | |
| Weekly | 15 (39.47) | 19 (50) | 11 (28.95) | |
| Occasionally | 15 (39.47) | 11 (28.95) | 15 (39.47) | |
| p | 0.81 | 0.1 | ||
| Tobacco use | Ex-smoker | 8 (21.05) | 8 (21.05) | 13 (34.21) |
| No | 30 (78.95) | 29 (76.32) | 23 (60.53) | |
| Yes | 0 | 1 (2.63) | 2 (5.25) | |
| p | 0.9 | 0.8 | ||
| Medical Cannabis use | No | 38 (100) | 38 (100) | 37 (97.37) |
| Yes | 0 | 0 | 1 (2.63) | |
| p | 0.91 | 0.82 | ||
| Recreational Cannabis use | No | 37 (97.37) | 34 (89.47) | 37 (97.37) |
| Yes | 1 (2.63) | 4 (10.53) | 1 (2.63) | |
| p | 0.81 | 0.72 |
Data are presented as means ± standard deviations (SDs) or frequencies (percentages).
*The p values are presented from comparisons between RCT Fem™ UI, PollenBerry®, and placebo; continuous and categorical outcomes were evaluated using an analysis of variance post hoc test and Fisher exact test, respectively.
†Indicates significant difference between groups.
BMI = body mass index; UI = urinary incontinence.
3.2. UI Severity
The RCT Fem™ UI, PollenBerry®, and placebo groups had significant improvement in UI severity from baseline at weeks 6, 12, 18, and 24. However, the greatest improvement in ICIQ-SF score after 24 weeks of supplementation occurred in the RCT Fem™ UI group (−4.07 ± 3.4) (Fig. 3). No significant differences were observed between the groups.
Figure 3.
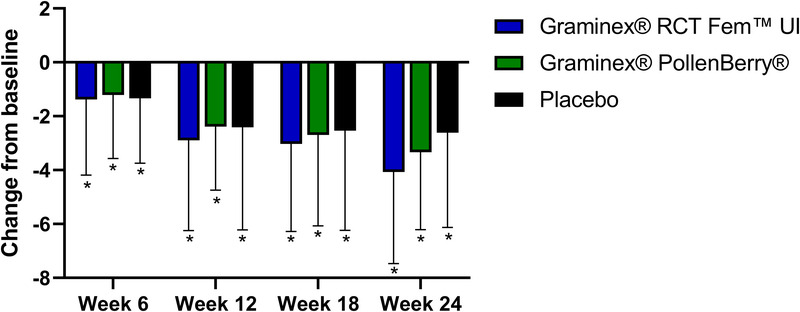
Change in International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form scores from baseline at weeks 6, 12, 18, and 24. * indicates a significant difference from baseline.
3.3. Degree of bother
In all the groups, OAB-q scores significantly improved from baseline at weeks 6, 12, 18, and 24. The greatest improvements in OAB-q scores at week 24 were reported by participants in the PollenBerry® group with a reduction of 16.78 ± 13.38 (47.22%; p < 0.001), followed by the RCT Fem™ UI (16.33 ± 17.16; 42.60%; p < 0.001) and placebo groups (13.51 ± 14.16; 41.85%; p < 0.001). No significant differences were observed between the groups.
3.4. Frequency of UI
Participants supplemented with RCT Fem™ UI had significantly greater frequency of UI than those on placebo at weeks 6 and 12 (p ≤ 0.04). No other significant differences were identified, except for a significant increase in frequency of UI for participants supplemented with RCT Fem™ UI from baseline at week 6. However, this increase was not sustained until 24 weeks (Table 2).
Table 2.
Change in frequency of urinary incontinence at weeks 6, 12, 18, and 24.
| Study time point | RCT Fem™ UI Mean ± SD (n) Within-group p* | PollenBerry® Mean ± SD (n) Within-group p* | Placebo Mean ± SD (n) Within-group p* | p RCT Fem™ UI vs. placebo* PollenBerry® vs. placebo* |
|---|---|---|---|---|
| Change from baseline at week 6 | 0.84 ± 0.9 (32) 0.02† |
−0.3 ± 0.7 (33) 0.95 |
0.21 ± 1.4 (38) 0.28 |
0.62 0.90 |
| Change from baseline at week 12 | 0.42 ± 1.37 (29) 0.22 |
−0.03 ± 1.08 (32) 0.8 |
−0.21 ± 1.52 (38) 0.78 |
0.50 0.34 |
| Change from baseline at week 18 | 0.4 ± 1.3 (30) 0.21 |
0 ± 0.7 (29) 0.65 |
−0.36 ± 1 (38) 0.12 |
0.09 0.34 |
| Change from baseline at week 24 | 0.17 ± 2.09 (29) 0.52 |
−0.22 ± 1.40 (29) 0.72 |
−0.42 ± 1.83 (38) 0.3 |
0.09 0.34 |
*The p values for between-group and within-group differences were generated using linear mixed-effects models.
†Indicates a significant within-group difference from baseline.
SD = standard deviation; UI = urinary incontinence.
3.5. Frequency of nocturia
At baseline, participants in the RCT Fem™ UI group had significantly greater frequency of nocturia than the placebo group (p = 0.05). Participants supplemented with RCT Fem™ UI and those on placebo had significant 38.0% and 42.7% improvements in the frequency of nocturia at week 24, respectively, whereas participants supplemented with PollenBerry® had an 18.8% improvement, although this was not significant (Table 3).
Table 3.
Change in frequency of nocturia at weeks 6, 12, 18, and 24.
| Study time point | RCT Fem™ UI Mean ± SD (n) Within-group p* | PollenBerry® Mean ± SD (n) Within-group p* | Placebo Mean ± SD (n) Within-group p* | p RCT Fem™ UI vs. placebo* PollenBerry® vs. placebo* |
|---|---|---|---|---|
| Change from baseline at week 6 | −0.06 ± 1.11 (32) 0.75 | 0.15 ± 0.87 (33) 0.32 | 0.18 ± 1.45 (38) 0.44 | 0.42 0.91 |
| Change from baseline at week 12 | −0.32 ± 0.98 (29) 0.08 | 0.16 ± 0.93 (31) 0.34 | −0.24 ± 0.88 (38) 0.11 | 0.71 0.08 |
| Change from baseline at week 18 | −0.17 ± 2.22 (29) 0.68 | −0.25 ± 0.75 (28) 0.09 | −0.21 ± 0.81 (38) 0.12 | 0.93 0.84 |
| Change from baseline at week 24 | −0.52 ± 1.26 (25) 0.05† | −0.12 ± 0.74 (24) 0.42 | −0.38 ± 0.89 (37) 0.01† | 0.63 0.23 |
*The p values for between-group and within-group differences were generated using linear mixed-effects models.
†Indicates a significant within-group difference from baseline.
SD = standard deviation; UI = urinary incontinence.
3.6. Daily urinary leakage volume
The changes in the daily urinary leakage volume are illustrated in Figure 4. Participants supplemented with RCT Fem™ UI or PollenBerry® had 65.1% (p = 0.02) and 28% (p = 0.08) improvements in daily urinary leakage volume at week 24, respectively. At week 6, the RCT Fem™ UI group demonstrated a significant improvement in daily urinary leakage volume compared to the placebo group, although this significant difference between groups was not sustained at week 24. Those receiving placebo exhibited a 36% improvement (p = 0.01) in daily urinary leakage volume at the end of the study.
Figure 4.
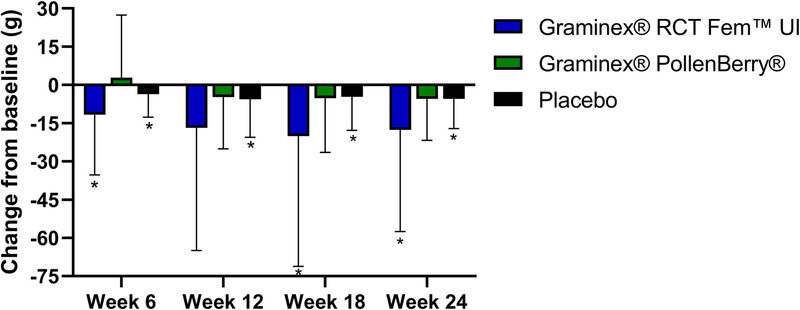
Change in daily urinary leakage volume (as assessed by pad weight) at weeks 6, 12, 18, and 24. * indicates a significant difference from baseline.
3.7. Stress-induced urinary leakage volume
The changes in stress-induced urinary leakage volume are illustrated in Figure 5. Participants in the PollenBerry® group had a significant improvement in stress-induced urinary leakage volume after 24 weeks of supplementation (p = 0.02). The RCT Fem™ UI and placebo groups exhibited improvements in stress-induced urinary leakage volume at week 24, although these changes were not statistically significant. Moreover, no significant differences were observed between the groups.
Figure 5.
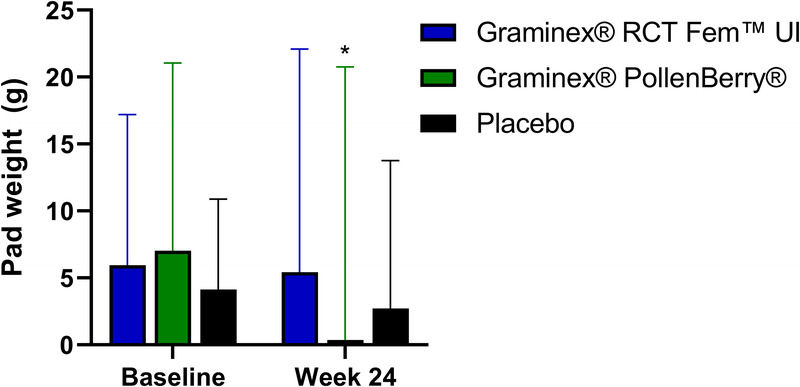
Stress-induced urinary leakage volume (as assessed by pad weight) at baseline and week 24. * indicates a significant change from baseline.
3.8. Frequency of daytime urination
No significant differences in daytime urination frequency were observed between the groups. Participants in the RCT Fem™ UI group had a 0.24 ± 3.06 decrease at week 24 (2.58%; p = 0.7), whereas those supplemented with PollenBerry® had a 0.38 ± 2.9 decrease (4.47%; p = 0.66). No changes in the frequency of daytime urination were observed in the placebo group.
3.9. Incontinence-related quality of life
No significant differences in incontinence-related QoL scores were noted between the groups. After 24 weeks of supplementation, participants in the RCT Fem™ UI group had an improvement in I-QoL score of 1.89 ± 11.93 (4.02%; p = 0.39), whereas those in the PollenBerry® and placebo groups had improvements of 2.04 ± 11.53 (4.39%; p = 0.35) and 1.35 ± 15.09 (2.90%; p = 0.59), respectively.
3.10. Lower urinary tract symptoms
Participants supplemented with PollenBerry® and those on placebo had significant 7.0% (2.86 ± 6.27) and 6.96% (2.82 ± 8.14) increases in LUTSS after 24 weeks, respectively. Lower urinary tract symptom scores were also increased in the RCT Fem™ UI group (1.17 ± 6.68; 2.79%), although this increase was not significant compared to baseline (p = 0.35). No significant differences were noted between the groups.
3.11. Sleep quality
Participants in the RCT Fem™ UI group had a significant decrease in sleep efficiency ([6.51 ± 22.65]%; p = 0.01) and a significant improvement in subjective sleep quality (0.43 ± 0.9; 15.4%; p = 0.01) after 24 weeks of supplementation. The placebo group also had a significant improvement in subjective sleep quality (0.35 ± 0.82; 13.06%; p = 0.01). No other significant differences in sleep quality parameters were observed between the study groups (data not shown).
3.12. Bone density
No significant differences in bone density were observed after 24 weeks of supplementation. Participants supplemented with RCT Fem™ UI had a 0.01 ± 0.13 g/cm2 increase in bone density from baseline at week 24 (p = 0.11), whereas those supplemented with PollenBerry® and those on placebo had no change.
3.13. Safety
Supplementation with RCT Fem™ UI and PollenBerry® for 24 weeks was safe and well tolerated in the studied population. All hematology and clinical chemistry values outside the normal laboratory range were deemed clinically irrelevant, as assessed by the MD. A total of 151 postemergent AEs were reported by 60 participants: 35 by 21 participants in the RCT Fem™ UI group, 75 by 22 participants in the PollenBerry® group, and 41 by 17 participants in the placebo group. No AEs were categorized as “most probable” or “probably” related. In total, 11 AEs were listed as “possibly related,” with 7 reported by participants in the PollenBerry® group, 3 in the placebo group, and 1 in the RCT Fem™ UI group. For the PollenBerry® group, “possibly related” AEs included 1 incidence each of headache, constipation, oral pain, tiredness, and gastroesophageal reflux disease, and 2 incidences of bloating. In the RCT Fem™ UI group, 1 incidence of nausea was deemed “possibly related.” In the placebo group, 3 incidences of abdominal discomfort were classified as “possibly related.” All “possibly related” AEs resolved by the end of the study period except for 1 participant who reported experiencing constipation with moderate intensity. This participant was lost to follow-up and was advised to contact his/her general practitioner.
4. Discussion
Both RCT Fem™ UI and PollenBerry® supplementation significantly improved UI severity at week 24. However, only RCT Fem™ UI resulted in reductions that met the definition of a minimal clinically important difference (MCID). An MCID is used to evaluate meaningful and valuable changes in UI severity.[25] An MCID of at least 4 points was established in 120 women who received nonsurgical interventions to reduce the severity of UI symptoms.[26] The clinically meaningful reduction in ICIQ-SF scores at week 24 in the RCT Fem™ UI group was driven by sustained improvement over time, with a decrease in ICIQ-SF score of 1.04 points from week 18 to week 24. This reduction in ICIQ-SF score for participants in the RCT Fem™ UI group was 1.5 times greater than the improvement experienced by participants supplemented with PollenBerry® (0.66 point reduction) and more than 14 times the improvement of those on placebo (0.07 point reduction). The results of the ICIQ-SF data were consistent with significant improvements in UI symptom bother. This was an expected finding, as the OAB-q measured how much participants were bothered by UI symptoms over the past 4 weeks and was directly related to UI severity. A significant placebo effect was observed in the ICIQ-SF and OAB-q data at the end of the study, which is consistent with UI literature.
Previous research has demonstrated that UI treatment effects are accompanied with a placebo effect ranging from 32% to 65% for self-reported incontinence episodes, and between 9% and 34% for a reduction in symptom scores.[27] Although a larger placebo effect (72%) was present at the end of the study, the placebo effect associated with the RCT Fem™ UI ICIQ-SF scores waned over time. If participants were supplemented and followed up for >6 months, a significant difference in ICIQ-SF scores between RCT Fem™ UI and placebo may have been present with the continued waning of the placebo effect. The expected duration of the placebo effect in UI treatment has not been well-described in the literature. However, pharmacological treatment of other urological disorders has demonstrated that the placebo effect reaches a maximum in the first 4–6 months, followed by a stabilization period.[28] Interestingly, the placebo effect remained after 1 year.[28] Furthermore, participants in the RCT Fem™ UI group reported a greater severity of UI symptoms at baseline compared to the placebo group. Despite both groups being characterized as having “moderate” severity based on falling within a mean ICIQ-SF range of 6–12,[16] the greater severity reported by participants supplementing with RCT Fem™ UI may have contributed to a lack of significant change between groups. Previous studies have demonstrated that less severe UI at baseline is associated with more successful treatment outcomes.[29] The ICIQ-SF total severity scores are the summation of subscores in the categories of frequency, leakage volume, and impact on the overall QoL. The overall severity may improve without decreasing the frequency of UI episodes if corresponding improvements in daily urinary leakage volume are also present, as evidenced by the findings of the current study.
The lack of a statistically significant change in the frequency of UI at the end of the study may be indicative of the fact that all study groups reported less than 1 episode of UI/day at baseline or may suggest that participants underestimated their frequency of UI. Based on the baseline levels of daily urinary leakage volume across all groups, the latter may be the most plausible explanation. More than 91% of the participants required the use of at least 4 pads on the day before their baseline visit. It is possible that the self-reported frequency of UI was likely underreported compared to the actual number of UI episodes. Considering the relationship between subjective measures and larger placebo effects,[27] a fully objective measure of daily urinary leakage volume may be a better indicator of UI symptom improvement. RCT Fem™ UI supplementation resulted in a significant decrease of 65% in daily urinary leakage volume at week 24, compared to a 36% decrease in the placebo group. Further supporting the efficacy of RCT Fem™ UI in relieving UI symptom severity was the benefit to participants after they had fallen asleep, with significant reductions in the frequency of nocturia.
The frequency of nocturia is an aspect of UI that has been reported to significantly affect QoL.[30] Participants supplemented with RCT Fem™ UI and those on placebo had significant reductions in the frequency of nocturia episodes of 0.52 and 0.38 at week 24, respectively. The RCT Fem™ UI group reported a significantly higher frequency of nocturia than the placebo group at baseline, suggesting that the decrease experienced by those supplementing with RCT Fem™ UI may be more clinically relevant. Nocturia has been ranked as the most bothersome symptom in patients with lower urinary tract symptoms,[30] with a negative correlation between the number of nocturia episodes and QoL.[31] Considering the direct relationship between frequency of nocturia and QoL, the 0.52 reduction (equivalent to a half-trip to the bathroom) in frequency of nocturia experienced by participants supplemented with RCT Fem™ UI may have contributed to improvements in overall UI symptoms.
The majority of evidence supporting the use of Graminex® Nonsolvent Flower Pollen Extracts for reducing the severity of UI symptoms in this study pertained to RCT Fem™ UI. However, PollenBerry® supplementation significantly improved stress-induced urinary leakage volume, providing support for its use in a specific subset of the UI population. Stress UI has been estimated to affect up to 35% of adult women[32] and presents an additional challenge, as leaks occur spontaneously. The specific PollenBerry® formulation included spray dried cranberry powder, which may have provided enhanced urethral contractibility, providing better protection against stress-induced urinary leakage.[33] PollenBerry® should be further explored in a population of women who may be more prone to stress-induced urinary leakage, such as those working in manufacturing or other non-office work settings.[34,35]
Limitations
This study has some limitations. First, the current study did not include a follow-up period; therefore, the severity of UI in the participants after supplementation is unknown. Second, subgroup analyses based on UI type (stress, urgency, or mixed) were not performed. Therefore, whether the IPs may have been more efficacious in one type of UI compared to another, such as PollenBerry® supplementation in women with stress UI, is unclear. Future randomized controlled trials should consider stratification by UI type to further examine the efficacy of nonsolvent flower pollen extracts on UI. Finally, the current study enrolled a relatively small sample size, and larger clinical trials investigating the effects of nonsolvent flower pollen extracts should be considered in the future.
5. Conclusions
Supplementation with RCT Fem™ UI for 24 weeks resulted in significant and clinically meaningful reductions in UI severity in a healthy population of women aged 40–75 years old experiencing UI. Both RCT Fem™ UI and PollenBerry® were safe, well tolerated, and significantly improved ICIQ-SF scores at week 24. However, only RCT Fem™ UI resulted in a MCID in ICIQ-SF score,[26] with corresponding significant improvements in daily urinary leakage volume and frequency of nocturia. The placebo group demonstrated a significant improvement in ICIQ-SF score at the end of the study; however, this effect waned over the 24-week supplementation period. PollenBerry® improved stress-induced urinary leakage volume, demonstrating efficacy in a unique subset of the overall UI population. The findings of this study provide support for further investigation of RCT Fem™ UI in populations of women who experience moderate-to-severe UI and PollenBerry® in those who experience stress UI.
Acknowledgments
The authors wish to thank the participants for their compliance to the conduct of the study.
Statement of ethics
This study was approved by the Natural and Non-Prescription Health Products Directorate, Health Canada, Ottawa, Ontario, Canada, and ethical approval was granted by the IRB Services, Aurora, Ontario, Canada (Pro00033511). The study was conducted in compliance with the International Council for Harmonization of Technical Requirements for harmaceuticals for Human Use Guidelines for Good Clinical Practice guidelines and in accordance with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from all participants before study initiation. This study was registered at ClinicalTrials.gov (NCT05510999).
Funding source
This study was funded by Graminex L.L.C. (Saginaw, MI, USA).
Author contributions
MM: Data interpretation, visualization, writing—original draft preparation, review, and editing; EDL: Writing—original draft preparation, review, and editing;
DCC: Study supervision;
CEM: Study design, writing—review and editing;
ME: Study design, data interpretation, writing—original draft preparation, review and editing, and supervision;
All the authors have read and approved the final version of the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Footnotes
Supplemental Digital Content is available for this article.
How to cite this article: Moulin M, Lewis ED, Crowley DC, May CE, Evans M. Efficacy of nonsolvent flower pollen extracts in healthy women with urinary incontinence: A randomized, double-blind, placebo-controlled, parallel study. Curr Urol 2024;18(3):203–211. doi: 10.1097/CU9.0000000000000248
Contributor Information
Erin D. Lewis, Email: elewis@kgkscience.com.
David C. Crowley, Email: dcrowley@kgkscience.com.
Colleen E. May, Email: bugs@graminex.com.
Malkanthi Evans, Email: mevans@kgkscience.com.
Conflict of interest statement
CEM is an employee of Graminex, L.L.C. MM, EDL, DCC, and ME are employees of KGK Science Inc. and have no competing interests to declare.
References
- 1.Subak LL Brown JS Kraus SR, et al. The "costs" of urinary incontinence for women. Obstet Gynecol 2006;107(4):908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almousa S, Bandin van Loon A. The prevalence of urinary incontinence in nulliparous adolescent and middle-aged women and the associated risk factors: A systematic review. Maturitas 2018;107:78–83. [DOI] [PubMed] [Google Scholar]
- 3.Long JE, Khairat S, Chmelo E, Palmer MH. Mind over bladder: Women, aging, and bladder health. Geriatr Nurs 2018;39(2):230–237. [DOI] [PubMed] [Google Scholar]
- 4.Buckley BS Lapitan MCM, Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008 . Prevalence of urinary incontinence in men, women, and children—Current evidence: Findings of the fourth international consultation on incontinence. Urology 2010;76(2):265–270. [DOI] [PubMed] [Google Scholar]
- 5.Muth CC. Urinary incontinence in women. JAMA 2017;318(16):1622. [DOI] [PubMed] [Google Scholar]
- 6.Markland AD, Richter HE, Fwu CW, Eggers P, Kusek JW. Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol 2011;186(2):589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardsley A. An overview of urinary incontinence. Br J Nurs 2016;25(18):S14–S21. [DOI] [PubMed] [Google Scholar]
- 8.Capobianco G Madonia M Morelli S, et al. Management of female stress urinary incontinence: A care pathway and update. Maturitas 2018;109:32–38. [DOI] [PubMed] [Google Scholar]
- 9.Palacios S, Ramirez M, Lilue M, Vega B. Evaluation of Femaxeen® for control of urinary incontinence in women: A randomized, double-blind, placebo-controlled study. Maturitas 2020;133:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Kimura M, Kimura I, Nakase K, Sonobe T, Mori N. Micturition activity of pollen extract: Contractile effects on bladder and inhibitory effects on urethral smooth muscle of mouse and pig. Planta Med 1986;2:148–151. [DOI] [PubMed] [Google Scholar]
- 11.Locatelli M Macchione N Ferrante C, et al. Graminex pollen: Phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules 2018;23(5):1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai T, Verze P, La Rocca R, Anceschi U, De Nunzio C, Mirone V. The role of flower pollen extract in managing patients affected by chronic prostatitis/chronic pelvic pain syndrome: A comprehensive analysis of all published clinical trials. BMC Urol 2017;17(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugendorff EW, Weidner W, Ebeling L, Buck AC. Results of treatment with pollen extract (Cernilton N) in chronic prostatitis and prostatodynia. Br J Urol 1993;71(4):433–438. [DOI] [PubMed] [Google Scholar]
- 14.Wagenlehner FME, Schneider H, Ludwig M, Schnitker J, Brähler E, Weidner W. A pollen extract (Cernilton) in patients with inflammatory chronic prostatitis-chronic pelvic pain syndrome: A multicentre, randomised, prospective, double-blind, placebo-controlled phase 3 study. Eur Urol 2009;56(3):544–551. [DOI] [PubMed] [Google Scholar]
- 15.Moher D Hopewell S Schulz KF, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10(1):28–55. [DOI] [PubMed] [Google Scholar]
- 16.Klovning A, Avery K, Sandvik H, Hunskaar S. Comparison of two questionnaires for assessing the severity of urinary incontinence: The ICIQ-UI SF versus the incontinence severity index. Neurourol Urodyn 2009;28(5):411–415. [DOI] [PubMed] [Google Scholar]
- 17.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23(4):322–330. [DOI] [PubMed] [Google Scholar]
- 18.Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP. Quality of life of persons with urinary incontinence: Development of a new measure. Urology 1996;47(1):67–71; discussion 71–72. [DOI] [PubMed] [Google Scholar]
- 19.Coyne K Lai J Zyczynski T, et al. An overactive bladder symptom and quality-of-life short form: Development of the Overactive Bladder Questionnaire Short Form (OAB-q SF). 34th joint meeting of the International Continence Society and the International Urogynecological Association; 2004. [Google Scholar]
- 20.Blaivas JG Tsui JF Mekel G, et al. Validation of the lower urinary tract symptom score. Can J Urol 2015;22(5):7952–7958. [PubMed] [Google Scholar]
- 21.Moulin M, Lewis ED, Crowley DC, Langston J, Evans M. A randomized, double-blind, placebo-controlled, cross-over pilot study to investigate the efficacy of rest-ZZZ formula in healthy participants with occasional sleeplessness. Sleep Biol Rhythms 2022;21(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krhut J Zachoval R Smith PP, et al. Pad weight testing in the evaluation of urinary incontinence. NeurourolUrodyn 2014;33(5):507–510. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York: Routledge; 1988. [Google Scholar]
- 24.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 25.McGlothlin AE, Lewis RJ. Minimal clinically important difference: Defining what really matters to patients. JAMA 2014;312(13):1342–1343. [DOI] [PubMed] [Google Scholar]
- 26.Lim R, Liong ML, Lim KK, Leong WS, Yuen KH. The minimum clinically important difference of the international consultation on incontinence questionnaires (ICIQ-UI SF and ICIQ-LUTSqol). Urology 2019;133:91–95. [DOI] [PubMed] [Google Scholar]
- 27.van Leeuwen JH, Castro R, Busse M, Bemelmans BL. The placebo effect in the pharmacologic treatment of patients with lower urinary tract symptoms. Eur Urol 2006;50(3):440–452; discussion 453. [DOI] [PubMed] [Google Scholar]
- 28.Hansen BJ, Meyhoff HH, Nordling J, Mensink HJ, Mogensen P, Larsen EH. Placebo effects in the pharmacological treatment of uncomplicated benign prostatic hyperplasia. The ALFECH study group. Scand J Urol Nephrol 1996;30(5):373–377. [DOI] [PubMed] [Google Scholar]
- 29.Obloza A, Teo R, Marriott E, Parker G, Tincello D. Association of baseline severity of lower urinary tract symptoms with the success conservative therapy for urinary incontinence in women. Int Urogynecol J 2019;30(5):705–710. [DOI] [PubMed] [Google Scholar]
- 30.Stanley N. The underestimated impact of nocturia on quality of life. Eur Urol Suppl 2005;4(7):17–19. [Google Scholar]
- 31.Yu H-J, Chen F-Y, Huang P-C, Chen TH-H, Chie W-C, Liu C-Y. Impact of nocturia on symptom-specific quality of life among community-dwelling adults aged 40 years and older. Urology 2006;67(4):713–718. [DOI] [PubMed] [Google Scholar]
- 32.Luber KM. The definition, prevalence, and risk factors for stress urinary incontinence. Rev Urol 2004;6(Suppl 3(Suppl 3)):S3–S9. [PMC free article] [PubMed] [Google Scholar]
- 33.Norton P, Brubaker L. Urinary incontinence in women. Lancet 2006;367(9504):57–67. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald ST, Palmer MH, Kirkland VL, Robinson L. The impact of urinary incontinence in working women: A study in a production facility. Women Health 2002;35(1):1–16. [DOI] [PubMed] [Google Scholar]
- 35.Palmer MH, Fitzgerald S. Urinary incontinence in working women: A comparison study. J Womens Health (Larchmt) 2002;11(10):879–888. [DOI] [PubMed] [Google Scholar]


