Abstract
Despite the importance of cholesterol in the formation and function of caveolar microdomains in plasma membranes, almost nothing is known regarding the structural properties, cholesterol dynamics or intracellular factors affecting caveolar cholesterol dynamics. A non-detergent method was employed to isolate caveolae/raft domains from purified plasma membranes of murine fibroblasts. A series of fluorescent lipid probe molecules or a fluorescent cholesterol analogue, dehydroergosterol, were then incorporated into the caveolae/raft domains to show that: (i) fluorescence polarization of the multiple probe molecules {diphenylhexatriene analogues, DiI18 (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), parinaric acids and NBD-stearic acid {12-(N-methyl)-N-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-octadecanoic acid} indicated that acyl chains in caveolae/raft domains were significantly less ‘fluid’ (i.e. more rigid) and the transbilayer ‘fluidity gradient’ was 4.4-fold greater than in plasma membranes; (ii) although sterol was more ordered in caveolae/raft domains than plasma membranes, spontaneous sterol transfer from caveolae/raft domains was faster (initial rate, 32%; half-time, t1/2, 57%) than from the plasma membrane; (iii) although kinetic analysis showed similar proportions of exchangeable and non-exchangeable sterol pools in caveolae/raft domains and plasma membranes, addition of SCP-2 (sterol carrier protein-2) 1.3-fold more selectively increased sterol transfer from caveolae/raft domains by decreasing the t1/2 (50%) and increasing the initial rate (5-fold); (iv) SCP-2 was also 2-fold more selective in decreasing the amount of non-exchangeable sterol in caveolae/raft domains compared with plasma membranes, such that nearly 80% of caveolar/raft sterol became exchangeable. In summary, although caveolae/raft lipids were less fluid than those of plasma membranes, sterol domains in caveolae/rafts were more spontaneously exchangeable and more affected by SCP-2 than those of the bulk plasma membranes. Thus caveolae/raft domains isolated without the use of detergents display unique structure, cholesterol domain kinetics and responsiveness to SCP-2 as compared with the parent plasma membrane.
Keywords: caveola, cholesterol, plasma membrane, raft, sterol carrier protein-2 (SCP-2), sterol exchange assay
Abbreviations: DHE, dehydroergosterol; DiI1, 1,1′,3,3,3′,3′-hexamethylindodicarbocyanine iodide; DiI18, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DPH, 1,6-diphenyl-1,3,5-hexatriene; DPH-Pro, 3(DPH)-propionic acid; DPH-TMA, DPH-trimethylammonium; DRM, detergent-resistant membrane; FBS, foetal bovine serum; HDL, high-density lipoprotein; LDL, low-density lipoprotein; L-FABP, liver fatty-acid-binding protein; NBD-stearic acid, 12-(N-methyl)-N-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-octadecanoic acid; cis-parinaric acid, 9Z,11E,13E,15Z-octatetradecanoic acid; trans-parinaric acid, 9E,11E,13E,15E-octatetradecanoic acid; SCP-2, sterol carrier protein-2; t1/2, half-time
INTRODUCTION
Cells take up the majority of exogenous cholesterol in the form of LDL (low-density lipoprotein) cholesteryl esters via endocytosis through the LDL-receptor pathway mediated by clathrin-coated pits and lysosomes (reviewed in [1]). However, novel experiments have elucidated the presence of parallel pathways of non-endocytic lipid uptake via another plasma membrane microdomain, caveolae (reviewed in [2,3]). Caveolae contain the SRB1 (scavenger receptor B1) which binds HDL (high-density lipoprotein) and mediates the non-endocytic ‘selective’ uptake of cholesteryl esters (reviewed in [2]), cholesterol uptake [4], and cholesterol efflux (i.e. ‘reverse cholesterol transport’) [2,5]. Abnormalities in the uptake, intracellular trafficking, or efflux of cholesterol occur in human diseases, such as cardiovascular (reviewed in [2]), Niemann–Pick C [6] and Alzheimer's [7]. Thus it is essential to resolve not only how caveolae mediate cholesterol trafficking through the plasma membrane, but also what factors control this trafficking within the cells.
Despite the importance of plasma membrane caveolae to cellular cholesterol flux, molecular details of the structural organization of caveolar lipids, especially cholesterol, are not fully understood. As compared with the parent plasma membrane, caveolar microdomains isolated by use of detergents [i.e. DRMs (detergent-resistant membranes)] (reviewed in [2,8]) or non-detergent methods [7,9] are enriched in cholesterol and sphingomyelin. The lipids in DRMs are organized in a liquid-ordered phase whose structure is intermediate between the fluid liquid-crystalline phase and the solid-ordered phase [8]. The fluidity of DRM phospholipid acyl chains, determined by DPH (1,6-diphenyl-1,3,5-hexatriene) polarization, also suggests that caveolar lipids are organized in the liquid-ordered phase [10]. In contrast with studies with DRMs, almost nothing is known regarding cholesterol organization, acyl chain fluidity or structure of caveolae isolated by detergent-free methods. One recent report indicated that, despite the high level of cholesterol in caveolae, the cholesterol in detergent-free caveolae is primarily monomeric, with only a small amount (i.e. ≤0.3%) being in crystalline phase [11].
Although considerable progress has been made in our understanding of cholesterol dynamics in plasma membranes [12], molecular details of cholesterol dynamics within caveolae have not been reported. Plasma membrane cholesterol is distributed into at least two kinetically (reviewed in [12]) and structurally [13] resolvable sterol domains. Because of the rapid transbilayer migration rate of cholesterol across the plasma membrane (reviewed in [14]), the rapidly exchangeable (minutes to hours) and non-exchangeable sterol pools are both thought to reflect lateral cholesterol domains (reviewed in [12,15]). Although cholesterol uptake and efflux via caveolae are rapid [2,4,5], whether or not the rapidly transferable sterol domain detected in plasma membranes represents caveolar cholesterol is unclear. For example, model membranes rich in the types of lipids present in caveolae (i.e. cholesterol and sphingomyelin) exhibit slow cholesterol dynamics (reviewed in [16]). However, nothing is known regarding cholesterol dynamics in caveolae.
Although vesicular trafficking pathways to and from the plasma membrane have been studied in detail, factors mediating rapid, non-vesicular cholesterol transfer between membranes are only beginning to be understood [2,4,5]. The importance of non-vesicular pathways is illustrated by a recent real-time fluorescence-imaging study of sterol transport between plasma membrane domains of polarized hepatic cells [17], which, together with earlier studies with liver [18,19], indicate that the majority of liver cholesterol traffics from the basolateral membranes by non-vesicular pathways to the bile canalicular region. At least three candidate soluble proteins may be postulated to mediate non-vesicular lipid trafficking from plasma membrane caveolae: caveolin, SCP-2 (sterol carrier protein-2), and L-FABP (liver fatty-acid-binding protein) (reviewed in [15]) [20,21]. Caveolin itself binds cholesterol in vitro, and cytoplasm contains complexes of caveolin, chaperone protein, and either cholesterol [22] or cholesteryl esters [23]. SCP-2 binds cholesterol (reviewed in [24]), enhances sterol transfer from plasma membranes in vitro and from plasma membrane to the endoplasmic reticulum of sphingomyelinase-treated (i.e. disrupts caveolae) SCP-2-overexpressing cells, and redistributes cholesterol from the plasma membrane to lipid droplets in living cells (reviewed in [25]). Although L-FABP binds cholesterol more weakly [26] and enhances sterol transfer from plasma membranes in vitro and from plasma membrane to endoplasmic reticulum of sphingomyelinase-treated L-FABP-overexpressing cells (reviewed in [16,27]), the intracellular concentration of L-FABP in liver and enterocytes is two-orders of magnitude greater than that of SCP-2 or caveolin. Up-regulation of L-FABP in liver elicits hypersecretion of cholesterol into bile [18]. Despite these studies, it is not known whether sterol-binding proteins alter cholesterol dynamics directly within isolated caveolae.
To begin to resolve these issues, we now extend our investigation on cholesterol domains in purified plasma membranes (reviewed in [12]) to caveolae isolated by a non-detergent method [28]. We note that the method of isolation of caveolae from purified plasma membrane obtains both caveolar lipid rafts and non-caveolar lipid rafts. This isolate is essentially an enriched caveolae/lipid raft fraction. Implicit to the work of the present study is the understanding that the caveolar isolate is a combination of both caveolar lipid rafts and non-caveolar lipid rafts. Hence, we refer to this isolate as a caveolae/raft domain or a caveolae/raft. The present study was designed to: (i) employ fluorescence polarization/intensity analysis of a series of fluorescent probe molecules to determine whether or not caveolar/raft membranes differ in structure (i.e. membrane fluidity, transbilayer fluidity gradient) from the parent plasma membranes; (ii) investigate whether or not caveolae/raft domains, as plasma membrane lipid subdomains, could mediate the fast transfer of sterol; and (iii) determine whether cholesterol transfer in vitro from isolated caveolae/raft domains is fixed or whether it is sensitive to the presence of a cholesterol transfer protein (SCP-2) to account for overall sterol dynamics from the plasma membrane. Fluorescence polarization and intensity measurements of multiple fluorescent probe molecules {DPH analogues, DiI18 (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), parinaric acids, NBD-stearic acid {12-(N-methyl)-N-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-octadecanoic acid} showed that the acyl chains in caveolae/raft domains were less fluid, and hence more rigid, than those of the parent plasma membranes. Significantly, the transbilayer fluidity gradient was 4.4-fold greater in caveolae/raft domains than that in plasma membranes. Kinetic analysis of the sterol exchange data revealed that, as compared with the plasma membrane: (i) spontaneous sterol transfer from caveolae/raft domains was slightly quicker (faster initial rate), (ii) spontaneous sterol transfer from caveolae/raft domains and from plasma membranes was similarly distributed into two domains, a slowly exchangeable domain [t1/2 (half-time) near 2 h] and a non-exchangeable domain constituting half of total sterol, (iii) SCP-2 much more dramatically enhanced sterol transfer from caveolae/raft domains by decreasing the t1/2 of transfer from the slowly transferable domain nearly 2-fold and by redistributing caveolar/raft domain cholesterol from the non-exchangeable domain.
MATERIALS AND METHODS
Materials
Ergosterol (>99% pure) and cholesterol (>99% pure) were obtained from Steraloids (Wilmington, NH, U.S.A.). EDTA, Trisbase, sucrose, PBS, PMSF and Percoll were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Optiprep was purchased from Accurate Chemical Scientific Corporation (Westbury, NY, U.S.A.). A monoclonal antibody against the α-subunit of Na+,K+-ATPase was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, U.S.A.). Antisera against caveolin-1 were obtained from Affinity Bioreagents (Golden, CO, U.S.A.). DPH, DPH-TMA (DPH-trimethylammonium), DPH-Pro [3(DPH)-propionic acid], cis-parinaric acid (9Z,11E,13E,15Z-octatetradecanoic acid), trans-parinaric acid (9E,11E,13E,15E-octatetradecanoic acid), NBD-stearic acid, DiI1 (1,1′,3,3,3′,3′-hexamethyl-indodicarbocyanine iodide) and DiI18 were all obtained from Molecular Probes (Eugene, OR, U.S.A.). All solutions in which water was used required milliQ deionized water. Protein concentrations were measured using a Bradford protein assay [29] kit available from Bio-Rad Laboratories (Hercules, CA, U.S.A.).
DHE (dehydroergosterol) synthesis
DHE, a fluorescent cholesterol analogue molecule, was synthesized from ergosterol (>99% pure). The synthesized DHE was of a high degree of purity (>99%).
Cell culture
Murine L-cells (L aprt−tk−) acquired from Dr David Chaplin (Washington University, St. Louis, MO, U.S.A.) were cultured for 90 h in 10% FBS (foetal bovine serum)-containing Higuchi medium as described previously [30–33]. For preparation of donor membranes in sterol-exchange assays, the cells were cultured as above for 72 h in 10% FBS-containing medium, before the medium was removed and cells were washed with PBS. Serum-free medium containing DHE (15 μg/ml in serum-free medium) was added, and cells were cultured for an additional 18 h before subcellular fractionation as described previously [30–33]. Acceptor membranes were obtained similarly, except that the cells were cultured in serum-free medium containing cholesterol (15 μg/ml in serum-free medium). This ensured that, although donor and acceptor membranes differed in the type of sterol (DHE compared with cholesterol), the sterol/phospholipid ratio of both donor and acceptor membranes was the same [30–33]. As a result, the assay determined sterol exchange, rather than net transfer down a concentration gradient [30–33]. Cholesterol and DHE were added to the culture medium from stock solutions of cholesterol (10 mg/ml) and DHE (5 mg/ml) prepared in 95% ethanol containing 1 molar percent BHT (butylated hydroxytoluene) and stored at −70 °C. The low level of ethanol (<0.3%) added had no effect on cell growth.
Cellular subfractionation and plasma membrane vesicle isolation
Cells were cultured with DHE or cholesterol, as described in the preceding section, followed by isolation of plasma membrane vesicles exactly as described previously [12]. Plasma membrane purity was monitored as follows: (i) Western blot analysis and probing with antisera against Na+,K+-ATPase basically as described for other membrane markers [30]; (ii) potential contamination by adherent crystalline sterol added to the culture medium was determined as described previously [11]. After measurement of protein concentration, the final purified plasma membranes were divided into 3 μg protein aliquots for donors (i.e. containing DHE) and 30 μg protein quantities for acceptors (i.e. containing cholesterol). The aliquots were quick-frozen for long-term storage (>2 weeks).
Isolation of caveolae/raft domains from purified plasma membrane vesicles
After cells were cultured with DHE or cholesterol as described in the preceding section, caveolae/raft domains were isolated using the detergent-free isolation method of Smart et al. [28], modified as described previously [11]. Briefly, after 90 h of total culture, the medium was removed from the L-cell fibroblasts, and the cells were rinsed four times with 25 ml of PBS each time. The cells were then harvested in a PMSF/PBS solution, centrifuged for 5 min at 1000 g with a JA25.5 fixed-angle rotor on an Avanti J25 Centrifuge (Beckman, Fullerton, CA, U.S.A.), resuspended in a known volume of PMSF/PBS, and re-sedimented at 1000 g for 5 min again, and resuspended in a known quantity of buffer labelled as buffer A, which contained 500 ml of 2.5 M sucrose, 2 mM EDTA, 40 mM Tris base, diluted further with water at a ratio of 1:1 (v/v), resulting in a total pH of about 7.4. This cell suspension was disrupted with a N2 Bomb Cell Disrupter (Parr Instrument Co., Moline, IL, U.S.A.) set at approx. 40 p.s.i. (1 p.s.i.=6.9 kPa) of N2 for approx. 13 min. The post-nuclear supernatant was in turn collected following centrifugation at 1000 g for 10 min with a JA25.5 fixed-angle rotor on an Avanti J25 Centrifuge. This supernatant was loaded on to a sucrose gradient comprising 35% and 20% (w/v) sucrose, and then in turn was centrifuged at 39000 rev./min for 100 min using an SW40 Ti rotor and Model UL60 Ultracentrifuge (Beckman). The band containing plasma membrane vesicles was collected at the 20%/35% interface and placed in a tube filled to the top with cold buffer A, and then centrifuged for 90 min at 39000 rev./min with a SW40 Ti rotor and Model UL60 Ultracentrifuge. The resulting plasma membrane vesicle pellet was resuspended in a known quantity of cold buffer A, sonicated in a model 550 Sonic Dismembrator (Fisher Scientific, Pittsburgh, PA, U.S.A.) at 15 s pulses with 1 min intervals, with a total of six pulses, to give a total of 90 s of pulsing. After determination of the sample volume, a 50% (v/v) Optiprep solution was added to make a resultant 23% Optiprep solution. Then 3.5 ml of 10% and 20% Optiprep solutions were combined by 50:50 linear mixing and added on top of the 23% Optiprep sample mixture, followed by centrifugation at 17500 rev./min at 4 °C for 88 min with a SW40 Ti rotor and Model UL60 Ultracentrifuge. The top 5 ml (called the fractionate) was collected from along the wall of the tube and transferred to a new tube. More 50% Optiprep solution was added to the sample to give approximately a 25% Optiprep layer at the bottom of the tube. Approx. 1 ml of 5% Optiprep was placed on top of the 25% layer, and the tube was centrifuged at 17500 rev./min at 4 °C for 88 min with a SW40 Ti rotor and Model UL60 Ultracentrifuge. The result was an opaque band located just above the 5%/25% Optiprep interface. This band (enriched in caveolae/raft domains) was collected in as little volume as possible. After measurement of protein concentration, the final purified caveolae/raft domains were divided into 3 μg protein aliquots for donors (i.e. containing DHE) and 30 μg protein aliquots for acceptors (i.e. containing cholesterol). The aliquots were quick-frozen for long-term storage (>2 weeks). The purity of the caveolae/raft domains was determined by three methods: (i) Western blot analysis and probing with antisera against caveolin-1 as described for other membrane markers [30]; (ii) in the case of caveolae/rafts isolated from cells cultured with DHE, the DHE fluorescence/mg of protein was determined by excitation at 324 nm and monitoring DHE emission intensity at 375 nm with a PC1 photon-counting spectrofluorimeter (ISS Instruments, Champaign, IL, U.S.A.); this intensity was compared with that of plasma membranes isolated from cells supplemented with DHE; (iii) potential contamination by adherent crystalline sterol from the culture medium was determined as described previously [11].
Incorporation of fluorescent probes to measure membrane fluidity with steady-state fluorescence polarization in caveolae/raft domains and plasma membranes isolated from L-cell fibroblasts
The steady-state fluorescence polarization and fluorescence emission intensity of DHE and other fluorescent probe molecules were determined using a PC1 spectrofluorimeter with photon-counting electronics. The instrument was configured in the T-format with the emission monochromator set at 375 nm, with 16 nm spectral slit widths. A 300 W xenon arc lamp was the source for excitation with a monochromator (16 nm spectral slit width) set at 324 nm. The samples were stirred continuously, and light intensity was minimized through the use of neutral-density filters in the excitation light path and narrow (0.5 mm) entrance and exit slits on the excitation monochromator. This was done in order to ensure smooth data sampling and reduced photobleaching. In order to account for artifacts due to light scatter, these were minimized by monitoring the emission through low-fluorescence cut-off filters (Schott Glass Technologies, Duryea, PA, U.S.A.). DHE was excited at 324 nm and emission was monitored through KV-389 filters (Schott Glass Technologies). DPH, DPH-TMA and DPH-Pro were excited at 350 nm, 355 nm and 354 nm respectively, and emission was monitored through GG 435, GG 400 and GG 400 longpass filters respectively (Edmund Industrial Optics, Barrington, NJ, U.S.A.). cis- and trans-parinaric acids were excited at 303 nm and 290 nm respectively, and emission was monitored through GG 385 longpass filters (Edmund Industrial Optics). NBD-stearic acid was excited at 467 nm, and emission was monitored through OG 530 longpass filters (Edmund Industrial Optics). DiI18 and DiI1 were excited at 550 and 639 nm respectively, and emission was monitored through OG 570 and RG 665 longpass filters (Edmund Industrial Optics). Any remaining residual light-scatter contribution to polarization data with caveolae/raft domains or plasma membranes in the case of DHE exchange (from both donor and acceptor membranes) was corrected by converting polarization into anisotropy according to the relation r=2P/(3−P), and subtracting the residual fluorescence anisotropy of both donor and acceptor membranes from all experimental data. Absorbance of sample solutions at the excitation wavelengths were kept below 0.15 in order to avoid artifacts due to inner filter effects. Samples were resuspended in filtered (0.2 μm pore-size) 10 mM Pipes buffer solution at pH 7.4, and 2 ml samples in quartz cuvettes were thermostatically controlled at 37±0.3 °C through the use of a water heating bath (Fisher Scientific, Pittsburgh, PA, U.S.A.).
Measurement of membrane sterol transfer
Sterol transfer between the isolated organelles was determined by using a fluorescent sterol (DHE) exchange assay previously described by our laboratory for determination of sterol transfer between plasma membranes [12]. DHE is used as a probe for cholesterol transfer (reviewed in [25,34–36]) because it: (i) is a close structural analogue of cholesterol, (ii) exhibits the same exchange kinetics as cholesterol, (iii) is a naturally occurring fluorescent sterol, (iv) is taken up by cultured L-cells, such that >80% of endogenous sterol is replaced by DHE without altering membrane lipid composition or sterol-sensitive enzymes, (v) is non-toxic to cultured cells or animals, and (vi) co-distributes with cholesterol in model and biological membranes. The underlying premise of the DHE-exchange assay is that fluorescence self-quenching of DHE occurs in the donor membrane, which contains high levels of DHE, and thus results in low DHE fluorescence polarization values. However, on addition of 10-fold excess acceptor membranes, which contain cholesterol and no DHE, the donor membrane DHE exchanges one-for-one with acceptor membrane cholesterol, thereby resulting in release from self-quenching of DHE, thus resulting in an increase in DHE polarization.
In all sterol exchanges, DHE fluorescence polarization of the donor sample was measured for 10 min in 2 ml of 10 mM Pipes buffer to ensure a stable signal baseline and to obtain an initial value for the fluorescence polarization. This was followed by the addition of a 10-fold excess of acceptor (i.e. no DHE) membrane. The protein concentration of the donor plasma membrane in the 2 ml of 10 mM Pipes buffer sample was 7 μg/ml, whereas the protein concentration of the acceptor plasma membrane in the 2 ml sample was 70 μg/ml. For the caveolae/raft domains, the requisite protein concentration of the donor caveolae/raft domains in the 2 ml sample was 1.5 μg/ml, whereas the protein concentration of the acceptor caveolae/raft domains in the 2 ml sample was 15 μg/ml. This was because the donor sample isolations were both dilute and contained a small amount of protein. The DHE polarization was subsequently recorded at 20 s intervals for either 3 or 4 h to monitor sterol transfer between membranes.
Standard curves for the sterol-exchange assay
The standard curves used for the current investigation are those derived for plasma membranes in L-cell fibroblasts in previous studies [12]. Although caveolae/raft domains are relatively lipid-rich and cholesterol-rich compared with the plasma membrane, the ratio of sterol/phospholipid in caveolae/raft domains isolated by detergent-free methods [7,9,11] is basically similar to that of plasma membranes [12]. Since the standard curve is determined primarily by the sterol/phospholipid ratio, the caveolae/raft domain–caveolae/raft domain sterol exchange followed the same standard curve as the plasma membrane. The standard curve that calculates the fraction of DHE remaining in the donor membranes during an exchange is a polynomial equation involving polarization, P, of the exchange of the form:
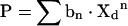 |
(1) |
where Xd is the mole fraction of DHE left in the donor.
For sterol exchange between plasma membrane donors and plasma membrane acceptors, a polynomial with two terms yielded a fit with r2=0.9999, i.e. eqn (2):
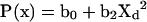 |
(2) |
where b0=0.3155 and b2=−0.131.
Calculation of the initial rate of sterol transfer
The initial rate of DHE exchange between plasma membranes was estimated from the first 10 min of exchange data by using the standard curve described above in eqn (2) [31]. In essence, eqn (2) is the definitive relation which describes the exchange between donor and acceptor membranes. Taking the time derivative of eqn (2) yields:
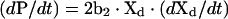 |
(3) |
As t→0, Xd→1 (i.e. initial rate criteria) and rearranging eqn (3), then the following expression is obtained:
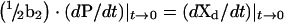 |
(4) |
To obtain the molar transfer rate of DHE (d[DHE]/dt) from plasma membrane donors to plasma membrane acceptors, dXd/dt was transformed into d[DHE]/dt by factoring in the initial plasma membrane donor protein concentration (7 μg of protein/ml), the total sterol/protein concentration in the plasma membrane donors (1011.02 pmol of total sterol/μg of protein), the molar percentage of DHE in the donor membranes (8%), and the value of b2 (−0.131).
Combining this information with eqn (4) yielded eqn (5):
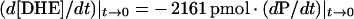 |
(5) |
The initial rate of DHE transfer was estimated directly by substituting the initial measured rate of fluorescence polarization change per unit time (i.e. minutes) for (dP/dt)|t→0.
Similarly, for DHE transfer from caveolae/raft domain donors to caveolae/raft domain acceptors, dXd/dt was transformed into d[DHE]/dt by factoring in the initial donor caveolae/raft domain protein concentration (1.5 μg of protein/ml), the total sterol/protein concentration in the caveolar/raft domain membranes, the molar percentage of DHE in the donor caveolae/raft domains, and the value of b2. Combining this information with eqn (4) yielded eqn (6):
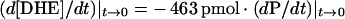 |
(6) |
Calculation of the kinetic parameters of sterol exchange
The kinetic parameters of exchange between plasma membrane donor/acceptor pairs and between caveolae/raft domain donor/acceptor pairs were determined by use of the standard-curve equation, i.e. eqn (2), and the equation for a one-exponential exchange:
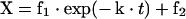 |
(7) |
where f1 and f2 are the exchangeable and non-exchangeable fractions respectively of the sterol in the exchange assay, and k is the rate constant of the exchange. The expression for X in eqn (7) was substituted into eqn (2) to obtain the expression describing the exchange:
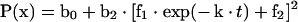 |
(8) |
The exchange curves were fitted to eqn (8) with r2 values varying from 0.97 to 0.99. The t1/2 of the exchanges was defined by the following equation:
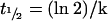 |
(9) |
Data and statistical analyses
All curve-fitting and data analyses in the present study were performed by use of SigmaPlot (Jandel Scientific, San Rafael, CA, U.S.A.) scientific data analysis and graphing software.
RESULTS
Purification of caveolae/raft domains from L-cell fibroblast plasma membranes
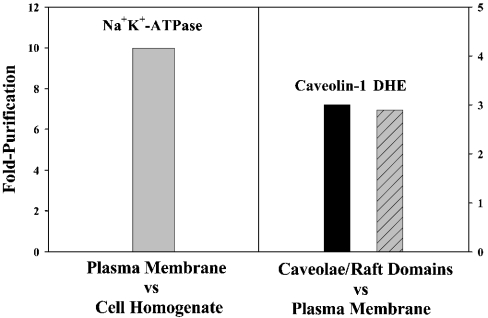
Consistent with earlier findings [11,12], Western blotting of purified L-cell plasma membranes indicated that the plasma membrane fraction was enriched approx. 10-fold in Na+K+-ATPase as compared with the cell homogenate (Figure 1). Potential contamination of plasma membranes with adherent microcrystalline sterol (cholesterol or DHE), which had been added to the culture medium, was quantified from emission spectra of DHE in the purified plasma membranes. Monomeric and crystalline DHE emit maximally at 373 and 426 nm respectively [11]. In the purified plasma-membrane fraction, essentially all of the DHE was monomeric, indicating the absence of adherent microcrystalline sterol. Western blotting of caveolae/rafts isolated from purified L-cell plasma membranes showed that caveolae/raft domains were enriched approx. 3-fold in caveolin-1 as compared with the plasma membrane (Figure 1). Likewise, measurement of the DHE fluorescence emission intensity/μg of protein revealed that caveolae/raft domains were enriched 2.9-fold in DHE as compared with the plasma membrane (Figure 1). These approx. 3-fold enrichments in caveolin-1 and sterol in the purified caveolae/raft domains as compared with the plasma membrane were similar to those reported for caveolin-1 and cholesterol in caveolae/raft domains isolated by this non-detergent method from other cells and tissues [7,9]. Examination of DHE emission spectral characteristics in caveolae/raft domains as described in the Materials and methods section showed that sterol in purified caveolae/raft domains was >99% monomeric, again indicating very little contamination with adherent microcrystalline sterol.
Figure 1. Fold-purification of plasma membranes and caveolae/raft domains.
Left-hand panel: plasma membranes were isolated and the purity was compared with that of cell homogenates by analysis of Western blots with anti-Na+,K+-ATPase as described in the Materials and methods section. Right-hand panel: caveolae/raft domains were isolated from the plasma membranes without the use of detergents as described in the Materials and methods section. The purity of caveolae/rafts was compared with that of plasma membranes by analysis of Western blots with anti-caveolin-1 or measurement of DHE/mg of protein as described in the Materials and methods section.
Fluorescence polarization of probe molecules that preferentially localize in caveolae/raft domains: DHE and DiI18
As indicated in the preceding section, DHE was enriched 3-fold in caveolae/raft domains as compared with plasma membranes. Under non-self-quenching conditions, the polarization of DHE in caveolae/raft domains was 0.3255±0.0022, significantly (P<0.01) lower than that of DHE in plasma membranes, 0.3391±0.0016 (Table 1). Thus sterols appeared to be less highly ordered in caveolae/raft domains than in the plasma membrane. Since long-chain-length DiI18 preferentially partitions into lipid rafts [8,37], the polarization of this probe incorporated into caveolae/raft domains and plasma membranes was determined. The fluorescence intensity, as well as the polarization of DiI18, were significantly greater in caveolae/raft domains than in plasma membranes (Table 1); this suggested that caveolae/raft domains contained more rigid lipid phases.
Table 1. Structural parameters of lipidic probes that preferentially distribute in caveolae/raft domains: DHE and DiI18.
DHE, DiI1, and DiI18 were incorporated at low, non-self-quenching levels into purified plasma membranes and caveolae/raft domains as described in the Materials and methods section. Relative fluorescence intensity and polarization values are means±S.D. (n=7).
| Membrane | Relative fluorescence intensity | Polarization |
|---|---|---|
| DHE | ||
| Caveolae/rafts | 95.0±2.3 | 0.3255±0.0022* |
| Plasma membranes | 100.0±3.1 | 0.3391±0.0016 |
| DiI18 | ||
| Caveolae/rafts | 100.0±0.4*† | 0.3871±0.0031*† |
| Plasma membranes | 84.4±0.6† | 0.3684±0.0027† |
| DiI1 | ||
| Caveolae/rafts | 84.5±0.4* | 0.3482±0.0013* |
| Plasma membranes | 68.6±0.5 | 0.3304±0.0024 |
* P<0.01 compared with plasma membrane.
† P<0.01 compared with DiI1.
Fluorescence polarization and emission intensity probe molecules that preferentially distribute in ‘solid/gel’ or fluid/liquid-crystalline lipid phases: DiI18 and parinaric acids
Long-chain-length DiI18 [38], as well as other C18 straight-chain probes (e.g. trans-parinaric acid or NBD-stearic acid) [39,40], preferentially partition into solid/gel phases in the lateral plane of the lipid bilayer. These probes were incorporated into caveolae/raft domains and plasma membranes. Both fluorescence intensity and polarization of DiI18 (Table 1), trans-parinaric acid (Table 2) and NBD-stearic acid were significantly greater in caveolae/raft domains than in plasma membranes. This suggests the presence of rigid lipid phase in caveolae/raft domains as well as plasma membranes, and furthermore indicates that caveolae/raft domains may be enriched in more rigid, gel-phase lipid. In contrast, short-chain-length DiI1 [38] and cis-parinaric acid [40] do not exhibit a preference for solid/gel phases. When these probes were incorporated into caveolae/raft domains and plasma membranes, both fluorescence intensity as well as the polarization of DiI1 (Table 1) and cis-parinaric acid (Table 2) were significantly greater in caveolae/raft domains than in plasma membranes.
Table 2. Lateral structure of caveolae/raft domains and plasma membrane: selectively probing ‘solid’ versus ‘fluid’ domains with trans- and cis-fatty acids.
trans-Parinaric acid, cis-parinaric acid and NBD-stearic acid were incorporated at low, non-self-quenching levels into purified plasma membranes and caveolae/raft domains as described in the Materials and methods section. Relative fluorescence intensity and polarization values are means±S.D. (n=7).
| Membrane | Relative fluorescence intensity | Polarization |
|---|---|---|
| trans-Parinaric acid | ||
| Caveolae/rafts | 76.5±1.1*† | 0.3183±0.0009*† |
| Plasma membranes | 73.6±0.8† | 0.3071±0.0020† |
| cis-Parinaric acid | ||
| Caveolae/rafts | 72.1±1.0* | 0.3478±0.0021* |
| Plasma membranes | 69.2±0.8 | 0.3386±0.0017 |
| NBD-stearic acid | ||
| Caveolae/rafts | 100.0±0.7* | 0.3299±0.0018* |
| Plasma membranes | 95.7±0.8 | 0.3190±0.0010 |
* P<0.05 compared with plasma membrane.
† P<0.05 compared with cis-parinaric acid.
Fluorescence polarization and emission intensity of a probe molecule that preferentially distributes in ‘intermediate liquid-ordered’ lipid phases: DPH
The fact that the fluorescence intensity and polarization of both gel-phase-preferring (i.e. DiI18 and trans-parinaric acid) and non-gel-phase-preferring (DiI1 and cis-parinaric acid) probes were higher in caveolae/raft domains than in plasma membranes (Tables 1 and 2) suggested that: (i) both gel and liquid-crystalline phases were more rigid in caveolae/raft domains; and/or (ii) both types of probes sensed an intermediate lipid phase enriched in caveolae/raft domains as compared with plasma membranes. The latter possibility was examined further through the use of DPH. Since DPH has no preference for coexisting gel compared with fluid liquid-crystalline phases [41], the fluorescence polarization of DPH has been used to show that acyl chains in caveolae isolated by the use of detergents (DRMs) are organized in the liquid-ordered phase [10]. To determine whether or not this was also true for caveolae/raft domains isolated without the use of detergents, DPH polarization was measured in plasma membranes and caveolae/raft domains. Both types of membranes were incubated with DPH (at a DPH/membrane protein ratio of 1:1000) at a temperature of 37 °C for 30 min to maximally incorporate DPH as described in the Materials and methods section. Three lines of evidence suggested that the acyl chain fluidity in caveolae/raft domains was less than that in plasma membranes: (i) DPH polarization in caveolae/raft domains was 0.3085±0.0008, significantly higher than in plasma membranes (0.2689±0.0009; P<0.01) (Table 3); (ii) maximal DPH fluorescence emission intensity was higher in caveolae/raft domains than in plasma membranes, i.e. (1.14±0.13)×107 compared with (7.61±0.24)×106 (Table 3); and (iii) excitation maxima of DPH in caveolae/raft domains were near 372 nm, slightly red-shifted as compared with those of DPH in the plasma membrane near 362 nm (results not shown). These findings were consistent with DPH sensing a ‘liquid-ordered domain’ enriched in caveolae/raft domains similar to that in DRMs reported previously [10].
Table 3. Transbilayer structure of caveolae/raft domains and plasma membranes: DPH probes (DPH, TMA-DPH and DPH-Pro).
DPH, TMA-DPH, and DPH-Pro were incorporated at low, non-self-quenching levels into purified plasma membranes and caveolae/rafts as described in the Materials and methods section. Relative fluorescence intensity and polarization values are means±S.D. (n=7).
| Membrane | Relative fluorescence intensity | Polarization |
|---|---|---|
| DPH | ||
| Caveolae/rafts | 95.2±2.7* | 0.3085±0.0008*† |
| Plasma membranes | 87.7±1.9 | 0.2689±0.0009 |
| DPH-TMA | ||
| Caveolae/rafts | 98.5±2.8* | 0.2577±0.0.0007*‡ |
| Plasma membranes | 87.9±1.8 | 0.2658±0.0025 |
| DPH-Pro | ||
| Caveolae/rafts | 100.0±1.9* | 0.2896±0.0032* |
| Plasma membranes | 88.2±2.5 | 0.2731±0.0011 |
* P<0.01 compared with plasma membrane.
† P<0.01 compared with DPH-TMA.
‡ P<0.01 compared with DPH-Pro.
Fluorescence polarization of probe molecules that preferentially distribute into outer and inner leaflets of the membrane: DPH-TMA and DPH-Pro
Studies with L-cell plasma membranes show that DPH polarization is lower in the outer (exofacial) leaflet than in the inner (cytofacial) leaflet, suggesting that the outer leaflet acyl chains are more fluid than those of the inner leaflet (reviewed in [42,43]). Based on its positive charge, DPH-TMA appears to selectively localize in the outer leaflet (enriched in positively charged lipids, but essentially devoid of negatively charged lipids), while the negatively charged DPH-Pro appears to localize in the plasma membrane inner leaflet (which contains most negatively charged lipids) [43,44]. Consistent with this pattern, the DPH-TMA polarization was significantly less (P<0.01) than that of DPH-Pro in L-cell plasma membranes (Table 3). Furthermore, the polarizations of DPH-TMA and DPH-Pro in plasma membranes were the same or only slightly larger than those of DPH (Table 3). This indicated that the acyl chain order sensed by the DPH attached to charged surface-anchors (DPH-TMA and DPH-Pro) was only slightly less mobile or similar to non-anchored DPH, which partitions more near the centre of the bilayer of the plasma membrane.
In contrast, properties of the DPH probes in caveolae differed significantly from those in plasma membranes. In caveolae/raft domains, DPH-TMA polarization was lower (P<0.01) than that of DPH-Pro, but the difference (i.e. 0.0319) was 4.4-fold larger than that observed with plasma membranes (Table 3). This was because the polarization of DPH-TMA in caveolae/raft domains was 0.0081 polarization unit smaller than that in plasma membranes, while that of DPH-Pro was 0.0165 polarization unit larger than that in plasma membranes. Taken together, these data suggest that, as for plasma membranes, the inner leaflet lipids of caveolae/raft domains were more ordered than those of the outer leaflet. However, this fluidity gradient across the bilayer was much greater in caveolae/raft domains as compared with the plasma membrane. The data suggested further that this greater difference was due to caveolae/raft domains having a more fluid outer leaflet and a more rigid inner leaflet.
Spontaneous sterol transfer between plasma membranes
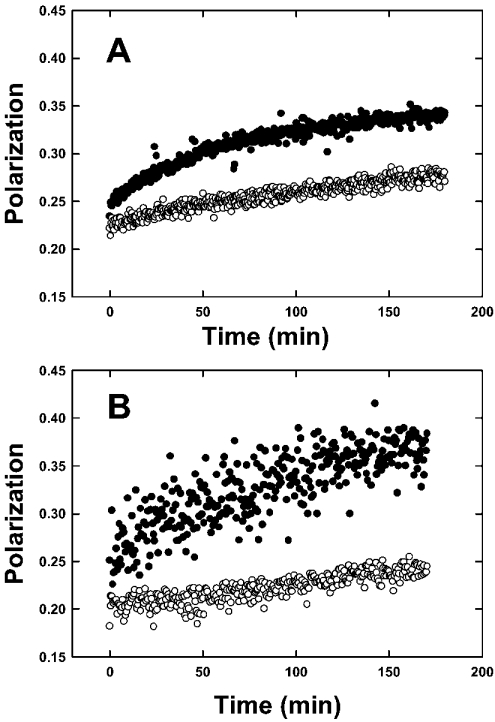
To determine the spontaneous transfer of sterol from plasma membranes, L-cells were cultured in the presence of cholesterol or DHE followed by isolation of donor plasma membranes (which contain DHE) and acceptor plasma membranes (which contain cholesterol, but not DHE) as described in the Materials and methods section. The initial polarization of DHE in the donor plasma membranes was 0.2218±0.0092, consistent with self-quenching due to the presence of high levels of DHE (Figure 2A, open circles). In the absence of acceptor plasma membrane (which contain only cholesterol), DHE polarization in plasma membrane donors did not significantly change over several hours (results not shown). In contrast, the addition of a 10-fold excess of acceptor plasma membranes resulted in a slow, spontaneous sterol exchange between DHE-containing plasma membrane donors and cholesterol-containing plasma membrane acceptors, as indicated by the increase in DHE polarization (Figure 2A, open circles). The initial rate of spontaneous molecular sterol transfer between plasma membranes was 0.702±0.059 pmol/min (Table 4). Kinetic analysis of spontaneous sterol-exchange curves as described in the Materials and methods section indicated that the data best fit two components. A rapidly transferable component, with a t1/2 of 147±11 min, represented an exchangeable fraction of 0.482±0.026 (Table 4). A second very slowly exchangeable component (a t1/2 of several days) represented a non-exchangeable fraction of 0.518±0.019 (Table 4). Thus spontaneous sterol transfer from plasma membranes is relatively slow, with a t1/2>2 h, and does not require the assistance of a sterol-transfer protein. However, over half of the total plasma membrane sterol was localized in an essentially non-exchangeable domain in the absence of a mediating protein.
Figure 2. Sterol exchange between membranes.
(A) Effects of SCP-2 on sterol transfer from plasma membranes. DHE exchange between plasma membrane donors and plasma membrane acceptors was measured by monitoring polarization as described in the Materials and methods section. Open circles show the spontaneous sterol transfer from donor plasma membranes after the addition of a 10-fold excess of acceptor plasma membranes. Closed circles show the effects of 1.5 μM SCP-2 on the sterol transfer from donor plasma membranes to a 10-fold excess of acceptor plasma membranes. (B) Effects of SCP-2 on sterol transfer from caveolae/raft domains. DHE exchange was measured as in (A), except that caveolar/raft domain donor and caveolar/raft domain acceptor membranes were used as described in the Materials and methods section. Open circles show the spontaneous sterol transfer from donor caveolae after addition of a 10-fold excess of acceptor caveolae/raft domains. Closed circles show the effect of 1.5 μM SCP-2 on the sterol transfer from donor caveolae/rafts to a 10-fold excess of acceptor caveolae/rafts.
Table 4. Initial rates and kinetic multiexponential analysis of molecular sterol exchange: effect of SCP-2.
Fluorescence-polarization-exchange curves for DHE sterol transfer from plasma membrane donors to plasma membrane acceptors (PM–PM) as well as DHE transfer from caveolae/raft domain donors to caveolae/raft domain acceptors (CAV–CAV) were measured in the absence or presence of SCP-2 (1.5 μM) followed by determination of initial rates and kinetic analysis as described in the Materials and methods section. Values are means±S.D. (n=3–4). f1 and f2 represent the fractions due to the exchangeable and non-exchangeable components respectively. Values are means±S.D. (n=3–4).
| Donor–acceptor | Protein added | Initial rate (pmol/min) | t1/2 (min) | f1 | f2 |
|---|---|---|---|---|---|
| PM–PM | None | 0.702±0.059 | 147±11 | 0.482±0.026 | 0.518±0.029 |
| SCP-2 | 4.03±0.52 | 102±4* | 0.563±0.019* | 0.437±0.023 | |
| CAV–CAV | None | 1.10±0.32 | 111±15 | 0.490±0.031 | 0.510±0.038 |
| SCP-2 | 5.06±0.61 | 61±17*† | 0.787±0.022*† | 0.213±0.027*† |
* P<0.05 compared with no protein added.
† P<0.05 compared with PM.
Probing transfer of sterol from plasma membranes by SCP-2
SCP-2 is a ubiquitous protein present in all mammalian tissues examined and, with erythrocytes being a rare exception [45], enhances sterol transfer between almost all membranes examined to date. Hence, SCP-2 was used to probe the cholesterol dynamics between plasma membranes. SCP-2 itself did not change the fluorescence polarization of DHE when added to the donor membrane. However, in the presence of a 10-fold excess of acceptor plasma membranes, SCP-2 induced rapid sterol transfer, as indicated by dramatic increase in DHE polarization (Figure 2A, closed circles). SCP-2-mediated sterol exchange between plasma membranes exhibited an initial rate of 4.03±0.52 pmol/min (Table 4). Thus SCP-2 increased the initial rate of molecular sterol transfer from plasma membrane donors almost 6-fold over the spontaneous sterol transfer (Table 4). Kinetic analysis of SCP-2-mediated sterol-transfer curves from plasma membranes indicated that SCP-2 did not alter the number of sterol domains, but decreased the t1/2 of the exchangeable sterol pool by 30% from 147±11 to 102±4 (P<0.05) (Table 4). Furthermore, SCP-2 increased the size of the exchangeable sterol pool by 17% from 0.482±0.026 to 0.563±0.019 (P<0.05) (Table 4).
In summary, SCP-2 significantly increased sterol transfer between plasma membrane donor and acceptor in vitro by enhancing both the kinetics (initial rate and t1/2) and size of the exchangeable sterol domain.
Spontaneous transfer of sterol between caveolae/raft domains
To determine the spontaneous sterol dynamics in caveolae/raft domains, L-cells were cultured in the presence of cholesterol or DHE, plasma membranes were isolated and donor caveolae/raft domains (which contain DHE) and acceptor caveolae/raft domains (which contain cholesterol) were obtained as described in the Materials and methods section. The initial polarization of DHE in the donor caveolae/raft domains was 0.2068±0.0084 (Figure 2B, open circles), not significantly different from that in donor plasma membranes (0.2218±0.0092; Figure 2A, open circles). Thus DHE was self-quenched to essentially the same extent in donor caveolae/raft domains as in the donor plasma membranes. In the absence of acceptor caveolae/raft domains (which contain only cholesterol), DHE polarization in caveolae/raft domain donors was stable and did not significantly change over several hours (results not shown).
Upon the addition of a 10-fold excess of acceptor caveolae/raft domains, DHE spontaneously transferred from the donor to acceptor caveolae/raft domains as indicated by increased polarization (Figure 2B, open circles). The initial rate of spontaneous molecular sterol transfer from caveolae/raft domain donors was 1.10±0.32 pmol/min, 57% faster (P<0.05) as compared with spontaneous molecular sterol transfer from plasma membrane donors (Table 4). Kinetic analysis, as described in the Materials and methods section, showed that the exchange curves for spontaneous molecular sterol transfer best fit two components: an exchangeable sterol pool exhibited a t1/2 of 111±15 min and represented 0.490±0.031 of total sterol (Table 4). Neither the t1/2 nor fractional contribution of the exchangeable pool in caveolae/raft domains differed from those in the plasma membrane (Table 4). In addition, caveolae/raft domains contained a very slowly (t1/2 of days), essentially non-exchangeable sterol pool representing 0.510±0.038 of total caveolar/raft domain sterol. Again, the size of this non-exchangeable pool did not differ from that of the plasma membrane (Table 4). Thus spontaneous sterol dynamics of caveolae/rafts resolved two sterol domains whose properties were similar to those in plasma membranes. The spontaneous exchangeable sterol pool in plasma membranes was relatively slow (t1/2 of hours) and could not be ascribed to being due exclusively to a rapidly exchangeable sterol domain in caveolae/rafts. Caveolae/rafts also contain both exchangeable and non-exchangeable spontaneously transferable sterol domains.
Probing transfer of sterol from caveolae/raft domains by SCP-2
When sterol transfer from donor to acceptor caveolae/raft domains was probed with SCP-2, the DHE polarization increased markedly (Figure 2B, solid circles), qualitatively similar to that observed with SCP-2 and plasma membranes (Figure 2A, solid circles). SCP-2 enhanced the initial rate of molecular sterol transfer from caveolae/raft domains nearly 5-fold from 1.10±0.32 to 5.06±0.61 pmol/min, P<0.01 (Table 4). Again, this enhancement was nearly the same as that from the plasma membrane (Table 4). Kinetic analysis of the SCP-2-mediated sterol-exchange curves showed that SCP-2 enhanced the sterol transfer from caveolae/raft domains by decreasing the t1/2 of exchange by nearly 50% from 111±15 to 61±17 min, P<0.05, and by increasing the fraction of exchangeable sterol by 61% from 0.490±0.031 to 0.787±0.022, P<0.05 (Table 4). Thus SCP-2 much more dramatically altered the sterol dynamics of caveolae/raft domains than plasma membranes. This suggested that SCP-2 may selectively enhance sterol trafficking through caveolae/raft domains as compared with the rest of the plasma membrane.
DISCUSSION
Almost nothing is known regarding the structure or cholesterol dynamics of caveolae/raft domains. To begin to address this problem, the method of Smart et al. [28] was adapted to isolate caveolae/raft domains without the use of detergents that may disrupt the functional properties of the caveolae/rafts. It is important to emphasize that this technique of caveolae isolation does not distinguish caveolar lipid rafts from non-caveolar lipid rafts. Two types of markers were used to determine the relative fold-purification of caveolae/rafts from L-cell plasma membranes: protein (i.e. caveolin-1) and lipid (i.e. sterol).
Caveolae/raft domains isolated from L-cell fibroblasts were purified 3–4-fold in caveolin-1 as compared with plasma membranes in the same range purification reported by others using protein markers (caveolin-1, flotillin, epithelial NO synthase) and the same isolation technique [7,9,46]. These studies suggested that caveolae/raft domains represented approx. 20–30% of the L-cell fibroblast plasma membrane. We note that in the original work by Smart et al. [28], caveolin-1 enrichment was first described to be 2200 times that of the plasma membrane. While this suggested that caveolae/rafts represent only a very minor component of the total plasma membrane, most recent reports indicate otherwise (reviewed in [7–9,46,47]). Furthermore, Smart et al. [28] also noted that their caveolin-1 enrichment was anomalously high. By using immunoblotting, they found that the intensity of the glycosylphosphatidylinositol-anchored folate receptor and epidermal growth factor receptor bands in the caveolae/raft domain fraction were enriched 42-fold compared with the plasma membranes, demonstrating wide variation. Although the molecular basis for this high degree of variability in fold-purification of caveolae/raft domain markers is not yet completely clear, we note that commonly used caveolae/lipid raft markers (e.g. caveolin-1, flotillin and epithelial NO synthase) occur not only in the plasma membrane caveolae/lipid rafts, but also in non-caveolar lipid rafts (e.g. flotillin) and in subcellular locations, including the trans-Golgi network, caveolar vesicles and caveolin-1–chaperone complexes [20]. Therefore the fold-purification of protein markers in caveolae/raft domains isolated by the method of Smart et al. [28] may be highly sensitive to: (i) the relative amount of caveolar lipid rafts and non-caveolar lipid rafts; (ii) the distribution of caveolin-1 within the plasma membrane; and (iii) the distribution of caveolin-1 between the plasma membrane caveolae/lipid rafts and intracellular cytoplasmic vesicles, chaperone complexes and the trans-Golgi network.
In contrast with fold-purification obtained using protein markers, lipid markers (i.e. sterol) indicated that caveolae/raft domains isolated from L-cell fibroblasts were purified 3-fold as compared with plasma membranes. Very recently, Eckert et al. [7], using the non-detergent method of caveolae/raft domain isolation, obtained 3- and 2-fold enrichment in cholesterol and total phospholipid respectively. Similarly, also using the method of Smart et al. [28] for isolating caveolae/raft domains, Pike et al. [9] found that in KBC cells (KB cells which express caveolin-1), cholesterol was enriched 4.8-fold compared with the plasma membrane, while sphingomyelin, total phospholipids and total lipids were enriched 2.5-fold, 1.7-fold and 2.2-fold respectively. The same four entities were found to be enriched 4.4-fold, 2.9-fold, 2-fold and 2.3-fold in lipid rafts compared with plasma membranes of KB cells (which do not express caveolin-1). These data: (i) suggest that the fold-enrichment of lipid markers were essentially the same in caveolae/lipid rafts and non-caveolar lipid rafts, and (ii) imply that caveolae/raft domains represent a larger fraction of plasma membrane than based on protein markers such as caveolin-1, the distribution of which to intracellular sites may significantly affect the apparent fold-purification. In summary, the fold-purification of caveolae/raft domains obtained from L-cell plasma membranes by the Smart et al. [28] non-detergent method was in the same range as that obtained by many other investigators using different cell types. The proportion of the plasma membrane represented by caveolae/raft domains in L-cells represented 25–33% of the plasma membrane: in the range of that reported for a variety of other cell types (5–33%) [2,7,9,48,49].
In view of the paucity of information regarding lipid structure (transbilayer and lateral), cholesterol dynamics and regulation of cholesterol dynamics in caveolae [8,50], the present study was undertaken to begin to address issues in caveolae isolated without the use of detergents.
First, the acyl chain fluidity of caveolae/raft domains purified without the use of detergents was significantly lower (i.e. more rigid) than that of the plasma membrane. Interestingly, the fluorescence polarization of DPH in detergent-free caveolae/raft domains noted in the present paper was consistent with the acyl chain fluidity of caveolae being in that of the liquid-ordered phase [10]. DPH polarization in the detergent-free caveolae/raft domains was 0.3085±0.0008 (Table 3), very similar to that of DPH in the liquid-ordered phase of model membranes (0.281) [10]. In contrast, DPH polarization in model membranes in the non-fluid gel phase and in the liquid-crystalline phase was 0.381 and 0.095 respectively [10]. Significantly, the DPH polarization in the detergent-free caveolae/raft domains, 0.3085±0.0008 (Table 3), was very similar to that of DPH in DRMs isolated from model membranes [10]. Finally, the data with leaflet-selective DPH-TMA and DPH-Pro probes indicated that the caveolae/raft domains exhibited a 4.4-fold greater transbilayer fluidity difference as compared with the plasma membrane. Transbilayer fluidity gradients are known to be important in the function of plasma membrane receptors and transporters (reviewed in [51,52]), the action of anaesthetics (reviewed in [51–53]) and disease (reviewed in [54–56]).
Secondly, it was shown for the first time that the spontaneous transfer of sterol from isolated caveolae/raft domains was still significantly faster than from bulk plasma membranes. This was consistent with the apparent higher fluidity of the microenvironment wherein sterol was localized in the caveolae/raft domains as compared with plasma membranes. Nevertheless, the relatively long t1/2 of spontaneous sterol transfer from isolated caveolae/raft domains or plasma membranes (1.7 compared with 2.5 h) must still be considered in view of the very rapid (in minutes) cholesterol uptake or efflux via the HDL-receptor pathway mediated by caveolae in intact cells [2,4,5]. Since transmembrane cholesterol migration across both model membranes and plasma membranes [14,57,58] is fast (in minutes), additional factors within the cell must participate in mediating rapid cholesterol transfer out of the cell through the plasma membrane. Indeed, cells have evolved very rapid (1–2 min) non-vesicular and rapid (10–20 min) vesicular intracellular cholesterol-trafficking pathways to and from the plasma membrane [2,4,5].
Thirdly, although plasma membranes contain two kinetically resolvable sterol domains, an exchangeable and a non-exchangeable domain, the exchangeable sterol domain did not equate with caveolae/raft domains. On the contrary, caveolar/raft domain sterol kinetics were resolved into essentially the same exchangeable and non-exchangeable sterol domains present in approximately equal amounts as in the plasma membrane. When the transbilayer migration rate of sterol across model membranes and plasma membranes [14,57] was taken into account, these data suggested that caveolar/raft domain sterol organization was non-uniform. Half of total sterol in caveolae/raft domains was essentially non-exchangeable. Although some caveolar sterol is closely associated with proteins such as caveolin [22] and the oxytocin receptor [59], this is unlikely to account for half of total sterol in the caveolae/rafts domains. Instead, model-membrane studies have shown that cholesterol spontaneously organizes itself into multiple domains with the proportion of non-exchangeable domains depending on the specific lipid composition (reviewed in [60]).
Fourthly, probing of caveolar/raft domain sterol dynamics with SCP-2 revealed that SCP-2 selectively enhanced sterol transfer from caveolae/raft domains much more than from plasma membranes. This was not predicted based only on lipid composition of caveolae/raft domains. As compared with the plasma membrane, caveolar microdomains isolated by the use of detergents (i.e. DRMs) [2,8] or non-detergent methods [7,9] are enriched in cholesterol and sphingomyelin. However, SCP-2 is essentially ineffective in transferring sterol from cholesterol- and sphingomyelin-rich model membranes [16] or from cholesterol- and sphingomyelin-rich erythrocyte membranes [45]. The greater sensitivity of caveolar/raft domain sterol dynamics to SCP-2 as compared with plasma membranes may be accounted for by the fact that SCP-2 co-localizes with caveolin-1 at the plasma membrane [61], possibly due to the fact that caveolae are also rich in anionic phospholipids (phosphatidylinositol, polyphosphoinositides and phosphatidylserine) (reviewed in [2]) and SCP-2 preferentially interacts with anionic-phospholipid-rich membranes [62,63] to mediate and transfer sterol (reviewed in [60]). Untransfected L-cell fibroblasts were used in the present work. These cells do not express detectable levels of SCP-2 [25]. Hence, the response of cellular fractions to SCP-2 in these sterol exchanges was not masked by an intrinsic response due to the presence of any endogenous SCP-2 in the membranes.
Fifthly, SCP-2 dramatically increased the size of the exchangeable compared with the non-exchangeable sterol domain in caveolae/raft domains. In the absence of SCP-2, the ratio of exchangeable/non-exchangeable sterol was essentially 1.0 in both caveolae/raft domains and plasma membranes. In contrast, in the presence of SCP-2, the ratio of exchangeable/non-exchangeable sterol was 3.7 in caveolae/raft domains and only 1.3 in plasma membranes. These findings were consistent with studies on anionic-phospholipid-containing model membranes, which demonstrate that SCP-2 preferentially increased the size of the exchangeable sterol domain (reviewed in [60]). The potential importance of intracellular sterol-binding proteins to the mediation of sterol transfer from plasma membrane domains in cells such as hepatocytes is supported by the fact that the majority of sterol transport from basolateral plasma membrane domains into the hepatocyte, and from there to the bile canalicular region, occurs by non-vesicular pathways [17–19]. SCP-2 binds cholesterol (reviewed in [24]) and is known to enhance sterol transfer from plasma membranes in vitro and in living cells (reviewed in [25,60]).
In summary, although caveolar/raft domain lipid acyl chain fluidity was lower than in the plasma membrane, sterol was less ordered, and spontaneous sterol transfer from caveolae/raft domains was faster than from plasma membranes. Nevertheless, spontaneous sterol transfer from isolated caveolae/raft domains was still relatively slow (1–2 h) as compared with that exhibited by intact cells. SCP-2 dramatically altered the sterol domain dynamics of caveolae/raft domains much more than those of plasma membrane. This would suggest that the presence of intracellular sterol-transfer proteins may account, at least in part, for the rapid sterol transfer through plasma membrane caveolae in intact cells.
Acknowledgments
This work was supported in part by the USPHS (United States Public Health Service), National Institutes of Health grant GM31651 (F.S.) and by a Mentored Quantitative Research Career Development Award (K25) DK062812 (A.M.G.).
References
- 1.Goldstein J. L., Brown M. S. Lipoprotein receptors and the control of plasma LDL cholesterol levels. Eur. J. Biochem. 1992;13:34–36. doi: 10.1093/eurheartj/13.suppl_b.34. [DOI] [PubMed] [Google Scholar]
- 2.Smart E. J., van der Westhuyzen D. R. In: Intracellular Cholesterol Trafficking. Chang T. Y., Freeman D. A., editors. Boston: Kluwer Academic Publishers; 1998. pp. 253–272. [Google Scholar]
- 3.Anderson R. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 4.Frolov A., Petrescu A., Atshaves B. P., So P. T. C., Gratton E., Serrero G., Schroeder F. High density lipoprotein mediated cholesterol uptake and targeting to lipid droplets in intact L-cell fibroblasts. J. Biol. Chem. 2000;275:12769–12780. doi: 10.1074/jbc.275.17.12769. [DOI] [PubMed] [Google Scholar]
- 5.Atshaves B. P., Starodub O., McIntosh A. L., Roths J. B., Kier A. B., Schroeder F. Sterol carrier protein-2 alters HDL-mediated cholesterol efflux. J. Biol. Chem. 2000;275:36852–36861. doi: 10.1074/jbc.M003434200. [DOI] [PubMed] [Google Scholar]
- 6.Neufeld E. B. In: Intracellular Cholesterol Trafficking. Chang T. Y., Freeman D. A., editors. Boston: Kluwer Academic Publishers; 1998. pp. 93–121. [Google Scholar]
- 7.Eckert G. P., Igbavboa U., Muller W., Wood W. G. Lipid rafts of purified mouse brain synaptosomes prepared with or without detergent reveal different lipid and protein domains. Brain Res. 2003;962:144–150. doi: 10.1016/s0006-8993(02)03986-0. [DOI] [PubMed] [Google Scholar]
- 8.Brown D. A., London E. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 9.Pike L. J., Han X., Chung K.-N., Gross R. W. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder R., London E., Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntosh A., Gallegos A., Atshaves B. P., Storey S., Kannoju D., Schroeder F. Fluorescence and multiphoton imaging resolve unique structural forms of sterol in membranes of living cells. J. Biol. Chem. 2003;278:6384–6403. doi: 10.1074/jbc.M205472200. [DOI] [PubMed] [Google Scholar]
- 12.Gallegos A. M., Atshaves B. P., Storey S., McIntosh A., Petrescu A. D., Schroeder F. Sterol carrier protein-2 expression alters plasma membrane lipid distribution and cholesterol dynamics. Biochemistry. 2001;40:6493–6506. doi: 10.1021/bi010217l. [DOI] [PubMed] [Google Scholar]
- 13.Nemecz G., Schroeder F. Time-resolved fluorescence investigation of membrane cholesterol heterogeneity and exchange. Biochemistry. 1988;27:7740–7749. doi: 10.1021/bi00420a024. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder F., Nemecz G., Wood W. G., Joiner C., Morrot G., Ayraut-Jarrier M., Devaux P. F. Transmembrane distribution of sterol in the human eurythrocyte. Biochim. Biophys. Acta. 1991;1066:183–192. doi: 10.1016/0005-2736(91)90185-b. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder F., Frolov A., Schoer J., Gallegos A., Atshaves B. P., Stolowich N. J., Scott A. I., Kier A. B. In: Intracellular Cholesterol Trafficking. Chang T. Y., Freeman D. A., editors. Boston: Kluwer Academic Publishers; 1998. pp. 213–234. [Google Scholar]
- 16.Schroeder F., Jefferson J. R., Kier A. B., Knittell J., Scallen T. J., Wood W. G., Hapala I. Membrane cholesterol dynamics: cholesterol domains and kinetic pools. Proc. Soc. Exp. Biol. Med. 1991;196:235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- 17.Wustner D., Herrmann A., Hao M., Maxfield F. R. Rapid nonvesicular transport of sterol between the plasma membrane domains of polarized hepatic cells. J. Biol. Chem. 2002;277:30325–30336. doi: 10.1074/jbc.M202626200. [DOI] [PubMed] [Google Scholar]
- 18.Hafer A., Katzberg N., Münch C., Scheibner J., Stange E. F., Seedorf U., Fuchs M. Studies with sterol carrier protein-2 (SCP-2) gene knockout mice identify liver fatty acid binding protein (FABP1) as intracellular cholesterol transporter contributing to biliary cholesterol hypersecretion and gallstone formation. Gastroenterology. 2000;118(suppl. 2):135. [Google Scholar]
- 19.Fuchs M., Hafer A., Münch C., Kannenberg F., Teichmann S., Scheibner J., Stange E. F., Seedorf U. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J. Biol. Chem. 2001;276:48058–48065. doi: 10.1074/jbc.M106732200. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder F., Gallegos A. M., Atshaves B. P., Storey S. M., McIntosh A., Petrescu A. D., Huang H., Starodub O., Chao H., Yang H., et al. Recent advances in membrane cholesterol microdomains: rafts, caveolae, and intracellular cholesterol trafficking. Exp. Biol. Med. 2001;226:873–890. doi: 10.1177/153537020122601002. [DOI] [PubMed] [Google Scholar]
- 21.Sviridov D. Intracellular cholesterol trafficking. Histol. Histopathol. 1999;14:305–319. doi: 10.14670/HH-14.305. [DOI] [PubMed] [Google Scholar]
- 22.Uittenbogaard A., Ying Y. S., Smart E. J. Characterization of a cytosolic heat-shock protein–caveolin chaperone complex. J. Biol. Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- 23.Uittenbogaard A., Everson W. V., Matveev S. V., Smart E. J. Cholesteryl ester is transported from caveolae to internal membranes as part of a caveolin–annexin II lipid–protein complex. J. Biol. Chem. 2002;277:4925–4931. doi: 10.1074/jbc.M109278200. [DOI] [PubMed] [Google Scholar]
- 24.Stolowich N. J., Petrescu A. D., Huang H., Martin G., Scott A. I., Schroeder F. Sterol carrier protein-2: structure reveals function. Cell. Mol. Life Sci. 2002;59:193–212. doi: 10.1007/s00018-002-8416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallegos A. M., Atshaves B. P., Storey S. M., Starodub O., Petrescu A. D., Huang H., McIntosh A., Martin G., Chao H., Kier A. B., Schroeder F. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog. Lipid Res. 2001;40:498–563. doi: 10.1016/s0163-7827(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 26.Nemecz G., Schroeder F. Selective binding of cholesterol by recombinant fatty acid-binding proteins. J. Biol. Chem. 1991;266:17180–17186. [PubMed] [Google Scholar]
- 27.Schroeder F., Frolov A. A., Murphy E. J., Atshaves B. P., Jefferson J. R., Pu L., Wood W. G., Foxworth W. B., Kier A. B. Recent advances in membrane cholesterol domain dynamics and intracellular cholesterol trafficking. Proc. Soc. Exp. Biol. Med. 1996;213:150–177. doi: 10.3181/00379727-213-44047. [DOI] [PubMed] [Google Scholar]
- 28.Smart E. J., Ying Y., Mineo C., Anderson R. G. W. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 30.Schoer J., Gallegos A., Starodub O., Petrescu A., Roths J. B., Kier A. B., Schroeder F. Lysosomal membrane cholesterol dynamics: role of sterol carrier protein-2 gene products. Biochemistry. 2000;39:7662–7677. doi: 10.1021/bi992686h. [DOI] [PubMed] [Google Scholar]
- 31.Gallegos A. M., Schoer J., Starodub O., Kier A. B., Billheimer J. T., Schroeder F. A potential role for sterol carrier protein-2 in cholesterol transfer to mitochondria. Chem. Phys. Lipids. 2000;105:9–29. doi: 10.1016/s0009-3084(99)00128-0. [DOI] [PubMed] [Google Scholar]
- 32.Frolov A., Woodford J. K., Murphy E. J., Billheimer J. T., Schroeder F. Spontaneous and protein-mediated sterol transfer between intracellular membranes. J. Biol. Chem. 1996;271:16075–16083. doi: 10.1074/jbc.271.27.16075. [DOI] [PubMed] [Google Scholar]
- 33.Frolov A. A., Woodford J. K., Murphy E. J., Billheimer J. T., Schroeder F. Fibroblast membrane sterol kinetic domains: modulation by sterol carrier protein 2 and liver fatty acid binding protein. J. Lipid Res. 1996;37:1862–1874. [PubMed] [Google Scholar]
- 34.Schroeder F. Fluorescent sterols: probe molecules of membrane structure and function. Prog. Lipid Res. 1984;23:97–113. doi: 10.1016/0163-7827(84)90009-2. [DOI] [PubMed] [Google Scholar]
- 35.Gimpl G., Burger K., Fahrenholz F. Cholesterol as modulator of receptor function. Biochemistry. 1997;36:10959–10974. doi: 10.1021/bi963138w. [DOI] [PubMed] [Google Scholar]
- 36.Matyash V., Geier C., Henske A., Mukherjee S., Hirsh D., Thiele C., Grant B., Maxfield F. R., Kurzchalia T. V. Distribution and transport of cholesterol in Caenorhabditis elegans. Mol. Biol. Cell. 2001;12:1725–1736. doi: 10.1091/mbc.12.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas J. L., Holowka D., Baird B., Webb W. W. Large scale co-aggregation of fluorescent lipid probes with cell surface proteins. J. Cell Biol. 1994;125:795–802. doi: 10.1083/jcb.125.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spink C. H., Yeager M., Feigenson G. W. Partitioning behavior of indocarbocyanine probes between coexisting gel and fluid phases in model membranes. Biochim. Biophys. Acta. 1990;1023:25–33. doi: 10.1016/0005-2736(90)90005-9. [DOI] [PubMed] [Google Scholar]
- 39.Sklar L. A., Miljanich G. P., Dratz E. A. Phospholipid lateral phase separation and the partition of cis-parinaric acid and trans-parinaric acid among aqueous, solid lipid, and fluid lipid phases. Biochemistry. 1979;18:1707–1716. doi: 10.1021/bi00576a012. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder F., Soler-Argilaga C. Calcium modulates fatty acid dynamics in rat liver plasma membranes. Eur. J. Biochem. 1983;132:517–524. doi: 10.1111/j.1432-1033.1983.tb07392.x. [DOI] [PubMed] [Google Scholar]
- 41.Florine-Casteel K., Feigenson G. W. On the use of partition coefficients to characterize the distribution of fluorescent membrane probes between coexisting gel and fluid lipid phases: an analysis of the partition behavior of 1,6-diphenyl-1,3,5-hexatriene. Biochim. Biophys. Acta. 1988;941:102–106. doi: 10.1016/0005-2736(88)90218-0. [DOI] [PubMed] [Google Scholar]
- 42.Sweet W. D., Schroeder F. Polyunsaturated fatty acids alter sterol transbilayer domains in LM fibroblast plasma membrane. FEBS Lett. 1988;229:188–192. doi: 10.1016/0014-5793(88)80824-x. [DOI] [PubMed] [Google Scholar]
- 43.Schroeder F., Wood W. G., Kier A. B. In: Cell Physiology Sourcebook: A Molecular Approach. Sperelakis N., editor. New York: Academic Press; 2001. pp. 81–94. [Google Scholar]
- 44.Daleke D. L., Lyles J. V. Identification and purification of aminophospholipid flippases. Biochim. Biophys. Acta. 2000;1486:108–127. doi: 10.1016/s1388-1981(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 45.Kavecansky J., Joiner C. H., Schroeder F. Erythrocyte membrane lateral sterol domains: a dehydroergosterol fluorescence polarization study. Biochemistry. 1994;33:2880–2890. doi: 10.1021/bi00176a018. [DOI] [PubMed] [Google Scholar]
- 46.Oh P., Schnitzer J. E. Immunoisolation of caveolae with high affinity antibody binding to the oligomeric caveolin cage. J. Biol. Chem. 1999;274:23144–23154. doi: 10.1074/jbc.274.33.23144. [DOI] [PubMed] [Google Scholar]
- 47.Anderson R. G. W., Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 48.Wu C., Butz S., Ying Y., Anderson R. G. W. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J. Biol. Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- 49.Schnitzer J. E., Oh P., Jacobson B. S., Dvorak A. M. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca2+-ATPase, and inositol trisphosphate receptor. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1759–1763. doi: 10.1073/pnas.92.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu P., Rudick M., Anderson R. G. W. Multiple functions of caveolin-1. J. Biol. Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 51.Sweet W. D., Wood W. G., Schroeder F. Charged anesthetics selectively alter plasma membrane order. Biochemistry. 1987;26:2828–2835. doi: 10.1021/bi00384a026. [DOI] [PubMed] [Google Scholar]
- 52.Sweet W. D., Schroeder F. In: Advances in Membrane Fluidity: Lipid Domains and the Relationship to Membrane Function. Aloia R. C., Cirtain C. C., Gordon L. M., editors. New York: Alan R. Liss, Inc.; 1988. pp. 17–42. [Google Scholar]
- 53.Wood W. G., Schroeder F., Rao A. M., Igbavboa U., Avdulov N. A. In: Pharmacological Effects of Ethanol on the Nervous System. Dietrich R., Erwin V. G., editors. Boca Raton: CRC Press; 1996. pp. 13–27. [Google Scholar]
- 54.Wood W. G., Schroeder F., Avdulov N. A., Chochina S. V., Igbavboa U. Recent advances in brain cholesterol dynamics: transport, domains, and Alzheimer's disease. Lipids. 1999;34:225–234. doi: 10.1007/s11745-999-0357-9. [DOI] [PubMed] [Google Scholar]
- 55.Wood W. G., Eckert G. P., Igbavboa U., Mueller W. E. Amyloid β-protein interactions with membranes and cholesterol: causes or casualties of Alzheimer's disease. Biochim. Biophys. Acta. 2003;1610:281–290. doi: 10.1016/s0005-2736(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 56.Wood G. W., Schroeder F., Igbavboa U., Avdulov N. A., Chochina S. V. Brain membrane cholesterol domains, aging and amyloid β-peptides. Neurobiol. Aging. 2002;23:685–694. doi: 10.1016/s0197-4580(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 57.John K., Kubelt J., Muller P., Wustner D., Herrmann A. Rapid transbilayer movement of the fluorescent sterol dehydroergosterol in lipid membranes. Biophys. J. 2002;83:1525–1534. doi: 10.1016/S0006-3495(02)73922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boesze-Battaglia K., Clayton S. T., Schimmel R. J. Cholesterol redistribution within human platelet plasma membrane: evidence for a stimulus-dependent event. Biochemistry. 1996;35:6664–6673. doi: 10.1021/bi951846w. [DOI] [PubMed] [Google Scholar]
- 59.Gimpl G., Fahrenholz F. Human oxytocin receptors in cholesterol-rich vs cholesterol-poor microdomains of the plasma membrane. Eur. J. Biochem. 2000;267:2483–2497. doi: 10.1046/j.1432-1327.2000.01280.x. [DOI] [PubMed] [Google Scholar]
- 60.Schroeder F., Gallegos A. M., Atshaves B. P., McIntosh A., Petrescu A. D., Huang H., Chao H., Yang H., Frolov A., Kier A. B. Recent advances in membrane microdomains: rafts, caveolae and intracellular cholesterol trafficking. Exp. Biol. Med. 2001;226:873–890. doi: 10.1177/153537020122601002. [DOI] [PubMed] [Google Scholar]
- 61.Schroeder F., Zhou M., Swaggerty C. L., Atshaves B. P., Petrescu A. D., Storey S., Martin G. G., Huang H., Helmkamp G. M., Ball J. M. Sterol carrier protein-2 functions in phosphatidylinositol transfer and signaling. Biochemistry. 2003;42:3189–3202. doi: 10.1021/bi026904+. [DOI] [PubMed] [Google Scholar]
- 62.Huang H., Ball J. A., Billheimer J. T., Schroeder F. Interaction of the N-terminus of sterol carrier protein-2 with membranes: role of membrane curvature. Biochem. J. 1999;344:593–603. [PMC free article] [PubMed] [Google Scholar]
- 63.Huang H., Ball J. A., Billheimer J. T., Schroeder F. The sterol carrier protein-2 amino terminus: a membrane interaction domain. Biochemistry. 1999;38:13231–13243. doi: 10.1021/bi990870x. [DOI] [PubMed] [Google Scholar]