Abstract
The key insulin-regulated gluconeogenic enzyme G6Pase (glucose-6-phosphatase) has an important function in the control of hepatic glucose production. Here we examined the inhibition of G6Pase gene transcription by TNF (tumour necrosis factor) in H4IIE hepatoma cells. TNF decreased dexamethasone/dibtuyryl cAMP-induced G6Pase mRNA levels. TNFα, but not insulin, led to rapid activation of NFκB (nuclear factor κB). The adenoviral overexpression of a dominant negative mutant of IκBα (inhibitor of NFκB α) prevented the suppression of G6Pase expression by TNFα, but did not affect that by insulin. The regulation of G6Pase by TNF was not mediated by activation of the phosphoinositide 3-kinase/protein kinase B pathway, extracellular-signal-regulated protein kinase or p38 mitogen-activated protein kinase. Reporter gene assays demonstrated a concentration-dependent down-regulation of G6Pase promoter activity by the transient overexpression of NFκB. Although two binding sites for NFκB were identified within the G6Pase promoter, neither of these sites, nor the insulin response unit or binding sites for Sp proteins, was necessary for the regulation of G6Pase promoter activity by TNFα. In conclusion, the data indicate that the activation of NFκB is sufficient to suppress G6Pase gene expression, and is required for the regulation by TNFα, but not by insulin. We propose that NFκB does not act by binding directly to the G6Pase promoter.
Keywords: diabetes, gluconeogenesis, glucose-6-phosphatase, insulin, liver, sepsis
Abbreviations: AP-1, activator protein-1; ATF-2, activating transcription factor-2; Bt2cAMP, N6,2′-O-dibutyryl cAMP; CBP, cAMP response element binding protein binding protein; DMEM, Dulbecco's modified Eagle's medium; EMSA, electrophoretic mobility shift assay; ERK, extracellular-signal-regulated kinase; FCS, fetal calf serum; G6Pase, glucose-6-phosphatase; GSK3, glycogen synthase kinase 3; IκB, inhibitor of NFκB; IRU, insulin response unit; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; NFκB, nuclear factor κB; PGC-1α, peroxisome proliferator-activated receptor γ co-activator-1α; PEPCK, phosphoenolpyruvate carboxykinase; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; TNF, tumour necrosis factor
INTRODUCTION
G6Pase (glucose-6-phosphatase) catalyses the final step of the metabolic pathways that are central to hepatic glucose production, i.e. gluconeogenesis and glycogen breakdown [1]. Thus G6Pase has an important role in blood glucose homoeostasis. G6Pase activity is regulated predominantly at the level of gene expression. The molecular mechanisms mediating the regulation of G6Pase gene expression have been studied intensively, as the inappropriate control of G6Pase activity is associated with metabolic alterations such as diabetes and glycogen storage disease [2]. Glucagon (via cAMP) and glucocorticoids induce G6Pase gene transcription during starvation, whereas insulin inhibits gene expression post-prandially. The co-activator protein PGC-1α (peroxisome proliferator-activated receptor γ co-activator-1α) and the transactivating transcription factor Foxo1 (also named FKHR) play a central role in the regulation of G6Pase by these hormones [3–9]. PGC-1α expression is up-regulated by glucagon, and induces G6Pase transcription via interaction with hepatocyte nuclear factor-4, the glucocorticoid receptor and Foxo1. Insulin inhibits G6Pase expression at least in part by activation of the PI3K (phosphoinositide 3-kinase)/PKB (protein kinase B) pathway and the subsequent phosphorylation of Foxo1. In addition, activation of the MEK [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase]/ERK pathway and the pharmacological inhibition of GSK3 (glycogen synthase kinase 3) are sufficient to suppress G6Pase gene expression [10,11].
In addition to insulin, the pro-inflammatory cytokine TNF (tumour necrosis factor) is also able to suppress G6Pase expression in vitro and in vivo [12]. This could be of pathophysiological importance during septic shock, where the TNF level increases rapidly and G6Pase expression decreases in parallel [13]. The suppression of G6Pase expression probably contributes to the diminished hepatic glucose production and the hypoglycaemia observed in the later stages of sepsis [14]. Receptor binding of TNF leads to the activation of transcription factors such as NFκB (nuclear factor κB) and AP-1 (activator protein-1), via the recruitment of signal transducers and the stimulation of several complex signalling cascades, such as the NFκB pathway and the MAPK pathway [15,16]. NFκB is formed by a heterodimer of proteins that belong to the Rel family, the predominant members of which are p50 and p65/RelA. In the absence of a stimulus, IκBα (inhibitor of NFκB α) retards NFκB within the cytoplasm. An agonist of the NFκB pathway, such as TNF, leads to the activation of the IκB kinase complex and the subsequent phosphorylation of IκBα on Ser-32 and Ser-36. Phosphorylated IκBα releases NFκB and is degraded rapidly by an ubiquitin-dependent pathway. Free NFκB translocates into the nucleus, where it regulates the expression of genes important for cellular defence and inflammation, either by direct binding to promoter elements or in a DNA-independent fashion by the sequestration of co-activator proteins, e.g. CBP (cAMP response element binding protein binding protein) [16–18]. TNF also activates the stress-activated protein kinase p38 MAPK (also known as SAPK2) [19,20]. The activation of p38 MAPK leads to the phosphorylation of several transcription factors, including ATF-2 (activating transcription factor-2), and is essential for the regulation of genes encoding pro-inflammatory cytokines (such as interleukin-6) and inflammation-related enzymes (such as inducible nitric oxide synthase) by TNF [19,20].
In the present study, we demonstrate that activation of the NFκB pathway is central to the inhibition of G6Pase gene expression by TNFα, but not by insulin.
EXPERIMENTAL
Plasmids
The G6Pase reporter gene constructs G6Pase(−1227/+57), G6Pase(−1100/+57), G6Pase(−499/+57), G6Pase(−161/+57) and G6Pase(−150/+57) were created by cloning the human G6Pase promoter fragments −1227/+57, −1100/+57, −499/+57, −161/+57 and −150/+57 respectively into the promoterless luciferase reporter gene vector pGL3-Basic (Promega) [21]. The plasmid G6Pase(−1227/+57/IRUmut) is not regulated by forkhead proteins and PKB because of a mutated IRU (insulin response unit) between positions −196 and −156 within the plasmid G6Pase(−1227/+57), as described in [5]. The plasmid G6Pase(−1227/+57/SpA,Bmut) was generated by mutating Sp-binding sites A (−19/−11) and B (−63/−55) within the plasmid G6Pase(−1227/+57) [22]. In order to generate the plasmid G6Pase(−499/+57/NF2mut), the NFκB-binding site 2 between positions −155 and −145 was mutated from 5′-GTAAATCACCCT-3′ to 5′-GTAAATCATCTA-3′ within G6Pase(−499/+57). The plasmid G6Pase(−151/+57/NF2mut) was derived from G6Pase(−151/+57) by mutation of the promoter sequence between −151 and −145 to 5′-ATCATCTA-3′, in order to destroy NFκB-binding site 2 completely. The plasmid pGL-TK-2×NFκB was created by cloning a doublestranded oligonucleotide with the sequence 5′-CCGGGGACTTTCCCGGATCCAGGGGACTTTCCCTC-3′, which contains two NFκB consensus binding sites (underlined), into the pGL-TK vector, which contains the luciferase gene under the control of the herpes simplex thymidine kinase minimal promoter [21]. Dr N. Perkins (Department of Biochemistry, University of Dundee, Dundee, Scotland, U.K.) provided the plasmid pRSVNFκB, which expresses the p65/RelA subunit, and the control plasmid pRSV-βgal, which expresses β-galactosidase [16]. The plasmids pRSV-NFκB-Y36F and pRSV-NFκB-S276A were cloned by changing the NFκB sequence from 5′-CCGCTACAAGTG-3′ and 5′-GCCTTCCGACCG-3′ within pRSV-NFκB to 5′-CCGCCTCAAGTG-3′ and 5′-GCCTGCCGACCG-3′ respectively. The conversion of Tyr-36 into Phe leads to an impairment of DNA binding [23], whereas the mutation of Ser-276 to Ala reduces the interaction of NFκB with co-activator proteins, for instance CBP [17,18].
Transient transfections
H4IIE cells were cultured in DMEM (Dulbecco's modified Eagle's medium)/10% (v/v) FCS (fetal calf serum) in the presence of 5.5 mM glucose. The cells were transfected with 8.5 μg/dish of the reporter gene vector, 0.5 μg/dish of pRL-TK control plasmid (Promega) and the expression vector for either pRSV-NFκB or pRSV-βgal as indicated using the calcium phosphate/DNA co-precipitation method followed by a glycerol shock. The transfected cells were serum-starved for 1 h and then incubated in the presence or absence of dexamethasone (1 μM), Bt2cAMP (N6,2′-O-dibutyryl cAMP; 500 μM), TNFα (10 ng/ml) and insulin (10 nM), as indicated. After 20 h, cell extracts were prepared and luciferase activities were determined as described [10]. All experiments were performed at least three times in triplicate with at least two different DNA preparations. Statistical analysis was performed using Student's t test for unpaired data.
Viral infections
A cDNA encoding full-length IκBα and another encoding IκBα in which Ser-32 and Ser-36 were mutated to Ala were subcloned into the pACCMV plasmid [24]. These plasmids were then cotransfected with the pJM17 plasmid (which encodes the adenovirus genome) into HEK293 cells to construct recombinant adenoviruses by homologous recombination as described previously [25]. The cells were incubated in DMEM/2% (v/v) FCS for 4–5 days until virus production began. The adenoviruses were named Ad-IκBα wt and Ad-IκBα S32,36A respectively, and were amplified in HEK293 cells. The construction of a recombinant adenovirus containing the cDNA encoding the Escherichia coli β-galactosidase gene (Ad-β-Gal) has been described previously [26]. The amount of Ad-β-Gal virus required was determined first by finding the volume of clarified HEK293 cell lysate that stained more than 80% of a monolayer of H4IIE cells after fixation and treatment with 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside [25]. The amounts of the Ad-IκBα wt and Ad-IκBα S32,36A viruses used were determined from a concentration–response experiment, in which the maximum amount of virus that was not toxic to the cells was established. Overexpression of protein from both viruses was confirmed by Western blotting. Expression levels of wild-type IκBα and IκBα S32,36A were approximately equal, as determined by this method. H4IIE cells were infected in serum-free DMEM essentially as described in [25]. After 36 h, cells were incubated as indicated and lysed for further analyses. In order to infect cells that had been transiently transfected with reporter gene constructs, the adenoviruses were added 30 min after the calcium phosphate/DNA co-precipitate. The cells were incubated in serum-free medium for 24 h after the glycerol shock and subsequently for 6 h in the presence or absence of TNFα, insulin, dexamethasone and Bt2cAMP, as indicated.
RNA isolation and Northern blot
Serum-starved H4IIE cells were incubated in the presence or absence of dexamethasone (1 μM), Bt2cAMP (500 μM), TNFα (10 ng/ml), insulin (10 nM), SB203580 (20 μM), PD98059 (50 μM) and LY294002 (100 μM), as indicated, for 3 h. RNA isolation and Northern blot analysis were carried out essentially as described in [10]. The blots were rehybridized using a random-primed 32P-labelled probe for actin.
Western blot analysis
H4IIE cell lysates were prepared as described in [10]. Equal amounts of protein were separated by SDS/PAGE and transferred on to nitrocellulose membranes. Even transfer was verified by Ponceau staining. Membranes were blocked with Roti-Block (Roth, Karlsruhe, Germany). The blots were incubated with antibodies against IκBα, ERK1/2, Thr-202/Thr-204-phosphorylated ERK1/2, GSK3α/β, Ser-9/Ser-21-phosphorylated GSK3α/β, PKB or Ser-473-phosphorylated PKB (all from Cell Signaling, Beverly, MA, U.S.A.). After washing the blots with TBST [15 mM Tris/HCl, pH 7.4, 150 mM NaCl, 0.05% (v/v) Tween 20], they were incubated with peroxidase-conjugated anti-(rabbit IgG) for 1 h and then washed again with TBST. Detection was performed using LumiGLO (Cell Signaling). p38 MAPK activity was determined using the p38 MAP Kinase Assay Kit (Cell Signaling) according to the manufacturer's instructions.
EMSAs (electrophoretic mobility shift assays)
EMSAs were performed essentially as described in [5] using 8 μg of nuclear extract protein from H4IIE cells. As probes, 32P-labelled double-stranded oligonucleotides with the following sequences were used (NFκB-binding sites underlined): 5′-AGTTGAGGGGACTTTCCCAGGC-3′ (NFcons), 5′-GGGCAACATGGAAAAACCCCATCTCTA-3′ (NF1), 5′-TACGTAAATCACCCTGAACATGTTTGC-3′ (NF2) and 5′-TACGTAAATCATCTAGAACATGTTTGC-3′ (NF2mut).
NFκB ELISA
The activation of NFκB in infected cells was determined using 2 μg of nuclear extract protein with the TransAM NFκB p65 Chemi ELISA Kit (Active Motif, Rixensart, Belgium) according to the manufacturer's instructions. The specificity of the assay was confirmed by competition experiments using double-stranded oligonucleotides having the sequence of the NFκB consensus site or an unrelated sequence.
RESULTS
Inhibition of G6Pase expression by TNFα
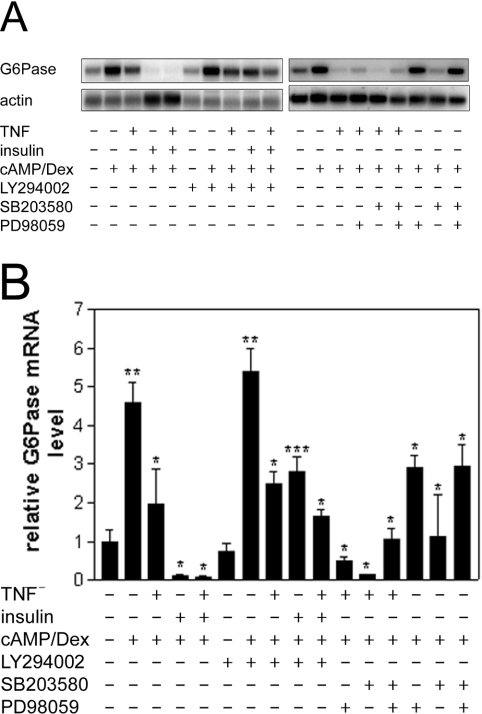
TNFα decreases G6Pase mRNA levels in vivo and in primary hepatocytes [12]. In agreement with this, we observed a TNFα-mediated reduction in dexamethasone/Bt2cAMP-induced G6Pase mRNA levels of approx. 60% in rat hepatoma H4IIE cells (Figure 1), although this was less than that observed following insulin treatment.
Figure 1. TNFα suppresses the Bt2cAMP/dexamethasone-induced increase in G6Pase mRNA levels in H4IIE cells.
Serum-starved H4IIE cells were preincubated in the presence or absence of the PI3K inhibitor LY294002 (100 μM), the MEK inhibitor PD98059 (50 μM) and/or the p38 MAPK inhibitor SB203580 (20 μM) for 1 h. Then, in the presence or absence of TNFα (10 ng/ml), insulin (10 nM) and Bt2cAMP (cAMP; 500 μM)/dexamethasone (Dex; 1 μM) were added, as indicated. After an additional incubation of 3 h, cells were lysed and RNA was isolated. RNA (15 μg) was electrophoresed, transferred on to nylon membranes and hybridized using a random-primed 32P-labelled probe for G6Pase. Actin was used as a normalization control. (A) Northern blots, (B) densitometric analysis of three Northern blots (means±S.E.M.; n=3). The G6Pase mRNA level is shown relative to that under basal conditions, which was set as 1. Significance of differences: *P<0.05 compared with expression in the presence of dexamethasone and Bt2cAMP alone; **P<0.05 compared with basal expression; ***P<0.05 compared with expression in the presence of insulin, dexamethasone and Bt2cAMP.
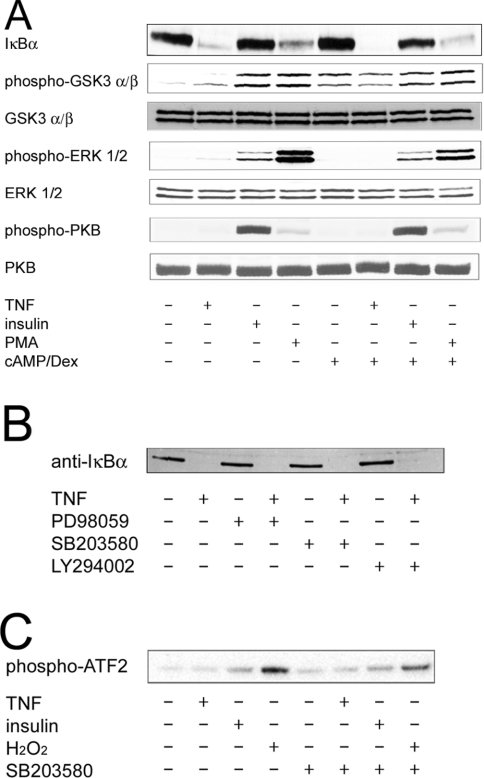
In order to characterize the pathways involved in the suppression of G6Pase expression by TNFα, we studied signalling events that have been shown either to suppress G6Pase gene expression or to be stimulated by TNFα. This was performed by determination of the activation of these pathways, as well as by the use of pharmacological inhibitors. The regulation of G6Pase gene expression by TNFα was not affected by LY294002, an inhibitor of PI3K. In parallel incubations, this compound impaired the regulation by insulin, which confirms previous reports that PI3K is important for the regulation of G6Pase by insulin [5,24]. Furthermore, in contrast with insulin, TNFα failed to induce the phosphorylation of PKB and GSK3 (Figure 2A), which are downstream events of PI3K signalling. Therefore the data demonstrate that TNFα does not regulate G6Pase gene expression via activation of the PI3K pathway. Activation of the MEK/ERK pathway is sufficient to suppress G6Pase gene expression, since PD98059, an inhibitor of MEK, blocks the inhibition of G6Pase gene expression by a phorbol ester [10]. PD98059 alone decreased G6Pase mRNA levels slightly. This effect was synergistic with the inhibition by TNFα (Figure 1). Furthermore, TNFα activated ERK1/2 very weakly compared with the stimulation observed with the phorbol ester PMA (Figure 2A). This suggests that activation of the MEK/ERK pathway plays, at most, a minor role in the inhibition of G6Pase by TNFα. SB203580, an inhibitor of p38 MAPK, also decreased G6Pase mRNA levels by itself and acted synergistically with TNFα (Figure 1). In contrast with insulin or oxidative stress, TNFα did not stimulate p38 MAPK activity, which was measured by the phosphorylation of ATF-2 (Figure 2C). Therefore TNFα does not suppress G6Pase gene expression by stimulation of p38 MAPK.
Figure 2. Regulation of signalling pathways by TNFα.
Serum-starved H4IIE cells were incubated for 20 min with TNFα (10 ng/ml), insulin (10 nM), the phorbol ester PMA (1 μM), H2O2 (2 mM), the PI3K inhibitor LY294002 (100 μM), the MEK inhibitor PD98059 (50 μM) and/or the p38 MAPK inhibitor SB203580 (20 μM), as indicated. (A, B) Cell lysates were electrophoresed by SDS/PAGE and transferred on to nylon membranes. Western blots were performed using the respective specific antibodies. Levels of unphosphorylated PKB, GSK and ERK are shown as controls for equal loading. (C) p38 MAPK was immunoprecipitated from cell lysates and assayed with ATF-2 as substrate. Phosphorylation of ATF-2 was determined by Western blotting.
TNFα, but not insulin, activates NFκB in H4IIE cells
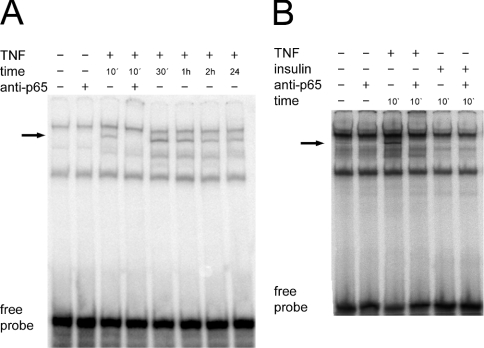
In contrast with insulin, TNFα and the phorbol ester PMA stimulated the degradation of IκBα (Figure 2A). SB203580, LY294002 and PD98059 did not affect significantly the levels of IκBα or the degradation of IκBα induced by TNFα (Figure 2B). Next, we measured the activation of NFκB in H4IIE cells by a gel shift assay using a double-stranded oligonucleotide comprising the consensus sequence of a NFκB-binding site as a probe. TNFα treatment of cells resulted in the formation of a nuclear complex, which was recognized by an antibody against the p65/RelA subunit of NFκB (Figure 3A). This NFκB-containing complex appeared within 10 min of TNFα administration and was still apparent, although at slightly reduced levels, after 24 h. Insulin was not able to significantly promote formation of this NFκB complex (Figure 3B). The administration of dexamethasone and Bt2cAMP did not affect complex formation (results not shown). Overall, the data indicate that TNFα activates the NFκB pathway to a much greater extent than does insulin in H4IIE cells.
Figure 3. Activation of NFκB by TNFα and insulin.
Double-stranded labelled oligonucleotides with the sequence of a consensus NFκB-binding site (NFcons) were incubated with 8 μg of nuclear extract protein from H4IIE cells treated with TNFα (A, B) or insulin (B) for the indicated times. Supershift experiments were carried out by preincubation of the nuclear extracts with 100 ng of anti-p65/RelA antibody. The arrow indicates the NFκB-containing complex.
Activation of NFκB is necessary for the suppression of G6Pase by TNFα
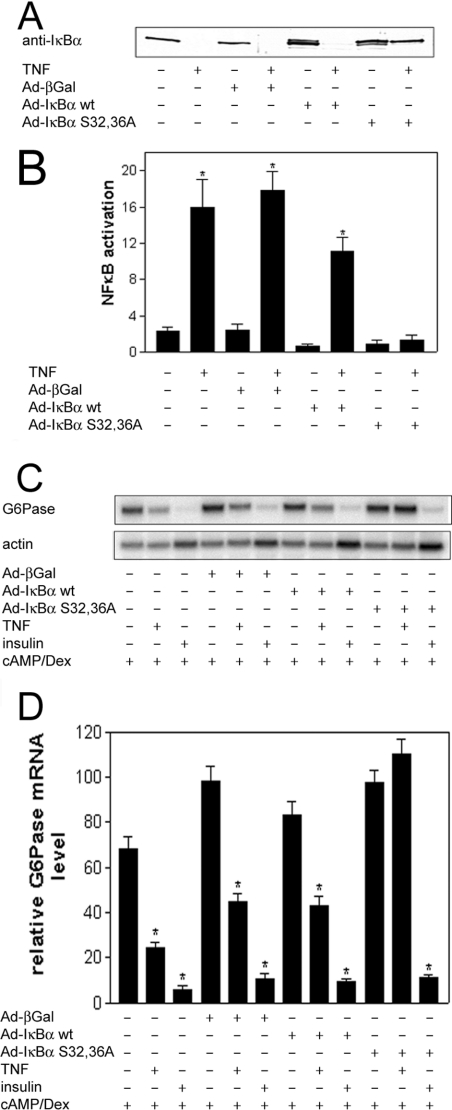
In order to investigate whether TNFα decreases G6Pase expression via activation of NFκB, H4IIE cells were infected with an adenovirus overexpressing wild-type IκBα, IκBα S32,36A or β-galactosidase. The levels of overexpressed IκBα protein in the cells infected with virus expressing either wild-type IκBα or IκBα S32,36A were similar in the absence of TNFα (Figure 4A). TNFα stimulated the degradation of endogenous IκBα in cells overexpressing β-galactosidase, and also of both endogenous and overexpressed wild-type IκBα in cells infected with the adenovirus expressing wild-type IκBα (Figure 4A). However, the cytokine did not induce the complete degradation of IκBα in cells overexpressing the IκBα S32,36A mutant. This indicates that the administration of TNFα does not stimulate the degradation of the IκBα S32,36A mutant, which may therefore still be able to sequester NFκB in the presence of TNFα. This was demonstrated by the failure of TNFα to activate NFκB in cells infected with adenovirus expressing the IκBα S32,36A mutant. However, TNFα led to the activation of NFκB in non-infected cells, as well as in cells overexpressing either wild-type IκBα or β-galactosidase (Figure 4B). The data show that IκBα S32,36A acts as a dominant negative inhibitor of the NFκB pathway. The overexpression of neither wild-type IκBα nor β-galactosidase affected the regulation of G6Pase by TNFα and insulin, compared with non-infected cells (Figures 4C and 4D). However, TNFα failed to suppress G6Pase mRNA levels in cells overexpressing the dominant negative IκBα S32,36A. The regulation of G6Pase by insulin was unchanged by IκBα S32,36A. These data show that TNFα, but not insulin, decreases G6Pase expression by the activation of NFκB.
Figure 4. Effects of adenoviral-mediated overexpression of wild-type IκBα, IκBα S32,36A and β-galactosidase on the regulation of the NFκB pathway and G6Pase gene expression.
H4IIE cells were infected with virus expressing wild-type IκBα (Ad-IκBα wt), IκBα S32,36A (Ad-IκBα S32,36A) or β-galactosidase (Ad-β-Gal) and incubated with TNFα, insulin, Bt2cAMP (cAMP) and dexamethasone (Dex), as indicated, prior to the isolation of cell lysates, nuclear extracts and RNA. (A) Western blot of cell lysates using anti-IκBα antibodies. (B) ELISA of activated NFκB in nuclear extracts isolated from H4IIE cells treated with or without TNFα. Data are expressed relative to the absorbance with Ad-IκBα wt in the absence of TNFα, which was set as 1; *P<0.05 compared with relative activation in the absence of TNFα. (C) Northern blot. RNA (15 μg) isolated from cells infected and incubated as indicated was electrophoresed, transferred on to nylon membranes and hybridized using a random-primed 32P-labelled probe for G6Pase. Actin was used as a normalization control. (D) Densitometric analysis of three Northern blots (means±S.E.M.; n=3); *P<0.05 compared with expression in the absence of insulin or TNFα. The level of expression of Ad-β-Gal-infected cells in the presence of dexamethasone and Bt2cAMP was set as 100.
TNFα and NFκB inhibit G6Pase gene transcription
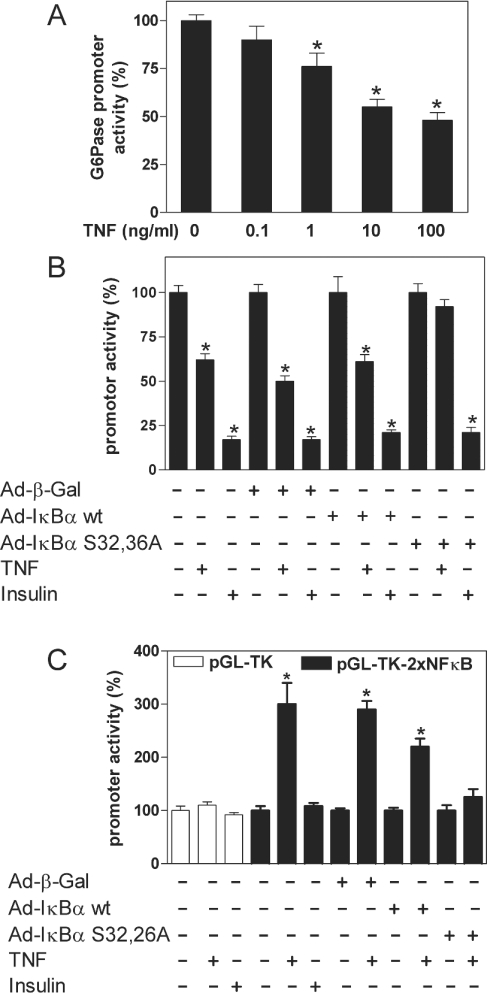
In order to address the mechanism of G6Pase suppression by NFκB, we carried out transient transfection studies using G6Pase promoter–luciferase reporter gene constructs. TNFα decreased dexamethasone/Bt2cAMP-induced G6Pase promoter activity in a concentration-dependent manner (Figure 5A). Similar to endogenous G6Pase mRNA levels, the regulation of G6Pase promoter activity by TNFα was not affected by LY294002 or SB203580, and was inhibited only slightly by PD98059 (results not shown). TNFα and insulin decreased G6Pase promoter activity in H4IIE cells that had been infected with adenovirus expressing β-galactosidase or wild-type IκBα prior to transient transfection with the reporter gene construct. However, adenoviral overexpression of the dominant negative IκBα S32,36A mutant blocked the inhibition of G6Pase promoter activity by TNFα, but not that by insulin (Figure 5B). TNFα had no effect on the activity of the thymidine kinase minimal promoter (pGL-TK), but it induced luciferase expression strongly in cells transfected with pGL-TK-2×NFκB, which contains two binding sites for NFκB (Figure 5C). In contrast, insulin did not affect luciferase expression significantly in pGL-TK-2×NFκB-transfected cells. This suggests that NFκB is not activated to an appreciable extent by insulin in H4IIE cells. The induction of pGL-TK-2×NFκB by TNFα was blocked in cells infected with Ad-IκBα S32,36A (Figure 5C). Overall, the data show that, similar to the regulation of endogenous G6Pase mRNA levels, TNFα regulates the G6Pase promoter–luciferase reporter gene construct and pGL-TK-2×NFκB via the activation of NFκB.
Figure 5. Inhibition of G6Pase promoter activity by TNFα.
H4IIE cells were co-transfected with 8.5 μg/dish of (A, B) the promoter plasmid G6Pase(−1227/+57) or (C) pGL-TK-2×NFκB (▪) and pGL-TK (□) and 0.5 μg/dish pRL control plasmid. In (B) and (C) H4IIE cells were infected with virus expressing wild-type IκBα (Ad-IκBα wt), IκBα S32,36A (Ad-IκBα S32,36A) or β-galactosidase (Ad-β-gal) 36 h prior to transient transfection with the reporter gene constructs. The transfected cells were incubated with or without the indicated concentration of TNFα or insulin (10 nM) in the presence (A, B) or absence (C) of Bt2cAMP (500 μM) and dexamethasone (1 μM) for 20 h (A) or 6 h (B, C). Data are presented as means±S.E.M. (n=3) relative to either induced (A, B) or basal (C) luciferase activity, which was set as 100; *P<0.05 compared with expression in the absence of TNFα or insulin.
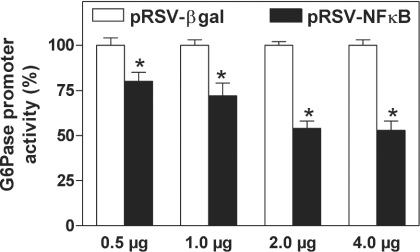
Meanwhile, co-transfection of NFκB decreased G6Pase promoter activity in a concentration-dependent manner (Figure 6). This indicates that NFκB activation is sufficient to suppress G6Pase gene transcription.
Figure 6. Concentration-dependent inhibition of G6Pase promoter activity by the overexpression of NFκB.
H4IIE cells were co-transfected with 8.5 μg/dish of the reporter gene plasmid G6Pase(−1227/+57) and 0.5 μg/dish of the pRL control plasmid together with the indicated amount of DNA per dish of either pRSV-NFκB (▪) or pRSV-βgal (□) in the presence of Bt2cAMP (500 μM) and dexamethasone (100 nM). Data are presented as means±S.E.M. (n=3) relative to the luciferase activity in the presence of pRSV-βgal, which was set as 100; *P<0.05 compared with expression in the presence of pRSV-βgal.
Characterization of potential TNFα-responsive elements within the G6Pase promoter
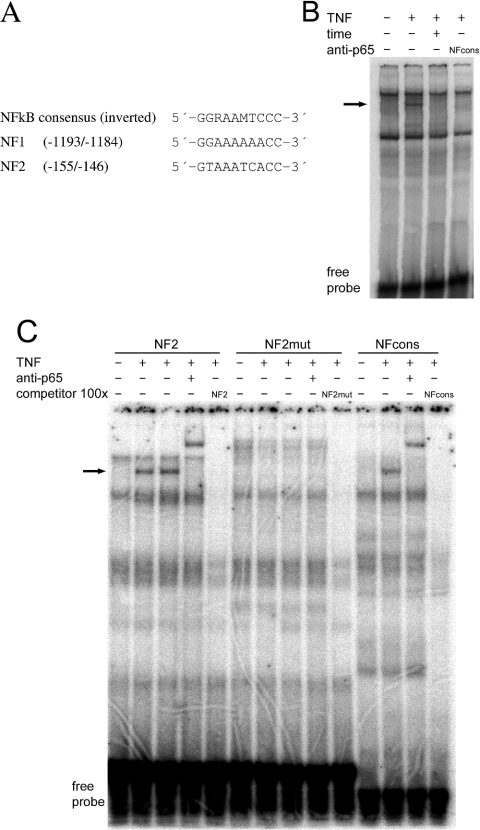
Next we examined the 5′ sequence from positions −1227 to +57 of the G6Pase gene in order to identify potential NFκB-binding site(s) that could mediate the regulation of gene expression by TNFα. Two sequences with similarity to the inverted consensus sequence of a NFκB-binding site were located between positions −1193 and −1184 (NF1) and −155 and −146 (NF2) (Figure 7A). The ability of NFκB to bind to these motifs was studied in an EMSA using double-stranded oligonucleotides having the relevant sequences (Figures 7B and 7C). Nuclear extracts that had been isolated from TNFα-treated H4IIE cells resulted in the formation of a complex using the NF1 probe that was absent when nuclear extracts of untreated cells were used. The complex was not observed after preincubation of the nuclear extracts with antibodies against p65/RelA or p50, or in the presence of an excess of unlabelled oligonucleotide with the consensus sequence of a NFκB-binding site as competitor. The data indicate that NFκB can bind as a heterodimer to the NF1 site. Similarly, an extra band was observed using a probe with the sequence of NF2 and nuclear extracts isolated from TNFα-treated H4IIE cells. Again, this band could be supershifted by an antibody against p65/RelA (Figure 7C). This band was also observed using a probe with the sequence of the consensus NFκB-binding site, but not with a probe with the sequence of a mutated NF2 site. In conclusion, NFκB can bind to both sequence motifs, NF1 and NF2.
Figure 7. Identification of NFκB-binding sites within the G6Pase promoter.
(A) Comparison of the inverted consensus sequence of a NFκB-binding site with the G6Pase promoter sequences −1193/−1184 (NF1) and −155/−146 (NF2). (B, C). EMSAs using double-stranded oligonucleotides with the sequence of (B) NF1 and (C) NF2, NF2mut or NFcons as probes. Nuclear extracts were isolated from H4IIE cells treated with or without TNFα, as indicated. Supershift experiments were carried out by preincubation of the nuclear extracts with 100 ng of anti-p65/RelA or anti-p50 antibodies. In competition experiments, a 100-fold molar excess of the unlabelled double-stranded probe was used. Arrows indicate NFκB-containing complexes.
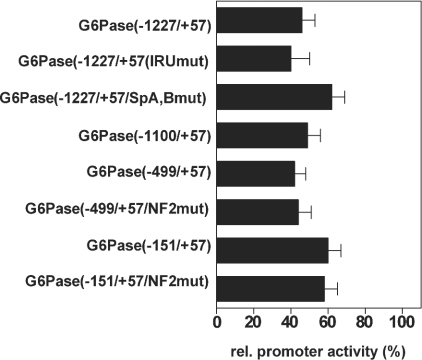
We addressed the functional significance of these sites within the G6Pase gene for the regulation by TNFα using G6Pase promoter constructs in which these regions were deleted or mutated. As shown in Figure 8, the promoter activities of the constructs G6Pase(−1100/+57) and G6Pase(−499/+57), which both lack NF1, were controlled by TNFα in a manner similar to that of construct G6Pase(−1227/+57). In the construct G6Pase(−499/+57/NF2mut), NF1 was deleted and NF2 was mutated. Nevertheless, regulation of this construct by TNFα was unchanged. Furthermore, the administration of TNFα was able to decrease the promoter activity of plasmid G6Pase(−151/+57). In construct G6Pase(−151/+57/NF2mut), NF2, which was partially deleted in G6Pase(−151/+57), was completely mutated. However, this construct was still regulated by TNFα. The apparently reduced inhibition of the promoter activity of the constructs G6Pase(−151/+57) and G6Pase(−151/+57/NF2mut) by TNFα was due to the lower expression in the absence of TNFα compared with that of the longer constructs. The further deletion of the promoter sequence decreased the G6Pase promoter activity to such a low level that it was not possible to determine a potential influence of TNFα on promoter activity. However, no region with sequence similarity to a NFκB-binding site could be detected within the G6Pase promoter sequence between positions −145 and +57.
Figure 8. Effects of different mutations of the G6Pase promoter on regulation by TNFα.
H4IIE cells were co-transfected with 8.5 μg/dish of the indicated reporter gene plasmids and 0.5 μg/dish of pRL control plasmid in the presence or absence of TNFα (10 ng/ml) and the presence of Bt2cAMP (500 μM) and dexamethasone (1 μM). Data are presented as means±S.E.M. (n=3) of promoter activity in the presence of TNFα compared with the luciferase activity of the respective construct in the absence of TNFα, which was set as 100. All constructs show a significant inhibition by TNFα.
Because the cis-active IRU within the G6Pase promoter mediates in part the suppression of G6Pase expression by insulin [5], we examined its role in the regulation by TNFα. Mutation of the IRU [construct G6Pase(−1227/+57/IRUmut)] did not affect the control of promoter activity by TNFα (Figure 8), whereas it significantly reduced the suppression by insulin [5]. The data provide further evidence that the PI3K/PKB/Foxo pathway is not involved in the regulation of G6Pase by TNFα, because the IRU mediates control of the gene by this pathway [5]. Binding sites for Sp proteins can mediate the action of TNFα [27], and the G6Pase promoter contains two binding sites for Sp1 and Sp3 [22]. However, mutation of these binding sites [construct G6Pase(−1227/+57/SpA,Bmut)] did not abolish the inhibition of promoter activity by TNFα. Again, the slightly lower effect of TNFα was due to the lower basal activity after mutation of the Sp-binding elements [22]. Furthermore, the administration of TNFα prior to the isolation of nuclear extracts did not change the ability of Sp1 and Sp3 to bind to double-stranded oligos having the sequence of the Sp-binding sites of the G6Pase promoter (results not shown). Thus the Sp-binding sites are not necessary for the inhibition of G6Pase expression by TNFα.
Inhibition by NFκB depends on its transactivation and DNA-binding ability
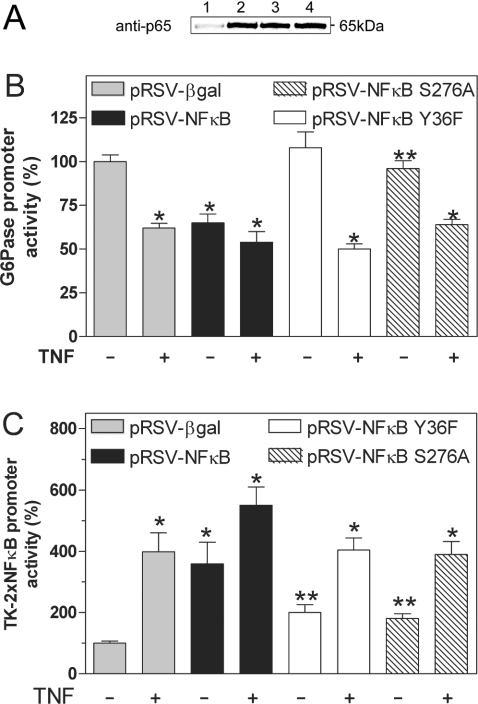
NFκB decreases the expression of PEPCK (phosphoenolpyruvate carboxykinase) in a DNA-independent fashion by the sequestration of a co-activator protein such as CBP [17]. In order to address whether a similar mechanism might contribute to the suppression of G6Pase expression, we created the p65/RelA mutants NFκB-S276A and NFκB-Y36F. The mutation of Ser-276 to Ala decreases the interaction of p65/RelA with co-activator proteins and impairs the regulation of PEPCK by NFκB [17]. The conversion of Tyr-36 to Phe decreases the DNA-binding ability of p65/RelA [23]. In transfected HEK293 cells, mutated and wild-type NFκB were overexpressed to similar levels (Figure 9A). The co-expression of NFκB-Y36F or NFκB-S276A suppressed the expression of the G6Pase promoter significantly less than did wild-type NFκB (Figure 9B). In the presence of co-expressed wild-type NFκB, TNFα did not inhibit G6Pase promoter activity further. However, the cytokine decreased G6Pase promoter activity significantly when NFκB-Y36F or NFκB-S276A was coexpressed. This indicates that these mutants are not acting in a dominant negative manner. Both mutants of NFκB induced the NFκB-dependent control plasmid pGL-TK-2×NFκB significantly less than did wild-type NFκB (Figure 9C). TNFα was able to increase the promoter activity significantly in the presence of wild-type NFκB, NFκB-Y36F or NFκB-S276A. The results suggest that the ability of NFκB to suppress G6Pase expression depends on its ability to bind to DNA and to interact with co-activator proteins.
Figure 9. Role of DNA binding and protein–protein interactions on regulation of G6Pase promoter activity by NFκB.
(A) Western Blot analysis of HEK293 cells transiently transfected with 10 μg of empty vector (lane 1), wild-type pRSV-NFκB (lane 2), pRSV-NFκB-S276A (lane 3) or pRSV-NFκB-Y36F (lane 4). Western blotting was performed using an antibody against the p65/RelA subunit of NFκB. (B, C) H4IIE cells were co-transfected with 8.5 μg/dish of the reporter gene plasmids G6Pase(−1227/+57) (B) or pGL-TK-2×NFκB (C) together with 0.5 μg/dish of pRL control plasmid and 2 μg/dish of pRSV-NFκB, pRSV-NFκB-Y36F, pRSV-NFκB-S276A or pRSV-βgal in the presence or absence of TNFα as indicated. Data are presented as means±S.E.M. (n=3) of promoter activity compared with the respective luciferase activity in cells transfected with pRSV-βgal without TNFα, which was set as 100; *P<0.05 compared with expression in the presence of pRSV-βgal and in the absence of TNFα; **P<0.05 compared with expression in the presence of pRSV-βgal and pRSV-NFκB.
DISCUSSION
In the present paper we show that the activation of NFκB is central and sufficient for the suppression of G6Pase gene expression by TNFα. We found no contribution of p38 MAPK to the regulation of G6Pase expression by TNFα. Thus regulation of the expression of the G6Pase gene by TNFα is different from that of several pro-inflammatory cytokines, such as interleukin-6 [19,20]. SB203580 and PD98059, inhibitors of p38 MAPK and MEK respectively, decreased G6Pase expression by themselves. The reason for this is not known; however, the mechanism of this effect is different from that mediating the regulation by TNFα, as these compounds did not affect signalling via the NFκB pathway. Furthermore, TNFα inhibited G6Pase expression synergistically with these compounds.
In addition, the data show that the PI3K pathway and the IRU within the G6Pase promoter, which participate in the regulation of the G6Pase gene by insulin [6], are not involved in the suppression mediated by TNFα. In addition, we did not find evidence for the participation of NFκB in the regulation of G6Pase by insulin. Therefore TNFα and insulin regulate G6Pase gene expression via different signalling pathways and different cis-acting sequences. The conclusion that insulin does not regulate G6Pase expression via NFκB is based on the observations that insulin failed to increase the levels of free NFκB or to activate a NFκB-dependent promoter construct. In addition, overexpression of a dominant negative IκBα construct did not affect the ability of insulin to suppress G6Pase expression. In a previous study, insulin was shown to activate NFκB in H4IIE cells, and it has been postulated that this transcription factor mediates in part regulation of the PEPCK gene [17]. The reason for this discrepancy regarding the activation of NFκB by insulin between [17] and the present study is not known, but may be due to differences in strain.
NFκB is proposed to inhibit expression of the gluconeogenic enzyme PEPCK by sequestration of a co-activator protein such as CBP [17]. In the present study, we found that the ability of NFκB to interact with other proteins was also critical for its suppressive effect on G6Pase expression, as the mutant NFκB-S276A had a reduced effect on G6Pase promoter activity, similar to that described for PEPCK expression. However, we provide evidence that the inhibition of G6Pase expression by NFκB also depends on DNA binding, because mutation of an amino acid that is important for DNA binding impaired the suppression of G6Pase. Although we failed to characterize a cis-active element within the G6Pase promoter that mediates the regulation by TNFα, we were able to identify binding sites for NFκB within the G6Pase promoter. However, these sequences were not relevant for the regulation by TNFα. Furthermore, mutation of neither the forkhead-binding motifs, which mediate suppression of G6Pase expression, nor the Sp-binding sites, which have been linked to suppression of genes by NFκB [27], affected the regulation by TNFα. It is therefore possible that the ability of TNFα to suppress G6Pase gene expression via NFκB might also be mediated indirectly by regulation of a different gene that, for instance, encodes a transcription factor or a co-activator protein involved in G6Pase expression.
The acute actions of TNFα and the NFκB pathway described here seem to be different from their chronic, pro-diabetic effects in obesity, which lead to increased G6Pase expression [28–31]. A possible explanation for this could be that prolonged activation of the IκB kinase complex by adipocyte-derived TNFα or by fatty acids impairs insulin signalling [28–32]. Furthermore, there is evidence that in obesity the elevation of non-esterified fatty acid levels induces G6Pase expression directly [33]. Therefore either insulin resistance or fatty acid-induced G6Pase expression could overcome the acute suppression of G6Pase by TNFα and NFκB activation in vivo. In conclusion, the regulation of G6Pase by NFκB described here provides further evidence that a network of signalling events controls G6Pase expression.
Acknowledgments
We thank Dr S. Patel for help with the adenovirus work, Dr B. Bröker for providing S2 facilities, Mrs B. Parlow for excellent technical assistance and Dr P. Oppermann for the synthesis of oligonucleotides. This work was supported by the Deutsche Forschungsgemeinschaft (Schm 1125/4-1 and GK 840 ‘Host-pathogen-interactions in generalized bacterial infections’) and a FEBS Summer Fellowship to R.G.
References
- 1.van Schaftingen E., Gerin I. The glucose-6-phosphatase system. Biochem. J. 2002;362:513–532. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthel A., Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2003;285:E685–E692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- 3.Yoon J. C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C. R., Granner D. K., et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature (London) 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 4.Herzig S., Long F., Jhala U. S., Hedrick S., Quinn R., Bauer A., Rudolph D., Schutz G., Yoon C., Puigserver P., et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature (London) 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 5.Schmoll D., Walker K. S., Alessi D. R., Grempler R., Burchell A., Guo S., Walther R., Unterman T. G. Regulation of glucose-6-phosphatase gene expression by protein kinase Bα and the forkhead transcription factor FKHR. J. Biol. Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 6.Dickens M., Svitek C. A., Culbert A. A., O'Brien R. M., Tavare J. M. Central role for phosphatidylinositide 3-kinase in the repression of glucose-6-phosphatase gene transcription by insulin. J. Biol. Chem. 1998;273:20144–20149. doi: 10.1074/jbc.273.32.20144. [DOI] [PubMed] [Google Scholar]
- 7.Boustead J. N., Stadelmaier B. T., Eeds A. M., Wiebe P. O., Svitek C. A., Oeser J. K., O'Brien R. M. Hepatocyte nuclear factor-4 alpha mediates the stimulatory effect of peroxisome proliferator-activated receptor gamma co-activator-1 alpha (PGC-1 alpha) on glucose-6-phosphatase catalytic subunit gene transcription in H4IIE cells. Biochem. J. 2003;369:17–22. doi: 10.1042/BJ20021382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puigserver P., Rhee J., Donovan J., Walkey C. J., Yoon J. C., Oriente F., Kitamura Y., Altomonte J., Dong H., Accili D., Spiegelman B. M. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature (London) 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 9.Nakae J., Biggs W. H., III, Kitamura T., Cavenee W. K., Wright C. V., Arden K. C., Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 10.Schmoll D., Grempler R., Barthel A., Joost H. G., Walther R. Phorbol ester-induced activation of mitogen-activated protein kinase/extracellular-signal-regulated kinase kinase and extracellular-signal-regulated protein kinase decreases glucose-6-phosphatase gene expression. Biochem. J. 2001;357:867–873. doi: 10.1042/0264-6021:3570867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lochhead P. A., Coghlan M., Rice S. Q., Sutherland C. Inhibition of GSK-3 selectively reduces glucose-6-phosphatase and phosphoenolypyruvate carboxykinase gene expression. Diabetes. 2001;50:937–946. doi: 10.2337/diabetes.50.5.937. [DOI] [PubMed] [Google Scholar]
- 12.Metzger S., Begleibter N., Barah V., Drize O., Peretz T., Shiloni E., Chajek-Shaul T. Tumor necrosis factor inhibits the transcriptional rate of glucose-6-phosphatase in vivo and in vitro. Metab. Clin. Exp. 1997;46:579–583. doi: 10.1016/s0026-0495(97)90197-9. [DOI] [PubMed] [Google Scholar]
- 13.Deutschman C. S., Andrejko K. M., Haber B. A., Bellin L., Elenko E., Harrison R., Taub R. Sepsis-induced depression of rat glucose-6-phosphatase gene expression and activity. Am. J. Physiol. 1997;273:R1709–R1718. doi: 10.1152/ajpregu.1997.273.5.R1709. [DOI] [PubMed] [Google Scholar]
- 14.Dhainaut J.-F., Marin N., Mignon A., Vinsonneau C. Hepatic response to sepsis: Interaction between coagulation and inflammatory processes. Crit. Care Med. 2001;29:S42–S47. doi: 10.1097/00003246-200107001-00016. [DOI] [PubMed] [Google Scholar]
- 15.Baud V., Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 16.Perkins N. D. The Rel/NFκB family: friend and foe. Trends Biochem. Sci. 2000;25:434–440. doi: 10.1016/s0968-0004(00)01617-0. [DOI] [PubMed] [Google Scholar]
- 17.Waltner-Law M., Daniels M. C., Sutherland C., Granner D. K. NFκB inhibits glucocorticoid and cAMP-mediated expression of the phosphoenolpyruvate carboxykinase gene. J. Biol. Chem. 2000;275:31847–31856. doi: 10.1074/jbc.M003656200. [DOI] [PubMed] [Google Scholar]
- 18.Zhong H., Voll R. E., Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 19.Ono K., Han J. The p38 signal transduction pathway: Activation and function. Cell. Signalling. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 20.Beyaert R., Cuenda A., Vanden Berghe W., Plaisance S., Lee J. C., Haegeman G., Cohen P., Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- 21.Schmoll D., Wasner C., Hinds C. J., Allan B. B., Walther R., Burchell A. Identification of a cAMP response element within the glucose-6-phosphatase hydrolytic subunit gene promoter which is involved in the transcriptional regulation by cAMP and glucocorticoids in H4IIE hepatoma cells. Biochem. J. 1999;338:457–463. [PMC free article] [PubMed] [Google Scholar]
- 22.Wasner C., Grempler R., Walther R., Schmoll D. Basal level glucose-6-phosphatase gene transcription requires binding sites for Sp family proteins within the gene promoter. Biochim. Biophys. Acta. 2001;1521:126–129. doi: 10.1016/s0167-4781(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu J., Sodeoka M., Lane W. S., Verdine G. L. Evidence for a non-helical DNA-binding motif in the Rel homology region. Proc. Natl. Acad. Sci. U.S.A. 1994;91:908–912. doi: 10.1073/pnas.91.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker T. C., Noel R. J., Coats W. S., Gomez-Foix A. M., Alam T., Gerard R. D., Newgard C. B. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 25.Wang J. C., Stafford J. M., Scott D. K., Sutherland C., Granner D. K. The molecular physiology of hepatic nuclear factor 3 in the regulation of gluconeogenesis. J. Biol. Chem. 2000;275:14717–14721. doi: 10.1074/jbc.275.19.14717. [DOI] [PubMed] [Google Scholar]
- 26.Herz J., Gerard R. D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson H. D., Rahmutula D., Gardner D. G. Tumor necrosis factor-alpha inhibits endothelial nitric oxide synthase gene promoter activity in bovine aortic endothelial cells. J. Biol. Chem. 2004;279:963–969. doi: 10.1074/jbc.M309552200. [DOI] [PubMed] [Google Scholar]
- 28.Moller D. E. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 29.Hundal R. S., Petersen K. F., Mayerson A. B., Randhawa P. S., Inzucchi S., Shoelson S. E., Shulman G. I. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan M., Konstantopoulos N., Lee J., Hansen L., Li Z.-W., Karin M., Shoelson S. E. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKKβ. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 31.Lam T. K., Carpentier A., Lewis G. F., van de Werve G., Fantus I. G., Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am. J. Physiol. Endocrinol. Metab. 2003;284:E863–E873. doi: 10.1152/ajpendo.00033.2003. [DOI] [PubMed] [Google Scholar]
- 32.Jiang G., Dallas-Yang Q., Liu F., Moller D. E., Zhang B. B. Salicylic acid reverses phorbol 12-myristate-13-acetate (PMA)- and tumor necrosis factor α (TNFα)-induced insulin receptor substrate 1 (IRS1) serine 307 phosphorylation and insulin resistance in human embryonic kidney 293 (HEK293) cells. J. Biol. Chem. 2003;278:180–186. doi: 10.1074/jbc.M205565200. [DOI] [PubMed] [Google Scholar]
- 33.Massillon D., Barzilai N., Hawkins M., Prus-Wertheimer D., Rossetti L. Induction of hepatic glucose-6-phosphatase gene expression by lipid infusion. Diabetes. 1997;46:153–157. doi: 10.2337/diab.46.1.153. [DOI] [PubMed] [Google Scholar]