Abstract
The relationship between protonmotive force and superoxide production by mitochondria is poorly understood. To address this issue, the rate of superoxide production from complex I of rat skeletal muscle mitochondria incubated under a variety of conditions was assessed. By far, the largest rate of superoxide production was from mitochondria respiring on succinate; this rate was almost abolished by rotenone or piericidin, indicating that superoxide production from complex I is large under conditions of reverse electron transport. The high rate of superoxide production by complex I could also be abolished by uncoupler, confirming that superoxide production is sensitive to protonmotive force. It was inhibited by nigericin, suggesting that it is more dependent on the pH gradient across the mitochondrial inner membrane than on the membrane potential. These effects were examined in detail, leading to the conclusions that the effect of protonmotive force was mostly direct, and not indirect through changes in the redox state of the ubiquinone pool, and that the production of superoxide by complex I during reverse electron transport was at least 3-fold more sensitive to the pH gradient than to the membrane potential.
Keywords: electron transport chain, hydrogen peroxide (H2O2), mitochondria, reactive oxygen species, skeletal muscle
Abbreviations: Δp, protonmotive force; FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; PHPA, p-hydroxyphenyl acetic acid; Q, ubiquinone; ROS, reactive oxygen species; SOD, superoxide dismutase; TPMP+, triphenylmethylphosphonium ion
INTRODUCTION
Reactive oxygen species (ROS), such as superoxide, hydrogen peroxide (H2O2) and hydroxyl radicals, are known to play a role in various pathological disorders [1], and there is evidence to suggest that they may also play a role in aging [2]. Despite an elaborate array of protection systems to neutralize ROS [including SOD (superoxide dismutase), catalase and glutathione peroxidase], it appears that oxidative damage to macromolecules such as proteins and DNA can accumulate and lead to cellular dysfunction. The main source of ROS in animal cells is the mitochondria (in plants, it is the plastids), and in vitro studies indicate that superoxide is the primary ROS produced as a result of the single electron reduction of oxygen [3–5]. The importance of superoxide removal from the mitochondrial matrix in vivo is particularly demonstrated by manganese-SOD nullizygous mice, which have only a 10-day lifespan and exhibit several severe pathological disorders [6,7]. In addition to the recognized deleterious action of ROS, there is growing evidence that they can serve as specific signalling molecules [8].
Within the mitochondria, the main sites of superoxide production have been localized to the electron transport chain. The ‘normal’ function of the chain is to pump protons across the inner membrane, driven by the energy released during the transfer of electrons from reduced substrates through cytochrome oxidase (complex IV) to oxygen. Complex IV reduces oxygen to water using electrons from cytochrome c in four tightly controlled, one-electron steps, and produces little or no superoxide. However, during electron transport, electron leaks, primarily at complexes I and III, can pass single electrons to oxygen and give rise to superoxide. The mechanism of superoxide production by complex III is relatively well understood, since it is linked to the operation of the Q (ubiquinone) cycle [9]. However, the mechanism of superoxide production by complex I is less clear, probably because the exact sequence of electron transfers and how they are coupled to proton transfer is not known [10–12]. For instance, it is unclear which site(s) within complex I are responsible for generating superoxide. The flavin group [13–15], the N-1a iron–sulphur cluster [16], the N-2 iron–sulphur cluster [17], the iron–sulphur clusters in general [13,15,18] and ubisemiquinone [18–20] have each been implicated.
An interesting observation reported in several studies is that mitochondria respiring on succinate, the substrate for complex II (in the absence of rotenone, an inhibitor of complex I), have a greater rate of superoxide production than they do when respiring on complex I-linked substrates [13,14,16,21,22]. Most of the superoxide production during oxidation of succinate occurs during reverse electron transport into complex I [14,21–23], and thus superoxide production during reverse electron transport is greater than during forward electron transport. The mechanism and physiological relevance of this phenomenon are not known.
Over the course of the last 7 years, it has become apparent that the rate of superoxide production by the electron transport chain in vitro is sensitive to the mitochondrial protonmotive force (Δp) [21,22,24,25]. This conclusion is based on observations that addition of either uncouplers (which increase the consumption of Δp) or inhibitors (which inhibit formation of Δp) decreases the rate of superoxide production by mitochondria respiring on succinate in the absence of rotenone. Reverse electron transport depends on the thermodynamic forces across complex I and is, therefore, favoured by a high Δp and a high reduction state of the Q pool. However, in the intact electron transport chain, Δp will have both a direct effect on complex I and an indirect effect through the Q pool, because of its downstream effects on complex III and complex IV. Lowering Δp will tend to oxidize the Q pool and decrease electron supply into complex I, and indirectly lower superoxide production. These complications make it difficult to assess from the published studies the relative importance of the direct and indirect effects of Δp on superoxide production by complex I.
Δp consists of two components: Δψ (the membrane potential, i.e. the electrical component) and ΔpH (the pH gradient, i.e. the concentration component). Their relative importance in determining superoxide production by complex I is unknown. The observation by Liu [25] that conversion of ΔpH into Δψ by addition of nigericin lowered ROS production in isolated liver mitochondria suggests that ΔpH may be more important than is currently recognized.
The purpose of the present study was to explore the relationships between superoxide production by complex I and the components of Δp, to gain an insight into the mechanism and regulation of complex I superoxide production.
MATERIALS AND METHODS
Materials
Piericidin A was kindly given by Dr Mauro Degli Esposti (University of Manchester, U.K.). All other chemicals were purchased from Sigma.
Measurement of mitochondrial superoxide production
Mitochondria from skeletal muscle of female Wistar rats (aged between 5 and 8 weeks) were isolated by differential centrifugation, as described previously [26]. The superoxide production rate was assessed by measurement of the H2O2 generation rate, determined fluorimetrically by measurement of oxidation of PHPA (p-hydroxyphenyl acetic acid) coupled to the enzymic reduction of H2O2 by horseradish peroxidase. Mitochondria were incubated at 0.35 mg of mitochondrial protein·ml−1 in standard buffer containing 120 mM KCl, 3 mM Hepes, 1 mM EGTA and 0.3% (w/v) BSA (pH 7.2 at 37 °C). All incubations also contained 50 μg·ml−1 PHPA, 4 units·ml−1 horseradish peroxidase, 30 units·ml−1 SOD and 1.875 μM TPMP+ (triphenylmethylphosphonium) bromide. The reaction was initiated by addition of respiratory substrates, and, after 1 min, the increase in fluorescence at an excitation wavelength of 320 nm and emission wavelength of 400 nm was followed on a computer-controlled Shimadzu RF5301 spectrofluorimeter for 2–3 min. Appropriate correction for background signals [27] and standard curves generated using known amounts of H2O2 were used to calculate the rate of H2O2 production in nmol·min−1·mg of mitochondrial protein−1. Essentially, all the superoxide from complex I is generated on the matrix side of the inner membrane, and then converted by endogenous processes into H2O2, which leaks out and is measured in the assay [27].
Measurement of mitochondrial Δp
The mitochondrial Δψ was determined using an electrode sensitive to TPMP+, as described previously [28]. Skeletal muscle mitochondria were incubated at 37 °C in standard buffer with PHPA, horseradish peroxidase and SOD. The electrode was calibrated by sequential 0.375 μM additions of TPMP+ up to 1.875 μM. The reaction was initiated by addition of respiratory substrate, and Δψ was measured upon reaching the steady state (approx. 1 min). The chemical component of the protonmotive force, ΔpH, was then measured as the change in Δψ after ΔpH was converted into Δψ following addition of 100 nM nigericin. After each run, the uncoupler FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone) was added to a concentration of 2 μM to release the TPMP+ and allow correction for any small drift in the TPMP+ electrode. Potentials were calculated as described previously [28] on the basis that Δp=Δψ+ΔpH (all in mV, giving positive signs to electrical potentials that were positive outside and pH gradients that were acid outside).
Measurement of reverse electron transport
The rate of reverse electron transport from succinate to NAD+ was assessed by monitoring the rate of NADH formation fluorimetrically at excitation and emission wavelengths of 365 and 450 nm respectively, as described previously [29]. Incubation and buffer conditions were similar to those used for measurement of H2O2 and Δp. The NADH production rate was quantified using a standard curve.
Statistics
Results are presented as means±S.E.M, with n being the number of separate mitochondrial preparations. The significance of differences between means was assessed by an unpaired Student's t test; the minimum level of significance chosen was P<0.05.
RESULTS
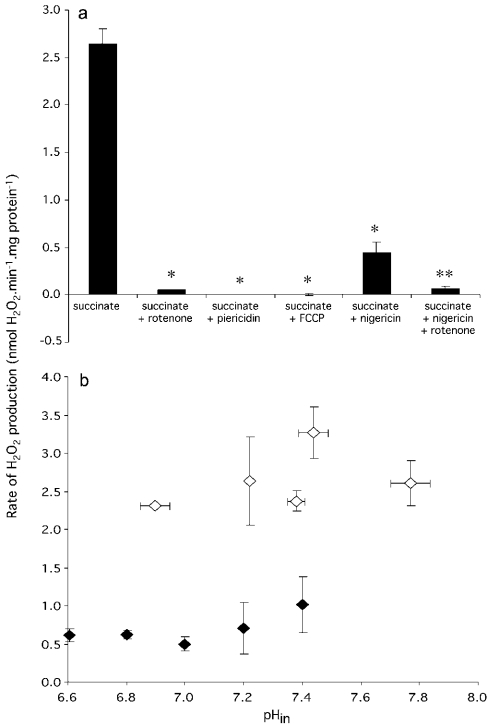
The rate of H2O2 production by rat skeletal muscle mitochondria respiring under various conditions is shown in Figure 1(a). In the presence of succinate alone, a relatively large rate of H2O2 production was observed (2.65 nmol·min−1·mg of protein−1). This rate was almost abolished in the presence of rotenone or piericidin (specific complex I inhibitors), indicating that the large rate of superoxide production was due to reverse electron transport from succinate through complex II into complex I. In the presence of complex I inhibitors, the rate of superoxide production from succinate dehydrogenase, complex III and other downstream sites was less than 4% of the rate from complex I during reverse electron transport. Reverse electron transport is known to be dependent on Δp, which provides the necessary driving force. Neither rotenone nor piericidin significantly affected Δp, Δψ or ΔpH with succinate as substrate (Table 1), and therefore inhibition by rotenone and piericidin was direct, and not acting through Δp or its components.
Figure 1. (a) Rates of superoxide production (measured as H2O2 production) by isolated rat skeletal mitochondria respiring on succinate and (b) effects of internal (matrix) pH (pHin) and ΔpH on H2O2 production rate.
(a) Standard incubation conditions were 120 mM KCl, 3 mM Hepes, 1 mM EGTA, 0.3% (w/v) BSA (pH 7.2 and 37 °C), 50 μg PHPA·ml−1, 4 units·ml−1 horseradish peroxidase, 30 units·ml−1 SOD and 1.875 μM TPMP+. Where shown, 4 mM succinate, 4 μM rotenone, 2 μM piericidin, 2 μM FCCP and 100 nM nigericin were also present. *Significant (P<0.0001) difference from succinate; **significant (P<0.05) difference from succinate+nigericin. Bars show means±S.E.M. of measurements on four to ten separate mitochondrial preparations. (b) Incubation conditions were as for (a), except that the pH of the buffer was adjusted to 6.6–7.4 in steps of 0.2 pH units. Open symbols, nigericin absent; closed symbols, plus 100 nM nigericin. pHin in the absence of nigericin was determined in parallel incubations using the TPMP+ electrode, as described in the Materials and methods section. In the presence of nigericin, pHin=pHout. Points represent means±S.E.M. of measurements on four separate mitochondrial preparations.
Table 1. Values of ΔpH, Δψ and Δp under various conditions.
Potentials were determined using a TPMP+ electrode as described in the Materials and methods section. Standard incubation conditions were 120 mM KCl, 3 mM HEPES, 1 mM EGTA, 0.3% (w/v) BSA (pH 7.2 and 37 °C), 50 μg of PHPA·ml−1, 4 units·ml−1 horseradish peroxidase, 30 units·ml−1 SOD and 1.875 μM TPMP. Where shown, 4 mM succinate, 4 μM rotenone, 2 μM piericidin, 2 μM FCCP and 100 nM nigericin were added. Values are means±S.E.M. for measurements on four to ten separate mitochondrial preparations. *Significant (P<0.001) difference from succinate. We define ΔpH as zero in the presence of nigericin.
| Δψ (mV) | ΔpH (mV) | Δp (mV) | |
|---|---|---|---|
| Succinate | 141±4 | 40±5 | 181±5 |
| Succinate+rotenone | 139±4 | 41±4 | 180±4 |
| Succinate+piericidin | 147±3 | 40±3 | 187±3 |
| Succinate+FCCP | 0±0* | 0±0* | 0±0* |
| Succinate+nigericin | 181±5* | 0 | 181±5 |
To examine the characteristics of reverse electron transport-driven superoxide production by complex I, uncouplers and ionophores were used to alter the components of Δp. In the presence of the uncoupler FCCP, where Δp was zero (Table 1), reverse electron transport from succinate to NAD+ was abolished, as expected (results not shown). For this condition, superoxide production with succinate as substrate was not measurable (Figure 1a), confirming that superoxide production is sensitive to Δp. Nigericin is an ionophore that exchanges potassium ions for protons across the mitochondrial inner membrane. In the presence of nigericin, if [K+]in=[K+]out, then [H+]in=[H+]out and ΔpH=0. The concentration of [K+]out in the buffer was 120 mM, which approximates to [K+]in. Since ΔpH is abolished by nigericin in this medium, the electron transport chain compensates for the drop in ΔpH by pumping more protons, so that Δψ increases and ΔpH is quickly converted entirely into Δψ (Table 1). In the presence of nigericin, the superoxide production rate was decreased from 2.65 to 0.45 nmol·min−1·mg of protein−1 (Figure 1a). This leads to the important qualitative conclusion that superoxide production by complex I was much more inhibited by the 40 mV drop in ΔpH than it was stimulated by the equal 40 mV rise in Δψ. The residual superoxide production under these conditions was still sensitive to rotenone (Figure 1a), showing that both the nigericin-sensitive and nigericin-insensitive components originated in complex I by reverse electron transport from complex II.
These results suggest that most of the large rate of superoxide production from complex I is dependent on the pH difference across the mitochondrial inner membrane. However, since nigericin collapses the ΔpH component of Δp to zero, it will also change the internal (matrix) pH (pHin), and this might provide an alternative explanation for the lowering of the superoxide generation rate. This possibility was explored by measuring ΔpH and H2O2 production in a series of buffers with different pH values, in the presence and absence of nigericin. In the presence of nigericin, ΔpH=0, and so pHin will equal pHout; thus pHin is easily manipulated by using buffers of different pH in the presence of nigericin. In the absence of nigericin, ΔpH is normally approx. 30–40 mV (the exact value is calculated from the relationship ΔpH=Δp−Δψ, determined using the TPMP+ electrode), and pHin can be calculated as pHout+ΔpH (in pH units). As shown in Figure 1(b), H2O2 production did not depend strongly on pHin, regardless of the absence or presence of nigericin. However, it did depend on ΔpH: at any given pHin, the rate of H2O2 production was always severalfold higher in the absence of nigericin than in its presence. This indicates that the superoxide production rate during reverse electron transport is dependent on ΔpH, and not on pHin.
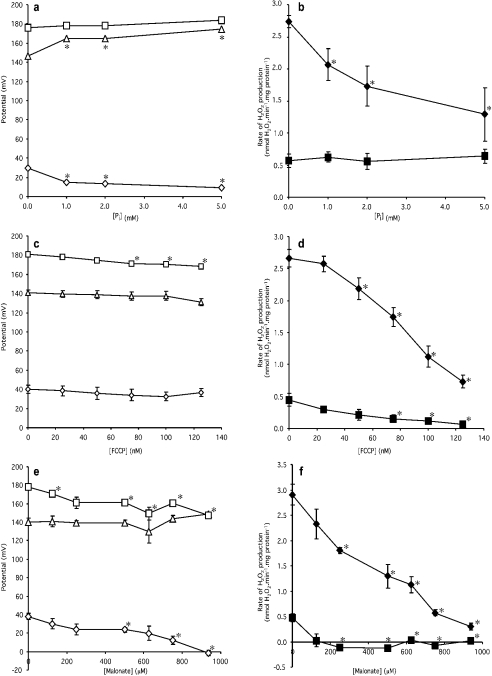
Having established that the rate of superoxide production by reverse electron transport into complex I depends more strongly on ΔpH than on Δψ, we investigated the relationship in more detail. To vary ΔpH over a range of values, we could have titrated with nigericin, but it is difficult to obtain consistent results with submaximal concentrations of nigericin, so instead, a phosphate titration was performed. Inorganic phosphate (Pi) enters the mitochondrial matrix effectively in symport with protons, and thus lowers ΔpH, leading to a compensatory rise in Δψ. The effect of increasing Pi concentration on Δp, Δψ and ΔpH is shown in Figure 2(a). As expected, with increasing Pi concentrations, ΔpH progressively declined, Δψ increased and Δp remained almost constant. Figure 2(b) shows the corresponding effect of Pi titration on the H2O2 production rate: increasing Pi concentration caused a sharp reduction in H2O2 production rate. In the presence of nigericin, increasing Pi concentration had no effect on H2O2 production, as expected, since ΔpH was already abolished. Once again, qualitatively, superoxide production is more sensitive to small decreases in ΔpH than to the equivalent small increases in Δψ.
Figure 2. Effects of phosphate, FCCP and malonate on the components of Δp and on the H2O2 production rate.
Incubation conditions were the same as those described in the legend to Figure 1(a). Open symbols (a,c,e) represent potentials (squares, Δp; triangles, Δψ; diamonds, ΔpH). Closed symbols (b,d,f) represent H2O2 production rate (squares, 100 nM nigericin present; diamonds, nigericin absent). Data points represent means±S.E.M. for measurements on four to six separate mitochondrial preparations. Phosphate (Pi) was added as KH2PO4 and malonate was added as the K+ salt. *Significant (P<0.05) difference from the datum point at zero concentration of Pi/FCCP/malonate for each respective panel.
Another way to vary ΔpH is to uncouple progressively with FCCP. In this case, the supply of electrons to complex I may also decline as uncoupling lowers Δp, stimulates downstream electron transport and tends to oxidize the Q pool. Figure 2(c) shows that with increasing FCCP concentration, Δp, Δψ and ΔpH all declined slightly. Even though the changes in ΔpH with FCCP were not significant, there was a strong effect of FCCP on superoxide production rate, again indicating qualitatively that superoxide production is very sensitive to ΔpH (Figure 2d). The effect may be slightly exaggerated, due to reduced electron supply into complex I resulting from oxidation of the Q pool. In the presence of nigericin, the rate of H2O2 production also declined with increasing FCCP concentration, unlike the titration with phosphate shown in Figure 2(b). This indicates that, as well as its effect through ΔpH, there was an additional effect of FCCP on H2O2 production rate. This could be via the redox state of the Q pool due to the drop in Δp as FCCP increased, or a direct effect of Δψ on complex I. The relatively small absolute magnitude of this additional effect suggests that alterations in the redox state of the Q pool or in Δψ are less important than alterations in ΔpH in causing the decrease in H2O2 production in response to uncoupling.
ΔpH can also be varied by inhibiting succinate oxidation with malonate, a competitive inhibitor of succinate dehydrogenase. In this case, the supply of electrons to the Q pool and to complex I will also decline as succinate dehydrogenase is progressively inhibited. The effect of malonate concentration on Δp, Δψ and ΔpH is shown in Figure 2(e). With increasing malonate concentration, Δp and ΔpH declined and Δψ remained almost constant. Figure 2(f) shows the corresponding effect of malonate titration on H2O2 production rate. In both the presence and absence of nigericin, the H2O2 production rate declined with increasing malonate concentration. The steep slope of the line in the absence of nigericin, where ΔpH declined but Δψ remained almost constant, once again suggests that superoxide production is very sensitive to ΔpH, whereas the effect in the presence of nigericin echoes the indirect effect through the Q pool and the direct effect through Δψ also seen in Figure 2(d), and leads to the same interpretation.
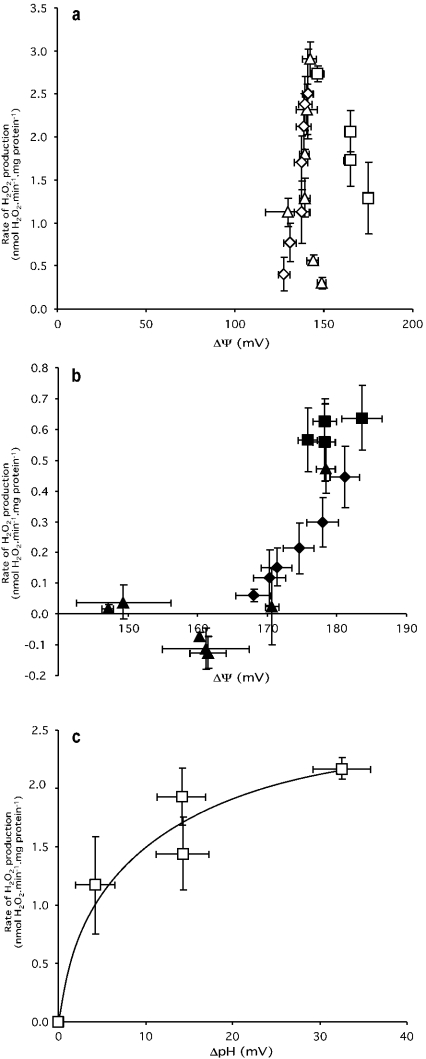
The data in Figure 2 allow a quantitative analysis of the effects of Δψ and ΔpH on the rate of superoxide production by complex I to be made, as shown in Figure 3. Figure 3(a) shows that there is no unique dependence of H2O2 production on Δψ in the absence of nigericin; the relationship depends on the contribution of ΔpH, which varies in different ways in the different titrations. During titration with phosphate, ΔpH and Δψ change in different directions, but during titration with FCCP they vary in the same direction, whereas malonate titration leads to greater changes in ΔpH than in Δψ.
Figure 3. Dependence of H2O2 production rate on Δψ and ΔpH.
Data are taken from Figure 2. (a) Membrane potential (Δψ) where ΔpH is present; (b) Δψ where ΔpH is zero; and (c) ΔpH, with the effect due to Δψ in panel (b) subtracted. ΔpH and Δψ were titrated with Pi (squares), FCCP (diamonds) or malonate (triangles) in the presence (closed symbols) or absence (open symbols) of 100 nM nigericin. Points represent means±S.E.M. of measurements on four to six separate mitochondrial preparations.
In the presence of nigericin, ΔpH is clamped at zero, and these complications are eliminated. Figure 3(b) shows the dependence of H2O2 production on Δψ when nigericin is present and ΔpH is zero. Because the redox state of the Q pool, and hence the electron supply to complex I, is compromised during FCCP and malonate titrations (see above), the true dependence on Δψ may be much less steep than the relationship shown here.
Figure 3(c) shows the dependence of H2O2 production on ΔpH during the phosphate titration, after correction for effects of Δψ (results for the FCCP and malonate titrations were unsuitable, because Δp changed and compromised the Q pool redox state). For each point on the phosphate titration, we took the rate of H2O2 production in the absence of nigericin in Figure 3(a) and subtracted the small rate at the same value of Δψ in the presence of nigericin in Figure 3(b), and then plotted the result against the appropriate ΔpH value shown in Figure 2(a). The result in Figure 3(c) shows quantitatively how superoxide production by complex I depends on ΔpH.
Comparison of Figure 3(b) with Figure 3(c) shows how superoxide production from complex I during reverse electron transport is much more dependent on ΔpH than on Δψ. An increase in ΔpH from 0 to 15 mV (at a Δp of approx. 170–180 mV) gave an increase of approx. 2 nmol·min−1·mg of protein−1 in the H2O2 production rate (Figure 3c). The same 15 mV increase in Δψ at the same overall Δp gave an increase of 0.7 nmol·min−1·mg of protein−1 in H2O2 (Figure 3b). Because of compromised electron supply in the FCCP and malonate titrations in Figure 3(b), the true increase in H2O2 production in response to Δψ is likely to be rather less. Thus the H2O2 production rate is at least 3-fold more sensitive to changes in ΔpH than changes in Δψ.
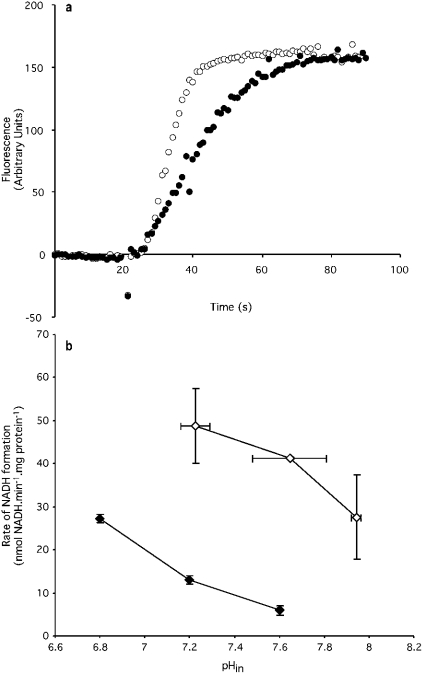
We next investigated the dependence of reverse electron transport on ΔpH, since the inhibitory effect of nigericin on the superoxide production rate might reflect inhibition of reverse electron transport through complex I. Figure 4(a) indicates that nigericin did decrease the rate of reverse electron transport from succinate to NAD+ by approx. 40%. This effect might have been due to changes in ΔpH, or to changes in pHin (as discussed previously for the effect of nigericin on superoxide production). Therefore the rate of reverse electron transport was determined at different values of pHin, as in Figure 1(b). Unlike the superoxide production rate, the rate of reverse electron transport did depend on pHin; it declined with increasing pH, as shown in Figure 4(b). However, like superoxide production, reverse electron transport rate also depended on ΔpH; at any pHin the rate of reverse electron transport was higher in the absence of nigericin than in its presence. Thus the different effects of pHin show that superoxide production from complex I during reverse electron transport is not just a simple function of reverse electron transport rate, while the similar effects of ΔpH show that the two processes do have some common features.
Figure 4. Effects of nigericin and pHin on reverse electron transport.
(a) Reverse electron transport measured by NADH production with succinate as substrate. The fluorescence signal was set to zero at time zero. Points represent means for measurements on three separate mitochondrial preparations, with error bars omitted for clarity. Succinate (4 mM) was added at 20 s. (b) Effect of internal (matrix) pH (pHin) on reverse electron transport rate. pHin in the absence of nigericin was determined in parallel incubations using the TPMP+ electrode, as described in the Materials and methods section. In the presence of nigericin, pHin=pHout. Open symbols, nigericin absent; closed symbols, plus 100 nM nigericin.
DISCUSSION
High rates of superoxide production by complex I during reverse electron transport have been reported for brain and heart mitochondria from the rat [14,16,21] and for mitochondria from the fruit fly Drosophila melanogaster [30]. In the present study, we show that the same is true for mitochondria isolated from rat skeletal muscle: succinate-supported superoxide production is high, and it is rotenone- and piericidin-sensitive, showing that it depends on reverse electron transport into complex I, and explaining its high dependence on protonmotive force.
The relationship between the high rate of superoxide production from complex I during reverse electron transport and protonmotive force has not previously been investigated in detail. Previous reports [22,24,25] have used uncouplers and/or inhibitors to show that superoxide production is sensitive to Δψ, but under conditions in which electron supply and ΔpH will also be altered. We have confirmed these observations using FCCP and malonate (Figure 3a). We show here that protonmotive force alters superoxide production mostly by a direct effect on complex I, and only to a small extent through an indirect effect on the redox state of the Q pool, and hence on electron supply. We confirm the earlier finding [25] that addition of nigericin, which lowers ΔpH and raises Δψ at constant Δp, strongly decreases superoxide production, showing that it is more strongly inhibited by the drop in ΔpH than it is stimulated by the rise in Δψ.
We demonstrate using a phosphate titration (where Δp and electron supply are relatively constant) that a high ΔpH across the mitochondrial inner membrane during reverse electron transfer is required for high rates of superoxide production. Quantitatively, superoxide production by complex I is at least 3-fold more sensitive to ΔpH than to Δψ (Figure 3). The published results showing sensitivity to Δψ [22,24,25] are probably better interpreted as reflecting mostly sensitivity to ΔpH and reduced electron supply.
Whilst many biochemical processes are dependent on pH, it is those that directly or indirectly move protons across a membrane, such as the reactions catalysed by complexes III and IV [31,32], that can be dependent on ΔpH. It seems likely, therefore, that the ΔpH-sensitive generation of superoxide from complex I is linked mechanistically to the transport of protons by the enzyme. The mechanism of proton pumping by complex I remains almost completely unknown, partly because of the large size of the complex, and partly because of the lack of easily studied intermediates in the coupling reaction. In other respiratory complexes, the behaviour of intermediates has led to mechanistic insights: the oxidant-induced reduction of the b cytochromes led to the formulation of the Q cycle in complex III [33], and analysis of the different states of the binuclear cluster has allowed considerable progress in understanding the mechanism of complex IV [34]. Superoxide production during reverse electron transport in complex I is strongly dependent on the components of Δp (Figure 3), implying that the reductant of oxygen at this site is intimately involved in the coupling mechanism. Superoxide production by complex I may provide a new tool for the elucidation of the mechanism of proton pumping by this complex.
In intact cells, the pH gradient across the mitochondrial inner membrane is generally high, despite high concentrations of phosphate. Estimates range from 30 mV in hepatocytes [35,36] and adipocytes [37] to 73 mV in thymocytes [38]. Whether high rates of superoxide production from complex I occur in cells will depend on the current supply of electrons from NADH and ubiquinone, on the current value of ΔpH (which may be regulated by calcium uptake [39], the mitochondrial permeability transition pore [40] or uncoupling proteins [41]) and on other cellular regulators of complex I superoxide production [42,43]. Fatty acid oxidation may be particularly prone to cause superoxide production from this site, since it is regulated mostly by fatty acid supply, leads to reduction of both NADH and Q, and is associated with high ΔpH. One test of whether the site is active in cells under any particular condition is to measure the sensitivity of ROS production to rotenone: some studies have reported that ROS production in intact cells is inhibited by rotenone [44–47], although other studies find that rotenone increases cellular ROS production [48–51].
Acknowledgments
This work was supported by the Medical Research Council and the Wellcome Trust. We thank Steven Roebuck, Helen Boysen and Julie Buckingham for excellent technical assistance.
References
- 1.Halliwell B., Gutteridge J. M. C. Free radicals in biology and medicine. NY: Oxford University Press Inc; 1999. [Google Scholar]
- 2.Cadenas E., Davies K. J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 3.Boveris A., Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975;54:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- 4.Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984;105:429–435. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- 5.Turrens J. F. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 6.Melov S., Coskun P., Patel M., Tuinstra R., Cottrell B., Jun A. S., Zastawny T. H., Dizdaroglu M., Goodman S. I., Huang T. T., et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Huang T. T., Carlson E. J., Melov S., Ursell P. C., Olson J. L., Noble L. J., Yoshimura M. P., Berger C., Chan P. H., et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 8.Allen R. G., Tresini M. Oxidative stress and gene regulation. Free Radical Biol. Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 9.Turrens J. F., Alexandre A., Lehninger A. L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 10.Degli Esposti M., Ghelli A. The mechanism of proton and electron transport in mitochondrial complex I. Biochim. Biophys. Acta. 1994;1187:116–120. doi: 10.1016/0005-2728(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 11.Brandt U. Proton translocation by membrane–bound NADH:ubiquinone–oxidoreductase (complex I) through redox-gated ligand conduction. Biochim. Biophys. Acta. 1997;1318:79–91. doi: 10.1016/s0005-2728(96)00141-7. [DOI] [PubMed] [Google Scholar]
- 12.Dutton P. L., Moser C. C., Sled V. D., Daldal F., Ohnishi T. A reductant-induced oxidation mechanism for complex I. Biochim. Biophys. Acta. 1998;1364:245–257. doi: 10.1016/s0005-2728(98)00031-0. [DOI] [PubMed] [Google Scholar]
- 13.Turrens J. F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Fiskum G., Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 15.Young T. A., Cunningham C. C., Bailey S. M. Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch. Biochem. Biophys. 2002;405:65–72. doi: 10.1016/s0003-9861(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 16.Kushnareva Y., Murphy A. N., Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation–reduction state. Biochem. J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genova M. L., Ventura B., Giuliano G., Bovina C., Formiggini G., Parenti Castelli G., Lenaz G. The site of production of superoxide radical in mitochondrial complex I is not a bound ubisemiquinone but presumably iron–sulfur cluster N2. FEBS Lett. 2001;505:364–368. doi: 10.1016/s0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- 18.Herrero A., Barja G. Localization of the site of oxygen radical generation inside the complex I of heart and nonsynaptic brain mammalian mitochondria. J. Bioenerg. Biomembr. 2000;32:609–615. doi: 10.1023/a:1005626712319. [DOI] [PubMed] [Google Scholar]
- 19.Boveris A., Cadenas E., Stoppani A. O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem. J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genova M. L., Pich M. M., Biondi A., Bernacchia A., Falasca A., Bovina C., Formiggini G., Castelli G. P., Lenaz G. Mitochondrial production of oxygen radical species and the role of coenzyme Q as an antioxidant. Exp. Biol. Med. 2003;228:506–513. doi: 10.1177/15353702-0322805-14. [DOI] [PubMed] [Google Scholar]
- 21.Hansford R. G., Hogue B. A., Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- 22.Votyakova T. V., Reynolds I. J. ΔΨm-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 23.Han D., Canali R., Rettori D., Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol. Pharmacol. 2003;64:1136–1144. doi: 10.1124/mol.64.5.1136. [DOI] [PubMed] [Google Scholar]
- 24.Korshunov S. S., Skulachev V. P., Starkov A. A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 25.Liu S. S. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci. Rep. 1997;17:259–272. doi: 10.1023/a:1027328510931. [DOI] [PubMed] [Google Scholar]
- 26.Rolfe D. F. S., Hulbert A. J., Brand M. D. Characteristics of mitochondrial proton leak and control of oxidative phosphorylation in the major oxygen-consuming tissues of the rat. Biochim. Biophys. Acta. 1994;1188:405–416. doi: 10.1016/0005-2728(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 27.St-Pierre J., Buckingham J. A., Roebuck S. J., Brand M. D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 28.Brand M. D. Measurement of mitochondrial protonmotive force. In: Brown G. C., Cooper C. E., editors. Bioenergetics: A Practical Approach. Oxford: IRL Press; 1995. pp. 39–62. [Google Scholar]
- 29.Ernster L., Lee C.-P. Energy–linked reduction of NAD+ by succinate. Methods Enzymol. 1967;10:729–738. [Google Scholar]
- 30.Miwa S., St–Pierre J., Partridge L., Brand M. D. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radical Biol. Med. 2003;35:938–948. doi: 10.1016/s0891-5849(03)00464-7. [DOI] [PubMed] [Google Scholar]
- 31.Moroney P. M., Scholes T. A., Hinkle P. C. Effect of membrane potential and pH gradient on electron transfer in cytochrome oxidase. Biochemistry. 1984;23:4991–4997. doi: 10.1021/bi00316a025. [DOI] [PubMed] [Google Scholar]
- 32.Papa S., Lorusso M., Izzo G., Capuano F. Control of electron transfer in the cytochrome system of mitochondria by pH, transmembrane pH gradient and electrical potential. The cytochromes b-c segment. Biochem. J. 1981;194:395–406. doi: 10.1042/bj1940395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trumpower B. L. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J. Biol. Chem. 1990;265:11409–11412. [PubMed] [Google Scholar]
- 34.Michel H. Cytochrome c oxidase: catalytic cycle and mechanisms of proton pumping – a discussion. Biochemistry. 1999;38:15129–15140. doi: 10.1021/bi9910934. [DOI] [PubMed] [Google Scholar]
- 35.Nobes C. D., Brown G. C., Olive P. N., Brand M. D. Non–ohmic proton conductance of the mitochondrial inner membrane in hepatocytes. J. Biol. Chem. 1990;265:12903–12909. [PubMed] [Google Scholar]
- 36.Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J. Biol. Chem. 1980;255:1458–1464. [PubMed] [Google Scholar]
- 37.Davis R. J., Brand M. D., Martin B. R. The effect of insulin on plasma-membrane and mitochondrial-membrane potentials in isolated fat-cells. Biochem. J. 1981;196:133–147. doi: 10.1042/bj1960133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brand M. D., Felber S. M. The intracellular calcium antagonist TMB-8 [8-(NN-diethylamino)octyl-3,4,5-trimethoxybenzoate] inhibits mitochondrial ATP production in rat thymocytes. Biochem. J. 1984;224:1027–1030. doi: 10.1042/bj2241027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernardi P., Azzone G. F. ΔpH induced calcium fluxes in rat liver mitochondria. Eur. J. Biochem. 1979;102:555–562. doi: 10.1111/j.1432-1033.1979.tb04272.x. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch T., Marzo I., Kroemer G. Role of the mitochondrial permeability transition pore in apoptosis. Biosci. Rep. 1997;17:67–76. doi: 10.1023/a:1027339418683. [DOI] [PubMed] [Google Scholar]
- 41.Talbot D. A., Lambert A. J., Brand M. D. Production of endogenous matrix superoxide from mitochondrial complex I leads to activation of uncoupling protein 3. FEBS Lett. 2004;556:111–115. doi: 10.1016/s0014-5793(03)01386-3. [DOI] [PubMed] [Google Scholar]
- 42.Taylor E. R., Hurrell F., Shannon R. J., Lin T. K., Hirst J., Murphy M. P. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 43.Raha S., Myint A. T., Johnstone L., Robinson B. H. Control of oxygen free radical formation from mitochondrial complex I: roles for protein kinase A and pyruvate dehydrogenase kinase. Free Radical Biol. Med. 2002;32:421–430. doi: 10.1016/s0891-5849(01)00816-4. [DOI] [PubMed] [Google Scholar]
- 44.Vrablic A. S., Albright C. D., Craciunescu C. N., Salganik R. I., Zeisel S. H. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl-rac-glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J. 2001;15:1739–1744. doi: 10.1096/fj.00-0300com. [DOI] [PubMed] [Google Scholar]
- 45.Schuchmann S., Heinemann U. Increased mitochondrial superoxide generation in neurons from trisomy 16 mice: a model of Down's syndrome. Free Radical Biol. Med. 2000;28:235–250. doi: 10.1016/s0891-5849(99)00226-9. [DOI] [PubMed] [Google Scholar]
- 46.Li Y., Trush M. A. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem. Biophys. Res. Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 47.Parthasarathi K., Ichimura H., Quadri S., Issekutz A., Bhattacharya J. Mitochondrial reactive oxygen species regulate spatial profile of proinflammatory responses in lung venular capillaries. J. Immunol. 2002;169:7078–7086. doi: 10.4049/jimmunol.169.12.7078. [DOI] [PubMed] [Google Scholar]
- 48.Barrientos A., Moraes C. T. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J. Biol. Chem. 1999;274:16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura K., Bindokas V. P., Kowlessur D., Elas M., Milstien S., Marks J. D., Halpern H. J., Kang U. J. Tetrahydrobiopterin scavenges superoxide in dopaminergic neurons. J. Biol. Chem. 2001;276:34402–34407. doi: 10.1074/jbc.M103766200. [DOI] [PubMed] [Google Scholar]
- 50.Li N., Ragheb K., Lawler G., Sturgis J., Rajwa B., Melendez J. A., Robinson J. P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 51.Siraki A. G., Pourahmad J., Chan T. S., Khan S., O'Brien P. J. Endogenous and endobiotic induced reactive oxygen species formation by isolated hepatocytes. Free Radical Biol. Med. 2002;32:2–10. doi: 10.1016/s0891-5849(01)00764-x. [DOI] [PubMed] [Google Scholar]