Abstract
The COQ2 gene in Saccharomyces cerevisiae encodes a Coq2 (p-hydroxybenzoate:polyprenyl transferase), which is required in the biosynthetic pathway of CoQ (ubiquinone). This enzyme catalyses the prenylation of p-hydroxybenzoate with an all-trans polyprenyl group. We have isolated cDNA which we believe encodes the human homologue of COQ2 from a human muscle and liver cDNA library. The clone contained an open reading frame of length 1263 bp, which encodes a polypeptide that has sequence homology with the Coq2 homologues in yeast, bacteria and mammals. The human COQ2 gene, when expressed in yeast Coq2 null mutant cells, rescued the growth of this yeast strain in the absence of a non-fermentable carbon source and restored CoQ biosynthesis. However, the rate of CoQ biosynthesis in the rescued cells was lower when compared with that in cells rescued with the yeast COQ2 gene. CoQ formed when cells were incubated with labelled decaprenyl pyrophosphate and nonaprenyl pyrophosphate, showing that the human enzyme is active and that it participates in the biosynthesis of CoQ.
Keywords: CoQ biosynthesis, human COQ2 gene, polyprenyl transferase, yeast mutant complementation
Abbreviations: PHB, p-hydroxybenzoate; Coq2, PHB:polyprenyl transferase; EST, expressed sequence tag; MTE, multiple tissue expression; poly(A)+, polyadenylated; SD, synthetic glucose minimal medium; TM-HMM, transmembrane hidden Markov model; Ura, uracil; YNB, yeast nitrogen base
INTRODUCTION
CoQ (ubiquinone) serves as a redox carrier in the mitochondrial respiratory chain and is the only lipid-soluble antioxidant that is endogenously synthesized in both unicellular and multicellular organisms [1]. Recent studies have shown that CoQ is also involved in a broad range of metabolic processes, in addition to its involvement in redox processes and its role as antioxidant. CoQ participates in the electron-transport systems of plasma membranes and lysosomes, in the regulation of mitochondrial permeability transition pores and in the modulation of β2 integrins that are present on the surface of blood monocytes [2–4]. Improvement of endothelial dysfunction by CoQ has also been observed as a result of increased production of nitric oxide [5]. Furthermore, CoQ in yeast participates in the oxidation of sulphide, whereas in bacteria it is involved in the introduction of disulphide bonds into proteins [6,7].
Genes that are involved in biosynthetic processes are often modified in human diseases and under experimental pathological conditions [8], and it is therefore important to identify such genes. Tissue concentrations of CoQ are substantially decreased in cardiomyopathy, cancer and Niemann–Pick type C disease, whereas the concentrations are increased in Alzheimer's disease, prion diseases, preneoplastic liver noduli and in Type II diabetes. The concentration of CoQ in tissues increases in humans during the first 20–30 years of life, followed by a continuous decrease in all organs. Many cases of genetic deficiency of CoQ synthesis in children have been well documented [9]. In these cases, lipid is missing from tissues in varying degrees, leading to severe neuronal and muscular symptoms. Supply of lipid in the diet improves cellular functions considerably.
The genes involved in the final reactions of the CoQ biosynthetic pathway have been cloned in bacteria, and some of these genes have been cloned in yeast [10]. Information about these enzymes in mammalian tissues is, however, limited [10]. In particular, details of the terminal synthesis of CoQ in mammalian cells are not known, and it has been questioned whether the sequence of reactions that occurs in yeast and in bacteria also occurs in mammals [11]. Only two mammalian genes have been isolated, and this has been achieved by employing their ability to rescue CoQ biosynthesis in Saccharomyces cerevisiae [12,13]. These genes are COQ3, which catalyses two O-methylations, and COQ7, which mediates hydroxylation of the benzoquinone ring. 4-p-Hydroxybenzoate:polyprenyl transferase (PHB:polyprenyl transferase), also referred to as the ‘Coq2 enzyme’, mediates the second step in the final reaction sequence of CoQ biosynthesis, which is the condensation of the polyisoprenoid side chain with PHB. The length of the polyprenyl side chain is different in different species. S. cerevisiae, Escherichia coli, rodents and humans have 6, 8, 9 and 10 isoprene units respectively [8]. However, the specificity is not complete, and all rodent organs contain some CoQ10, whereas human tissues have low amounts of CoQ9. It has been shown that the rat transferase uses solanesyl-PPi to generate CoQ9, whereas the yeast transferase uses hexaprenyl-PPi to produce CoQ6 as the final product [10]. The gene encoding the transferase has been cloned and sequenced in S. cerevisiae (COQ2) [14], in E. coli (UBIA) [15] and in Schizosaccharomyces pombe (PPT1) [16]. There is a high degree of sequence homology among these genes, and they all contain two motifs that are rich in conserved aspartic acid residues, which bind polyprenyl diphosphate. All the sequences contain six potential membrane-spanning domains and an N-terminal mitochondrial leader sequence. Some aspects of the enzyme activity have been studied in isolated mitochondria, using partially purified protein, and in membranes of E. coli that overexpress the UBIA gene [17].
In the present study, we have cloned and sequenced the human COQ2 gene and used it for complementation of mutant S. cerevisiae cells that are deficient in this gene. The restored cells were analysed in accordance to their capacity for growth and CoQ synthesis, to confirm the identity and functional capacity of the isolated human Coq2 enzyme.
EXPERIMENTAL
Chemicals
all-trans-Nonaprenyl (solanesyl) diphosphate and all-trans-decaprenyl (spadicyl) diphosphate were prepared in parallel by a two-step synthesis performed as described previously with some modifications [18]. Both alcohols were first oxidized with pyridinium chlorochromate (Aldrich) and the aldehydes produced were reduced with [3H]NaBH4 (60 Ci/mmol; Amersham Biosciences). The products were carefully purified on a silica gel column (240–400 mesh; Merck). Elution was monitored by TLC (silica gel plate 60; Merck), the plates being developed in benzene/ethyl acetate (95:5, v/v). Pure all-trans-[1-3H]solanesol and [1-3H]decaprenol were phosphorylated according to the method described earlier [19] with minor modifications, namely the separation of [1-3H]polyprenyl monophosphate from [1-3H]polyprenyl diphosphate was performed on DEAE-Sephadex (acetate form; Amersham Biosciences) with the application of a linear gradient of ammonium acetate from 0 to 300 mM in methanol. Fractions containing the diphosphate were pooled, evaporated and freeze-dried after the addition of water. The diphosphates were dissolved in benzene/ethanol (7:3, v/v).
Yeast strains and growth media
The S. cerevisiae null mutant strain CENΔCoq2 (MATa, his3-Δ1, leu2-3, 112, tpr-289, ura3-52, MAL2-8 c, MAL3, SUC3, COQ2::HIS3) and the JM8 yeast strain Rho− (MATα, ade1, r0) were gifts from Dr C. Ocana (University of Seville, Seville, Spain). Media for growth of yeast included YPD [1% (w/v) yeast extract, 2% (w/v) peptone and 2% (w/v) dextrose], YPG [1% yeast extract, 2% peptone and 3% (w/v) glycerol], YPGal [1% yeast extract, 2% peptone and 2% (w/v) galactose], YNB-D [0.18% YNB (yeast nitrogen base) without amino acids and without (NH4)2SO4, 0.014% NaH2PO4 and 0.5% (NH4)2SO4 and 2% (w/v) dextrose] and synthetic glucose minimal medium without uracil [SD-ura: 0.17% YNB, 2% dextrose, 0.5% (NH4)2SO4, and a complete amino acid supplement lacking uracil]. All yeast strains were grown at 30 °C with shaking at 250 rev./min.
Cloning of the human COQ2 gene
Preparations of mRNA from human liver and skeletal muscle (ClonTech) were used as templates for preparing cDNA with the SMART™ RACE cDNA amplification kit and the Advantage2 polymerase mix (ClonTech). For the 3′-cloning, the public sequence AF091086 was used to design the PCR primer BV-1 (5′-AGTCAACGGCTCTGCGGTTCGGAGA-3′). PCR amplification was performed according to the manufacturer's instructions using a universal primer mix and BV-1 primers. A band of approx. 700 bp in length was obtained. The fragment was subcloned into pCR2.1-TOPO and subsequently sequenced. For the 5′-cloning, the gi29824575 genomic sequence was used to design two specific primers, BV-2 (5′-GTATTCCTCCCTAGAGTAAGCGACCACGATGACCC-3′) and BV-3 (5′-TCTCCGAACCGCAGAGCCGTTGACT-3′). PCR amplification was performed according to the manufacturer's instructions, using 5% (v/v) DMSO. A band of approx. 1000 bp in length was obtained and subsequently purified by gel electrophoresis. The purified PCR fragment containing the 5′-end of the human COQ2 gene was joined in a PCR to the plasmid DNA containing the 3′-part of this gene described above. PCR was performed as before, although in this case the universal primer mix was used instead of BV-3. A band of 1700 bp in length was purified by gel electrophoresis, cloned into pCR2.1-TOPO and sequenced. Five amino acid mutations were detected. These were subsequently replaced by digestion with restriction enzymes and religated to generate pBV134.
Cloning of the yeast COQ2 gene
S. cerevisiae poly(A)+ (polyadenylated) RNA (ClonTech) was used as a template using the ThermoScript reverse transcriptase–PCR system (Life Technologies). The best result was obtained using 100 ng of yeast poly(A)+ RNA, and the resulting cDNA was used as a template to amplify the COQ2 gene by PCR, using BV-4 (5′-CAACTAATGTTTATTTGGCAGAG-3′) and BV-5 (5′-CAAGAATCCAAACAGTCTCA-3′) primers and PLATINIUM Taq DNA Polymerase High Fidelity (Gibco BRL, Basel, Switzerland). A fragment of approx. 1250 bp in length was purified by gel electrophoresis and subcloned into pYes2.1/V5-His as described below.
Construction of yeast expression vectors
The subcloning of the human and yeast COQ2 genes in a yeast expression vector was performed with a pYES2.1 TOPO TA cloning kit (Invitrogen). Construction of the hCOQ2 began by a PCR amplification using BV6 (5′-AGAACCATGACCCCAATTTCA-3′) and BV7 (5′-AGAACCATGACCCCAATTTCA-3′) primers. PCR was performed using pfu-turbo DNA polymerase (Stratagene), 5% DMSO and pBV134 as template. A fragment of approx. 1265 bp in length was purified by gel electrophoresis and subsequently incubated with Taq DNA polymerase (Boehringer Mannheim, Mannheim, Germany) in the presence of 200 μM dATP at 72 °C for 15 min. This incubation was necessary to incorporate the A base pair that was required for the TOPO cloning. The fragment was subsequently cloned into pYES2.1/V5-His, and the insert analysed by restriction analysis and sequencing to generate pBV277. Two further plasmids were constructed in which 50 (Δ1–50) and 127 (Δ1–127) N-terminal amino acids respectively of the human COQ2 gene had been deleted. Construction began with PCR amplification using BV8 (5′-AGCGCCATGCTGGGC-3′) and BV7 for the amplification of h(Δ1–50)COQ2, and using BV9 (5′-CGCCTCATGCGGTTGGACAAG-3′) and BV7 for the amplification of h(Δ1–127)COQ2. After PCR amplification, fragments of approx. 1115 and 884 bp in length respectively were obtained, and these fragments were subsequently cloned into pYES2.1/V5-His as described above to create pBV601 and pBV600. pBV601 carried the (Δ1–50) deleted hCOQ2 genes and pBV600 carried the (Δ1–127) deleted hCOQ2 genes. The yeast COQ2 gene was cloned from a yeast cDNA library as described above. Subsequently, a 1250 bp fragment was subcloned into pYes2.1/V5-His to generate pBV275.
Expression tissue profiling
A commercial dot-blot MTE (multiple tissue expression) array (ClonTech) was used in a hybridization experiment with a 283 bp human COQ2 gene-specific probe. This probe, which corresponded to the C-terminal region of the human COQ2 gene, was prepared by digestion of the 1700 bp fragment described above with DraIII and HindII, followed by gel purification of the resulting fragment. The purified fragment (25 ng) was used in a 32P-labelling reaction performed as recommended in the Strip-EZ DNA probe synthesis instruction manual (Ambion, Austin, TX, U.S.A.). Hybridization and washing conditions were those recommended by the ClonTech manual PT3307-1. The MTE array was scanned with a STORM860 phosphorImager after 48 h exposure. ImageQuant was used to measure the hybridization signal. Northern blotting was performed with the probe described above on poly(A)+ RNA from human pancreas, skeletal muscle, liver and fetal brain (ClonTech). Hybridization and washing were performed as recommended by ClonTech.
Complementation of a S. cerevisiae Coq2 null mutant
The yeast null mutant strain CENΔCoq2, which carries a truncated Coq2 gene, was grown overnight in 10 ml of YPD medium, during which time it reached the stationary phase. The cells were made competent and transformed separately with pBV275, pBV277, pBV600 and pBV601 plasmid DNA according to the manufacturer's instructions (Invitrogen). Isolated recombinant yeast colonies carrying the yeast and human COQ2 genes were obtained after growth on SD/−ura plates. The recombinant yeast Rho+ genotype was determined by mating with JM8 yeast. Diploid cells were selected by 2–3 days growth in YNB-glucose medium. Cells were replica-plated into YPG medium. Positive growth after 2–3 days at 30 °C indicated a Rho+ genotype. Growth complementation of recombinant yeast cells was determined by detecting growth at 30 °C in liquid or solid YPG media. This growth was detected by following the absorbance at 600 nm and the appearance of plate growth respectively.
Cell culture and activity measurement
Recombinant yeasts separately carrying pBV277 and pBV275 plasmids coding for the yeast and human COQ2 genes respectively were picked as single colonies following incubation for 3 days on SD-ura agar plates. The cells picked were cultured under continuous shaking at 250 rev./min in SD-ura medium for 30 h at 30 °C. The cells were then cultured in YPGal medium to induce expression in the cells of the pYES vector containing either the yeast or human COQ2 gene. Accordingly, cells were diluted into YPGal (A600=0.1) and cultured for another 20 h. The cell suspension was then centrifuged at 1500 g for 10 min and resuspended in 4 ml of S-buffer (10 mM Tris/HCl, pH 7.4, and 1 M sorbitol) containing 3 mg of zymolase (ICN Biomedicals, Chemicon, Malmö, Sweden). The cells were incubated for 30 min at 30 °C with gentle shaking. After incubation, the spheroplasts were pelleted by centrifugation at 1500 g for 10 min, and the pellet resuspended in 25 ml of YPGal medium. This medium was complemented with PHB to a final concentration of 40 mM and with 1 mCi of [3H]decaprenyl-PPi or [3H]solanesyl-PPi (specific activity 3.6 and 3.9 Ci/mmol respectively). The cells were incubated at 30 °C for 19 h under continuous shaking (250 rev./min).
Product extraction and HPLC analyses
The incubation mixture was centrifuged at 1500 g for 10 min, and the pellet was then dissolved in a chloroform/methanol/water mixture (10:10:3) at 50 °C for 30 min. The phases were separated by adding chloroform and water to give a ratio of chloroform/methanol/water of 3:2:1. The phase in the solution with a lower chloroform content was passed through a silica gel column (silica gel 60, 230–400 mesh; Sharlau, Barcelona, Spain) and neutral lipids were eluted with chloroform. The chloroform phase was evaporated under nitrogen and resuspended in chloroform/methanol mixture (2:1) and analysed by reversed-phase HPLC using a Hewlett–Packard Hypersil ODS 3 μm C18 column. A linear gradient was used that started from methanol/water (9:1, v/v) and progressed to methanol/2-propanol (4:1, v/v) with a programme time of 30 min and a flow rate of 1.5 ml/min. The absorption of eluate was monitored at 210 nm, and the eluted radioactivity was measured using a radioactivity flow detector. The products were identified using synthetic standards.
Bioinformatics analyses
The TM-HMM (transmembrane hidden Markov method) method was used to model and predict the location and orientation of α-helices in membrane-spanning proteins [20]. The TargetP tool [21] was used to predict the protein subcellular localization, and PFAM analysis [22] was used to identify protein domains in the amino acid sequence of the hCoq2 protein. The Est2genome software for predicting gene sequences by sequence homology [23] was used to align hCoq2 ESTs (expressed sequence tags) to the genomic DNA sequence. The program performs a local alignment scan to align a set of spliced nucleotide sequences (ESTs, cDNAs or mRNAs) to an unspliced genomic DNA sequence, inserting introns when needed.
RESULTS
Cloning and sequencing of the human COQ2 gene
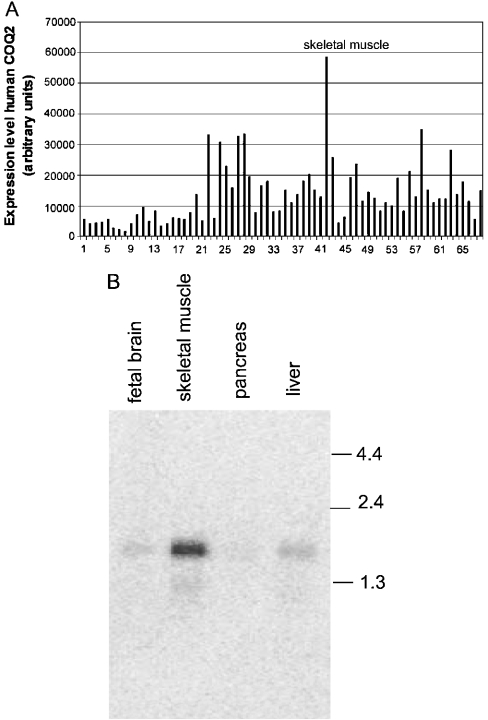
We used reverse transcriptase–PCR methods to obtain the 5′- and 3′-sequences of the human COQ2 gene. The 3′-sequence was obtained using first-strand SMART-RACE (where SMART stands for simple modular architecture research tool and RACE for rapid amplification of cDNA ends) cDNA from human liver as template. The clone was 700 bp in length, and it included approx. 400 bp of the 3′-untranslated region sequence. We tried to use the template described above to clone the 5′-end. However, we failed to obtain 5′-sequences, since the polymerase extension always stopped at a position approximately 110 bp into the sequence in Figure 1. Analysis of the genomic sequence (gi29824575) revealed that this was a GC-rich region and it contained two more methionine residues upstream of this position. Addition of DMSO did not improve the PCR. We concluded therefore that this ATG is the first translation codon. This conclusion was supported by the fact that the first of these two methionine residues is followed by a Kozac-like sequence (GCGACC). This led us to design a primer (BV-2) based on the genomic sequence containing this ATG (see Figure 1) and, together with BV-3, we amplified the 5′-end. The tissue expression data (Figure 2) showed that the hCOQ2 gene is expressed highly in muscle, and for this reason we used SMART-RACE cDNA from human skeletal muscle as template. We obtained a clone of approx. 1000 bp in length, the sequence of which overlapped with the 3′-end of the clone described above.
Figure 1. Human Coq2 nucleotide and amino acid sequence (EMBL accession number AJ621061).
The underlined amino acid sequence corresponds to what we believe to be the allylic polyprenyl diphosphate substrate-binding site. Boxes indicate the transmembrane regions predicted by computer modelling. The 28 bp in italics preceding the ATG are part of the BV-2 primer (see the Experimental section) designed to clone the 5′-end based on a genomic sequence (gi29824575). A sequence that is similar to the Kozac-like sequence (GCGACC) is indicated in bold.
Figure 2. The expression profile analysis of hCOQ2 gene in various human tissues.
(A) Multiple tissue expression dot-blot analysis of hCOQ2. The background signal was 10373 arbitrary units and it has been subtracted from all results. The human tissues tested are: 1, whole brain; 2, cerebral cortex; 3, frontal lobe; 4, parietal lobe; 5, occipital lobe; 6, temporal lobe; 7, P.g. of cerebral cortex; 8, pons; 9, cerebellum left; 10, cerebellum right; 11, corpus callosum; 12, amygdala; 13, caudate nucleus; 14, hippocampus; 15, medulla oblongata; 16, putamen; 17, substantia nigra; 18, accubens nucleus; 19, thalamus; 20, pituitary gland; 21, spinal cord; 22, heart; 23, aorta; 24, atrium left; 25, atrium right; 26, ventriculum left; 27, ventriculum right; 28, interventricular septum; 29, apex of the heart; 30, oesophagus; 31, stomach; 32, duodenum; 33, jejunum; 34, ileum; 35, ileocaecum; 36, appendix; 37, colon ascending; 38, colon transverse; 39, colon descending; 40, rectum; 41, kidney; 42, skeletal muscle; 43, spleen; 44, thymus; 45, peripheral blood leucocytes; 46, lymph node; 47, bone marrow; 48, trachea; 49, lung; 50, placenta; 51, bladder; 52, uterus; 53, prostate; 54, testis; 55, ovary; 56, liver; 57, pancreas; 58, adrenal gland; 59, thyroid gland; 60, salivary glands; 61, mammary gland; 62, fetal brain; 63, fetal heart; 64, fetal kidney; 65, fetal liver; 66, fetal spleen; 67, fetal thymus; 68 fetal lung. (B) Northern-blot analysis of hCOQ2 mRNA performed on various human tissues. Each line was loaded with 2 μg of poly(A)+ RNA from a commercial source. The numbers represent size standards in kb.
Figure 1 shows the nucleotide sequence and the resulting amino acid sequence of what we believe to be the human COQ2 gene. The cDNA contained an open reading frame of length 1263 nt encoding a polypeptide of 421 amino acids (EMBL accession number AJ621061). The gene is located on chromosome 4 and is composed of seven exons that extend over 20700 nt in the chromosome 4q21–4q22 regions. The human COQ2 gene sequence is partially supported by several EST sequences that predict the seven exons in the cDNA, with the exception of exon 1. This exon contains an additional 111 bp at the 5′-end that are not present in any of the known EST sequences.
The amino acid sequence of human COQ2 is 46% identical with that of the Drosophila melanogaster protein CG9613, and it is approx. 30 and 22% identical with the yeast (M81869) and bacterial (P26601) protein homologues respectively. The sequence identity to a predicted PHB:polyprenyl transferase protein from mouse (AK009092) is approx. 60%. The underlined sequence in Figure 1 indicates a possible polyprenyl diphosphate substrate-binding site. The sequence of this site is 60% identical with that of the equivalent site in the yeast Coq2 protein.
Prediction sorting signals and localization sites
We used TM-HMM analysis to determine if the human COQ2 gene product contains potential transmembrane sequences. A total of six transmembrane segments were identified in the human Coq2 protein and these are indicated by boxes in Figure 1. TargetP analysis of the sequence of the hCOQ2 gene predicted that the protein will be located in the mitochondrion, although we could not identify a distinctive N-terminal signal peptide. PFAM analysis was also performed on the sequence of the hCOQ2 gene. The analysis revealed the presence of an UbiA domain, which is also present in several other polyprenyl transferases. The dbSNP database was screened, and this showed that there is no polymorphism in the hCOQ2 gene between nt 151 and 1263.
Tissue distribution of the hCOQ2 gene
We performed a dot-blot MTE-array analysis to determine the relative expression levels of the human COQ2 gene in different human tissues. Figure 2(A) shows that this transcript is present in all of the tissues tested. This agrees with the broad distribution of CoQ biosynthesis observed in animals. The expression levels were highest in skeletal muscle, adrenal glands and the heart. In a separate experiment (Figure 2B), the same probe was used in hybridization Northern-blot experiments against human liver, pancreas, skeletal muscles and fetal brain mRNA, and a similar relative expression pattern was obtained. The size of the transcript (approx. 1.8 kb) agreed with the size expected.
Complementation of a S. cerevisiae COQ2 mutant
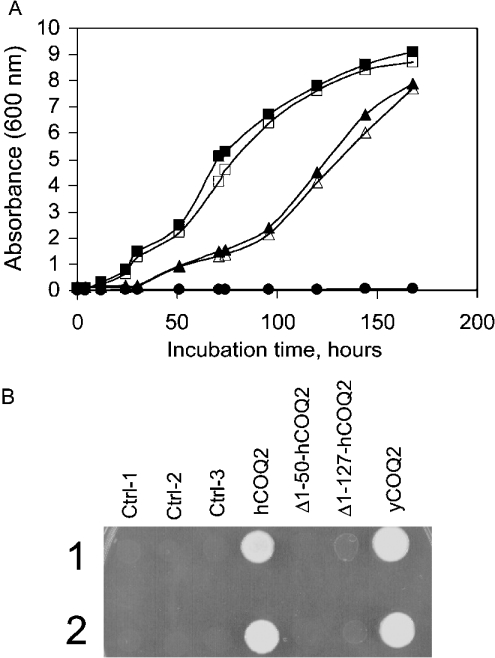
We introduced a plasmid containing the hCOQ2 cDNA into S. cerevisiae yeast cells that had a disrupted endogenous COQ2 gene. This enabled us to study the functional activity of the human Coq2 gene. The Coq2-truncated strain of yeast cannot grow on a non-fermentable carbon source (such as glycerol or ethanol), as the wild-type can. Growth was restored after the yeast had been complemented by the human COQ2 gene. Figure 3(A) shows that the expression of both yeast and human Coq2 could rescue the growth of CENΔCoq2 yeast cells when glycerol was the only carbon source, albeit with growth at a slower rate for the hCoq2 when compared with that for the yeast homologue. Control CENΔCoq2 cells were included to confirm that these cells cannot grow in the presence of glycerol as sole carbon source. These results show that the human PHB:polyprenyl transferase has a similar function to that of the known yeast COQ2 gene product.
Figure 3. Complementation of yeast COQ2 null mutant by yeast and human COQ2 genes.
(A) Null mutant strain CENΔCoq2 (•) and the same yeast strain harbouring vectors containing human (▵) or yeast (□) COQ2 from two isolated clones were grown overnight in 15 ml of YPD and SD-ura respectively. The cultures were diluted into 50 ml YPG (A600=0.1). Growth was monitored by A600 measurements. Open and closed symbols represent measurement of two independent clones except for the control null mutant strain CENΔCoq2, which is one clone (A600=0.003–0.05). (B) Effect of N-terminal sequence deletions of the human COQ2 gene. Ctrl-1, null mutant strain CENΔCoq2; Ctrl-2 and Ctrl-3, yeast harbouring control plasmids. Cells were grown in YPD and SD-ura. A spot test for growth was performed by dropping 2.5 μl of each culture on to YPG agar plates and incubating at 30 °C for 5 days. Two independent spots were applied.
The database contains two human EST sequences, AAY27589 and AAG93279, which are similar to the sequence of the hCOQ2 gene presented here. These sequences lack 50 and 127 amino acids respectively from the N terminus. We have constructed two modified forms of the human COQ2 gene to investigate the minimal sequence required for a functionally active human Coq2 protein. These two forms had deletions from the N terminus of 50 and 127 amino acids respectively. They were introduced into S. cerevisiae CENΔCoq2 yeast cells in separate experiments, as was the full-length gene. CENΔCoq2 and yCOQ2CENΔCoq2 cells were included as controls. Figure 3(B) shows that only the expression of the yeast protein and the full-length human Coq2 protein could rescue the growth of CENΔCoq2 yeast cells when glycerol was the only carbon source. Neither of the truncated hCoq2 proteins, (Δ1–50) and (Δ1–127), could complement the growth of the CENΔCoq2 yeast strain. Non-transformed and transformed cells with a plasmid backbone were included as controls to illustrate the lack of growth of these cells when glycerol is the only carbon source available. These results show that the first 50 amino acids from the N terminus of the human Coq2 protein (Figure 1) are required for the functional activity of the protein (such as protein folding, catalytic residues etc.). We could not identify a classical N-terminal import signal in the functionally active hCoq2 sequence, but it is known that mitochondrial proteins can also be imported into the mitochondria using a system of non-cleavable targeting and sorting signals distributed throughout the protein sequence itself [24]. Misguided cellular localization may explain the lack of complementation observed in the null mutant yeast cells with the deleted forms.
Biosynthesis of CoQ
[3H]Decaprenyl pyrophosphate was synthesized and used as substrate for the human Coq2 enzyme expressed in CENΔCoq2 cells, to test the biosynthetic capacity of these cells. The labelled compound does not penetrate yeast cell walls, and so the cell walls were removed by zymolase treatment before the substrate was incubated with the cells. The zymolase treatment gives cells that do not divide, but are viable. In these cells, therefore, CoQ is not produced for cell division, but some CoQ is produced as part of the protein turnover. The control mutant yeast cells, and cells complemented with the human COQ2 gene, were cultured in the YP medium that contained galactose for 16 h to allow expression of the gene product before zymolase treatment. The spheroplasts that formed under these conditions were incubated in the presence of [3H]decaprenyl pyrophosphate for 19 h, and then the labelled lipids were extracted and analysed by reversed-phase HPLC (Table 1). As expected, the limited rate of CoQ synthesis resulted in an accumulation of the intermediate [3H]decaprenyl-4-hydroxybenzoate, the amount of which was eight times higher in cells complemented with human COQ2 than it was in cells complemented with the yeast gene. Furthermore, the cells complemented with the yeast COQ2 gene, which thus possessed the yeast transferase for hexaprenyl-PPi, produced less CoQ10 than the cells complemented with the human transferase, which thus used decaprenyl-PPi. The spheroplasts were also incubated in the presence of [3H]solanesyl-PPi under the same conditions as those used for decaprenyl-PPi. Labelled CoQ9 was detected by HPLC both in cells complemented with the human gene and in cells complemented with the yeast gene, indicating that these transferases lack, to a certain extent, substrate specificity. The lipid profile of the yeast Coq2 null mutant was analysed and CoQ6 was not detected (results not shown).
Table 1. Incubation of yeast spheroplasts with [3H]decaprenyl-PPi and [3H]solanesyl-PPi.
Spheroplasts were prepared by zymolase treatment of yeast cells harbouring the indicated genes. The cells were kept in culture for 19 h in the presence of the radioactive substrates. The lipids were extracted and the products were analysed by reversed-phase HPLC and a radioactivity flow detector. For hCoq2 cells, the recovery of the added radioactivity was 1.8% for decaprenyl-4-hydroxybenzoate and 0.2% in CoQ10. The recovery of the other determinations was 0.3–0.6%. The values, expressed as c.p.m./109 cells, are the means±SD for three independent experiments. yCoq2 and hCoq2 are cells that overexpress the yeast and the human Coq2 protein respectively. n.d., not determined.
| Cells | Substrate | Decaprenyl-4-hydroxybenzoate | CoQ9 | CoQ10 |
|---|---|---|---|---|
| yCoq2 | [3H]Decaprenyl-PPi | 33800±2750 | n.d. | 14500±960 |
| hCoq2 | [3H]Decaprenyl-PPi | 276000±31120 | n.d. | 29900±2480 |
| yCoq2 | [3H]Solanesyl-PPi | n.d. | 5430±550 | n.d. |
| hCoq2 | [3H]Solanesyl-PPi | n.d. | 7870±640 | n.d. |
It has been suggested previously that the ring structure in the initial state of CoQ synthesis is not exclusively PHB. It may be either the CoA-activated form of PHB or a partially substituted form following hydroxylation and methylation [25,26]. We performed incubations in the presence of PHB-CoA, protocatechuic acid and vanilic acid as ring acceptors (results not shown). It appeared, however, that the situation is similar to that observed in rat liver, with endogenous PHB present in excess. CoQ10 was also formed in the absence of added PHB and there was no significant change in product formation after the addition of other ring structures.
DISCUSSION
PHB:polyprenyl transferase mediates the conjugation of the benzoquinone ring with the completed side chain, and thus plays a central role in the biosynthesis of CoQ. The intermediate that is formed is the only compound in the biosynthetic sequence that is accumulated and can be detected by HPLC. The six subsequent enzymes in the CoQ synthesis pathway are products of the COQ3–COQ8 genes, and these enzymes are organized into a multisubunit complex in yeast [27]. Since all six proteins may be required for the functioning of the complex, the biosynthetic intermediate that accumulates in mutants or in various modifications of metabolism is polyprenyl-4-hydroxybenzoate. These enzymes are also probably organized as a complex in animal tissues. The accumulation of polyprenyl-4-hydroxybenzoate as an intermediate both in perfused beating rat heart and in mitochondria prepared from rat heart supports this hypothesis [28,29].
The COQ2 gene restores growth of the complemented S. cerevisiae mutant strain, indicating that CoQ biosynthesis is restored in this strain. Direct evidence for this is provided by the experiments in which the culture medium was supplemented with [3H]decaprenyl diphosphate. Both decaprenyl-4-hydroxybenzoate and CoQ10 were produced, which shows that the protein product in the reconstituted strain is Coq2. The human transferase prefers decaprenyl-PPi as substrate but it can also use solanesyl-PPi, as is the case for the enzyme from other species [8]. Control S. cerevisiae cells that produce CoQ6 under natural conditions also show the same lack of specificity, but these cells also synthesize CoQ9 and CoQ10. Human tissues, however, contain small amounts of CoQ9 and, therefore, the production of this derivative in our experiments is not necessarily the result of a lack of specificity.
Preparation of spheroplasts was found to be necessary for the highly negatively charged substrates to pass through the cell wall. The use of spheroplasts, however, is not optimal. The cells were viable after overnight incubation but the cells did not divide. CoQ in animal tissues has a half-life of approx. 100 h [30], which probably is also the half-life of CoQ in our experimental system. Consequently, we expect only a limited amount of lipid synthesis to take place during the time used for incubation, which makes it difficult to analyse the biosynthetic mechanism. We attempted various permeabilizing procedures that do not interfere with growth, but all of these experiments were unsuccessful. Furthermore, incubation of cells with labelled decaprenol, which does penetrate the cell wall, did not result in CoQ labelling. Thus alcohol is not phosphorylated in yeast. Farnesol and geranylgeraniol are phosphorylated in rat liver and in tobacco plant cells [31,32].
The yeast Coq2 enzyme contains a typical N-terminal mitochondrial leader sequence, but subcellular fractionation showed that the microsomal enzyme activity was nearly as high as the mitochondrial activity [14]. The location of the enzyme in S. pombe was shown by fluorescent protein fusion to be the mitochondria [16]. In rat liver and to some extent also in spinach, enzyme activity is present in the mitochondria, the endoplasmic reticulum–Golgi system and (as has recently been discovered) in peroxisomes [33–36]. Both the endoplasmic reticulum–Golgi and the peroxisomal transferase activities are up-regulated when rats are treated with peroxisomal inducers. The products of the mevalonate pathway, CoQ, cholesterol and dolichol, are synthesized at several subcellular locations, just as protein farnesylation also occurs at several locations [37–39]. It is possible that several enzymes are produced for the PHB:polyprenyl transferase, and these enzymes are located in various subcellular compartments. It is known that mitochondrial proteins can be imported into the mitochondria using a system of non-cleavable targeting and sorting signals distributed throughout the protein sequence [24]. Further studies are required to understand fully the localization of Coq2.
The product of the COQ2 gene is important not only in the study of the specificity of the polyisoprenoid side chain, but also when considering alternative pathways concerning ring structure. The sequence of reactions is well established for yeast and bacteria, but not for animal tissues. In fact, the validity of the mechanism that occurs in yeast had been questioned for animal cells [13]. The possibility was raised that PHB, similar to fatty acids, must be activated by CoA to be able to condense with polyprenyl pyrophosphates [25]. An even more interesting suggestion is that this benzoquinone ring is not the only substrate. Rings modified by hydroxylation and methylation, which are supposed to take place later, may also serve as substrates in the condensation reaction [26]. These possibilities are of great interest from a metabolic point of view, since some of these products are generated during catecholamine catabolism. The products could then be utilized for CoQ biosynthesis. We could not test these possibilities since the yeast cells, similar to most animal tissues, contain an excess of PHB, and the conjugation product is synthesized even when external ring structures are not added. It will be necessary to isolate the transferase protein and study its enzymic properties, to evaluate possible interactions with more complex ring structures.
Acknowledgments
We thank K. Bergdahl, K. Larsson and M. Warolén for their technical support, and C. Sörving, S. James and B. Cannon for their critical review. This work was supported in part by the Family E. Persson Foundation (to J.G. and G.D.) and the Polish State Committee for Scientific Research, Project no. 6P04A 077 21 (to E.S.).
References
- 1.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Morré D. J., Morré D. M. Cell surface NADH oxidases (ECTO-NOX proteins) with roles in cancer, cellular time-keeping, growth, aging and neurodegenerative diseases. Free Radic. Res. 2003;37:795–808. doi: 10.1080/1071576031000083107. [DOI] [PubMed] [Google Scholar]
- 3.Fontaine E., Ichas F., Bernardi P. A ubiquinone-binding site regulates the mitochondrial permeability transition pore. J. Biol. Chem. 1998;273:25734–25740. doi: 10.1074/jbc.273.40.25734. [DOI] [PubMed] [Google Scholar]
- 4.Turunen M., Wehlin M., Sjöberg J., Lundahl J., Dallner G., Brismar K., Sindelar P. J. β2-Integrin and lipid modifications indicate a non-antioxidant mechanism for the anti-atherogenic effect of dietary coenzyme Q10. Biochem. Biophys. Res. Commun. 2002;296:255–260. doi: 10.1016/s0006-291x(02)00871-9. [DOI] [PubMed] [Google Scholar]
- 5.Watts G. F., Playford D. A., Croft K. D., Ward N. C., Mori T. A., Burke V. Coenzyme Q (10) improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia. 2002;45:420–426. doi: 10.1007/s00125-001-0760-y. [DOI] [PubMed] [Google Scholar]
- 6.Vande Weghe J. G., Ow D. W. A fission yeast gene for mitochondrial sulfide oxidation. J. Biol. Chem. 1999;274:13250–13257. doi: 10.1074/jbc.274.19.13250. [DOI] [PubMed] [Google Scholar]
- 7.Bader M. W., Xie T., Ty C. A., Bardwell C. A. Disulfide bonds are generated by quinone reduction. J. Biol. Chem. 2000;275:26082–26088. doi: 10.1074/jbc.M003850200. [DOI] [PubMed] [Google Scholar]
- 8.Ernster L., Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 9.Rustin P., Munnich A., Rötig A. Mitochondrial respiratory chain dysfunction due to coenzyme Q deficiency. Methods Enzymol. 2004;382:81–88. doi: 10.1016/S0076-6879(04)82005-6. [DOI] [PubMed] [Google Scholar]
- 10.Jonassen T., Clarke C. F. Genetic analysis of Coenzyme Q biosynthesis. In: Kagan V. E., Quinn P. J., editors. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton, FL: CRC Press; 2001. pp. 185–208. [Google Scholar]
- 11.Kang D., Takeshige K., Isobe R., Minakami S. Evidence that the decarboxylation reaction occurs before the first methylation in ubiquinone biosynthesis in rat liver mitochondria. Eur. J. Biochem. 1991;198:599–605. doi: 10.1111/j.1432-1033.1991.tb16056.x. [DOI] [PubMed] [Google Scholar]
- 12.Vajo Z., King L. M., Jonassen T., Wilkin D. J., Ho N., Munnich A., Clarke C. A. Conservation of the Caenorhabditis elegans timing gene clk-1 from yeast to human: a gene required for ubiquinone biosynthesis with potential implications for aging. Mamm. Genome. 1999;10:1000–1004. doi: 10.1007/s003359901147. [DOI] [PubMed] [Google Scholar]
- 13.Jonassen T., Clarke C. F. Isolation and functional expression of human COQ3, a gene encoding a methyltransferase required for ubiquinone biosynthesis. J. Biol. Chem. 2000;275:12381–12387. doi: 10.1074/jbc.275.17.12381. [DOI] [PubMed] [Google Scholar]
- 14.Ashby M. N., Kutsunai S. Y., Ackerman S., Tzagoloff A., Edwards P. A. COQ2 is a candidate for the structural gene encoding para-hydroxybenzoate:polyprenyltransferase. J. Biol. Chem. 1992;267:4128–4136. [PubMed] [Google Scholar]
- 15.Siebert M., Bechthold A., Melzer M., May U., Berger U., Schröder G., Schröder J., Severin K., Heide L. Ubiquinone biosynthesis: cloning of the genes coding for chorismate pyruvate-lyase and 4-hydroxybenzoate octaprenyl transferase from Escherichia coli. FEBS Lett. 1992;307:347–350. doi: 10.1016/0014-5793(92)80710-x. [DOI] [PubMed] [Google Scholar]
- 16.Uchida N., Suzuki K., Saiki R., Kainou T., Tanaka K., Matsuda H., Kawamukai M. Phenotypes of fission yeast defective in ubiquinone production due to disruption of the gene for p-hydroxybenzoate polyprenyl diphosphate transferase. J. Bacteriol. 2000;182:6933–6939. doi: 10.1128/jb.182.24.6933-6939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melzer M., Heide L. Characterization of polyprenyldiphosphate: 4-hydroxybenzoate polyprenyltransferase from Escherichia coli. Biochim. Biophys. Acta. 1994;1212:93–102. doi: 10.1016/0005-2760(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 18.Swiezewska E. Ubiquinone and plastoquinone metabolism in plants. Methods Enzymol. 2004;378:124–131. doi: 10.1016/S0076-6879(04)78007-6. [DOI] [PubMed] [Google Scholar]
- 19.Danilov L. L., Druzhinina T. N., Kalinchuk N. A., Maltsev S. S., Schibayev V. N. Polyprenyl phosphates: synthesis and structure–activity relationship for a biosynthetic system of Salmonella anatum O-specific polysaccharide. Chem. Phys. Lipids. 1989;51:191–204. doi: 10.1016/0009-3084(89)90006-6. [DOI] [PubMed] [Google Scholar]
- 20.Sonnhammer E. L., von Heijne G., Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998;6:175–182. [PubMed] [Google Scholar]
- 21.Emanuelsson O. Predicting protein subcellular localisation from amino acid sequence information. Briefings in Bioinformatics. 2002;3:361–376. doi: 10.1093/bib/3.4.361. [DOI] [PubMed] [Google Scholar]
- 22.Bateman A., Birney E., Cerruti L., Durbin R., Etwiller L., Eddy S. R., Griffiths-Jones S., Howe K. L., Marshall M., Sonnhammer E. L. The Pfam protein families database. Nucleic Acids Res. 2002;30:276–280. doi: 10.1093/nar/30.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mott R. EST_GENOME: a program to align spliced DNA sequences to unspliced genomic DNA. Comput. Applic. 1997;13:477–478. doi: 10.1093/bioinformatics/13.4.477. [DOI] [PubMed] [Google Scholar]
- 24.Pfanner N., Geissler A. Versatility of the mitochondrial protein import machinery. Nature Rev. Mol. Biol. 2001;2:339–349. doi: 10.1038/35073006. [DOI] [PubMed] [Google Scholar]
- 25.Dialameh G. H., Nowicki H. G., Yekundi K. G., Olson R. E. Involvement of p-OH-benzoyl-coenzyme A in the biosynthesis of ubiquinone-9 in the rat. Biochem. Biophys. Res. Commun. 1970;40:1063–1069. doi: 10.1016/0006-291x(70)90902-2. [DOI] [PubMed] [Google Scholar]
- 26.Olson R. E., Rudney H. Biosynthesis of ubiquinone. Vitam. Horm. 1983;40:1–43. doi: 10.1016/s0083-6729(08)60431-8. [DOI] [PubMed] [Google Scholar]
- 27.Hsu A. Y., Do T. Q., Lee P. T., Clarke C. F. Genetic evidence for a multi-subunit complex in the O-methyltransferase steps of coenzyme Q biosynthesis. Biochim. Biophys. Acta. 2000;2000:287–297. doi: 10.1016/s1388-1981(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto T., Shimizu S., Sugawara H., Momose K., Rudney H. Identification of regulatory sites in the biosynthesis of ubiquinone in the perfused rat heart. Arch. Biochem. Biophys. 1989;269:86–92. doi: 10.1016/0003-9861(89)90089-1. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T., Sugawara H., Shimizu S., Momose K. Possible existence of an intermediate pool of ubiquinone in rat heart mitochondria. Int. J. Biochem. 1990;22:89–91. doi: 10.1016/0020-711x(90)90082-e. [DOI] [PubMed] [Google Scholar]
- 30.Thelin A., Schedin S., Dallner G. Half-life of ubiquinone-9 in rat tissues. FEBS Lett. 1992;313:118–120. doi: 10.1016/0014-5793(92)81425-l. [DOI] [PubMed] [Google Scholar]
- 31.Bentinger M., Grünler J., Peterson E., Swiezewska E., Dallner G. Phosphorylation of farnesol in rat liver microsomes: properties of farnesol kinase and farnesyl phosphate kinase. Arch. Biochem. Biophys. 1998;353:191–198. doi: 10.1006/abbi.1998.0611. [DOI] [PubMed] [Google Scholar]
- 32.Thai L., Rush J. S., Maul J. E., Devarenne T., Rodgers D. L., Chappell J., Waechter C. J. Farnesol is utilized for isoprenoid biosynthesis in plant cells via farnesyl pyrophosphate formed by successive monophosphorylation reactions. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13080–13085. doi: 10.1073/pnas.96.23.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Momose K., Rudney H. 3-Polyprenyl-4-hydroxybenzoate synthesis in the inner membrane of mitochondria from p-hydroxybenzoate and isopentenylpyrophosphate. A demonstration of isoprenoid synthesis in rat liver mitochondria. J. Biol. Chem. 1972;247:3930–3940. [PubMed] [Google Scholar]
- 34.Kalén A., Appelkvist E. L., Chojnacki T., Dallner G. Nonaprenyl-4-hydroxybenzoate transferase, an enzyme involved in ubiquinone biosynthesis, in the endoplasmic reticulum–Golgi system of rat liver. J. Biol. Chem. 1990;265:1158–1164. [PubMed] [Google Scholar]
- 35.Swiezewska E., Dallner G., Andersson B., Ernster L. Biosynthesis of ubiquinone and plastoquinone in the endoplasmic reticulum–Golgi membranes of spinach leaves. J. Biol. Chem. 1993;268:1494–1499. [PubMed] [Google Scholar]
- 36.Tekle M., Bentinger M., Nordman T., Appelkvist E. L., Chojnacki T., Olsson J. Ubiquinone biosynthesis in rat liver peroxisomes. Biochem. Biophys. Res. Commun. 2002;291:1128–1133. doi: 10.1006/bbrc.2002.6537. [DOI] [PubMed] [Google Scholar]
- 37.Thompson S. L., Burrows R. J., Laub R. J., Krisans S. K. Cholesterol synthesis in rat liver peroxisomes. Conversion of mevalonic acid to cholesterol. J. Biol. Chem. 1987;262:17420–17425. [PubMed] [Google Scholar]
- 38.Ericson J., Appelkvist E. L., Thelin A., Chojnacki T., Dallner G. Isoprenoid biosynthesis in rat liver peroxisomes. Characterization of cis-prenyltransferase and squalene synthetase. J. Biol. Chem. 1992;267:18707–18714. [PubMed] [Google Scholar]
- 39.Grünler J., Parmryd I. Subcellular distribution of farnesyl protein transferase in rat liver. FEBS Lett. 1999;455:233–237. doi: 10.1016/s0014-5793(99)00892-3. [DOI] [PubMed] [Google Scholar]