Abstract
Background
Allergic bronchopulmonary aspergillosis (ABPA) is a rare airway disorder primarily affecting patients with asthma and cystic fibrosis. Persistent airway inflammation brought on by Aspergillus fumigatus exacerbates the underlying condition and can cause significant respiratory damage. Treatments center on reducing inflammation with the use of corticosteroids and antifungals. PANoptosis is a new concept in the field of cell death and inflammation that posits the existence of cross talk and a master control system for the 3 programmed cell death (PCD) pathways, namely, apoptosis, pyroptosis, and necroptosis. This concept has revolutionized the understanding of PCD and opened new avenues for its exploration. Studies show that Aspergillus is one of the pathogens that is capable of activating PANoptosis via the Z-DNA binding protein 1 (ZBP1) pathway and plays an active role in the inflammation caused by this organism.
Objective
This article explores the nature of inflammation in ABPA and ways in which PCD could lead to novel treatment options.
Method
PubMed was used to review the literature surrounding Aspergillus infection–related inflammation and PANoptosis.
Results
There is evidence that apoptosis and pyroptosis protect against Aspergillus-induced inflammation, whereas necroptosis promotes inflammation.
Conclusion
Experimental medications, in particular, necroptosis inhibitors such as necrosulfonamide and necrostatin-1, should be studied for use in the treatment of ABPA.
Key words: ABPA, allergic bronchopulmonary aspergillosis, apoptosis, Aspergillus fumigatus, necroptosis, PANoptosis, programmed cell death, pyroptosis, TAK1, ZBP1
Allergic bronchopulmonary aspergillosis (ABPA) is a rare airway disorder characterized by persistent inflammation in response to infection by fungi from the genus Aspergillus. Although other fungi, such as Candida albicans and Shizophyllum commune, are capable of causing ABPM, Aspergillus is the most common and best-studied fungus associated with this disease.1,2 ABPA has an estimated prevalence of 2.5% in subjects with asthma and 8.9% in subjects with cystic fibrosis. These numbers may be as high as 22.4% and 25%, respectively, depending on the diagnostic criteria used, and underdiagnosis of the disease is speculated.3, 4, 5, 6 ABPA primarily affects patients with chronic airway diseases, although cases without underlying respiratory problems do occur.7, 8, 9 ABPA exacerbates airway symptoms and causes hemoptysis, a productive cough with mucus plugs, and dyspnea.5 Its diagnosis is based on laboratory and radiologic findings, including blood eosinophilia, elevated level of specific IgG to A fumigatus, elevated total and specific levels of IgE, and bronchiectasis on computed tomography.5,6,10 These criteria make ABPA significantly easier to diagnose than the other ABPMs. Treatment strategies include systemic glucocorticosteroids and antifungals, which reduce inflammation and fungal load, with targeted biologics emerging as alternative therapeutic options.11,12
ABPA-associated inflammation occurs via the TH2 cell inflammatory pathway, in which TH2 cells produce IL-4, IL-5, and IL-13, which in turn activate downstream pathways.13,14 IL-33, which is located in the nuclei of epithelial cells, activates cytokines and enhances production of IL-4, IL-5, and IL-13 in conjunction with the other critical epithelial-derived TH2 inflammatory cytokines, IL-25 and thymic stromal lympopoietin (TSLP).14, 15, 16, 17 IL-33 binds to IL-1 receptor-like 1 (IL1RL1 [also known as ST2]) during allergic inflammation, signaling the formation of a heterodimer between ST2L, the transmembrane isoform of ST2, and IL-1 receptor accessory protein (IL-1 RAcP). The result is a signaling cascade that activates the transcription of proinflammatory molecules, including nuclear factor-κB.18 This leads to the activation of eosinophils and other cells involved in allergic inflammation.
Aspergillus and other fungal allergens contain proteases capable of cleaving IL-33 into a more mature form, increasing its bioactivity compared with that of the full-length form.19 Increased levels of IL-33 in ABPA and other Aspergillus spp infections make it a potential target for future ABPA therapies.20,21 Inhibition of the IL-33 signaling pathway in a mouse model of ABPA significantly reduces inflammation in invasive aspergillosis and Aspergillus–sensitive asthma, making it likely that the increased bioactivity from IL-33 cleavage is the source of this inflammation.22,23 Thus, it can reasonably be concluded that inhibition of this signaling pathway may similarly reduce ABPA inflammation.
Methods
PCD pathways
Apoptosis is the best-known and best-understood programmed cell death (PCD) pathway. It proceeds through 2 methods: intrinsic and extrinsic. The intrinsic pathway is activated when there is an absence of prosurvival molecules, such as growth factors, or the presence of proapoptotic molecules, such as reactive oxygen species. These molecules trigger the release of mitochondrial proteins into the cytoplasm capable of activating apoptotic protease activating factor 1 (APAF1), which assembles into an oligomer known as the apoptosome. The apoptosome activates procaspase-9, which subsequently activates procaspase-3, the final executioner of apoptosis. The extrinsic pathway involves death signals released from natural killer cells and macrophages, such as TNF-α and Fas ligand (Fas-L). Binding of these death ligands to a death receptor allows the recruitment and activation of procaspase-8, which is able to dimerize and activate procaspase-3.24
Pyroptosis is governed by inflammasomes, which form when pattern-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are detected by NOD-like receptors (NLRs), absent in melanoma 2 (AIM2), and other pattern recognition receptors (PRRs).25 Interaction of these PRRs with adapter apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and procaspase-1 generates an inflammasome. The inflammasome can produce activated caspase-1 molecules, which cleave gasdermin D (GSDMD). The N-terminal of GSDMD inserts pores into the cellular membrane, causing secretion of the proinflammatory molecules IL-1β and IL-18, thereby activating pyroptosis.26
Necroptosis is the least-understood cell death pathway. Initiation is similar to the extrinsic pathway of apoptosis in that it relies on the death signal, TNF, to activate TNF receptor 1 (TNFR1). This causes the formation of a complex that includes the molecules TNFR-associated factors (TRAF), receptor-interacting serine/threonine-protein kinase-1 (RIPK1), cellular inhibitor of apoptosis protein 1 (cIAP1), cellular inhibitor of apoptosis protein 2 (cIAP2), Fas-associated via death domain (FADD), and TNFR superfamily member 1A -associated via death domain (TRADD). The cIAPs and TRAF work together to ubiquitinate RIPK1. FADD recruits procaspase-8, and RIPK1 recruits receptor-interacting serine/threonine-protein kinase-3 (RIPK3).26 The ubiquitination process is opposed by the deubiquitinating molecule, cylindromatosis (CYLD). If RIPK1 is deubiquitinated and caspase-8 is not present, RIPK1 and RIPK3 will complex to activate mixed-lineage kinase domain-like (MLKL), which permeabilizes the cell membrane and causes cell death.26 The requirement that caspase-8 (CASP8) be absent has led to the hypothesis that necroptosis is meant to be a backup cell death pathway for cells incapable of undergoing apoptosis. Table I summarizes the molecules involved in apoptosis, pyroptosis, and necroptosis.
Table I.
Molecules involved in PCD
| Molecule name | Abbreviation | Pathway | Function |
|---|---|---|---|
| Absent in melanoma 2 | AIM2 | Pyroptosis | Pattern recognition receptor that starts the pyroptosis cascade |
| Apoptotic protease activating factor 1 | APAF1 | Apoptosis (intrinsic) | Activator of procaspase-9 |
| Apoptosis-associated speck-like protein containing a caspase recruitment domain | ASC | Pyroptosis | Recruits procaspase-1 to the inflammasome |
| Caspase-1 | CASP1 | Pyroptosis | Cleaves GSDMD |
| Caspase-3 | CASP3 | Apoptosis | Executioner caspase that triggers apoptosis |
| Caspase-8 | CASP8 | Apoptosis (extrinsic), necroptosis | Initiator caspase of the extrinsic apoptosis pathway; activates procaspase-3; its presence inhibits necroptosis |
| Caspase-9 | CASP9 | Apoptosis (intrinsic) | Initiator caspase of intrinsic apoptosis pathway; activates procaspase-3 |
| Cellular inhibitor of apoptosis protein 1 | cIAP1 | Apoptosis (extrinsic), necroptosis | Ubiquitinates caspase-8, allowing necroptosis to proceed |
| Cellular inhibitor of apoptosis protein 2 | cIAP2 | Apoptosis (extrinsic), necroptosis | Ubiquitinates caspase-8, allowing necroptosis to proceed |
| Cylindromatosis | CYLD | Apoptosis (extrinsic), necroptosis | Deubiquitinates caspase-8 to inhibit necroptosis |
| Fas-associated via death domain | FADD | Apoptosis (extrinsic), necroptosis | Recruits procaspase-8 |
| Fas ligand | Fas-L | Apoptosis (extrinsic), necroptosis | Binds death receptor to initiate extrinsic apoptosis or necroptosis |
| Gasdermin D | GSDMD | Pyroptosis | Perforates cell membrane to allow release of IL-1β and IL-18 |
| Mixed-lineage kinase domain like | MLKL | Necroptosis | Final executor of necroptosis; permeabilizes cell membrane |
| Receptor-interacting serine/threonine-protein kinase 1 | RIPK1 | Apoptosis (extrinsic), necroptosis | Recruits RIPK3; can form apoptotic complex |
| Receptor-interacting serine/threonine-protein kinase 3 | RIPK3 | Necroptosis | Activates MLKL |
| TNF-α | TNF-α | Apoptosis (extrinsic), necroptosis | Binds death receptors to trigger extrinsic apoptosis or necroptosis |
| TNF receptor 1 | TNFR1 | Apoptosis (extrinsic), necroptosis | Activated by TNF-α to initiate extrinsic apoptosis or necroptosis |
| TNF receptor–associated factor | TRAF | Apoptosis (extrinsic), necroptosis | Ubiquitinates caspase-8, allowing necroptosis to proceed |
| TNF receptor superfamily member 1A–associated via death domain | TRADD | Necroptosis | Recruits procaspase-8 |
The PANoptosome
The term PANoptosis, which was first coined in 2019 (with the initial data dating back to as early as 2016), is an emerging concept in understanding cell death pathways.27, 28, 29 Underlying this concept is the hypothesis that pyroptosis, apoptosis, and necroptosis involve cross talk regulated by a master controlling complex, the PANoptosome.29 Research on PANoptosis has opened up many avenues in cell death related–research, in particular, infectious organisms such as A fumigatus.30,31
Two critical molecules at the top of the PANoptosis pathway are Z-DNA binding protein 1 (ZBP1) and TGF-β–activated kinase 1 (TAK1).29 A 2020 study demonstrates that the ZBP1 molecule plays a role in inflammation related to Aspergillus infections.32
ZBP1 contains a Zα domain capable of detecting Z-nucleic acids derived from infectious pathogens.29 This domain is rarely found in mammals and is shared only endogenously, with the molecule adenosine deaminase acting on ribonucleic acid (RNA) 1 (ADAR1), an inhibitor of ZBP1.33 Activation of this domain in concert with type 1 interferon signaling is essential for its activation.34 After activation, ZBP1 can then interact via its RIP homotypic activation motif (RHIM) with RIPK3, which forms a complex with CASP8 to induce downstream PCD pathways.29 RIPK3 interacts with MLKL to induce necroptosis, CASP8 induces apoptosis, and the complex interacting with nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) induces pyroptosis.29 Evidence suggests that deletion of the ZBP1 gene prevents the activation of PCD pathways in influenza A–infected cells.34 In contrast, deletion of the gene in the presence of C albicans and Aspergillus fumigatus infection results in reduced, but not absent, PCD.32 Therefore, either alternative PANoptosis pathways are involved in activating the PANoptosome or activation of the individual PCD pathways by variance virulence factors plays a significant role in fungal infections.
TAK1 has an effect opposite that of ZBP1 by inhibiting the PANoptosis pathway. Activity of this molecule prevents formation of RIPK1-FADD-CASP8 complexes, which function as a PANoptosome similar to ZBP1-RIPK3-CASP8, activating PCD pathways in the same way.29 Inactivation of this molecule results in unregulated activity of PCD, allowing exploitation by infectious organisms, such as Yersinia. Yersinia spp produce toxins capable of blocking TAK1 activity to promote their pathogenesis.29 Experiments in a Drosophila model indicate that Aspergillus may produce a cyclopentanediol analog capable of acting on this pathway (Fig 1). However, further investigation is necessary to confirm this fact.35 If such a virulence factor were discovered, it could become a target for future therapeutic agents with the benefit of having a low side effect profile because of its target specificity.
Fig 1.
PANoptosis is suspected to be induced by A fumigatus. Evidence suggests there may be a cyclopentanediol analog produced by A fumigatus that serves as an inhibitor of TAK1. In addition, direct activation can occur through an unknown Z-DNA ligand binding to ZBP1. The PANoptosome complex formed from either pathway can then go on to induce the 3 modalities of cell death, namely, apoptosis, pyroptosis, and necroptosis. ASC, Adapter apoptosis-associated speck-like protein containing a caspase recruitment domain; CASP8, caspase-8; FADD, FAS-associated via death domain.
Results
PCD and A fumigatus
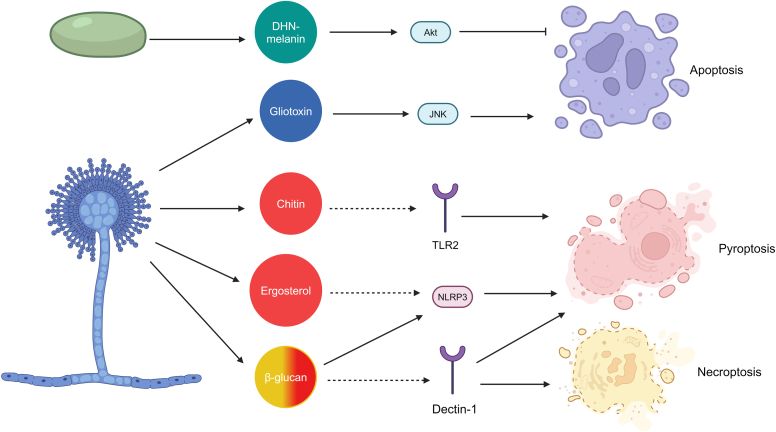
A fumigatus contains multiple virulence factors involved in inducing PCD. The 2 major players in apoptosis are dihydroxynapthalene-melanin and gliotoxin.31 Dihydroxynapthalene-melanin activates protein kinase B (PKB/Akt), an inhibitor of caspase-9 and other molecules involved in the extrinsic apoptosis pathway, allowing Aspergillus to produce conidia. Once they germinate and grow hyphae, A fumigatus produces gliotoxin, a molecule capable of activating the intrinsic pathway via c-Jun N-terminal kinase (JNK).31 The pyroptosis pathway is induced via interaction of the fungal cell wall component β-glucan with NLRP3 accompanied by activation of the PRR dectin-131,36 Toll-like receptor 2 (TLR2) associates with dectin-1 in A fumigatus recognition and influences Treg cell differentiation.37 Chitin is a common component of fungal cell walls and binds Toll-like receptor 2 to trigger inflammation.38 Because this antigen is also found in A fumigatus, it is most likely an important component of the pyroptosis pathway in ABPA.39 On the basis of the mechanisms of inflammasome activation seen in other fungi, it is also suspected that ergosterol is involved in activating pyroptosis.31,40 On the basis of studies with Candida, it is hypothesized that dectin-1 activation plays a role in necroptosis that is similar to its role in pyroptosis (Fig 2).31,41
Fig 2.
A fumigatus–induced cell death. Solid lines represent known relationships, dashed lines represent suspected relationships based on studies in C albicans. A fumigatus conidia inhibits apoptosis through the secretion of dihydroxynapthalene-melanin (DHN-melanin). Once it has germinated, A fumigatus has multiple virulence factors capable of inducing the 3 different cell death pathways. Gliotoxin serves as an activator of apoptosis. Ergosterol and chitin are known virulence factors of A fumigatus but have not been directly shown to activate the pyroptosis pathway in aspergillosis. Activation of dectin-1 via β-glucan has been demonstrated to play a role in the pyroptosis pathway but is currently only suspected in necroptosis.
The varying activation of the PCD pathways by A fumigatus influences the level of inflammation that the organism causes, particularly through varying amounts of IL-33 released. Death by apoptosis results in decreased IL-33 signaling owing to caspase 3– and caspase 7–induced cleavage of the C-terminal portion of IL-33, rendering it inert.17 Alternatively, necroptosis induces the release of uncleaved IL-33.42 Although less active than the mature form seen following fungal protease cleavage, the full-length IL-33 can produce an immune response. Finally, although a direct connection between IL-33 release in Aspergillus infection and pyroptosis is not established, there is evidence that it plays a protective role. There is increased susceptibility to invasive aspergillosis in AIM2 and NLRP3 knockout mice (AIM2 and NLRP3 being 2 genes involved in the pyroptosis pathway).43 These findings suggest that whereas pyroptosis and apoptosis promote clearance of Aspergillus infection, necroptosis plays a pivotal role in promoting Aspergillus infections, and thus ABPA. Whether targeting the PANoptosis pathway would be beneficial or deleterious is unclear. Today, there is no evidence that deletion of ZBP1 increases or decreases the incidence of aspergillosis. It is possible that inhibition of PANoptosis would block most inflammation and thus allow asymptomatic colonization, given the fact that Aspergillus, a ubiquitous organism, often colonizes the respiratory tract without inducing any pathology. However, reduced ability to clear the pathogen may allow for unchecked proliferation, eventually causing more severe disease. One thing is clear: therapies directed toward inhibition of the necroptosis pathway should be considered.
Discussion
Necroptosis inhibitors as novel therapeutics to treat ABPA
Therapeutic agents that target the necroptosis pathway are available and are being evaluated to treat cancer, stroke, acute coronary syndrome, and other diseases.30,44,45 Necrosulfonamide (NSA) is an inhibitor of MLKL that demonstrates efficacy in slowing breast tumor growth in xenografted mice.46 Because MLKL is involved only in necroptosis and not in apoptosis or pyroptosis, it would be an ideal target for therapy. However, the preclinical animal model studies required before human testing would be difficult to perform because NSA does not bind to the mouse variant of MLKL.47 Additionally, NSA may also bind GSDMD from the pyroptosis pathway, inhibiting its activation.48, 49, 50 There are also data to suggest that NSA has an inhibitory effect on apoptosis, further reducing the potential clinical significance of this compound.51
Necrostatin-1 (Nec-1) is a drug that targets necroptosis via inhibiting autophosphorylation of RIPK1. RIPK1 dimerizes and autophosphorylates to form a complex with RIPK3 to activate MLKL.52 Because it is upstream in the necroptosis pathway, targeting RIPK1 over MLKL has its own issues. Namely, RIPK1 also serves as a driving force in the apoptosis pathway, although there is an RIPK1-independent apoptotic pathway.52 The inhibitory effect of Nec-1 may be a promising area for clinical trials in the treatment of ABPA. There is evidence to suggest that in the absence of TAK1, necroptosis can also be activated in an RIPK1-independent manner.53 This pathway may reduce the potential efficacy of Nec-1 as a treatment option for ABPA.
Although these 2 compounds are not the only PANoptosis regulators that may treat ABPA, they demonstrate the potential of this pathway and its complexity. Because of the cross talk among the 3 PCD pathways seen in PANoptosis, even drugs targeting a particular PCD pathway may show unexpected effects, as demonstrated by NSA potentially influencing apoptosis despite targeting a relatively downstream necroptosis molecule.49 These cascading effects spilling over into the other PCD pathways also highlights the importance of targeted localization of these drugs. Although this article highlights necroptosis inhibitors as potential treatments, apoptosis and pyroptosis inhibit the release of IL-33 and could therefore be targeted by activators rather than by inhibitors. This would be associated with a greater risk of triggering cell death in otherwise healthy cells if sufficient drug localization could not be achieved. mAbs are also becoming treatment options for managing ABPA.54,55 The mAb targeting IL-33 tozorakimab may be another promising candidate to treat ABPA.56 Advancements in research open the door to development of new therapeutic options, and PANoptosis may be the next step in research on ABPA.
Conclusion
ABPA is a rare disease of the airways primarily affecting subjects with asthma and cystic fibrosis. Research demonstrates that PCD pathways play a role in ABPA; however, the emerging concept of PANoptosis and cross talk between these pathways provides a new avenue for understanding and possibly treating this disease. Studies show that pyroptosis and apoptosis reduce disease burden whereas necroptosis promotes pathology. Novel drugs that target elements of these pathways are under study and show promise as new treatment options for ABPA. The drugs NSA and Nec-1 are being explored to treat of other diseases, such as intervertebral disk degeneration and ischemia-reperfusion injury; they demonstrate how necroptosis inhibitors could be effective to treat ABPA. Additionally, other elements in these pathways, such as Aspergillus-produced virulence factors that influence TAK1 activity, are worth exploring. Blocking a potential TAK1 inhibitor may be a way of targeting PANoptosis as a whole order to inhibit the capacity of Aspergillus to induce inflammation. For this reason, depending on what future experiments in ZBP1-deficient mice show, ZBP1 also may be a worthy therapeutic target. As research on PANoptosis progresses, even more options for targetable pathways may be discovered.
Clinical implications.
Necroptosis inhibitors and other therapeutic agents targeting PANoptosis should be explored as treatment options for ABPA.
Disclosure statement
The N. Kolliputi laboratory is funded by the Joy McCann Culverhouse endowment to the Division of Allergy and Immunology.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Acknowledgments
We thank Jennifer D. Newcomb, MS (University of South Florida), for comments on and revisions of our article.
Footnotes
Data availability statement: No new data were created or analyzed in this study. Data sharing is not applicable to this article.
References
- 1.Harada T., Inui G., Ishikawa H., Kato R., Sueda Y., Funaki Y., et al. The clinical characteristics of allergic bronchopulmonary mycosis differ among pathogenic fungi. Yonago Acta Med. 2023;66:257–262. doi: 10.33160/yam.2023.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano K., Hebisawa A., Ishiguro T., Takayanagi N., Nakamura Y., Suzuki J., et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. 2021;147:1261–1268.e5. doi: 10.1016/j.jaci.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Denning D.W., Pleuvry A., Cole D.C. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013;51:361–370. doi: 10.3109/13693786.2012.738312. [DOI] [PubMed] [Google Scholar]
- 4.Maturu V.N., Agarwal R. Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: systematic review and meta-analysis. Clin Exp Allergy. 2015;45:1765–1778. doi: 10.1111/cea.12595. [DOI] [PubMed] [Google Scholar]
- 5.Roboubi A., Audousset C., Frealle E., Brun A.L., Laurent F., Vitte J., et al. Allergic bronchopulmonary aspergillosis: a multidisciplinary review. J Mycol Med. 2023;33 doi: 10.1016/j.mycmed.2023.101392. [DOI] [PubMed] [Google Scholar]
- 6.Greenberger P.A. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2002;110:685–692. doi: 10.1067/mai.2002.130179. [DOI] [PubMed] [Google Scholar]
- 7.Shah A., Maurya V., Panjabi C., Khanna P. Allergic bronchopulmonary aspergillosis without clinical asthma caused by Aspergillus niger. Allergy. 2004;59:236–237. doi: 10.1046/j.1398-9995.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 8.Koh W.J., Han J., Kim T.S., Lee K.S., Jang H.W., Kwon O.J. Allergic bronchopulmonary aspergillosis coupled with broncholithiasis in a non-asthmatic patient. J Korean Med Sci. 2007;22:365–368. doi: 10.3346/jkms.2007.22.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Y.T., Greco A., Garimella B.V., Kumley B. Case report of allergic bronchopulmonary aspergillosis in a patient without asthma or bronchiectasis. Chest. 2022;162:18a–a. [Google Scholar]
- 10.Sisodia J., Bajaj T. StatPearls Publishing; 2024. Allergic bronchopulmonary aspergillosis. Treasure Island, FL. [PubMed] [Google Scholar]
- 11.Greenberger P.A. Defining the outcome markers and therapeutic role for omalizumab in treatment of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2023;11:906–907. doi: 10.1016/j.jaip.2023.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Jin M.L., Douglass J.A., Elborn J.S., Agarwal R., Calhoun W.J., Lazarewicz S., et al. Omalizumab in allergic bronchopulmonary aspergillosis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2023;11:896–905. doi: 10.1016/j.jaip.2022.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Latge J.P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokubo K., Onodera A., Kiuchi M., Tsuji K., Hirahara K., Nakayama T. Conventional and pathogenic Th2 cells in inflammation, tissue repair, and fibrosis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.945063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saenz S.A., Taylor B.C., Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraishi Y., Yamaguchi S., Yoshizaki T., Nambu A., Shimura E., Takamori A., et al. IL-33, IL-25 and TSLP contribute to development of fungal-associated protease-induced innate-type airway inflammation. Sci Rep. 2018;8 doi: 10.1038/s41598-018-36440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cayrol C., Girard J.P. Interleukin-33 (IL-33): a critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine. 2022;156 doi: 10.1016/j.cyto.2022.155891. [DOI] [PubMed] [Google Scholar]
- 18.Pusceddu I., Dieplinger B., Mueller T. ST2 and the ST2/IL-33 signalling pathway-biochemistry and pathophysiology in animal models and humans. Clin Chim Acta. 2019;495:493–500. doi: 10.1016/j.cca.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Cayrol C., Duval A., Schmitt P., Roga S., Camus M., Stella A., et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol. 2018;19:375–385. doi: 10.1038/s41590-018-0067-5. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y., Takazono T., Obase Y., Fukahori S., Ashizawa N., Hirayama T., et al. Serum cytokines usefulness for understanding the pathology in allergic bronchopulmonary aspergillosis and chronic pulmonary aspergillosis. J Fungi (Basel) 2022;8:436. doi: 10.3390/jof8050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bojanowski C.M., Bitoun J.P., Kolls J.K. An ALARMINg Type 2 Response in cystic fibrosis-the key to understanding ABPA? Am J Respir Crit Care Med. 2023;207:1418–1419. doi: 10.1164/rccm.202303-0580ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramaprakash H., Shibata T., Duffy K.E., Ismailoglu U.B., Bredernitz R.M., Moreira A.P., et al. Targeting ST2L potentiates CpG-mediated therapeutic effects in a chronic fungal asthma model. Am J Pathol. 2011;179:104–115. doi: 10.1016/j.ajpath.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garth J.M., Reeder K.M., Godwin M.S., Mackel J.J., Dunaway C.W., Blackburn J.P., Steele C. IL-33 Signaling regulates innate IL-17A and IL-22 production via suppression of prostaglandin E(2) during lung fungal infection. J Immunol. 2017;199:2140–2148. doi: 10.4049/jimmunol.1602186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Arcy M.S. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 25.Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ketelut-Carneiro N., Fitzgerald K.A. Apoptosis, pyroptosis, and necroptosis-oh my! The many ways a cell can die. J Mol Biol. 2022;434 doi: 10.1016/j.jmb.2021.167378. [DOI] [PubMed] [Google Scholar]
- 27.Kuriakose T., Man S.M., Malireddi R.K., Karki R., Kesavardhana S., Place D.E., et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu P., Ke Z.R., Chen J.X., Li S.J., Ma T.L., Fan X.L. Advances in mechanism and regulation of PANoptosis: prospects in disease treatment. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malireddi R.K.S., Kesavardhana S., Kanneganti T.D. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis) Front Cell Infect Microbiol. 2019;9:406. doi: 10.3389/fcimb.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan W.T., Zhao W.J., Hu X.M., Ban X.X., Ning W.Y., Wan H., et al. PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons. Neural Regen Res. 2023;18:357–363. doi: 10.4103/1673-5374.346545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams T.J., Gonzales-Huerta L.E., Armstrong-James D. Fungal-induced programmed cell death. J Fungi (Basel) 2021;7:231. doi: 10.3390/jof7030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banoth B., Tuladhar S., Karki R., Sharma B.R., Briard B., Kesavardhana S., et al. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis) J Biol Chem. 2020;295:18276–18283. doi: 10.1074/jbc.RA120.015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karki R., Kanneganti T.D. ADAR1 and ZBP1 in innate immunity, cell death, and disease. Trends Immunol. 2023;44:201–216. doi: 10.1016/j.it.2023.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samir P., Malireddi R.K.S., Kanneganti T.D. The PANoptosome: a deadly protein complex driving pyroptosis, apoptosis, and necroptosis (PANoptosis) Front Cell Infect Microbiol. 2020;10:238. doi: 10.3389/fcimb.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekiya M., Ueda K., Okazaki K., Kikuchi H., Kurata S., Oshima Y. A cyclopentanediol analogue selectively suppresses the conserved innate immunity pathways, Drosophila IMD and TNF-alpha pathways. Biochem Pharmacol. 2008;75:2165–2174. doi: 10.1016/j.bcp.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Briard B., Karki R., Malireddi R.K.S., Bhattacharya A., Place D.E., Mavuluri J., et al. Fungal ligands released by innate immune effectors promote inflammasome activation during Aspergillus fumigatus infection. Nat Microbiol. 2019;4:316–327. doi: 10.1038/s41564-018-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan W., Zhao Y.S., Xie K., Xing Y., Xu F. Aspergillus fumigatus influences gasdermin-D-dependent pyroptosis of the lung via regulating Toll-like receptor 2-mediated regulatory T cell differentiation. J Immunol Res. 2021;2021 doi: 10.1155/2021/5538612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs K., Cardona Gloria Y., Wolz O.O., Herster F., Sharma L., Dillen C.A., et al. The fungal ligand chitin directly binds TLR2 and triggers inflammation dependent on oligomer size. EMBO Rep. 2018;19 doi: 10.15252/embr.201846065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abad A., Fernandez-Molina J.V., Bikandi J., Ramirez A., Margareto J., Sendino J., et al. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol. 2010;27:155–182. doi: 10.1016/j.riam.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues M.L. The multifunctional fungal ergosterol. mBio. 2018;9 doi: 10.1128/mBio.01755-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao M., Wu Z., Lou Q., Lu W., Zhang J., Li Q., et al. Dectin-1-induced RIPK1 and RIPK3 activation protects host against Candida albicans infection. Cell Death Differ. 2019;26:2622–2636. doi: 10.1038/s41418-019-0323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shlomovitz I., Erlich Z., Speir M., Zargarian S., Baram N., Engler M., et al. Necroptosis directly induces the release of full-length biologically active IL-33 in vitro and in an inflammatory disease model. FEBS J. 2019;286:507–522. doi: 10.1111/febs.14738. [DOI] [PubMed] [Google Scholar]
- 43.Karki R., Man S.M., Malireddi R.K.S., Gurung P., Vogel P., Lamkanfi M., Kanneganti T.D. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe. 2015;17:357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khoury M.K., Gupta K., Franco S.R., Liu B. Necroptosis in the pathophysiology of disease. Am J Pathol. 2020;190:272–285. doi: 10.1016/j.ajpath.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong X., Tang R., Xiao M., Xu J., Wang W., Zhang B., et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174. doi: 10.1186/s13045-022-01392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X., Zhou M., Mei L., Ruan J., Hu Q., Peng J., et al. Key roles of necroptotic factors in promoting tumor growth. Oncotarget. 2016;7:22219–22233. doi: 10.18632/oncotarget.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun L., Wang H., Wang Z., He S., Chen S., Liao D., et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 48.Rathkey J.K., Zhao J., Liu Z., Chen Y., Yang J., Kondolf H.C., et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J., Wei K. Necrosulfonamide reverses pyroptosis-induced inhibition of proliferation and differentiation of osteoblasts through the NLRP3/caspase-1/GSDMD pathway. Exp Cell Res. 2021;405 doi: 10.1016/j.yexcr.2021.112648. [DOI] [PubMed] [Google Scholar]
- 50.Yang W., Tao K., Wang Y., Huang Y., Duan C., Wang T., et al. Necrosulfonamide ameliorates intestinal inflammation via inhibiting GSDMD-medicated pyroptosis and MLKL-mediated necroptosis. Biochem Pharmacol. 2022;206 doi: 10.1016/j.bcp.2022.115338. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q.X., Guo D., Wang F.C., Ding W.Y. Necrosulfonamide (NSA) protects intervertebral disc degeneration via necroptosis and apoptosis inhibition. Eur Rev Med Pharmacol Sci. 2020;24:2683–2691. doi: 10.26355/eurrev_202003_20538. [DOI] [PubMed] [Google Scholar]
- 52.Cao L., Mu W. Necrostatin-1 and necroptosis inhibition: pathophysiology and therapeutic implications. Pharmacol Res. 2021;163 doi: 10.1016/j.phrs.2020.105297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malireddi R.K.S., Gurung P., Kesavardhana S., Samir P., Burton A., Mummareddy H., et al. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med. 2020;217 doi: 10.1084/jem.20191644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewington-Gower E., Chan L., Shah A. Review of current and future therapeutics in ABPA. Ther Adv Chronic Dis. 2021;12 doi: 10.1177/20406223211047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Reilly A., Dunican E. The use of targeted monoclonal antibodies in the treatment of ABPA-a case series. Medicina (Kaunas) 2021;58:53. doi: 10.3390/medicina58010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.England E., Rees D.G., Scott I.C., Carmen S., Chan D.T.Y., Chaillan Huntington C.E., et al. Tozorakimab (MEDI3506): an anti-IL-33 antibody that inhibits IL-33 signalling via ST2 and RAGE/EGFR to reduce inflammation and epithelial dysfunction. Sci Rep. 2023;13:9825. doi: 10.1038/s41598-023-36642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]