Abstract
Recent studies by our group and others have disclosed the presence of ceramides in mitochondria, and the activities of ceramide synthase and reverse ceramidase in mitochondria have also been reported. Since a possible contamination with the ER (endoplasmic reticulum)-related compartment MAM (mitochondria-associated membrane) could not be ruled out in previous studies, we have re-investigated the presence of the enzymes of ceramide metabolism in mitochondria and MAM highly purified from rat liver. In the present paper, we show that purified mitochondria as well as MAM are indeed able to generate ceramide in vitro through both ceramide synthase or reverse ceramidase, whereas the latter enzyme activity is barely detectable in microsomes. Moreover, ceramide synthase activities were recovered in outer mitochondrial membranes as well as in inner mitochondrial membranes. Using radiolabelled sphingosine as a substrate, mitochondria could generate ceramide and phytoceramide. However, the in vitro sensitivity of ceramide synthase toward FB1 (fumonisin B1) in mitochondria as well as in MAM was found to depend upon the sphingoid base: whereas dihydrosphingosine N-acyltransferase was inhibited by FB1 in a concentration-dependent manner, FB1 actually activated the ceramide synthase when using sphingosine as a substrate. Acylation of sphingosine 1-phosphate and dihydrosphingosine 1-phosphate, generating ceramide 1-phosphate, was also shown with both subcellular fractions. Moreover, the same difference in sensitivity towards FB1 for the ceramide synthase activities was seen between the two phosphorylated sphingoid bases, raising the possibility that distinct base-specific enzymes may be involved as ceramide synthases. Collectively, these results demonstrate the involvement of mitochondria in the metabolism of ceramides through different pathways, thereby supporting the hypothesis that topology of ceramide formation could determine its function.
Keywords: ceramide, ceramide 1-phosphate, fumonisin B1, mitochondrion, mitochondria-associated membrane (MAM), sphingoid base
Abbreviations: ER, endoplasmic reticulum; FB1, fumonisin B1; GFP, green fluorescent protein; HPTLC, high-performance TLC; LAG1, longevity-assurance gene 1; MAM, mitochondria-associated membrane; SPP-1, sphingosine-1-phosphate phosphohydrolase; uog1, upstream of growth and differentiation 1
INTRODUCTION
Sphingolipid metabolites are now recognized as important components in signal transduction in numerous cell lines where they are implicated in cellular responses to stress. Among these metabolites, ceramide has been shown to play a role in cell cycle arrest, differentiation and apoptosis [1–3]. Signal transduction of stress via ceramide mainly occurs after its generation from sphingomyelin by the action of neutral and/or acidic sphingomyelinases [4]. Increases in cellular ceramide levels often precede the mitochondrial phase of apoptosis [5,6]. Mitochondria are believed to be the targets in ceramide-mediated apoptosis and are known to play a major regulatory role in cell death via apoptosis [7,8]. Whereas the generation of ceramide from a sphingomyelin source is generally thought to be the norm in ceramide signalling pathways, de novo ceramide biosynthesis has also been demonstrated to occur in response to many agents of cellular stress leading to apoptosis [9–12]. Ceramide is synthesized at the cytosolic face of the endoplasmic reticulum [13,14] and can then serve as a precursor for the biosynthesis of glycolipids in the Golgi apparatus [15], as well as sphingomyelin through the action of sphingomyelin synthase [16]. It has recently been appreciated that mitochondria contain a variety of sphingolipids and ceramide [17]. Although ceramide may be directly imported from the ER (endoplasmic reticulum) via intimate membrane contact between the two organelles [18,19], mitochondria have been reported to contain ceramide synthase [20] and reverse ceramidase [21] activities, which could also explain the presence of ceramides within mitochondrial membranes. The presence of such enzymes in mitochondria would be of particular interest, since ceramides have been demonstrated to interact directly with the mitochondrial electron-transport chain either in inhibiting complex I [22] and complex III [23] of the respiratory chain or increasing generation of hydrogen peroxide in isolated mitochondria [24]. Nevertheless, purification of ceramide synthase was achieved to date from a bovine liver mitochondrion-rich fraction [20], which was not characterized in terms of marker enzyme activities. Mitochondria obtained by the classical fractionation procedures are well known to contain a subcompartment of the ER termed the MAM (mitochondrial-associated membrane) fraction [25], which has been recently reported to contain ceramide synthase [13] and highly active glycosyltransferases involved in the synthesis of glycolipids [26]. In the same way, the subcellular localization of a reverse ceramidase in mitochondria has been ascertained using a GFP (green fluorescent protein)–ceramidase construct, and confirmation was obtained with confocal microscopy by co-localization of GFP–ceramidase with the Mitotracker Red as a specific mitochondrial probe [21]. Again, since no biochemical data using purified mitochondria have been presented until now to confirm these results, it seems quite possible that these enzymic activities might be preferentially associated with the MAM fraction instead of with mitochondria. In order to gain insight into the existence of enzymes involved in the metabolism of ceramide within mitochondria, we decided to re-investigate this problem by using purified and well-defined organelles in vitro.
EXPERIMENTAL
Materials
Leupeptin, pepstatin, PMSF, bicinchoninic acid, sphingoid bases and ceramides type III were from Sigma (L'Isle d'Abeau, France). Phytoceramides purified from plants were kindly provided by Coletica (Lyon, France). Dihydrosphingosine 1-phosphate was supplied by Biomol Research Laboratories (Paris, France). LC-NH2 cartridges were from Supelco (L'Isle d'Abeau, France). Analytical grade solvents and HPTLC (high-performance TLC) silica gel 60 plates were from Merck (Nogent, Paris). Percoll was from Amersham Biosciences (Paris, France). [3H]Sphingosine (20 Ci/mmol), [14C]palmitoyl-CoA (56 mCi/mmol) and [14C]palmitic acid (55 mCi/mmol) were purchased from NEN PerkinElmer (Paris, France). [3H]Dihydrosphingosine (60 Ci/mmol) was obtained from American Radiolabelled Chemicals (St. Louis, MO, U.S.A.). Biomax MR films were from Kodak.
Isolation of subcellular fractions from rat liver
Crude mitochondria and microsomes were isolated from the livers of Sprague–Dawley rats as reported previously [18]. Briefly, livers were immersed in ice-cold medium containing 250 mM sucrose in 10 mM Hepes buffer, pH 7.4, and 10 mM EDTA. Crude mitochondria were obtained from the cellular homogenate by differential centrifugations and were purified further on a 30% Percoll gradient following the procedure described by Vance [25]. After a 30 min centrifugation at 27500 rev./min in a Beckman SW28 rotor, a dense band containing purified mitochondria recovered from approximately two-thirds of the way down the tube was removed from the gradient, diluted with the isolation medium and washed four times at 7500 rev./min in Beckman JA14 rotor in order to remove Percoll. The MAM fraction was collected from the top of the Percoll gradient [25] and was centrifuged twice at 30000 rev./min for 1 h in a Beckman Ti-70 rotor in the presence of 1 μM leupeptin, 1 μM pepstatin and 0.1 mM PMSF. Outer and inner mitochondrial membranes were prepared from purified mitochondria after two swelling procedures as previously reported [18], purified on discontinuous sucrose gradients and assayed for marker enzyme activities.
Marker enzyme assays
The determination of glucose-6-phosphatase (EC 3.1.3.9) as a marker for the MAM fraction, of monoamine oxidase (EC 1.4.3.4) for the outer mitochondrial membranes, of cytochrome c oxidase (EC 1.9.3.1) for the inner mitochondrial membranes and of NADPH:cytochrome c reductase (EC 1.6.2.5) for the whole microsomes were carried out as reported previously [17].
Ceramide synthase assays
Ceramide synthase(s) were assayed by measuring the acylation of [3H]sphingosine or [3H]dihydrosphingosine with unlabelled palmitoyl-CoA as described by Wang and Merrill [27]. The assay mixture (total volume 200 μl) contained 25 mM potassium phosphate buffer (pH 7.4), 0.5 mM dithiothreitol, 5 μM [3H]sphingosine or [3H]dihydrosphingosine, 100 μg of protein and 50 μM palmitoyl-CoA. [3H]Sphingosine or [3H]dihydrosphingosine was added from a concentrated stock solution, dried under a stream of nitrogen and either resuspended in a small volume of ethanol (less than 1%) or sonicated in phosphate buffer. The reaction was initiated by the addition of the fatty acyl-CoA and carried out for 15 min at 37 °C. The reaction was stopped by the addition of 1 ml of methanol and 0.5 ml of chloroform. After addition of 25 μg of unlabelled ceramide as a carrier, 1 ml of chloroform was added and the mixture was vortex-mixed before the addition of 3 ml of water. The aqueous layer was discarded and the organic phase was fractionated further on LC-NH2 cartridges as described by Bodennec et al. [28]. Alternatively, when unlabelled sphingoid bases, phosphorylated sphingoid bases and [14C]palmitoyl-CoA were used, glycerolipids were cleaved by mild alkaline hydrolysis before purification on LC-NH2 cartridges.
Reverse ceramidase assays
Reverse ceramidase assay was performed as described by El Bawab et al. [21]. Briefly, the substrates [14C]palmitic acid and sphingosine were first dried under nitrogen. The mixture was then resuspended by sonication in 200 μl of 0.1 M Hepes buffer (pH 7.0) containing 0.4% (v/v) Triton X-100 and 100 μg of protein. The reaction was terminated by adding 2 ml of Dole's solution (propan-2-ol/heptane/1 M NaOH; 4:1:0.1, by vol.). After centrifugation at 2000 rev./min for 10 min in a Beckman 6 KR rotor, the upper phase with heptane containing the labelled ceramide was collected, and the lower phase was washed once more with 2 ml of heptane. The heptane phases were pooled and dried before fractionation on LC-NH2 cartridges [28].
Characterization of the reaction products
For ceramide synthase and reverse ceramidase activities, the organic phases were evaporated to dryness under reduced pressure, taken up in 200 μl of chloroform and fractionated using solid-phase extraction on LC-NH2 cartridges [28]. The fractions containing ceramides (fractions 2) were concentrated and analysed further by TLC in the presence of unlabelled standards using chloroform/methanol (12.5:1, v/v) as a developing solvent. Radioactive ceramides were scraped from the plate and quantified by liquid-scintillation counting. Identification of phytoceramides was performed according to the method of Karlsson and Pascher [29]: after the first run, radioactive spots co-migrating with standard ceramide and phytoceramide were scraped from the plate, applied to a silica gel plate that was pre-impregnated by spraying with sodium meta-arsenite (1% in methanol), and run in the solvent system chloroform/methanol (19:1, v/v). The radioactive areas on the plate were localized by scraping each 0.5 cm up to the solvent front, then counting the radioactivity by liquid scintillation using Emulsifier Scintillator Plus™ (Packard).
Protein assay
Protein concentration was determined using the bicinchoninic acid method [30].
RESULTS
Characterization of the subcellular fractions
As reported in Table 1, estimation of marker enzyme activities show that purified mitochondria are only slightly contaminated by ER-related compartments (up to 2% with microsomes and 8% with MAM respectively). As reported previously [18], outer and inner mitochondrial membranes are also devoid of contamination by the ER markers. These results make our mitochondrial preparations, as well as mitochondrial membranes, suitable fractions to study the presence of ceramide synthase(s) within these subcellular compartments.
Table 1. Specific marker enzyme activities in subcellular fractions from rat liver (n=3).
NADPH:cytochrome c reductase activity in nmol of cytochrome c reduced/mg of protein per min; glucose-6-phosphatase activity in μmol of Pi formed/mg of protein per min; monoamine oxidase activity in nmol of benzylamine oxidized/mg of protein per min; cytochrome c oxidase activity in nmol of cytochrome c oxidized/mg of protein per min. ND, not determined.
| Fraction | NADPH:cytochrome c reductase (microsomes) | Glucose-6-phosphatase (MAM) | Monoamine oxidase (outer membranes) | Cytochrome oxidase (inner membranes) |
|---|---|---|---|---|
| Microsomes | 35.2±8.2 | 139±33 | ND | ND |
| MAM | 14.1±2.8 | 298.5±76 | 0.69±0.12 | 17.3±2.5 |
| Whole mitochondria | 0.70±0.08 | 23.9±2.7 | 11.5±0.9 | 427±23 |
| Outer membranes | 1.05±0.10 | 15.2±0.9 | 36.8±2.7 | 27.1±3.3 |
| Inner membranes | 0.15±0.02 | 1.25±0.11 | 1.84±0.12 | 1750±160 |
Ceramide synthase activities in mitochondria
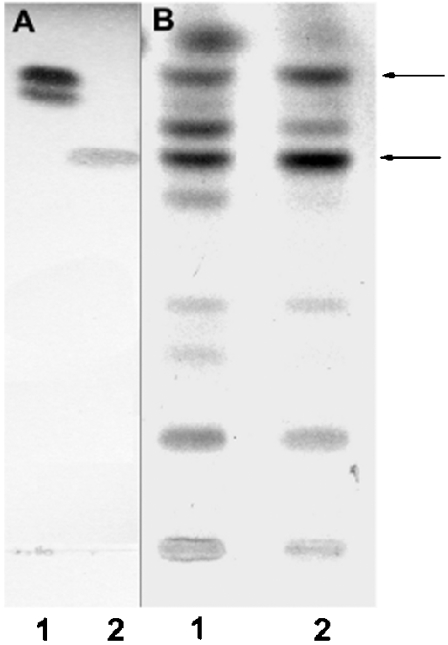
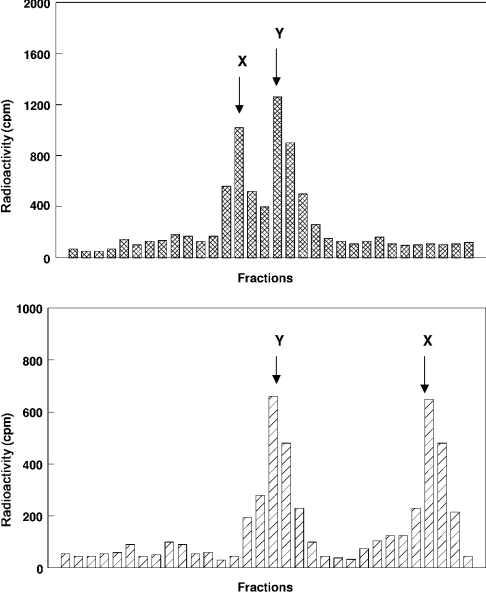
Purified mitochondria and MAM were incubated at 37 °C for 15 min with palmitoyl-CoA and [3H]sphingosine. As depicted in Figure 1, HPTLC analysis performed in the presence of authentic standards revealed in both subcellular fractions the presence of two major products co-migrating with ceramide and phytoceramide respectively. In lane 1 in Figure 1(B) (mitochondria), the product co-migrating with ceramide separated into two poorly demarcated bands, which presumably correspond to ceramides with different fatty-acid-chain lengths. In order to confirm the presence of phytoceramide as the other major radioactive product in Figure 1, the spots co-migrating with standard ceramide and phytoceramide were scraped from the plate, eluted and re-run either on silica gel plates (Figure 2, upper panel) or on 1% arsenite-impregnated HPTLC plates (Figure 2, lower panel). According to Karlsson and Pascher [29], the arsenite ion binds the two hydroxy groups at positions 3 and 4 of phytosphingosine, and the formed complex hinders the interaction of the α-glycol with the silica gel, resulting in a much faster migration of phytosphingosine-containing ceramides on thin-layer plates. As expected, the product previously identified as phytoceramide (X) migrated up to the solvent front (RF=0.95) on arsenite plates, whereas ceramide (RF=0.56) did not move ahead, thus confirming the presence of phytosphingosine as the sphingoid base in compound X. The time-course study of ceramide formation (Figure 3) showed that in both fractions (MAM and mitochondria), the amount of ceramide formed increased in a non-linear fashion to reach a maximum at 30 min, then continued at a constant rate throughout the experiment.
Figure 1. Ceramide biosynthesis by purified mitochondria and MAM in vitro.
Purified mitochondria and MAM were incubated with [3H]sphingosine and unlabelled palmitoyl-CoA under the conditions described in the Experimental section. After purification of the total lipid extract on solid-phase LC-NH2 columns, the fractions corresponding to ceramides (fractions 2 according to [28]) were analysed on silica gel TLC in the solvent system chloroform/methanol (12.5:1, v/v), along with standards. (A) Standards visualized at 150 °C after spraying with copper acetate (3%) in phosphoric acid (8%). Lane 1, ceramides type III; lane 2, phytoceramides. (B) Autoradiogram of fractions 2 eluted from LC-NH2 columns. Lane 1, mitochondria; lane 2, MAM. Arrows indicate the spots that were scraped and re-run in Figure 2.
Figure 2. TLC of the radiolabelled products obtained through ceramide synthase activity.
Upper panel: the spots co-migrating in Figure 1 respectively as standard ceramide type III and phytoceramide were scraped from the plate and re-run on an HPTLC plate in chloroform/methanol (12.5:1, v/v). The plate was then cut into 0.5 cm portions, extracted with chloroform/methanol (2:1, v/v) and counted for radioactivity after evaporation of the organic solvents. X, phytoceramide; Y, ceramide. Lower panel: the radioactive spots from the upper panel were scraped from the plate, extracted with chloroform/methanol (2:1, v/v) and re-run in chloroform/methanol (19:1, v/v) on a silica gel plate pre-coated with 1% sodium meta-arsenite. The plate was scraped into 0.5 cm portions, extracted with chloroform/methanol (2:1, v/v) and the radioactivity was counted after evaporation of the organic solvents. X, phytoceramide; Y, ceramide.
Figure 3. Time course of conversion of radioactive [3H]sphingosine into ceramide.
Incubation conditions were as described in the Experimental section, except that time was varied as indicated. Radioactive ceramides were purified as in Figure 1 and were separated on TLC, before scraping and liquid-scintillation counting. Inset, increasing protein concentrations were used for 5 min incubations. Results are means±S.D. for three distinct experiments.
Influence of FB1 (fumonisin B1) on mitochondrial ceramide synthase
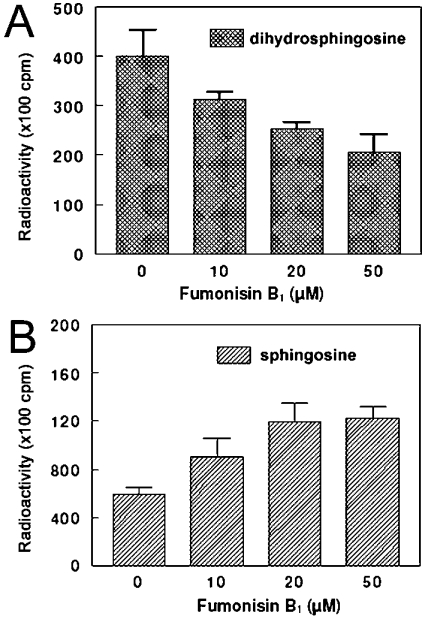
As suggested previously [20], we checked the ability of purified mitochondria to catalyse the N-acylation of [3H]sphingosine and [3H]dihydrosphingosine in vitro. As depicted in Figure 4, both sphingoid bases were effectively good substrates for the mitochondrial ceramide synthase(s). However, the sensitivity of the enzyme toward FB1, a well-known inhibitor of ceramide synthase, was surprisingly very different: sphinganine N-acyltransferase was inhibited in a concentration-dependent manner with FB1 (Figure 4A), whereas sphingosine N-acyltransferase was slightly stimulated, also in a concentration-dependent manner (Figure 4B). A similar result was obtained with the MAM fraction and rat liver microsomes (results not shown). Whatever the concentrations used (<75 μM), we never succeeded in obtaining the total inhibition of ceramide synthase(s) that was previously reported for rat brain microsomes [32].
Figure 4. Effect of FB1 on mitochondrial ceramide synthase in vitro.
Purified mitochondria were pre-incubated for 10 min at 37 °C in the presence of 20 μM FB1, after which ceramide synthase assays were performed as described in the Experimental section with [3H]sphingosine or [3H]dihydrosphingosine as substrates. After a 15 min incubation time, total lipids were fractionated on LC-NH2 columns and analysed by silica gel TLC and autoradiography. Radioactive ceramides co-migrating with authentic standards were scraped and radioactivity was estimated by liquid-scintillation counting. Results are means±S.D. for four distinct experiments. (A) Dihydrosphingosine N-acyltransferase. (B) Sphingosine N-acyltransferase.
Submitochondrial localization of ceramide synthase(s)
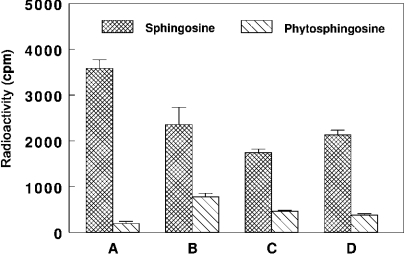
As shown in Figure 5, both outer and inner mitochondrial membranes were found to acylate exogenous sphingosine to approximately the same extent. The recovered ceramide synthase activity was 50% in outer mitochondrial membranes and 60% in the inner membranes of that measured in the MAM fraction respectively. As labelled phytoceramide was recovered in purified mitochondria incubated with [3H]sphingosine and palmitoyl-CoA (Figure 1), we checked the ability of the submitochondrial fractions and MAM to acylate exogenous phytosphingosine. Figure 5 shows that exogenous phytosphingosine is a less efficient substrate for mitochondrial ceramide synthase(s) when compared with exogenous sphingosine (30% of that recovered in mitochondria in the presence of added sphingosine), whereas MAM is not able to acylate exogenous phytosphingosine significantly.
Figure 5. Substrate specificity of ceramide synthase in MAM and submitochondrial compartments in vitro.
Purified subcellular fractions (100 μg of protein) were incubated for 15 min at 37 °C with 10 μM sphingosine or phytosphingosine and 2 nmol of [14C]palmitoyl-CoA. After purification of the total lipid extract on solid-phase LC-NH2 columns, fractions corresponding to ceramides were submitted to mild alkaline hydrolysis and analysed by silica gel TLC and autoradiography. Radioactive components co-migrating with authentic ceramides were scraped from the plate and radioactivity was estimated by liquid-scintillation counting. Results are means±S.D. for three distinct experiments. (A) MAM. (B) Purified mitochondria. (C) Purified outer mitochondrial membranes. (D) Purified inner mitochondrial membranes.
Other ways to synthesize ceramides in purified mitochondria
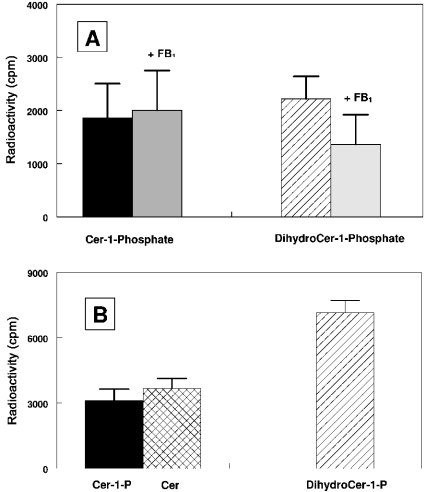
Among the different possibilities to generate ceramides in subcellular organelles, the reverse reaction of a neutral ceramidase has been recently characterized as a CoA-independent and FB1-insensitive ceramide synthase [21,31]. On the basis of molecular cloning and confocal-microscopy data, this activity has been ascribed to mitochondria [21]. We confirm that purified mitochondria, once freed from contamination by the MAM fraction, did indeed exhibit reverse ceramidase activity. This activity was also recovered in the MAM fraction (up to 70% of that recovered in purified mitochondria), whereas no significant activity (with regard to the cross-contamination by MAM) was detected in the whole microsomes under our experimental conditions. Another way to generate ceramides within the cell has been recently pointed out by Spiegel and co-workers [32], who presented evidence for the involvement of SPP-1 (sphingosine-1-phosphate phosphohydrolase), presumably located in the ER, in generating sphingosine as a substrate for ceramide synthase. On the basis of these data, purified mitochondria and MAM were incubated in the presence of unlabelled sphingosine 1-phosphate or dihydrosphingosine 1-phosphate and [14C]palmitoyl-CoA, and the products were purified further on LC-NH2 columns. Fraction 2 (containing free ceramides) and fraction 6 (containing ceramide 1-phosphate or dihydroceramide 1-phosphate) were eluted from the column and analysed by TLC in the presence of authentic standards. Figure 6 shows that mitochondria (Figure 6A) and MAM (Figure 6B) can acylate both phosphorylated sphingoid bases to generate the corresponding phosphorylated ceramides. The newly synthesized ceramide 1-phosphate can be converted further into ceramide in the MAM, but not in mitochondria. Although no significant differences were obtained for the mitochondrial sphingosine-1-phosphate acyltransferase in the presence of 20 μM FB1, dihydrosphingosine N-acyltransferase was inhibited up to 40% under the same experimental conditions.
Figure 6. Comparative acylation of exogenous phosphorylated sphingoid bases in mitochondria (A) and MAM (B).
Subcellular fractions (100 μg of protein) were incubated for 30 min at 37 °C in the presence of either 10 μM sphingosine 1-phosphate or dihydrosphingosine 1-phosphate and 2 nmol of [14C]palmitoyl-CoA (with or without 20 μM FB1). The radioactive lipids were submitted to mild alkaline hydrolysis and purified further on LC-NH2 columns. Ceramides (eluted in fraction 2) and ceramide 1-phosphate or dihydroceramide 1-phosphate (eluted in fraction 6) was applied on TLC and run in chloroform/methanol (12.5:1, v/v) for ceramides or chloroform/methanol/ethanoic acid (6.5:1.5/1, by vol.) for ceramide 1-phosphate. After autoradiography, radioactive spots co-migrating with authentic standards were scraped from the plate and radioactivity was estimated by liquid-scintillation counting. Results are the means±S.D. for three distinct experiments. Cer, ceramide.
DISCUSSION
The purpose of the present study was to establish without ambiguity the involvement of mitochondria in the subcellular metabolism of ceramides. Previous studies suggesting the presence of both ceramide synthase and reverse ceramidase within mitochondria lack convincing approaches in order to rule out a possible [21] or even certain [20] cross-contamination by the MAM fraction.
Our data show that purified mitochondria are able to acylate both dihydrosphingosine or sphingosine in vitro, thus generating either ceramide or phytoceramide. In addition, we were able to confirm that MAM also contains ceramide synthase(s), as suggested previously by Merrill [13]. Although phytoceramides are predominantly recovered in plants [33] and yeast [34], mammalian cells have also been reported to contain phytoceramide [35–37] in neutral glycolipids or sphingomyelins. Synthesis of 4-hydroxysphinganine (phytosphingosine) apparently occurs by the direct hydroxylation of sphinganine in plants [38], yeast [34] and mammals [39], but the subcellular localization of this synthesis is still a matter of debate. Nevertheless, a recent study demonstrated ceramide hydroxylation in plant microsomes [38], but almost 20% of the sphinganine hydroxylase activity was also recovered in the 10000 g pellet, thus suggesting a possible involvement of mitochondria in the metabolism of phytoceramide. As phytosphingosine was recently demonstrated to induce apoptotic cell death in cancer cells including mitochondrial alterations [40], our results underline phytoceramide as an intriguing molecule which could at the mitochondrial level also play a regulatory role in the execution of programmed cell death.
In addition, our in vitro study revealed surprising opposite effects of FB1 on mitochondrial ceramide synthase(s) whether dihydrosphingosine or sphingosine was used as substrates. FB1 has been demonstrated as a specific inhibitor of ceramide synthase both in vivo [41,42] and in vitro using rat liver microsomes [41]. Our intriguing results are not in accordance with those reported by the study of Merrill and co-workers [41] in isolated rat liver microsomes, which demonstrated an IC50 of 0.1 μM FB1 for inhibition of ceramide synthase. However a recent report presented evidence for the existence of a mammalian homologue (uog1, for upstream of growth and differentiation 1) of the yeast gene LAG1 (longevity-assurance gene 1) required for the regulation of a specific (dihydro)ceramide synthase [43]. In this earlier study, a resistance to FB1 was observed in uog1-transfected cells in vivo, whereas an inhibition of the synthase was obtained in microsomes from transfected cells. ASC1 (Alternaria stem canker), a tomato gene homologue of LAG1, has also been reported to encode a FB1-insensitive dihydroceramide synthase [45]. Although we are at present unable to explain the differential sensitivity of mitochondrial ceramide synthase(s) toward FB1 in vitro, such differences are becoming increasingly evident and lead us to agree with the idea [43,44] that more than one dihydroceramide synthase and/or ceramide synthase, based on either their fatty acid specificity or their difference in FB1 sensitivity, may coexist within the cell and in various subcellular organelles.
The further submitochondrial investigation of ceramide synthases revealed that both isolated outer and inner mitochondrial membranes are able to synthesize ceramides, emphasizing the idea proposed by Van Blitterswijk et al. [3] that the topology of ceramide formation should determine its function. The capacity of outer mitochondrial membrane to generate ceramide, as well as its presence as intrinsic components of this membrane [17], could suggest an involvement of this molecule in the alteration of the properties of the outer membrane during apoptosis. This consideration should be valid as well for inner mitochondrial membranes, since ceramide has been shown to interact directly with respiratory-chain components [22–24]. The elevated levels of ceramide found in mitochondria during CD95-, TNF-α (tumour necrosis factor α)- and radiation-induced apoptosis [24,25] should therefore imply this local production of ceramides.
Exogenous phytosphingosine was not a good substrate for ceramide synthase(s) when compared with sphingosine (Figure 5) or dihydrosphingosine, whatever the subcellular fractions used in the present study (MAM or mitochondria). This is apparently in contradiction with the results presented in Figure 1 for mitochondria, but such contradictory results have already been described in plants [38], where long-chain base hydroxylation also occurred following ceramide formation. The lack of competition between dihydrosphingosine and dihydroceramide for the hydroxylase led the authors of this previous study to propose the existence of separate hydroxylation isoenzymes.
From the pioneering work of Gatt [46], several lines of evidence have suggested that there is an alternative synthetic pathway of ceramide catalysed by reverse hydrolysis reaction of ceramidase [31,47,48]. The enzyme acted through a CoA-independent mechanism and was not inhibited by FB1 [31]. The recovery of a reverse ceramidase activity in purified mitochondria, as well as in MAM, confirms that the re-investigation of the mitochondrial localization of this enzyme with highly purified organelles was absolutely necessary to avoid misleading evidence.
Sphingosine 1-phosphate was recently shown to be also a potential substrate to generate ceramide within the cell [32]. Using exogenous sphingosine 1-phosphate or dihydrosphingosine 1-phosphate, it was shown that purified mitochondria are able to generate ceramide 1-phosphate in vitro, therefore pointing out another way for mitochondria to potentially increase ceramide levels within the organelle. MAM was also found to use both exogenous substrates to generate ceramide 1-phosphate, which in part was recovered as free ceramide, whereas it was not the case with purified mitochondria. Although the mammalian SPP-1 has been shown to co-localize with the ER marker calnexin [32], ceramide-1-phosphate phosphatase is a poorly documented enzyme, which has been mainly localized to plasma membrane [49,50], but also in a rat liver microsomal fraction [50]. Here again, the differential sensitivity of mitochondrial (or MAM) sphingosine-1-phosphate and dihydrosphingosine-1-phosphate acyltransferases to FB1 in vitro is in contradiction with that previously reported in SPP1-overexpressing cells in vivo, therefore suggesting additional regulatory processes in the control of ceramide levels in vivo.
Collectively, the results presented in the present paper give a clear indication of the involvement of mitochondria through different ways in the metabolism of ceramide. This participation cannot be ascribed to a cross-contamination by the MAM fraction, therefore strengthening the idea that endogenous mitochondrial ceramide may have a regulatory role in the execution of programmed cell death [51].
References
- 1.Hannun Y. A., Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 2.Mathias S., Peña L. A., Kolesnick R. N. Signal transduction of stress via ceramide. Biochem. J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Blitterswijk W., Van der Luit A., Veldman R. J., Verheij M., Borst J. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 2003;369:199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levade T., Jaffrezou J. P. Signaling sphingomyelinases: which, where, how and why? Biochim. Biophys. Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 5.Perry D. K., Carton J., Shah A. K., Meredith F., Uhlinger D. J., Hannun Y. A. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J. Biol. Chem. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- 6.Charles A. G., Han T. Y., Liu Y. Y., Hansen N., Giuliano A. E., Cabot M. C. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer Chemother. Pharmacol. 2001;47:444–450. doi: 10.1007/s002800000265. [DOI] [PubMed] [Google Scholar]
- 7.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;342:233–249. [PMC free article] [PubMed] [Google Scholar]
- 8.Susin S. A., Zamzani N., Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochim. Biophys. Acta. 1998;1366:151–165. doi: 10.1016/s0005-2728(98)00110-8. [DOI] [PubMed] [Google Scholar]
- 9.Xu J., Yeh C. H., Chen S., He L., Sensi S. L., Canzoniero L. M. T., Choi D. W., Hsu C. Y. Involvement of de novo ceramide biosynthesis in tumour necrosis factor-α/cycloheximide-induced cerebral endothelial cell death. J. Biol. Chem. 1998;273:16521–165426. doi: 10.1074/jbc.273.26.16521. [DOI] [PubMed] [Google Scholar]
- 10.Perry D. K. The role of de novo ceramide synthesis in chemotherapy-induced apoptosis. Ann. N.Y. Acad. Sci. 2000;905:91–96. doi: 10.1111/j.1749-6632.2000.tb06541.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Giuliano A. E., Cabot M. C. Enhanced de novo ceramide generation through activation of serine palmitoyltransferase by the P-glycoprotein antagonist SDZ PSC 833 in breast cancer cells. Mol. Cancer Ther. 2002;1:719–726. [PubMed] [Google Scholar]
- 12.Uchida Y., Nardo A. D., Collins V., Elias P. M., Holleran W. H. De novo ceramide synthesis participates in the ultraviolet B irradiation-induced apoptosis in undifferentiated cultured human keratinocytes. J. Invest. Dermatol. 2003;120:663–669. doi: 10.1046/j.1523-1747.2003.12098.x. [DOI] [PubMed] [Google Scholar]
- 13.Merrill A. H., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous pathway. J. Biol. Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 14.Mandon E. C., Ehses I., Rothen J., van Echten G., Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase and sphinganine N-acyltransferase in mouse liver. J. Biol. Chem. 1992;267:11144–11148. [PubMed] [Google Scholar]
- 15.Kolter T., Proia R. L., Sandhoff K. Combinatorial ganglioside biosynthesis. J. Biol. Chem. 2002;277:25859–25862. doi: 10.1074/jbc.R200001200. [DOI] [PubMed] [Google Scholar]
- 16.Futerman A. H., Steiger B., Hubbard A. L., Pagano R. E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of Golgi apparatus. J. Biol. Chem. 1990;265:8650–8657. [PubMed] [Google Scholar]
- 17.Ardail D., Popa I., Alcantara K., Pons A., Zanetta J. P., Louisot P., Thomas L., Portoukalian J. Occurrence of ceramides and neutral glycolipids with unusual long-chain base composition in purified rat liver mitochondria. FEBS Lett. 2001;488:160–164. doi: 10.1016/s0014-5793(00)02332-2. [DOI] [PubMed] [Google Scholar]
- 18.Ardail D., Gasnier F., Lermé F., Simonot C., Louisot P., Gateau-Roesch O. Involvement of mitochondrial contact sites in the subcellular compartmentalization of phospholipid biosynthetic enzymes. J. Biol. Chem. 1993;268:25985–25992. [PubMed] [Google Scholar]
- 19.Marsh B. J., Mastronarde D. N., Buttle K. F., Howell K. E., McIntosh J. R. Organellar relationships in the Golgi region of the pancreatic β-cell line HIT-T15, visualized by high resolution electron tomography. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2399–2406. doi: 10.1073/pnas.051631998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimeno H., Soeda S., Sakamoto M., Kouchi T., Kowakame T., Kihara T. Partial purification and characterization of sphingosine N-acyltransferase (ceramide synthase) from bovine liver mitochondrion-rich fraction. Lipids. 1998;33:601–605. doi: 10.1007/s11745-998-0246-2. [DOI] [PubMed] [Google Scholar]
- 21.El Bawab S., Roddy P., Qian T., Bielawska A., Lemasters J. J., Hannun Y. A. Molecular cloning and characterization of a human mitochondrial ceramidase. J. Biol. Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 22.Di Paola M., Cocco T., Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39:6660–6668. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 23.Gudz T. I., Tserng K. Y., Hoppel C. L. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Ruiz C., Colell A., Mari M., Morales A., Fernandez-Checa J. C. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J. Biol. Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 25.Vance J. E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 26.Ardail D., Popa I., Bodennec J., Louisot P., Schmitt D., Portoukalian J. The mitochondria-associated endoplasmic reticulum subcompartment (MAM fraction) of rat liver contains highly active sphingolipid-specific glycosyltransferases. Biochem. J. 2003;371:1013–1019. doi: 10.1042/BJ20021834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang E., Merrill A. H., Jr Ceramide synthase. Methods Enzymol. 2000;311:15–21. doi: 10.1016/s0076-6879(00)11062-6. [DOI] [PubMed] [Google Scholar]
- 28.Bodennec J., Koul O., Agado L., Brichon G., Zwingelstein G., Portoukalian J. A procedure for fractionation of sphingolipid classes by solid-phase extraction on aminopropyl cartridges. J. Lipid Res. 2000;41:1524–1531. [PubMed] [Google Scholar]
- 29.Karlsson K.-A., Pascher I. Thin-layer chromatography of ceramides. J. Lipid Res. 1971;12:466–472. [PubMed] [Google Scholar]
- 30.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gurtner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olsol B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 31.El Bawab S., Birbes H., Roddy P., Szulc Z. M., Bielawska A., Hannun Y. A. Biochemical characterization of the reverse activity of rat brain ceramidase: a CoA-independent and fumonisin B1-insensitive ceramide synthase. J. Biol. Chem. 2001;276:16758–16766. doi: 10.1074/jbc.M009331200. [DOI] [PubMed] [Google Scholar]
- 32.Le Stunff H., Galve-Roperh I., Peterson C., Milstein S., Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J. Cell. Biol. 2002;158:1039–1049. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbas H. K., Tanaka T., Duke S. O., Porter J. K., Wray E. M., Hodge L., Sessions A. E., Wang E., Merrill A. H., Jr, Riley A. T. Fumonisin- and AAL-toxin-induced disruption of sphingolipid metabolism and accumulation of free sphingoid bases. Plant Physiol. 1994;106:1085–1093. doi: 10.1104/pp.106.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickson R. C., Lester R. L. Sphingolipid functions in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2002;1583:13–25. doi: 10.1016/s1388-1981(02)00210-x. [DOI] [PubMed] [Google Scholar]
- 35.Crossman M. W., Hirschberg C. B. Biosynthesis of phytosphingosine by the rat. J. Biol. Chem. 1977;252:5815–5819. [PubMed] [Google Scholar]
- 36.Sato E., Uezado T., Fujita H., Nishimura K. Development profiles of glycolipids in mouse small intestine. J. Biochem. (Tokyo) 1982;91:2013–2019. doi: 10.1093/oxfordjournals.jbchem.a133894. [DOI] [PubMed] [Google Scholar]
- 37.Madison K. C., Swartzendruber D. C., Wertz P. W, Downing D. T. Sphingolipid metabolism in organotypic mouse keratinocyte cultures. J. Invest. Dermatol. 1990;95:657–664. doi: 10.1111/1523-1747.ep12514333. [DOI] [PubMed] [Google Scholar]
- 38.Wright B. S., Snow J. W., O'Brian T. C., Lynch D. V. Synthesis of 4-hydroxysphinganine and characterization of sphinganine hydroxylase activity in corn. Arch. Biochem. Biophys. 2003;415:184–192. doi: 10.1016/s0003-9861(03)00261-3. [DOI] [PubMed] [Google Scholar]
- 39.Crossman M. W., Hirschberg C. B. Biosynthesis of 4D-hydroxysphinganine by the rat: en bloc incorporation of the sphinganine carbon backbone. Biochim. Biophys. Acta. 1984;795:411–416. doi: 10.1016/0005-2760(84)90092-4. [DOI] [PubMed] [Google Scholar]
- 40.Park M. T., Kang J. A., Choi J. A., Kim T. H., Bae S., Kang S., Kim S., Choi W. I., Cho C. K., Chung H. Y., et al. Phytosphingosine induces apoptotic cell death via caspase-8 activation and Bax translocation in human cancer cells. Clin. Cancer Res. 2003;9:878–885. [PubMed] [Google Scholar]
- 41.Wang E., Norred W. P., Bacon C. W., Riley R. T., Merrill A. H., Jr Inhibition of sphingolipid biosynthesis by fumonisins: implication for diseases associated with Fusarium monoliforme. J. Biol. Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- 42.Merrill A. H., Jr, van Echten G., Wang E., Sandhoff K. Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J. Biol. Chem. 1993;268:27299–27306. [PubMed] [Google Scholar]
- 43.Venkataraman K., Riebeling C., Bodennec J., Riezman H., Allegood J. C., Sullards M. C., Merrill A. H., Jr, Futerman A. H. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J. Biol. Chem. 2002;277:35642–25649. doi: 10.1074/jbc.M205211200. [DOI] [PubMed] [Google Scholar]
- 44.Riebeling C., Allegood J. C., Wang E., Merrill A. H., Jr, Futerman A. H. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J. Biol. Chem. 2003;278:43452–43459. doi: 10.1074/jbc.M307104200. [DOI] [PubMed] [Google Scholar]
- 45.Brandwagt B. F., Meslah L. A., Takken F. L., Laurent P. L., Kneppers T. J., Hille J., Nijkamp H. J. A longevity assurance gene homolog of tomato mediates resistance to Alternaria alternata f. sp. lycopersici toxins and fumonisin B1. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4961–4966. doi: 10.1073/pnas.97.9.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatt S. Enzymatic hydrolysis of sphingolipids: I hydrolysis and synthesis of ceramides by an enzyme from rat brain. J. Biol. Chem. 1966;241:3274–3230. [PubMed] [Google Scholar]
- 47.Mao C., Xu R., Bielawska A., Szulc Z. M., Obeid L. M. Cloning and characterization of a Saccharomyces cerevisiae alkaline ceramidase with specificity for dihydroceramide. J. Biol. Chem. 2000;275:31369–31378. doi: 10.1074/jbc.M003683200. [DOI] [PubMed] [Google Scholar]
- 48.Tani M., Okino N., Mori K., Tanigawa T., Izu H., Ito M. Molecular cloning of the full-length cDNA encoding mouse neutral ceramidase: a novel but highly conserved gene family of neutral alkaline ceramidases. J. Biol. Chem. 2000;275:11229–11234. doi: 10.1074/jbc.275.15.11229. [DOI] [PubMed] [Google Scholar]
- 49.Shinghal R., Scheller R. H., Bajjalieh S. M. Ceramide-1-phosphate phosphatase activity in brain. J. Neurochem. 1993;61:2279–2285. doi: 10.1111/j.1471-4159.1993.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 50.Boudker O., Futerman A. H. Detection and characterization of ceramide-1-phosphate phosphatase activity in rat liver plasma membrane. J. Biol. Chem. 1993;268:22150–22155. [PubMed] [Google Scholar]
- 51.Birbes H., El Bawab S., Hannun Y. A., Obeid L. M. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]