Abstract
Background
APG-1387 is a novel second mitochondrial-derived activator of caspases mimetic, small-molecule inhibitor targeting inhibitor of apoptosis proteins. We report results from two phase I trials evaluating the tolerability, safety, and antitumor activity of APG-1387 monotherapy and APG-1387 plus toripalimab [a programmed cell death 1 (PD-1) inhibitor] for advanced solid tumors.
Patients and methods
Participants aged ≥18 years who had histologically confirmed advanced solid tumors with no appropriate standard of care (or refractory to standard care) were eligible. Patients received escalating intravenous doses of APG-1387 alone or combined with fixed-dose toripalimab (240 mg every 3 weeks) in a ‘3 + 3’ design. Primary endpoints were dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD) in the monotherapy trial, and recommended phase II dose (RP2D) in the combination therapy trial. Secondary endpoints included the pharmacokinetic and pharmacodynamic profiles and preliminary efficacy in both trials.
Results
In the monotherapy trial, 28 subjects were enrolled and received ≥1 treatment cycle. No DLT was reported among the 28 subjects, and the MTD was not reached. One participant (3.6%) had a grade ≥3 treatment-related adverse event (TRAE) of alanine aminotransferase elevation. In efficacy analysis of 23 participants, none achieved an objective response, and the disease control rate was 21.7%. In the combination trial, 22 subjects were enrolled and included in all analyses. There was one DLT of grade 3 lipase elevation. The MTD was not reached. Four grade ≥3 TRAEs occurred in three participants (13.6%), with the most common being lipase elevation (n = 2). The RP2D was 45 mg weekly. The objective response rate was 13.6%, with complete response achieved in one subject, and the disease control rate was 54.5%.
Conclusions
APG-1387 45 mg weekly plus toripalimab was well tolerated and is recommended for further study, with preliminary clinical activity observed in study participants with advanced solid tumors.
Key words: antitumor activity, PD-1 inhibitor, phase I trial, IAP inhibitor, SMAC mimetic
Highlights
-
•
APG-1387 has good safety profile; no DLTs were reported.
-
•
APG-1387 45 mg every week plus toripalimab was well tolerated and thus chosen as the recommended phase II dose.
-
•
APG-1387 plus toripalimab showed activity in anti-PD-1/PD-L1 treatment-naïve patients with NPC.
Introduction
The global cancer burden is both formidable and burgeoning. According to the GLOBOCAN 2020 estimates of cancer incidence and mortality produced by the International Agency for Research on Cancer, 19 million new cancer cases and 10 million cancer deaths occurred worldwide in 2020, and 28 million incident cases are projected in 2040 because of the growing economy and population aging all over the world.1,2 Advanced cancers, including lung cancer, gastric cancer, colorectal cancer (CRC), and nasopharyngeal carcinoma (NPC), have dismal prognoses, and there is no drug available after standard systemic therapy. Therefore, exploring new antitumor therapies has become a research hotspot.
Resisting cell death by apoptosis is a fundamental hallmark of oncogenesis.3 Apoptosis is a form of programmed cell death that is initiated by either intrinsic or extrinsic apoptotic signals and is highly regulated by different proapoptotic and antiapoptotic proteins. The inhibitor of apoptosis protein (IAP) family functions as the main inhibitors of caspase activity.4 Eight human IAPs, namely, X-linked-IAP (XIAP), cellular IAP1 (cIAP1), cIAP2, livin (ML-IAP), neuronal apoptosis inhibitory protein (NAIP), IAP-like protein (ILP2), survivin, and baculoviral IAP repeat (BIR)-containing ubiquitin conjugating enzyme (BRUCE/Apollon), have been identified.5, 6, 7 IAPs are the only known endogenous proteins that negatively regulate the apoptotic pathway by inhibiting both initiator and effector caspases.8,9 IAPs are also involved in regulation of nuclear factor (NF)-kB,10 mitogen-activated protein kinase (MAPK)11 and transforming growth factor-β (TGF-β) signaling12 as well as innate and adaptive immunity signaling pathways.13 IAP overexpression has been found in various human cancers and is associated with advanced disease stage, chemoresistance, and poor prognosis.14 Considering the salient roles of IAPs in regulating tumor cell apoptosis, mediating drug resistance, and influencing patients’ prognoses, targeting IAPs is a potential therapeutic method to treat cancers. Over decades, targeting IAPs has been studied and developed as novel cancer treatments. The antiapoptotic effects of IAPs can be blocked by specific proteins, including second mitochondrial-derived activator of caspases/Diablo (SMAC), Omi/HtrA2, and XAF1 (XIAP-associated factor 1), by binding to the BIR domains of IAPs.15, 16, 17, 18
APG-1387 is a novel bivalent SMAC mimetic that binds to both BIR2 and BIR3 domains, promoting the rapid degradation of cIAP1, cIAP2, and XIAP. APG-1387 showed antitumor activities in vivo and in vitro by inducing proteasomal degradation of IAPs and interrupting IAP-mediated inhibition of caspases, hence promoting apoptosis of cancer cells.19, 20, 21 APG-1387 also induced activation of natural killer (NK) cells in vitro, and its combination with tumor necrosis factor-alpha (TNF-α) or TNF-related apoptosis-inducing ligand (TRAIL) significantly inhibited tumor growth by promoting apoptosis in hepatocellular carcinoma (HCC) cells.22 Further, studies have demonstrated that IAP antagonists can induce antitumor immunity by promoting the noncanonical NF-κB pathway, in turn causing nonmutated tumor cells to secrete type I interferon (IFN), then inducing macrophages to phagocytose multiple myeloma cells23 as well as enhancing in vivo prophylactic and therapeutic antitumor vaccines,24 oncolytic viruses,25 and chimeric antigen receptor T-cell (CAR-T)26 in solid tumors. Kearney and colleagues27 reported that an IAP antagonist combined with programmed cell death 1 (PD-1) blockade markedly enhanced antitumor activity independently of perforin-mediated tumor cell death. Mechanistically, APG-1387 had a synergistic antitumor effect with immune checkpoint inhibitors in preclinical models.28
Therefore, we conducted two phase I trials to evaluate the tolerability, safety, preliminary antitumor activity, and pharmacologic profile of APG-1387 monotherapy, or APG-1387 in combination with the PD-1 inhibitor toripalimab, in subjects with advanced solid tumors.
Patients and methods
Methods and protocol
This report presents the results of two phase I, open-label, single-arm studies of APG-1387 alone (internal study identifier, APG-1387-CH101; Chinese Clinical Trial Registry identifier, CTR20150161), or in combination with toripalimab (Coherus BioSciences, Inc., Redwood City, CA), in subjects with advanced solid tumors (internal study identifier, APG-1387-XC101; ClinicalTrials.gov identifier, NCT04284488). Baseline demographics and disease characteristics of these subjects are summarized in Table 1. The two studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines, and applicable local regulations. The protocols were approved by institutional review boards at Sun Yat-sen University Cancer Center and the First People’s Hospital of Foshan. Written informed consent was obtained from all subjects before enrollment.
Table 1.
Baseline characteristics of subjects in the two trials
| Characteristic | APG-1387 monotherapy trial |
APG-1387 combination trial |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤12 mg | 20 mg | 30 mg | 45 mg | Overall | 20 mg QW∗2 | 30 mg QW∗2 | 30 mg QW∗3 | 45 mg QW∗3 | Overall | |
| n | 15 | 5 | 3 | 5 | 28 | 3 | 8 | 6 | 5 | 22 |
| Age, year median (range) | 57.0 (29-71) | 49.0 (43-66) | 45.0 (29-59) | 45.0 (32-59) | 53.0 (29-71) | 44.0 (39-57) | 47.0 (37-65) | 60.0 (46-70) | 49.0 (31-69) | 49.0 (31-70) |
| Sex, n (%) | ||||||||||
| Female | 4 (26.7) | 2 (40.0) | 0 | 1 (20.0) | 7 (25.0) | 1 (33.3) | 2 (25.0) | 1 (16.7) | 0 | 4 (18.2) |
| Male | 11 (73.3) | 3 (60.0) | 3 (100.0) | 4 (80.0) | 21 (75.0) | 2 (66.7) | 6 (75.0) | 5 (83.3) | 5 (100.0) | 18 (81.8) |
| ECOG performance status, n (%) | ||||||||||
| 0 | 7 (46.7) | 2 (40.0) | 0 | 1 (20.0) | 10 (35.7) | 3 (100.0) | 6 (75.0) | 2 (33.3) | 5 (100.0) | 16 (72.7) |
| 1 | 8 (53.3) | 3 (60.0) | 3 (100.0) | 4 (80.0) | 18 (64.3) | 0 | 2 (25.0) | 4 (66.7) | 0 | 6 (27.3) |
| Tumor type, n (%) | ||||||||||
| Colorectal cancer | 8 (53.3) | 2 (40.0) | 1 (33.3) | 2 (40.0) | 13 (46.4) | 2 (66.7) | 2 (25.0) | 0 | 1 (20.0) | 5 (22.7) |
| Esophageal cancer | 3 (20.0) | 1 (20.0) | 0 | 0 | 4 (14.3) | 0 | 0 | 0 | 0 | 0 |
| Gastric cancer | 1 (6.7) | 0 | 0 | 1 (20.0) | 2 (7.1) | 0 | 0 | 0 | 0 | 0 |
| Hepatobiliary cancer | 0 | 0 | 1 (33.3) | 1 (20.0) | 2 (7.1) | 0 | 0 | 0 | 0 | 0 |
| Nasopharyngeal carcinoma | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 3 (37.5) | 2 (33.3) | 2 (40.0) | 8 (36.4) |
| Non-small-cell lung cancer | 0 | 0 | 0 | 0 | 0 | 0 | 2 (25.0) | 4 (66.7) | 2 (40.0) | 8 (36.4) |
| Other carcinomas | 3 (20.0) | 2 (40.0) | 1 (33.3) | 1 (20.0) | 7 (25.0) | 0 | 1 (12.5) | 0 | 0 | 1 (4.5) |
| Metastasis organs, n (%) | ||||||||||
| 1 | 3 (20.0) | 1 (20.0) | 0 | 2 (40.0) | 6 (21.4) | 1 (33.3) | 2 (25.0) | 1 (16.7) | 1 (20.0) | 5 (22.7) |
| 2 | 6 (40.0) | 1 (20.0) | 1 (33.3) | 3 (60.0) | 11 (39.3) | 1 (33.3) | 3 (37.5) | 2 (33.3) | 1 (20.0) | 7 (31.8) |
| ≥3 | 6 (40.0) | 3 (60.0) | 2 (66.7) | 0 | 11 (39.3) | 1 (33.3) | 3 (37.5) | 3 (50.0) | 3 (60.0) | 10 (45.5) |
| Metastasis site, n (%) | ||||||||||
| Liver | 11 (73.3) | 3 (60.0) | 2 (66.7) | 2 (40.0) | 18 (64.3) | 1 (33.3) | 6 (75.0) | 3 (50.0) | 3 (60.0) | 13 (59.1) |
| Lung | 11 (73.3) | 4 (80.0) | 2 (66.7) | 2 (40.0) | 19 (67.9) | 0 | 3 (37.5) | 2 (33.3) | 3 (60.0) | 8 (36.4) |
| Other | 10 (66.7) | 4 (80.0) | 3 (100.0) | 3 (60.0) | 20 (71.4) | 3 (100.0) | 6 (75.0) | 6 (100.0) | 4 (80.0) | 19 (86.4) |
| Prior lines of systemic therapy, n (%) | ||||||||||
| <3 | 3 (20.0) | 0 | 2 (66.7) | 3 (60.0) | 8 (28.6) | 0 | 1 (12.5) | 2 (33.3) | 1 (20.0) | 4 (18.2) |
| ≥3 | 12 (80.0) | 5 (100.0) | 1 (33.3) | 2 (40.0) | 20 (71.4) | 3 (100.0) | 7 (87.5) | 4 (66.7) | 4 (80.0) | 18 (81.8) |
| Prior PD-1/PD-L1 inhibitor treatment, n (%) | ||||||||||
| No | 15 (100.0) | 5 (100.0) | 3 (100.0) | 5 (100.0) | 28 (100.0) | 2 (66.7) | 2 (25.0) | 2 (33.3) | 1 (20.0) | 7 (31.8) |
| Yes | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 6 (75.0) | 4 (66.7) | 4 (80.0) | 15 (68.2) |
Continuous data are presented as median (interquartile range). Categorical data are presented as number (percentage) of subjects.
ECOG, Eastern Cooperative Oncology Group; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1.
Study design and treatments
In the APG-1387 monotherapy trial, doses of APG-1387 were increased according to an escalation scheme: 0.3, 0.6, 4.0, 7.0, 12, 20, 30, and 45 mg according to a ‘3 + 3’ design. The Safety Monitoring Committee (SMC) guidance was followed to determine the dose escalation based on the previously obtained safety and pharmacokinetic (PK) data for that dose group. APG-1387 was administered by intravenous (i.v.) infusion over 30 min each week at a starting dose of 0.3 mg on days 1, 8, and 15 (D1/8/15), followed by a 1-week rest period in a 28-day treatment cycle.
In the APG-1387 combination trial, toripalimab 240 mg was administered i.v. over at least 30 min every 3 weeks (Q3W), and the combined dose exploration of APG-1387 was conducted in two stages. The first stage was a 3 + 3 scheme. The initial dose of APG-1387 was 20 mg, administered i.v. on D1/8 Q3W, and the highest dose of APG-1387 was 30 mg. According to the APG-1387-US001 study, APG-1387 45 mg QW combined with pembrolizumab was well tolerated.29 Based on these findings, the second stage was added, and the starting dose of APG-1387 was 45 mg i.v. (D1/D8/D15, Q3W) weekly. The dose was decreased to 30 mg according to a single dose-limiting toxicity (DLT) event of grade 3 lipase increase in the 45-mg dose cohort. If no DLT occurred, no less than six study participants could be enrolled at the highest dose. The drug was administered and efficacy observed until any a priori ‘stopping criteria’ were met.
Participants
Subjects ≥18 years old were eligible for both the APG-1387 monotherapy and combination trials if they had (i) histologically confirmed locally advanced or metastatic solid tumors with no appropriate standard of care (or failed standard antitumor therapy), (ii) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, (iii) an estimated life expectancy of ≥3 months, (iv) adequate organ function, and (v) any adverse event (AE) from previous treatment for cancer that had resolved to grade ≤1 per National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0 (APG-1387 monotherapy trial) or 5.0 (APG-1387 combination trial, including two sites, Sun Yat-sen University Cancer Center and the First People’s Hospital of Foshan).
In the APG-1387 combination trial, at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was required. Key exclusion criteria included (i) receiving anticancer treatment or any investigational treatment within 28 days before the first dose of APG-1387; (ii) clinically significant tumor embolization or tumor lysis syndrome (TLS); (iii) previous treatment with an IAP inhibitor; (iv) allergy to APG-1387 or any component used in its preparation; (v) active central nervous system metastases; (vi) active autoimmune disease or a history of autoimmune disease or immunodeficiency; (vii) active infection within 2 weeks before enrollment; (viii) a history of interstitial lung disease; (ix) a history of chronic viral hepatitis or human immunodeficiency virus infection; (x) clinically significant cardiovascular, hepatic, renal, gastrointestinal, or metabolic disease; (xi) pregnant or lactating status in female participants; or (xii) other uncontrolled chronic comorbidities. In the APG-1387 combination trial, study participants with allergies to toripalimab were excluded.
Assessments
Safety assessments included vital signs, physical examination, hematology tests, blood biochemistry, thyroid function, coagulation function, carcinoembryonic antigen, electrocardiogram, pregnancy test for women, and clinical symptoms. Adverse events were graded according to the CTCAE version 4.0 (APG-1387 monotherapy trial) or 5.0 (APG-1387 combination trial) and were documented from the time informed consent was obtained until 90 days after the last dose of study drug was administered, or the participant started treatment with a new clinical regimen, died, or was lost to follow-up, whichever occurred first.
In both trials, any of the following AEs evaluated by the investigator as related to study drug was considered a DLT during the first treatment cycle: (i) grade ≥3 nonhematologic toxicity, including nausea, vomiting, or diarrhea refractory to maximum antiemetics/diarrhea treatment; (ii) grade ≥3 alanine aminotransferase (ALT), aspartate aminotransferase (AST), or total bilirubin elevations lasting >7 days; (iii) grade 4 neutropenia lasting for 7 days; (iv) grade ≥3 neutropenia with oral temperature ≥38.5°C lasting >4 days; (v) grade ≥3 neutropenia with grade ≥3 infection; (vi) thrombocytopenia of any grade with clinically significant bleeding; and (vii) grade 4 thrombocytopenia lasting ≥7 days. In the APG-1387 combination trial, DLTs also included grade ≥3 lipase elevation and grade ≥2 pneumonitis/interstitial lung disease that could not be resolved by dose delay and systemic steroid therapy. The dose level below the dose at which DLTs occurred in at least two of six subjects in cycle 1 was defined as the maximum tolerated dose (MTD).
Tumors were evaluated by computed tomography (CT) or magnetic resonance imaging according to RECIST criteria version 1.1 after the first cycle and then every 8 weeks in the APG-1387 monotherapy trial and modified RECIST 1.1 for immune-based therapeutics (termed iRECIST)30 every 6 weeks in the APG-1387 combination trial.
Endpoints
The primary endpoints in the APG-1387 monotherapy trial were DLT and MTD, whereas in the APG-1387 combination trial the primary outcome measures were MTD and recommended phase II dose (RP2D). Secondary endpoints in both trials included the PK and pharmacodynamic (PD) profiles of APG-1387, as well as the preliminary efficacy of APG-1387 monotherapy or APG-1387 combined with toripalimab in individuals with advanced solid tumors. The antitumor activity parameters included complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), objective response rate (ORR, defined as the proportion of study participants who achieved a CR or PR), disease control rate (DCR, defined as the proportion of subjects with a CR, PR, or SD), and duration of response (DoR, defined as the time from first documented CR or PR to the first assessment of PD or death, whichever occurred first).
PK and PD assessments
In the APG-1387 monotherapy trial, blood samples for PK analyses were collected at the following time points: before dosing and 0.25, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 12, and 24 h after dosing on D1/15. Plasma concentrations of APG-1387 were analyzed using liquid chromatography-tandem mass spectrometry (LC/MS/MS), with a lower limit of quantification of 0.1 ng/ml for APG-1387. The PK parameters were calculated for APG-1387 using the Phoenix WinNonlin software version 8.2 (Certara USA, Inc., Princeton, NJ).
IAPs are important regulators of cytokinesis, proliferation, differentiation, apoptosis, and signal transduction,31 and IAP antagonists have demonstrated divergent immunomodulatory properties in human immune subsets and cytokine production.32 The latter have also been observed in APG-1387 preclinical studies. To further verify the immunomodulatory effects of APG-1387 at the clinical stage, plasma specimens for cytokines in the APG-1387 combination trial testing were collected on C1D1 before dosing and 4 and 24 h after the first APG-1387 dose and on C2D1 and C3D1 before toripalimab dosing by the Meso Scale Discovery assay method. Because of large interindividual variability, post-dose interferon-γ (IFN-γ), interleukin-12 (IL-12), IL-5, and IL-6 levels were normalized by dividing values by baseline levels of each subject. A total of 3 ml of peripheral blood was collected on C1D1, C2D1, and C3D1, and at the end of treatment for lymphocyte subset detection by flow cytometry. Post-dose cell proportions were normalized by dividing values by the corresponding study participant’s baseline level.
Quantification and statistical analyses
No formal hypotheses were tested in this study. Sample sizes in both trials were determined by the SMC according to observed toxicities and the PK profile. All subjects who received at least one dose of APG-1387 or APG-1387 combined with toripalimab were included in the safety and efficacy analyses. The 95% confidence intervals (CIs) of DCR and DoR were assessed by the Clopper–Pearson method. For categorical data, numbers and percentages of individuals are presented. All statistical analyses were carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Monotherapy trial—baseline characteristics
For the APG-1387 monotherapy trial, 36 study participants were screened between 23 March 2015, and 21 March 2017. Of these, 28 were enrolled and received at least one cycle of APG-1387. Hence, 28 participants were included in the safety population. Of these 28 subjects, 23 were included in the efficacy evaluation population; five individuals did not receive at least one tumor evaluation because of safety issues related to primary disease. Participants received the following doses: 0.3 mg (n = 3), 0.6 mg (n = 3), 4 mg (n = 3), 7 mg (n = 3), 12 mg (n = 3), 20 mg (n = 5), 30 mg (n = 3), and 45 mg (n = 5) (Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2024.103651).
Baseline demographics and disease characteristics of these subjects are summarized in Table 1. The major tumor type was CRC (n = 12, 43%), followed by esophageal (n = 4, 14%), gastric (n = 3, 11%), hepatobiliary (n = 2, 7%), and other cancers (n = 7, 25%). In all, 11 (39.3%) of 28 study participants had >3 metastatic organs, and 20 (71.4%) of 28 received ≥3 prior lines of systemic therapy. No subject received prior PD-1 or programmed death-ligand 1 (PD-L1) inhibitor treatment.
Monotherapy trial—safety analysis
Of 28 participants in the safety population, 25 (89.3%) experienced treatment-emergent adverse events, most commonly grade 1 or 2 abdominal pain, anemia, headache, and elevated aspartate aminotransferase (AST). In all, treatment-related adverse events (TRAEs) occurred in six (21.4%) subjects. The most common TRAE was facial-nerve disorder (n = 3, 10.7%), all of which occurred in the APG-1387 45-mg group, followed by elevated alanine transaminase (ALT) (n = 2, 7.1%) and elevated AST (n = 2, 7.1%), which occurred in the 30- and 45-mg groups. Pruritus (n = 1, 3.6%) and asthenia (n = 1, 3.6%) occurred in the 20- and 45-mg groups, respectively (Table 2, Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103651). A grade 3 TRAE of elevated ALT was recorded in one individual within the 45-mg group. Grade 2 facial-nerve disorder associated with APG-1387 occurred in one study participant in the 45-mg group during the first cycle. No DLTs occurred, and the MTD was not reached.
Table 2.
Treatment-related adverse events (TRAEs)
| n (%) | APG-1387 monotherapy trial (n = 28) |
APG-1387 combination trial (n = 22) |
||
|---|---|---|---|---|
| All grade | Grade ≥3 | All grade | Grade ≥3 | |
| Any TRAE | 6 (21·4) | 1 (3·6) | 17 (77·3) | 3 (13·6) |
| Headache | 0 (0) | 0 (0) | 1 (4·5) | 0 (0) |
| Hypogeusia | 0 (0) | 0 (0) | 2 (9·1) | 0 (0) |
| Facial-nerve disorder | 3 (10·7) | 0 (0) | 0 (0) | 0 (0) |
| Alanine aminotransferase elevation | 2 (7·1) | 1 (3·6) | 2 (9·1) | 0 (0) |
| Aspartate aminotransferase elevation | 2 (7·1) | 0 (0) | 1 (4·5) | 0 (0) |
| Asthenia | 1 (3·6) | 0 (0) | 0 (0) | 0 (0) |
| Pruritus | 1 (3·6) | 0 (0) | 1 (4·5) | 0 (0) |
| Lipase elevation | 0 (0) | 0 (0) | 8 (36·4) | 2 (9·1) |
| Platelet count decrease | 0 (0) | 0 (0) | 1 (4·5) | 1 (4·5) |
| Immune-mediated pancreatitis | 0 (0) | 0 (0) | 1 (4·5) | 1 (4·5) |
| Rash | 0 (0) | 0 (0) | 6 (27·3) | 0 (0) |
| Hypoesthesia | 0 (0) | 0 (0) | 2 (9·1) | 0 (0) |
| Amylase elevation | 0 (0) | 0 (0) | 4 (18·2) | 0 (0) |
| Blood creatinine elevation | 0 (0) | 0 (0) | 2 (9·1) | 0 (0) |
| Leukopenia | 0 (0) | 0 (0) | 2 (9·1) | 0 (0) |
| Infusion site reaction | 0 (0) | 0 (0) | 2 (9·1) | 0 (0) |
| Hypothyroidism | 0 (0) | 0 (0) | 2 (9·1) | 0 (0) |
| Decreased appetite | 0 (0) | 0 (0) | 2 (9·1) | 0 (0) |
Data are presented as number (percentage) of patients.
Monotherapy trial—efficacy analysis
As of the data cut-off of 21 March 2017, all 28 subjects had discontinued study treatment, primarily because of disease progression (n = 21, 75%). No study participant achieved a CR or PR, and five experienced SD at the first post-baseline scan, including one each in the 0.6-, 7-, 20-, 30-, and 45-mg groups. The remaining 18 subjects had PD, resulting in a DCR of 21.7%. One individual with CRC in the 20-mg group achieved SD with tumor reduction for 16 weeks (Table 3, Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103651).
Table 3.
Pooled clinical activity
| APG-1387 monotherapy trial | APG-1387 combination trial | |
|---|---|---|
| n | 23 | 22 |
| Best overall response, n (%) | ||
| Complete response | 0 (0) | 1 (4·5) |
| Partial response | 0 (0) | 2 (9·1) |
| Stable disease | 5 (21·7) | 9 (40·9) |
| Progressive disease | 18 (78·3) | 10 (45·5) |
| Not evaluable | 5 (21·7) | 0 (0) |
| Objective response rate | 0 (0) | 3 (13·6) |
| 95% confidence interval | 0-0 | 2·9-34·9 |
| Disease control rate | 5 (21·7) | 12 (54·5) |
| 95% confidence interval | 7·46-43·70 | 32·2-75·6 |
| Duration of response (months) | NA | NR [8·4-NR] |
| 95% confidence interval | NA | NR |
Data are presented as number (percentage of patients) or median (interquartile range) unless otherwise specified.
NA, not applicable; NR, not reached.
Monotherapy trial—pharmacokinetic analysis
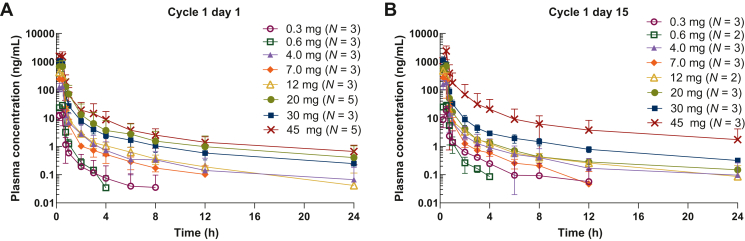
Following weekly (QW) i.v. infusion of APG-1387 for 30 min, plasma concentrations of the study drug declined in a biphasic manner, with an initial rapid distribution followed by a slower elimination phase. The median time to maximum plasma concentration (Cmax) was 0.25-0.50 h. Both Cmax and area under the plasma concentration time curve (AUC0-t) increased in an approximately dose-proportional manner. The mean steady-state volume of distribution (Vss) was 10.1-60.1 l, the mean rate of clearance was 15.9-60.7 l/h, and the mean terminal half-life was 0.742-11.2 h. No significant accumulation of APG-1387 was observed with the QW dosing schedule after multiple dosing (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103651, Figure 1).
Figure 1.
APG-1387 pharmacokinetics in plasma. APG-1387 concentration–time profiles (mean ± SD) on (A) cycle 1 day 1 and (B) cycle 1 day 15.
Combined therapy trial—baseline characteristics
For the combined therapy trial (APG-1387 and toripalimab), 27 study participants were screened between 16 April 2020 and 15 September 2021, and 22 were enrolled. All 22 participants were included in the safety and efficacy evaluation populations. Because MTD was not reached in the APG-1387 monotherapy trial, and the safety profile indicated that the dose of 45 mg QW was well tolerated, subjects received the following doses: APG-1387 20 mg D1/D8 every 3 weeks (Q3W) (n = 3), 30 mg D1/D8 Q3W (n = 3), 30 mg QW (n = 3), and 45 mg QW (n = 3), along with toripalimab 240 mg D1 Q3W (Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2024.103651).
Baseline demographics and disease characteristics for the 22 subjects are summarized in Table 1. The tumor types were CRC (n = 5, 22.7%), NPC (n = 8, 36.4%), non-small-cell lung cancer (NSCLC) (n = 8, 36.4%), and adenoid cystic carcinoma (n = 1, 4.5%). A total of 10 (45.5%) of 22 study participants had >3 metastatic organs. Of 22 participants, 18 (81.8%) had received ≥3 prior lines of systemic therapy, and 15 (68.2%) of 22 individuals had received prior PD-1 or PD-L1 inhibitor treatment.
Combined therapy trial—safety analysis
Adverse events were experienced by 20 (90.9%) of 22 subjects (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103651), including TRAEs in 17 (77.3%). The most common TRAEs were elevated lipase (n = 8, 36.4%), rash (n = 6, 27.3%), and elevated amylase (n = 4, 18.2%). Grade 3 TRAEs were elevated lipase (n = 2, 9.1%), decreased platelet count (n = 1, 4.5%), and immune-mediated pancreatitis (n = 1, 4.5%) (Table 2). The APG-1387-related grade 3 TRAEs comprised elevated lipase, which occurred in the 30- and 45-mg groups (n = 2, 9.1%). Grade 3 lipase elevation in the 45-mg group was also considered a DLT (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103651). Lipase elevation related to APG-1387 occurred in the first treatment cycle and recovered rapidly. According to the safety data of 22 subjects in four dose groups, only 1 of 5 subjects in the 45 mg QW∗3 dose group developed G3 TRAE (transient lipase elevation), and the safety of 45 mg QW∗3 group and 30 mg QW∗3 group was comparable. APG-1387 45 mg QW∗3 in combination with toripalimab has a good safety profile. Therefore, the MTD was not reached, and the recommended phase II dose (RP2D) for APG-1387 was 45 mg QW which was determined by SMC guidance.
Combined therapy trial—efficacy analyses
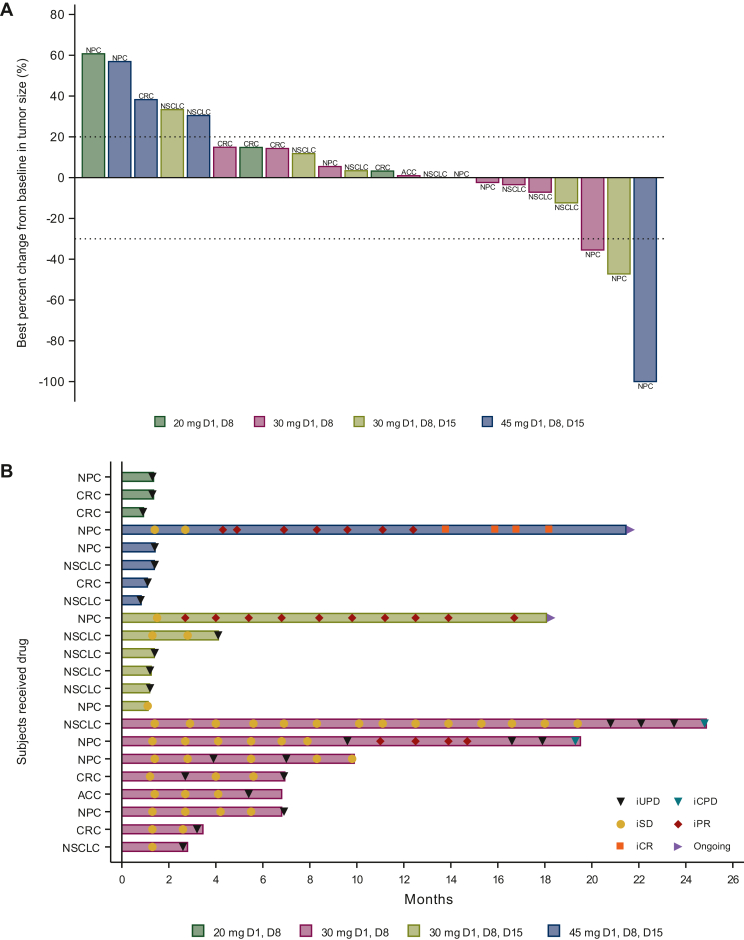
At data cut-off on 15 March 2023, 20 (90.9%) participants had discontinued study treatment, primarily because of disease progression (n = 17, 77.3%). One subject with nasopharyngeal cancer (NPC) who had received one prior line of systemic chemotherapy without a PD-1 inhibitor achieved a CR in the APG-1387 45-mg QW plus toripalimab group. Two study participants with NPC experienced PRs: one participant in the APG-1387 30 mg D1/D8 Q3W plus toripalimab group (after having received two prior lines of systemic chemotherapies along with a PD-1 inhibitor) and another in the 30-mg QW plus toripalimab group (after having received four prior lines of systemic chemotherapies without a PD-1 inhibitor) (Figure 2A).
Figure 2.
Tumorresponse in the APG-1387 combination trial. (A) Waterfall plot of the best percent change in target lesion diameter from baseline. (B) Swimmer plots of individual subject responses. ACC, adenoid cystic carcinoma; CPD, confirmed progressive disease; CR, complete response; CRC, colorectal cancer; NPC, nasopharyngeal carcinoma; NSCLC, non-small-cell lung cancer; PR, partial response; SD, stable disease; UPD, unconfirmed progressive disease.
In addition, nine participants experienced SD, and 10, PD (Table 3). Therefore, in the APG-1387-toripalimab combination trial, the ORR was 13.6%, DCR was 54.5%, and DoR not reached (95% CI 8.43 months-not reached) (Table 3). One subject with NSCLC who had received eight prior lines of systemic chemotherapies and antitumor immunotherapies, including a PD-1 inhibitor, an anti-CD137 monoclonal IgG4 antibody, and a bispecific antibody targeting PD-L1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), had durable SD for 2.6 years, and two participants were still receiving therapy on the data cut-off day (Figure 2B).
In all, 69 plasma samples from 16 subjects in the combination therapy trial were evaluated for plasma levels of cytokines. The results showed that the pattern of pre- and postdosing cytokine changes in different subjects were inconsistent. After 4 and 24 h of APG-1387 dosing, plasma levels of IFN-γ, IL-12/IL-23p40, IL-5, and IL-6 were significantly increased (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.103651). However, NK cell (CD3, −CD16+, and CD56+) populations did not change significantly at low doses of APG-1387 but increased at the 45-mg dose (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2024.103651).
In the APG-1387 combination trial, a 49-year-old man with NPC receiving APG-1387 45 mg experienced a durable response after failure on first-line systemic treatment with gemcitabine and capecitabine before enrollment. Baseline CT imaging showed metastatic lesions in the right lung (target lesions, Supplementary Figure S5A and B, available at https://doi.org/10.1016/j.esmoop.2024.103651). The study participant experienced gradual shrinkage of the target lesions. By iRECIST assessment, the tumor response was classified as iSD on cycle 2 day 19 (C2D19) (Supplementary Figure S5C and D, available at https://doi.org/10.1016/j.esmoop.2024.103651), iPR on C6D16 (30 September 2021), and iCR on C20D19 (Supplementary Figure S5E and F, available at https://doi.org/10.1016/j.esmoop.2024.103651). He experienced grade 3 immune-mediated pancreatitis and grade 4 lipase elevation, which were both related to toripalimab, on C18D20. These recovered after high-dose corticosteroids, which were tapered over 5 weeks. However, grade 3 immune-mediated pancreatitis recurred 15 days after cessation of corticosteroids and was again managed with high-dose corticosteroids. Administration of APG-1387 and toripalimab was reintroduced after 3 months. During the period, the subject experienced a CR and continues to receive APG-1387 D1 and toripalimab D1 Q3W up to the data cut-off date.
Discussion
These phase I studies evaluated APG-1387 monotherapy and APG-1387 combined with toripalimab in participants with advanced/metastatic solid tumors who had failed standard care treatment. In the APG-1387 monotherapy trial, no DLT occurred, the MTD was not reached, and the DCR was 21.7%. The APG-1387 combination therapy also demonstrated favorable safety and tolerability, the MTD was not reached, and the RP2D was 45 mg QW. The ORR was 13.6% and DCR, 54.5%. In general, APG-1387 was well tolerated at doses of 45 mg or lower. The most common TRAEs of APG-1387 were facial-nerve disorder as well as elevations in lipase and ALT/AST. One DLT was a grade 3 lipase elevation, which occurred in the 45-mg group. The highest TRAE rate was <15%, and grade 3 TRAEs were manageable by drug interruption and reduction.
With another oral SMAC mimetic (LCL161), the most common AEs were vomiting, nausea, fatigue, diarrhea, and decreased appetite.33 In addition, LCL161 exhibited grade 3-4 cytokine release syndrome (CRS), which was not observed with APG-1387. This disparity might be explained by LCL161-induced degradation of cIAP1 and cleavage of caspase 3, which functions by regulating the ubiquitination of key proteins in the NF-kB signaling pathway,34 and CRS occurred at high doses of 1800, 2100, and 3000 mg, all of which were grades 3-4, whereas APG-1387 promotes the rapid degradation of cIAP1/2 and XIAP, and the highest dose was 45 mg. Clinical trials of the oral SMAC mimetic IAP antagonist Debio 1143 reported that the most common any-grade TRAEs were nausea, diarrhea, and febrile neutropenia, with grade 3-4 TRAEs of dysphagia and mucositis.35,36 Both LCL161 and Debio 1143 showed gastrointestinal toxicity, whereas APG-1387 had little impact, possibly because the latter was administered by i.v. infusion. All three cases of facial-nerve disorder occurred in the 45-mg group, with two being grade 1 and one grade 2, and the patients recovered spontaneously after discontinuation of APG-1387. Facial-nerve disorder also occurred with the SMAC mimetic birinapant37 and was believed to be related to reactivation of herpes simplex virus and varicella zoster virus through an unknown mechanism.38 Nevertheless, the participants in our study did not undergo tests of herpes virus or any other virus such as Epstein–Barr virus. In general, APG-1387 has a favorable safety profile and manageable AEs.
Compared with APG-1387 monotherapy, the combination with toripalimab showed more frequent AEs of any grade, including hematologic toxicity, hepatic toxicity, pancreatic toxicity, and dermatologic toxicity. However, most of these were toripalimab-related, with the main APG-1387-related AE being grade 3 lipase elevation. For context, a preclinical study found that APG-1387 could induce cytoprotective autophagy,21 which might partially explain lipase elevations with this agent. Lipase elevation and immune-mediated pancreatitis were more common than previously reported with toripalimab monotherapy,39 warranting further attention in future trials. Findings from a study of IAP inhibitor Debio 1143 combined with the PD-L1 inhibitor avelumab in patients with advanced solid malignancies may add further context. The results showed that 44% of participants experienced ALT elevation; 50%, AST elevation; and 100%, increased creatinine; these events were more frequent than what were observed with the combination of APG-1387 and toripalimab therapy. Lipase elevation occurred in 31% of participants receiving Debio 1143 plus avelumab, which was similar to the results of the current study.40 In a clinical trial of birinapant and the PD-1 inhibitor pembrolizumab, one DLT was ALT elevation, and two (of 19) participants experienced grade ≥2 lipase increase.41 There was no CRS or facial-nerve disorder with the combination of the IAP and the immune checkpoint inhibitors. Interestingly, facial-nerve disorder did not occur with the combination of APG-1387 and toripalimab therapy, most likely due to a lower dose of APG-1387 being adopted for the combination cohort.
In the APG-1387 monotherapy trial, no objective responses were achieved, five study participants experienced SD, and the DCR was 21.7%. In both LCL161 and birinapant monotherapy phase I trials, no participant had a CR or PR with monotherapy, and 19% and 28% of subjects had a best response of SD, respectively.33,42 The absence of objective responses in these trials of IAP inhibitor treatment may be partly related to the fact that study participants had received multiple systemic therapies before enrollment and had heavy tumor burdens, which also indicates a necessity for combination therapy. On the other hand, DCR suggested preliminary clinical benefits.
In vitro experiments showing that APG-1387 promoted NK cell proliferation and enhanced natural antitumor immunity43 formed a rationale to evaluate the potential synergy of APG-1387 combined with the PD-1 antibody toripalimab. In our study, this combination showed evidence of clinical benefit, with an ORR of 13.6% and a DCR of 54.5%. Furthermore, 22.7% (5/22) of participants in our study had CRC, and three showed durable disease control. Clinical benefit has been observed in subjects receiving 30 mg of APG-1387, suggesting that a relatively low dose of this agent can sensitize certain tumors to anti-PD-1 treatment, even in previously PD-1-resistant subjects.
In the trial of Debio 1143 combined with avelumab, there was one confirmed PR and five SD in 15 assessable participants as per RECIST v1.1.40 Birinapant combined with pembrolizumab conferred an ORR of 5.3% (1/19) by RECIST v1.1: two participants achieved a PR, and seven achieved SD as defined by best target lesion response.41 Compared with the two combination trials, APG-1387 combined with toripalimab treatment showed a higher response rate and longer duration of tumor control. Mechanistically, APG-1387 may not only induce apoptotic tumor cell death through engagement of TNF receptor-1 by the TNF-α signaling pathway21 but also positively regulate T-cell function43 and enhance TNF-α- and TRAIL-mediated cell killing activities.22 Taken together, our results suggest potential synergy of the combination therapy, and a CR was achieved in one study participant with NPC and two PRs (also in subjects with NPC). Therefore, a phase II trial combining APG-1387 and toripalimab is ongoing for treatment-refractory NPC patients to further evaluate efficacy (ClinicalTrials.gov identifier, NCT04284488).
APG-1387 plasma concentrations declined in a biphasic manner, with an initial rapid distribution followed by a slower elimination phase. Plasma exposure increased in an approximately dose-proportional manner across the dose levels tested. Up-regulation of IFN-γ was previously observed in a preclinical study of APG-1387.43 Consistent with these findings, the LCL161 phase I clinical study also demonstrated up-regulation of TNF-α, IL-8, and C-C motif chemokine ligand 2 (CCL2).33 Collectively, the above clinical data suggest that APG-1387 and other SMAC mimetics play a potential role in stimulating host immune responses. This is consistent with the results observed in in vitro experiments that APG-1387 can promote NK cell number and enhance natural antitumor immunity.43
There are several possible limitations to our study. Firstly, the two phase I trials included only subjects of Chinese descent, potentially reducing the generalizability of the findings. Secondly, the tumor types were limited; 43% of subjects enrolled in the APG-1387 monotherapy trial had CRC, and the chief tumor types were CRC, NPC, and NSCLC in the combination trial. Thirdly, although preliminary efficacy of APG-1387 plus toripalimab treatment was observed in patients with prior anti-PD-1 therapy, some of the subjects in our study had not received anti-PD-1 therapy. A larger study is needed to determine the effect of this combination in subjects who have previously received PD-1 therapy. Finally, the sample size in the combination trial was small. The aforementioned ongoing phase II trial (ClinicalTrials.gov identifier, NCT04284488) with a larger number of subjects may help to identify specific individuals who would benefit from this combination regimen.
In summary, weekly APG-1387 (45 mg i.v.) plus toripalimab (240 mg Q3W) showed a manageable safety profile in individuals with advanced solid tumors and is recommended for further study. The combination treatment indicated preliminary clinical activity in participants with advanced solid tumors, especially in NPC.
Acknowledgements
We thank all patients and their families for participating in the trial. Ashutosh K. Pathak, MD, PhD, MBA, FRCP (Edin.), Stephen W. Gutkin, Ndiya Ogba, PhD, and Paul Fletcher, PhD, employees of Ascentage Pharma Group Inc., provided substantive input in manuscript research and preparation.
Funding
This study was supported by the National Natural Science Foundation of China [grant number 82073377]; Natural Science Foundation of Guangdong [grant number 2021A1515012439]; and Ascentage Pharma Group Corp Ltd (Hong Kong).
Disclosure
WP, XT, LM, EL, HW, and CW are employed by Ascentage Pharma (Suzhou) Co., Ltd or its affiliate, Ascentage Pharma Group Inc., and are stockholders in Ascentage Pharma Group International, the ultimate parent of both corporations. YZ is employed by Ascentage Pharma (Suzhou) Co., Ltd and Ascentage Pharma Group Inc. and holds a leadership position in these companies. DY is employed by Ascentage Pharma (Suzhou) Co., Ltd, Ascentage Pharma Group Inc., and Ascentage Pharma Group International and holds a Fiduciary Officer position in these companies. YZ and DY are stockholders in Ascentage Pharma Group International. YZ and DY are patent holders of the following patent related to the APG-1387-XC101 study: WO2020024932A1, PCT/CN2019/098331, ‘Method for treating cancer by combination of IAP inhibitor and modulator of immune checkpoint molecule’. All other authors have declared no conflicts of interest.
Data sharing
The individual patient data reported in this study cannot be deposited in a public repository because these data are confidential medical records. To request access, please contact lead contact, RHX (xurh@sysucc.org.cn), MZQ (qiumzh@sysucc.org.cn), and YFZ (yzhai@ascentage.com) for de-identified summary data.
Footnotes
Description for Social Media: APG-1387 is a novel SMAC mimetic with a safety profile. Combined with toripalimab showed activity in NPC patients.
Contributor Information
Y. Zhai, Email: yzhai@ascentage.com.
M.-Z. Qiu, Email: qiumzh@sysucc.org.cn.
R.-H. Xu, Email: xurh@sysucc.org.cn.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Xia C., Dong X., Li H., et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135(5):584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Deveraux Q.L., Reed J.C. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13(3):239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 5.Salvesen G.S., Duckett C.S. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3(6):401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 6.Donepudi M., Grutter M.G. Structure and zymogen activation of caspases. Biophys Chem. 2002;101-102:145–153. doi: 10.1016/s0301-4622(02)00151-5. [DOI] [PubMed] [Google Scholar]
- 7.Duckett C.S., Li F., Wang Y., Tomaselli K.J., Thompson C.B., Armstrong R.C. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol Cell Biol. 1998;18(1):608–615. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baig S., Seevasant I., Mohamad J., Mukheem A., Huri H.Z., Kamarul T. Potential of apoptotic pathway-targeted cancer therapeutic research: where do we stand? Cell Death Dis. 2016;7(1) doi: 10.1038/cddis.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silke J., Meier P. Inhibitor of apoptosis (IAP) proteins-modulators of cell death and inflammation. Cold Spring Harb Perspect Biol. 2013;5(2) doi: 10.1101/cshperspect.a008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulda S., Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11(2):109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 11.Varfolomeev E., Goncharov T., Maecker H., et al. Cellular inhibitors of apoptosis are global regulators of NF-κB and MAPK activation by members of the TNF family of receptors. Sci Signal. 2012;5(216) doi: 10.1126/scisignal.2001878. [DOI] [PubMed] [Google Scholar]
- 12.Birkey Reffey S., Wurthner J.U., Parks W.T., Roberts A.B., Duckett C.S. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-beta signaling. J Biol Chem. 2001;276(28):26542–26549. doi: 10.1074/jbc.M100331200. [DOI] [PubMed] [Google Scholar]
- 13.Beug S.T., Cheung H.H., LaCasse E.C., Korneluk R.G. Modulation of immune signalling by inhibitors of apoptosis. Trends Immunol. 2012;33(11):535–545. doi: 10.1016/j.it.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed M.S., Bishr M.K., Almutairi F.M., Ali A.G. Inhibitors of apoptosis: clinical implications in cancer. Apoptosis. 2017;22(12):1487–1509. doi: 10.1007/s10495-017-1429-4. [DOI] [PubMed] [Google Scholar]
- 15.Du C., Fang M., Li Y., Li L., Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102(1):33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 16.Verhagen A.M., Ekert P.G., Pakusch M., et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102(1):43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 17.Hegde R., Srinivasula S.M., Zhang Z., et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J Biol Chem. 2002;277(1):432–438. doi: 10.1074/jbc.M109721200. [DOI] [PubMed] [Google Scholar]
- 18.Yin W., Cheepala S., Clifford J.L. Identification of a novel splice variant of X-linked inhibitor of apoptosis-associated factor 1. Biochem Biophys Res Commun. 2006;339(4):1148–1154. doi: 10.1016/j.bbrc.2005.11.128. [DOI] [PubMed] [Google Scholar]
- 19.Li N., Feng L., Han H.Q., et al. A novel Smac mimetic APG-1387 demonstrates potent antitumor activity in nasopharyngeal carcinoma cells by inducing apoptosis. Cancer Lett. 2016;381(1):14–22. doi: 10.1016/j.canlet.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Ji J., Yu Y., Li Z.L., et al. XIAP limits autophagic degradation of Sox2 and is a therapeutic target in nasopharyngeal carcinoma stem cells. Theranostics. 2018;8(6):1494–1510. doi: 10.7150/thno.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B.X., Wang H.B., Qiu M.Z., et al. Novel smac mimetic APG-1387 elicits ovarian cancer cell killing through TNF-alpha, ripoptosome and autophagy mediated cell death pathway. J Exp Clin Cancer Res. 2018;37(1):53. doi: 10.1186/s13046-018-0703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z., Chen J., Liu H., et al. The SMAC mimetic APG-1387 sensitizes immune-mediated cell apoptosis in hepatocellular carcinoma. Front Pharmacol. 2018;9:1298. doi: 10.3389/fphar.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesi M., Mirza N.N., Garbitt V.M., et al. IAP antagonists induce anti-tumor immunity in multiple myeloma. Nat Med. 2016;22(12):1411–1420. doi: 10.1038/nm.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dougan M., Dougan S., Slisz J., et al. IAP inhibitors enhance co-stimulation to promote tumor immunity. J Exp Med. 2010;207(10):2195–2206. doi: 10.1084/jem.20101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai J., Lin Y., Zhang H., et al. Selective replication of oncolytic virus M1 results in a bystander killing effect that is potentiated by Smac mimetics. Proc Natl Acad Sci U S A. 2017;114(26):6812–6817. doi: 10.1073/pnas.1701002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michie J., Beavis P.A., Freeman A.J., et al. Antagonism of IAPs enhances CAR T-cell efficacy. Cancer Immunol Res. 2019;7(2):183–192. doi: 10.1158/2326-6066.CIR-18-0428. [DOI] [PubMed] [Google Scholar]
- 27.Kearney C.J., Lalaoui N., Freeman A.J., Ramsbottom K.M., Silke J., Oliaro J. PD-L1 and IAPs co-operate to protect tumors from cytotoxic lymphocyte-derived TNF. Cell Death Differ. 2017;24(10):1705–1716. doi: 10.1038/cdd.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan W., Luo Q., Liang E., et al. Synergistic effects of Smac mimetic APG-1387 with anti-PD-1 antibody are attributed to increased CD3 + NK1.1 + cell recruitment secondary to induction of cytokines from tumor cells. Cancer Cell Int. 2024;24(1):181. doi: 10.1186/s12935-024-03373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasco D.W., Lakhani N.J., Tang Y., et al. Phase Ib study of a novel bivalent IAP antagonist APG-1387 in combination of pembrolizumab for patients with advanced solid tumors. J Clin Oncol. 2020;38:3508. [Google Scholar]
- 30.Seymour L., Bogaerts J., Perrone A., et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baud V., Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8(1):33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cetraro P., Plaza-Diaz J., MacKenzie A., Abadía-Molina F. A review of the current impact of inhibitors of apoptosis proteins and their repression in cancer. Cancers (Basel) 2022;14(7):1671. doi: 10.3390/cancers14071671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Infante J.R., Dees E.C., Olszanski A.J., et al. Phase I dose-escalation study of LCL161, an oral inhibitor of apoptosis proteins inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2014;32(28):3103–3110. doi: 10.1200/JCO.2013.52.3993. [DOI] [PubMed] [Google Scholar]
- 34.Firestone B., Conway C., Yang G., et al. Correlation between TNFα and LCL161 anti-tumor activity in patient derived xenograft models of human cancer. Mol Cancer Ther. 2009;8:B27. [Google Scholar]
- 35.DiPersio J.F., Erba H.P., Larson R.A., et al. Oral Debio1143 (AT406), an antagonist of inhibitor of apoptosis proteins, combined with daunorubicin and cytarabine in patients with poor-risk acute myeloid leukemi—results of a phase I dose-escalation study. Clin Lymphoma Myeloma Leuk. 2015;15(7):443–449. doi: 10.1016/j.clml.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Tourneau C., Tao Y., Gomez-Roca C., et al. Phase I trial of debio 1143, an antagonist of inhibitor of apoptosis proteins, combined with cisplatin chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck. Clin Cancer Res. 2020;26(24):6429–6436. doi: 10.1158/1078-0432.CCR-20-0425. [DOI] [PubMed] [Google Scholar]
- 37.Condon S.M., Mitsuuchi Y., Deng Y., et al. Birinapant, a smac-mimetic with improved tolerability for the treatment of solid tumors and hematological malignancies. J Med Chem. 2014;57(9):3666–3677. doi: 10.1021/jm500176w. [DOI] [PubMed] [Google Scholar]
- 38.Eviston T.J., Croxson G.R., Kennedy P.G., Hadlock T., Krishnan A.V. Bell's palsy: aetiology, clinical features and multidisciplinary care. J Neurol Neurosurg Psychiatry. 2015;86(12):1356–1361. doi: 10.1136/jnnp-2014-309563. [DOI] [PubMed] [Google Scholar]
- 39.Wang F.H., Wei X.L., Feng J., et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02) J Clin Oncol. 2021;39(7):704–712. doi: 10.1200/JCO.20.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juergens R.A., Chu Q., Renouf D., et al. A dose-finding study of the SMAC mimetic Debio 1143 when given in combination with avelumab to patients with advanced solid malignancies. J Clin Oncol. 2019;37:2599. [Google Scholar]
- 41.Schilder R.J., Albertella M., Strauss J.F., et al. Determination of the recommended phase II dose of birinapant in combination with pembrolizumab: results from the dose-escalation phase of BPT-201. J Clin Oncol. 2019;37:2506. [Google Scholar]
- 42.Amaravadi R.K., Schilder R.J., Martin L.P., et al. A phase I study of the SMAC-mimetic birinapant in adults with refractory solid tumors or lymphoma. Mol Cancer Ther. 2015;14(11):2569–2575. doi: 10.1158/1535-7163.MCT-15-0475. [DOI] [PubMed] [Google Scholar]
- 43.Pan W., Luo Q., Yan X., et al. A novel SMAC mimetic APG-1387 exhibits dual antitumor effect on HBV-positive hepatocellular carcinoma with high expression of cIAP2 by inducing apoptosis and enhancing innate anti-tumor immunity. Biochem Pharmacol. 2018;154:127–135. doi: 10.1016/j.bcp.2018.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.