Abstract
Gab2 (Grb2-associated binder-2), a member of the IRS (insulin receptor substrate)/Gab family of adapter proteins, undergoes tyrosine phosphorylation in response to cytokine or growth factor stimulation and serves as a docking platform for many signal transduction effectors, including the tyrosine phosphatase SHP-2 [SH2 (Src homology 2)-domain-containing tyrosine phosphatase]. Here, we report that, following IL-2 (interleukin-2) stimulation of human T lymphocytes, SHP-2 binds tyrosine residues 614 and 643 of human Gab2 through its N- and C-terminal SH2 domains respectively. However, the sole mutation of Tyr-614 into phenylalanine is sufficient to prevent Gab2 from recruiting SHP-2. Expression of the Gab2 Tyr-614→Phe (Y614F) mutant, defective in SHP-2 association, prevents ERK (extracellular-signal-regulated kinase) activation and expression of a luciferase reporter plasmid driven by the c-fos SRE (serum response element), indicating that interaction of SHP-2 with Gab2 is required for ERK activation in response to IL-2. Further investigation of IL-2-dependent induction of SRE showed that expression of a constitutively active mutant of the RhoA GTPase synergizes with IL-2 for SRE-driven transcription, whereas a dominant-negative mutant reduces the IL-2 response. Thus, in response to IL-2, full induction of the SRE requires ERK-dependent as well as Rho-dependent signals that target the Ets-box and the CArG-box respectively. We also report that the synergy between Gab2/SHP-2 and RhoA for IL-2-dependent CArG-box-driven transcription depends upon MEK (mitogen-activated protein kinase/ERK kinase) activation, and is likely to involve regulation of the serum response factor co-activator MAL. Our studies thus provide new insights into the role of Gab2 and SHP-2 in IL-2 signal transduction.
Keywords: c-fos, Grb2-associated binder 2 (Gab2), interleukin 2 (IL-2), mitogen-activated protein (MAP) kinase, Rho GTPase, serum response element (SRE), SH2-domain-containing tyrosine phosphatase (SHP-2)
Abbreviations: ECL, enhanced chemiluminescence; ERK, extracellular-signal-regulated kinase; Gab2, Grb2-associated binder-2; GST, glutathione S-transferase; Ha, haemagglutinin; IL, interleukin; IPTG, isopropyl β-D-thiogalactoside; IRS, insulin receptor substrate; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; PDGF-R, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase; SRE, serum response element; SRF, serum response factor; SH2, Src homology 2; SHP-2, SH2-domain-containing tyrosine phosphatase; STAT, signal transduction and activators of transcription; TCF, ternary complex factor; Y614F, etc., a mutant bearing a replacement of Tyr-614 with phenylalanine, etc
INTRODUCTION
SHP-2 [SH2 (Src homology 2)-domain-containing tyrosine phosphatase] is a ubiquitously expressed tyrosine phosphatase characterized by two SH2 domains in tandem in its N-terminal region (N-SH2 and C-SH2). These domains are followed by a tyrosine phosphatase domain and a C-terminal tail, which contains two phosphorylatable tyrosine residues and a proline-rich domain (see [1] for a review). Activation of SHP-2 requires occupancy of its tandem SH2 domains by phosphotyrosine residues, which is achieved by binding either to receptors or to scaffold proteins of the IRS (insulin receptor substrate)/Gab family, and induces conformational changes in the enzyme [2,3]. The sequence of molecular events in SHP-2 activation has been resolved by analysis of the crystal structure of the protein [3]. In the inactive folded conformation, the N-SH2 domain blocks the catalytic site by intramolecular interaction, and this interaction must be broken to activate the enzyme. SHP-2 is recruited through interaction of its C-SH2 domain to one site of a bisphosphotyrosyl ligand, which brings the N-SH2 domain into a position to associate with the second site of the ligand, thus disrupting the intramolecular bond and ‘unmasking’ the phosphatase activity. SHP-2 activation may also be regulated through phosphorylation of tyrosine residues Tyr-542 and Tyr-580 in its C-terminal tail, which would relieve basal inhibition of the phosphatase [4]. In keeping with these structural features, constitutively activated mutants of SHP-2 have been obtained either by deleting its N-SH2 domain or by mutating critical residues (Asp-61 or Glu-76) within this domain [5,6].
SHP-2 is thought to play a critical positive role in many signalling pathways (see [7,8] for reviews). The mechanisms whereby SHP-2 is involved in signalling serve at least two functional roles: on the one hand, SHP-2 provides an adapter function necessary, amongst other pathways, for MAPK (mitogen-activated protein kinase) activation. For example, upon stimulation of the PDGF-R (platelet-derived growth factor receptor), SHP-2 binds Tyr-1009 of the PDGF-R and becomes itself phosphorylated on Tyr-542 [9]. Phosphorylation of this residue creates a binding site for Grb2, leading eventually to Ras activation [10]. The adapter function of SHP-2 has also been demonstrated in response to insulin, IL (interleukin)-6 and erythropoietin [11–13]. On the other hand, the phosphatase activity of SHP-2 can dephosphorylate important components of signalling pathways, as demonstrated by the use of a phosphatase-dead mutant of SHP-2, Cys-459→Ser (C459S). Indeed, numerous studies have demonstrated, by this means, that SHP-2 positively regulates activation of the MAPK pathway induced by epidermal growth factor, insulin and hepatocyte growth factor [14–16]. However, the phosphatase activity of SHP-2 may also be involved in negative regulation, leading to termination of signalling, by dephosphorylating for instance the JAKs (Janus kinases) or STAT (signal transduction and activators of transcription) proteins [17]. Recent investigations have identified mammalian Gab2 (Grb2-associated binder 2) as a major substrate of SHP-2 [18]. Gab family proteins (Gab1, Gab2 and Gab3) contain a pleckstrin homology domain in the N-terminal region, central proline-rich sequences and several tyrosine-based motifs, which are binding sites for SH3- or SH2-domain-containing proteins respectively. Gab proteins function as molecular scaffolds by interacting with various signalling partners, such as SHP-2, p85, PI3K (phosphoinositide 3-kinase), CrkL, phospholipase C-γ1 and Grb2 in response to a variety of growth factors and cytokines [19].
IL-2 is one of the major growth and differentiation factors for T lymphocytes, and the signalling pathways whereby it mediates its biological activity have been extensively studied [20–22]. In previous studies, we have demonstrated that Gab2 is tyrosine-phosphorylated and interacts with the SH2 domains of SHP-2, PI3K and CrkL in response to IL-2 in T lymphocytes [23,24]. Although Gab2 appears to be the major binding partner and substrate of SHP-2 in cytokine signalling, the role of SHP-2 itself in IL-2 signalling has not been fully elucidated. In the present study, we show data clarifying the mode of interaction between Gab2 and SHP-2, and analyse further the signalling cascades that regulate ERK (extracellular-signal-regulated kinase) activation and expression of the immediate early c-fos gene promoter. Indeed, as with many other growth factors, IL-2 induces expression of c-fos in stimulated lymphocytes, and this induction has long been known to depend upon signalling events that are initiated within the acidic region of the IL-2Rβ chain [25,26]. Of interest, Taniguchi and co-workers [27] reported that IL-2-induced tyrosine phosphorylation of SHP-2 depended upon the same region of IL-2Rβ, raising the possibility that SHP-2 might be involved in the complex regulation of c-fos expression. One critical regulatory sequence in the c-fos promoter is represented by a 20-nucleotide sequence known as the SRE (serum response element), which co-operatively binds TCF (ternary complex factor) and SRF (serum response factor) transcription factors [28,29]. In addition, previous studies have identified the SRE present within the c-fos promoter as a target for regulation by the Rho family of Ras-related GTPases [30]. Indeed, these GTPases, and particularly RhoA, acting through a set of effector proteins are critical to the dynamics of the actin-based cytoskeleton, which in turn is required for proper activation of SRF [31]. Here, we provide evidence for a role of the Gab2–SHP-2 interaction in regulating both ERK-dependent and Rho-dependent signals, leading to c-fos promoter activation in IL-2-stimulated T lymphocytes.
EXPERIMENTAL
Cell lines and culture conditions
The human T-cell chronic lymphocytic leukaemia-derived, IL-2-dependent Kit 225 cell line was kindly provided by Dr T. Hori (Kyoto University, Japan) [32]. Cells were maintained in RPMI 1640 culture medium containing 2 mM L-glutamine, 0.1 mg/ml streptomycin, 100 units/ml penicillin, 2% sodium pyruvate, and 10% fetal calf serum, which was supplemented with 0.5 nM recombinant human IL-2 (Proleukin; Chiron Corp., Emeryville, CA, U.S.A.). For some experiments, we used a subclone of the Kit 225 line (Tetoff), which expresses the tetracycline-regulated transactivator (pUHD15-1) in combination with pUHD10-3 heptamerized tet-operator-driven expression plasmids [33].
Reagents and antibodies
Polyclonal antibody (C-18) against SHP-2 protein was from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Monoclonal antibody against SHP-2 (PTP1D) was from Transduction Laboratories (Lexington, KY, U.S.A.); anti-phosphotyrosine (4G10) and anti-p85 PI3K antiserum (06-195) were from Upstate Biotechnology Inc. (Lake Placid, NY, U.S.A.), and anti-phospho-p42/p44 MAP kinases (ref. 9102) were from Cell Signaling (OZYME; Saint Quentin en Yvelines, France). Monoclonal anti-GST (glutathione S-transferase), anti-Ha (haemagglutinin; 12CA5) and anti-Myc (9E10) antibodies were produced and purified in our laboratory. A monoclonal antibody against Gab2 was obtained by immunization of Balb/c mice with purified GST–Gab2 (amino acids 601–676). Hybridoma generation, selection and screening were performed according to standard protocols in use at Diaclone [34]. One antibody (B-D39) with good reactivity against Gab2 in immunoprecipitation and immunoblot analysis was selected and characterized further. This anti-Gab2 mAb (IgG1) does not immunoprecipitate the closely related Gab1 protein; nor does it recognize Gab1 in immunoblot analysis. Epitope mapping of this antibody indicated that it recognizes a sequence that exists between amino acids 601–621 of human Gab2.
GSH–Sepharose beads were from Amersham Biosciences (Les Ulis, France), nickel–agarose beads were from Qiagen, and streptavidin–agarose beads and lyophilized D-biotin were obtained from Sigma-Aldrich (St Louis, MO, U.S.A.).
Horseradish-peroxidase-conjugated secondary antibodies were purchased from Amersham Biosciences or Dako (Copenhagen, Denmark) and detected using ECL (enhanced chemiluminescence).
Plasmid constructs
cDNA for full-length human Gab2 was obtained as described previously [24] and inserted in-frame into pcDNA 3.1 vector. Gab2 C-terminal domain (amino acids 601–676) was excised from the previously described pGad vector [24], and inserted inframe in the pGEX-4T1 vector (Amersham Biosciences) to obtain the GST fusion protein. Mutations of tyrosine residues into phenylalanine were performed with oligonucleotides containing appropriate TAC-to-TTC codon changes using the Quikchange® mutagenesis kit (Stratagene, La Jolla, CA, U.S.A.). cDNAs for full-length SHP-2, catalytically inactive (C459S) and constitutively active (Glu-76→Ala; E76A) mutants were kindly provided by Dr Benjamin G. Neel and Dr Haihua Gu (Beth Israel Deaconess Medical Center, Boston, MA, U.S.A.), amplified by PCR, Ha-tagged and subcloned into pcDNA 3.1 vector. Subdomains of human SHP-2 (N-SH2, amino acids 1–106; C-SH2, amino acids 106–220; and (N+C)-SH2, amino acids 1–220) were obtained by PCR using oligonucleotides designed after the full-length SHP-2 sequence and inserted in-frame into the p6xHis-Myc vector.
pUHD-GST–[(N+C)-SH2] construct, allowing expression in Tetoff cells of the [(N+C)-SH2] subdomain of human SHP-2 as a GST fusion protein, was constructed by inserting the coding sequence for the (N+C)-SH2 subdomain of human SHP-2 into a previously constructed pUHD-GST vector.
For Rho expression, the cDNAs for human RhoA containing either the activating Gly-14→Val (G14V) or the inactivating Ser-19→Asn (S19N) mutations were inserted in-frame into pCMV-Flag vector (Stratagene).
For MEK (MAPK/ERK kinase) expression, we used pECE-MEK1 Ser-218→Asp/Ser-222→Asp (S218D/S222D), a Hatagged constitutively active mutant of MEK1 (MEKA), which has been described previously [35] and was kindly provided by Dr J. Pierre, and pMCL-MEK1 Lys-97→Met (K97M), a Ha-tagged dead mutant of MEK1 (shown as MEK DN in the Figures), originally from Dr N. G. Ahn [36] and kindly provided by J. Raingeaud.
Luciferase reporter plasmid pFR-Luc (5×Gal4 binding site) was from Stratagene, and pSG424-Gal4-Elk, described previously by Dr R. Treisman [37], was kindly provided by J. Raingeaud.
Luciferase (firefly) reporter plasmids containing one copy of the SRE of human c-fos promoter (SRE Luc), in front of a minimal c-fos promoter (−90 to +42 bp with reference to the transcription start site), and its derivatives (ETS Luc and CArG Luc) were kindly provided by Dr A. Harel-Bellan (CNRS UPR 9079, France) and have been described previously [38].
pCMX-MAL WT is a cytomegalovirus-based vector containing the MAL-coding sequence [39], in-frame with an Ha tag. pCMX-MALΔAct plasmid was obtained by truncating the transactivation domain of MAL following amino acid 721.
For an internal control, and because most commonly used viral promoters respond to some extent to IL-2 stimulation in Kit 225 cells, we constructed a novel Renilla luciferase reporter plasmid by insertion of the composite SRα promoter [40] into pRL-null vector (Promega, Madison, WI, U.S.A.).
All constructs, whether wild-type or mutated, were verified by DNA sequencing.
Fusion proteins
GST fusion proteins were produced in Escherichia coli strain BL21(DE3) by induction for 4 h at 37 °C with 1 mM IPTG (isopropyl β-D-thiogalactoside) and purified by adsorption on to GSH–Sepharose beads. For production of phosphorylated GST proteins, the various pGEX constructs were transformed into BL21(DE3) bacteria containing a pBC plasmid coding for the catalytic domain of the Elk receptor tyrosine kinase, as described previously [41]. His6-Myc-tagged proteins were produced in E. coli strain BL21(DE3) by induction for 4 h at 37 °C with 1 mM IPTG, and purified using nickel–agarose beads.
Immunoprecipitation and GST pull-down experiments
For immunoprecipitation experiments, Kit 225 cells were deprived of IL-2 for 48 h, and then stimulated for 10 min (or as indicated) with 1 nM human recombinant IL-2 (Sanofi, Labège, France). Stimulation was then blocked with cold PBS, cells were collected by centrifugation (400 g, 10 min at 4 °C) and incubated for 30 min on ice in cold lysis buffer [50 mM Tris/HCl (pH 7.5)/150 mM NaCl/0.5% Triton X-100/10 mM NaF/1 mM EDTA/1 mM EGTA/1 mM PMSF/1 mM vanadate/1 μg/ml each of leupeptin, pepstatin and aprotinin]. Cell lysates were clarified by centrifugation at 15000 g for 20 min at 4 °C. Post-nuclear cell lysates were pre-cleared with Protein G–Sepharose beads for 30 min at 4 °C, and then incubated with 2–5 μg of antibodies overnight at 4 °C. Immune complexes were collected on Protein G–Sepharose beads, and the beads were washed five times with lysis buffer. Bound proteins were resolved by SDS/PAGE and analysed by immunoblotting.
For in vitro GST pull-down experiments, Kit 225 cell lysates (4 mg of protein) were pre-cleared with GST-0 protein adsorbed on to GSH–Sepharose beads. Equivalent amounts of the various GST fusion proteins were adsorbed on to GSH–Sepharose beads for 1 h at 4 °C. Pre-cleared lysates were shaken with coupled beads for 2 h at 4 °C. The beads were then washed five times, and bound proteins were analysed by immunoblotting.
For in vitro protein interactions, His6-Myc-tagged fusion proteins [0.5 μg of subdomain (N+C)-SH2, 0.5 μg of C-SH2 or 2 μg of N-SH2] were added to GST-fusion-protein-coupled beads and shaken in lysis buffer supplemented with 1.5% BSA for 2 h at 4 °C. Beads were then washed, and bound proteins were analysed by immunoblotting.
An N-terminal biotinylated and tyrosine-phosphorylated peptide designed after the sequence of human Gab2 surrounding Tyr-614 [STGSVD(pT)LALDFQPSSPSPHR, where pT represents phosphotyrosine] was synthesized by Sigma-Genosys (Cambridge, U.K.). For peptide interaction with purified His6-Myc-tagged fusion proteins, 10 μg of biotinylated peptide was bound to streptavidin–agarose beads overnight, and then beads were washed in PBS buffer and blocked for 1 h with 1 mg/ml D-biotin. Following additional washes in PBS containing BSA, 1 μg of purified His6-Myc-tagged fusion proteins was added on beads and shaken for 2 h at 4 °C. Beads were then washed, and bound proteins were analysed by immunoblotting.
Far-Western blotting and immunoblotting
For immunoblotting, membranes were blocked for 2 h at room temperature with either 5% non-fat dried milk or 3% BSA in TBS-T buffer [50 mM Tris/HCl (pH 7.5)/150 mM NaCl/0.5% (v/v) Tween 20]. Membranes were washed four times in TBS-T and incubated for 1 h with optimal concentrations of primary antibodies diluted in TBS-T. Following four washes in TBS-T, the membranes were incubated for 45 min with horseradish-peroxidase-conjugated secondary antibodies. Bound antibodies were detected using Amersham ECL reagents and autoradiographic films.
For far-Western experiments, membranes were blocked for 2 h at room temperature with 5% non-fat dried milk in TBS-T. Membranes were washed four times in TBS-T and incubated for 45 min with His6-Myc-tagged fusion proteins (5 μg/ml for Myc (N+C)-SH2 and Myc C-SH2; 20 μg/ml for Myc N-SH2). Following four additional washes in TBS-T, the membranes were revealed as described above for the immunoblotting experiments.
Blots were stripped between probing with a 30 min, 50 °C incubation in 62.5 mM Tris/HCl, pH 6.8, containing 100 mM 2-mercaptoethanol and 1% SDS. Membranes were then washed extensively and blocked for at least 2 h with either 5% non-fat dried milk or 3% BSA in TBS-T.
Transient transfections and luciferase assays
Transfections were performed by electroporation using a gene pulser apparatus (Bio-Rad Laboratories, Hercules, CA, U.S.A.) set at 250 V and 960 μF. For analysis of Gab2–SHP-2 interactions, transiently transfected cells were cultured 24 h without IL-2 after electroporation, and then stimulated without (control) or with 1 nM recombinant IL-2 (or as indicated) for 10 min. Cells were then lysed and processed for immunoprecipitation and immunoblot analysis as described above.
For luciferase assays, exponentially growing Kit 225 cells (107) were washed in RPMI 1640 medium, resuspended in 150 μl of RPMI 1640 and electroporated with 30 μg of plasmid DNA as follows: plasmids coding for the desired proteins were generally used at 10 μg (unless otherwise indicated); for the Elk transactivation assay, 2 μg of Gal4-Elk, 5 μg of 5×Gal4-luciferase reporter and 0.05 μg of pSRα-Renilla reporter, included as an internal transfection control to normalize luciferase reporter activity, was used; for c-fos promoter activation studies, 2 μg of SRE Luc vectors was used in combination with 0.02 μg of the pSRα-Renilla reporter. The total amounts of transfected DNA were kept constant by addition of empty control vector. Following electroporation, Kit 225 cells were incubated for 16 h without IL-2, and then 50% of the cells were stimulated with IL-2 for 8 h and lysed in 25 μl of passive lysis buffer (Promega) to proceed to a dual luciferase assay, following the manufacturer's instructions. Luciferase activity in 5 μl of the lysates was measured on a MicroLumat Plus LB 96 V luminometer (Berthold Technologies, Pforzheim, Germany). Results were analysed by dividing firefly signals by Renilla signals, and expressed as the fold increase relative to the basal activity measured in unstimulated cells transfected with empty control vector. The remaining 20 μl of the cell lysates was used to assess expression of the desired proteins by immunoblotting. For all experiments, the results shown are representative of at least three independent experiments.
RESULTS
Gab2 residues Tyr-614 and Tyr-643 bind SHP-2 N- and C-SH2 domains respectively
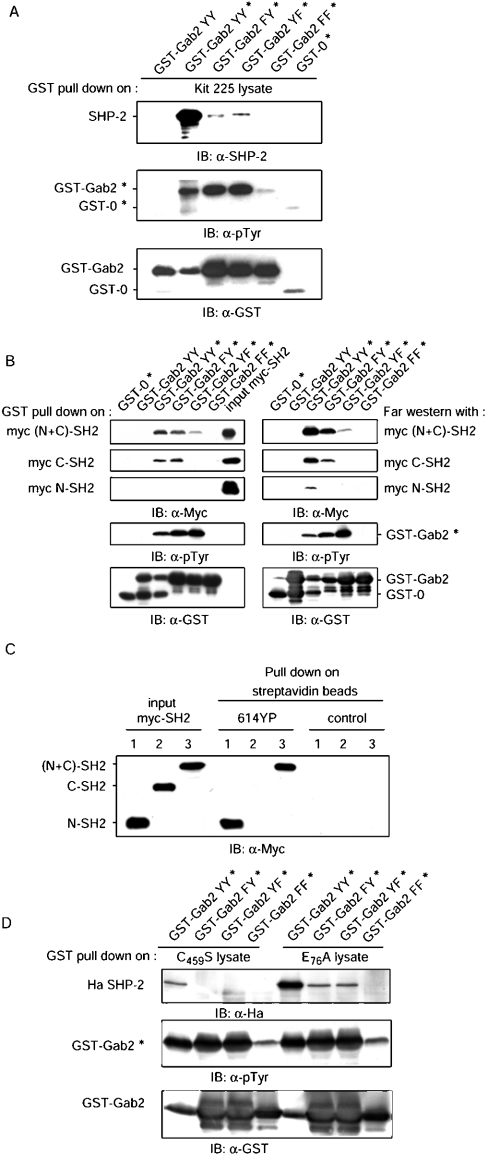
We and others [18,24,42,43] have previously reported that Gab2 associates with SHP-2 through two tyrosine residues (Tyr-614 and Tyr-643) that are present at its C-terminus. To analyse this interaction in more detail, the C-terminal end of Gab2 (amino acids 601–676) was expressed as GST fusion proteins in E. coli strain BL21, or in E. coli that had been transformed with a plasmid encoding the catalytic domain of the Elk tyrosine kinase, resulting in the production of phosphorylated GST fusion proteins, as described previously [41]. Anti-phosphotyrosine (4G10) immunoblot analysis showed that GST–Gab2 wild-type or single-tyrosine mutant proteins produced under these conditions are indeed tyrosine-phosphorylated, whereas the double phenylalanine mutant of Gab2 is not. We then used tyrosine-phosphorylated GST–Gab2 proteins to study their interaction with SHP-2. GST–Gab2 proteins, whether phosphorylated or not, were immobilized on GSH–Sepharose beads and used to pull-down interacting proteins from Kit 225 cell lysates. Bound proteins were separated by SDS/PAGE and analysed by immunoblotting with anti-SHP-2 antibodies. Results of a representative experiment, shown in Figure 1(A), indicated that SHP-2 binding to GST–Gab2 was only detected when GST–Gab2 was tyrosine-phosphorylated. Replacing both Tyr-614 and Tyr-643 with phenylalanine completely abrogated the binding, whereas a small amount of SHP-2 binding was still detectable with single-tyrosine mutants. To study how each of the SHP-2 SH2 domains contributed to the interaction with Gab2, Myc-tagged recombinant proteins comprising the N-terminal SH2 domain (Myc N-SH2), the C-terminal SH2 domain (Myc C-SH2) or the entire N-terminus of SHP-2 [Myc (N+C)-SH2] were produced and purified as described in the Experimental section. Binding of these proteins to GST–Gab2 was first studied in pull-down and far-Western experiments, as shown in Figure 1(B). The Myc (N+C)-SH2 protein interacted strongly with phosphorylated WT GST–Gab2 in pull-down and far-Western experiments, and no binding was detectable with either the non-phosphorylated GST–Gab2 or the double-tyrosine mutant proteins. Although Myc (N+C)-SH2 association with single-tyrosine mutants was still detectable, intensity of the binding was less on Gab2 Tyr-643→Phe (Y643F) than on Gab2 Tyr-614→Phe (Y614F; top panels). In this series of experiments, the C-SH2 domain was clearly shown to interact with tyrosine residue Tyr-643, and not with Tyr-614. In contrast, the N-SH2 domain could only be shown to bind the WT Gab2 protein in far-Western analysis, which appeared to be slightly more sensitive than the pull-down assay. However, the results shown above indicating that the (N+C)-SH2 subdomain binds both Tyr-614 and Tyr-643, and that the C-SH2 subdomain binds Tyr-643, strongly suggest that the N-SH2 domain of SHP-2 might be targeting Tyr-614. This was clearly established by the use of a biotinylated tyrosine-phosphorylated peptide containing Tyr-614 that, when bound to streptavidin–agarose beads, was found to associate very efficiently with the N-SH2 and (N+C)-SH2 recombinant proteins, but not with the C-SH2 protein (Figure 1C).
Figure 1. The N and C-terminus SH2 domains of SHP-2 interact respectively with Tyr-614 and Tyr-643 of Gab2.
(A) GST–Gab2 proteins were expressed in E. coli (BL21DE3) or in Elk tyrosine kinase-containing E. coli (denoted by an asterisk) so that tyrosine residues are phosphorylated (Gab2 YY=Tyr-614/Tyr-643, Gab2 FY=Phe-614/Tyr-643, Gab2 YF=Tyr-614/Phe-643 and Gab2 FF=Phe-614/Phe-643). Immobilized GST–Gab2 proteins (≈15 μg) were used to pull-down SHP-2 from Kit 225 cell lysates (4 mg of protein). Bound proteins were analysed by immunoblotting (IB) with SHP-2 antibodies (top panel). Phosphorylation of GST–Gab2 proteins was controlled with anti-phosphotyrosine 4G10 antibodies (α-pTyr; middle panel) and following stripping, loading was verified with anti-GST antibodies (bottom panel). (B) Immobilized GST–Gab2 proteins (≈15 μg), phosphorylated on tyrosine or not, were incubated with 0.5 μg of Myc (N+C)-SH2 or Myc C-SH2 subdomain, or 2 μg of Myc N-SH2 (left-hand panels). Purified Myc-SH2 and bound proteins were analysed by immunoblotting with anti-Myc (9E10) antibodies (three uppermost panels). Phosphorylation and loading of GST–Gab2 proteins were controlled as above with 4G10 and anti-GST antibodies. For far-Western experiments (right-hand panels), GST–Gab2 proteins were resolved by SDS/PAGE and electrotransferred on to PVDF membranes. Membranes were then incubated with 5 μg/ml Myc (N+C)-SH2 or Myc C-SH2, or 20 μg/ml Myc N-SH2, and bound proteins were analysed by immunoblotting with Myc antibodies (three uppermost panels). Phosphorylation and loading of GST–Gab2 proteins were controlled as above with anti-pTyr and anti-GST antibodies. (C) A biotinylated and tyrosine-phosphorylated peptide, designated 614YP and designed after the human Gab2 sequence, was immobilized on streptavidin–agarose beads and incubated with 2 μg of Myc N-SH2 (lanes 1), 1 μg of Myc C-SH2 (lanes 2) or Myc (N+C)-SH2 (lanes 3). Uncoated streptavidin beads were used as a control. Bound proteins and input Myc-SH2 were analysed by immunoblotting with anti-Myc antibodies. (D) Kit 225 cells were transfected with pcDNA-Ha SHP-2 C459S or pcDNA-Ha SHP-2 E76A plasmids. After 24 h, cell extracts were prepared, and immobilized GST–Gab2 proteins (≈15 μg) were used to pull-down Ha-SHP-2 from cell lysates (1 mg of protein). Bound proteins were analysed by immunoblotting with Ha antibodies (top panel). Phosphorylation and loading of GST–Gab2 proteins were controlled as above with 4G10 and anti-GST antibodies. All experiments are representative of at least three independent experiments.
To gain further information about this interaction, we made use of recently described mutants of SHP-2. Mutation of Glu-76 in the N-SH2 domain has been shown to disrupt the intramolecular bond, but not the ability of the N-SH2 to interact with phosphotyrosyl ligands [5]. Single-tyrosine GST–Gab2 proteins were used to pull down either the catalytically inactive (C459S) or the activated (E76A) SHP-2 mutants expressed in Kit 225 cells. As shown in Figure 1(D), SHP-2 C459S behaves similarly to endogenous SHP-2, whereas SHP-2 E76A bound as efficiently to Gab2 Y643F as to Gab2 Y614F, but not to the double mutant Gab2 Y614F/Y643F, indicating that, due to the constitutive open conformation of this mutant, the N-SH2 domain is indeed capable of binding to phosphorylated single-tyrosine mutants of Gab2.
Taken together, the results presented above clearly established that human Gab2 Tyr-643 associates with the C-SH2 domain of SHP-2, whereas Tyr-614 is a ligand for the N-SH2 domain.
Gab2 Tyr-614 is required for SHP-2 binding in IL-2-stimulated cells
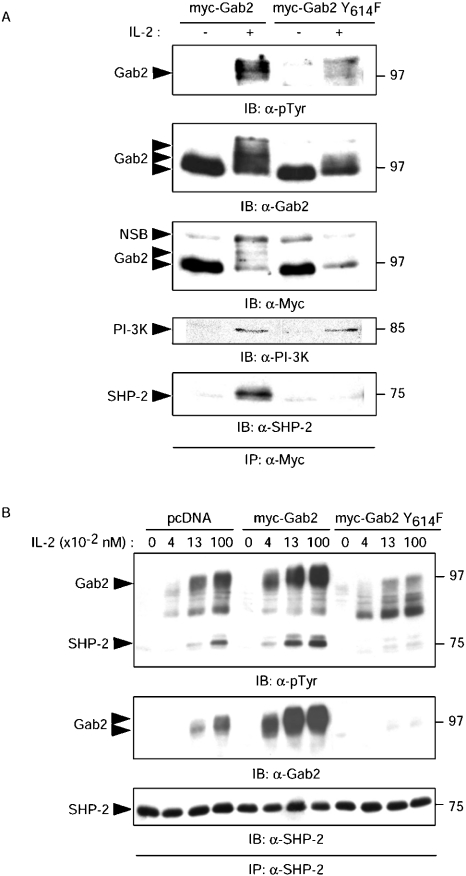
Both Tyr-614 and Tyr-643 in vitro are required for efficient binding of SHP-2 to Gab2. However, our earlier studies in a modified yeast two-hybrid assay have indicated that, in such an in vivo setting, Tyr-614 was more important than Tyr-643 for interaction between Gab2 and SHP-2 [24]. In addition, it has clearly been shown that SHP-2 has no enzymic activity unless its N-SH2 subdomain is ligated [2]. We therefore sought to investigate the effects of a single mutation in position 614 of Gab2 on its ability to recruit SHP-2 in IL-2-stimulated Kit 225 cells. To this end, Myc-tagged full-length Gab2 constructs, either WT or Y614F, were expressed in transient transfection experiments in Kit 225 cells. The IL-2-induced tyrosine phosphorylation of Gab2 and its association with SHP-2 were investigated in immunoprecipitation experiments with anti-Myc (Figure 2A) or anti SHP-2 antibodies (Figure 2B). As shown in Figure 2(A), Gab2 WT was phosphorylated and interacted with SHP-2 in response to IL-2. Although the single Y614F mutation slightly reduced the tyrosine phosphorylation of Gab2 and its characteristic migration shift, it did not affect association with PI3K, but completely prevented association with SHP-2 in response to IL-2. In the reverse experiment (Figure 2B), and consistent with the results shown above, Gab2 Y614F was undetectable in SHP-2 immunoprecipitates. It is noteworthy that, in cells that express Gab2 Y614F, SHP-2 immunoprecipitates did not contain any significant amount of endogenous Gab2 as compared with cells transfected with empty vector, suggesting that Gab2 Y614F prevents association of SHP-2 with endogenous Gab2 and may therefore behave as a dominant-negative mutant. In addition, IL-2-induced tyrosine phosphorylation of SHP-2 appeared to be increased in Gab2-transfected cells, and was dramatically reduced in the presence of Gab2 Y614F, suggesting that tyrosine phosphorylation of SHP-2 depends critically on its interaction with Gab2. Thus the tyrosine-to-phenylalanine mutation in position 614 is enough to prevent binding of SHP-2 to Gab2 in IL-2-stimulated cells, and this mutant interferes with SHP-2 recruitment by endogenous Gab2.
Figure 2. Mutation of Gab2 Tyr-614 to phenylalanine abrogates IL-2-induced association of SHP-2.
(A) Tyr-614 of Gab2 is required for Gab2–SHP-2 complex formation in response to IL-2. Kit 225 cells were transiently transfected with pcDNA Myc-Gab2 or pcDNA Myc-Gab2 Y614F, cultured 24 h without IL-2, and then stimulated for 10 min with (+) or without (−) 1 nM IL-2. Cells were then lysed and Myc-tagged proteins were immunoprecipitated with anti-Myc antibody (IP: α-Myc). Proteins were resolved by SDS/PAGE and immunoblotted with anti-phosphotyrosine (α-pTyr), anti-Gab2, anti-Myc, anti PI3K and anti-SHP-2 antibodies, as indicated. (B) The Y614F mutant interferes with SHP-2 recruitment by endogenous Gab2. Kit 225 cells were transiently transfected with pcDNA, pcDNA Myc-Gab2 or pcDNA Myc-Gab2 Y614F, cultured 24 h without IL-2, and then stimulated for 10 min with 0.04 nM, 0.13 nM or 1 nM of IL-2, or not (0). Cells were then lysed and SHP-2 was immunoprecipitated with anti-SHP-2 antibody (IP: α-SHP-2). Proteins were resolved by SDS/PAGE, transferred to a PVDF membrane and immunoblotted with α-pTyr, anti-Gab2 and anti-SHP-2 antibodies, as indicated.
Interaction of SHP-2 with Gab2 regulates MAPK in IL-2-stimulated T lymphocytes
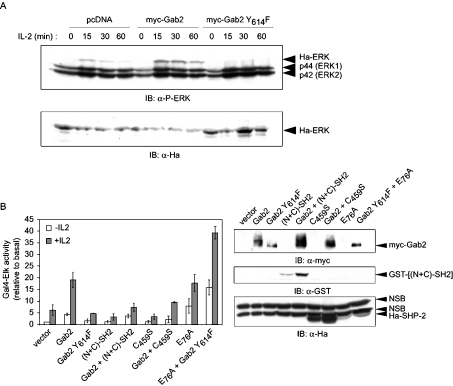
Recruitment of SHP-2 by Gab family proteins has been shown to be required for ERK activation and subsequent phosphorylation of the Elk transcription factor in various cell models [16,18,43–45]. It was therefore of interest to study how Gab2 participated in IL-2 signalling in T lymphocytes from this point of view. To do so, we first analysed the IL-2-dependent phosphorylation of an exogenous Ha-ERK co-expressed with Gab2 WT or Gab2 Y614F (Figure 3A). Indeed, the relatively low transfection efficiency in Kit 225 cells has generally prevented an interpretation of the effects on endogenous proteins, whereas analysis of Ha-ERK allowed us to focus on cells that effectively co-express Gab2 constructs. Gab2 WT potentiated ERK phosphorylation induced by IL-2 stimulation, whereas Gab2 Y614F prevented this phosphorylation, clearly indicating that Gab2–SHP-2 interaction is involved in ERK activation. Since ERK phosphorylation does not always reflect full activation of ERK, we also analysed regulation of the MAPK pathway by monitoring Elk transactivation by IL-2. Kit 225 cells were co-transfected with Gal4-Elk and 5×Gal4 luciferase reporter in the presence or absence of the indicated expression plasmids (Figure 3B). Expression of WT Gab2 resulted in an approx. 4-fold increase in luciferase activity induced by IL-2, suggesting that Gab2 is a limiting factor in this pathway (Figure 3B). In contrast, the Gab2 Y614F construct did not display this enhancing effect, and was even slightly (and reproducibly) inhibitory when compared with the control vector, suggesting that the function of Gab2 in this assay depended upon its ability to bind SHP-2. We then tested an alternative means of preventing this interaction, i.e. by overexpressing SHP-2 tandem SH2 domains fused to GST {GST–[(N+C)-SH2]}, which compete with endogenous SHP-2 for Gab2 binding (results not shown). Expression of this construct significantly reduced the IL-2 response and abrogated the enhancing effect of Gab2 overexpression. In addition, co-expression of the catalytically inactive C459S SHP-2 mutant with WT Gab2 similarly inhibited the Gal4-Elk-induced luciferase activity in response to IL-2, thus indicating that phosphatase activity of SHP-2 is required in the pathway leading from Gab2 to Elk activation. Furthermore, the E76A constitutively active mutant of SHP-2 induced a strong increase in luciferase activity and, unexpectedly, co-expression of Gab2 Y614F and SHP-2 E76A induced a greater response than expression of SHP-2 E76A alone, even in absence of IL-2. A similar response was also obtained with a Gab2 Y643F mutant, but not with the double tyrosine-to-phenylalanine Gab2 mutant (results not shown). One explanation, consistent with our biochemical analysis shown in Figure 1(D), could be that due to its open conformation SHP-2 E76A is indeed recruited by Gab2 Y614F. In a series of additional experiments (results not shown), Kit 225 cells were transfected with combinations of SHP-2 and MEK-activated or dominant-negative mutants. The strong response of the Gal4 reporter induced by constitutively active SHP-2 (SHP-2 E76A) was blocked by the K97M dead mutant of MEK (MEK DN). In contrast, the effect of constitutively active MEK was unaltered in the presence of SHP-2 C459S.
Figure 3. Interaction of SHP-2 with Gab2 is required for ERK activation in response to IL-2.
(A) Gab2/SHP-2 is involved in ERK phosphorylation. Kit 225 cells were transiently co-transfected with Ha-ERK1 and pcDNA, pcDNA-Myc-Gab2 or pcDNA-Myc-Gab2 Y614F, cultured 12 h without IL-2 and stimulated for indicated times. Cells were lysed with hot Laemmli sample buffer, and proteins were then resolved by SDS/PAGE and subjected to immunoblot analysis with anti-phospho-ERK (IB: α-P-ERK) or anti-Ha antibodies (IB: α-Ha), as indicated. (B) Interaction of SHP-2 with Gab2 and phosphatase activity of SHP-2 are involved in Elk transactivation. Kit 225 cells were transfected with 10 μg of plasmids coding for the desired proteins, 2 μg of Gal4-Elk, 5 μg of 5×Gal4 luciferase reporter and 0.05 μg of pSRα-Renilla standardization reporter. The cells were left 16 h without IL-2, and then half of the cells were stimulated with IL-2 (0.5 nM) for 8 h. Results are expressed as the means for at least three independent experiments. Expression of the desired proteins was verified by immunoblotting with anti-Myc, anti-GST and anti-Ha antibodies (NSB, non-specific band), as indicated (for additional details, see the Experimental section).
Taken together, the results described above indicate that, in the IL-2-induced pathway leading to ERK activation, and depending upon Gab2, SHP-2 participates in a step that lies upstream of MEK activation.
Regulatory role of SHP-2 on activation of the c-fos SRE
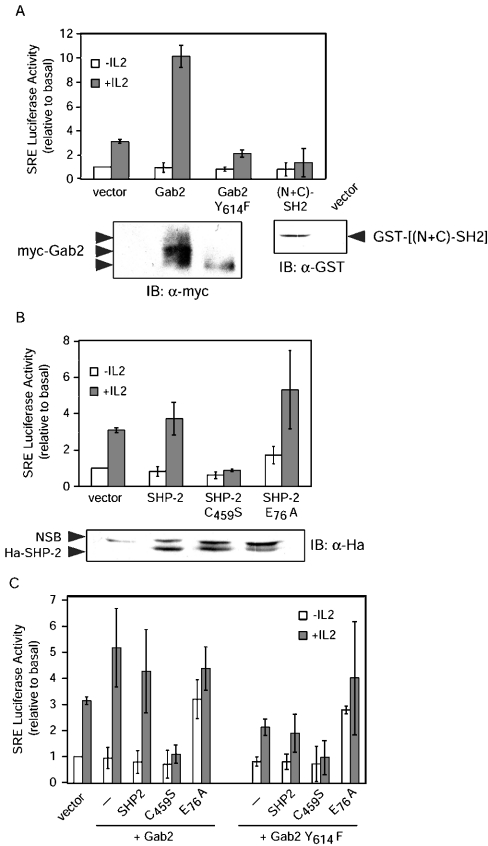
One of the best characterized early-response genes regulated by many growth factors, including IL-2 in T lymphocytes, is c-fos, and it has been suggested that SHP-2 and/or ERK activation might be involved in c-fos regulation by IL-2 [25–27]. We therefore investigated whether the recruitment of SHP-2 on Gab2 played any role in IL-2 regulation of the c-fos SRE. Co-expression of WT Myc-Gab2 in Kit 225 cells evoked a strong increase in IL-2-induced activity of a c-fos SRE luciferase reporter plasmid (Figure 4A). Conversely, Myc-Gab2 Y614F was not only unable to stimulate IL-2-induced SRE activity, but slightly reduced the response as compared with empty control vector. Expression of GST–[(N+C)-SH2] resulted in inhibition of the IL-2-induced SRE response in a way similar to expression of Gab2 Y614F. Completely identical results were obtained in CTLL-2 cells, another IL-2-dependent cell line of murine origin (results not shown), indicating that these results do not reflect a unique feature of the Kit 225 cell line. Thus the ability of Gab2 to transmit a signal necessary for SRE activation depends upon recruitment of SHP-2.
Figure 4. Interaction of SHP-2 with Gab2 is required for c-fos promoter SRE response to IL-2.
(A) Kit 225 cells were transfected with 10 μg of the indicated expression plasmids, 2 μg of SRE Luc and 0.02 μg of pSRα-Renilla standardization reporter. The cells were left 16 h without IL-2, and then half of the cells were stimulated with IL-2 (0.5 nM) for 8 h. (B) Kit 225 cells were transfected with the indicated SHP-2 expression and reporter plasmids, and treated as described in (A). (C) Kit 225 cells were transfected with the indicated expression and reporter plasmids and treated as described in (A). Luciferase assay and control immunoblotting were performed as described in the Experimental section (NSB, non-specific band). For all experiments, the data shown are the means for three independent experiments, and error bars indicate the standard deviation. SRE Luc activities are expressed relative to basal (i.e. control vector without IL-2) after correction for Renilla luciferase activities.
To directly investigate the role of SHP-2 in signalling to the SRE, cells were transiently transfected with expression vectors encoding WT SHP-2 or its dominant-negative C459S or constitutively active E76A mutants. The results shown in Figure 4(B) demonstrate a critical role for SHP-2 in this pathway. Whereas SHP-2 WT had little detectable effect, indicating that it is not limiting in Kit 225 cells, the dominant-negative mutant completely abrogated SRE-driven luciferase expression in response to IL-2. On the other hand, the activated mutant of SHP-2, which shows little effect on basal levels of luciferase expression, significantly and reproducibly increased the IL-2 response.
We then investigated the effects of these SHP-2 mutants when expressed in combination with either Gab2 or Gab2 Y614F (Figure 4C). The striking observation made in this series of experiments was that the C459S mutant completely abrogated the effect of Gab2 WT, whereas expression of SHP-2 E76A in combination with Gab2, either WT or Y614F, resulted in increased luciferase activity even in the absence of IL-2 stimulation, thus drawing parallels with the data described above for the Gal4-Elk assay (Figure 3B). Thus the phosphatase activity of SHP-2 and its interaction with Gab2 are involved in IL-2 induction of the c-fos promoter.
The Gab2/SHP-2/MEK pathway regulates the SRE CArG box
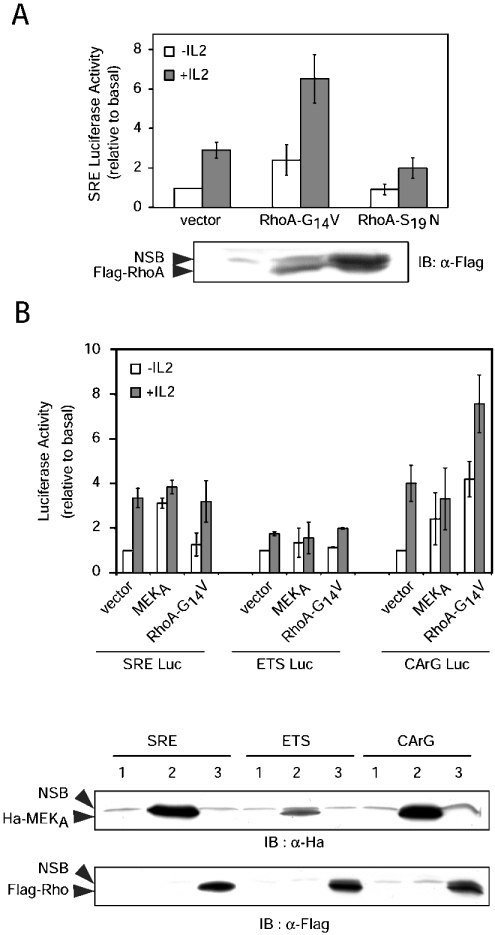
The c-fos SRE is a composite element containing an Ets box (5′-CAGGATG), which is targeted by transcription factors of the TCF family, such as Elk-1, and a CArG box (5′-CCATATTAGG), which binds SRF and has been shown to be regulated by Rho family GTPase-dependent pathways [31,46]. To investigate whether the GTPase RhoA might be involved in SRE activation in IL-2-stimulated T lymphocytes, the response of the SRE Luc construct was analysed in Kit 225 cells in the presence of expression plasmids for activated (G14V) or inactive (S19N) mutants of RhoA. As shown in Figure 5(A), RhoA-G14V significantly increased luciferase activity, whereas RhoA-S19N reduced the response to IL-2.
Figure 5. RhoA- and ERK-dependent regulation of SRE ETS and CArG boxes.
(A) Kit 225 cells were transfected with 10 μg of Rho expression plasmids, 2 μg of SRE Luc and 0.02 μg of pSRα-Renilla standardization reporter. The cells were left for 16 h without IL-2, then half of the cells were stimulated with IL-2 (0.5 nM) for 8 h. Luciferase assay and control immunoblotting were done as described in the Experimental section (NSB, non-specific band). The data shown are the means for three independent experiments, and error bars indicate the standard deviation. SRE Luc activities are expressed relative to basal (i.e. control vector without IL-2) after correction for Renilla luciferase activities. (B) Kit 225 cells were transfected with 10 μg of plasmids coding for the desired proteins, 2 μg of SRE or mutated SRE reporter plasmids (ETS Luc=SREΔCArG, CArG Luc=SREΔETS) and 0.02 μg of pSRα-Renilla standardization reporter. The cells were left for 16 h without IL-2, then half of the cells were stimulated with IL-2 (0.5 nM) for 8 h. Luciferase assay and immunoblotting were performed as described in the Experimental section (1=vector; 2=MEKA; 3=RhoA-G14V). The data shown are the means for three independent experiments; the error bars indicate the standard deviation. Luciferase activities are expressed relative to basal (i.e. control vector without IL-2) after correction for Renilla luciferase activities.
To delineate further whether Gab2/SHP-2 regulation of the MEK/ERK cascade might affect the Rho pathway, we used SRE reporter plasmids mutated either in the ERK-responsive ETS box (CArG Luc) or in the SRF-responsive CArG box (ETS Luc). In keeping with another study [46], WT SRE deprived of its CArG box (ETS Luc) did not respond to any stimulation. In contrast, the (SREΔETS) CArG Luc reporter (with the ETS box deleted) responded both to IL-2 stimulation and to activated MEK at a level comparable with that of WT SRE, but displayed a much stronger response to RhoA-G14V (Figure 5B). These results confirmed that indeed RhoA-G14V targets the CArG box of the SRE.
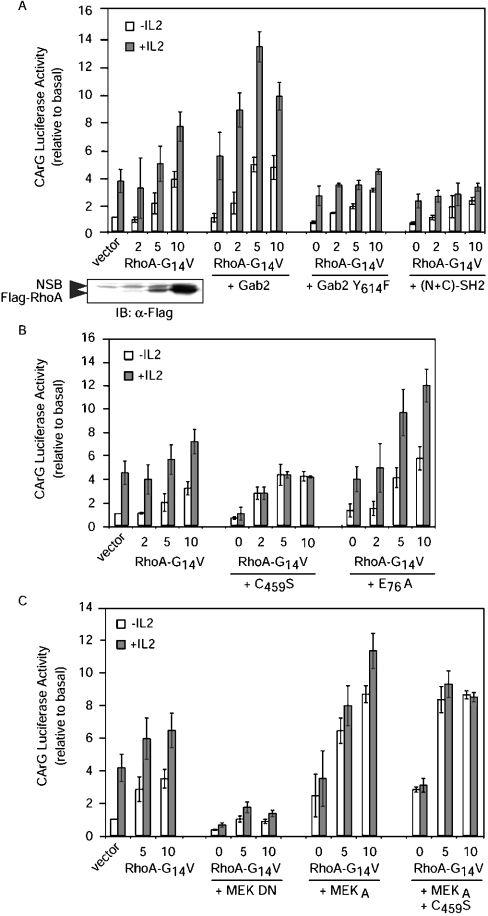
However, since the CArG Luc construct still responded significantly to active MEK, it was possible that at least part of the IL-2 effect depended on the Gab2/SHP-2/MEK pathway described above. We therefore investigated whether Gab2/SHP-2 might also regulate the SRE CArG box. Overexpression of Gab2 alone enhanced the CArG Luc response and synergized its effect efficiently with RhoA-G14V (Figure 6A). Disrupting the interaction between Gab2 and SHP-2 with either Gab2 Y614F or GST[(N+C)-SH2] prevented IL-2-induced luciferase response in the presence of RhoA-G14V. In addition, SHP-2 C459S completely abolished the IL-2 response of the CArG Luc reporter, but showed no effect on the RhoA-G14V response in the absence of IL-2. On the other hand, SHP-2 E76A by itself did not affect the response of the CArG Luc construct, but potentiated the IL-2 response when co-expressed with RhoA-G14V (Figure 6B). These results therefore suggest that Gab2 acts via SHP-2 to regulate events that synergize with the Rho pathway in activating the SRE CArG box. The possible involvement of MEK in these events was then investigated further. The experiments shown in Figure 6(C) indicated that dominant-negative MEK blunted the response of the CArG reporter to either IL-2 or RhoA-G14V, whereas activated MEK potently synergized with RhoA-G14V, in a way that was insensitive to the co-expression of C459S SHP-2.
Figure 6. Effect of Gab2, SHP-2 and MEK on Rho-dependent activation of the SRE CArG box in IL-2-stimulated cells.
(A) Gab2–SHP-2 interaction is required for activation of the CArG box. (B) Effects of SHP-2 on Rho-dependent activation of the CArG box. For these two panels, Kit 225 cells were transfected with 2 μg of CArG Luc, 0.02 μg of pSRα-Renilla standardization reporter and 10 μg of plasmid coding for the desired proteins, except for RhoA-G14V, which was analysed in dose–response experiments (0, 2, 5 and 10 μg). The total amounts of transfected DNA were kept constant by addition of empty control vector. The cells were left for 16 h without IL-2, then half of the cells were stimulated with IL-2 (0.5 nM) for 8 h. Luciferase activity and immunoblotting were performed as described in the Experimental section (NSB, non-specific band). (C) The Gab2/SHP-2/MEK pathway is involved in Rho-dependent activation of the CArG box. Kit 225 cells were transfected with 2 μg of CArG Luc, 0.02 μg of pSRα-Renilla standardization reporter and 10 μg of plasmid coding for the desired proteins, except for RhoA-G14V, which was analysed in dose–response experiments (0, 5 and 10 μg). The total amount of transfected DNA was kept constant by addition of empty control vector. The cells were left for 16 h without IL-2, then half of the cells were stimulated with IL-2 for 8 h. Luciferase section: results are expressed as the means for three independent experiments. Luciferase activities are expressed relative to basal (i.e. control vector without IL-2) after correction for Renilla luciferase activities.
Taken together, these results led us to the conclusion that the Gab2/SHP-2/MEK pathway might not be required for basal induction of the CArG box by activated Rho, but is nevertheless critical for full induction by IL-2.
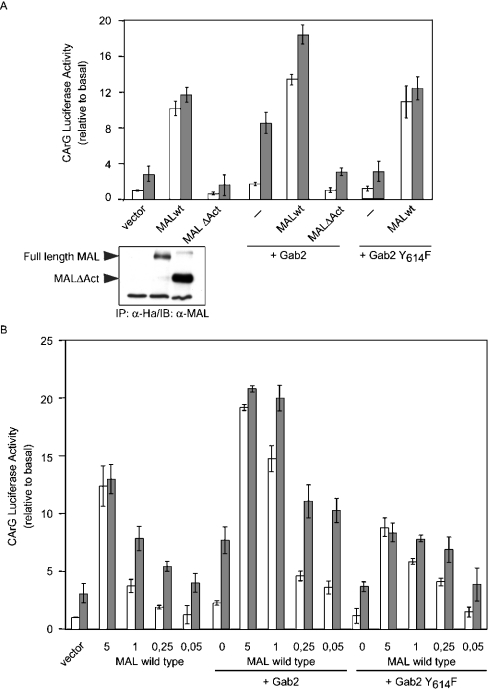
While this work was in progress, Treisman and colleagues [47] identified MAL as a necessary co-activator of SRF, whose shuttling to the nucleus and activity depended upon Rho-actin signalling, as well as MEK/ERK-dependent phosphorylation. These two features would make MAL an interesting candidate to integrate Gab2-initiated signals and explain their synergy with Rho, as described above. This hypothesis was investigated directly by assessing the effects of expressing either WT MAL or a MAL protein deleted of its activation domain (MALΔAct) upon Gab2 stimulation of SRF. Expression of wt MAL alone (5 μg of plasmid) induced robust activity from the CarG-Luc reporter independently of IL-2 stimulation, and MALΔAct reduced the response (Figure 7A). MALΔAct also completely abolished the potentiating effect of Gab2, whereas Gab2 Y614F did not antagonize the effect of WT MAL. We then performed a titration of the MAL expression plasmid together with a constant amount of either Gab2 or Gab2 Y614F (Figure 7B). These experiments clearly demonstrated that the ability of Gab2 to potentiate the CArG Luc response to low amounts (1 μg or less) of MAL expression plasmid depended upon the presence of Tyr-614. Thus MAL is likely to represent a target for the Gab2/SHP-2/MEK/ERK pathway in IL-2-stimulated T lymphocytes.
Figure 7. Gab2 potentiates the effects of MAL on CarG-driven transcription.
(A) Kit 225 cells were transfected with 2 μg of CArG Luc, 0.02 μg of pSRα-Renilla standardization reporter, MAL (5 μg) and/or Gab2 (10 μg) expression plasmids. The cells were left for 16 h without IL-2, then half of the cells were stimulated with IL-2 for 8 h. Insert: expression of WT MAL and MALΔAct proteins was assessed in parallel samples following anti-Ha immunoprecipitation and anti-Mal immunoblotting. (B) Same as in (A) except that the MAL expression plasmid was used at various concentrations. Total amount of transfected DNA was kept constant by addition of empty control vector. Results are expressed as the means for two independent experiments. Luciferase activities are expressed relative to basal (i.e. control vector without IL-2) after correction for Renilla luciferase activities.
DISCUSSION
In the past few years, impressive progress has been made in understanding the signalling pathways whereby IL-2 mediates its biological activities. Both the IL-2Rγ and β chains are critical to signal transduction, with an essential role of the γ chain in activating the JAK kinases. The IL-2Rβ chain then becomes tyrosine-phosphorylated and initiates two major signalling pathways: the Ras/MAPK pathway is activated following binding of Shc to IL-2Rβ Yp338, which recruits the Grb2/Sos complex to activate Ras, and the JAK/STAT pathway is activated upon recruitment of STAT5 to IL-2Rβ Yp510, followed by JAK-dependent phosphorylation of STAT and its translocation to the nucleus, where it acts as a potent transcription factor to regulate expression of a wide array of genes [21,25,41,48]. In addition, although either chain of the receptor lacks consensus sequences for binding of the p85 subunit of PI3K, IL-2 activates PI3K, which itself via protein kinase B (PKB)/Akt regulates various aspects of cell survival and cell cycle progression [49]. The identification and characterization of Gab2, a major tyrosine kinase substrate in cytokine signalling, provided the missing link in PI3K activation by IL-2, as it contains three functional binding sites (pYXXM, where ‘pY’ represents phosphotyrosine and ‘X’ any amino acid) for recruitment of the tandem SH2 domains of the PI3K subunit [24,50]. Furthermore, Gab2 contains additional binding sites, e.g. for the CrkL SH2 domain, and for SHP-2, an important tyrosine phosphatase, that had been described to be involved in IL-2 signalling, but whose mode of activation and function had remained elusive [18,23,51,52].
All three Gab family members known today share a bisphosphotyrosyl motif at their C-terminal end that has been shown to be necessary for SHP-2 binding. For instance, C-terminal-deletion mutants of Gab2, or Gab2 mutated in these two tyrosine residues, no longer associate with SHP-2 and inhibit various aspects of cytokine or receptor tyrosine kinase signalling [18,44]. We have shown in the present study that mutating Tyr-614 alone destabilizes IL-2-induced complex formation between Gab2 and SHP-2. This is consistent with a recent report [43] where a similar mutant of Gab2 was introduced into Bcr-Abl-expressing cells and shown to inhibit the ERK pathway leading to Elk activation. Our finding that, when phosphorylated, Tyr-614 of Gab2 is a ligand for the N-SH2 domain of SHP-2 implied that, even if residual low-affinity binding would occur through C-SH2 recognition of Tyr-643 of Gab2, a Gab2 Y614F mutant would not activate SHP-2. This is indeed what we observed, since this mutant lost its ability to induce ERK activation and Elk transactivation in IL-2-stimulated T lymphocytes. Notably, previous studies have demonstrated a similar scheme in the interaction between Gab1 and SHP-2, in which Tyr-627 of Gab1, the residue equivalent to Tyr-614 of Gab2, binds the N-SH2 of SHP-2, and is required for signalling [16,53]. Further support for the critical role of SHP-2 recruitment by Gab2 is provided by our results showing that, probably because of its open conformation, constitutively active SHP-2 E76A is able to interact with Gab2 Y614F and synergizes with Gab2 Y614F in Elk and SRF activation.
We also report here that the recruitment of SHP-2 to Gab2 is required for its tyrosine phosphorylation in response to IL-2. Although the role of SHP-2 tyrosine phosphorylation in regulating its catalytic activation remains controversial, it has been shown to provide a binding site for the Grb2 SH2 domain [4,10]. Thus SHP-2 is involved in signalling either through its phosphatase activity or through its adapter function, or both. The relative importance of Gab2/SHP-2-dependent events may vary depending on cell type and on the receptor system that is addressed [14]. Indeed, different receptors recruit SHP-2 in different ways; for instance, PDGF-R, the EPO receptor as well as gp130 bind SHP-2 directly [54–56], whereas other receptors might use the Shc/Grb2 adapters or proteins of the IRS/Gab family [8]. These differences might explain some of the discrepancies that can be found in the literature regarding the role of the Gab/SHP-2 connection in regulating the MAPK pathway. Indeed, whereas catalytically inactive (C459S) SHP-2 has generally been found to inhibit MAPK activation, the use of Gab mutants unable to associate with SHP-2 has generated contradictory results, suggesting that requirement for Gab2/SHP-2 in MAPK activation may depend on whether potent alternative and more direct pathways (Grb2/Sos) are engaged by the specific growth factor/cell-type combination studied [18,43,44]. In contrast, all publications seem to agree upon a critical role of Gab proteins in signalling, through SHP-2, to activation of the Ets family transcription factor Elk-1. We report here that Gab2 Y614F is able to block not only Elk-1 transactivation, but also ERK phosphorylation in response to IL-2. Thus our results demonstrate that, in T lymphocytes, activation of ERK in response to IL-2 depends upon the ability of Gab2 to recruit SHP-2. Although this has not been formally established, this dominant-negative effect of Gab2 Y614F probably reflects its ability to compete with endogenous Gab2 for membrane or receptor association. Furthermore, catalytically inactive or constitutively active SHP-2 respectively inhibits or increases Elk transactivation, and additional experiments (results not shown) with combinations of MEK and SHP-2 mutants clearly positioned SHP-2 upstream of MEK in this pathway. These results suggest that SHP-2 acts by dephosphorylating a critical, as-yet-unidentified substrate required for MEK activation, rather than through its adaptor function.
We were then interested in trying to understand how c-fos, an immediate-early gene induced upon IL-2 stimulation and known to depend in part on the MEK/ERK pathway, might be regulated through the Gab2/SHP-2 connection. Studies reported here using a WT c-fos SRE Luc construct are consistent with the Gab2/SHP-2 regulation of Elk, as discussed above. Indeed, Gab2 Y614F and tandem SH2 of SHP-2 blocked IL-2-induced activation of the SRE reporter, suggesting that the SRE is a major target for Gab2 regulation of c-fos expression. Moreover, we have shown that SHP-2 C459S inhibited the response of SRE Luc to IL-2, and prevented the enhancing effect of overexpressed Gab2, whereas SHP-2 E76A strongly increased the response when co-expressed with Gab2. However, full response of the c-fos SRE requires co-operativity between Elk-1/TCF and SRF. At this stage in our studies, it remained to be investigated whether the IL-2-induced interaction between Gab2 and SHP-2 might also influence transcriptional activity of SRF. We therefore used an SRE reporter plasmid mutated in the ERK-responsive ETS-box, but with a functional SRF-responsive CArG-box (CArG Luc). SRF has been reported to be regulated by Rho family GTPase-induced changes in actin dynamics and cytoskeleton remodelling [31,57,58]. Although to our knowledge it has not yet been formally established that IL-2 activates the GTPase RhoA, IL-2 induces morphological changes and reorganization of the actin-based cytoskeleton that strongly suggest the involvement of Rho-dependent pathways ([59] and results not shown). We have not directly addressed this question in the present study, but our data indicate that an activated mutant of RhoA synergizes with IL-2 for regulation of SRE- and CArG-driven transcription, and that the IL-2 response of these reporter plasmids is reduced by the dominant-negative RhoA-S19N. From the results presented here, a major role appears to be attributable to MEK/ERK in the synergistic effects of activated Rho and IL-2 in regulating SRF-driven transcription of the CArG box Luc construct. SRF activity depends critically on its interaction either with TCF (Elk1) or with MAL, a recently described co-activator. It is of interest that the SRF co-activator function of MAL is modulated by both RhoA and MAPK signalling, suggesting that MAL may integrate signals from different signalling pathways [47]. Our finding that Gab2 synergizes with MAL in controlling SRF-driven transcription indicates that such mechanisms may indeed be playing a role in IL-2-stimulated lymphocytes.
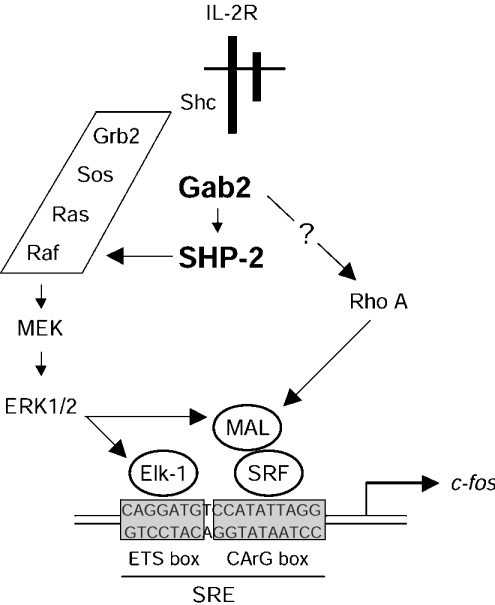
Thus we report here that IL-2 activates SHP-2 via Gab2, and that the phosphatase activity of SHP-2 is required to regulate c-fos SRE induction via MEK/ERK-dependent pathways. ERK then leads to TCF (Elk1) phosphorylation to regulate the ETS box, and participates in regulation of the SRF co-activator MAL, itself acting on the CArG box of the SRE (Scheme 1). Whether these represent synergistic or alternative effects remains to be investigated. A better understanding of the molecular mechanisms at play in these events will have to await identification of the critical SHP-2 substrates and Rho effectors which are involved in these pathways.
Scheme 1. IL-2-induced regulation of c-fos promoter involves Gab2 and SHP-2.
IL-2 induces phosphorylation of Gab2, which recruits and activates SHP-2. The Gab2–SHP-2 complex acts upstream of MEK to activate ERK. ERK regulates c-fos SRE induction in two ways: on the one hand, ERK phosphorylates Elk-1, thus targeting the ETS box of SRE; on the other hand, ERK may also phosphorylate MAL, which, depending on additional signals mediated by the GTPase Rho, allows SRF to drive transcription from the CArG box of the SRE.
Acknowledgments
We thank Dr J. Raingeaud and Dr J. Pierre (INSERM U461), Dr A. Harel-Bellan (CNRS UPR 9079, France), Dr B.G. Neel and Dr H. Gu (Beth Israel Deaconess Medical Center, Boston, MA, U.S.A.) for generously providing us with the plasmids, and J. Raingeaud, J. Pierre and M. David for a critical reading of this manuscript. This work was supported by INSERM, and in part by grants from ARC (grant 4605 and 5608), Ligue nationale contre le cancer (Equipe labellisée 2003) and Ministère de l'Education Nationale de la Recherche et de la Technologie (grant 97C0113).
References
- 1.Feng G. S. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell. Res. 1999;253:47–54. doi: 10.1006/excr.1999.4668. [DOI] [PubMed] [Google Scholar]
- 2.Barford D., Neel B. G. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure. 1998;6:249–254. doi: 10.1016/s0969-2126(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 3.Hof P., Pluskey S., Dhe-Paganon S., Eck M. J., Shoelson S. E. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 4.Lu W., Gong D., Bar-Sagi D., Cole P. A. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol. Cell. 2001;8:759–769. doi: 10.1016/s1097-2765(01)00369-0. [DOI] [PubMed] [Google Scholar]
- 5.O'Reilly A. M., Pluskey S., Shoelson S. E., Neel B. G. Activated mutants of SHP-2 preferentially induce elongation of Xenopus animal caps. Mol. Cell. Biol. 2000;20:299–311. doi: 10.1128/mcb.20.1.299-311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu C. K., Shi Z. Q., Shen R., Tsai F. Y., Orkin S. H., Feng G. S. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol. Cell. Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huyer G., Alexander D. R. Immune signalling: SHP-2 docks at multiple ports. Curr. Biol. 1999;9:R129–R132. doi: 10.1016/s0960-9822(99)80080-3. [DOI] [PubMed] [Google Scholar]
- 8.Qu C. K. Role of the SHP-2 tyrosine phosphatase in cytokine-induced signaling and cellular response. Biochim. Biophys. Acta. 2002;1592:297–301. doi: 10.1016/s0167-4889(02)00322-1. [DOI] [PubMed] [Google Scholar]
- 9.Li W., Nishimura R., Kashishian A., Batzer A. G., Kim W. J., Cooper J. A., Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol. Cell. Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett A. M., Tang T. L., Sugimoto S., Walsh C. T., Neel B. G. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor β to Ras. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharitonenkov A., Schnekenburger J., Chen Z., Knyazev P., Ali S., Zwick E., White M., Ullrich A. Adapter function of protein-tyrosine phosphatase 1D in insulin receptor/insulin receptor substrate-1 interaction. J. Biol. Chem. 1995;270:29189–29193. doi: 10.1074/jbc.270.49.29189. [DOI] [PubMed] [Google Scholar]
- 12.Kim H., Baumann H. Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol. Cell. Biol. 1999;19:5326–5338. doi: 10.1128/mcb.19.8.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tauchi T., Feng G. S., Shen R., Hoatlin M., Bagby G. C., Jr, Kabat D., Lu L., Broxmeyer H. E. Involvement of SH2-containing phosphotyrosine phosphatase Syp in erythropoietin receptor signal transduction pathways. J. Biol. Chem. 1995;270:5631–5635. doi: 10.1074/jbc.270.10.5631. [DOI] [PubMed] [Google Scholar]
- 14.Bennett A. M., Hausdorff S. F., O'Reilly A. M., Freeman R. M., Neel B. G. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol. Cell. Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noguchi T., Matozaki T., Horita K., Fujioka Y., Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell. Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maroun C. R., Naujokas M. A., Holgado-Madruga M., Wong A. J., Park M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 2000;20:8513–8525. doi: 10.1128/mcb.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stofega M. R., Herrington J., Billestrup N., Carter-Su C. Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B. Mol. Endocrinol. 2000;14:1338–1350. doi: 10.1210/mend.14.9.0513. [DOI] [PubMed] [Google Scholar]
- 18.Gu H., Pratt J. C., Burakoff S. J., Neel B. G. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Rohrschneider L. R. The gift of Gab. FEBS Lett. 2002;515:1–7. doi: 10.1016/s0014-5793(02)02425-0. [DOI] [PubMed] [Google Scholar]
- 20.Gesbert F., Delespine-Carmagnat M., Bertoglio J. Recent advances in the understanding of interleukin-2 signal transduction. J. Clin. Immunol. 1998;18:307–320. doi: 10.1023/a:1023223614407. [DOI] [PubMed] [Google Scholar]
- 21.Nelson B. H., Willerford D. M. Biology of the interleukin-2 receptor. Adv. Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 22.Ellery J. M., Nicholls P. J. Alternate signalling pathways from the interleukin-2 receptor. Cytokine Growth Factor Rev. 2002;13:27–40. doi: 10.1016/s1359-6101(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 23.Gesbert F., Guenzi C., Bertoglio J. A new tyrosine-phosphorylated 97-kDa adaptor protein mediates interleukin-2-induced association of SHP-2 with p85-phosphatidylinositol 3-kinase in human T lymphocytes. J. Biol. Chem. 1998;273:18273–18281. doi: 10.1074/jbc.273.29.18273. [DOI] [PubMed] [Google Scholar]
- 24.Crouin C., Arnaud M., Gesbert F., Camonis J., Bertoglio J. A yeast two-hybrid study of human p97/Gab2 interactions with its SH2 domain-containing binding partners. FEBS Lett. 2001;495:148–153. doi: 10.1016/s0014-5793(01)02373-0. [DOI] [PubMed] [Google Scholar]
- 25.Gaffen S. L., Lai S. Y., Ha M., Liu X., Hennighausen L., Greene W. C., Goldsmith M. A. Distinct tyrosine residues within the interleukin-2 receptor beta chain drive signal transduction specificity, redundancy, and diversity. J. Biol. Chem. 1996;271:21381–21390. doi: 10.1074/jbc.271.35.21381. [DOI] [PubMed] [Google Scholar]
- 26.Hatakeyama M., Kawahara A., Mori H., Shibuya H., Taniguchi T. c-fos gene induction by interleukin 2: identification of the critical cytoplasmic regions within the interleukin 2 receptor β chain. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2022–2026. doi: 10.1073/pnas.89.6.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adachi M., Ishino M., Torigoe T., Minami Y., Matozaki T., Miyazaki T., Taniguchi T., Hinoda Y., Imai K. Interleukin-2 induces tyrosine phosphorylation of SHP-2 through IL-2 receptor β chain. Oncogene. 1997;14:1629–1633. doi: 10.1038/sj.onc.1200981. [DOI] [PubMed] [Google Scholar]
- 28.Shaw P. E., Schroter H., Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989;56:563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- 29.Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988;55:989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 30.Hill C. S., Wynne J., Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 31.Gineitis D., Treisman R. Differential usage of signal transduction pathways defines two types of serum response factor target gene. J. Biol. Chem. 2001;276:24531–24539. doi: 10.1074/jbc.M102678200. [DOI] [PubMed] [Google Scholar]
- 32.Hori T., Uchiyama T., Tsudo M., Umadome H., Ohno H., Fukuhara S., Kita K., Uchino H. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987;70:1069–1072. [PubMed] [Google Scholar]
- 33.Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijdenes J., Heinrich P. C., Muller-Newen G., Roche C., Gu Z. J., Clement C., Klein B. Interleukin-6 signal transducer gp130 has specific binding sites for different cytokines as determined by antagonistic and agonistic anti-gp130 monoclonal antibodies. Eur. J. Immunol. 1995;25:3474–3481. doi: 10.1002/eji.1830251240. [DOI] [PubMed] [Google Scholar]
- 35.Brunet A., Pages G., Pouyssegur J. Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene. 1994;9:3379–3387. [PubMed] [Google Scholar]
- 36.Mansour S. J., Matten W. T., Hermann A. S., Candia J. M., Rong S., Fukasawa K., Van de Woude G. F., Ahn N. G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 37.Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 38.Ramirez S., Ait-Si-Ali S., Robin P., Trouche D., Harel-Bellan A., Ait Si Ali S. The CREB-binding protein (CBP) cooperates with the serum response factor for transactivation of the c-fos serum response element. J. Biol. Chem. 1997;272:31016–31021. doi: 10.1074/jbc.272.49.31016. [DOI] [PubMed] [Google Scholar]
- 39.Mercher T., Coniat M. B., Monni R., Mauchauffe M., Khac F. N., Gressin L., Mugneret F., Leblanc T., Dastugue N., Berger R., Bernard O. A. Involvement of a human gene related to the Drosophila spen gene in the recurrent t(1;22) translocation of acute megakaryocytic leukemia. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5776–5779. doi: 10.1073/pnas.101001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR α promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol. Cell. Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delespine-Carmagnat M., Bouvier G., Bertoglio J. Association of STAT1, STAT3 and STAT5 proteins with the IL-2 receptor involves different subdomains of the IL-2 receptor beta chain. Eur. J. Immunol. 2000;30:59–68. doi: 10.1002/1521-4141(200001)30:1<59::AID-IMMU59>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Ali S. Recruitment of the protein-tyrosine phosphatase SHP-2 to the C-terminal tyrosine of the prolactin receptor and to the adaptor protein Gab2. J. Biol. Chem. 2000;275:39073–39080. doi: 10.1074/jbc.M007478200. [DOI] [PubMed] [Google Scholar]
- 43.Dorsey J. F., Cunnick J. M., Mane S. M., Wu J. Regulation of the Erk2-Elk1 signaling pathway and megakaryocytic differentiation of Bcr-Abl+ K562 leukemic cells by Gab2. Blood. 2002;99:1388–1397. doi: 10.1182/blood.v99.4.1388. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y., Jenkins B., Shin J. L., Rohrschneider L. R. Scaffolding protein Gab2 mediates differentiation signaling downstream of Fms receptor tyrosine kinase. Mol. Cell. Biol. 2001;21:3047–3056. doi: 10.1128/MCB.21.9.3047-3056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunnick J. M., Meng S., Ren Y., Desponts C., Wang H. G., Djeu J. Y., Wu J. Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J. Biol. Chem. 2002;277:9498–9504. doi: 10.1074/jbc.M110547200. [DOI] [PubMed] [Google Scholar]
- 46.Charvet C., Auberger P., Tartare-Deckert S., Bernard A., Deckert M. Vav1 couples T cell receptor to serum response factor-dependent transcription via a MEK-dependent pathway. J. Biol. Chem. 2002;277:15376–15384. doi: 10.1074/jbc.M111627200. [DOI] [PubMed] [Google Scholar]
- 47.Miralles F., Posern G., Zaromytidou A.-I., Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 48.Lin J. X., Leonard W. J. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 49.Brennan P., Babbage J. W., Burgering B. M., Groner B., Reif K., Cantrell D. A. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 50.Gu H., Maeda H., Moon J. J., Lord J. D., Yoakim M., Nelson B. H., Neel B. G. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol. Cell. Biol. 2000;20:7109–7120. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gadina M., Stancato L. M., Bacon C. M., Larner A. C., O'Shea J. J. Involvement of SHP-2 in multiple aspects of IL-2 signaling: evidence for a positive regulatory role. J. Immunol. 1998;160:4657–4661. [PubMed] [Google Scholar]
- 52.Gadina M., Sudarshan C., Visconti R., Zhou Y. J., Gu H., Neel B. G., O'Shea J. J. The docking molecule gab2 is induced by lymphocyte activation and is involved in signaling by interleukin-2 and interleukin-15, but not other common γ chain-using cytokines. J. Biol. Chem. 2000;275:26959–26966. doi: 10.1074/jbc.M004021200. [DOI] [PubMed] [Google Scholar]
- 53.Rocchi S., Tartare-Deckert S., Murdaca J., Holgado-Madruga M., Wong A. J., Van Obberghen E. Determination of Gab1 (Grb2-associated binder-1) interaction with insulin receptor-signaling molecules. Mol. Endocrinol. 1998;12:914–923. doi: 10.1210/mend.12.7.0141. [DOI] [PubMed] [Google Scholar]
- 54.Bazenet C. E., Gelderloos J. A., Kazlauskas A. Phosphorylation of tyrosine 720 in the platelet-derived growth factor α receptor is required for binding of Grb2 and SHP-2 but not for activation of Ras or cell proliferation. Mol. Cell. Biol. 1996;16:6926–6936. doi: 10.1128/mcb.16.12.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tauchi T., Damen J. E., Toyama K., Feng G. S., Broxmeyer H. E., Krystal G. Tyrosine 425 within the activated erythropoietin receptor binds Syp, reduces the erythropoietin required for Syp tyrosine phosphorylation, and promotes mitogenesis. Blood. 1996;87:4495–4501. [PubMed] [Google Scholar]
- 56.Fuhrer D. K., Feng G. S., Yang Y. C. Syp associates with gp130 and Janus kinase 2 in response to interleukin-11 in 3T3-L1 mouse preadipocytes. J. Biol. Chem. 1995;270:24826–24830. doi: 10.1074/jbc.270.42.24826. [DOI] [PubMed] [Google Scholar]
- 57.Sotiropoulos A., Gineitis D., Copeland J., Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 58.Geneste O., Copeland J. W., Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. J. Cell Biol. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arrieumerlou C., Donnadieu E., Brennan P., Keryer G., Bismuth G., Cantrell D., Trautmann A. Involvement of phosphoinositide 3-kinase and Rac in membrane ruffling induced by IL-2 in T cells. Eur. J. Immunol. 1998;28:1877–1885. doi: 10.1002/(SICI)1521-4141(199806)28:06<1877::AID-IMMU1877>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]