Highlights
-
•
FUBP1 protein is a single stranded nucleic acid binding protein that consists of an N-terminal domain, a central domain comprising four KH subdomains, and a C-terminal domain.
-
•
FUBP1 regulates DNA transcription, RNA biogenesis, and translation by binding to DNA and RNA.
-
•
Aberrant expression or mutation of FUBP1 is linked to development of various tumors.
-
•
FUBP1 is a potential prognostic biomarker for various cancer types, and targeting it may enhance drug therapy efficacy and overcome drug resistance.
Keywords: Far upstream element binding protein 1, Cancer, Transcription, RNA splicing, Clinical significance
Abstract
Far upstream element-binding protein 1 (FUBP1) is a single-stranded nucleic acid-binding protein that binds to the Far Upstream Element (FUSE) sequence and is involved in important biological processes, including DNA transcription, RNA biogenesis, and translation. Recent studies have highlighted the significance of aberrant expression or mutations in FUBP1 in the development of various tumors, with FUBP1 overexpression often indicating oncogenic roles in different tumor types. However, it is worth noting that recent research has discovered its tumor-suppressive role in cancer, which is not yet fully understood and appears to be tissue- or context-dependent. This review summarizes the association between FUBP1 and diverse cancers and discusses the functions of FUBP1 in cancer. In addition, this review proposes potential clinical implications and outlines future research directions to pave the way for the development of targeted therapeutic strategies focusing on FUBP1.
Graphical abstract
FUBP1 acts as an oncogene in multiple tumors via DNA/RNA binding, yet mutations in oligodendroglioma may suppress tumor growth.
Introduction
The far upstream element-binding protein (FUBP) family is a class of highly conserved single-stranded nucleic acid-binding proteins that are crucial for multiple cellular processes, such as transcriptional regulation, RNA splicing, RNA transport, and translational regulation. This family comprises three members: FUBP1 (initially called FBP1), FUBP2 (also called KH-type splicing regulatory protein, KHSRP), and FUBP3 [[1], [2], [3], [4]]. Although FUBP family members exhibit significant sequence and structure conservation, their functions and expression patterns diverge [2,5,6]. FUBP1 is important for regulating the transcription and translation of its target genes, acting as either an activator or repressor [4]. FUBP2 plays a crucial role in diverse post-transcriptional processes, including alternative splicing, RNA biogenesis, and decay [1,7]. FUBP3 is involved in both transcriptional and post-transcriptional regulation, although its specific biological function is still not well understood [8,9]. Of note, developmental functions of FUBP1 have been shown to include hematopoiesis [10,11]. Dysregulation of FUBP1 expression and genetic alterations in FUBP1 have been identified in various cancers, indicating a potential role for FUBP1 in the development of human cancers. Understanding the role of FUBP1 in human cancers is crucial for elucidating the disease mechanisms and identifying potential therapeutic targets.
This review aimed to comprehensively outline the role of FUBP1 in human cancers, explore its association with various cancer types, and explore the underlying mechanisms by which FUBP1 influences disease pathogenesis. By highlighting the current understanding of FUBP1 in tumors, this review provides insights into potential clinical implications and future research avenues for targeting FUBP1 in cancer treatment.
Domain structure of the FUBP1 protein
The FUBP1 gene is located on chromosome 1p31.1 and was initially cloned from a cDNA library derived from the human undifferentiated monoblastic line U937 in 1994 [12]. FUBP1 comprises three distinct domains: an N-terminal domain with an amphipathic helical structure, a central domain responsible for DNA and RNA binding, and a C-terminal domain (Fig.1) [3]. The N-terminal region, with amino acid residues 1–106, is a repression domain that is believed to inhibit the function of the C-terminal domain; however, the mechanism behind this phenomenon remains unclear [3,5]. The central part of FUBP1 comprises four KH subdomains spanning amino acid residues 107–447 that can bind to DNA. These KH domains recognize and bind to the Far Upstream Element Sequence (FUSE) located in the promoter region of the target genes. This binding is crucial for the gene expression regulated by FUBP1. Through its interaction with the FUSE sequence, FUBP1 recruits other transcriptional regulators and chromatin-remodeling factors to modulate the transcriptional activity of target genes. Moreover, studies have revealed that FUBP1 can bind to RNA to regulate alternative splicing or RNA stability and can interact with non-coding RNA [13,14]. The C-terminal region of FUBP1, specifically amino acid residues 448–644, contains three tyrosine-rich motifs. These motifs play crucial role in the transcriptional activation of FUBP1 [3,5]. FUBP1 also possesses nuclear localization signals (NLSs) that regulate its subcellular localization. Specifically, the N-terminus harbors a standard bipartite NLS, whereas NLSs found in the central and C-terminal domains are non-canonical [15]. Thus, FUBP1 can shuttle between the nucleus and cytoplasm, allowing it to dynamically interact with its target genes and exert its transcriptional regulatory functions [[16], [17], [18]].
Fig.1.
Structural overview of human FUBP1 protein and its interactions with DNA and RNA molecules. The human FUBP1 protein is composed of distinct functional domains: a repression domain (amino acid residues 1–106), a central domain (amino acid residues 107–447), and a transactivation domain (amino acid residues 448–644). Specifically, FUBP1 interacts with a variety of genes, including C-MYC, DVL1, USP29, C-KIT, CDKN1A, CCNA1, VHL, and PTGES. Additionally, FUBP1 binds to several RNA molecules, encompassing mRNAs such as MDM2, DMD, PKD2, GAP43, COX2, Nrf2, and P27, as well as long non-coding RNA LCAT3 and circular RNA ACTN4.
Molecular functions of FUBP1
However, the precise mechanisms by which FUBP1 exerts its effects are still being investigated. Several key mechanisms have been identified to clarify their roles in cellular processes and disease pathogenesis.
Binding to DNA
FUBP1 primarily functions as a single-stranded nucleic acid-binding protein by binding to the FUSE sequence in the promoter region of target genes, leading to the recruitment and assembly of transcriptional complexes. FUBP1 contains KH domains that can bind DNA or RNA to form stable complexes [12]. The interaction between the c-MYC gene and FUBP1 is widely recognized and is largely attributed to the FUSE element containing an AT-rich sequence. The 29-nucleotide FUSE element sequence is 5′-TATATTCCCTCGGGATTTTTTATTTTGTG-3′. It is located approximately 1.5 kb upstream of the P1 promoter (or 1.7 kb upstream of the P2 promoter) on the c-MYC gene [19,20]. The two subdomains KH4 and KH3 of FUBP1 each recognize specific short sequences. KH4 is specific to the sequence 5′-TATTCC-3′, while KH3 recognizes the sequence 5′-ATTTTT-3′ [21,22]. This interaction leads to the recruitment and activation of the transcription factor TFIIH, ultimately enhancing transcription of the c-MYC gene [23]. FUBP1 recruits FBP-interacting repressor (FIR), forming an inhibitory complex with FUSE and TFIIH. Ultimately, this complex suppresses the transcription of c-MYC. The interplay and coordination between FUSE, FUBP1, and FIR are crucial for regulating c-MYC expression [24]. In addition to its interaction with the FUSE sequence of the c-MYC gene, FUBP1 can interact with a variety of sequences with different affinities (Table1) [25]. For instance, FUBP1 binds to the "P3″ segment (−1422 ∼ −1261) located 2 kb downstream of DVL1, with the specific sequence "TTCCCCTGATTT" serving as its binding site to enhance the transcription of DVL1 [26,27]. FUBP1 interacts with the transcription start sites (TSS). It binds to a T- and GT-rich sequence, TTGCATTACTTTTTTTTTTGTTTGTTTTTGAGATGGAGTTTTGCTCTTGTTGCCC, located −2.5 kb upstream of the TSS on USP29 gene to activate transcription by cooperating with P38 [28]. Additionally, FUBP1 binds to an A/T-rich sequence, AGTTTATTCCTATGGGGATATAAAAGTGTGTCAG, located +30 kb upstream of the TSS on c-KIT, to activate transcription in cooperation with the major hematopoietic regulator RUNX1 [29]. FUBP1 also interacts with DNA sequences to repress transcription (Table 1). For example, FUBP1 can bind with a T- and GT-rich sequence, CTGGCTTTTTGTTTTCATTTTGTTTTTTTGTTTTGTTTTGTTTTTTGAGACAA, located −2.7 kb upstream of TSS on CDKN1A gene to repress P21 expression [30]. A summary of FUBP1 as a DNA-binding protein is listed in Table1. These A/T-rich DNA sequences are prone to melting into single-stranded forms under torsional stress. Changes in DNA shape occur in response to the twisting force of transcription, leading to melting of the B-form of DNA in certain regions, unconventional connections between base pairs, and the formation of alternative DNA structures. In the natural environment, transcriptional activation can alter the DNA structure to regulate the speed of transcription. FUBP1 is required to remodel DNA topology [19,20,31].
Table 1.
FUBP1 acts as a DNA-binding protein.
| Gene | Binding location | Binding sites | Function | Involved model | References |
|---|---|---|---|---|---|
| MYC | 1.5 kb upstream of the P1 promoter (or 1.7 kb upstream of the P2 promoter) | GTATATTCCCTCGGGATTTTTTATTTTGTGTTATTCCACGGCATGAAAAA | Promoting transcription | In vitro, U-937 leukemia cells | [19,20,22,32] |
| DVL1 | P3 segment (−1422 ∼ −1261) located 2 kb downstream | TTCCCCTGATTT | Promoting transcription | LoVo cells | [26,27] |
| USP29 | −2.5 kb upstream of TSS | TTGCATTACTTTTTT TTTTGTTTGTTTTTG AGATGGAGTTTTGCTCTTGTTGCCC | Promoting transcription | MCF7 cells | [28] |
| c-KIT | +30 kb from TSS | AGTTTATTCCTATGGGGATATAAAAGTGTGTCAG | Promoting transcription | HEK293 cells, Nalm6 cells | [29] |
| PTGES | Not clear, from 3000 bp upstream to 500 bp downstream | N/A | Promoting transcription | MG63 osteosarcoma cells | [30,33] |
| CCNA1 | −1991∼−1889 and −1691∼−1561 upstream of TSS | N/A | Promoting transcription | Lewis lung carcinoma cell | [34] |
| VHL | − 1833 bp ∼ − 1734 bp of VHL promoter sequence | TTTCACCATGTTGTCAGGCTGGTCTAGAACTCCTGACCTTGTGATCAG | Suppressing transcription | SH-EP cells | [35] |
| CDKN1A | −2.7 kb upstream of TSS | CTGGCTTTTTGTTTT CATTTTGTTTTTTTG TTTTGTTTTGTTTTT TGAGACAA | Suppressing transcription | In vitro, Hep3B cell | [30] |
Binding to RNA
FUBP1 is involved in multiple aspects of RNA regulation, including splicing. They interact with AU-rich elements (AREs) via their KH subdomains. AREs are specific cis-regulatory RNA elements that usually span 50–150 nucleotides and contain repeated AUUUA pentamer or UUAUUUAUU nonamer sequences [36,37]. Selective splicing plays a significant role in generating genetic diversity in eukaryotes, with approximately 95 % of human multiexon genes undergoing this process [38]. FUBP1 has been observed to interact with an exonic splicing silencer present in exon 10 of the cardiac protein triadin. This interaction results in the exclusion or skipping of exon 10 during splicing process [39]. The splicing process involves two consecutive reactions that occur before introns are released. During the second-step reaction of splicing, which typically involves the attack of a hydroxyl (-OH) group at the 3′ end of an exon on the phosphodiester bond at the 3′ splice site, FUBP1 plays a role in inducing splicing repression [39,40]. FUBP1 inhibits protein factors that are essential for the second step of splicing and binds to an AU-rich sequence located on exon 10 of triadin. This binding prevents the proper ligation of exons [39,40]. FUBP1 is also involved in other pre-mRNA splicing (Table 2). For example, FUBP1 has been identified as a positive regulator of splicing in MDM2 which is an oncogene known for its role in ubiquitinating p53 and promoting its degradation [41,42]. FUBP1 directly binds to the AAUUCCCUUUCUUGUGUGUAUGGU sequence on the intronic regions of exon 11 of MDM2, facilitating the enhancement of spliceosome recruitment or positive regulatory factors. FUBP1 masks or competes with negative regulatory factors in this context [41]. One study provided evidence for the involvement of FUBP1 in facilitating the appropriate inclusion of DMD pre-mRNA exon 39 in splicing [43]. This is accomplished through FUBP1′s interaction with an intronic splicing enhancer element positioned approximately 80 bp upstream of the DMD exon 39 [43]. Additionally, FUBP1 has been identified as an auxiliary splicing factor for U2AF2. For specific pre-mRNA targets, FUBP1 enhances the stability of U2AF2 binding, thereby exerting control over selective splicing. An illustrative example is FUBP1’s role in promoting the inclusion of exon 10 in PTBP2. By binding to at least one distal intronic site, FUBP1 strengthens the interaction between U2AF2 and this site [44].
Table 2.
FUBP1 acts as a RNA-binding protein.
| RNA type | RNA | Binding sites | Function | Involved model | References | |
|---|---|---|---|---|---|---|
| Pre-mRNA | Triadin | AU-rich sequence (AUAUAUGAU) located on exon 10 | Repressing splicing | HeLa cells | [39,40] | |
| MDM2 | Sequence of AAUUCCCUUUCUUGUGUGUAUGGU on exon 11 | Enhancing splicing | HeLa S3 cells | [41,42] | ||
| DMD | Sequence of UUGUGUGUGUUUA AAUAACAUGU intron 38 | Facilitating inclusion | C25cl48 cells | [43] | ||
| PTBP2 | Exon 10 | Promoting inclusion | HeLa cells | [44] | ||
| mRNA | PKD2 | 3′UTR | Inhibiting mRNA translation | HeLa cells, HEK cells, Zebrafish | [45] | |
| GAP43 | 3′UTR | Inducing mRNA degradation | Rat brain | [46] | ||
| COX2 | 3′UTR | NA | HeLa cells | [47] | ||
| Nrf2 | 5′UTR | Promoting mRNA translation | HeLa cells | [48] | ||
| P27 | 5′UTR | Promoting mRNA translation | MCF7 cells | [49] | ||
| NPM | 3′ UTR | Inhibiting mRNA translation | Tsc−/−p53−/−MEF cells | [51] | ||
| Non-coding RNA | LncRNA-LCAT3 | Stem-loop region (208–342 nt) | LCAT3 recruits FUBP1 to activate MYC transcription | Calu1 cells | [14] | |
| CircACTN4 | N/A | Co-operating to activate transcription of MYC | MCF-7 cell | [13] | ||
| tiRNA-Val-CAC-2 | N/A | Enhancing stability of FUBP1 | AsPC-1 cell | [50] | ||
Additionally, FUBP1 can bind to 3′ untranslated (3′UTR) of mRNA. The inhibitory effect of FUBP1 on PKD2 mRNA translation involves binding to the 3′UTR region of PKD2, which is rich in AU and ARES motifs, hindering efficient mRNA translation [45]. By binding to a uridine-rich sequence spanning 26 nucleotides located at the downstream end of the coding region FUBP1 influences the stability of GAP-43 mRNA. GAP43 encodes a membrane phosphoprotein that plays an essential role in axonal growth and the establishment of neuronal connections [46]. Additionally, FUBP1 has been observed to bind to the 3′UTR of COX2 mRNA. This region contains a 76-nucleotide sequence that houses six AREs. However, the precise role of FUBP1 remains unclear [47].
FUBP1 has the potential to increase mRNA stability by interacting with the 5′UTR to regulate translation, as shown in Table 2. FUBP1 binding to the Nrf2 5′UTR region plays a role in regulating Nrf2 5′UTR activity. Under oxidative stress conditions, FUBP1 recruits the 40S ribosomal subunit to Nrf2 mRNA, facilitating the formation of the 43S pre-initiation complex, thereby promoting the initiation of Nrf2 protein synthesis [48]. Moreover, FUBP1 binds to the internal ribosome entry site located on the 5′UTR of P27 mRNA, enhancing its translation. The mechanism involves the central domain of FUBP1 interacting with a specific 8-nucleotide sequence, 5′-GCGAAGAG-3′, located upstream of the codon start of P27 [49].
Recent studies have shown that FUBP1 can interact with various non-coding RNAs, such as circRNAs, lncRNAs, and small RNAs, expanding its functional roles (Table 2) [13,14,50].
Other underlying mechanisms
Under stressful conditions, FUBP1 has been shown to bind to the DNA-binding domain of p53, a well-known tumor suppressor protein, inhibiting its function, particularly in viral replication [[52], [53], [54]]. In hepatitis C virus (HCV) infection, FUBP1 can directly interact with p53 protein to inhibit its DNA binding ability of p53, and FUBP1 immunoprecipitation also co-immunoprecipitates the viral proteins NS5A and NS5B from the cell lysates of MH14 cells [53]. Another study reported that FUBP1 the direct interation with E1A of the adenovirus results in stabilization of the FUBP1-p53 complex, preventing p53 from binding to its promoter [54]. Additionally, FUBP1 is implicated in a direct binding interaction with the eukaryotic translation initiation factor 4E-binding protein 1, suggesting a connection with the translation initiation complex [45].
Overall, FUBP1 functions through various mechanisms, such as transcriptional and alternative splicing regulation. FUBP1 dysregulation interferes with these processes, resulting in cellular dysfunction and disease development. Understanding the specific mechanisms by which FUBP1 functions in diverse cellular environments will provide valuable insights into its involvement in human diseases, and may offer potential therapeutic targets.
Functions of FUBP1 in human cancers
FUBP1 is a multifaceted protein that is involved in various biological processes. These processes encompass biological rhythms [55], viral replication [53,54,56,57], cell self-renewal [58] and cell differentiation [59,60]. It is worth noting that abnormal overexpression of FUBP1 has been found in various types of tumors, such as colorectal cancer [26], breast cancer [13], pancreatic adenocarcinoma [61], cervical carcinoma [61], and others. Functions of FUBP1 in human cancers are summarized in Table 3.
Table 3.
The functions of FUBP1 in human cancers.
|
Cancer type |
Expression | Utilized assays | Function | Mechanism | Reference |
|---|---|---|---|---|---|
| Colorectal cancer (CRC) | Upregulation in tumor tissues and tumor cell lines | IHC, Western blot | Overexpression of FUBP1 enhances colorectal cancer cell migration, invasion, tumor sphere formation, and CD133 as well as ALDH1 expression in vitro, and tumorigenicity in xenograft models in vivo. | FUBP1 activates Wnt/β-catenin signaling via directly binding to the promoter of DVL1, thereby increasing pluripotent transcription factors, including c-Myc, NANOG, and SOX2. | [26] |
| N/A | N/A | Silencing FUBP1 inhibits the ability of HCT116 cells to proliferate, migrate, invade and glycolysis, and enhances its apoptosis in vitro. | May by affecting c-MYC. | [62] | |
| Pancreatic cancer | Upregulation in tumor tissues | Bioinformatics analysis, RT-qPCR | FUBP1 promotes cell proliferation, migration and invasion; and regulates expression levels of EMT‑related markers in vitro. | FUBP1 regulates the TGFβ/Smad signaling cascade by upregulating phosphorylated‑Smad2/3 and TGFβ1 expression levels. | [61] |
| Upregulation in tumor tissues | RT-qPCR, Western blot | FUBP1 promotes tumor cell proliferation, migration and PD-L1 expression in vitro. | N/A | [63] | |
| Cervical cancer | Upregulation in tumor tissues | Bioinformatics analysis, IHC | Knockdown of FUBP1 inhibits cell proliferation and migration in vitro. | The sequence of FUBP1 contains a PY-NLS (proline-tyrosine) motif, which is recognized by Transportin 1 (TNPO1). Furthermore, TNPO1 mediates the nuclear import of FUBP1 by interacting with FUBP1. | [64] |
| Upregulation in tumor tissues and cell lines | Bioinformatics analysis, IHC, RT-qPCR, | FUBP1 promotes cervical carcinoma cell proliferation and inhibits cell apoptosis in vitro. | Bioinformatics analysis reveales that FUBP1 promotes the biological function of cervical carcinoma cells via enhancing DNA repair signal pathways. | [65] | |
| Lung cancer | Upregulation in tumor tissues and cell lines | Bioinformatics analysis, IHC, Western blot | Knockdown of FUBP1 inhibits cell migration, invasion, and proliferation in lung squamous carcinoma cell in vitro; increases CD4+ as well as CD8+ cells and reduces PD-L1 protein expression in xenograft tumor in vivo. | N/A | [66] |
| Upregulation in tumor tissues | Bioinformatics analysis | FUBP1 silencing inhibits proliferation and survival of lung cancer cells in vitro. | FUBP1 is recruited by long non-coding RNA LCAT3 to activate c-MYC transcription | [14] | |
| N/A | N/A | FUBP1 deficiency slows down cell cycle progression and promote cell survival in Lewis lung carcinoma cell in vitro. | FUBP1 binds to CCNA1 promoter to promote its transciption. | [34] | |
| Neuroblastoma | Upregulation in tumor cell lines | Western blot | FUBP1 promotes cell proliferation and inhibits cell apoptosis in SH-EP and SK-N-BE cell lines in vitro. | FUBP1 downregulates Von Hippel-Lindau (VHL) by binding to VHL promoter to inhibit VHL transcription, inhibiting the degradation of HIF1α. FUBP1 might also bind to the HIF1α promoter to upregulate HIF1α mRNA levels. | [35] |
| Glioblastoma | Upregulation in tumor tissues | RT-qPCR, | FUBP1 silencing increases the growth inhibitory rate and apoptosis rate of the U251 cells, and enhances the chemotherapy sensitivity of U251 cells to DDP (cisplatin) in vitro. | N/A | [67] |
| Hepatocellular carcinoma (HCC) | Upregulation in tumor tissues | Bioinformatics analysis | Overexpression of FUBP1 promotes HCC proliferation, invasion, and metastasis in vitro and in vivo. | by activating transforming growth factor-β (TGF-β)/Smad pathway and enhancing epithelial-mesenchymal transition (EMT) | [68] |
| N/A | N/A | FUBP1 promotes cell proliferation in vitro. | FUBP1 induces the abnormal expression of lnc-LYZ-2. | [69] | |
| Upregulation in tumor tissues | IHC | Loss of FUBP1 expression leads to increased apoptosis sensitivity and decreased proliferation in the HCC cell line Hep3B in vitro, abrogate tumor growth in HCC xenograft transplantation model in vivo. | Binds to p21 promoter to promote its transcription | 31 | |
| Leukemia | Upregulation in tumor cellls | Bioinformatics analysis | Knockdown of FUBP1 decreases CML progenitor cells survival, cell cycle activity, and increases apoptosis as well as DNA damage in vitro. | N/A | [70] |
| N/A | N/A | Contribute to cell proliferation in Nalm6 cells in vitro. | Binds to c-KIT promoter to promote its transcription | 30 | |
| B lymphoblastic leukemia | N/A | N/A | FUBP1 contributes to cell proliferation and renders cells more resistant to the c-KIT inhibitor imatinib mesylate in vitro, and leads to poorer survival in vivo. | That FUBP1 protein interacts with RUNX1 regulates c-KIT transcription by binding to enhancer in the c-KIT oncogene. | [29] |
| Chordoma | Upregulation in tumor tissues | IHC | FUBP1 promotes cell proliferation and invasion in vitro. | 3′UTR of FUBP1 is the target of miR-193a, and FUBP1 expression is negatively regulated by miR-193a downregulated by lncRNA KRT8P41. | [71,72] |
| Esophageal cancer | Upregulation in tumor tissues | IHC, Western blot | FUBP1 promotes cell proliferation in vitro. | Activating c-Myc. | [73] |
| Tongue cancer | Upregulation in tumor tissues | IHC, RT-qPCR, Western blot | FUBP1 knockdown inhibits cell proliferation, and induced cell cycle arrest and apoptosis in vitro. | N/A | [74] |
| Nasopharyngeal cancer | Upregulation in tumor tissues and cell line | Western blot, IHC, RT-qPCR | Knockdown of FUBP1 reduces cell proliferation, clonogenicity and the ratio of side populations in vitro, as well as tumorigenesis in nude mice in vivo. | N/A | [75] |
| Gastric Cancer | Upregulation in tumor tissues | IHC, RT-qPCR | Silencing of FUBP1 inhibits cell proliferation and affect the distribution of the cell cycle, resulting in S-phase arrest and cell growth inhibition in vitro. | N/A | [76,77] |
| Renal cancer | Upregulation in tumor tissues and cell lines | Western blot, IHC, RT-qPCR | FUBP1 knockdown inhibits cell proliferation and induces cell cycle arrest and apoptosis in vitro. | N/A | [78] |
| Osteosarcoma | Upregulation in tumor cell lines and tumor tissues | Western blot, IHC, RT-qPCR | Overexpression of FUBP1 confers lobaplatin resistance, whereas the inhibition of FUBP1 sensitizes osteosarcoma cells to lobaplatin-induced cytotoxicity both in MG63 and SOSP‐9607 cell lines in vitro, as well as in xenografts models in vivo. | The binding of FUBP1 to the PTGES promoter to transcriptionally activates PTGES. | [33] |
| Breast cancer | Upregulation in tumor tissues | Western blot, IHC | Knockdown of FUBP1 decreases cell proliferation by arresting the cell cycle at the G2 phase, cell migration and metastasis by downregulating matrix metalloproteinase 2 expression, and enhances the sensitivity of TNBC cells to cisplatin by inducing apoptosis in vitro. | N/A | [79] |
Cell proliferation and cell cycle
FUBP1 plays a crucial role in promoting the transcription of c-MYC, a key regulator of cell proliferation and cell cycle processes in various human cancers. FUBP1 activation enhances the proliferation of esophageal squamous cell carcinoma cells by activation of c-MYC [73]. Conversely, Qian et al. demonstrated that suppression of FUBP1 expression inhibits the proliferation and survival of lung cancer cells. Mechanistically, FUBP1 can be recruited by the long non-coding RNA LCAT3 to activate the transcription of c-MYC [14]. Furthermore, FUBP1, a single-stranded nucleic acid-binding protein, has been found to affect cell proliferation and the cell cycle by influencing the expression of other genes. For instance, Kang et al. discovered that FUBP1 binds to the CCNA1 promoter and promotes its transcription, thereby enhancing cell cycle progression and cell survival in lung carcinoma [34]. FUBP1 also regulates cell proliferation by modulating non-coding RNA. Inhibition of lnc-LYZ-2 significantly reduces the proliferative effect of FUBP1 on hepatocellular carcinoma cells, indicating a partial contribution of lnc-LYZ-2 to FUBP1’s proliferative regulation [69].
Cell migration and invasion
Cell migration and invasion are essential steps in tumor cell metastasis [80,81]. FUBP1 is closely associated with tumor metastasis. Research has shown that FUBP1 promotes cell migration by activating the transcription of c-MYC. For instance, Wang et al. discovered that c-MYC overexpression reverses the inhibitory effects of FUBP1 knockdown on the migratory and invasive abilities of colon cancer cells [62]. Additionally, FUBP1 can active the TGFβ/Smad signaling cascade by upregulating the expression levels of phosphorylated-Smad2/3 and TGFβ1. This promotes cell migration and invasion and upregulates the expression levels of mesenchyme-related markers, while downregulating epithelial marker expression in pancreatic adenocarcinoma and hepatocellular carcinoma [61,68]. Furthermore, Liu et al. found that the reduction in FUBP1 inhibited cell migration and metastasis by decreasing the expression of matrix metalloproteinase 2 in triple-negative breast cancer (TNBC) [79].
Cell apoptosis
Cell apoptosis, also known as programmed cell death, is a critical process that helps maintain normal physiological conditions, control disease progression, and preserve tissue structure and function [82,83]. Dysregulation of apoptosis in tumors can lead to abnormal cell growth and survival, and studies have demonstrated that FUBP1 plays a role in influencing tumor cell apoptosis and promoting tumor progression. Hoang et al. discovered that the knockdown of FUBP1 led to increased apoptosis and reduced cell cycle activity in acute myeloid leukemia (AML) cells [70]. Nuclear localization of FUBP1 is associated with apoptosis. Multiple regions of NORAD (a highly conserved and abundantly expressed lncRNA) act as platforms bound to the central domain of the anti-apoptotic factor FUBP1, which weakens the localization of FUBP1 in the nucleus, thereby affecting FUBP1 occupation of the promoter of its target pro-apoptotic genes (BIK, NOXA, TRAIL, and TNFA) and leading to apoptosis induction in endometrial cancer [84], suggesting that the presence of FUBP1 can inhibit the expression of these four target pro-apoptotic genes. Moreover, FUBP1 inhibits apoptosis in other cancers (Table 1), including colon cancer [62], tongue squamous cell carcinoma [74], and cervical carcinoma [65].
Other functions as an oncogene
With advances in the understanding of tumors, tumor hallmarks have evolved from 6 features in 2000 to 14 features in 2022 [[85], [86], [87]], including maintaining proliferative signaling, resisting cell death, promoting tumor inflammation, and activating invasion and metastasis. Recent studies have shown that, besides influencing cell growth and metastasis, FUBP1 can also affect other aspects of tumor biology. Tumor cell metabolism encompasses the metabolic processes and molecular interactions that support growth and survival. It enables tumor cells to obtain the necessary energy and resources for their rapid proliferation and division and is intricately connected to tumor progression [88,89]. FUBP1 is involved in tumor metabolism. For example, FUBP1 can upregulate two hexokinase expressions, Hk1 and Hk2, by interacting with the promoter region of Hk1 and Hk2, to mediate lactate production, thereby activating the Akt-mTOR axis [90]. Another study found that FUBP1 could enhance glycolysis and ATP production via VHL/ HIF1α/ LDHB axis in neuroblastoma. Mechanistically, FUBP1 binds to VHL promoter to downregulate VHL expression, the E3 ligase for HIF1α, thereby inhibiting the degradation of HIF1α, resulting in upregulation of LDHB expression to enhance glycolysis [35]. In terms of immune infiltration, Yu et al. observed a decrease in PD-L1 protein expression and an increase in CD4+ and CD8+ cells when FUBP1 was knocked down in lung squamous cancer [66]. Similarly, another study found that FUBP1 promotes the expression of PD-L1 in pancreatic ductal adenocarcinoma [63]. However, the underlying mechanism requires further investigation. Recent investigations have highlighted the role of FUBP1 in the DNA damage response. FUBP1 knockdown leads to elevated levels of DNA damage compared to leukemia cells with normal FUBP1 levels, suggesting that FUBP1 participates in DNA repair processes, potentially contributing to the maintenance of genomic stability [70].
FUBP1 as a tumor suppressor gene
FUBP1 is phenotypically characterized as an oncogene in most tumors, including high-grade gliomas [91]. Recent studies have identified FUBP1 mutations in oligodendrogliomas [[92], [93], [94], [95], [96], [97]]. Oligodendrogliomas, the second most prevalent malignant brain tumors in adults, are characterized by the loss of chromosomes 1p and 19q. Analysis of 27 oligodendrogliomas revealed that 3 and 12 tumors harbored mutations in FUBP1 and CIC (homolog of the Drosophila gene capicua), respectively, with approximately 58 % of these mutations predicted to generate a truncated form [97]. Several other studies identified FUBP1 mutations in oligodendrogliomas [95,98]. Thus far, all documented FUBP1 mutations have been anticipated to inactivate FUBP1, suggesting a tumor suppressor role for FUBP1 in oligodendrogliomas, which is different from most other tumors [98]. Several recent studies have attempted to explain this mechanism. The loss of the cellular terminal differentiation ability caused by mutations is a selective genetic event in tumor development. Hwang et al. found that the expression of FUBP1 is dynamically regulated during neurogenesis and that its downregulation in neural progenitor cells, in conjunction with the expression of IDH1R132H, synergistically affects terminal differentiation and promotes tumorigenesis [99]. Mechanistically, the collaborative interaction between SRRM4 and FUBP1 is required for neurospecific microexon splicing of the LSD1+8a isoform. In neural progenitor cells, the loss of FUBP1 leads to the downregulation of LSD1+8a expression, impairing the differentiation and maturation of terminal neurons. In FUBP1-downregulated neural progenitor cells, enhanced expression of LSD1+8a restored terminal differentiation and inhibit tumorigenesis [99]. This study emphasized the significance of LSD1+8a as a crucial mediator of FUBP1-dependent neuronal differentiation. Moreover, a study has illustrated that FUBP1 interacts with the 3′ UTR of NPM mRNA to inhibits NPM translation in mouse embryonic fibroblasts (MEFs). NPM is a multifunctional oncoprotein and its expression level of NPM directly influences cell growth and proliferation. FUBP1 knockout leads to a significant increase in NPM translation, promoting overall cell proliferation in MEFs [51], which is in contrast to its predicted functions as an oncoprotein. This phenomenon remains poorly understood and may vary depending on the tissue or circumstances. These studies revealed the role of FUBP1 as a tumor suppressor. These various functions of FUBP1 highlight its importance in cellular processes and tumor pathogenesis. FUBP1 dysregulation can disrupt these processes, leading to cellular dysfunction and tumor development (Fig.2).
Fig.2.
The model that depicts the diverse mechanistic aspects of FUBP1 as both an oncogene and a tumor suppressor. As an oncogene, FUBP1 could promote proliferation, migration as well as invasion, and inhibit apoptosis of tumor cells by regulating transcription, RNA splicing and translation via binding to DNA or RNA. Furthermore, the mutations of FUBP1 leads to the inactivation of FUBP1, thereby impairing the differentiation and maturation of terminal neurons to promote tumorigenesis by down-regulating LSD1+8a expression in oligodendroglioma.
Notably, Klener et al. found FUBP1 gene mutations in a patient with clonally related pleomorphic highly aggressive mantle cell lymphoma, which developed after five cycles of fludarabine-based second-line therapy for the first relapse of chronic lymphocytic leukemia [100]. However, it remains unclear whether mutations in FUBP1 in chronic lymphocytic leukemia, similar to those observed in oligodendrogliomas, lead to the inactivation of FUBP1, and further research is warranted.
Clinical application
Prognostic biomarker
Several studies have shown that high FUBP1 expression is associated with tumor malignancy and poor prognosis (Table 4). Elevated FUBP1 expression is positively correlated with lymph node metastasis and clinical stage and negatively associated with overall survival in colorectal cancer [26]. In patients with cervical carcinoma, high FUBP1 expression correlated with patient age, tumor T classification, N classification, recurrence, Ki67 expression, and poor prognosis. FUBP1 expression has been identified as an independent unfavorable predictor of both overall survival and disease-free survival [65]. Similarly, in patients with osteosarcoma, high FUBP1 expression correlated with a more aggressive phenotype and poor prognosis [33]. These findings underscore the clinical significance of FUBP1 as a prognostic biomarker across different cancer types, and highlight its potential for predicting patient outcomes and guiding treatment strategies.
Table 4.
The Correlation of FUBP1 to prognosis of cancer patients.
| Cancer type | Correlation to prognosis | Reference |
|---|---|---|
| Colorectal cancer | Elevated FUBP1 is positively correlated with lymph node metastasis as well as clinical stage, and negatively associated with overall survival. | [26] |
| Pancreatic cancer | FUBP1 upregulated expression is significantly associated with poor survival | [63,101] |
| Cervical cancer | Elevated FUBP1 expression is positively correlated with age, T classification, N classification, tumor recurrence, Ki67 expression, and poor prognosis in cervical carcinoma patients. Besides, elevated FUBP1 expression acta as an independent unfavorable predictor for overall survival and disease-free survival. | [64,65] |
| Lung cancer | High expression of FUBP1 is associated with shorter survival time. | [14] |
| Neuroblastoma | FUBP1 expression is negatively correlated with patient survival rate. | [35] |
| Acute myeloid leukemia | Higher FUBP1 expression is observed with a trend toward correlation with shorter overall survival. | [70] |
| Esophageal cancer | Upregulation of FUBP1 is associated with poor survival. | [73] |
| Tongue squamous cell cancer | FUBP1 expression is an independent predictor for overall survival and is closely related to poor prognosis. | [74] |
| Renal cancer | High levels of FUBP1 mRNA expression are associated with higher tumor stage and tumor size. | [78] |
| Hepatocellular cancer | High level of FUBP1 expression predicts poor prognosis after surgery. | [68] |
| Osteosarcoma | High expression of FUBP1 is correlated with a more aggressive phenotype and a poor prognosis. | [33] |
| Chordoma | FUBP1 levels is associated with the patients' local recurrence-free survival but not with the overall survival. | [72] |
| Nasopharyngeal cancer | High FUBP1 expression had a significantly poorer prognosis compared with the patients with low expression. | [75] |
Improve tumor sensitivity to anti-tumor drugs
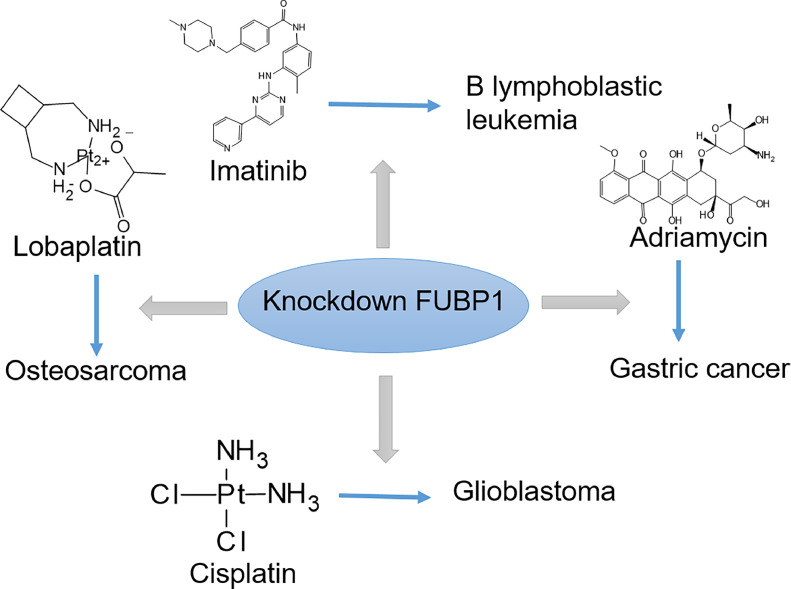
Tumor drug resistance is a phenomenon in which tumor cells become less responsive to therapeutic drugs, allowing them to survive and multiply. This resistance hinders the effectiveness of cancer treatment and lowers patient survival rates [102,103]. Recent studies have shown a strong correlation between FUBP1 and the development of drug resistance in various types of tumors [79,104]. In B lymphoblastic leukemia, FUBP1 has been found to confers resistance to the c-KIT inhibitor imatinib mesylate, a commonly used therapeutic drug [29]. Additionally, FUBP1 knockdown improves tumor sensitivity to anti-tumor drugs. In glioblastoma, silencing FUBP1 has been shown to increase the sensitivity of U251 cells to the chemotherapeutic drug DDP (cisplatin) [67]. Furthermore, in osteosarcoma, research has shown that inhibiting FUBP1 can increase the effectiveness of lobaplatin, a third-generation platinum-based anti-tumor agent, both in vivo and in vitro [33,105]. In gastric cancer, silencing FUBP1 can lower the half-inhibitory concentration of adriamycin, an antineoplastic agent effective against a wide range of malignant conditions, in the SGC7901/AR cell line and significantly enhance chemotherapy sensitivity [106]. These findings emphasize the important role of FUBP1 in the development and maintenance of drug resistance across virous types of cancer, in different tumor types, suggesting that targeting FUBP1 could be a promosing approach to overcome drug resistance and improve the efficacy of drug therapies (Fig.3).
Fig.3.
Knockdown of FUBP1 enhances the effectiveness of anti-tumor dugs, including lobaplatin in osteosarcoma, cisplatin in glioblastoma, adriamycin in gastric cancer, and imatinib in B lymphoblastic leukemia.
Conclusion and perspective
FUBP1 has emerged as a significant factor in human cancers and plays varying phenolytic roles in different tumor types. In most cancers, FUBP1 acts as an oncogene, promoting tumor progression and is correlated with higher malignancy grades and poor prognoses. Its interaction with key oncogenic pathways such as c-MYC, as well as its involvement in cell proliferation, further solidifies its role in tumorigenesis. However, recent studies have discovered FUBP1 mutations in oligodendrogliomas, suggesting a potential tumor suppressive role for this specific cancer type. Future research on FUBP1 in human cancer should prioritize understanding its precise molecular mechanisms and identifying downstream targets. Exploring the regulatory networks involving FUBP1 and its crosstalk with other oncogenic pathways could provide insights into potential therapeutic strategies. Additionally, investigating the functional implications of FUBP1 mutations in oligodendrogliomas and their effect on tumorigenesis could clarify the dual role as both an oncogene and a tumor suppressor. Furthermore, the development of specific inhibitors targeting FUBP1 shows promise for therapeutic intervention in FUBP1-dependent tumors (Table 5). Various drugs are known to inhibit FUBP1. Benzoyl anthranilic acid interacts with the hydrophobic region of the KH domain of FUBP1, leading to impaired DNA- binding [107]. Other inhibitors, including pyrazolo [1,5a] pyrimidine, compound 9, camptothecin, and its analog SN-38, hinder the binding between FUBP1 and FUSE element [[108], [109], [110]]. GSK343 acts as a competitive inhibitor of FUBP1 [111]. Additionally, there are activators of FUBP1, including SMN-C2 and SMN-C3, that can interact with the AGGAAG motif on exon 7 of SMN2 pre-mRNA to form a novel functional binding site for FUBP1, leading to the activation of SMN2 splicing [112]. Moreover, the identification of biomarkers associated with FUBP1 expression or mutations could assist in the diagnosis, prognosis, and personalized treatment of patients with cancer. Delving deeper into the intricate role of FUBP1 in human cancers presents significant opportunities to understand tumorigenesis and develop innovative therapeutic strategies.
Table 5.
Inhibitors or activators of FUBP1.
| Compound | Structure | Cancer type | Function | Reference |
|---|---|---|---|---|
| Benzoyl anthranilic Acid (Compound 1) |  |
N/A | Interacts with the hydrophobic region of the KH domain of FUBP1, leading to impaired DNA-binding ability. | [107] |
| Pyrazolo[1,5a] pyrimidine |  |
Hepatocellular carcinoma | Diminishes the binding of FUBP1 to the FUSE of p21, thereby reducing cell proliferation. | [108] |
| Camptothecin |  |
Hepatocellular carcinoma | Prevents the binding of FUBP1 to its single-stranded target DNA FUSE, and induces deregulation of FUBP1 target genes. | [109] |
| Camptothecin derivative 7-ethyl-10-hydroxycamptothecin (SN-38) |  |
Hepatocellular carcinoma | Prevents the binding of FUBP1 to its single-stranded target DNA FUSE, and induces deregulation of FUBP1 target genes. | [109] |
| Anthranilic Acid derivatives (compound 9) |  |
Pancreatic cancer | Inhibits the FUBP1-FUSE interaction, reduces both c-Myc mRNA and protein expression, increases p21 mRNA and protein expression, and depletes intracellular polyamines. | [110] |
| GSK343 |  |
Osteosarcoma | Inhibits FUBP1 protein expression, and FUBP1 could interact with EZH2. | [111] |
| SMN-C2 |  |
N/A | Increases the affinity of FUBP1 and KH-type splicing regulatory protein (KHSRP) to the SMN2 pre-mRNA complex. | [112] |
| SMN-C3 |  |
N/A | Increases the affinity of FUBP1 and KH-type splicing regulatory protein (KHSRP) to the SMN2 pre-mRNA complex. | [112] |
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Funding
This research was funded by Shaanxi Natural Science Foundation (2022JQ-923), Young Talents Fund of Association for Science and Technology in Shaanxi (20240341), Scientific and Technological Talents support Program Foundation of Shaanxi Provincial People's Hospital (2021JY-26), and Incubation Fund Program of Shaanxi Provincial People's Hospital (2023YJY-04).
CRediT authorship contribution statement
Fan Zhang: Writing – original draft, Conceptualization. Qunli Xiong: Writing – review & editing, Writing – original draft, Investigation. Min Wang: Writing – original draft, Validation. Ximing Cao: Writing – review & editing. Congya Zhou: Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
References
- 1.Briata P., Bordo D., Puppo M., Gorlero F., Rossi M., Perrone-Bizzozero N., et al. Diverse roles of the nucleic acid-binding protein KHSRP in cell differentiation and disease. Wiley. Interdiscip. Rev. RNa. 2016;7:227–240. doi: 10.1002/wrna.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung H.J., Liu J., Dundr M., Nie Z., Sanford S., Levens D. FBPs are calibrated molecular tools to adjust gene expression. Mol. Cell Biol. 2006;26:6584–6597. doi: 10.1128/MCB.00754-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan R., Collins I., Tomonaga T., Zhang T., Levens D. A unique transactivation sequence motif is found in the carboxyl-terminal domain of the single-strand-binding protein FBP. Mol. Cell Biol. 1996;16:2274–2282. doi: 10.1128/mcb.16.5.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J., Chen Q.M. Far upstream element binding protein 1: a commander of transcription, translation and beyond. Oncogene. 2013;32:2907–2916. doi: 10.1038/onc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis-Smyth T., Duncan R.C., Zheng T., Michelotti G., Levens D. The far upstream element-binding proteins comprise an ancient family of single-strand DNA-binding transactivators. J. Biol. Chem. 1996;271:31679–31687. doi: 10.1074/jbc.271.49.31679. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y., Dubois W., Benham C., Batchelor E., Levens D. FUBP1 and FUBP2 enforce distinct epigenetic setpoints for MYC expression in primary single murine cells. Commun. Biol. 2020;3:545. doi: 10.1038/s42003-020-01264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gherzi R., Lee K.Y., Briata P., Wegmuller D., Moroni C., Karin M., et al. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol. Cell. 2004;14:571–583. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Gau B.H., Chen T.M., Shih Y.H., Sun H.S. FUBP3 interacts with FGF9 3′ microsatellite and positively regulates FGF9 translation. Nucl. Acids Res. 2011;39:3582–3593. doi: 10.1093/nar/gkq1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber A., Kristiansen I., Johannsen M., Oelrich B., Scholmann K., Gunia S., et al. The FUSE binding proteins FBP1 and FBP3 are potential c-myc regulators in renal, but not in prostate and bladder cancer. BMC Cancer. 2008;8:369. doi: 10.1186/1471-2407-8-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debaize L., Troadec M.B. The master regulator FUBP1: its emerging role in normal cell function and malignant development. Cell. Mol. Life Sci. 2019;76:259–281. doi: 10.1007/s00018-018-2933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou W., Chung Y.J., Parrilla Castellar E.R., Zheng Y., Chung H.J., Bandle R., et al. Far upstream element binding protein plays a crucial role in embryonic development, hematopoiesis, and stabilizing Myc expression levels. Am. J. Pathol. 2016;186:701–715. doi: 10.1016/j.ajpath.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan R., Bazar L., Michelotti G., Tomonaga T., Krutzsch H., Avigan M., et al. A sequence-specific, single-strand binding protein activates the far upstream element of c-myc and defines a new DNA-binding motif. Genes Dev. 1994;8:465–480. doi: 10.1101/gad.8.4.465. [DOI] [PubMed] [Google Scholar]
- 13.Wang X., Xing L., Yang R., Chen H., Wang M., Jiang R., et al. The circACTN4 interacts with FUBP1 to promote tumorigenesis and progression of breast cancer by regulating the expression of proto-oncogene MYC. Mol. Cancer. 2021;20:91. doi: 10.1186/s12943-021-01383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian X., Yang J., Qiu Q., Li X., Jiang C., Li J., et al. LCAT3, a novel m6A-regulated long non-coding RNA, plays an oncogenic role in lung cancer via binding with FUBP1 to activate c-MYC. J. Hematol. Oncol. 2021;14:112. doi: 10.1186/s13045-021-01123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He L., Weber A., Levens D. Nuclear targeting determinants of the far upstream element binding protein, a c-myc transcription factor. Nucl. Acids. Res. 2000;28:4558–4565. doi: 10.1093/nar/28.22.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chien H.L., Liao C.L., Lin Y.L. FUSE binding protein 1 interacts with untranslated regions of Japanese encephalitis virus RNA and negatively regulates viral replication. J. Virol. 2011;85:4698–4706. doi: 10.1128/JVI.01950-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q., Xia W., Ji Q., Ni R., Bai J., Li L., et al. Role of far upstream element binding protein 1 in colonic epithelial disruption during dextran sulphate sodium-induced murine colitis. Int. J. Clin. Exp. Pathol. 2014;7:2019–2031. [PMC free article] [PubMed] [Google Scholar]
- 18.Jang M., Park B.C., Kang S., Chi S.W., Cho S., Chung S.J., et al. Far upstream element-binding protein-1, a novel caspase substrate, acts as a cross-talker between apoptosis and the c-myc oncogene. Oncogene. 2009;28:1529–1536. doi: 10.1038/onc.2009.11. [DOI] [PubMed] [Google Scholar]
- 19.Michelotti G.A., Michelotti E.F., Pullner A., Duncan R.C., Eick D., Levens D. Multiple single-stranded cis elements are associated with activated chromatin of the human c-myc gene in vivo. Mol. Cell Biol. 1996;16:2656–2669. doi: 10.1128/mcb.16.6.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomonaga T., Levens D. Activating transcription from single stranded DNA. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5830–5835. doi: 10.1073/pnas.93.12.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braddock D.T., Baber J.L., Levens D., Clore G.M. Molecular basis of sequence-specific single-stranded DNA recognition by KH domains: solution structure of a complex between hnRNP K KH3 and single-stranded DNA. EMBO J. 2002;21:3476–3485. doi: 10.1093/emboj/cdf352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braddock D.T., Louis J.M., Baber J.L., Levens D., Clore G.M. Structure and dynamics of KH domains from FBP bound to single-stranded DNA. Nature. 2002;415:1051–1056. doi: 10.1038/4151051a. [DOI] [PubMed] [Google Scholar]
- 23.Liu J., He L., Collins I., Ge H., Libutti D., Li J., et al. The FBP interacting repressor targets TFIIH to inhibit activated transcription. Mol. Cell. 2000;5:331–341. doi: 10.1016/s1097-2765(00)80428-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu J., Kouzine F., Nie Z., Chung H.J., Elisha-Feil Z., Weber A., et al. The FUSE/FBP/FIR/TFIIH system is a molecular machine programming a pulse of c-myc expression. EMBO J. 2006;25:2119–2130. doi: 10.1038/sj.emboj.7601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin L.R., Chung H.J., Sanford S., Kouzine F., Liu J., Levens D. Hierarchical mechanisms build the DNA-binding specificity of FUSE binding protein. Proc. Natl. Acad. Sci. U.S.A. 2008;105:18296–18301. doi: 10.1073/pnas.0803279105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin H., Gao T., Xie J., Huang Z., Zhang X., Yang F., et al. FUBP1 promotes colorectal cancer stemness and metastasis via DVL1-mediated activation of Wnt/beta-catenin signaling. Mol. Oncol. 2021;15:3490–3512. doi: 10.1002/1878-0261.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu S., Luo C., Chen D., Tang L., Cheng Q., Chen L., et al. circMMD reduction following tumor treating fields inhibits glioblastoma progression through FUBP1/FIR/DVL1 and miR-15b-5p/FZD6 signaling. J. Exp. Clin. Cancer Res. 2023;42:64. doi: 10.1186/s13046-023-02642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J., Chung H.J., Vogt M., Jin Y., Malide D., He L., et al. JTV1 co-activates FBP to induce USP29 transcription and stabilize p53 in response to oxidative stress. EMBO J. 2011;30:846–858. doi: 10.1038/emboj.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debaize L., Jakobczyk H., Avner S., Gaudichon J., Rio A.G., Serandour A.A., et al. Interplay between transcription regulators RUNX1 and FUBP1 activates an enhancer of the oncogene c-KIT and amplifies cell proliferation. Nucleic. Acids. Res. 2018;46:11214–11228. doi: 10.1093/nar/gky756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabenhorst U., Beinoraviciute-Kellner R., Brezniceanu M.L., Joos S., Devens F., Lichter P., et al. Overexpression of the far upstream element binding protein 1 in hepatocellular carcinoma is required for tumor growth. Hepatology. 2009;50:1121–1129. doi: 10.1002/hep.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaytseva O., Quinn L.M. DNA conformation regulates gene expression: the MYC promoter and beyond. Bioessays. 2018;40 doi: 10.1002/bies.201700235. [DOI] [PubMed] [Google Scholar]
- 32.Avigan M.I., Strober B. Levens D. A far upstream element stimulates c-myc expression in undifferentiated leukemia cells. J. Biol. Chem. 1990;265:18538–18545. [PubMed] [Google Scholar]
- 33.Ma Q., Sun J., Wang H., Zhou C., Li C., Wu Y., et al. Far upstream element-binding protein 1 confers lobaplatin resistance by transcriptionally activating PTGES and facilitating the arachidonic acid metabolic pathway in osteosarcoma. MedComm. 2023;4:e257. doi: 10.1002/mco2.257. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang M., Kim H.J., Kim T.J., Byun J.S., Lee J.H., Lee D.H., et al. Multiple Functions of Fubp1 in cell cycle progression and cell survival. Cells. 2020;9 doi: 10.3390/cells9061347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang P., Huang M., Qi W., Wang F., Yang T., Gao T., et al. FUBP1 promotes neuroblastoma proliferation via enhancing glycolysis-a new possible marker of malignancy for neuroblastoma. J. Exp. Clin. Cancer Res. 2019;38:400. doi: 10.1186/s13046-019-1414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc. Natl. Acad. Sci. U.S.A. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T., Kruys V., Huez G., Gueydan C. AU-rich element-mediated translational control: complexity and multiple activities of trans-activating factors. Biochem. Soc. Trans. 2002;30:952–958. doi: 10.1042/bst0300952. [DOI] [PubMed] [Google Scholar]
- 38.Pan Q., Shai O., Lee L.J., Frey B.J., Blencowe B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 39.Li H., Wang Z., Zhou X., Cheng Y., Xie Z., Manley J.L., et al. Far upstream element-binding protein 1 and RNA secondary structure both mediate second-step splicing repression. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E2687–E2695. doi: 10.1073/pnas.1310607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Will C.L., Luhrmann R. Spliceosome structure and function. Cold. Spring. Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacob A.G., Singh R.K., Mohammad F., Bebee T.W., Chandler D.S. The splicing factor FUBP1 is required for the efficient splicing of oncogene MDM2 pre-mRNA. J. Biol. Chem. 2014;289:17350–17364. doi: 10.1074/jbc.M114.554717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W., Qin J.J., Rajaei M., Li X., Yu X., Hunt C., et al. Targeting MDM2 for novel molecular therapy: beyond oncology. Med. Res. Rev. 2020;40:856–880. doi: 10.1002/med.21637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miro J., Laaref A.M., Rofidal V., Lagrafeuille R., Hem S., Thorel D., et al. FUBP1: a new protagonist in splicing regulation of the DMD gene. Nucl. Acids. Res. 2015;43:2378–2389. doi: 10.1093/nar/gkv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutandy F.X.R., Ebersberger S., Huang L., Busch A., Bach M., Kang H.S., et al. In vitro iCLIP-based modeling uncovers how the splicing factor U2AF2 relies on regulation by cofactors. Genome Res. 2018;28:699–713. doi: 10.1101/gr.229757.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng W., Shen F., Hu R., Roy B., Yang J., Wang Q., et al. Far upstream element-binding protein 1 binds the 3′ untranslated region of PKD2 and suppresses its translation. J. Am. Soc. Nephrol. 2016;27:2645–2657. doi: 10.1681/ASN.2015070836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irwin N., Baekelandt V., Goritchenko L., Benowitz L.I. Identification of two proteins that bind to a pyrimidine-rich sequence in the 3′-untranslated region of GAP-43 mRNA. Nucl. Acids Res. 1997;25:1281–1288. doi: 10.1093/nar/25.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sully G., Dean J.L., Wait R., Rawlinson L., Santalucia T., Saklatvala J., et al. Structural and functional dissection of a conserved destabilizing element of cyclo-oxygenase-2 mRNA: evidence against the involvement of AUF-1 [AU-rich element/poly(U)-binding/degradation factor-1], AUF-2, tristetraprolin, HuR (Hu antigen R) or FBP1 (far-upstream-sequence-element-binding protein 1) Biochem. J. 2004;377:629–639. doi: 10.1042/BJ20031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai W., Qu H., Zhang J., Thongkum A., Dinh T.N., Kappeler K.V., et al. Far upstream binding protein 1 (FUBP1) participates in translational regulation of Nrf2 protein under oxidative stress. Redox. Biol. 2021;41 doi: 10.1016/j.redox.2021.101906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Y., Miskimins W.K. Far upstream element binding protein 1 activates translation of p27Kip1 mRNA through its internal ribosomal entry site. Int. J. Biochem. Cell Biol. 2011;43:1641–1648. doi: 10.1016/j.biocel.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong Q., Zhang Y., Xu Y., Yang Y., Zhang Z., Zhou Y., et al. tiRNA-Val-CAC-2 interacts with FUBP1 to promote pancreatic cancer metastasis by activating c‑MYC transcription. Oncogene. 2024 doi: 10.1038/s41388-024-02991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olanich M.E., Moss B.L., Piwnica-Worms D., Townsend R.R., Weber J.D. Identification of FUSE-binding protein 1 as a regulatory mRNA-binding protein that represses nucleophosmin translation. Oncogene. 2011;30:77–86. doi: 10.1038/onc.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dixit U., Liu Z., Pandey A.K., Kothari R., Pandey V.N. Fuse binding protein antagonizes the transcription activity of tumor suppressor protein p53. BMC Cancer. 2014;14:925. doi: 10.1186/1471-2407-14-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixit U., Pandey A.K., Liu Z., Kumar S., Neiditch M.B., Klein K.M., et al. FUSE binding protein 1 facilitates persistent hepatitis C virus replication in hepatoma cells by regulating tumor suppressor p53. J. Virol. 2015;89:7905–7921. doi: 10.1128/JVI.00729-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frost J.R., Mendez M., Soriano A.M., Crisostomo L., Olanubi O., Radko S., et al. Adenovirus 5 E1A-mediated suppression of p53 via FUBP1. J. Virol. 2018:92. doi: 10.1128/JVI.00439-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim T.J., Sung J.H., Shin J.C., Kim D.Y. CRISPR/Cas-mediated Fubp1 silencing disrupts circadian oscillation of Per1 protein via downregulating Syncrip expression. Cell Biol. Int. 2020;44:424–432. doi: 10.1002/cbin.11242. [DOI] [PubMed] [Google Scholar]
- 56.Hung C.T., Kung Y.A., Li M.L., Brewer G., Lee K.M., Liu S.T., et al. Additive promotion of viral internal ribosome entry site-mediated translation by far upstream element-binding protein 1 and an enterovirus 71-induced cleavage product. PLoS. Pathog. 2016;12 doi: 10.1371/journal.ppat.1005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang P.N., Lin J.Y., Locker N., Kung Y.A., Hung C.T., Lin J.Y., et al. Far upstream element binding protein 1 binds the internal ribosomal entry site of enterovirus 71 and enhances viral translation and viral growth. Nucl. Acids Res. 2011;39:9633–9648. doi: 10.1093/nar/gkr682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabenhorst U., Thalheimer F.B., Gerlach K., Kijonka M., Bohm S., Krause D.S., et al. Single-stranded DNA-binding transcriptional regulator FUBP1 is essential for fetal and adult hematopoietic stem cell self-renewal. Cell Rep. 2015;11:1847–1855. doi: 10.1016/j.celrep.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 59.Steiner M., Schneider L., Yillah J., Gerlach K., Kuvardina O.N., Meyer A., et al. FUSE binding protein 1 (FUBP1) expression is upregulated by T-cell acute lymphocytic leukemia protein 1 (TAL1) and required for efficient erythroid differentiation. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0210515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wesely J., Steiner M., Schnutgen F., Kaulich M., Rieger M.A., Zornig M. Delayed mesoderm and erythroid differentiation of murine embryonic stem cells in the absence of the transcriptional regulator FUBP1. Stem Cells Int. 2017;2017 doi: 10.1155/2017/5762301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Chen J., Zhou N., Lu Y., Lu J., Xing X., et al. FUBP1 mediates the growth and metastasis through TGFbeta/Smad signaling in pancreatic adenocarcinoma. Int. J. Mol. Med. 2021:47. doi: 10.3892/ijmm.2021.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S., Wang Y., Li S., Nian S., Xu W., Liang F. Far upstream element -binding protein 1 (FUBP1) participates in the malignant process and glycolysis of colon cancer cells by combining with c-Myc. Bioengineered. 2022;13:12115–12126. doi: 10.1080/21655979.2022.2073115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan P., Ma J., Jin X. Far upstream element-binding protein 1 is up-regulated in pancreatic cancer and modulates immune response by increasing programmed death ligand 1. Biochem. Biophys. Res. Commun. 2018;505:830–836. doi: 10.1016/j.bbrc.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Yang B., Chen J., Teng Y. TNPO1-mediated nuclear import of FUBP1 contributes to tumor immune evasion by increasing NRP1 expression in cervical cancer. J. Immunol. Res. 2021;2021 doi: 10.1155/2021/9994004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma C., Huang Z., Wu Z., Di C., Lin X., Huang M., et al. Overexpression of FUBP1 is associated with human cervical carcinoma development and prognosis. Life Sci. 2021;269 doi: 10.1016/j.lfs.2021.119098. [DOI] [PubMed] [Google Scholar]
- 66.Yu J., Peng W., Xue Y., Li Y., Yang L., Geng Y. FUBP1 promotes the proliferation of lung squamous carcinoma cells and regulates tumor immunity through PD-L1. Allergol. Immunopathol. (Madr) 2022;50:68–74. doi: 10.15586/aei.v50i5.659. [DOI] [PubMed] [Google Scholar]
- 67.Hong Y., Shi Y., Shang C., Xue Y., Liu Y. Influence of far upstream element binding protein 1 gene on chemotherapy sensitivity in human U251 glioblastoma cells. Arch. Med. Sci. 2016;12:156–162. doi: 10.5114/aoms.2016.57592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu P.Y., Hu B., Ma X.L., Tang W.G., Yang Z.F., Sun H.X., et al. Far upstream element-binding protein 1 facilitates hepatocellular carcinoma invasion and metastasis. Carcinogenesis. 2020;41:950–960. doi: 10.1093/carcin/bgz171. [DOI] [PubMed] [Google Scholar]
- 69.Li X., Yu H., Xu F., Wu Y., Sheng J. Differentially Expressed Long Noncoding RNAs Involved in FUBP1 Promoting Hepatocellular Carcinoma Cells Proliferation. Biomed. Res. Int. 2021;2021 doi: 10.1155/2021/6664519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoang V.T., Verma D., Godavarthy P.S., Llavona P., Steiner M., Gerlach K., et al. The transcriptional regulator FUBP1 influences disease outcome in murine and human myeloid leukemia. Leukemia. 2019;33:1700–1712. doi: 10.1038/s41375-018-0358-8. [DOI] [PubMed] [Google Scholar]
- 71.Wen H., Fu Y., Zhu Y., Tao S., Shang X., Li Z., et al. Long non-coding RNA KRT8P41/miR-193a-3p/FUBP1 axis modulates the proliferation and invasion of chordoma cells. J. Bone Oncol. 2021;31 doi: 10.1016/j.jbo.2021.100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wen H., Ma H., Li P., Zheng J., Yu Y., Lv G. Expression of far upstream element-binding protein 1 correlates with c-Myc expression in sacral chordomas and is associated with tumor progression and poor prognosis. Biochem. Biophys. Res. Commun. 2017;491:1047–1054. doi: 10.1016/j.bbrc.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Yang L., Zhu J.Y., Zhang J.G., Bao B.J., Guan C.Q., Yang X.J., et al. Far upstream element-binding protein 1 (FUBP1) is a potential c-Myc regulator in esophageal squamous cell carcinoma (ESCC) and its expression promotes ESCC progression. Tumour. Biol. 2016;37:4115–4126. doi: 10.1007/s13277-015-4263-8. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y., Liu J., Geng N., Feng C. Upregulation of far upstream element-binding protein 1 (FUBP1) promotes tumor proliferation and unfavorable prognosis in tongue squamous cell carcinoma. Int. J. Biol. Markers. 2020;35:56–65. doi: 10.1177/1724600820912252. [DOI] [PubMed] [Google Scholar]
- 75.Liu Z.H., Hu J.L., Liang J.Z., Zhou A.J., Li M.Z., Yan S.M., et al. Far upstream element-binding protein 1 is a prognostic biomarker and promotes nasopharyngeal carcinoma progression. Cell Death Dis. 2015;6:e1920. doi: 10.1038/cddis.2015.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang F., Zhang Y., Wang Y. Effect of RNA Interference Inhibiting the Expression of the FUBP1 Gene on Biological Function of Gastric Cancer Cell Line SGC7901. Turk. J. Gastroenterol. 2021;32:923–931. doi: 10.5152/tjg.2020.19513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang F., Tian Q., Wang Y. Far upstream element-binding protein 1 (FUBP1) is overexpressed in human gastric cancer tissue compared to non-cancerous tissue. Onkologie. 2013;36:650–655. doi: 10.1159/000355659. [DOI] [PubMed] [Google Scholar]
- 78.Duan J., Bao X., Ma X., Zhang Y., Ni D., Wang H., et al. Upregulation of far upstream element-binding protein 1 (FUBP1) promotes tumor proliferation and tumorigenesis of clear cell renal cell carcinoma. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0169852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu W., Xiong X., Chen W., Li X., Hua X., Liu Z., et al. High expression of FUSE binding protein 1 in breast cancer stimulates cell proliferation and diminishes drug sensitivity. Int. J. Oncol. 2020;57:488–499. doi: 10.3892/ijo.2020.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Trans. Targeted Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang Y., Hong W., Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022;15:129. doi: 10.1186/s13045-022-01347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng X., Ferrell J.E., Jr. Apoptosis propagates through the cytoplasm as trigger waves. Science. 2018;361:607–612. doi: 10.1126/science.aah4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fadeel B. Programmed cell clearance. Cell. Mol. Life Sci. 2003;60:2575–2585. doi: 10.1007/s00018-003-3145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han T., Wu Y., Hu X., Chen Y., Jia W., He Q., et al. NORAD orchestrates endometrial cancer progression by sequestering FUBP1 nuclear localization to promote cell apoptosis. Cell Death Dis. 2020;11:473. doi: 10.1038/s41419-020-2674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanahan D. Hallmarks of Cancer: new Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 86.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 87.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 88.Zanotelli M.R., Zhang J., Reinhart-King C.A. Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab. 2021;33:1307–1321. doi: 10.1016/j.cmet.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pavlova N.N., Zhu J., Thompson C.B. The hallmarks of cancer metabolism: still emerging. Cell Metab. 2022;34:355–377. doi: 10.1016/j.cmet.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang M., Lee S.M., Kim W., Lee K.H., Kim D.Y. Fubp1 supports the lactate-Akt-mTOR axis through the upregulation of Hk1 and Hk2. Biochem. Biophys. Res. Commun. 2019;512:93–99. doi: 10.1016/j.bbrc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Ding Z., Liu X., Liu Y., Zhang J., Huang X., Yang X., et al. Expression of far upstream element (FUSE) binding protein 1 in human glioma is correlated with c-Myc and cell proliferation. Mol. Carcinog. 2015;54:405–415. doi: 10.1002/mc.22114. [DOI] [PubMed] [Google Scholar]
- 92.Jiao Y., Killela P.J., Reitman Z.J., Rasheed A.B., Heaphy C.M., de Wilde R.F., et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3:709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bettegowda C., Papadopoulos N., Agrawal N. Mapping genes for oligodendroglioma. Per. Med. 2012;9:311–313. doi: 10.2217/pme.12.15. [DOI] [PubMed] [Google Scholar]
- 94.Klink B., Miletic H., Stieber D., Huszthy P.C., Campos Valenzuela J.A., Balss J., et al. A novel, diffusely infiltrative xenograft model of human anaplastic oligodendroglioma with mutations in FUBP1, CIC, and IDH1. PLoS ONE. 2013;8:e59773. doi: 10.1371/journal.pone.0059773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu G., Bu C., Guo G., Zhang Z., Sheng Z., Deng K., et al. Genomic alterations of oligodendrogliomas at distant recurrence. Cancer Med. 2023;12:17171–17183. doi: 10.1002/cam4.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Labreche K., Simeonova I., Kamoun A., Gleize V., Chubb D., Letouze E., et al. TCF12 is mutated in anaplastic oligodendroglioma. Nat. Commun. 2015;6:7207. doi: 10.1038/ncomms8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bettegowda C., Agrawal N., Jiao Y., Sausen M., Wood L.D., Hruban R.H., et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333:1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baumgarten P., Harter P.N., Tonjes M., Capper D., Blank A.E., Sahm F., et al. Loss of FUBP1 expression in gliomas predicts FUBP1 mutation and is associated with oligodendroglial differentiation, IDH1 mutation and 1p/19q loss of heterozygosity. Neuropathol. Appl. Neurobiol. 2014;40:205–216. doi: 10.1111/nan.12088. [DOI] [PubMed] [Google Scholar]
- 99.Hwang I., Cao D., Na Y., Kim D.Y., Zhang T., Yao J., et al. Far upstream element-binding protein 1 regulates LSD1 alternative splicing to promote terminal differentiation of neural progenitors. Stem Cell Rep. 2018;10:1208–1221. doi: 10.1016/j.stemcr.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klener P., Fronkova E., Berkova A., Jaksa R., Lhotska H., Forsterova K., et al. Mantle cell lymphoma-variant Richter syndrome: detailed molecular-cytogenetic and backtracking analysis reveals slow evolution of a pre-MCL clone in parallel with CLL over several years. Int. J. Cancer. 2016;139:2252–2260. doi: 10.1002/ijc.30263. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y., Chen J., Zhou N., Lu Y., Lu J., Xing X., et al. FUBP1 mediates the growth and metastasis through TGFβ/Smad signaling in pancreatic adenocarcinoma. Int. J. Mol. Med. 2021:47. doi: 10.3892/ijmm.2021.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vasan N., Baselga J., Hyman D.M. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X., Jiang W., Du Y., Zhu D., Zhang J., Fang C., et al. Targeting feedback activation of signaling transduction pathways to overcome drug resistance in cancer. Drug Resist. Updat. 2022;65 doi: 10.1016/j.drup.2022.100884. [DOI] [PubMed] [Google Scholar]
- 104.Venturutti L., Cordo Russo R.I., Rivas M.A., Mercogliano M.F., Izzo F., Oakley R.H., et al. MiR-16 mediates trastuzumab and lapatinib response in ErbB-2-positive breast and gastric cancer via its novel targets CCNJ and FUBP1. Oncogene. 2016;35:6189–6202. doi: 10.1038/onc.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oude Munnink T., van der Meer A., de Haan J., Touw D., van Kruchten M. Reversible impaired methotrexate clearance after platinum-based chemotherapy for osteosarcoma. Ther. Drug Monit. 2019;41:693–695. doi: 10.1097/FTD.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 106.Zhao D., Zhang Y., Song L. MiR-16-1 targeted silences far upstream element binding protein 1 to advance the chemosensitivity to adriamycin in gastric cancer. Pathol. Oncol. Res. 2018;24:483–488. doi: 10.1007/s12253-017-0263-x. [DOI] [PubMed] [Google Scholar]
- 107.Huth J.R., Yu L., Collins I., Mack J., Mendoza R., Isaac B., et al. NMR-driven discovery of benzoylanthranilic acid inhibitors of far upstream element binding protein binding to the human oncogene c-myc promoter. J. Med. Chem. 2004;47:4851–4857. doi: 10.1021/jm0497803. [DOI] [PubMed] [Google Scholar]
- 108.Hauck S., Hiesinger K., Khageh Hosseini S., Achenbach J., Biondi R.M., Proschak E., et al. Pyrazolo[1,5a]pyrimidines as a new class of FUSE binding protein 1 (FUBP1) inhibitors. Bioorg. Med. Chem. 2016;24:5717–5729. doi: 10.1016/j.bmc.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 109.Khageh Hosseini S., Kolterer S., Steiner M., von Manstein V., Gerlach K., Trojan J., et al. Camptothecin and its analog SN-38, the active metabolite of irinotecan, inhibit binding of the transcriptional regulator and oncoprotein FUBP1 to its DNA target sequence FUSE. Biochem. Pharmacol. 2017;146:53–62. doi: 10.1016/j.bcp.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 110.Dobrovolskaite A., Moots H., Tantak M.P., Shah K., Thomas J., Dinara S., et al. Discovery of anthranilic acid derivatives as difluoromethylornithine adjunct agents that inhibit far upstream element binding protein 1 (FUBP1) function. J. Med. Chem. 2022;65:15391–15415. doi: 10.1021/acs.jmedchem.2c01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiong X., Zhang J., Liang W., Cao W., Qin S., Dai L., et al. Fuse-binding protein 1 is a target of the EZH2 inhibitor GSK343, in osteosarcoma cells. Int. J. Oncol. 2016;49:623–628. doi: 10.3892/ijo.2016.3541. [DOI] [PubMed] [Google Scholar]
- 112.Wang J., Schultz P.G., Johnson K.A. Mechanistic studies of a small-molecule modulator of SMN2 splicing. Proc. Natl. Acad. Sci. U.S.A. 2018;115:E4604–E4E12. doi: 10.1073/pnas.1800260115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.