Abstract
S-nitrosothiols (RSNOs) regulate several aspects of platelet physiology including inhibition of activation, adhesion and aggregation. PDI (protein disulphide-isomerase) has recently been found to be localized to the cell surface, where it exhibits both disulphide-exchange and denitrosation activities. The disulphide-exchange activity of PDI has been linked to aspects of platelet aggregation. The present study suggests that the metabolism of RSNOs by platelets is a function of PDI denitrosation activity. Exposure of washed human platelets to increasing concentrations of GSNO (S-nitrosoglutathione) resulted in saturable denitrosation kinetics. The presence of known PDI inhibitors phenylarsine oxide and anti-PDI antibodies prevented GSNO denitrosation. The fact that, in the presence of GSNO plus the cell-permeable guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxaline-1-one, the initial rates of ADP-induced platelet aggregation and the maximum ΔOD were diminished by ∼40% shows that RSNOs have dual inhibitory effects on platelets, which are mediated through PDI. First, PDI denitrosates RSNOs, releasing NO that, via the guanylate cyclase/G-kinase route, attenuates platelet activation. Secondly, RSNOs are denitrosated at the same PDI-active site that catalyses the disulphide bond formation between integrins and their ligands, thereby attenuating irreversible aggregation.
Keywords: cell-surface protein disulphide-isomerase, disulphide exchange, denitrosation, platelet, S-nitrosoglutathione
Abbreviations: ACD, acid citrate dextrose; GC, guanylate cyclase; GSNO, S-nitrosoglutathione; ODQ, 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxaline-1-one; PAO, phenylarsine oxide; PDI, protein disulphide-isomerase; csPDI, cell-surface PDI; RSNO, S-nitrosothiol; SNPL, S-nitroso-DL-penicilamine
INTRODUCTION
Protein disulphide-isomerase (PDI) is a homodimeric protein widely distributed across eukaryotic tissues, making up ∼1% of the total protein content of cells [1]. One of the most studied functions of PDI is its ability to catalyse isomerization and rearrangement of disulphide bonds in the endoplasmic reticulum [2]. In recent years, PDI has been found localized on the surface of mammalian cells and platelets and is termed csPDI (cell-surface PDI) [3,4].
The role of csPDI in platelet physiology was first established by Chen et al. [5]. They observed that PDI mediated the cell adhesion properties of thrombospondin through the intramolecular isomerization of its disulphide bonds [5]. Subsequent work by Essex et al. [6,7] and Hogg and co-workers [8] used thiol alkylating agents and inhibitors of PDI to prevent platelet activation and aggregation. Recent studies have identified two distinct activities for csPDI.
The first activity involves thiol-disulphide-exchange activity. Lahav et al. [9–11] showed that csPDI catalyses disulphide bond formation between integrins and their ligands, thus promoting covalently linked adhesion of platelets to other platelets and to other vascular cells. The second activity of csPDI, termed denitrosation, is catalysed by the same active-site thiols involved in the thiol-disulphide-exchange activity of the enzyme [12–14]. In this process, csPDI catalyses the release of NO (nitric oxide) from RSNOs (S-nitrosothiols).
RSNOs are involved in many physiological processes including neurotransmission, smooth-muscle relaxation, non-specific immune response and inhibition of platelet activation and aggregation [15]. NO inhibits platelet activation through activation of GC (guanylate cyclase)/G-kinase resulting in a reduction in agonist-induced [Ca2+]i. Myosin light chain phosphorylation is then inhibited, decreasing the ability to aggregate [16].
Previous research has linked the inhibition of csPDI to the inhibition of platelet aggregation [9–11]. Furthermore, RSNOs have also been shown to inhibit platelet aggregation [17,18]. In the present study, we show that csPDI plays a dual role in the RSNO-dependent inhibition of platelet aggregation.
MATERIALS AND METHODS
Preparation of GSNO (S-nitrosoglutathione)
GSNO was synthesized by first determining [free thiol] in the GSH with Ellman's reagent [19]. A stoichiometric amount of acidified NaNO2 was reacted for 30 min at 4 °C. The pH of the solution was then adjusted to 7.4. The NO2− contamination in 1 mM GSNO prepared in this manner was determined by the Griess reagent to be 4.8 μM [20]. Since the highest [GSNO] utilized in these studies was 0.1 mM, the maximum NO2− contamination would be ∼0.5 μM, which is ∼10-fold lower than that reported in human serum [NO2−] (5–20 μM) [21]. GSNO was then recrystallized by the slow addition of ice-cold acetone, which resulted in [NO2−] levels that were below the detection limit of the Griess assay.
Purification of PDI
PDI was isolated from two separate sources. First, PDI was purified from bovine liver as described by Hillson et al. [22]. Secondly, recombinant human PDI was expressed using Escherichia coli strain BL21(DE3) and expression vector pET-28a. This plasmid encodes a fusion protein containing the entire human PDI sequence with an N-terminal His6 tag [23]. Recombinant PDI was purified from the soluble fraction of the cell lysate using Ni-CAM™ HC Resin (Sigma), which is a high-capacity nickel-affinity matrix. Bound PDI was eluted using 250 mM imidazole in 50 mM Tris/HCl (pH 8.0) and collected in 2.0 ml fractions. The fractions containing PDI were pooled and dialysed against 0.1 M potassium phosphate buffer (pH 7.0). Protein quantification was performed using the Bradford assay [24].
PDI disulphide-exchange assay
PDI thiol-disulphide-exchange activity was monitored using the turbidimetric assay of insulin disulphide reduction [25]. Briefly, 0.25 μM purified bovine PDI was added to a solution of insulin (0–250 μM) and GSH (500 μM) in a 0.1 M K2HPO4 buffer containing 2 mM EDTA (pH 7.0). The increase in turbidity was monitored (λ=630) at 30 s intervals for 30 min. This assay was repeated in the presence of known PDI inhibitors, 200 μM PAO (phenylarsine oxide) and 20 μg/ml anti-PDI antibodies [10,26]. The disulphide-exchange activity of bovine PDI was then challenged by the presence of GSNO (0–625 μM). To demonstrate the similarity between human and bovine PDI, the disulphide-exchange activity of 0.25 μM recombinant human PDI was also monitored using the insulin turbidity assay in the presence of increasing concentrations of GSNO (0–625 μM).
Platelet isolation
Suspensions of washed human platelets were obtained by the method of Mustard et al. [27]. Briefly, samples of peripheral venous blood were mixed 6:1 with ACD (acid citrate dextrose; 25 g/l trisodium citrate dihydrate, 15 g/l citric acid monohydrate and 20 g/l dextrose). Whole blood was centrifuged (15 min at 190 g at 37 °C) to yield platelet-rich plasma. Platelets were isolated by centrifugation (15 min at 2000 g at 37 °C) and washed three times in Tyrode-albumin solution (pH 7.4). The first wash contained heparin (2 units/ml) and apyrase (1 unit/ml); the second only apyrase (1 unit/ml); and the third wash contained Tyrode's solution without apyrase and heparin. Platelets were quantified using a haemocytometer.
GSNO consumption by platelets
The consumption of GSNO (0–250 μM) by washed human platelets was monitored first spectrophotometrically using a 96-well plate reader [28]. The decrease in absorbance (λ=340 nm) was monitored in the presence of 2×106 platelets/ml. The role of platelet csPDI in GSNO degradation was assessed by treating platelets with the PDI inhibitors 200 μM PAO and 20 μg/ml anti-PDI antibodies for 30 min before the addition of GSNO [10,26]. Denitrosation was also monitored by using ISO-NO Mark II equipped with a WPI MKII NO electrode. The addition of 500 μM CuSO4 to known concentrations of GSNO (0–250 μM) was used to generate a standard curve to quantify the release of NO [29]. Solutions of washed human platelets (2×106 ml−1) were prepared in ACD buffer. The NO electrode was immersed in ACD buffer containing increasing concentrations of GSNO (0–250 μM), and the NO release was initiated by the addition of platelets. The role of platelet csPDI was assessed by incubating platelets for 30 min with 20 μg/ml anti-PDI antibodies and monitoring the amount of NO released in the presence of 40 μM GSNO. As a control, the amount of NO released from 40 μM GSNO by platelets treated for 30 min with 20 μg/ml non-PDI-specific IgG was also monitored.
Platelet aggregation studies
Samples of washed human platelets were prepared in Tyrode's solution (10×108 ml−1). Aggregation was induced with 2.5 μM ADP and monitored spectrophotometrically in a 96-well plate at 630 nm for loss of turbidity [30]. Platelet aggregation was monitored in the presence of PDI inhibitors PAO (200 μM), anti-PDI antibodies (20 μg/ml) as well as GSNO (20, 40 and 100 μM) [10,21]. Platelets (10×107 ml−1) were also treated with 2.5 μM of the GC inhibitor ODQ (1H-[1,2,4]oxadiazolo-[4,3-a]quinoxaline-1-one) [31]. After 30 min of incubation, aggregation was induced using 2.5 μM ADP and monitored in the presence of 100 μM GSNO.
RESULTS
Disulphide-exchange activity of PDI
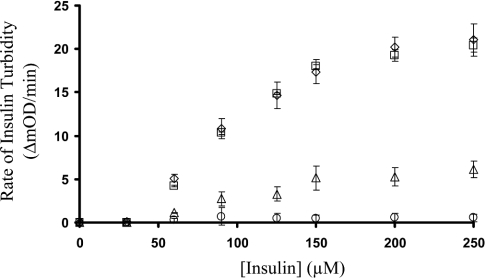
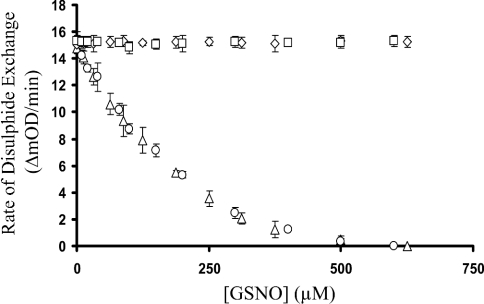
The disulphide-exchange activity of 0.25 μM PDI first monitored using the insulin turbidity assay resulted in a KM of 65 μM (Figure 1). Repeating this assay in the presence of 200 μM PAO resulted in complete loss of disulphide-exchange activity. The presence of 20 μg/ml anti-PDI antibodies resulted in ∼75% loss of activity, whereas the use of 20 μg/ml non-PDI-specific IgG caused no inhibition of disulphide exchange (Figure 1). The presence of increasing concentrations (1–600 μM) of GSNO with 0.25 μM PDI, 65 μM insulin and 500 μM GSH resulted in a concentration-dependent inhibition with an apparent Ki of 120 μM. Bovine PDI and recombinant human PDI exhibited identical disulphide-exchange activities and inhibition by GSNO (Figure 2).
Figure 1. Inhibition of PDI-catalysed insulin turbidity assay.
The disulphide-exchange activity of 0.25 μM PDI monitored in the presence of 0–250 μM insulin and 500 μM GSH resulted in a KM of 65 μM (⋄). Repeating this assay in the presence of 200 μM PAO resulted in the complete loss of disulphide-exchange activity (○). The presence of 20 μg/ml anti-PDI antibodies resulted in ∼75% decrease in activity (▵), whereas the presence of 20 μg/ml IgG (□) had no effect on the disulphide-exchange activity of PDI. The results are the average (n=8) and the error bars represent S.D.
Figure 2. GSNO inhibition of PDI-catalysed disulphide exchange.
The initial rates of PDI disulphide-exchange activity were monitored using bovine PDI (▵) as well as recombinant human PDI (○) in the presence of increasing concentrations (1–625 μM) of GSNO. In both cases, the disulphide-exchange activity of PDI was inhibited with an apparent Ki=120 μM. The presence of increasing concentrations (1–625 μM) of GSH as a control had no effect on the disulphide activity of bovine PDI (⋄) as well as the recombinant human PDI (□). The error bars represent S.D. (n=8).
GSNO consumption by platelets
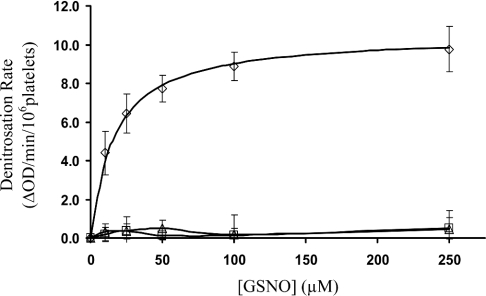
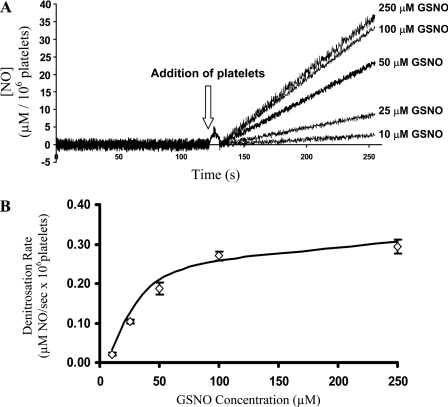
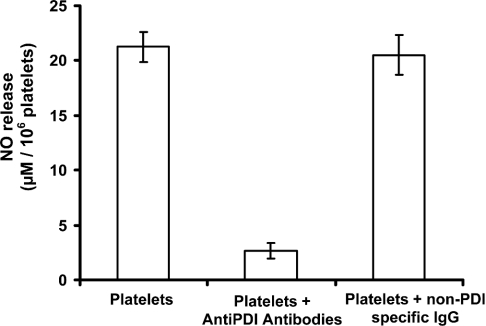
The consumption of GSNO by human platelets was first monitored spectrophotometrically (λ=340 nm, loss of S–NO absorbance) using a 96-well plate reader. The initial rates of S–NO bond loss displayed saturation kinetics with an apparent KM=16.5±3.8 μM (Figure 3). GSNO consumption was completely inhibited by the presence of known PDI inhibitors PAO [26] and anti-PDI antibodies [10], indicating that PDI is responsible for the observed denitrosation activity (Figure 3). To determine whether the product of csPDI-dependent GSNO denitrosation was NO, the GSNO platelet reaction was monitored with an NO electrode. The electrode was placed in an ACD buffer containing increasing [GSNO] (0–250 μM). There was no appreciable NO produced even at the highest [GSNO]. After the addition of platelets to the GSNO solutions, NO was released (Figure 4A). The initial rates of NO production as a function of [GSNO] once again displayed saturation kinetics with an apparent KM=22.5±1.2 μM (Figure 4B). Incubating platelets with anti-PDI antibodies for 30 min before the addition of 40 μM GSNO resulted in 85% reduction in the amount of NO released, whereas platelets incubated with non-PDI-specific IgG had no reduction in the amount of NO released (Figure 5).
Figure 3. GSNO consumption by platelets.
The consumption of GSNO (0–250 μM) by washed human platelets displayed saturation kinetics with an apparent KM=16.5±3.8 μM (⋄). The initial rates of denitrosation were completely inhibited by the presence of known PDI inhibitors PAO (▵) and anti-PDI antibodies (□). The error bars represent S.D. (n=4).
Figure 4. GSNO consumption by platelets.
In the absence of platelets for the first 120 s, there was no appreciable amount of NO released from the GSNO solutions (10–250 μM) as evident by the overlaid traces with approx. zero slope. The initial rate of NO release subsequent to the addition of platelets (arrow) to ACD buffers containing increasing concentrations of GSNO (0–250 μM) was monitored (130–250 s) and used for KM determination (A). The actual [NO] was obtained from the standard curve as described in the Materials and methods section. (B) GSNO consumption by platelets. The consumption of GSNO (0–250 μM) by washed human platelets determined by the use of an NO meter displayed saturation kinetics with an apparent KM=22.5±1.2 μM (⋄). The results are the average (n=4) and the error bars represent S.D.
Figure 5. Inhibition of denitrosation via anti-PDI antibodies.
Denitrosation of 40 μM GSNO by washed human platelets was monitored using an NO electrode. The release of NO by washed human platelets was inhibited ∼90% after 30 min incubation with anti-PDI antibodies. Incubation with non-PDI-specific IgG had no effect on the amount of NO released. NO release from GSNO in the absence of platelets was insignificant (see Figure 4A). The error bars represent S.D. (n=4).
Platelet aggregation studies
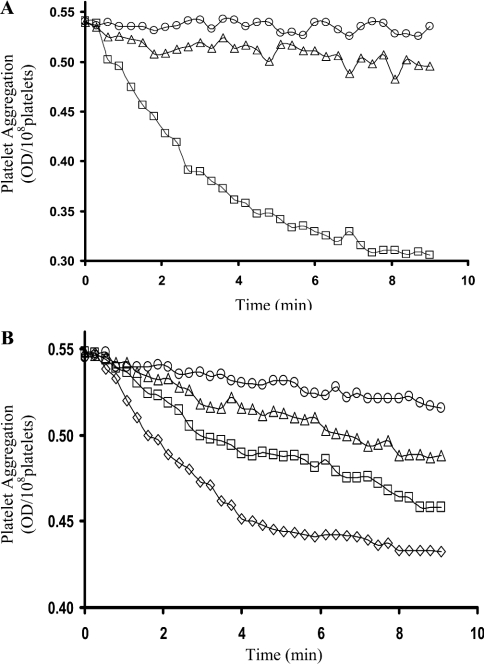
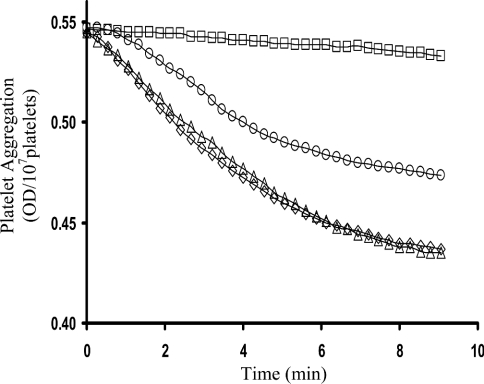
Platelet aggregation was induced using 2.5 μM ADP. The initial rate of aggregation was 98% inhibited after a treatment with 200 μM PAO and 92% inhibited with 20 μg/ml anti-PDI antibodies (Figure 6A). Furthermore, the presence of 20, 40 and 100 μM GSNO resulted in 38, 56 and 80% inhibition of platelet aggregation respectively (Figure 6B). GSNO could be inhibiting platelet aggregation in, at least, two ways: NO produced from PDI-dependent GSNO denitrosation could inhibit platelet activation via the GC/G-kinase route [16]; alternatively, GSNO could act as a competitive inhibitor of PDI-catalysed disulphide-bond formation between integrins and their ligands [9–11]. To test this, ADP-induced platelet aggregation was performed in the presence of GSNO±ODQ, a cell-permeable GC inhibitor [31] (Figure 7). In the absence of GSNO, ODQ had no effect on aggregation. In the presence of GSNO (100 μM), aggregation was totally inhibited. After inclusion of ODQ and elimination of the GC/G-kinase route, both the initial rates of aggregation and the total ΔOD decreased by ∼40%, indicating that GSNO can also inhibit platelets via the PDI-mediated integrin-disulphide-exchange route.
Figure 6. Inhibition of platelet aggregation.
(A) Aggregation of washed human platelets was induced using 2.5 μM ADP (□). Incubation of platelets with PAO (○) or anti-PDI antibodies (▵) resulted in 98 and 92% inhibition of the initial rate of aggregation respectively. (B) Inhibition of platelet aggregation with GSNO. Platelet aggregation was induced using 2.5 μM ADP (□). No GSNO (⋄), 20 μM GSNO (□), 40 μM GSNO (▵) and 100 μM GSNO (○) resulted in 38, 56 and 80% inhibition of the initial rates of platelet aggregation respectively.
Figure 7. Platelet aggregation in the presence of GSNO and ODQ.
Platelet aggregation was induced using 2.5 μM ADP (⋄). In the absence of GSNO, 2.5 μM ODQ had no effect on aggregation (▵). In the presence of GSNO (100 μM), aggregation was totally inhibited (□). On inclusion of ODQ and GSNO, both the initial rates of aggregation and the total ΔOD decreased by ∼40% (○).
DISCUSSION
Results from the present study support findings from a number of previous studies involving platelet aggregation, csPDI and denitrosation. It has been shown that platelets have the ability to metabolize GSNO [32]. Other studies have shown that inhibiting PDI disulphide-exchange activity resulted in complete inhibition of platelet aggregation [9–11]. In addition, results from this study coincide with the preceding work, which identified the ability of GSNO to prevent platelet aggregation [17,18]. It is well established that NO inhibits platelet aggregation through a GC/G-kinase-dependent process [16]. Our results show that the denitrosation activity of platelets exhibits saturation kinetics indicative of an enzyme-mediated process. The inhibition of this activity through the use of both anti-PDI antibodies and PAO identifies the participation of PDI in this process. PAO is a small tervalent arsenical that selectively binds to proteins containing vicinal dithiols such as those found in the PDI active site [26,33]. This study also indicates that RSNOs can inhibit platelet activation via two PDI-mediated routes. As shown here, RSNOs are a substrate for csPDI. The product of this reaction is NO, which can prevent aggregation through the GC/G-kinase route. Furthermore, since in the presence of extracellular RSNOs, the PDI-active site is occupied by the denitrosation reaction, PDI cannot participate in integrin–ligand disulphide formation which leads to irreversible aggregation. Here, we showed this by performing the platelet aggregation in the presence of ODQ, a soluble GC inhibitor, plus GSNO. GSNO (100 μM) totally inhibited platelet aggregation. On the other hand, in the presence of GSNO (100 μM) plus ODQ (2.5 μM), the aggregation was inhibited by ∼40%. At the concentration of GSNO employed, csPDI active sites would be ∼80% saturated with GSNO (KM, GSNO∼20 μM) assuming that csPDI has a similar KM for integrins and their ligands on the platelet surface, ∼40% inhibition of aggregation should result. This is in contrast with the results obtained by Moro et al. [31], where ODQ plus SNPL (S-nitroso-DL-penicillamine; 3 μM) totally reversed inhibitory effect of the NO donor. The different results obtained in the present study and by Moro et al. are supposed to be dependent on the RSNO concentrations employed and also on the relative stabilities of GSNO and SNPL. In the Moro et al. study, 3 μM SNPL was employed. Assuming that SNPL and GSNO have similar KM values, the csPDI active site would only be 13% saturated. Once again, in the presence of competing integrin–ligand, the SNPL saturation in the active site would be ∼4%. In addition, GSNO and SNPL have decomposition half-lives of 2.7 h and 13.7 min respectively [34]. As a result, the effective [SNPL] is probably much lower than 3 μM in the Moro et al. study.
The significance of the present study can be summarized as follows: we have shown that platelet csPDI catalyses the denitrosation of GSNO. The product of this reaction is NO. Our results also indicate that platelet csPDI-dependent metabolism of GSNO inhibits platelets by two pathways: first, the NO released by PDI-dependent denitrosation of GSNO inhibits platelets via the GC/G-kinase route; in the second pathway, GSNO acts as a competitive inhibitor of the PDI-dependent integrin–ligand disulphide formation, thus attenuating irreversible platelet activation.
Platelet csPDI may also be involved in the release of NO from low-molecular-mass RSNOs in NO-producing cells (endothelial) [35] and NO storage cells (RBCs) [36]. These possibilities are currently under investigation.
Acknowledgments
This project was supported by a grant in aid from the Canadian Diabetes Association (CDA) and a discovery grant from Natural Sciences and Engineering Research Council (NSERC) to B.M. Equipment used in these studies were purchased with grants from the Canada Foundation for Innovation and the Ontario Innovation Trust.
References
- 1.Goldberg R. F., Epstein C. J., Anfinsen C. B. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J. Biol. Chem. 1963;238:628–635. [PubMed] [Google Scholar]
- 2.Noiva R., Lennarz W. J. Protein disulphide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum. J. Biol. Chem. 1992;267:3553–3556. [PubMed] [Google Scholar]
- 3.Chen K., Detwiler T. C., Essex D. W. Characterization of protein disulphide isomerase released from activated platelets. Br. J. Haematol. 1995;90:425–431. doi: 10.1111/j.1365-2141.1995.tb05169.x. [DOI] [PubMed] [Google Scholar]
- 4.Essex D. W., Chen K., Swiatowska M. Localization of protein disulphide isomerase to the external surface of the platelet plasma membrane. Blood. 1995;86:2168–2173. [PubMed] [Google Scholar]
- 5.Chen K., Lin Y., Detwiler T. C. Protein disulfide isomerase activity is released by activated platelets. Blood. 1992;79:2226–2228. [PubMed] [Google Scholar]
- 6.Essex D. W., Li M. A polyclonal antibody to protein disulphide isomerase induces platelet aggregation and secretion. Thromb. Res. 1999;96:445–450. doi: 10.1016/s0049-3848(99)00133-4. [DOI] [PubMed] [Google Scholar]
- 7.Essex D. W., Li M., Miller A., Feinman R. D. Protein disulphide isomerase and sulfhydryl-dependent pathways in platelet activation. Biochemistry. 2001;40:6070–6075. doi: 10.1021/bi002454e. [DOI] [PubMed] [Google Scholar]
- 8.Burgess J. K., Hotchkiss K. A., Suter C., Dudman N. P., Szollosi J., Chesterman C. N., Chong B. H., Hogg P. J. Physical proximity and functional association of glycoprotein 1bα and protein-disulfide isomerase on the platelet plasma membrane. J. Biol. Chem. 2000;275:9758–9766. doi: 10.1074/jbc.275.13.9758. [DOI] [PubMed] [Google Scholar]
- 9.Lahav J., Gofer-Dadosh N., Luboshitz J., Hess O., Shaklai M. Protein disulphide isomerase mediates integrin-dependent adhesion. FEBS Lett. 2000;475:89–92. doi: 10.1016/s0014-5793(00)01630-6. [DOI] [PubMed] [Google Scholar]
- 10.Lahav J., Jurk K., Hess O., Barnes M. J., Farndale R. W., Luboshitz J., Kehrel B. E. Sustained integrin ligation involves extracellular free sulfhydryls and enzymatically catalyzed disulphide exchange. Blood. 2002;100:2472–2478. doi: 10.1182/blood-2001-12-0339. [DOI] [PubMed] [Google Scholar]
- 11.Lahav J., Wijnen E. M., Hess O., Hamaia S. W., Griffiths D., Makris M., Knight C. G., Essex D. W., Farndale R. W. Enzymatically catalyzed disulfide exchange is required for platelet adhesion to collagen via integrin α2β1. Blood. 2003;102:2085–2092. doi: 10.1182/blood-2002-06-1646. [DOI] [PubMed] [Google Scholar]
- 12.Zai A., Rudd M. A., Scribner A. W., Loscalzo J. Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J. Clin. Invest. 1999;103:393–399. doi: 10.1172/JCI4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran N., Root P., Jiang X. M., Hogg P. J., Mutus B. Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide isomerase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9539–9544. doi: 10.1073/pnas.171180998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raturi A., Mutus B. Use of 2,3 di-aminonapthalene for studying denitrosation activity of protein disulfide isomerase. Anal. Biochem. 2004;326:281–283. doi: 10.1016/j.ab.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Upchurch G. R., Welch G. N., Loscalzo J. S-nitrosothiols: chemistry, biochemistry, and biological actions. Adv. Pharmacol. 1995;34:343–349. doi: 10.1016/s1054-3589(08)61096-0. [DOI] [PubMed] [Google Scholar]
- 16.Lincoln T. M., Cornwell L. T., Taylor A. E. cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells. Am. J. Physiol. 1990;258:C399–C407. doi: 10.1152/ajpcell.1990.258.3.C399. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann F., Ammendola A., Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J. Cell Sci. 2000;113:1671–1676. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- 18.Wang G. R., Zhu Y., Halushka P. V., Lincoln T. M., Mendelsohn M. E. Mechanism of platelet inhibition by nitric oxide: in vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4888–4893. doi: 10.1073/pnas.95.9.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddles P. W., Blakeley R. L., Zerner B. Ellman's reagent: 5,5′-dithiobis(2-nitrobenzoic acid) – a reexamination. Anal. Biochem. 1979;94:75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- 20.Munoz-Fuentes R. M., Vargas F., Bobadilla N. A. Assay validation for determining nitrites and nitrates in biological fluids. Rev. Invest. Clin. 2003;55:670–676. [PubMed] [Google Scholar]
- 21.Pinto P. C., Lima J. L., de Sousa Saraiva M. L. Sequential injection analysis of nitrites and nitrates in human serum using nitrate reductase. Clin. Chim. Acta. 2003;337:69–76. doi: 10.1016/j.cccn.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Hillson D. A., Lambert N., Freedman R. B. Formation and isomerization of disulfide-bonds in proteins: protein disulfide isomerase. Methods Enzymol. 1984;107:281–294. doi: 10.1016/0076-6879(84)07018-x. [DOI] [PubMed] [Google Scholar]
- 23.Pihlajaniemi T., Helaakoski T., Tasanen K., Myllyla R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the β-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987;6:643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 1979;254:9627–9632. [PubMed] [Google Scholar]
- 26.Bennett T., Edwards B., Sklar L., Rogelj S. Sulfhydryl regulation of L-selectin shedding: phenylarsine oxide promotes activation-independent L-selectin shedding from leukocytes. J. Immunol. 2000;164:4120–4129. doi: 10.4049/jimmunol.164.8.4120. [DOI] [PubMed] [Google Scholar]
- 27.Mustard J. F., Kinlough-Rathborne R. L., Packham M. A. Isolation of human platelets from plasma by centrifugation and washing. Methods Enzymol. 1989;169:3–11. doi: 10.1016/0076-6879(89)69045-3. [DOI] [PubMed] [Google Scholar]
- 28.Cook J. A., Kim S. Y., Teague D., Krishna M. C., Pacelli R., Mitchell J. B., Vodovotz Y., Nims R. W., Christodoulou D., Miles A. M., et al. Convenient colorimetric and fluorometric assays for S-nitrosothiols. Anal. Biochem. 1996;238:150–158. doi: 10.1006/abio.1996.0268. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer S., Schrammel A., Schmidt K., Mayer B. Electrochemical determination of S-nitrosothiols with a Clark-type nitric oxide electrode. Anal. Biochem. 1998;258:68–73. doi: 10.1006/abio.1998.2562. [DOI] [PubMed] [Google Scholar]
- 30.Krause S., Scholz T., Temmler U., Losche W. Monitoring the effects of platelet glycoprotein IIb/IIIa antagonists with a microtiter plate method for detection of platelet aggregation. Platelets. 2001;12:423–430. doi: 10.1080/09537100120071040. [DOI] [PubMed] [Google Scholar]
- 31.Moro M. A., Russel R. J., Cellek S., Lizasoain I., Su Y., Darley-Usmar V. M., Radomski M. W., Moncada S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah C. M., Locke I. C., Chowdrey H. S., Gordge M. P. Rapid S-nitrosothiol metabolism by platelets and megakaryocytes. Biochem. Soc. Trans. 2003;31:1450–1452. doi: 10.1042/bst0311450. [DOI] [PubMed] [Google Scholar]
- 33.Donoghue N., Yam P. T. W., Jiang X., Hogg P. J. Presence of closely spaced protein thiols on the surface of mammalian cells. Protein Sci. 2000;9:2436–2445. doi: 10.1110/ps.9.12.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnelle D. R., Stamler J. S. NO+, NO, and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 35.Govers R., Bevers L., de Bree P., Rabelink T. J. Endothelial nitric oxide synthase activity is linked to its presence at cell–cell contacts. Biochem. J. 2002;361:193–201. doi: 10.1042/0264-6021:3610193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawloski J. R., Stamler J. S. Nitric oxide in RBCs. Transfusion. 2002;42:1603–1609. doi: 10.1046/j.1537-2995.2002.00278.x. [DOI] [PubMed] [Google Scholar]