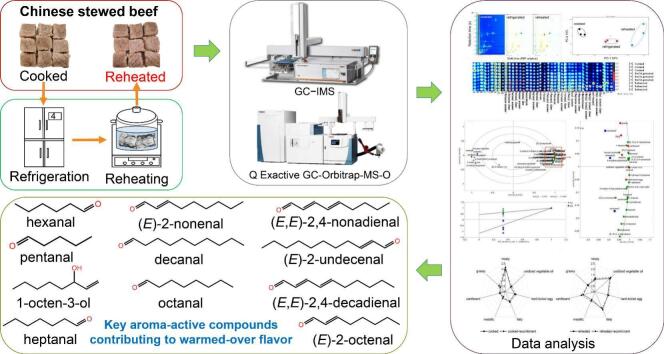
Abstract
The key odorants contributing to the warmed-over flavor (WOF) of reheated precooked stewed beef were characterized using a sensomics approach. Overall, 36 odorants were identified, and based on flavor dilution factors, odor activity values, aroma recombination, and omission test, 11 compounds mainly derived from lipid oxidation were characterized as the key odorants contributing to the formation of WOF. In particular, 3-(methylthio)propanal, which was positively correlated with meaty aroma, was implicated in an overall increase in WOF. Thus, these odorants were elected as potential markers of WOF in the reheated precooked stewed beef. In summary, the WOF of the precooked stewed beef could be attributed to an overall increase in lipid oxidation products and a decrease in the odorants with desirable aromas. The characterization of WOF in precooked stewed beef will aid in the flavor quality control of precooked stewed beef dishes.
Keywords: Precooked stewed beef, Warmed-over flavor, Key aroma-active compounds, Sensomics approach, Lipid oxidation, Interaction
Graphical abstract
Highlights
-
•
Key aroma-active compounds of Chinese stewed beef were first characterized.
-
•
The aroma profiles of Chinese stewed beef after reheating considerably varied.
-
•
(E)-2-undecenal was selected as an indicator of WOF in reheated Chinese stewed beef.
-
•
The reduction of 3-(methylthio) propanal might contribute to the increase in WOF.
1. Introduction
Stewed beef, a famous Chinese dish, contains beef and other auxiliary ingredients like vegetables and spices. It is particularly popular for its rich nutrition and attractive flavor. In China, the precooked Chinese dish industry is growing rapidly. Precooked stewed beef dishes, typical precooked Chinese dishes, are now widely developed owing to their more stable and simpler cooking processes compared with Chinese cooking methods such as stir-frying and pan-frying. Most of the precooked stewed beef dishes are refrigerated and need to be reheated before eating. Nevertheless, even after a short period of refrigeration within their shelf-life, they still develops a particular warmed-over flavor (WOF), which is considered an off-flavor that affects consumer acceptance (Tims & Watts, 1958). Simultaneously, the meaty attribute of precooked stewed beef gradually weakens, affecting their sensory quality of (O'Sullivan, Byrne, Jensen, Andersen, & Vestergaard, 2003).
Tims and Watts (1958) were the first to introduce the concept of WOF, and described it as an oxidized aroma such as rancid or stale. Subsequently, researchers described WOF using aroma profile evaluations, with descriptors such as wet cardboard, linseed oil, paint, sour, hard-boiled egg, and fatty notes (An et al., 2022; Lage et al., 2012). According to Tims and Watts (1958), the heat processing of uncured meats caused lipid oxidation, resulting in the loss of palatability during later storage. Thereafter, numerous studies have reported that WOF is mainly caused by lipid oxidation, as the development of WOF was related to lipid oxidation products (Pegg, Kerrihard, & Shahidi, 2014; Ruenger, Reineccius, & Thompson, 1978; Zhang et al., 2022). O'Sullivan et al. (2003) found that hexanal, 1-octen-3-ol, 2-pentylfuran, octanal, pentanal, and nonanal were associated with sensory data in cooked samples of two pork muscles using GC–MS. These compounds proved to be valid indicators of lipid oxidation. Recently, (E,E)-2,4-heptadienal, heptanal, (E)-2-octenal, octanal, (E)-2-nonenal, nonanal, (E)-2-decenal, decanal, (E,E)-2,4-decadienal, and 2,3-pentanedione were selected as the key odorants of the WOF in surimi gels by An et al. (2022) using aroma extract dilution analysis (AEDA), aroma recombination, and omission test.
In addition to lipid oxidation, protein degradation contributes to WOF by causing the loss of desirable aroma (Pegg et al., 2014; Zhang et al., 2022). Previous studies have revealed that WOF might be caused by a loss of desirable odorants, which are related to meaty notes attributable to 4,5-dimethyl-3-hydroxy-2(5H)-furanone, 2,5-dimethyl-4-hydroxy-3(2H)-furanone, 2-propylpyridine, 2,6-dimethylpyrazine, benzothiazole, 2-furfurylthiol, and 2-methoxybenzenethiol (An et al., 2022; Kerler & Grosch, 1996). In addition, WOF varies in different animal species, processing methods, and heating temperatures, to name a few. Heating is the leading cause of oxidation in meat (Khan, Jo, & Tariq, 2015). Unlike most meat products, Chinese stewed beef have unique production processes. They are produced via a three-stage thermal process, including blanching (20 °C -100 °C), boiling (100 °C), and braising (without heating, with the temperature reduced from 100 °C to approximately 75 °C). Nonetheless, the odorants responsible for the generation of the WOF in precooked Chinese stewed beef remain unclear. The objectives of the present work were to (1) characterize the key aroma-active compounds contributing to WOF in reheated precooked stewed beef using a sensomics approach and (2) elucidate the changes in the aroma profiles of precooked stewed beef during cooking-refrigeration-reheating. The study will provide information for off-flavor correction using the target-oriented flavor editing (TOFE) technology proposed by Wang et al. (2023) and Wang et al. (2023), which may be used to maintain and improve of the flavor quality of precooked stewed beef dishes.
2. Materials and methods
2.1. Preparation of stewed beef
All animal procedures in this study were approved by the Animal Care and Use Committee of the Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, and were performed according to the animal welfare and ethics guidelines. Thirty-six batches of beef chuck (seventy-two pieces) weighing 2.3 ± 0.2 kg per piece were obtained from 48-month-old Simmental cattle at Hebei Fucheng Wufeng Food Co., Ltd. (Hebei, China). All cattle had the same genetic background and were fed the same diet (complete formula feed). Raw beef chucks were vacuum-packed in the factory and transported to the laboratory via cold-chain logistics. Thirty-six batches of chucks were randomly divided into three groups, with twelve cattle in each group. All visible fat was removed, and beef chucks were washed, and cut into approximately 2 cm × 2 cm × 1.5 cm cubes. The raw beef cubes (500 g) were added to 1500 g of water, heated to boil, kept for 3 min, and then drained for use. These drained cubes were first added to 1500 g of water, heated to boil, and held for 5 min using an induction heater (Midea Group Co., Ltd., Guangdong, China) at 1200 W. The heat was subsequently reduced to 600 W and 6 g (1.2% per raw beef cube) of salt was added. The samples were then simmered for 45 min, maintaining a faint boil. Following that, the heat was turned off and the samples were braised for 20 min. Subsequently, the samples were drained, divided into aluminum foil bags, vacuum-packed, cooled with running water, and then refrigerated at 4 °C for 6 days to develop the WOF. For reheating, the refrigerated stewed beef was reheated at 100 °C for 10 min in a water bath using the induction heater. The stewed beef samples were assigned into three sets: Group 1-Cooked: freshly cooked stewed beef. Group 2-Refrigerated: precooked stewed beef refrigerated for six days. Group 3-Reheated: precooked stewed beef refrigerated for 6 days and then reheated. All samples were stored at −80 °C before analysis.
2.2. Chemicals
The following reference compounds, which are listed in Table 1, were provided by Sigma-Aldrich, Shanghai, China (no. 1, 4, 5, 7, 10, 11, 13, 16–18, 21, 23, 25–27, 28, and 31) and Macklin Biochemical Co., Ltd., Shanghai, China (no. 2, 3, 6, 8, 9, 12, 14, 15, 19, 20, 22–24, 29, 30, 32, and 33–36). The following chemicals were supplied by various companies: liquid nitrogen (Beijing Shangtong Hong Chemical Co. Ltd., Beijing, China), ultrahigh-purity helium and nitrogen (Beijing Jiangbo Environmental Technology Co., Ltd., Beijing, China), HPLC grade dichloromethane (Mereda Technology Co., Ltd., Beijing, China), a series of normal alkanes C7-C40 (o2si Smart Solutions, Shanghai, China), a series of n-ketones C4-C9 (Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China), 1, 2-dichlorobenzene and 2-methyl-3-heptanone (Sigma-Aldrich, Shanghai, China), and anhydrous sodium sulfate (Lab Gou e-mall, Beijing, China).
Table 1.
Comparison of FD factors of the aroma-active compounds in cooked and reheated Chinese stewed beef.
| no.a | aroma-active compoundb | odor qualityc | RId |
FD factorse |
dentification methodf | extraction methodg | ||
|---|---|---|---|---|---|---|---|---|
| VF-WAXms | DB-5 | cooked | reheated | |||||
| 1 | pentanal | fermented | 983 | 701 | 2 | 1 | MS/RI/O/STD | SAFE/SPME |
| 2 | 2-methyl-3-Buten-2-ol | oily | 1034 | 1 | 1 | MS/RI/O/STD | SAFE | |
| 3 | dimethyl disulfide | sulfurous, cooked cabbage | 1081 | 745 | 2 | 1 | MS/RI/O/STD | SAFE/SPME |
| 4 | hexanal | grassy, fatty | 1087 | 800 | 4 | 8 | MS/RI/O/STD | SAFE/SPME |
| 5 | 2-methyl-thiophene | roasted | 1100 | 1 | 1 | MS/RI/O/STD | SAFE | |
| 6 | 1-butanol | sweet, whiskey | 1142 | 2 | 4 | MS/RI/O/STD | SAFE | |
| 7 | heptanal | fatty | 1191 | 906 | 4 | 8 | MS/RI/O/STD | SAFE/SPME |
| 8 | 2-pentylfuran | green | 1236 | 991 | 1 | 2 | MS/RI/O/STD | SAFE/SPME |
| 9 | 1-pentanol | oily, sweet, balsamic | 1248 | 770 | 1 | 4 | MS/RI/O/STD | SAFE/SPME |
| 10 | octanal | fresh, fatty | 1295 | 16 | 16 | MS/RI/O/STD | SAFE | |
| 11 | 1-octen-3-one | mushroom-like | 1306 | 2 | 16 | MS/RI/O/STD | SAFE | |
| 12 | (E)-2-heptenal | fatty, oily | 1333 | 957 | 1 | 1 | MS/RI/O/STD | SAFE/SPME |
| 13 | 1-hexanol | herbal, nutty | 1351 | 874 | 4 | 1 | MS/RI/O/STD | SAFE/SPME |
| 14 | (Z)-3-hexen-1-ol | herbal, fatty | 1371 | 4 | 8 | MS/RI/O/STD | SAFE | |
| 15 | 5-methyl-2-ethylpyrazine | coffee-like, nutty | 1391 | 1 | 8 | MS/RI/O/STD | SAFE | |
| 16 | nonanal | green, fatty | 1400 | 1094 | 32 | 16 | MS/RI/O/STD | SAFE/SPME |
| 17 | (E)-2-octenal | fatty, herbal | 1439 | 1061 | 8 | 8 | MS/RI/O/STD | SAFE/SPME |
| 18 | 1-octen-3-ol | mushroom-like | 1447 | 982 | 16 | 32 | MS/RI/O/STD | SAFE/SPME |
| 19 | 3-(methylthio)propanal | potato-like, meaty | 1467 | 902 | 2 | 1 | MS/RI/O/STD | SAFE/SPME |
| 20 | (E,E)-2,4-heptadienal | fatty | 1474 | 1 | 8 | MS/RI/O/STD | SAFE | |
| 21 | decanal | orange-like, fresh | 1505 | 1195 | 2 | 4 | MS/RI/O/STD | SAFE/SPME |
| 22 | benzaldehyde | bitter almond | 1540 | 954 | 4 | 4 | MS/RI/O/STD | SAFE/SPME |
| 23 | (E)-2-nonenal | cardboard, nutty | 1545 | 1155 | 1 | 4 | MS/RI/O/STD | SAFE/SPME |
| 24 | formic acid octyl ester | fruity, orange-like | 1557 | 1 | 8 | MS/RI/O/STD | SAFE | |
| 25 | (E)-2-decenal | fatty | 1653 | 8 | 4 | MS/RI/O/STD | SAFE | |
| 26 | (E,E)-2,4-nonadienal | fatty | 1672 | 1223 | 4 | 8 | MS/RI/O/STD | SAFE/SPME |
| 27 | dodecanal | soapy, waxy | 1716 | 1407 | 1 | 1 | MS/RI/O/STD | SAFE/SPME |
| 28 | 4-ethylbenzaldehyde | almond, bitter | 1725 | 1 | 1 | MS/RI/O/STD | SAFE | |
| 29 | (E)-2-undecenal | soapy, metallic | 1761 | 1 | 4 | MS/RI/O/STD | SAFE | |
| 30 | 2-acetyl-2-thiazoline | cooked rice-like | 1777 | 4 | 4 | MS/RI/O/STD | SAFE | |
| 31 | (E,E)-2,4-decadienal | fatty, nutty | 1822 | 1327 | 4 | 8 | MS/RI/O/STD | SAFE/SPME |
| 32 | benzyl alcohol | phenolic | 1888 | 16 | 8 | MS/RI/O/STD | SAFE | |
| 33 | phenylethyl alcohol | floral | 1925 | 1 | 4 | MS/RI/O/STD | SAFE | |
| 34 | methyleugenol | waxy | 2018 | 4 | 8 | MS/RI/O/STD | SAFE | |
| 35 | 3-phenyl-2-propenal | spice-like | 2040 | 32 | 64 | MS/RI/O/STD | SAFE | |
| 36 | 4-methyl-5-thiazoleethanol | fatty | 2323 | 4 | 1 | MS/RI/O/STD | SAFE | |
Aroma-active compounds were consecutively numbered according to RI values on the VF − WAXms column.
Aroma-active compounds were identified in cooked and reheated samples.
Odor perception of each aroma-active compound that was detected at the sniffing port.
Retention indices on VF − WAXms and DB-5 capillaries.
FD factors of aroma-active odorants that were obtained by AEDA.
Identification methods of each compound. MS, RI, O and STD were represented for identifying by mass spectra, retention indices, olfactometry, and authentic reference compounds, respectively.
Extraction methods of each aroma-active compound. SAFE, solvent–assisted flavor evaporation; SPME, solid space microextraction.
2.3. Lipid oxidation
According to Xia, Kong, Liu, and Liu (2009), thiobarbituric acid reactive substance (TBARS) values were used to assess the level of lipid oxidation. The data were given in mg malondialdehyde (MDA) per kg.
2.4. Aroma profiles characterized by headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS)
The aroma profiles of cooked, refrigerated, and reheated samples were analyzed using an HS-GC-IMS device (FlavorSpec®, Gesellschaft für Analytische Sensorsysteme mBH, G.A.S., Dortmund, Germany), equipped with an automatic sampling device (CTC Analytics AG, Zwingen, Switzerland). stewed beef samples (2 g each) were quickly placed into a 20 mL glass vial. The samples in headspace vials were heated at 50 °C with an oscillation rate of 500 rpm for 20 min. The detection conditions and identification methods were adopted from Wang et al. (2021).
2.5. Sensory aroma profile analysis (APA)
Sensory evaluation was performed by 12 trained panelists (8 females and 4 males aged 22–45) according to the methods described by Yang et al. (2022). They received an extra three hours of training to recognize and define the descriptive terms of cooked and reheated stewed beef. Possible aroma terms listed in Table 2 were provided by previous studies (Lage et al., 2012). Seven odor attributes (meaty, grassy, cardboard, metallic, fatty, hard-boiled egg, and oxidized vegetable oil) were selected, and their intensities were rated on a scale from 0 (not perceivable) to 3 (very high intensity) on the scale steps of 0.5. The final score for each aroma attribute was determined based on the scores of all assessors. The sensory analysis was performed in a sensory laboratory with individual booths at 20 ± 2 °C. Most importantly, participation was voluntary for all participants. We confirmed that every participant's rights and privacy were appropriately protected while the study was being conducted. We guaranteed that all samples were nontoxic, and harmless to the human body. The participants were fully informed of the requirements and risks of the study, and they gave their consent. We also promised to keep all details on the evaluators confidential. All data obtained through this evaluation were used only for the identification of key aroma-active compounds on the aspects of sensory studies.
Table 2.
Sensory descriptive terms with definitions developed for the evaluation of cooked and reheated Chinese stewed beef.
| descriptor | definition with reference material |
|---|---|
| meaty | freshly boiled beef lean |
| oxidized vegetable oil | soybean oil heated for 5 min at 198 °C |
| hard-boiled egg | boiled egg in boiling water for 20 min |
| fatty | roast beef fat in the oven for 5 min at 150 °C |
| metallic | metal products (metal key) |
| cardboard | shredded wet cardboard |
| grassy | grass |
| linseed oil | warmed linseed oil |
| nutty | crushed fresh hazel nuts |
| roasted | oven cooked beef steaks with surface browning |
2.6. Extraction of aroma compounds
2.6.1. Solid-phase microextraction (SPME)
All samples were cut into approximately 0.5 cm3 cubes, frozen in liquid nitrogen for 5 min, and ground into a fine powder. Samples (2 g each), 1 μL 1, 2-dichlorobenzene (0.653 μg/μL), and 1 μL 2-methyl-3-heptanone (0.408 μg/μL) were placed into a 20 mL glass vial. The vial was stored at 50 °C for 20 min. Then, a 50/30 μm DVB/CAR/PDMS fiber was inserted into the headspace to adsorb for 30 min, followed by desorption at the injector port of the Q Exactive GC-Orbitrap-MS-O system for 5 min.
2.6.2. Solvent-assisted flavor evaporation (SAFE)
Samples (50 g each), 0.653 μg/μL of 1, 2-dichlorobenzene (30 μL), 0.408 μg/μL of 2-methyl-3-heptanone (50 μL), and dichloromethane (150 mL) were mixed in a Teflon bottle, shaken at 120 rpm at 4 °C in an incubator shaker (Tianjin Honour Instrument Co., Ltd., Tianjin, China) for 8 h, and extracted as described by Sun et al. (2021).
2.7. Identification of aroma-active compounds of the cooked and reheated stewed beef
2.7.1. Q exactive GC-orbitrap-MS-O analysis
Aroma compounds in all samples were identified by a Q Exactive GC-Orbitrap-MS system (Trace 1310 GC System, TSQ9000 MSD, Thermo Scientific, Bremen, Germany) equipped with an olfactometer detector ODP4 (Gerstel, Inc., Linthicum, MD, U.S.A.). VF-WAXms (60 m × 0.25 mm, 0.25 μm) and DB-5 capillary columns (30 m × 0.25 mm, 0.25 μm) were used to separate the odorants. The temperature of the VF-WAXms column was 40 °C for 2 min, increased at 4 °C/min to 230 °C, and held for 5 min. The final column temperature of the DB-5 column was 250 °C for 5 min. The flow rate of the helium carrier gas (99.999% purity) was 1.5 mL/min. MS conditions: electron impact (EI) energy; 70 eV, ion source temperature, 230 °C; MS source temperature, 280 °C. The ODP temperature was 250 °C.
The compounds were identified based on odor attributes (O), retention indices (RIs), mass spectra (MS), and data obtained from authentic reference standards (STDs). The RI values were calculated using the retention times of a series of n-alkanes (C7-C40).
2.7.2. Detection frequency analysis (DFA)
DFA was used to obtain odor patterns of the cooked and reheated stewed beef as described by Pang et al. (2012). The detection frequency (DF) for an odor with the same retention index and a similar description was summed. Any odorant at the sniffing port with DF ≥ 2 was considered to have aroma potential activity, regarded as an aroma-active compound (Pang et al., 2012).
2.8. AEDA
AEDA was used to acquire a preliminary concept of which odorants should be significant for the overall aroma. The SAFE extracts were diluted stepwise by 1 + 1 (v + v) with dichloromethane and analyzed by the Q Exactive GC-Orbitrap-MS-O system. The results are represented as the flavor dilution (FD) factor of the maximum dilution for the perceived odor. AEDA was performed on the VF-WAXms column as more odorants were detected by VF-WAXms than DB-5.
2.9. Quantitation of the aroma-active compounds and calculation of odor activity values (OAVs)
As matrix effects were not considered in the DFA analysis, the aroma intensity of the aroma-active compounds detected at the sniffing port does not necessarily indicate the importance of a single aroma compound. Thus, quantitation and OAVs determination of odorants need to be analyzed (Yang et al., 2022). According to Sun et al. (2021), the quantitative analysis of aroma-active compounds was achieved by constructing external standard curves using an artificial odorless matrix with various concentrations of authentic flavor standards. The standard solutions were prepared by diluting the corresponding stock standard solution with dichloromethane. Certain concentrations of authentic flavor standards containing internal standards were added to the artificial odorless matrix extracted by SAFE, and then detected using the Q Exactive GC-Orbitrap-MS-O system in the selected ion monitoring mode. The linear regression equation for each compound was determined by plotting the ratio of the peak area of the target compound to that of the internal standard against the corresponding concentration. All analyses were repeated in triplicate.
To obtain the odorless matrix, the cooked and reheated stewed beef samples were frozen with liquid nitrogen, ground into fine powder, extracted with dichloromethane, and filtered. The filtrates were subjected to high vacuum distillation using SAFE to eliminate all aroma compounds until nothing was detected by the Q Exactive GC-Orbitrap-MS-O system. Finally, the filtration residues were dried to remove the solvent and form an odorless powder (Sun et al., 2021).
The contribution of a single aroma compound can be considered by its OAV (Yang et al., 2022). The OAVs were calculated by dividing the concentration of each odorant by its threshold in water found in literature or detected in the present study (Cerny & Grosch, 1993, Giri, Osako, & Ohshima, 2010, Van Gemert, 2011, Han, Zhang, Fauconnier, & Mi, 2020, Beldarrain, Moran, Sentandreu, Barron, & Aldai, 2022).
2.10. Aroma recombination and omission experiments
To confirm that the key aroma compounds were correctly identified and quantitated, the cooked-recombinant model (odorants with OAVs ≥1 and FD ≥ 4) and the reheated-recombinant model (odorants with OAVs ≥1 and FD ≥ 8) were constructed according to the method described by Sun et al. (2021). The contribution of individual odorants to the overall aroma was assessed through omission experiments, which were conducted by removing individual odorants from the respective recombinant models. The flavor similarity between the recombinant model and the original sample, as well as between the recombinant model and each omission model, was evaluated using a triangle test.
2.11. Statistical analysis
All experiments were conducted in triplicate. Analysis of variance (ANOVA) for statistical analysis were conducted using the Statistical Program of Social Science (SPSS 25.0, Chicago, IL, USA) software. Statistically significant differences between groups were determined using Duncan's multiple range test at P < 0.05. A completely randomized design was employed. The orthogonal partial least squares discrimination analysis (OPLS-DA) was performed using SMICA 14.1 software (Umetrics, UmeUmeå, Sweden). The radar chart, column chart, and heatmap were drawn using Origin 2021 (OriginLab, Northampton, Massachusetts, USA).
3. Results and discussion
3.1. Changes in TBARS in the precooked stewed beef during refrigeration-reheating
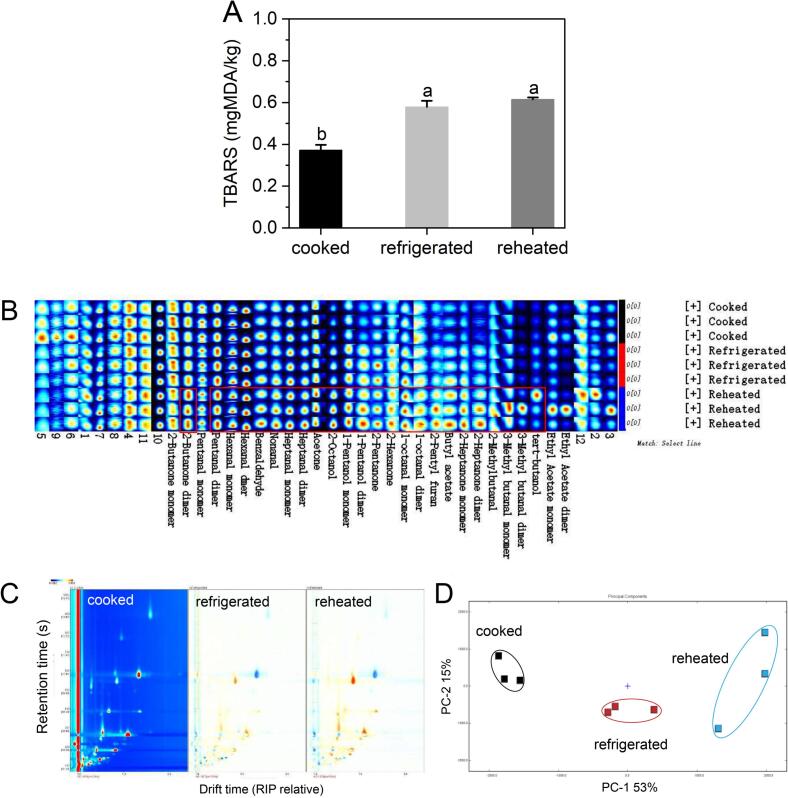
TBARS values can reflect the degree of lipid oxidation (Xia et al., 2009). The TBARS value of the precooked stewed beef significantly increased after refrigeration-reheating (P ≤ 0.05). There was no significant difference between refrigerated and reheated samples (P > 0.05) (Fig. 1A). This finding indicated that refrigeration-reheating induced significant lipid oxidation, which is recognized as often concomitant with the deterioration of flavor quality.
Fig. 1.
Thiobarbituric acid reactive substances (TBARS) value variation of Chinese stewed beef during cooking-refrigeration-reheating (different lowercase letters show significant differences at P ≤ 0.05) (A), fingerprint map of aroma compounds(B), two-dimensional differential map of HS − GC − IMS spectra (C), and principal component analysis based on the peak intensities of aroma compounds detected by HS − GC − IMS (D).
3.2. Changes of odorants in the precooked stewed beef during cooking-refrigeration-reheating by HS-GC-IMS
A total of 40 signal peaks were detected using HS-GC-IMS, and 28 typical aroma compounds were successfully identified via GC-IMS Library searches. The observed 40 peaks were analyzed using a fingerprint plot (Fig. 1B), including 13 aldehydes, 7 ketones, 4 alcohols, 3 esters, 1 furan, and 12 undefined compounds. Fig. 1B shows the intensities of each odorant. The signal intensities of most aldehydes in refrigerated and reheated samples were higher compared with those in cooked samples. Almost no new signals or spots were generated, suggesting that almost no new compounds were detected in the precooked stewed beef after refrigeration and reheating. Moreover, except for two compounds (2-butanone monomer and pentanal monomer), 26 odorants mainly produced via lipid oxidation (Merlo et al., 2021, Wang et al., 2021), such as hexanal, heptanal, 2-heptanone, and 2-pentylfuran, increased in intensity after refrigeration and reheating, suggesting an increasing trend of lipid oxidation. Additionally, 3-methylbutanal and 2-methylbutanal are Strecker aldehydes with malt and cocoa odors, respectively. According to a previous research from Zamora, Gallardo, and Hidalgo (2008), some carbonyls produced by lipid peroxidation, such as ketodienes and alkadienals, have been proven to facilitate the breakdown of specific amino acids to produce the corresponding Strecker aldehydes through Strecker-type reactions, which might lead to increased levels of 3-methylbutanal and 2-methylbutanal following refrigeration and reheating. Their increasing intensity suggested that Strecker degradation might be another reason for the aroma variation of precooked stewed beef after refrigeration and reheating. The differences in aroma compounds among the three samples were also visually distinguished by the two-dimensional differential topographic plots (Fig. 1C). Compared with the cooked samples, the signal intensities of most of the aromas were higher following refrigeration and reheating. Furthermore, PCA was conducted to inspect the clustering of the samples. As shown in Fig. 1D, the cumulative variance contribution was 68%. The PCA model can be regarded as the separation model (Wang et al., 2021). Three samples were separated in the distribution map, suggesting that after refrigeration and reheating, the aroma profiles of the precooked stewed beef considerably varied. The cooked samples were clustered in the upper left plot, the reheated samples were mainly located in the upper right plot, and the refrigerated samples were clustered in the bottom middle area closer to the reheated samples (Fig. 1D). The result demonstrated that the odorants of the cooked and reheated samples considerably differed. The differences between the refrigerated and reheated samples were smaller compared with the differences between the cooked and refrigerated samples (Fig. 1B, D), confirming the TBARS results. Thus, to characterize the indicators contributing to the WOF of reheated precooked stewed beef, the reheated and cooked stewed beef samples (control group) were further investigated in subsequent studies.
3.3. APA
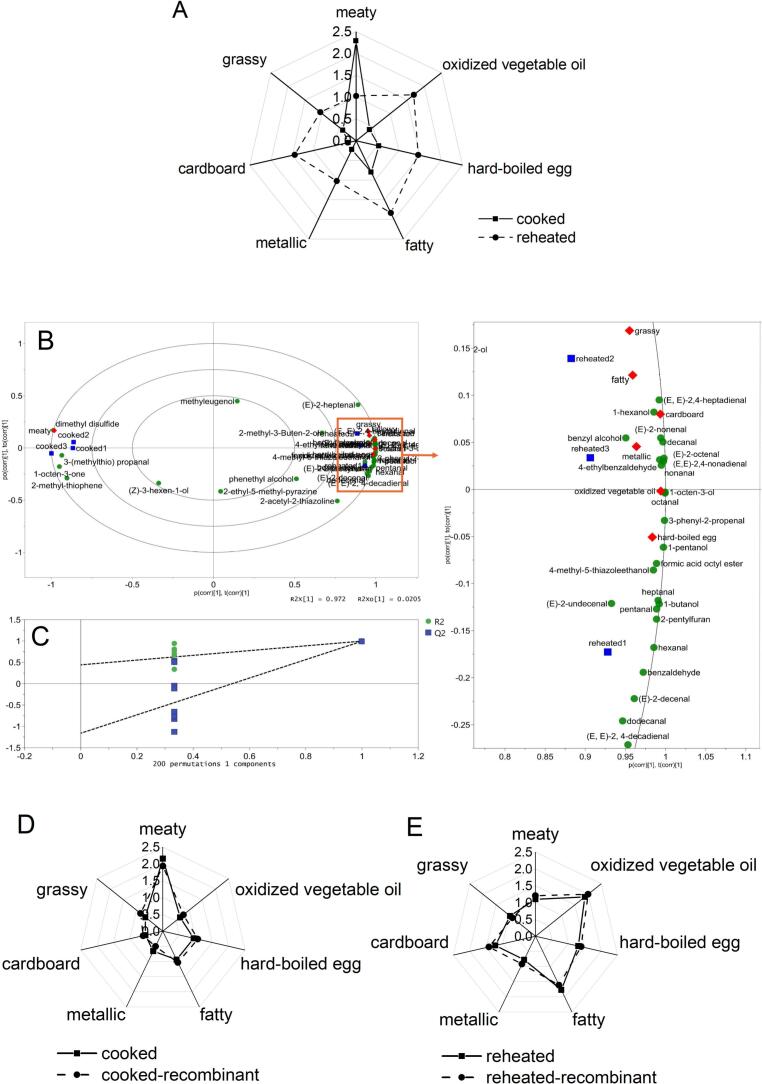
Sensory evaluation results of the cooked and reheated samples are shown in Fig. 2A, illustrating that the aroma profiles of the two samples were considerably different. The cooked samples had an intensely meaty odor. After refrigeration-reheating, the precooked stewed beef presented a strong WOF, manifesting as weaker intensities of meaty note, and considerably increased fatty and oxidized vegetable oil aroma. Moreover, the grassy, hard-boiled egg, metallic, and cardboard-like aroma significantly increased.
Fig. 2.
Aroma profiles of cooked (solid line) and reheated (broken line) Chinese stewed beef (A), OPLS-DA correlation bi-plot of the relationship between 36 aroma-active compounds (OAVs) (green plots) and sensory attributes (red plots) (B), OPLS-DA model permutation test plots (C), aroma profiles of cooked (solid line) and cooked-recombinant (broken line) (D), and aroma profiles of reheated (solid line) and reheated-recombinant (broken line) (E). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Composition of aroma-active compounds in cooked and reheated stewed beef
Overall, 36 active compounds were detected in the cooked and reheated samples, including 15 aldehydes, 6 alcohols, 6 benzene-containing compounds, 5 sulfur-containing compounds, 1 nitrogen-containing compound, 1 ketone, 1 ester, and 1 furan. Almost no new odorants were detected in the reheated samples, confirming the HS-GC-IMS result (Fig. 1B, C). These compounds exhibited FD factors between 1 and 64 (Table 1).
Hexanal (no. 4; grassy), heptanal (no. 7; fatty), octanal (no. 10; fresh), nonanal (no. 16; fresh), (E)-2-octenal (no. 17; fatty, herbal), (E,E)-2,4-heptadienal (no. 20; fatty), (E,E)-2,4-nonadienal (no. 26; fatty), and (E,E)-2,4-decadienal (no. 31; fatty) had FD factors of ≥8 in the reheated samples. Nos. 4, 7, 10, 16, 17, 26, 31, and 25 (fatty) were presented at FD factors of ≥4 in the cooked samples. Nos. 7, 10, 16, and 31 can be generated from oleic acid, and no. 4 is an oxidation product of linoleic and arachidonic acids (Merlo et al., 2021). The oxidation of oleic and linoleic acid can produce 2-alkenals (Elmore, Mottram, Enser, & Wood, 1999). Aldehydes were the dominant odorants in the cooked and reheated samples. They have a strong aroma and a lower threshold, contributing significantly to meat aroma (Wang et al., 2021).
In the cooked samples, 1-hexanol (no. 13; herbal, fatty), (Z)-3-hexen-1-ol (no. 14; fatty), and 1-octen-3-ol (no. 18; mushroom-like, oily) showed FD factors of ≥4. Nos. 14 and 18 had FD factors of ≥8 in the reheated samples. No. 18 can be generated from n-6 fatty acids (Elmore & Mottram, 2006). No. 14, a typical green leaf volatile, has been identified as a key odorant in some plant products (Tamura, Boonbumrung, Yoshizawa, & Varanyanond, 2000; Yang et al., 2022). It probably originated from grass and grain fed to the cattle.
Formic acid octyl ester (no. 24; fruity) had an FD of 8 in reheated samples. Nevertheless, it has a high odor threshold and might contribute less to the overall aroma profile of precooked stewed beef. In the reheated samples, the FD factor of 1-octen-3-one (no. 11; mushroom), which may be derived from omega-3 fatty acids (Lee, Suriyaphan, & Cadwallader, 2001), was 16. In addition, the FD factor of 5-methyl-2-ethylpyrazine (no. 15; coffee-like) was 8 in the reheated samples. Strecker products, such as aminoketones, may generate pyrazines (Liu et al., 2019). The thermal degradation of lipids and the Maillard reaction are the main reactions for the formation of aroma compounds in cooked meats (Khan et al., 2015).
3-phenyl-2-propenal (no. 35; spice-like) had the highest FD of 32 and 64 in cooked and reheated samples, respectively. Benzyl alcohol (no. 32; phenolic), methyleugenol (no. 34; waxy), and benzaldehyde (no. 22; bitter almond) had FD factors of ≥4 in cooked samples. Nos. 32 and 34 had FD factors of ≥8 in reheated samples. Nos. 34 and 35 might be developed in the stomachs of ruminants that consume green feed (Gąsior et al., 2021; Khan et al., 2015). Benzaldehyde is the Strecker degradation product of phenylalanine and the degradation product of α-linolenic acid (Elmore & Mottram, 2006; Feng et al., 2021).
In the cooked samples, 2-acetyl-2-thiazoline (no. 30; cooked rice) and 4-methyl-5-thiazoleethanol (no. 36; fatty) had an FD factor of 4. Sulfur-containing compounds can be generated from the Maillard reaction and amino acid and thiamine degradation (Khan, Jo and Tariq, 2015, Wang et al., 2021,Wang et al., 2021). They are considered important odorants contributing to the meaty aroma (Khan et al., 2015). Odorant no. 30 had the highest FD of 2048 in roasted duck (Straßer & Schieberle, 2014). No. 36 was reportedly the major aroma compound in pork broth (Zhao et al., 2017).
A comparison of aroma-active compounds suggested that the odorants detected in the sniffing port in the two samples had similar categories and amounts, indicating that desirable and undesirable odorants were generated by the same aroma compounds. The differences were reflected in their intensities (Table 1), such as nos. 4, 7, 26 (grassy and fatty) had increased FD factors from cooked to reheated samples, confirming the sensory results of the APA. Most of these odorants were reported to be the main contributors to the WOF in meat products (Konopka & Grosch, 1991; Pegg et al., 2014; Ruenger et al., 1978). In addition, 16 odorants in the reheated samples and only 7 in the cooked samples had FD factors of ≥8, showing a larger number of odorants with higher FD in the reheated samples than in the cooked samples (Table 1). The FD factors of three sulfur-containing compounds (nos. 3, 19, and 36) decreased from cooked to reheated samples, which might be the crucial factor for the reduced meaty attribute in the reheated samples (Fig. 2A).
3.5. Quantitation of the aroma-active compounds and calculation of OAVs
The concentrations and OAVs of aroma-active compounds are presented in Table 3. The highest concentration was measured for hexanal (no. 4) in the cooked (246.17 μg/kg) and reheated samples (1070.33 μg/kg), followed by nos. 14 and 1. Meanwhile, nos. 7, 16, 18, 10, and 17 had relatively high concentrations. Nevertheless, high concentrations do not consequently imply a significant influence on the overall aroma of food.
Table 3.
Concentrations, odor thresholds, and odor activity values (OAVs) of key aroma-active compounds in cooked and reheated Chinese stewed beef.
| no. | aroma-active compound | selected ions used for quantitation. | linear equations | R2 | ranges of concentration for provided linearity (μg/kg) | odor threshold in water (μg/kg) |
concentrations (μg/kg) |
OAV |
||
|---|---|---|---|---|---|---|---|---|---|---|
| cooked | reheated | cooked | reheated | |||||||
| 1 | pentanal | 44, 58, 86 | y = 0.4387× − 0.0282 | 0.9946 | 25.79–494.46 | 9b | 31.57 ± 1.14B | 102.38 ± 8.93 A | 4 | 11 |
| 2 | 2-methyl-3-buten-2-ol | 43, 59, 71 | y = 5.5312× + 0.0064 | 0.9919 | 0.01–1.25 | 51600c | 0.34 ± 0.01 A | 0.33 ± 0.01 A | <1 | <1 |
| 3 | dimethyl disulfide | 47, 79, 94 | y = 10.483× + 0.0002 | 0.9993 | 0.01–0.79 | 1.1a | 0.21 ± 0.00 A | 0.07 ± 0.02B | <1 | <1 |
| 4 | hexanal | 44, 56, 72 | y = 0.7435× − 0.01 | 0.9917 | 8.26–2401.08 | 1.00a | 246.17 ± 21.36B | 1070.33 ± 108.94 A | 246 | 1070 |
| 5 | 2-methyl-thiophene | 45, 97, 98 | y = 12.096× + 0.0036 | 0.9971 | 0.03–3.07 | 3000c | 0.20 ± 0.01 A | 0.13 ± 0.02B | <1 | <1 |
| 6 | 1-butanol | 31, 43, 56 | y = 1.003× + 0.0012 | 0.9997 | 0.05–39.42 | 459.20d | 4.31 ± 0.06B | 9.27 ± 0.91 A | <1 | <1 |
| 7 | heptanal | 44, 70, 114 | y = 0.2873× + 0.0018 | 0.9985 | 1.08–280.04 | 2.8a | 32.99 ± 0.25B | 69.46 ± 3.68 A | 12 | 25 |
| 8 | 2-pentylfuran | 53, 81, 138 | y = 17.305× + 0.015 | 0.9961 | 0.08–15.05 | 6b | 0.59 ± 0.01B | 2.35 ± 0.20 A | <1 | <1 |
| 9 | 1-pentanol | 42, 55, 70 | y = 2.2154× − 1 × 10−5 | 0.9998 | 0.22–65.46 | 4000a | 3.63 ± 0.01B | 14.95 ± 0.38 A | <1 | <1 |
| 10 | octanal | 41, 55, 84 | y = 2.0255× + 0.0012 | 0.9986 | 0.11–80.09 | 0.1c | 2.01 ± 0.28B | 17.89 ± 0.32 A | 20 | 179 |
| 11 | 1-octen-3-one | 27, 55, 70 | y = 2.4605× + 0.0007 | 0.999 | 0.01–9.63 | 0.1c | 1.75 ± 0.11 A | 1.41 ± 0.01B | 18 | 14 |
| 12 | (E)-2-heptenal | 55, 83, 112 | y = 3.0831× + 0.0017 | 0.9996 | 0.12–15.48 | 13a | 2.95 ± 0.41B | 8.30 ± 1.18 A | <1 | <1 |
| 13 | 1-hexanol | 43, 56, 105 | y = 7.5922× + 0.0062 | 0.9985 | 0.14–10.37 | 5.60a | 0.56 ± 0.10B | 4.06 ± 0.70 A | <1 | <1 |
| 14 | (Z)-3-hexen-1-ol | 41, 67, 100 | y = 0.0345× + 0.0019 | 0.992 | 27.96–972.3 | 200b | 104.75 ± 1.60 A | 97.91 ± 1.28B | <1 | <1 |
| 15 | 5-methyl-2-ethylpyrazine | 77, 106, 121 | y = 51.583× − 0.0019 | 0.9933 | 0.02–1.51 | 1000c | 0.10 ± 0.02 A | 0.09 ± 0.02 A | <1 | <1 |
| 16 | nonanal | 41, 57, 98 | y = 2.7061× + 0.0276 | 0.9928 | 3.10–123.20 | 1.00b | 16.52 ± 1.36B | 75.63 ± 6.96 A | 17 | 76 |
| 17 | (E)-2-octenal | 55, 70, 83 | y = 1.5036× + 0.0165 | 0.999 | 1.34–102.85 | 3.00a | 3.89 ± 0.05B | 13.49 ± 0.57 A | 1 | 5 |
| 18 | 1-octen-3-ol | 43, 57, 72 | y = 1.8271× + 0.0198 | 0.9988 | 3.14–150.82 | 1.5a | 7.00 ± 0.14B | 31.85 ± 0.48 A | 5 | 21 |
| 19 | 3-(methylthio)propanal | 48, 76, 104 | y = 0.3426× + 0.0001 | 0.995 | 0.01–3.14 | 0.04c | 0.74 ± 0.09 A | 0.42 ± 0.02B | 19 | 11 |
| 20 | (E,E)-2,4-heptadienal | 53, 81, 110 | y = 6.3891× + 0.0229 | 0.997 | 0.02–40.82 | 15.4a | 0.85 ± 0.02B | 2.18 ± 0.12 A | <1 | <1 |
| 21 | decanal | 41, 57, 70 | y = 0.2139× + 0.0004 | 0.997 | 0.15–20.01 | 2.00b | 2.56 ± 0.15B | 6.84 ± 0.21 A | 1 | 3 |
| 22 | benzaldehyde | 51, 77, 106 | y = 1.3412× + 0.0057 | 0.9995 | 1.70–152.35 | 41.70b | 26.51 ± 0.38B | 31.41 ± 1.02 A | <1 | <1 |
| 23 | (E)-2-nonenal | 43, 70, 83 | y = 0.2177× + 9 × 10−5 | 0.9963 | 0.25–9.99 | 0.08a | 2.93 ± 0.14B | 6.26 ± 0.19 A | 37 | 78 |
| 24 | formic acid octyl ester | 56, 70, 84 | y = 0.3159× + 9 × 10−5 | 0.9982 | 0.05–7.78 | 3132⁎ | 1.25 ± 0.20B | 3.63 ± 0.23 A | <1 | <1 |
| 25 | (E)-2-decenal | 55, 70, 154 | y = 0.2179× + 0.0001 | 0.9964 | 0.21–9.01 | 0.35a | 1.89 ± 0.05B | 2.33 ± 0.11 A | 5 | 7 |
| 26 | (E,E)-2,4-nonadienal | 67, 81, 138 | y = 1.1253× + 0.0012 | 0.9956 | 0.42–19.07 | 0.100a | 1.11 ± 0.01B | 5.75 ± 0.14 A | 11 | 58 |
| 27 | dodecanal | 41, 82, 109 | y = 0.3478× + 0.0002 | 0.9975 | 0.11–6.39 | 2.00b | 2.38 ± 0.13B | 4.08 ± 0.50 A | 1 | 2 |
| 28 | 4-ethyl benzaldehyde | 91, 119, 134 | y = 4.4924× + 5 × 10−5 | 0.9997 | 0.00–0.71 | 123.23d | 0.00 ± 0.00B | 0.02 ± 0.00 A | <1 | <1 |
| 29 | (E)-2-undecenal | 41, 70, 83 | y = 0.219× + 9 × 10−5 | 0.9952 | 0.11–5.90 | 1.40a | 1.83 ± 0.13B | 3.04 ± 0.56 A | 1 | 2 |
| 30 | 2-acetyl-2-thiazoline | 43, 60, 129 | y = 1.0494× + 3 × 10−5 | 0.9998 | 0.06–0.86 | 1e | 0.12 ± 0.01 A | 0.14 ± 0.01 A | <1 | <1 |
| 31 | (E,E)-2,4-decadienal | 41, 81, 152 | y = 77.564× + 0.0046 | 0.998 | 0.00–1.72 | 0.027a | 0.03 ± 0.00B | 0.21 ± 0.05 A | 1 | 8 |
| 32 | benzyl alcohol | 77, 79, 108 | y = 0.8016× + 0.0007 | 0.996 | 0.30–26.95 | 5500c | 3.76 ± 0.69B | 13.11 ± 2.55 A | <1 | <1 |
| 33 | phenethyl alcohol | 91, 92, 122 | y = 2.2791× + 0.0002 | 0.9998 | 0.02–1.55 | 4000c | 0.57 ± 0.05 A | 0.61 ± 0.04 A | <1 | <1 |
| 34 | methyleugenol | 147, 163, 178 | y = 2.41× + 0.002 | 0.9989 | 0.00–1.21 | 1250c | 0.09 ± 0.01 A | 0.10 ± 0.01 A | <1 | <1 |
| 35 | 3-phenyl-2-propenal | 103, 131, 132 | y = 1.3259× − 8 × 10−5 | 0.9999 | 0.01–3.91 | 90c | 0.15 ± 0.00B | 0.22 ± 0.00 A | <1 | <1 |
| 36 | 4-methyl-5-thiazoleethanol | 85, 112, 143 | y = 10.484× + 0.0502 | 0.9995 | 0.10–4.40 | 4748⁎ | 0.65 ± 0.06B | 1.19 ± 0.04 A | <1 | <1 |
Different uppercase letters show significant differences in concentrations of different samples in each row at P < 0.05.
Odor detection threshold in water according to ref. (Beldarrain, Moran, Sentandreu, Barron, & Aldai, 2022),
Odor detection threshold in water according to ref. (Han, Zhang, Fauconnier, & Mi, 2020),
Odor detection threshold in water according to ref. (Van Gemert, 2011),
Odor detection threshold in water according to ref. (Giri, Osako, & Ohshima, 2010),
Odor detection threshold in water according to ref. (Cerny & Grosch, 1993),
Represents that the odor threshold was detected in the present study.
To exhibit the contribution of a single aroma compound to the overall aroma, the OAVs were calculated. Overall, 16 identical compounds showed OAVs ≥1 in the cooked and reheated samples (Table 3). These compounds might have a significant contribution to the overall aroma of precooked stewed beef. By far, the OAV of hexanal is the highest (cooked, 246; reheated, 1070). It has been identified as a key odorant and a typical indicator of the WOF development in meat products (Konopka and Grosch, 1991, Liu et al., 2020). Kerler and Grosch (1996) reported similar results, revealing that the highest concentration of no. 4 in the reheated boiled beef was nine-fold higher than that in fresh-boiled beef. Meanwhile, the OAV of no. 4 considerably increased after refrigeration-reheating, manifesting that was no. 4 was the most potent odorant of reheated boiled beef. No. 31, which was responsible for the fatty aroma, showed a very low concentration (cooked, 0.03 μg/kg; reheated, 0.21 μg/kg). Nonetheless, it had OAVs (cooked, 1; reheated, 8) ≥1 owing to its lower threshold (0.027 μg/kg). In a previous study, no. 31 was identified as a contributor to WOF in precooked pork (Zang et al., 2019). Among odorants with an OAV of ≥1, although pentanal (no. 1) had a lower FD factor (cooked, 1; reheated, 2), its OAV was >1 because of higher concentration (cooked, 31.57 μg/kg; reheated, 102.38 μg/kg) and lower threshold (9 μg/kg). Due to lower concentrations or higher threshold, only one sulfur-containing compound, 3-(methylthio)propanal (methional) (no. 19), had an OAV of >1. The lower concentrations might be due to the fact that the precooked stewed beef in this study was cooked at a much lower temperature than the roasted and fried meats, thus producing a limited amount of Maillard reaction products (Khan et al., 2015). No. 19 with a roasted potato aroma had lower concentrations (cooked, 0.74 μg/kg; reheated, 0.42 μg/kg) and FD factors (cooked, 2; reheated, 1), presenting higher OAVs (cooked, 19; reheated, 11) because of their relatively lower thresholds (0.04 μg/kg). Similarly, decanal (no. 21, orange/fresh), dodecanal (no. 27, soapy/waxy), (E)-2-undecenal (no. 29, soapy/metallic), and (E)-2-nonenal (no. 23, cardboard/nutty), which are derived from lipid oxidation, had a lower FD factor, but OAVs ≥1. Nevertheless, most of the odorants with an OAV of >1 exhibited higher concentrations and FD factors. Meantime, they processed higher OAVs, covering no. 10 (cooked, 20; reheated, 179), no. 16 (cooked, 17; reheated, 76), no. 7 (cooked, 12; reheated, 25), and no. 18 (cooked, 5; reheated, 21). These aromas significantly contributed to the WOF of the reheated samples. In addition, nos. 11, 16, 26, 25, and 17 showed OAVs of ≥1 (Table 3) and mainly presented grassy, green, fatty, and metallic aromas. Their OAVs increased after reheating except for that of no. 19, which decreased in the reheated samples. The result was in line with the APA. These results suggested that the formation of WOF in the reheated precooked stewed beef was mainly caused by an increase in certain odorants and a decrease in certain odorants, which was similar to the findings of previous studies (An et al., 2022; Pegg et al., 2014).
Additionally, some aromas with higher FD factors showed lower OAV values. No. 35, with the highest FD, had an OAV of <1 attributing to its relatively low concentration and high threshold, including nos. 32, 34, 24, 14, 15, 13, 30, and 22. These odorants have a minor aroma contribution because of their low OAVs (< 1).
3.6. Correlation among samples, aroma-active compounds, and sensory attributes
To screen the effective aroma-active compounds contributing to sensory profiles, two data sets (Table 3) were analyzed by OPLS-DA, and the results are presented in Fig. 2B. The correlation biplot involving three ellipses indicated 50%, 75%, and 100% explained variance, respectively. Most variables (OAVs of the aroma-active compounds and intensities of the sensory attributes) were located within the 50%–100% ellipses. The derived OPLS-DA model with two principal components explained 99.6% of the validated variation. Excellent parameters (R2X = 0.996, R2Y = 0.999, and Q2 = 0.996) indicated a good model fit and acceptable predictability. In addition, in the 200-times permutation test (Fig. 2C), the values of R2 and Q2 of the original points on the right were higher than the values on the left, indicating that the OPLS-DA model was not overfitting. As shown in Fig. 2B, the two samples were located on the left side of the plot, and the reheated samples were located on the opposite side, exhibiting good separation on the PC1. Methyleugenol, (Z)-3-hexen-1-ol, and 5-methyl-2-ethylpyrazine with OAVs of <1 were located in the inner circle. These variables did not have adequate structured variation to be distinguished during processing (Li et al., 2020). The cooked sample was associated with meaty notes. The reheated sample was correlated with the other six sensory notes (grass, fatty, cardboard, metallic, oxidized vegetable oil, and hard-boiled egg), showing a strong WOF and weak meaty aroma, confirming the APA results. What's more, 3-(methylthio)propanal, dimethyl disulfide, 2-methyl-thiophene, and 1-octen-3-one, mainly sulfur-containing compounds, were positively correlated with the meaty aroma (P < 0.05). Other aroma-active compounds were relatively concentrated on the right side of the plot, close to the reheated samples and the other sensory attributes, except for 2-methyl-3-buten-2-ol, phenethyl alcohol, and 2-acetyl-2-thiazoline (OAVs <1). They were positively correlated with those undesirable sensory notes (grassy, fatty, cardboard, metallic, oxidized vegetable oil, and hard-boiled egg) (P < 0.05) (Fig. 2B). The result was consistent with that of a previous report (Pegg et al., 2014), revealing that the intensity of the off-flavor notes was positively correlated with the content of carbonyl compounds produced via lipid oxidation. Furthermore, hexanal, octanal, nonanal, (E,E)-2,4-nonadienal, and (E)-2-nonenal had a VIP of ≥1, P values of <0.05, indicating that they contributed significantly to the discrimination between the two groups. Moreover, they all exhibited higher OAVs (>10 in cooked; >50 in reheated), all of which increased in the reheated samples.
3.7. Aroma recombination and omission experiments
The cooked- and reheated-recombinant models exhibited very good similarities to the original food samples (Fig. 2D, E), confirming the correct identification and quantitation of all key aroma compounds of the cooked and reheated samples. The aroma contribution of a single odorant to the overall aroma of the cooked and reheated stewed beef was assessed using omission experiments. A total of 24 and 23 aroma omission models were built for the cooked and reheated samples, respectively. As shown in Table 4, all aroma-active compounds with an OAV of <1 exhibited no importance for the overall aroma of the cooked and reheated stewed beef. Omitting hexanal, (E)-2-decenal, pentanal, (E)-2-undecenal, octanal, 1-octen-3-ol, 3-(methylthio)propanal, (E,E)-2,4-nonadienal, 1-octen-3-one, and (E,E)-2,4-decadienal caused a significant change in the aroma of the cooked-recombinant model (P < 0.05), revealing that they contributed significantly to the overall aroma of the cooked stewed beef and were selected as the key aroma-active compounds. The results also showed that 11 aroma compounds, including hexanal, (E)-2-nonenal, pentanal, (E,E)-2,4-nonadienal, decanal, (E)-2-undecenal, 1-octen-3-ol, (E,E)-2,4-decadienal, octanal, (E)-2-octenal, and heptanal, were the key aroma-active compounds of reheated precooked stewed beef. They were positively correlated with oxidized vegetable oil, hard-boiled egg, fatty, metallic, grassy, and cardboard notes (P < 0.05) (Fig. 2B). These sensory notes are closely related to the WOF based on sensory evaluation and previous studies (An et al., 2022; Lage et al., 2012).
Table 4.
The triangle test results of each key aroma-active compounds by omission experiments in cooked and reheated Chinese stewed beef.
| cooked |
reheated |
||||
|---|---|---|---|---|---|
| key aroma-active compoundsa | n (cooked)b | significantc | key aroma-active compoundsa | n (reheated)b | significantc |
| hexanal | 12 | *** | hexanal | 12 | *** |
| (E)-2-decenal | 11 | *** | (E)-2-nonenal | 9 | ** |
| pentanal | 11 | *** | (E,E)-2, 4-nonadienal | 9 | ** |
| (E)-2-undecenal | 10 | *** | pentanal | 9 | ** |
| octanal | 9 | ** | decanal | 9 | ** |
| 3-(methylthio)propanal | 9 | ** | (E)-2-undecenal | 9 | ** |
| 1-octen-3-ol | 9 | ** | 1-octen-3-ol | 9 | ** |
| 1-octen-3-one | 8 | * | (E,E)-2, 4-decadienal | 8 | * |
| (E,E)-2,4-nonadienal | 8 | * | octanal | 8 | * |
| (E,E)-2,4-decadienal | 8 | * | heptanal | 8 | * |
| (E)-2-nonenal | 7 | NS | (E)-2-octenal | 8 | * |
| nonanal | 7 | NS | nonanal | 7 | NS |
| heptanal | 6 | NS | (E)-2-decenal | 6 | NS |
| (E)-2-octenal | 6 | NS | (E,E)-2,4-heptadienal | 4 | NS |
| decanal | 6 | NS | methyleugenol | 3 | NS |
| dodecanal | 5 | NS | (Z)-3-hexen-1-ol | 3 | NS |
| (Z)-3-hexen-1-ol | 5 | NS | 1-octen-3-one | 3 | NS |
| 1-hexanol | 3 | NS | dodecanal | 3 | NS |
| 2-acetyl-2-thiazoline | 3 | NS | 3-(methylthio)propanal | 2 | NS |
| 4-methyl-5-thiazoleethanol | 3 | NS | benzyl alcohol | 2 | NS |
| benzaldehyde | 3 | NS | 5-methyl-2-ethylpyrazine | 1 | NS |
| benzyl alcohol | 2 | NS | formic acid octyl ester | 1 | NS |
| methyleugenol | 1 | NS | 3-phenyl-2-propenal | 1 | NS |
| 3-phenyl-2-propenal | 1 | NS | |||
Aroma-active compounds involved in aroma recombination experiments.
Number of correct judgments from 12 assessors by the triangle test.
Significance: * significant (α ≤ 0.05), ** highly significant (α ≤ 0.01), *** very highly significant (α ≤ 0.001), NS, no significant different.
3.8. Changes in the concentrations of key aroma-active compounds of the precooked stewed beef during cooking-refrigeration-reheating
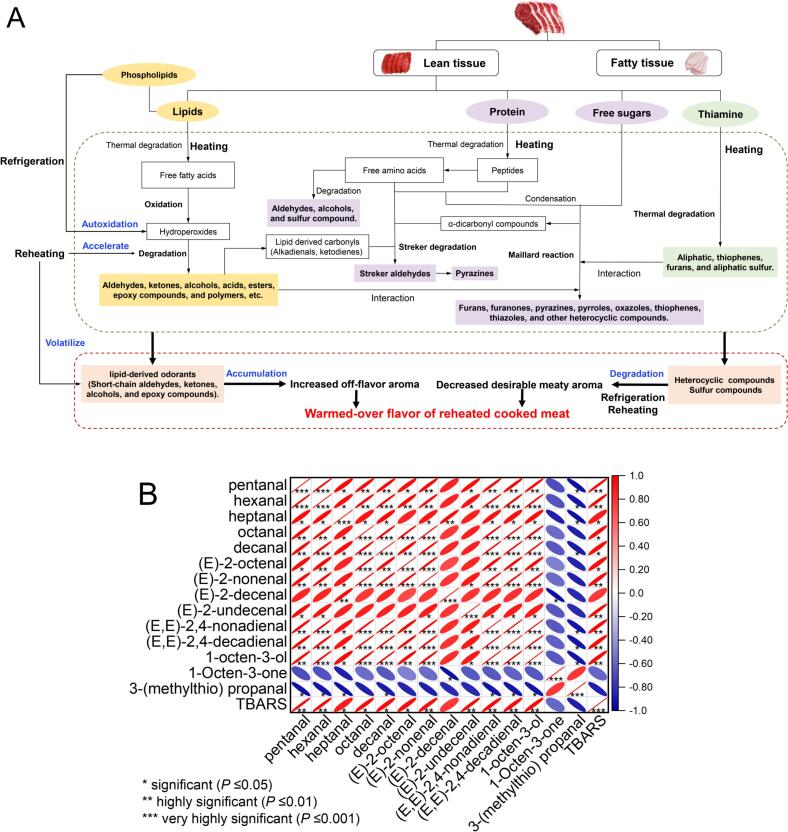
To clarify the patterns of variation in the WOF of the precooked stewed beef during the cooking-refrigeration-reheating, the aroma profiles of the refrigerated samples were detected by Q Exactive GC-Orbitrap-MS system. After 6 days of refrigeration, the concentrations of 10 key odorants were significantly increased (Table 5) (P < 0.05) because of the lipid autoxidation during chill storage. Notably, the increase rates decreased for pentanal, hexanal, heptanal, (E,E)-2,4-nonadienal, (E,E)-2,4-decadienal, and 1-octen-3-ol. This might be because on the one hand, the shorter reheating time produced limited oxidation products compared with the refrigeration period; on the other hand, reheating promoted the volatilization of the aroma compounds and increased the number of binding sites which promoted the irreversible covalent binding between aldehydes and proteins (Kuhn, Considine, & Singh, 2008; Wang, Yang, et al., 2023; Zhang, Kang, Zhang, & Lorenzo, 2021), thus reducing the formation rate of off-flavor substances. Unlike aldehydes, alcohols and proteins are predominantly bound via reversible noncovalent bonding (Wang & Arntfield, 2017). As a result, they tend to be released during subsequent reheating processes, which agrees with the result in our study that 1-octen-3-ol significantly increased after reheating. Nonetheless, the rate of increase in 1-octen-3-ol during reheating (71.84%) was lower than that during refrigeration (164.94%), illustrating that the WOF of the precooked stewed beef developed primarily during the refrigeration stage. Although double bonds may enhance the affinity of aroma compounds with proteins, potentially involving a “Michael addition” mechanism with the double bond (Kuhn et al., 2008) and resulting in the loss of aroma compounds, it was observed that the rate of concentration increase for (E)-2-octenal and (E)-2-nonenal was higher after reheating compared with that after refrigeration. This might be attributed to their pronounced formation induced by lipid oxidation during reheating. 3-(methylthio)propanal and 1-octen-3-one have been identified as key odorants in cooked meat (Aliani & Farmer, 2005; Kerscher & Grosch, 1997). They were positively correlated with the meaty aroma of the precooked stewed beef (P < 0.05) (Fig. 2B) and selected as key aroma-active compounds of the cooked stewed beef in our study. The concentration of 1-octen-3-one significantly decreased following refrigeration, subsequently increasing after reheating (P < 0.05). Its loss during refrigeration was probably because of its release to the environment and bonding to proteins (Wang, Yang, et al., 2023). However, ketones only form weak reversible hydrophobic contacts with proteins (Wang & Arntfield, 2016), which are weakened upon heating (Kuhn et al., 2008). Thus, after reheating, the concentration of 1-octen-3-one increased (P < 0.05), which also accounted for the continued lipid oxidation during reheating. Meanwhile, 1-octen-3-one was also selected as a WOF indicator for boiled beef by AEDA (Konopka & Grosch, 1991), but it was not identified as a key odorant for the formation of WOF in the reheated samples of our study. This might be due to the fact that OAV values were calculated, and the matrix effect was considered in this study. When meat is cooked, the primary reactions responsible for the development of the characteristic meat aroma are the oxidation of lipids, Maillard reaction between amino acids and sugars, Strecker reaction, and degradation of thiamine (Elmore & Mottram, 2006; Khan et al., 2015). During cooking, the reactions occur rapidly and provide a varied profile of aromas that contribute to desirable aromas. While such reactions also lead to off-flavors (such as WOF) during long-term storage (Mottram, 1998) (Fig. 3A). This was also corroborated in our study, where compounds such as, pentanal, hexanal, (E)-2-undecenal, (E,E)-2,4-nonadienal, (E,E)-2,4-decadienal, and 1-octen-3-ol, which are products of lipid oxidation, were characterized as the key aroma-active compounds of the freshly stewed beef and key contributors to the WOF of the reheated precooked stewed beef. The differences in their concentrations during cooking-refrigeration-reheating were remarkable. The concentration of 3-(methylthio)propanal decreased during cooking-refrigeration-reheating. 3-(methylthio)propanal, which is related to the Strecker degradation of methionine, serves as a valuable indicator for assessing the flavor acceptability in products (Han, Cai, Cheng, & Sun, 2021). The Maillard reaction produces heterocyclic compounds, sulfur-containing aroma compounds, furanones and their derivatives, etc., which are responsible for the desirable aroma of meat products (Elmore & Mottram, 2006; Khan et al., 2015) (Fig. 3A). Thus, the loss of 3-(methylthio)propanal led to the diminished meaty attribute of the precooked stewed beef, which was also verified via the omission experiment. Furthermore, 3-(methylthio)propanal decreased after reheating at a reduced rate (36.53%) compared with that after refrigeration (16.26%), suggesting that the degradation in the desirable aroma of the precooked stewed beef occurred primarily during the refrigeration period. In addition, 3-(methylthio)propanal is a labile aldehyde that may break down into low-boiling compounds (Drumm & Spanier, 2002). Furthermore, the binding of sulfur compounds to proteins can be categorized as an irreversible covalent interaction (Zhang et al., 2021). Numerous studies have proven that heating facilitates covalent binding as well as the volatilization of flavor compounds (Kuhn et al., 2008; Wang, Yang, et al., 2023). These contributed to the reduction of 3-(methylthio)propanal. Interestingly, despite 1-octen-3-one and 3-(methylthio) propanal exhibiting notable reductions after refrigeration, their impact on the development of WOF in the precooked stewed beef differed. The decrease in the former might alleviate the lipid-oxidized aroma, whereas the reduction in the latter accentuated the WOF by lessening the desirable meaty aroma. Similarly, they both underwent changes that might be associated with matrix effects, particularly those involving proteins in the matrix. Protein is an important component in meat products responsible for flavor loss (Zhang et al., 2021). The dynamic reaction between aroma compounds and the components of food matrices is complex and related to the chemical structure and functional groups of proteins and aroma compounds, as well as the stability and chemical activity of aroma compounds, impacting the overall aroma profile of the precooked stewed beef (Zhang et al., 2021). Wang, Yang, et al. (2023) detected the significant flavor dissipation of soy sauce–marinated beef during the air cooling stage, highlighting that the loss of the odorants might be caused by volatilization and the covalent binding of aroma compounds to proteins. In addition, Wang, Han, et al. (2023) pointed out that the binding ability of aroma substances to proteins differ with changing processing conditions. To date, the interaction mechanism of each key odorant to proteins during cooking-refrigeration-reheating is unclear, which will be the focus of our future research. Based on the above results, the WOF in the reheated precooked stewed beef was attributed to hexanal, (E,E)-2,4-decadienal, (E,E)-2,4-nonadienal, pentanal, decanal, octanal, heptanal, (E)-2-octenal, (E)-2-undecenal, 1-octen-3-ol, and (E)-2-nonenal, as well as increased aldehydes, which contributed to the oxidized aroma (An et al., 2022). This finding is consistent with those of previous reports (Konopka & Grosch, 1991; O'Sullivan et al., 2003; Pegg et al., 2014; Ruenger et al., 1978; Zang et al., 2019). (E)-2-undecenal, which was considered an off-flavor aldehyde (Chen, Wang, Cao, & Liu, 2019), was identified as a key aroma-active compound contributing to the WOF in the reheated precooked stewed beef for the first time in this study to the best of our knowledge. The significantly increased concentration of (E)-2-undecenal after reheating imparted the precooked stewed beef with a significantly enhanced metallic aroma, which confirmed the results of the APA. Moreover, our study revealed for the first time that the reduction of 3-(methylthio)propanal greatly contributed to an overall increase in WOF in the precooked stewed beef. In addition, the ratio of the total concentration of the 11 key odorants of WOF in the reheated precooked stewed beef to that amount of 3-(methylthio)propanal increased from 450.65 in the cooked samples to 1529.45 in the refrigerated samples and to 3657.45 in the reheated samples, with the increase rate of the concentration decreasing during reheating, which supported the aforementioned idea that the deterioration of the flavor quality of the precooked stewed beef occurred mainly during refrigeration.
Table 5.
Concentration changes of key aroma-active compounds in Chinese stewed beef during cooking-refrigeration-reheating.
| key aroma-active compoundsa | significantb |
concentrations (μg/kg)c |
increase rate (%)d |
||||
|---|---|---|---|---|---|---|---|
| cooked | reheated | cooked | refrigerated | reheated | cooked - refrigerated | refrigerated - reheated | |
| pentanal | *** | ** | 31.57 ± 1.14C | 59.81 ± 10.17B | 102.38 ± 8.93 A | 91.90 ± 29.44× | 67.02 ± 8.07y |
| hexanal | *** | *** | 246.17 ± 21.36C | 625.10 ± 47.22B | 1070.33 ± 108.94 A | 155.42 ± 25.35× | 71.00 ± 3.63y |
| heptanal | NS | * | 32.99 ± 0.25C | 62.56 ± 0.79B | 69.46 ± 3.68 A | 89.64 ± 0.87× | 10.99 ± 3.67y |
| octanal | ** | * | 2.01 ± 0.28C | 6.73 ± 0.88B | 17.89 ± 0.32 A | 263.84 ± 21.14× | 167.71 ± 21.29× |
| decanal | NS | ** | 2.56 ± 0.15C | 4.47 ± 0.12B | 6.84 ± 0.21 A | 74.72 ± 11.71× | 53.10 ± 1.86× |
| (E)-2-octenal | NS | * | 3.89 ± 0.05C | 5.99 ± 0.24B | 13.49 ± 0.57 A | 53.93 ± 4.40y | 125.23 ± 6.14× |
| (E)-2-nonenal | NS | ** | 2.93 ± 0.14C | 4.01 ± 0.15B | 6.26 ± 0.19 A | 36.92 ± 9.11y | 56.22 ± 1.29× |
| (E)-2-decenal | *** | NS | 1.89 ± 0.05B | 2.28 ± 0.07 A | 2.33 ± 0.11 A | 20.50 ± 5.53× | 5.01 ± 0.67y |
| (E)-2-undecenal | *** | ** | 1.83 ± 0.13C | 2.55 ± 0.03B | 3.04 ± 0.56 A | 39.57 ± 8.54× | 27.01 ± 6.66× |
| (E,E)-2,4-nonadienal | * | ** | 1.11 ± 0.01C | 3.14 ± 0.06B | 5.75 ± 0.14 A | 182.59 ± 7.34× | 83.37 ± 0.71y |
| (E,E)-2,4-decadienal | * | * | 0.03 ± 0.00C | 0.09 ± 0.00B | 0.21 ± 0.05 A | 194.50 ± 17.96× | 103.26 ± 4.63y |
| 1-octen-3-ol | ** | ** | 7.00 ± 0.14C | 18.54 ± 0.57B | 31.85 ± 0.48 A | 164.94 ± 10.65× | 71.84 ± 2.56y |
| 1-octen-3-one | * | NS | 1.75 ± 0.11 A | 1.28 ± 0.06B | 1.41 ± 0.01B | −26.78 ± 0.82y | 13.41 ± 1.14× |
| 3-(methylthio)propanal | ** | NS | 0.74 ± 0.09 A | 0.50 ± 0.00B | 0.42 ± 0.02B | −36.53 ± 0.13× | −16.26 ± 2.83y |
| concentration ratioe | 450.65 ± 28.10C | 1529.45 ± 126.94B | 3657.45 ± 613.30 A | 241.33 ± 42.25× | 137.57 ± 20.92y | ||
Key aroma-active compounds of cooked and reheated Chinese stewed beef.
Significance: * significant (α ≤ 0.05), ** highly significant (α ≤ 0.01), *** very highly significant (α ≤ 0.001), NS, no significant difference. Different uppercase letters show significant differences in concentrations in each row at P < 0.05. Different lowercase letters show significant differences in increase rates in each row at P < 0.05.
Concentrations of aroma-active compounds.
the rate of increase in the concentration of key odorants after refrigeration and reheating.
Ratio of total contents of the key aroma-active compounds contributing to WOF of reheated prepared stewed beef to the key odorants associated with meaty notes.
Fig. 3.
Mechanism of formation of warmer-over flavor (WOF) in meat products (A) and heat map representations of results of pearson correlation analysis between key aroma-active compounds and TBARS (B).
3.9. Correlation between TBARS and key aroma-active compounds
(E)-2-decenal and 1-octen-3-one, the key odorants of the cooked stewed beef, did not exhibit a significant correlation with TBARS (P > 0.05) (Fig. 3B). Although these two substances were lipid oxidation products, were not selected as key odorants of the reheated stewed beef in the omission experiments. Fig. 3B shows a significantly positive correlation between TBARS and the 11 key aroma-active compounds of the reheated precooked stewed beef (P ≤ 0.05). The outcome further validated that refrigeration and reheating promoted lipid oxidation, thus facilitating the formation of WOF. As shown in Fig. 3A, refrigeration promoted lipid autoxidation of the cooked stewed beef, resulting in the formation of hydroperoxides, which are quite unstable and tend to degrade. Their degradation generated large amounts of secondary oxidation products, such as alcohols, aldehydes, and ketones. Low-molecular-weight aldehydes (C3-C12) produced by the degradation of hydroperoxides, such as pentanal, hexanal, heptanal, octanal, (E,E)-2,4-nonadienal, and (E,E)-2,4-decadienal, are of great importance as they contribute to the formation of WOF (Pegg et al., 2014). Thereafter, reheating accelerated lipid oxidation and led to the volatilization of these active off-flavor compounds, contributing to more noticeable unpleasant WOF notes (Pegg et al., 2014).
4. Conclusions
The reheated precooked stewed beef exhibited a strong WOF, presenting with a diminished meaty aroma and enhanced fatty, oxidized vegetable oil, grassy, hard-boiled egg, metallic, and cardboard aroma. Hexanal, (E,E)-2,4-decadienal, (E,E)-2,4-nonadienal, pentanal, decanal, octanal, heptanal, (E)-2-octenal, (E)-2-undecenal, 1-octen-3-ol, and (E)-2-nonenal were identified as key aroma-active compounds contributing to the WOF in the reheated precooked stewed beef. Additionally, the reduction of 3-(methylthio)propanal might greatly contribute to an overall increase in WOF in the reheated precooked stewed beef. Thus, these odorants were elected as potential markers of WOF in the reheated precooked stewed beef. All in all, the increase in lipid oxidation products, interactions between odorants and protein, and volatilization of key odorants might largely affect the development of WOF. Further studies are required to reveal the mechanism of binding between the key odorants contributing to WOF and proteins during cooking-refrigeration-reheating will be taken into account, and TOFE-based off-flavor correction technologies will be combined for better handling of the flavor quality of precooked stewed beef dishes.
CRediT authorship contribution statement
Junmei Liu: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Siyang Deng: Writing – review & editing. Jingfan Wang: Writing – review & editing, Formal analysis. Feng Huang: Project administration, Funding acquisition, Conceptualization. Dong Han: Formal analysis. Ying Xu: Writing – review & editing, Formal analysis. Ping Yang: Project administration, Funding acquisition, Conceptualization. Chunhui Zhang: Supervision, Project administration, Funding acquisition, Conceptualization. Christophe Blecker: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors gratefully acknowledge the Innovation and Application of Smart Production Technology for the Whole Chain of Precooked Dishes (2022TZXD0021), the Diversified Processing of Precooked Dishes and Traditional Foods (G2022-IFST-05), the Central Public-interest Scientific Institution Basal Research Fund (S2022JBKY-08), and the "Tianchi Talent" Recruitment Program for the financial supports. We thank Shandong Hanon Scientific Instruments Co., Ltd., Beijing 100192, China for assistance on HS-GC-IMS measurements.
Contributor Information
Ping Yang, Email: yangping01@caas.cn.
Chunhui Zhang, Email: dr_zch@163.com.
Data availability
Data will be made available on request.
References
- Aliani M., Farmer L.J. Precursors of chicken flavor. II. Identification of key flavor precursors using sensory methods. Journal of Agricultural and Food Chemistry. 2005;53(16):6455–6462. doi: 10.1021/jf050087d. [DOI] [PubMed] [Google Scholar]
- An Y., Wen L., Li W., Zhang X., Hu Y., Xiong S. Characterization of warmed-over flavor compounds in surimi gel made from silver carp (Hypophthalmichthys molitrix) by gas chromatography-ion mobility spectrometry, aroma extract dilution analysis, aroma recombination, and omission studies. Journal of Agricultural and Food Chemistry. 2022;70(30):9451–9462. doi: 10.1021/acs.jafc.2c02119. [DOI] [PubMed] [Google Scholar]
- Beldarrain L.R., Moran L., Sentandreu M.A., Barron L.J.R., Aldai N. Effect of ageing time on the volatile compounds from cooked horse meat. Meat Science. 2022;184:108692. doi: 10.1016/j.meatsci.2021.108692. [DOI] [PubMed] [Google Scholar]
- Cerny C., Grosch W. Quantification of character-impact odour compounds of roasted beef. Zeitschrift fuer Lebensmittel-Untersuchung und -Forschung. 1993;196(5):417–422. doi: 10.1007/bf01190805. [DOI] [Google Scholar]
- Chen H., Wang Y., Cao P., Liu Y. Thermal oxidation rate of oleic acid increased dramatically at 140 °C studied using electron spin resonance and GC–MS/MS. Journal of the American Oil Chemists’ Society. 2019;96(8):937–944. doi: 10.1002/aocs.12213. [DOI] [Google Scholar]
- Drumm T.D., Spanier A.M. Changes in the content of lipid autoxidation and sulfur-containing compounds in cooked beef during storage. Journal of Agricultural and Food Chemistry. 2002;39(2):336–343. doi: 10.1021/jf00002a023. [DOI] [Google Scholar]
- Elmore J.S., Mottram D.S. The role of lipid in the flavour of cooked beef. Developments in Food Science. 2006;43:375–378. doi: 10.1016/S0167-4501(06)80089-0. [DOI] [Google Scholar]
- Elmore J.S., Mottram D.S., Enser M., Wood J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. Journal of Agricultural and Food Chemistry. 1999;47(4):1619–1625. doi: 10.1021/jf980718m. [DOI] [PubMed] [Google Scholar]
- Feng L., Tang N., Liu R., Gong M., Wang Z., Guo Y.…Chang M. The relationship between flavor formation, lipid metabolism, and microorganisms in fermented fish products. Food & Function. 2021;12(13):5685–5702. doi: 10.1039/d1fo00692d. [DOI] [PubMed] [Google Scholar]
- Gąsior R., Wojtycza K., Majcher M.A., Bielińska H., Odrzywolska A., Bączkowicz M., Migdał W. Aroma compounds in roasted white Kołuda goose. Journal of Agricultural and Food Chemistry. 2021;69(21):5986–5996. doi: 10.1021/acs.jafc.1c01475. [DOI] [PubMed] [Google Scholar]
- Giri A., Osako K., Ohshima T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chemistry. 2010;120(2):621–631. doi: 10.1016/j.foodchem.2009.10.036. [DOI] [Google Scholar]
- Han D., Zhang C.H., Fauconnier M.L., Mi S. Characterization and differentiation of boiled pork from Tibetan, Sanmenxia and Duroc x (Landrac x Yorkshire) pigs by volatiles profiling and chemometrics analysis. Food Research International. 2020;130:108910. doi: 10.1016/j.foodres.2019.108910. [DOI] [PubMed] [Google Scholar]
- Han Z., Cai M.J., Cheng J.H., Sun D.W. Effects of constant power microwave on the adsorption behaviour of myofibril protein to aldehyde flavour compounds. Food Chemistry. 2021;336 doi: 10.1016/j.foodchem.2020.127728. Article 127728. [DOI] [PubMed] [Google Scholar]
- Kerler J., Grosch W. Odorants contributing to warmed-over flavor (WOF) of refrigerated cooked beef. Journal of Food Science. 1996;61(6):1271–1275. doi: 10.1111/j.1365-2621.1996.tb10977.x. [DOI] [Google Scholar]
- Kerscher R., Grosch W. Comparative evaluation of potent odorants of boiled beef by aroma extract dilution and concentration analysis. Zeitschrift für Lebensmitteluntersuchung und-Forschung A. 1997;204(1):3–6. doi: 10.1007/Pl00005496. [DOI] [Google Scholar]
- Khan M.I., Jo C., Tariq M.R. Meat flavor precursors and factors influencing flavor precursors--a systematic review. Meat Science. 2015;110:278–284. doi: 10.1016/j.meatsci.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Konopka U.C., Grosch W. Potent odorants causing the warmed-over flavour in boiled beef. Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 1991;193:123–125. doi: 10.1007/BF01193360. [DOI] [Google Scholar]
- Kuhn J., Considine T., Singh H. Binding of flavor compounds and whey protein isolate as affected by heat and high pressure treatments. Journal of Agricultural and Food Chemistry. 2008;56(21):10218–10224. doi: 10.1021/jf801810b. [DOI] [PubMed] [Google Scholar]
- Lage M.E., Godoy H.T., Bolini H.M.A., de Oliveira R.R., Rezende C.S.M.E., Prado C.S. Sensory profile of warmed-over flavour in tenderloin from steers supplemented with alpha-tocopherol. Revista Brasileira de Zootecnia. 2012;41(8):1915–1920. doi: 10.1590/s1516-35982012000800016. [DOI] [Google Scholar]
- Lee G.H., Suriyaphan O., Cadwallader K.R. Aroma components of cooked tail meat of American lobster (Homarus americanus) Journal of Agricultural and Food Chemistry. 2001;49(9):4324–4332. doi: 10.1021/jf001523t. [DOI] [PubMed] [Google Scholar]
- Li C., Li X., Huang Q., Zhuo Y., Xu B., Wang Z. Changes in the phospholipid molecular species in water-boiled salted duck during processing based on shotgun lipidomics. Food Research International. 2020;132 doi: 10.1016/j.foodres.2020.109064. Article 109064. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang Z., Zhang D., Shen Q., Hui T., Ma J. Generation of key aroma compounds in Beijing roasted duck induced via Maillard reaction and lipid pyrolysis reaction. Food Research International. 2020;136:109328. doi: 10.1016/j.foodres.2020.109328. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang Z., Zhang D., Shen Q., Pan T., Hui T., Ma J. Characterization of key aroma compounds in Beijing roasted duck by gas chromatography-olfactometry-mass spectrometry, odor-activity values, and aroma-recombination experiments. Journal of Agricultural and Food Chemistry. 2019;67(20):5847–5856. doi: 10.1021/acs.jafc.9b01564. [DOI] [PubMed] [Google Scholar]
- Merlo T.C., Lorenzo J.M., Saldana E., Patinho I., Oliveira A.C., Menegali B.S.…Contreras-Castillo C.J. Relationship between volatile organic compounds, free amino acids, and sensory profile of smoked bacon. Meat Science. 2021;181 doi: 10.1016/j.meatsci.2021.108596. Article 108596. [DOI] [PubMed] [Google Scholar]
- Mottram D.S. Flavour formation in meat and meat products: a review. Food Chemistry. 1998;62(4):415–424. doi: 10.1016/s0308-8146(98)00076-4. [DOI] [Google Scholar]
- O’Sullivan M.G., Byrne D.V., Jensen M.T., Andersen H.J., Vestergaard J. A comparison of warmed-over flavour in pork by sensory analysis, GC/MS and the electronic nose. Meat Science. 2003;65(3):1125–1138. doi: 10.1016/S0309-1740(02)00342-X. [DOI] [PubMed] [Google Scholar]
- Pang X., Guo X., Qin Z., Yao Y., Hu X., Wu J. Identification of aroma-active compounds in Jiashi muskmelon juice by GC-O-MS and OAV calculation. Journal of Agricultural and Food Chemistry. 2012;60(17):4179–4185. doi: 10.1021/jf300149m. [DOI] [PubMed] [Google Scholar]
- Pegg R.B., Kerrihard A.L., Shahidi F. In: Encyclopedia of meat sciences. Dikeman M., Devine C., editors. Academic Press; 2014. Cooking of meat|warmed-over flavor; pp. 410–415. [DOI] [Google Scholar]
- Ruenger E.L., Reineccius G.A., Thompson D.R. Flavor compounds related to the warmed-over flavor of Turkey. Journal of Food Science. 1978;43(4):1198–1200. doi: 10.1111/j.1365-2621.1978.tb15268.x. [DOI] [Google Scholar]
- Straßer S., Schieberle P. Characterization of the key aroma compounds in roasted duck liver by means of aroma extract dilution analysis: Comparison with beef and pork livers. European Food Research and Technology. 2014;238(2):307–313. doi: 10.1007/s00217-013-2095-6. [DOI] [Google Scholar]
- Sun J., Ma M., Sun B., Ren F., Chen H., Zhang N., Zhang Y. Identification of characteristic aroma components of butter from Chinese butter hotpot seasoning. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.127838. Article 127838. [DOI] [PubMed] [Google Scholar]
- Tamura H., Boonbumrung S., Yoshizawa T., Varanyanond W. Volatile components of the essential oils in the pulp of four yellow mangoes (Mangifera indica L.) in Thailand. Food Science and Technology Research. 2000;6(1):68–73. doi: 10.3136/fstr.6.68. [DOI] [Google Scholar]
- Tims M.J., Watts B.M. Protection of cooked meats with phosphates. Food Technology. 1958;12:240–243. [Google Scholar]
- Van Gemert L.J. Odour thresholds: compilations of odour threshold values in air, water and other media. Oliemans Punter & Partners BV; The Netherlands: 2011. [Google Scholar]
- Wang F., Gao Y., Wang H., Xi B., He X., Yang X., Li W. Analysis of volatile compounds and flavor fingerprint in jingyuan lamb of different ages using gas chromatography-ion mobility spectrometry (GC-IMS) Meat Science. 2021;175:108449. doi: 10.1016/j.meatsci.2021.108449. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang P., Liu J., Yang W., Qiang Y., Jia W.…Fauconnier M.L. Study of the flavor dissipation mechanism of soy-sauce-marinated beef using flavor matrices. Food Chemistry. 2023;437(Pt 1) doi: 10.1016/j.foodchem.2023.137890. Article 137890. [DOI] [PubMed] [Google Scholar]
- Wang K., Arntfield S.D. Probing the molecular forces involved in binding of selected volatile flavour compounds to salt-extracted pea proteins. Food Chemistry. 2016;211:235–242. doi: 10.1016/j.foodchem.2016.05.062. [DOI] [PubMed] [Google Scholar]
- Wang K., Arntfield S.D. Effect of protein-flavour binding on flavour delivery and protein functional properties: A special emphasis on plant-based proteins. Flavour and Fragrance Journal. 2017;32(2):92–101. doi: 10.1002/ffj.3365. [DOI] [Google Scholar]
- Wang T., Han D., Zhao L., Huang F., Yang P., Zhang C. Binding of selected aroma compounds to myofibrillar protein, sarcoplasmic protein, and collagen during thermal treatment: Role of conformational changes and degradation of proteins. Journal of Agricultural and Food Chemistry, 71(46), 17860-17873. 2023 doi: 10.1021/acs.jafc.3c02618. [DOI] [PubMed] [Google Scholar]
- Xia X., Kong B., Liu Q., Liu J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze-thaw cycles. Meat Science. 2009;83(2):239–245. doi: 10.1016/j.meatsci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Yang P., Yu M., Song H., Xu Y., Lin Y., Granvogl M. Characterization of key aroma-active compounds in rough and moderate fire rougui wuyi rock tea (Camellia sinensis) by sensory-directed flavor analysis and elucidation of the influences of roasting on aroma. Journal of Agricultural and Food Chemistry. 2022;70(1):267–278. doi: 10.1021/acs.jafc.1c06066. [DOI] [PubMed] [Google Scholar]
- Zamora R., Gallardo E., Hidalgo F.J. Model studies on the degradation of phenylalanine initiated by lipid hydroperoxides and their secondary and tertiary oxidation products. Journal of Agricultural and Food Chemistry. 2008;56(17):7970–7975. doi: 10.1021/jf801409w. [DOI] [PubMed] [Google Scholar]
- Zang M., Wang L., Zhang Z., Zhang K., Li D., Li X., Wang S., Chen H. Changes in flavour compound profiles of precooked pork after reheating (warmed-over flavour) using gas chromatography–olfactometry–mass spectrometry with chromatographic feature extraction. International Journal of Food Science & Technology. 2019;55(3):978–987. doi: 10.1111/ijfs.14306. [DOI] [Google Scholar]
- Zhang J., Kang D., Zhang W., Lorenzo J.M. Recent advantage of interactions of protein-flavor in foods: Perspective of theoretical models, protein properties and extrinsic factors. Trends in Food Science & Technology. 2021;111:405–425. doi: 10.1016/j.tifs.2021.02.060. [DOI] [Google Scholar]
- Zhang K., Li D., Zang M., Zhang Z., Li X., Wang S.…Zhao B. Comparative characterization of fatty acids, reheating volatile compounds, and warmed-over flavor (WOF) of Chinese indigenous pork and hybrid pork. LWT-Food Science and Technology. 2022;155 doi: 10.1016/j.lwt.2021.112981. Article 112981. [DOI] [Google Scholar]
- Zhao J., Wang M., Xie J., Zhao M., Hou L., Liang J.…Cheng J. Volatile flavor constituents in the pork broth of black-pig. Food Chemistry. 2017;226:51–60. doi: 10.1016/j.foodchem.2017.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.