ABSTRACT
Previous studies suggest that the risk of human infection by hantavirus, a family of rodent-borne viruses, might be affected by different environmental determinants such as land cover, land use and land use change. This study examined the association between land-cover, land-use, land use change, and human hantavirus infection risk. PubMed and Scopus databases were interrogated using terms relative to land use (change) and human hantavirus disease. Screening and selection of the articles were completed by three independent reviewers. Classes of land use assessed by the different studies were categorized into three macro-categories of exposure (‘Agriculture’, ‘Forest Cover’, ‘Urban Areas’) to qualitatively synthesize the direction of the association between exposure variables and hantavirus infection risk in humans. A total of 25 articles were included, with 14 studies (56%) conducted in China, 4 studies (16%) conducted in South America and 7 studies (28%) conducted in Europe. Most of the studies (88%) evaluated land cover or land use, while 3 studies (12%) evaluated land use change, all in relation to hantavirus infection risk. We observed that land cover and land-use categories could affect hantavirus infection incidence. Overall, agricultural land use was positively associated with increased human hantavirus infection risk, particularly in China and Brazil. In Europe, a positive association between forest cover and hantavirus infection incidence was observed. Studies that assessed the relationship between built-up areas and hantavirus infection risk were more variable, with studies reporting positive, negative or no associations.
KEYWORDS: Public Health; epidemiology; disease outbreaks/prevention & control; disease vectors; disease transmission, infectious diseases
Introduction
Hantaviruses are negative-sensed, single-stranded RNA viruses belonging to the Orthohantavirus genus. Various species of rodents serve as reservoir hosts for hantaviruses [1]. Humans can be infected through aerosolized rodent urine, droppings, saliva, or particles containing viral quanta [2], while current evidence does not suggest that human-to-human transmission occurs [3]. Thus, the geographic distribution of human cases of hantavirus infection is closely related to the distributions of its rodent host species [4,5].
In general, there are three clinical syndromes that are caused by hantaviruses: i) Hemorrhagic Fever with Renal Syndrome (HFRS) is caused by Seoul, Dobrava, Saarema, and Hantan viruses and is mostly prevalent in Europe and Asia; ii) Nephropathia Epidemica (NE), a milder form of HFRS, that is mainly caused by Puumala hantavirus and occurs in Europe; iii) Hantavirus CardioPulmonary Syndrome (HCPS) or Hantavirus Pulmonary Syndrome (HPS) caused by Andes virus, Sin Nombre virus, and several other hantaviruses and occurs mostly in the Americas [6]. [7]. To date, more than 50 hantavirus species have been identified and only 24 of them are considered pathologically relevant to humans [8]. Globally, there are around 200 severe HCPS cases reported per year in the Americas and over 100,000 HFRS-NE cases reported per year in Europe and Asia with China accounting for 70%-90% of all cases [5].
Given the potential for multiple environment-rodent-pathogen-human interactions, rodent-borne diseases behave as dynamic systems that often adapt and respond to external perturbations, such as climatic and environmental factors [9]. Among different environmental drivers, land-use and land-use changes have a direct impact on rodent species survival and reproduction, influencing their spatio-temporal distributions [9,10]. Land use drivers include factors such as expansion of agricultural land, deforestation, sprawling of urban environments, habitat fragmentation, and other changes to the biophysical environment [11]. Land use and land use change might act as a selective force that favors the abundance and diversity of reservoir hosts and affects host – pathogen dynamics and prevalence [10]. The risk of zoonotic virus transmission has in fact been observed to be highest from domesticated land as competent species have globally increased in abundance or expanded in range by adapting to human-dominated landscapes [12]. In addition, it has been suggested that land-use drivers and future trajectories could influence the future risk of human infection by shaping rodent density and distribution, exacerbating virus transmission among reservoirs, and increasing rodent-human contacts [9]. However, these associations are likely dependent on the geographical location, reflecting the presence and differences in behaviors, ecological traits and population dynamics of different pathogens, vectors and human populations involved. The generality of the effect of land-use has been questioned as in some areas positive associations have been reported, while null or inverse associations were reported in other areas. For instance, agricultural land has been associated with increased risk of hantavirus infection in China [13–15] and Brazil [16,17], but not in Argentina [18,19] and Europe [20]. Similarly, forest cover has been repeatedly reported as an influential factor for Hantavirus infection risk in Europe [21,22], but not elsewhere [15,23]. In addition, the effect of land use on hantavirus infection risk, are of relevance under the current climate change scenarios. Indeed, climate change has been linked repeatedly to higher risk of hantavirus infection. Land use expansion (e.g. for agricultural purposes) can interact with changing of the climate conditions (e.g. increased temperature or extreme rainfall events) in shaping the hantavirus infection risk (e.g. increase rodent densities [24–26]. Gaining insight into the ways in which distinct land-covers and land-uses relate to the risk of zoonotic virus outbreaks across different regions of the world is essential to mitigate negative impacts on human health, healthcare systems, and economic development, as well as to devise targeted public health interventions that are adapted to different geographic contexts. Here, we present a systematic review combining spatial and spatial-temporal epidemiological studies that evaluate the relationship between HCPS, HFRS, or NE cases with land-cover and land-use, namely the proportion of a specific land cover/use in a given area, and land-use changes trend, namely the relative change of the proportion of a specific land use in a given area in two different time points, with no geographical and temporal limitation.
Methods
This systematic review was conducted following the PRISMA protocol [27].
Study selection
The population, exposures, and outcomes of interest are reported in Table 1, together with the inclusion and exclusion criteria. The search strategy was used to identify studies published between January 1973 and April 2023 and reported in two electronic academic literature databases: PubMed, and Scopus. Full search strings are shown in Supplementary Materials and were based on keywords related to our population, exposure, and outcome of interest. All titles and abstracts obtained were independently screened by three reviewers (MY, GM, AB) to check potential suitability for inclusion. Decision conflicts were resolved through discussions until consensus was reached, all discrepancies were documented, and each excluded article was labeled with reviewers’ rationales. Second, the reviewers independently checked the full text of identified articles by the abstract screening to identify articles fulfilling the inclusion criteria. Manual search of the references of the articles selected for full reading and systematic reviews previously published on the topic was also performed to identify additional articles that could match our inclusion criteria.
Table 1.
Population, exposure, outcomes, and criteria used to assess the eligibility of studies.
| Population | Human Population |
|---|---|
| Exposure | Agriculture, Urbanization, Land use, Land use change, Land cover |
| Outcome(s) | Human Hantavirus Infection: i) Haemorrhagic Fever with Renal Syndrome (HFRS), ii) Nephropathia Epidemica (NE), iii) Hantavirus CardioPulmonary Syndrome (HCPS) |
| Exclusion Criteria | Review and qualitative studies |
| Inclusion criteria | Articles in English |
Data extraction
Finally, the reviewers independently carried out the data extraction process using a predetermined data extraction sheet (Supplementary Materials, Table S1). From each study that met the eligibility criteria we extracted the following information: country and year of origin, study design, population size, exposure of interest, outcome of interest, statistical methodology, other covariates considered in the analyses, major findings, and the inclusion of the study’s limitations. In addition, for those studies that explicitly reported numeric measures of association between the exposure of interest (land cover, land use or land use change) and the outcome under study (prevalence, incidence, or presence of human hantavirus infection), we extracted the point estimate for regression coefficients (Odds Ratios or Relative Risks) as well as its uncertainty values (95% Confidence Intervals (CIs)).
Data analysis
In order to ensure consistency among studies reporting numeric estimates of association, regression coefficients and 95% confidence intervals (CIs) were re-expressed in a comparable unit: a 1% increase in land cover in the selected area. Coefficients extracted from studies using incidence rates (number of new cases divided by resident population in a given time interval) at the municipality/district level were expressed as relative risks (RRs), while coefficients extracted from studies using the presence of the disease in the municipality/district (i.e. occurrence of at least one case in the area) or the point pattern of cases (i.e. geocoded residences) compared to the point pattern of non-cases were expressed as odds ratios (ORs). In addition, commonly investigated land covers and land uses were categorized into three macro-categories: ‘Agriculture’ (including agricultural land uses, such as crops, pastures, and orchards, as well as agricultural land use changes, such as agricultural intensification and natural habitat conversion into crops), ‘Urban Areas’ (including built-up land cover, such as artificial surfaces and human settlements, and urbanization processes, such as active expansion of urban areas), and ‘Forest Cover’ (including land cover and natural habitat characterized by dense vegetation, such as coniferous or tropical forests). This categorization was done to summarize the results of identified studies, including those that did not explicitly report numeric estimates (e.g. studies with a predictive aim adopting machine learning techniques or ecological niche models). For each study and for each of the three macro-categories, we qualitatively evaluated the evidence of association between exposure and human hantavirus infection based on regression coefficients and 95% CIs or, if not reported, by the direction of associations as reported by the authors in the results and discussion sections. Study results were labeled as ‘Negative’ if at least one exposure belonging to the macro-category was inversely associated with the outcome (i.e. an increase in land cover corresponds to lower hantavirus incidence/occurrence), ‘Positive’ if at least one exposure belonging to the macro-category was positively associated with the outcome (i.e. an increase in land cover corresponds to higher hantavirus incidence/occurrence), ‘Bidirectional’ if some exposures belonging to the macro-category showed both positive and negative associations with the outcome within the same study, and ‘Null’ if no evidence of association between the investigated exposure and the outcome was found.
Results
Study characteristics
After exclusion of 67 duplicate records from the initial database search, we screened a total of 351 titles and abstracts for potential eligibility. Among 120 full-text reviewed articles, 22 eligible studies were identified. We identified 3 additional papers from references of identified papers as they fulfilled our eligibility criteria, leading to a final set of 25 studies. Figure 1 describes the eligible article identification process.
Figure 1.
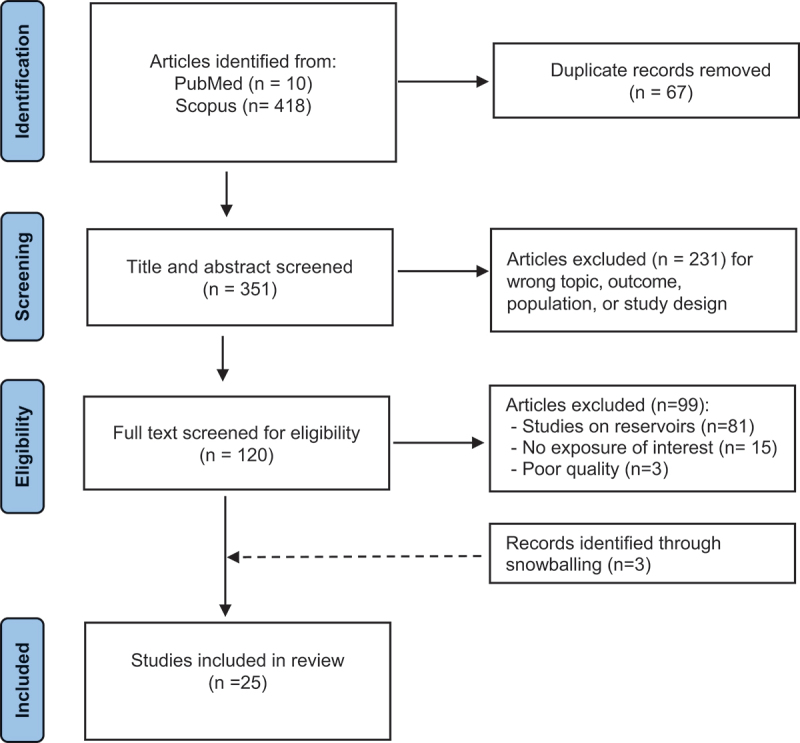
The 25 selected studies were published between April 2004 and April 2023. Data extracted from the selected articles are shown in Table 2. Of the 25 studies included in the review, 14 (56%) were conducted in China [13–15,23,28–37], 7 (28%) in Europe [20–22,38–41], and 4 (16%) in South America [16–19]. Outcome identification was homogeneous among different studies, since all identified studies retrieved data on human hantavirus infection from national health databases recorded by ministries of health or national epidemiological surveillance programs, targeting mainly symptomatic, laboratory-confirmed cases. Concerning the spatial resolution of study outcomes, 9 studies had information of the specific location of cases (point pattern), 4 studies had information at municipality level, and 12 studies had information at a coarser level (e.g. district/province). Concerning the type of exposure, 22 studies evaluated land cover and land use (expressed as the proportion of a specific land cover/use in a given area) while 3 studies explicitly evaluated dynamical land use change (expressed as the relative change of the proportion of a specific land cover in a given area in two different time points) [17,29,37]. More specifically, 16 studies evaluated at least one agricultural land use, 21 studies evaluated forest cover and 14 studies evaluated built-up and artificial land covers (Table 2). Concerning the type of analysis, 15 studies evaluated the relationship between the outcome and exposure of interest on a spatial scale (spatial studies) and 10 studies on a spatio-temporal scale (spatio-temporal studies). Among the 25 studies, 14 applied statistical regression models to determine the association between hantavirus incidence in humans and land-use exposures. the other 9 studies applied predictive models to identify which land-use and land cover features better explain the distribution of human cases (7 studies used machine learning based ecological niche models, and 2 studies used a predictive model based on animal, human, environment contact matrix). The remaining 2 studies applied both statistical regression models and boosted regression trees. Of the 16 studies that applied statistical regression models, extraction of regression coefficients was possible for 11 studies. The list of estimates (RR, OR) referred to specific land cover/use exposures are shown in Table 3. Concerning all studies included in the current review, relationships between land covers and land use macro-categories (‘Agriculture’, ‘Forest’, and ‘Built’) are summarized in Figures 2 and 3.
Table 2.
Study characteristics, methodology and main results.
| Ref, Author, Date | Dis | Location | Period | Spatial (S) vs Spatio-Temporal (ST) |
Spatial Resolution | # of Year | # of Ecological Unit | Exposures of interest for the current Review | Statistical Methods | Multivariable Analysis | Other covariates in the model | Response | Adjusted for Spatial/Temporal Correlation | # of Cases | Pop at risk | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Linard et al. [38] | NE (PUUV) | Belgium | 1994–2004 | S | Municipality Level | 11 | 581 | Forest cover, Built Areas |
Negative Binomial Regression | Yes | Average Income, % Pop Hunting |
Incidence | Yes | >1200 | ~10 million | NE incidence is positively associated with forest cover (especially broad-leaved forests), exposure risk is higher in forested and remote areas. |
| Schwarz et al. [40] | NE (PUUV) | Baden-Wuttenburg,Germany | 2001–2007 | ST | District Level | 8 | 44 | Forest cover | Poisson Regression | Yes | Temperatures, Population density, Year |
Incidence | No | 1,540 | ~10 million | NE incidence is associated with forest cover, especially for beech forest and seed plants forest. |
| Viel et al. [41] | NE (PUUV) | Franche-Comtè, France | 1999–2008 | S | District Level | 9 | 116 | Forest Cover | Poisson Regression | Yes | NDVI | Incidence | Yes | 113 | ~1 million | No evidence of association between NE and forest land cover, however an increased NDVI was associated with NE incidence. |
| Zeimes et al. [21] | NE (PUUV) | Sweden | 1991–1998 | S | Point pattern | 7 | / | Forest Cover | Logistic Regression, Ecological Niche Modelling | Yes | Distance to Forests, Distance to sea, Population density, Snow depth |
Presence/ Absence |
No | 1,726 | ~10 million | Presence of NE cases was associated with the presence of forest cover |
| Barrios et al. (2013) |
NE (PUUV) |
Belgium | 2000–2010 | S | Municipality Level | 11 | 581 | Croplands, Forest Cover, Built Areas, Other Land Use |
Regression Trees | Yes | Land Cover | Presence/ Absence |
No | / | ~10 million | Broad Leaf Forest cover was the most important landscape features determining the spatial spread of NE |
| Zeimes et al. [22] | NE (PUUV) |
Belgium, Finland, France, Netherlands, Norway, Sweden | 2003–2012 | S | Mixture ofPoint Pattern, Municipality and District Level | 10 | / | Forest Cover, Built Areas |
LogisticRegression, Boosted Regression Trees | Yes | Precipitations, Temperature, Contiguity of forests,Population, Enhanced Vegetation Index, Soil Water Index |
Presence/ Absence |
No | / | / | NE occurrence is positively associated with forest cover and built-up areas in forest ecotones. |
| Cunze et al. [39] | NE (PUUV) |
Germany | 2001–2015 | S | District Level | 15 | 402 | Forest Cover, Built areas |
Poisson Regression | Yes | Temperature, Precipitation |
Incidence | No | 573 | ~82 million | The percentage of forest area and the number of recorded PUUV infections is positively associated. |
| Busch et al. [18] | HPS (Andes virus) | Buenos Aires Province, Argentina | 1998–2001 | ST | District Level | 4 | 127 | Croplands | Poisson Regression | Yes | Population density, Rodent Abundance, Evapotranspiration |
Presence/ Absence |
Yes | 85 | ~12 million | No evidence of association between HPS presence and croplands. |
| Prist et al. [16] | HPS | Brazil | 1993–2012 | S | Municipality Level | 20 | 647 | Croplands, Forest Cover | Poisson Regression | Yes | Precipitations, Temperatures, % Rural workers, Human Development Index |
Incidence | Yes | 207 | ~17 million | HPS risk is associated with sugarcane crops, especially in areas covered by the Atlantic Forest. |
| Muylaert et al. (2019) | HPS | Brazil | 1993–2016 | ST | Municipality Level | 24 | 5570 | Forest Cover Change, Croplands Change |
Poisson Regression | Yes | Rodent diversity, Rainfall, Temperature, % Rural Workers | Incidence | Yes | 1795 | ~200 million | HPS risk is associated with maize sugarcane crops, and with forest cover. |
| Vadell et al. [19] | HPS (Andes virus) | Entre Rios, Argentina | 2004–2015 | S | District Level | 12 | 13 | Croplands, Forest Cover, Other Land Use |
Quasi-Poisson Regression, Logistic Regression |
Yes | Distance from Rivers, Rodent Composition |
Incidence & Presence/ Absence |
No | 60 | ~1.3 million | Theprobability of occurrence of HPS was higher in sites with a high percentage of tree cover. |
| Yan et al. [28] | HFRS | China | 1994–1998 | S | County Level | 5 | 1355 | Forest Cover, Built areas, Croplands, Other Land use |
Logistic regression | Yes | Temperature, Precipitation Soil types, Elevation |
Presence/ Absence |
No | 5–30 per year | ~1.2 billion | The logistic regression analysis showed that land for agriculture use, including paddy land, irrigated farmland, non-irrigated farmland, and orchard land, were the landscape elements with high probability of HFRS. |
| Fang et al. [13] | HFRS | BeijingMetropolitan Area, China |
1997–2006 | ST | Townships | 10 | 220 | Forest Cover, Built areas, Croplands, Other Land use |
Poisson Regression | No | / | Incidence | No | 852 | ~13.6 million | Inverse association between built-up land and HFRS incidence; Positive association between orchards, irrigable land, and rice paddies and HFRS incidence. No association between forest cover and HFRS incidence |
| Xiao et al. [23] | HFRS | Changsha, China | 2006–2015 | ST | Point Pattern | 10 | / | Forest Cover, Built Areas, Croplands |
Ecological Niche Modelling | Yes | Temperature, Precipitation, Elevation, Slope, Aspect, Human Footprint index, Population, NDVI | Presence/ Absence |
/ | 327 | ~6 million | The risk level of HFRS is correlated with an increase in area of cultivated and urban land, and a decrease in forested areas. |
| Li et al. [31] | HFRS | China | 2005–2012 | S | Province Level | 8 | 31 | Forest cover, Croplands |
Geographically Weighted Regression | Yes | Temperature, Precipitation, Grain Yield, NDVI, Elevation |
Incidence | / | / | 1.2 billion | HFRS was positively correlated with mosaic forest, shrub-land, grassland |
| Liu et al. (2014) | HFRS | Dongting Lake District, China |
2005–2010 | ST | Point Pattern | 6 | / | Croplands, Other Land Use |
Ecological Niche Modellling | Yes | Temperature, Precipitation, NDVI, Human Footprint Index, Slope, Distance to water sources, | Presence/ Absence |
/ | 296 | / | Cultivated land use and shrublands are associated with HFRS occurrence. |
| Liang et al. [30] | HFRS | Shaanxi Province, China |
2005–2016 | S | County Level | 12 | 80 | Built Area, Croplands, Forest cover, Other land use |
Boosted Regression Trees | Yes | Temperature, Precipitation, Humidity, Wind Speed, Elevation, GDP, Domestic Animal Density, Population Density | Incidence | / | 20,142 | ~37million | HFRS increased with increase of built areas, and croplands. No association with cover forest |
| Xiao et al. [14] | HFRS | Chenzhou, Hunan, China | 2006–2015 | S | Point Pattern | 10 | / | Forest cover, Built areas, Croplands, Other Land Use |
Predictive risk model | Yes | Temperature, Precipitation, Humidity, NDVI, TDVI, Rodent composition | Presence/absence | / | 723 | ~4.7 million | HFRS is positively associated with cultivated land, built-on land, and grassland. Fewer cases are found in forested areas and water-covered areas. |
| Xiao et al. [15] | HFRS | Loudi and Shaoyang, China | 2006–2013 | ST | Point Pattern | 8 | / | Forest cover, Built areas, Croplands, Other Land Use |
Predictive risk model | Yes | Rodent Composition | Presence/ Absence |
/ | 742 | ~10 million | Highest predicted risk of HFRS was found for cultivated land, following by forest and urban land. |
| Tian et al. [29] | HFRS | Hunan, China |
1993–2010 | ST | Province Level | 48 | 13 | Built Area | Poisson regression model | Yes | Rural-urban immigrants, GDP, Elevation, Epidemic Duration | Incidence | Yes | ~110,000 | ~60 million | HFRS incidence and urbanization followed an inverted U-shaped. At the beginning of urbanization, HFRS incidence increases, whereas in the second phase, HFRS incidence decreases. |
| She et al. [34] | HFRS | Shandong, China |
2010–2018 | S | District level | 9 | 137 | Croplands, Forest Cover, Built Areas |
Boosted Regression Trees | Yes | Elevation, GDP, Climatic Factors | Incidence | No | 11,432 | ~100 million | Proportion of cultivated land was positively associated with HFRS incidence. Forest cover shown a inverted U-shaped relationship with HFRS incidence. |
| Shen et al. [37] | HFRS | Xi’an, China | 2005–2018 | ST | Point Pattern | 14 | / | Built Area | Statistical Regression | No | / | Incidence | No | NA | ~10 million | HFRS incidence and urbanization followed an inverted U-shaped. At the beginning of urbanization, HFRS incidence increases, whereas in the second phase, HFRS incidence decreases. |
| Zhu et al. [35] | HFRS | Shaanxi Province, China | 2014–2016 | S | Point Pattern | 3 | / | Croplands, Forest Cover, Built Areas, Other Land Use |
Ecological Niche Modelling | Yes | Climatic Factors, NDVI, Elevation, Topography, Population Density |
Presence/ Absence |
No | 605 | ~1 million | Construction and cultivated were positively influencing HFRS occurrence; forests had low risk transmission. |
| Teng et al. [33] | HFRS | China | 2015–2018 | ST | Region | 4 | 31 | Forest Cover, Built Area | Poisson Regression | Yes | Climatic Factors, Economic Level | Incidence | Yes | NA | 1.2 billion | HFRS incidence was positively associated with Forest land cover and not with the urban indicators. |
| Zhu et al. [36] | HFRS | Weihe Basin, China |
2005–2020 | S | Point Patter | 16 | / | Croplands, Forest Cover, Built Areas, Other Land Use |
Ecological Niche Modelling | Yes | Climatic Factors, NDVI, Elevation, Topography, Population Density |
Presence/ Absence |
No | 26,307 | NA | Construction land was strongly influencing HFRS occurrence; cultivated land, had medium influence, woodland had the lowest transmission |
Table 3.
List of estimates and 95% CI from selected studies by land use macro-category.
| Ref, Author, Date | Disease | Continent | Outcome | RR vs OR |
Forest Exposure | Estimated Effects | Agricultural Exposure | Estimated Effect | Urban Exposure | Estimated Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| Linard et al. [38] | NE (PUUV) | Europe | Incidence | RR | 1 % Increase in Forest Cover | 1.048 (p-value <0.05) | Urbanization level Increase (5 levels) |
0.782 (p-value <0.05) | ||
| Schwarz et al. [40] | NE (PUUV) | Europe | Incidence | RR | 1% Increase in Beech Forest Cover | 1.142 (1.097–1.173) | ||||
| 1% Increase in Seed Plants Cover | 1.229 (1.182–1.277) | |||||||||
| Viel et al. (2010) | NE (PUUV) | Europe | Incidence | RR | 1 % increase in Broadleaf Forest Cover | 1.001 (0.985–1.033) | ||||
| 1 % increase in Coniferous Forest Cover | 1.023 (0.992–1.055) | |||||||||
| 1 % increase in Mixed Forest Cover | 1.048 (0.978–1.124) | |||||||||
| Zeimes et al. [21] | NE (PUUV) | Europe | Presence vs Absence | OR | 1% increase in Forest cover | 1.023 (p value < 0.05) | ||||
| Zeimes et al. [22] | NE (PUUV) | Europe | Presence vs Absence | OR | Built up areas in Forest Ecotones | 1.323 (p value < 0.05) | ||||
| Cunze et al. [39] | NE (PUUV) |
Europe | Incidence | RR | 1 % Increase in Forest Cover | 1.009 (1.005–1.014) | 1% Increase in Urban areas | 0.967 (0.969–1.025) | ||
| Prist et al. [16] | HPS | South America | Presence vs Absence | OR | 1 % Increase in Forest Cover (Cerrado Region) |
1.105 (0.548–1.822) | 1% Increase in Sugarcane (Cerrado Region) |
2.225 (1.419–3.158) | ||
| 1 % Increase in Forest Cover (Atlantic Region) |
1,284 (0.818–1.733) | 1% Increase in Sugarcane (Atlantic Region) |
1.491 (1.161–1.915) | |||||||
| Muylaert et al. (2019) | HPS | South America | Presence vs Absence | OR | 1 % Increase in Forest Cover | 1.336 (1.066–1.671) | 1% Increase in Sugarcane | 1.172 (1.002–1.434) | ||
| 1% Increase in Maize | 1.1996 (1.002–1.363) | |||||||||
| Yan et al. [28] | HFRS | Asia | Incidence | RR | 1 % Increase in Timber Forest Cover | 1.0739 (1.039–1.108) | 1% Increase in Orchards | 1.070 (1.016–1.126) | ||
| Fang et al. [13] | HFRS | Asia | Incidence | RR | 1 % Increase in Forest Cover | 1.006 (0.994–1.017) | 1% Increase in Orchards | 1.043 (1.017–1.070) | 1 % Built Area | 0.992 (0.985–1.001) |
| 1% Increase in Rice | 1.270 (1.040–1.530) | |||||||||
| 1% Increase in Irrigable Land | 1.012 (0.999–1.025) | |||||||||
| Teng et al. [33] | HFRS | Asia | Incidence | RR | 1 million Hectar of Forest | 1.357 (1.005,1.791) | Urbanization Level Principal Components 1 | 1.12 (0.87–1.43) |
Figure 2.
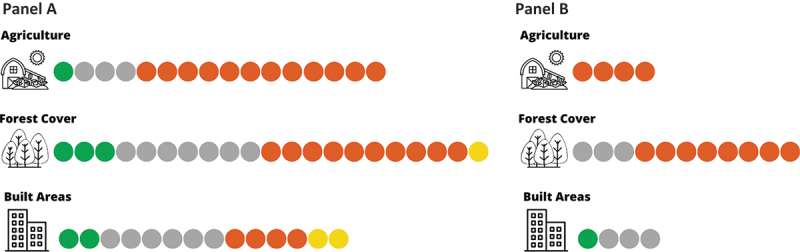
Number of studies suggesting a positive (orange), negative (green), both positive and negative (yellow) and null (gray) relationship between hantavirus infection in humans and macro-categories of land-cover/land-use. Panel A: all studies identified, Panel B: studies reporting coefficient estimates and 95% cis.
Figure 3.
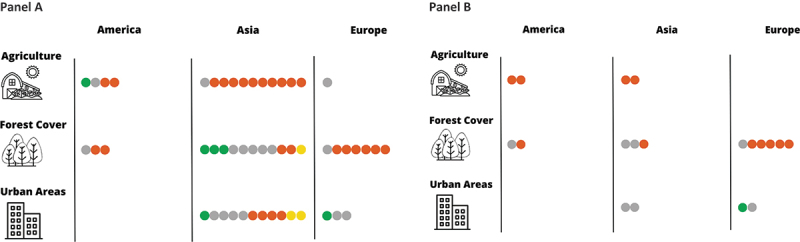
Number of studies suggesting a positive (orange), negative (green), both positive and negative (yellow) and null (gray) relationship between hantavirus infection in humans and macro-categories of land-cover/land-use stratified by geographical area. Panel A: all studies identified, Panel B: studies reporting coefficient estimates and 95% cis.
Agricultural land use
Sixteen studies explicitly evaluated the association between agricultural land uses and the risk of hantavirus infection among humans [13–20,23,28,30,31,34–36,42]. Eleven studies were conducted in Mainland China, four studies in South America, and one in Europe. Most of these studies (12 out of 16) suggested a positive relationship between agricultural land use and the risk of hantavirus infection.
Concerning studies conducted in China, 10 out of 11 studies found a positive association. Using data at county level from the whole of mainland China, Yan et al. [28] first suggested that agricultural land such as orchard cover could increase the risk of HFRS (RR for 1% increase in Orchards: 1.070; 95%CI: 1.016–1.126) [28]. Similarly, Fang et al. [13] found a positive association between HFRS incidence among municipalities of the Beijing metropolitan area and orchard land cover (RR 1% Increase in Orchards: 1.043; 95% CI: 1.017–1.070) and rice paddies cover (RR for 1% increase in Rice paddies: 1.270, 95% CI: 1.040–1.530), but no evidence of association with irrigable land cover (RR for 1% increase: 1.012; 95% CIs: 0.999–1.025) [13]. Xiao et al. [23] developed an Ecological Niche Model (ENM) on HFRS case locations applying a Genetic Algorithm for Rule-set Production (GARP) and detected a higher probability of cases among cultivated areas of the Hunan Province in China [23], while Liu et al. (2014), applying the same methodology, observed that among different land uses, cultivated land and shrublands were those affecting most the probability of HFRS occurrence in the Dongting Lake District, China [32]. Liang et al. [30], applying Boosted Regression Trees, identified both rainfed and irrigated croplands as major spatial drivers of HPRS incidence through the analysis of cases that occurred in the Shanxi province, China, between 2005–2017 [30], similar results were found by She et al. [34] by applying the same methodology in the Shandong area. Xiao et al. [14], using a predictive model based on animal, human, land-use contact matrices identified cultivated lands as the land use with higher risks of human HFRS infection in two different areas of China [14,15]. Zhu et al. [35,36] applying a Maximum Entropy Ecological Niche Model in two distinct areas of China, found that cultivated land positively influenced the spatial distribution of HFRS [35,36]. Li et al. [31], on the contrary did not report any association between cultivated land cover and HFRS cases but a positive association with grain yield at district level in some of the years under study [31]. Out of four studies focused on the agricultural land-use and risk of Hantavirus infection in South America, two found a positive association, one found an inverse association and one did not find strong evidence of association. The two studies conducted in Brazil found evidence of positive association between croplands, agricultural intensity and HPS risk. Among the municipalities belonging to the São Paulo state, Prist et al. [16] detected a positive relationship between municipalities reporting HPS cases and land use for sugarcane farming (OR 1% Increase in Sugarcane in Cerrado Region: 2.225: 95% CI: 1.419–3.158; 1% Increase in Sugarcane in the Atlantic Region: 1.491, 95% CI: 1.161–1.915) [43]. Similarly, Muylaert et al. (2019) observed that the probability of HPS occurrence across Brazil municipalities was associated with agricultural expansion of land dedicated to sugarcane farming (OR for 1% increase in sugarcane: 1.172, 95% CI: 1.002–1.434) and of land dedicated to maize farming (OR for 1% increase in maize 1.1996, 95% CI: 1.002–1.363) [17]. On the contrary, Busch et al. [18] did not find evidence of association between croplands and HPS in Buenos Aires province [18] and Vadell et al. [19] found an inverse association between agricultural land use and HPS risk in Entre Rios, Argentina [19]. The only study conducted in Europe (Belgium) did not identify the agricultural land use as an influencing variable for the distribution of NE cases [20].
Forest cover
Twenty-one studies explicitly evaluated the association between forest cover and the risk of hantavirus infection among humans [13–17,19–23,28,30,31,33–36,38–41]. These studies were conducted in Europe (n = 7), Asia (n = 11) and South America (n = 3). The direction of association between forest cover and hantavirus infection was heterogeneous among different geographic locations.
Six out of seven studies conducted in Europe clearly showed a positive association between forest cover and risk of hantavirus infection [20–22,38–40]. Linard et al. [38] found that NE incidence is positively associated with forest cover among Belgian municipalities, especially broad-leaved forests (RR for 1 % increase in land cover: 1.048, p-value <0.05) [38]. Similarly, applying a regression tree analysis, Barrios et al. [20] found that broad leaf forest coverage was the most influential land use predictor in explaining spatial distribution of NE cases in Belgium [20]. Schwarz et al. [40] detected that the incidence of NE infections in the southern districts of Germany was associated with the proportion of areas covered by beech forests (RR for 1% increase: 1.142, 95% CI: 1.097–1.173) and seed plants (e.g. pines, firs, yew, redwood) estimating a RR for 1% increase equal to 1.229 (95% CI: 1.182–1.277) [40]. Consistently, Cunze et al. [39] detected a positive association between NE incidence and forest cover analyzing data covering all districts of Germany (RR for 1 % increase: 1.009, 95%CI: 1.005–1.014) [39]. In Sweden, Zeimes at al. [21] reported an increased probability of observing NE human cases with forest cover (RR for 1% increase: 1.023, p value < 0.05) [21]. Another study conducted by Zeimes at al. [22] on NE occurrence at the European scale found that NE occurrence was more likely in forests and built-up areas in forest ecotones [22]. On the contrary Viel et al. [41] did not find evidence of association between NE and forest land cover; however, an increased NDVI was associated with NE incidence [41]. Among the eleven studies conducted in China, Yan et al. [28] did not find any evidence of association between timber forest cover and incidence of HFRS (RR for 1% increase: 1.073; 95% CIs: 1.039–1.108) [28]. Similarly, Fang et al. [13] did not find any evidence for forest cover (RR for 1 % increase: 1.006, 95% CIs: 0.994–1.017) [13]. Consistently, Liang et al. [30] as well as Xiao et al. [15] did not detect any significant contribution of covariates linked to forest cover in explaining spatial distribution of HFRS cases [14,30]. On the contrary, Li et al. (2014) analyzing at provincial level all incident cases recorded in China found a positive association between HFRS incidence and mosaic forest/shrublands land cover [31]. Similarly, on a national scale, Teng et al. [33] found that HFRS incidence was positively associated with Forest land cover (RR for 1 million hectare increase of forest: 1.357, 95% CIs:1.005–1.791). Finally, three study found that compared to artificial and croplands, the number of HFRS cases was lower in forest land areas [15,23,36] while one study reported an inverted U-shaped relationship with HFRS incidence and forest cover [37]. In south America, Mulayert et al. (2019) suggested that municipalities at higher probability of reporting HPC cases were those with high proportion of land covered by forests (OR for 1 % increase: 1.336; 95% CIS: 1.066–1.671) [17], while other studies found no evidence of association between natural forest cover and HPC presence both in the Cerrado Region (OR for 1 % increase: 1.105, 95% CIs: 0.548–1.822) and in the Atlantic Forest Region (RR for 1% increase: 1,284 (0.818–1.733) [16]. Lastly, Vadell et al. [19] found a positive association between HPC cases and tree cover in Entre Rios, Argentina [19].
Urban areas
We identified fourteen studies that explored the relationship between built-up areas and Hantavirus infection risk, which provided contrasting results [13–15,20,23,29,30,33–39]. Eleven studies were conducted in Mainland China, two studies in Europe, and none in South America. In China, Tian et al. [29] noted that HFRS incidence rate and urbanization share a u-shaped relationship over time, suggesting that hantavirus infection risk is positively correlated with urbanization in the first stage of urban development where land alteration and population growth are happening at a rapid rate, whereas it is negatively correlated in the second stage after urban population growth reaches a steady rate and little to no further land alteration occurs and sanitation measures are implemented [29]. A similar result was found by Shen et al. [37], who reported that at the beginning of urbanization (urban expansion), HFRS incidence increases, whereas in the second phase, HFRS incidence decreases [37]. Consistently, two studies conducted by Zhu et al. (35,36) found that active construction sites were positively associated with the spatial distribution of HFRS cases [35,36] Fours studies did not detect any association between built areas and HFRS among different areas of China [13,14,33,34]. Two other studies conducted in China [23,30] showed that HFRS increased with increases of built and urban areas. Conversely, two studies found that built areas were negatively correlated with hantavirus cases in the metropolitan area of Beijing [13,15]. Among the three studies conducted in Europe, one study conducted in Belgium [38] showed an inverse association between urbanization index and NE incidence (RR for level increase: 0.782, p-value <0.05), while two studies found no evidence of association [22,39].
Discussion
Emerging zoonotic diseases and reemerging infectious diseases are increasingly recognized as major global issues with potentially significant public health effects. In this study, we reviewed the available evidence in the literature on the association between landscape drivers such as land cover, land use and land-use change and human hantavirus infection, a rodent-borne disease.
Rodents are frequently implicated as hosts of zoonotic diseases [44] and their distribution can be affected by different environmental drivers such as land cover and land use (change) [9]. Overall, we found that different land-covers and land uses can drive and shape the risk of human hantavirus infection across different geographical areas. Hantaviruses are maintained in nature through horizontal transmission within competent rodent populations either through direct or indirect contact from their habitat. Spillovers or cross-species transmission, instead, occur when humans inhale particles containing virus quanta released in the environment [6]. Specifically, land-use could shape the risk of transmission of hantaviruses from the reservoir host to humans by influencing several potential factors including: i) the abundance of competent hosts, ii) the pathogen transmission and prevalence among competent hosts, iii) the probability of rodent-human contact. In this study, results provided by spatial and spatio-temporal epidemiological studies were stratified by land-cover/land-use category (‘Agriculture’, ‘Forest Cover’, ‘Urban Areas’) and geographical area to further elucidate potential mechanisms and explore consistency of associations across different geographical contexts.
Overall, studies included in the systematic review suggested a positive association between agricultural land use and human hantavirus infection. This association was particularly evident among studies conducted in China, with 91% of studies reporting a positive relationship between HFRS distribution and cultivated land, presence of rice paddies and rainfed crops. In South America the relationship was less clear but two studies conducted in Brazil suggested that increased sugarcane and maize production can increase HPS incidence especially in communities characterized by low socio-economic status. Agricultural land-use or land-use change has been repeatedly linked to infectious disease risks in humans [45]. Cultivated land, indeed, represents areas characterized by both sufficient food sources and shelter for rodent survival and human presence [46]. Rodent population density generally responds to levels of food availability, and location-specific rodent species occurrence is driven by changes in food resources. Additionally, human dominated ecosystems such as areas dedicated to cultivated land have been linked to a decrease of biodiversity but often a relative increase in abundance of some species (typically generalist species) [47]. ì Interestingly, rodents belonging to the Sigmodontinae genus, which are the main hantavirus reservoir in South America and Apodemus Agrarius and Rattus norvegicus which are considered the main hantavirus reservoirs in China, are considered generalist species [43,48,49]. In addition, agricultural areas, when compared to natural habitats are also linked to the presence of humans, including rural workers and their families, which may increase the probability of human-rodent contact and of potential exposure risks [16]. A recent meta-analysis including worldwide seroprevalence studies suggested that occupational exposure to agriculture is associated with a higher risk of hantavirus infection (Farmer Occupational Status: OR: 1.875, 95% CI 1.438–2.445) [50]. In addition, rural communities, are also characterized by poorer socio-economic conditions and, thus, may be more likely to be exposed to rodents or their excreta (e.g. for poor housing conditions and poor sewage systems) [51].
This systematic review found that natural land composition such as coniferous forest and seed plant forest could also shape human hantavirus infection risk. A positive association between increased human hantavirus incidence and forest cover, was found in the 86% of studies conducted in Europe (Belgium, Germany, and Poland, Sweden). In Europe, the main reservoir of the Puumala Virus, the most common hantavirus in Europe, is the bank vole (Myodes glareolus) which occurs in forests, especially deciduous and mixed woodland. Major individual risk factors for NE infection in Europe frequently include living close to forests, being employed as a forestry worker, and participating in outdoor activities [52,53]. Several studies have reported also that NE cases in Europe usually occur during the mast years, when climatic and environmental conditions favor beechnut production and, thus, food availability for rodent populations [40]. In South America, the positive association between forest cover and cases of human hantavirus infection was less clear, likely due to the heterogeneity of environmental factors, rodent population composition and different biomes evaluated in the different studies. For instance, Muyalaert et al. (2018) found a positive association between forest cover and HPCS risk analyzing HPCS diagnosed in all Brazilian municipalities, while Prist et al. [16] did not find any evidence of association between forest cover and HPCS incidence when focusing on the Sao Paolo State. Although it is hard to draw general conclusions about the association between hantavirus risk and forest composition in South America, it is apparent that rodent density and distribution are affected by the differential levels of biodiversity that characterize both native and human dominated forests [43].
Results on the relationship between artificial land use and hantavirus infection risk were less conclusive. Studies from Europe reported null results or evidence of decreased risk of hantavirus infection among urban areas. As mentioned before, the bank vole (Myodes glareolus), prefers habitat forests to human dominated landscapes. On the contrary, studies from China showed contrasting results, with studies finding both positive and negative associations with urban land cover. One possible explanation for this variation has been suggested by Tian et al. [29] proposing that the association between HFRS incidence rate and urbanization progression is characterized by a U-shaped relationship over time. This biphasic inverted U-shaped effect of urbanization on the HFRS epidemic was also observed by Shen [37]. Interestingly, two other studies conducted by Zhu [35] found that active construction sites were strongly associated with HFRS cases occurrence. These results might suggest that HFRS incidence is positively associated with urban development in the first stage where rural-to-urban land conversion and human population growth, poor socio-economic conditions increase drastically the interactions between humans and reservoirs able to proliferate in urban settings as the brown rat (Rattus norvegicus) and the black rats (Rattus rattus) [54]. Later, the negative association between HFRS cases and the second stage of urbanization process might be explained by the stabilization of the urban growth, when socio-economic and sanitation conditions improve. However, these findings were not replicated by other studies conducted in China. In addition, this hypothesis has not been tested by any of the studies conducted in South America, which has similarly been characterized by a high urbanization rate over the last few decades [55].
This study is subject to the limitations inherent to the primary studies making up the review. Most of the studies included relied on reported data from ministries of health, which usually rely on passive notification systems, rather than direct measures of hantavirus cases [56]. Aggregated data partially compromise the accuracy of spatial relationship estimation between exposure variables and human cases since the exact location of the probable site of infection cannot be obtained. Additionally, we observed a high heterogeneity between studies in the definition of exposures of interest and covariates, as well as in the dimension of spatial units used (e.g. point pattern, municipalities, provinces), temporal extension and in the statistical methods adopted (e.g. statistical regression vs machine learning techniques). Moreover, most studies applied an ecological study design whereby exposures assessments are made on population averages rather than the individual level. The majority of the studies(88%) adjusted for one or more potential confounders [16–19,21,22,28,29,31,33,38–41], while only two studies performed univariate regression [13,37]. Nine studies included some indicator of socio-economic characteristics of the population under study (e.g. average income, % Rural workers, population density) [16–18,21,22,29,33,38,40], twelve studies included some indicator of climatic parameters of the area under study (e.g. temperature, precipitation, relative humidity) and/or some landscape-related features (e.g. NDVI, EVI, slope, elevation, distance to water bodies) [16–19,21,22,28,29,33,39–41], and three studies included information on rodents population (e.g. rodent abundance, rodent composition) [17–19]. In contrast, the nine studies applying predictive models (i.e, Ecological Niche Modelling, Regression Trees) included several climatic, social, and environmental variables as predictors [14,15,20,23,30,32,34–36].
In addition, it is important to note that studies that apply machine learning predictive models are optimized for prediction and may not provide interpretable estimates of the relationships between exposures and outcomes. This is due to the complexity of the algorithms used, which can make it difficult to understand how specific factors contribute to predictions. As a result, classical regression models provide estimates easier to interpret as well as measures of uncertainty, making them more suitable for studies aimed at uncovering relationships between variables. For this reason, we additionally restrict the synthesis of evidence to the results from studies that reported interpretable coefficients and 95% confidence intervals (Figures 2 and 3: Panel B). Moreover, publication bias could not be excluded in this study. In this systematic review, efforts were made to include published studies; however, there is a possibility that unpublished studies with negative or nonsignificant results were not captured. Similarly, we only identified studies from three areas, namely Europe, China, and South America. Even if these areas account for the majority of the hantavirus infections reported worldwide, no eligible studies from other areas where hantavirus infection is documented were retrieved (e.g. United States (US) and Russia) [4]. While the absence of studies from these regions is a limitation, this is unlikely to be a direct result of publication bias because both countries have active scientific communities.
Assessing the quality of evidence from observational studies with standard quality tools [57] is a critical aspect of conducting systematic reviews. However, these tools are typically designed for use with studies that evaluate exposure and outcome data at the individual level, such as case-control or cohort studies, and are often focused on assessing the risk of bias in the individual studies. The heterogeneity of study design and statistical analysis in the studies included in our systematic review made it challenging to apply such standardized quality assessment tools. In particular, the studies included in our review evaluated the relationship between hantavirus risk and a wide range of environmental factors, including land use, land cover, and climate variables, using a variety of study designs and statistical methods. As a result, it was difficult to compare the quality of evidence across studies using a standardized tool. Nevertheless, in the current review we attempted to provide for each included study all important methodological features as the number of cases analyzed, the number of ecological units involved, the adoption of statistical methods to deal with spatio-temporal correlation of the data, and the use of multivariable regression techniques to partially remove the effect of confounding variables, as well as to compare results across studies to identify consistent findings and areas of uncertainty.
Overall, our systematic review suggested consistent evidence of a positive association between agricultural land use and human hantavirus infection in China and South America, and a positive association between forest cover and human hantavirus infection in Europe. However, specific mechanisms by which different land-covers and land-uses can affect the hantavirus emergence among humans are complex, and context or location specific. Further clarification of these associations taking into consideration specificities of different areas, communities potentially at risk and the temporal change of land use is needed to address the potential negative effects of anthropogenic environmental changes on hantavirus epidemiology.
Supplementary Material
Funding Statement
No funds were received for this review.
Disclosure statement
No conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20477724.2023.2272097
References
- [1].Laenen L, et al. Hantaviridae: current classification and future perspectives. doi: 10.3390/v11090788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schmaljohn C, Hjelle B.. Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3(2):95–104. doi: 10.3201/eid0302.970202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Toledo J, Haby MM, Reveiz L, et al. Evidence for human-to-human transmission of hantavirus: a systematic review. J Infect Dis. 2022;226(8):1362–1371. doi: 10.1093/infdis/jiab461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dearing MD, Dizney L. Ecology of hantavirus in a changing world. Ann N Y Acad Sci. 2010;1195(1):99–112. doi: 10.1111/j.1749-6632.2010.05452.x [DOI] [PubMed] [Google Scholar]
- [5].Jonsson CB, Figueiredo LTM, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev. 2010;23(2):412–441. doi: 10.1128/CMR.00062-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adam MN, Thomas GK, Pierre ER. Hantavirus pulmonary syndrome, United States, 1993-2009. Emerg Infect Dis. 2011;17(7):1195–201. doi: 10.3201/eid1707.101306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mir M Hantaviruses. doi: 10.1016/j.cll.2010.01.004. [DOI]
- [8].Krüger DH, Schönrich G, Klempa B. Human pathogenic hantaviruses and prevention of infection. Hum Vaccin. 2011;7(6):685–693. doi: 10.4161/hv.7.6.15197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].García-Peña GE, Rubio AV, Mendoza H, et al. Land-use change and rodent-borne diseases: hazards on the shared socioeconomic pathways. Philos Trans R Soc Lond B Biol Sci. 2021;376(1837):20200362. doi: 10.1098/rstb.2020.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mendoza H, Rubio AV, García-Peña GE, et al. Does land-use change increase the abundance of zoonotic reservoirs? Rodents say yes. Eur J Wildl Res. 2020;66(1):1–5. doi: 10.1007/s10344-019-1344-9 [DOI] [Google Scholar]
- [11].Patz JA, Daszak P, Tabor GM, et al. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112(10):1092–1098. doi: 10.1289/ehp.6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wegner GI, Murray KA, Springmann M, et al. Averting wildlife-borne infectious disease epidemics requires a focus on socio-ecological drivers and a redesign of the global food system. EClinicalMedicine. 2022;47:101386. doi: 10.1016/j.eclinm.2022.101386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fang LQ, Zhao W-J, de Vlas SJ, et al. Spatiotemporal dynamics of hemorrhagic fever with renal syndrome, Beijing, People’s Republic of China. Emerg Infect Dis. 2009;15(12):2043–2045. doi: 10.3201/eid1512.081078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xiao H, Tong X, Huang R, et al. Landscape and rodent community composition are associated with risk of hemorrhagic fever with renal syndrome in two cities in China, 2006–2013. BMC Infect Dis. 2018;18(1):1–10. doi: 10.1186/s12879-017-2827-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiao H, Tong X, Gao L, et al. Spatial heterogeneity of hemorrhagic fever with renal syndrome is driven by environmental factors and rodent community composition. PLoS Negl Trop Dis. 2018;12(10):e0006881. doi: 10.1371/journal.pntd.0006881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prist PR, Uriarte M, Tambosi LR, et al. Landscape, environmental and social predictors of hantavirus risk in São Paulo, Brazil. PLoS One. 2016;11(10):e0163459. doi: 10.1371/journal.pone.0163459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Muyalaert R, Sabino-Santos G, Prist P, et al. Spatiotemporal dynamics of hantavirus cardiopulmonary syndrome transmission risk in Brazil. Viruses. 2019;11(11):1008. doi: 10.3390/v11111008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Busch M, Cavia R, Carbajo AE, et al. Spatial and temporal analysis of the distribution of hantavirus pulmonary syndrome in Buenos Aires Province, and its relation to rodent distribution, agricultural and demographic variables. Trop Med Int Health. 2004;9(4):508–519. doi: 10.1111/j.1365-3156.2004.01218.x [DOI] [PubMed] [Google Scholar]
- [19].Vadell MV, Carbajo AE, Massa C, et al. Hantavirus pulmonary syndrome risk in Entre ríos, Argentina. Ecohealth. 2019;16(3):558–569. doi: 10.1007/s10393-019-01425-3 [DOI] [PubMed] [Google Scholar]
- [20].Barrios JM, Verstraeten WW, Maes P, et al. Relating land cover and spatial distribution of nephropathia epidemica and lyme borreliosis in Belgium. Int J Environ Health Res. 2012;23(2):132–154. doi: 10.1080/09603123.2012.708918 [DOI] [PubMed] [Google Scholar]
- [21].Zeimes CB, Olsson GE, Ahlm C, et al. Modelling zoonotic diseases in humans: comparison of methods for hantavirus in Sweden. Int J Health Geogr. 2012;11(1):39. doi: 10.1186/1476-072X-11-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zeimes CB, Quoilin S, Henttonen H, et al. Landscape and regional environmental analysis of the spatial distribution of hantavirus human cases in europe. Front Public Health. 2015;3: doi: 10.3389/fpubh.2015.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xiao H, Lin X, Gao L, et al. Ecology and geography of hemorrhagic fever with renal syndrome in Changsha, China. BMC Infect Dis. 2013;13(1). doi: 10.1186/1471-2334-13-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Douglas KO, Payne K, Sabino-Santos G, et al. Influence of climatic factors on human hantavirus infections in latin america and the Caribbean: a systematic review. Pathogens. 2022;11(1):15. doi: 10.3390/pathogens11010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Roda Gracia J, Schumann B, Seidler A. Climate variability and the occurrence of human Puumala hantavirus infections in Europe: a systematic review. Zoonoses Public Health. 2015;62(6):465–478. doi: 10.1111/zph.12175 [DOI] [PubMed] [Google Scholar]
- [26].Luo Y, Lv H, Yan H, et al. Meteorological change and hemorrhagic fever with renal syndrome epidemic in China, 2004–2018. Sci Rep. 2022;12(1):1–12. doi: 10.1038/s41598-022-23945-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Page MJ, Bossuyt PM, Boutron I, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://www.bmj.com/content/372/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yan L, Fang L-Q, Huang H-G, et al. Landscape elements and hantaan virus–related hemorrhagic fever with renal syndrome, People’s Republic of China. Emerg Infect Dis. 2007;13(9):1301–1306. doi: 10.3201/eid1309.061481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tian H, Hu S, Cazelles B, et al. Urbanization prolongs hantavirus epidemics in cities. Proc Natl Acad Sci U S A. 2018;115(18):4707–4712. doi: 10.1073/pnas.1712767115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liang W, Gu X, Li X, et al. Author correction: mapping the epidemic changes and risks of hemorrhagic fever with renal syndrome in Shaanxi Province, China, 2005–2016. Sci Rep. 2018;8(1). doi: 10.1038/s41598-018-24102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li S, Ren H, Hu W, et al. Spatiotemporal heterogeneity analysis of hemorrhagic fever with renal syndrome in China using geographically weighted regression models. Int J Environ Res Public Health. 2014;11(12):12129–12147. doi: 10.3390/ijerph111212129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu HN, Gao L-D, Chowell G, et al. Time-specific ecologic Niche models forecast the risk of hemorrhagic fever with renal syndrome in Dongting Lake District, China, 2005–2010. PLoS One. 2014;9(9):e106839. doi: 10.1371/journal.pone.0106839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Teng J, Ding S, Zhang H, et al. Bayesian spatiotemporal modelling analysis of hemorrhagic fever with renal syndrome outbreaks in China using R-INLA. Zoonoses Public Health. 2023;70(1):46–57. doi: 10.1111/zph.12999 [DOI] [PubMed] [Google Scholar]
- [34].She K, Li C, Qi C, et al. Epidemiological characteristics and regional risk prediction of hemorrhagic fever with renal syndrome in Shandong Province, China. Int J Environ Res Public Health. 2021;18(16):8495. doi: 10.3390/ijerph18168495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].ZHU LL, LI YP, LU L, et al. Spatial heterogeneity and influencing factors of HFRS epidemics in Rural and urban areas: a study in Guanzhong Plain of Shaanxi Province, China. Biomed Environ Sci. 2022;35(11):1012–1024. doi: 10.3967/bes2022.130 [DOI] [PubMed] [Google Scholar]
- [36].Zhu L, Lu L, Li S, et al. Spatiotemporal variations and potential influencing factors of hemorrhagic fever with renal syndrome: a case study in Weihe Basin, China. PLoS Negl Trop Dis. 2023;17(4):e0011245. doi: 10.1371/journal.pntd.0011245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shen L, Sun M, Wei X, et al. Spatiotemporal association of rapid urbanization and water-body distribution on hemorrhagic fever with renal syndrome: a case study in the city of Xi’an, China. PLoS Negl Trop Dis. 2022;16(1):e0010094. doi: 10.1371/journal.pntd.0010094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Linard C, Lamarque P, Heyman P, et al. Determinants of the geographic distribution of Puumala virus and lyme borreliosis infections in Belgium. Int J Health Geogr. 2007;6(1):15. doi: 10.1186/1476-072X-6-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cunze S, Kochmann J, Kuhn T, et al. Spatial and temporal patterns of human Puumala virus (PUUV) infections in Germany. PeerJ. 2018;6:e4255. doi: 10.7717/peerj.4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schwarz AC, Ranft U, Piechotowski I, et al. Risk factors for human infection with Puumala virus, southwestern Germany. Emerg Infect Dis. 2009;15(7):1032–1039. doi: 10.3201/eid1507.081413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Viel JF, LEFEBVRE A, MARIANNEAU P, et al. Environmental risk factors for haemorrhagic fever with renal syndrome in a French new epidemic area. Epidemiol Infect. 2011;139(6):867–874. doi: 10.1017/S0950268810002062 [DOI] [PubMed] [Google Scholar]
- [42].Liu Y, Gayle AA, Wilder-Smith A, et al. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;2020(2):1–4. doi: 10.1093/jtm/taaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Prist PR, D´andrea PS, Metzger JP. Landscape, climate and hantavirus cardiopulmonary syndrome outbreaks. Ecohealth. 2017;14:614–629. doi: 10.1007/s10393-017-1255-8 [DOI] [PubMed] [Google Scholar]
- [44].Gubler DJ, Reiter P, Ebi KL, et al. Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environ Health Perspect. 2001;109(2):223–233. doi: 10.1289/ehp.109-1240669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shah HA, Huxley P, Elmes J, et al. Agricultural land-uses consistently exacerbate infectious disease risks in Southeast Asia. Nat Commun. 2019;10(1). doi: 10.1038/s41467-019-12333-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Andreassen HP, Sundell J, Ecke F, et al. Population cycles and outbreaks of small rodents: ten essential questions we still need to solve. Oecologia. 2021;195(3):601–622. doi: 10.1007/s00442-020-04810-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gibb R, Redding DW, Chin KQ, et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature. 2020;584(7821):398–402. doi: 10.1038/s41586-020-2562-8 [DOI] [PubMed] [Google Scholar]
- [48].Khalil H, Ecke F, Evander M, et al. Declining ecosystem health and the dilution effect. Sci Rep. 2016;6(1). doi: 10.1038/srep31314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].de Oliveira RC, Guterres A, Fernandes J, et al. Hantavirus reservoirs: current status with an emphasis on data from Brazil. Viruses. 2014;6(5):1929–1973. doi: 10.3390/v6051929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Riccò M, Peruzzi S, Ranzieri S, et al. Occupational hantavirus infections in agricultural and Forestry workers: a systematic review and Metanalysis. Viruses. 2021;13(11):2150. doi: 10.3390/v13112150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Masi E, Pino FA, Santos MDGS, et al. Socioeconomic and environmental risk factors for urban rodent infestation in Sao Paulo, Brazil. J Pest Sci (2004). 2010;83(3):231–241. doi: 10.1007/s10340-010-0290-9 [DOI] [Google Scholar]
- [52].Sin MA, Stark K, van Treeck U, et al. Risk factors for hantavirus infection in Germany, 2005. Emerg Infect Dis. 2007;13(9):1364–1366. doi: 10.3201/eid1309.070552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Watson DC, Sargianou M, Papa A, et al. Epidemiology of hantavirus infections in humans: a comprehensive, global overview. Crit Rev Microbiol. 2014;40(3):261–272. doi: 10.3109/1040841X.2013.783555 [DOI] [PubMed] [Google Scholar]
- [54].Feng AYT, Himsworth CG. The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosyst. 2014;17(1):149–162. doi: 10.1007/s11252-013-0305-4 [DOI] [Google Scholar]
- [55].Jaitman L. Urban infrastructure in Latin America and the Caribbean: public policy priorities Research at the policy frontier in Latin America: health, Education, infrastructure and Housing and climate change Sebastian Galiani. Lat Am Econ Rev. 2015;24(1). doi: 10.1007/s40503-015-0027-5 [DOI] [Google Scholar]
- [56].Richmond-Bryant J, Long TC. Influence of exposure measurement errors on results from epidemiologic studies of different designs. J Expo Sci Environ Epidemiol. 2020;30(3):420–429. doi: 10.1038/s41370-019-0164-z [DOI] [PubMed] [Google Scholar]
- [57].Paul L. Reporting the quality/risk of bias - systematic reviews - Research Guides at George Washington University. https://guides.himmelfarb.gwu.edu/systematic_review/reporting-quality-risk-of-bias.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


