Abstract
Isopenicillin N synthase (IPNS) is a non-haem iron oxidase that catalyses the formation of bicyclic isopenicillin N from δ-(L-α-aminoadipoyl)-L-cysteinyl-D-valine (ACV). In this study we report a novel activity for the iron of the IPNS active site, which behaves as a Lewis acid to catalyse the elimination of HF from the fluorinated substrate analogue, δ-(L-α-aminoadipoyl)-L-cysteinyl-D-β-fluorovaline (ACβFV). X-Ray crystallographic studies of IPNS crystals grown anaerobically with ACβFV reveal that the valinyl β-fluorine is missing from the active site region, and suggest the presence of the unsaturated tripeptide δ-(L-α-aminoadipoyl)-L-cysteinyl-D-isodehydrovaline in place of substrate ACβFV. 19F NMR studies confirm the release of fluoride from ACβFV in the presence of the active IPNS enzyme. These results suggest a new mode of reactivity for the IPNS iron centre, a mechanism of action that has not previously been reported for any of the iron oxidase enzymes.
Keywords: fluoride elimination, β-lactam antibiotic, Lewis acid, non-haem iron oxidase, penicillin biosynthesis
Abbreviations: ACβFV, δ-(L-α-aminoadipoyl)-L-cysteinyl-D-β-fluorovaline; ACV, δ-(L-α-aminoadipoyl)-L-cysteinyl-D-valine; IPNS, isopenicillin N synthase
INTRODUCTION
Isopenicillin N synthase (IPNS) is the enzyme that catalyses the oxidative ring closure of δ-(L-α-aminoadipoyl)-L-cysteinyl-D-valine (ACV) to give isopenicillin N, the biosynthetic precursor to all of the penicillin and cephalosporin antibiotics. IPNS is a non-haem iron oxidase, which utilizes atmospheric dioxygen as the natural oxidant in a two-step bicyclization process [1].
The mechanism of action of IPNS has been under investigation for some years, with incubation studies [2,3] and crystallographic evidence [4–7] combining to create a detailed understanding of the transformations catalysed by this enzyme. In particular, the use of tripeptide substrate analogues to probe the enzyme mechanism has given a great deal of insight [2,3].
Initial studies focused on spectroscopic and biochemical identification of the incubation products arising from reaction of such substrates with IPNS in solution [2]. NMR spectroscopy and mass spectrometry were used extensively, in complement with analyses of β-lactamase induction and hole-plate assay tests, to characterize the products of these incubation experiments.
The isolation of the IPNS genes from Cephalosporium acremonium [8] and Aspergillus nidulans [5], subsequent determination of their sequences, and over-expression in Escherichia coli were vital in allowing the production of pure enzyme samples for this work [8–10].
The crystallization of A. nidulans IPNS in 1995 [11] opened the way for direct observation of the enzyme in the crystalline state using X-ray diffraction. Subsequent crystallographic studies have provided significant further insight into the enzyme mechanism [4–7].
In order to glean structural information on the IPNS mechanism, techniques were developed to initiate reaction in a concerted fashion throughout a protein crystal and to quench the reaction in a rapid and synchronous manner. Concerted initiation has been achieved by growing IPNS–substrate–iron(II) crystals in an anaerobic environment, then subjecting them to oxygen gas at hyperbaric pressures in a purpose-built oxygen ‘bomb’ [12,13]. Thus the enzyme is induced to turn over the substrate in the crystalline state, with the elevated oxygen pressures maximizing penetration and uniformity of the oxygen concentration throughout the protein crystal. Following oxygen exposure, reaction is ceased by flash-freezing in liquid nitrogen. Crystallographic data on the cryo-cooled crystals are then collected at 100 K using standard monochromatic X-ray diffraction methods.
In 1997, crystal structures for the enzyme complexed with ACV and with ACV plus nitric oxide (as a non-reactive O2 substitute) were solved, revealing the initial conformation of the enzyme–substrate complex [4]. Turnover experiments of the IPNS–ACV–Fe(II) complex with high-pressure oxygen allowed observation of an enzyme–product complex [7]. A monocyclic β-lactam species was isolated by oxygenation of IPNS crystals containing the substrate analogue, δ-(L-α-aminoadipoyl)-L-cysteinyl-D-S-methylcysteine, providing firm structural evidence for the sequence of the β-lactam and thiazolidine ring closures [7].
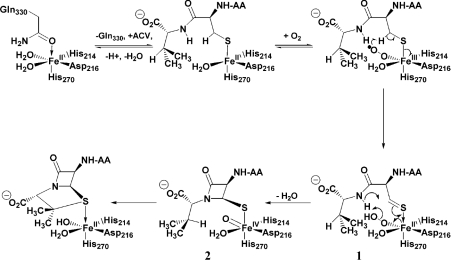
Using this methodology, the enzyme mechanism has been further probed with results that include novel turnover events and alternative oxidation pathways for a range of other ACV substrate analogues [6,14,15]. These studies combine with earlier spectroscopic work and incubation results to provide a comprehensive understanding of the mechanism of action of IPNS (Scheme 1). The reaction cycle is believed to involve a hydroperoxide (labelled 1 on Scheme 1) and a high-valent Fe(IV) oxo species (2), although direct evidence has yet to be obtained for these moieties. Such species, and in particular the oxo intermediate 2, would be highly unstable and thus extremely difficult to isolate. Hence the lack of direct evidence hitherto obtained for such intermediates. Iron(IV) oxo species have been observed in porphyrin-containing haem systems or in association with other stabilizing ligands [16–20]. There is a large body of information in the literature pertaining to high-valent iron in biological systems, but most of this has been derived from the haem-containing enzymes. However, interest in and understanding of the non-haem iron oxidase family of enzymes is rapidly expanding [21–25].
Scheme 1. Proposed mechanism of action of IPNS in reaction with its natural substrate, ACV.
The intermediate of direct interest and importance to this study is the high-valent iron(IV) oxo species 2. As shown in Scheme 1, the β-proton of the valinyl portion of ACV is abstracted by the oxo moiety 2 in the second stage of the proposed reaction cycle. Therefore, a substrate in which β-proton abstraction is prevented or strongly discouraged represents a useful probe for experiments aiming to structurally characterize an intermediate such as 2.
Replacement of the valinyl β-proton with a fluorine atom gives δ-(L-α-aminoadipoyl)-L-cysteinyl-D-β-fluorovaline (ACβFV) 3, which was envisaged as a suitable substrate for this study. Fluorine is a good hydrogen substitute for this purpose: it is similar in size to hydrogen [van der Waals radii 1.35 Å (F) versus 1.10 Å (H)] [26], it has a comparable C–X bond length (1.26–1.41 Å for F; 1.08–1.11 Å for H) [26] and yet the C–F bond is of greater strength than the C–H bond (108 kCal/mol for F-CH3 [27]; 105 kCal/mol for H–CH3 [28]). Furthermore, the electronegativity of fluorine (4.0) is significantly greater than that of hydrogen (2.1), imparting substantially different electronic properties to fluorinated molecules relative to their protiated analogues [26]. Thus the fluorinated tripeptide 3 should be accepted as a substrate at the IPNS active site on account of its steric similarity to ACV. However the increased C–F bond strength should hinder F• abstraction from substrate 3, thus inhibiting the second ring closure, and extending the lifetime of the iron(IV) oxo moiety.
Indeed, attempts have been made previously to study the reaction of substrate 3 with IPNS [29]. The incubation mixture showed no antibiotic activity; however, ACβFV was found to inhibit the formation of isopenicillin N from ACV, strongly suggesting that 3 does bind within the IPNS active site, although it is not turned over by the enzyme.
The aim of the current study was to crystallize the fluorinated substrate analogue 3 with IPNS and to use the IPNS–ACβFV–Fe(II) complex in mechanistic studies on the enzyme.
EXPERIMENTAL
Synthesis of ACβFV 3
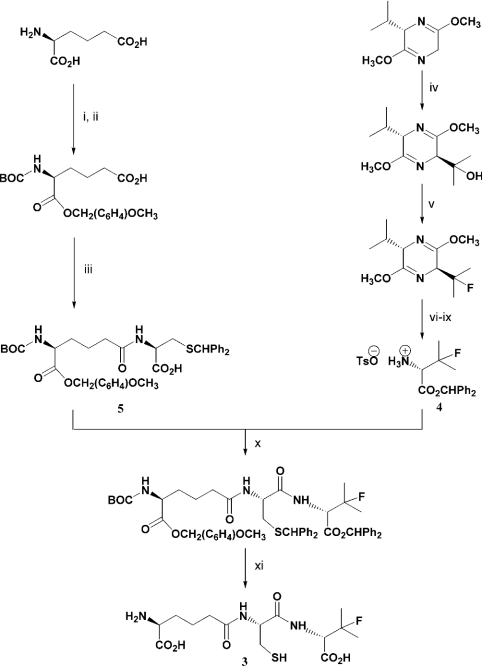
ACβFV 3 was prepared from appropriately protected D-β-fluorovaline 4 and δ-(L-α-aminoadipoyl)-L-cysteine 5 (Scheme 2). Rt (10 mM NH4. HCO3) 6 min 28 s; δH (2H2O, 400 MHz) 4.48 [2H, X of ABX, JXA 5.5, JXB 7 Hz, Hα(C)], 4.28 [1H, d, JH,F 20 Hz, Hα(βFV)], 3.64 [1H, t, J 6 Hz, Hα(A)], 2.85 (1H, A of ABX, JAX 5.5, JAB 14 Hz, 1 of CH2SH), 2.79 (1H, B of ABX, JBX 7, JBA 14 Hz, 1 of CH2SH), 2.31 [2H, t, J 7 Hz, 2×Hδ(A)], 1.83–1.72 [2H, m, 2×Hβ(A)], 1.67–1.52 [2H, m, 2×Hγ(A)], 1.34 [3H, d, JH,F 22.5 Hz, 1 of CF(CH3)2], 1.32 [3H, d, JH,F 22.5 Hz, 1 of CF(CH3)2] p.p.m.; δF (2H2O, 235 MHz) −146.17 (m, ACβFV), −146.00 [m, (ACβFV)2] p.p.m.; m/z (ES−) 380 ([M-H]−, 35%), 281 (28%), 255 (100%), 153 (33%); m/z (HRMS, ES−) 380.1294 ([M–H]−. C14H23FN3O6S requires 380.1292).
Scheme 2. Synthetic route to ACβFV 3.
(i) BOC2O, NaOH, tBuOH, H2O, 98%; (ii) p-ClCH2(C6H4)OCH3, Et3N, DMF, 99%; (iii) 1, iso-butylchloroformate, Et3N, THF, 2, S-benzhydryl-L-cysteine, Et3N, H2O, 71%; (iv) (CH3)2O, nBuLi, THF, −60 °C, quantitative; (v) DAST, DCM, −30 °C, 80%; (vi) HCl (aq), Δ; (vii) TsOH, H2O; (viii) Ph2CN2, CH3CN, Et2O; (ix) TsOH, H2O, 10% (over 4 steps); (x) EDCI, HOBt, Et3N, DCM, 87%; (xi) TFA (1), anisole; HPLC (2), 70%.
Crystallization experiments
Crystals of ACβFV 3, IPNS and Fe(II) were grown using the hanging drop method under anaerobic conditions at 17 °C in a Belle Technology Glovebox, according to the methodology developed in this laboratory [12,13]. Oxygen levels were maintained at or below 0.4 p.p.m. in an argon atmosphere. Thus, drops (7 μl) of seeded crystallization solution [2.6 mg/ml tripeptide; 2.0 mM FeSO4; 25 mg/ml IPNS; 1.0 M Li2SO4; 76 mM Tris/HCl; pH 8.5] were suspended on glass cover slips in the sealed wells of a Linbro plate over well buffer [1.8 M Li2SO4; 0.1 M Tris/HCl; pH 8.5] (750 μl) and maintained under an argon atmosphere for 3 days to give the requisite crystals for analysis.
Anaerobic crystals were prepared for data collection by exchanging into cryoprotectant buffer (1:1 well buffer/40% glycerol in saturated Li2SO4) and flash-freezing in liquid nitrogen. Oxygen exposures were conducted as described previously [13], by subjecting the crystals to hyperbaric oxygen (20 bar) for varying time periods (5 min to 5 h) in a high-pressure chamber before exchanging into the cryoprotectant (1:1 well buffer/40% glycerol in saturated Li2SO4) and flash-freezing in liquid nitrogen.
Data collection
Data were collected using synchrotron X-ray radiation [0.934 Å (where 1 Å=0.1 nm)], an ADSC Quantum 4 CCD detector and an Oxford Cryostream set for collection at 100 K at beamline ID14-EH2 of the European Synchrotron Research Facility (ESRF), Grenoble, France.
Structure determination
Data were processed using MOSFLM [30] and the CCP4 suite of programs [31]. An initial model was obtained by rigid body refinement of the protein atoms of the previously determined IPNS–ACV–Fe(II) structure [4] against the new data. Model refinement was carried out using REFMAC [31]. The electron density maps were interpreted using the program O [32]. The iron atom and water molecules were modelled in during the refinement process. Electron density for the substrate was clearly evident and was modelled in sequentially at advanced stages of the refinement. The crystallographic coordinates and structure factors have been deposited in the Brookhaven Protein Data Bank (PDB) under accession numbers 1uzw and r1uzwsf respectively.
Incubation experiments
Anaerobic incubations
(a) ACβFV with active IPNS
A solution (170 μl) of ACβFV 3 (3.1 mg/ml), FeSO4 (2.1 mM), IPNS (27 mg/ml), Li2SO4 (1.0 M) and Tris/HCl (76 mM) at pH 8.5 was incubated at 17 °C under anaerobic conditions for 3 days. Acetone (10 ml) was added, the mixture centrifuged at 3000 g for 30 min at 4 °C and the supernatant retained. The solvent was removed in vacuo and the residue taken up in 2H2O and analysed by NMR spectroscopy. δF (2H2O, 235 MHz) −122.84 (s, F−).
(b) ACβFV with denatured IPNS
An IPNS aliquot (75 μl, 60 mg/ml) was heated to 100 °C for 5 min and then allowed to cool to 17 °C prior to incubation with the tripeptide. The procedure outlined in (a) above was followed and the resulting solution analysed by NMR spectroscopy. δF (2H2O, 235 MHz) −145.92 (m, ACβFV).
(c) ACβFV in the absence of IPNS
A solution (500 μl) of ACβFV 3 (3.0 mg/ml), FeSO4 (2.2 mM), Li2SO4 (1.0 M) and Tris/HCl (76 mM) in 2H2O at pH 8.5 was incubated at 17 °C under anaerobic conditions for 3 days. Na2EDTA (1.0 mg, 2 equivalents) was added to complex the Fe(II) present in solution and the mixture immediately subjected to NMR analysis. δF (2H2O, 235 MHz) −145.93 (m, ACβFV).
Aerobic incubations
(a) ACβFV with IPNS
A solution (890 μl) of ACβFV 3 (1.1 mg/ml), IPNS (3.4 mg/ml), tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (11.2 mM), ascorbic acid (5.6 mM), FeSO4 (0.56 mM) and NH4HCO3 (48 mM) at pH 8.5 was incubated at 29 °C for 30 min. Additional TCEP (100 μl, 100 mM) and FeSO4 (10 μl, 50 mM) were added and the mixture further incubated at 29 °C for 30 min. Acetone (10 ml) was added and the solution cooled to −20 °C, then maintained at this temperature for 3 h. The mixture was centrifuged and the supernatant retained. The acetone was removed in vacuo, the remaining sample purified by reversed phase HPLC (5→50% methanol in 25 mM NH4HCO3 over 7 min) and the fractions analysed by NMR spectroscopy. 1H NMR analysis showed no evidence of β-lactam peaks, expected at δH approx. 5.0–5.5 p.p.m.
(b) ACV with IPNS
A solution (890 μl) of ACV (2.2 mg/ml), IPNS (3.4 mg/ml), TCEP (11.2 mM), ascorbic acid (5.6 mM), FeSO4 (0.56 mM) and NH4HCO3 (48 mM) at pH 8.5 was treated to the procedure outlined in (a) above. Peaks attributable to the β-lactam protons of isopenicillin N were observed in the 1H NMR spectrum: δH (2H2O, 500 MHz) 5.50 and 5.41 p.p.m.
RESULTS
Synthesis of ACβFV 3
An appropriately protected D-β-fluorovaline moiety was synthesized according to established methodology, using Schöllkopf's chiral cyclic dipeptide, (S)-2,5-dimethoxy-3-isopropyl-3,6-dihydropyrazine [33–36] to induce the required stereochemistry, and (diethylamino)sulphur trifluoride to introduce the fluorine at the desired β-position [37]. Subsequent hydrolysis and protecting group manipulations afforded the appropriately protected D-β-fluorovaline 4 (Scheme 2). The partially protected L-α-aminoadipoyl-L-cysteinyl fragment 5 was prepared by standard methods [15,38] and coupled to the D-β-fluorovaline portion using carbodiimide chemistry [39]. Acid-mediated global deprotection [15,40] and purification by HPLC yielded the desired ACβFV 3 (Scheme 2).
Anaerobic crystallization of ACβFV 3 with IPNS
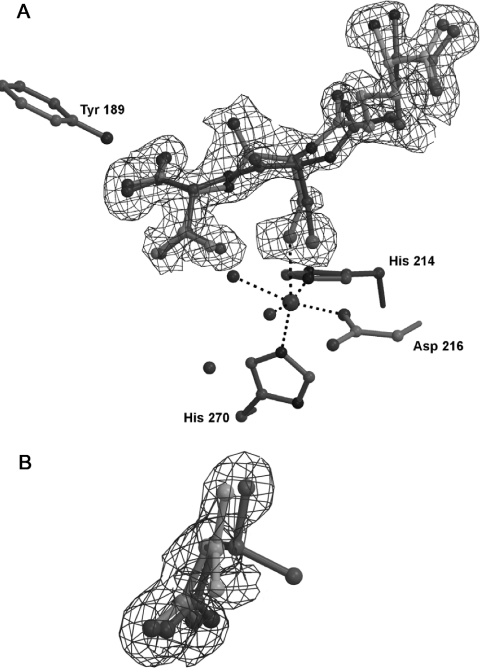
ACβFV 3 was crystallized anaerobically with IPNS and iron(II), and the resulting crystals were studied by X-ray crystallography. (Crystal structure data given in Table 1.) The crystal structure thus obtained (Figure 1A) shows a distinct lack of density for a fluorine atom attached to the β-carbon of ACβFV. The electron density map for this structure is also planar about the valinyl β-carbon, clearly indicating that this central atom is sp2 hybridized [Figure 1B]. Interestingly, it appears that the tripeptide occupies two orientations in the active site (with an occupancy ratio estimated at 7:3).
Table 1. Data collection and statistics for anaerobic crystal structure resulting from the IPNS–AcβFV–Fe(II) complex.
| X-ray source | ID14–EH2, ESRF | |
|---|---|---|
| Wavelength λ (Å) | 0.934 | |
| Space group | P212121 | |
| Unit cell (a Å, b Å, c Å) | 46.23, 70.60, 100.64 | |
| Resolution shell (Å) | 21.98–1.30 | 1.37–1.30 |
| Number of reflections | 390499 | 44384 |
| Number of unique reflections | 80218 | 11381 |
| Average I/σI | 17.1 | 3.9 |
| Completeness (%) | 98.4 | 97.1 |
| Rmerge (%)* | 6.1 | 35.0 |
| Rcryst (%)† | 17.35 | |
| Rfree (%)‡ | 19.43 | |
| RMS deviation§ | 0.018 Å (1.916°) | |
| B factors (Å2)∥ | 10.3, 12.6, 19.8, 9.2, 25.3 | |
| Number of residues | 329 | |
| Number of water molecules | 355 |
* Rmerge=ΣjΣh|Ih,j−〈Ih〉|/ΣjΣh〈Ih〉×100.
† Rcryst=Σ||Fobs|−|Fcalc||/Σ|Fobs|×100.
‡ Rfree based on 4% of total reflections.
§ RMS deviation from ideality for bond lengths (followed by RMS deviation from ideality for bond angles).
∥ Average B factors in order: main chain, side chain, substrate, iron and solvent.
Figure 1. X-ray crystal structure of the complex formed from anaerobic interaction of ACβFV 3 with IPNS, to 1.30 Å resolution.
(A) The two substrate orientations are shown separately in light and dark grey with a 7:3 occupancy ratio respectively. (B) The isopropyl region of ACβFV-derived product (light grey) overlaid with ACV (dark grey) for comparison (note the planarity in the electron density about the β-carbon of the ACβFV-derived structure). The 2mFo-DFc electron density map is contoured at 1.0σ.
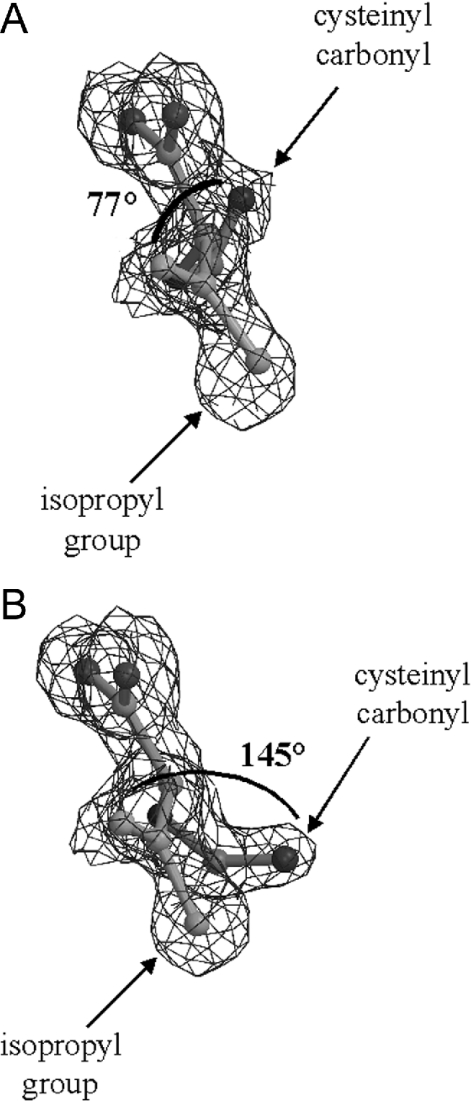
The two orientations (Figures 2A and 2B) show quite different torsion angles between the isopropyl region and the cysteinyl carbonyl of the substrate-derived molecule in the active site (there is a 68° difference between the two torsion angles). However, it is evident from these angles that the two groups are not co-planar in either conformation. Furthermore, the density corresponding to the α-carbon of the valinyl portion of the substrate is non-planar and consistent with the D-configuration of this amino acid. This evidence clearly indicates an sp3 hybridized centre at the valinyl α-position. In contrast with the native IPNS–ACV–Fe(II) structure [4], the sixth coordination site on the active site iron is occupied. There is clear electron density in the site trans to Asp216, presumably arising from a substrate-derived fluoride ligand or from an additional water molecule.
Figure 2. X-ray crystal structure of complex arising from anaerobic ACβFV–IPNS interaction, showing relative torsion angles between isopropyl group and cysteinyl carbonyl for conformations (A) and (B) (torsion angles indicated).
The validity of this X-ray crystal structure was confirmed by conducting a duplicate experiment, which revealed an essentially identical electron density map on repetition of this crystallization and X-ray diffraction study (results not shown).
Anaerobic reaction of ACβFV 3 with IPNS in solution
The interaction in solution between ACβFV 3 and IPNS has been studied extensively using NMR spectroscopy. These experiments were conducted primarily to confirm that the elimination of HF apparent by X-ray crystallographic analysis is also observable in solution. Furthermore, it was desirable to determine whether or not this elimination is an enzyme-mediated active site event.
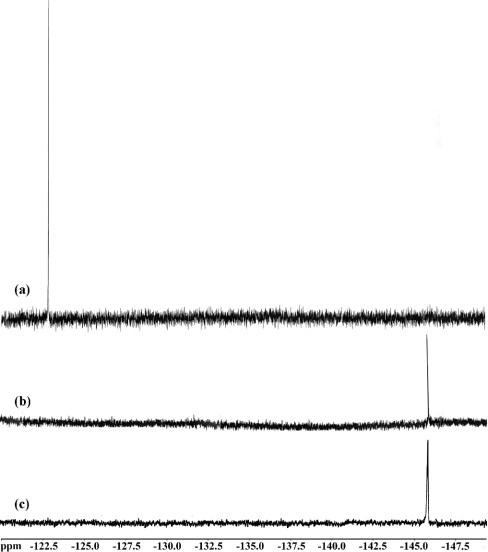
The anaerobic incubation of ACβFV 3 with IPNS was performed and monitored by 19F NMR spectroscopy (Figure 3). The 19F{1H} NMR spectrum following incubation for 3 days showed no evidence of remaining ACβFV (δF −146 p.p.m.). However, a new peak was observed at δF −123 p.p.m. in this spectrum (Figure 3a). This corresponds to fluoride anion, its identity being confirmed by doping experiments with sodium fluoride.
Figure 3. 19F{1H} NMR spectra (2H2O, 235 MHz) resulting from anaerobic incubation of ACβFV 3 with (a) active IPNS, (b) boiled IPNS, and (c) no IPNS, under crystallization conditions [ACβFV 3.1 mg/ml, FeSO4 2.1 mM, Li2SO4 1.0 M, Tris/HCl 76 mM, (IPNS 27 mg/ml), pH 8.5, 17 °C, 3 days].
As a control experiment, this incubation was repeated without the enzyme present. Thus ACβFV 3 was incubated anaerobically under similar conditions with a solution of iron(II) sulphate in the same solvent/buffer system. After 3 days, the starting material 3 was clearly evident by 19F NMR spectroscopy (δF −146 p.p.m.) and there was no evidence for fluoride release (Figure 3c). As a further control, the incubation of ACβFV was performed using IPNS that had been inactivated by heating to 100 °C. Upon incubation with the boiled enzyme (in the same solvent/buffer system) ACβFV 3 remained unreacted, as evidenced by the peak corresponding to substrate (δF −146 p.p.m.) in the 19F{1H} NMR spectrum and the absence of an observable fluoride anion peak (Figure 3b).
Aerobic interaction of ACβFV 3 with IPNS
Aerobic incubations of ACβFV 3 with IPNS were carried out in solution in an attempt to isolate any penam or cepham turnover products that might be formed. 1H NMR analysis was conducted following incubation for 1 h and showed no evidence of β-lactam peaks (expected δH∼5.5 p.p.m.). In a control experiment, ACV was incubated with IPNS under the same aerobic conditions and showed formation of a β-lactam product in the incubation mixture, by 1H NMR (δH 5.50 and 5.41 p.p.m.).
Oxygenation of the crystals grown from ACβFV 3, Fe(II) and IPNS for various periods of time [5 min, 20 min, 5 h; 20 bar O2] failed to give conclusive results (results not shown). The electron density maps arising from the oxygenated crystals were indistinct in the substrate-binding region, although there was some evidence for departure of the substrate/product(s) from the active site.
DISCUSSION
The cumulative evidence from the X-ray crystallography and NMR studies strongly suggests a novel mode of action for the active site of IPNS. The absence of valinyl-bound fluorine from the anaerobic crystal structure (Figure 1A) and the planar nature of the electron density for the valinyl β-carbon (Figure 1B) provide clear evidence that elimination has taken place, with HF being lost from the substrate analogue, ACβFV 3.
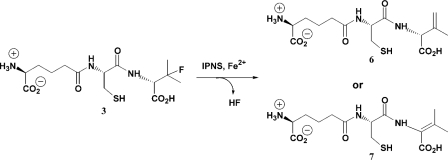
Such an elimination process would give a tripeptide containing an unsaturated valinyl residue, and this elimination event may proceed to afford either of two possible double bond orientations in the resulting alkene product. Elimination across the β,γ-bond would give δ-(L-α-aminoadipoyl)-L-cysteinyl-D-isodehydrovaline 6, whilst an α,β-elimination would afford δ-(L-α-aminoadipoyl)-L-cysteinyl-D-dehydrovaline 7 (Scheme 3). The position of the new double bond relative to the cysteinyl-valine amide bond in each of these molecules would confer decidedly different conformational properties to compounds 6 and 7.
Scheme 3. Alternative products obtainable by elimination of HF from ACβFV 3.
Compound 7 is an N-vinyl amide. The double bond is α,β to the amide nitrogen, so conjugation between the amide bond and the olefinic portion of the molecule would be expected. This would give considerable conformational rigidity to this part of the molecule, and the isopropyl-derived portion would therefore be expected to be coplanar with the cysteinyl carbonyl bond. Furthermore, the α- and β-carbons of 7 are both sp2 hybridized, which would give rise to planar electron density around both of these atoms.
Compound 6, however, has no extended conjugation system since the newly-formed double bond is positioned β,γ to the amide nitrogen. Thus, there would be no inherent electronic requirements to adopt a particular conformation, and the relative orientation of the isopropyl portion and the cysteinyl carbonyl would be dictated solely by steric and noncovalent interactions with the protein and nearby water molecules. The α-carbon of 6 is sp3 hybridized, whilst the β- and γ-carbons are sp2 hybridized. Thus the electron density arising from this structure would be planar about the β-carbon, but not the α-carbon.
The electron density map of the complex arising from crystallization of IPNS, ACβFV 3 and iron(II) shows no coplanarity between the cysteinyl carbonyl and valinyl isopropyl regions. This observation provides substantial evidence that δ-(L-α-aminoadipoyl)-L-cysteinyl-D-isodehydrovaline 6 is formed in the IPNS active site, rather than δ-(L-α-aminoadipoyl)-L-cysteinyl-D-dehydrovaline 7. This conclusion is further supported by the non-planar nature of the electron density at the valinyl α-carbon, which indicates the presence of an sp3 hybridized carbon atom at this position. Thus we propose that the elimination of HF from ACβFV 3 proceeds via the Hofmann orientation, to give a single product, the less substituted alkene 6.
It appears, therefore, that the two orientations of the tripeptide observed in the crystal structure arise not from two distinct elimination products, as might be expected, but rather from two alternative conformations of the one product 6. Dual occupancy in this region of the tripeptide has not been observed previously in other anaerobic IPNS–substrate–iron(II) structures. It presumably arises due to the particular structural demands of this unsaturated product, with different hydrogen bonding networks allowing both orientations to be favoured in the crystalline state.
The spectroscopic experiments performed using 19F NMR demonstrate that the HF elimination from ACβFV observed crystallographically also occurs in solution. Fluoride is not released in the absence of the enzyme, indicating that the elimination of HF from ACβFV 3 is an enzyme-mediated event. Moreover, the failure of the denatured enzyme to promote fluoride release suggests strongly that the elimination is mediated by the enzyme active site.
It is not surprising that the active site is intimately involved in this process considering the orientation of elimination observed in the X-ray crystal structure. Hofmann-type elimination of HF to give compound 6 would be most unusual if this event were simply a base-catalysed solution event rather than an enzymic process. If the process were occurring in solution, it would be expected to involve loss of the most acidic hydrogen situated vicinal to the fluoride leaving group. The most acidic proton in this region is the valinyl α-proton, due to its position α to the valinyl carbonyl group. Elimination involving abstraction of this proton would result in the formation of the alternative product, 7. As discussed above, the X-ray diffraction data point strongly towards formation of the β,γ-unsaturated tripeptide 6.
Given its close proximity to the site of elimination and its Lewis acid character, it is reasonable to suggest that the active site iron atom could promote this elimination process. Upon binding of the natural substrate ACV to the iron centre of IPNS, the iron adopts a five-coordinate, 16-electron configuration and is thus primed for reaction. In the reaction with ACV, dioxygen then binds trans to Asp216, initiating the oxidative turnover process (Scheme 1). In an analogous manner, coordination of the fluoride of ACβFV 3 to the active site iron at the vacant sixth coordination position would be an energetically favourable process, completing the 18-electron sphere of the iron centre. Thus the binding of fluoride from ACβFV to the active site iron may be considered to mirror the binding of dioxygen in the turnover of ACV by IPNS.
It therefore appears that the incomplete coordination environment and electron deficient nature of the iron atom in the IPNS active site are essential to its Lewis acid activity in the elimination of HF from ACβFV 3. In a similar way, the iron's incomplete ligand sphere is thought to facilitate the ready binding of dioxygen to the IPNS–ACV–iron(II) complex.
The non-polar nature of the valinyl sidechain of ACV plays an essential role both in keeping water from the sixth coordination site (thus reserving this position for oxygen) and in controlling the hydrophobicity of the oxygen binding pocket. With substrate analogues that bear smaller sidechains in this region, the sixth iron coordination site is often occupied by a water molecule [14,15]. The oxygen–water exchange equilibrium which is then required in order for turnover to commence has been offered as an explanation for the slower turnover of these substrate analogues by IPNS, as has been observed. It has also been noted that few analogues bearing polar sidechains in the valinyl region are substrates for IPNS [2,41,42]. This may be because the substrate hetero-atom binds to iron preventing coordination of dioxygen, or it may be attributable to other electronic factors.
In the present study, binding of the valinyl β-fluoride of ACβFV to iron would lead to weakening of the F–C bond, thus promoting elimination of hydrogen fluoride. Such iron-mediated elimination would result in the fluoride ion occupying the iron coordination site trans to Asp216, which is consistent with the electron density observed in the crystal structure. The appearance of fluoride ion in the NMR spectrum following incubation with active IPNS in solution presumably arises through ligand exchange with water in the incubation buffer. It is not possible to ascertain from the X-ray structure alone whether such an exchange process is occurring in the crystalline state, since fluoride and water are isoelectronic and hence indistinguishable in an electron density map. As previously discussed, the presence of a water molecule at the sixth coordination position, opposite Asp216, has been observed in crystal structures of IPNS complexes with several other substrates. These structures involve substrates of reduced steric bulk in the immediate vicinity of the iron centre (relative to the native ACV substrate), allowing space for water to bind at this position [14,15].
There are several amino acid residues in the vicinity of the active site, as well as a number of water molecules, that are potential candidates to abstract the γ-proton of ACβFV 3, leading to the formation of δ-(L-α-aminoadipoyl)-L-cysteinyl-D-isodehydrovaline 6. However, it is not clear which of these is responsible for this deprotonation step.
Attempts to gain solution phase data on aerobic incubations using 1H NMR spectroscopy met with considerable difficulty. It seems likely that the substrate is not turned over by the enzyme to a penicillin-type product, and so presumably remains bound to the iron via the strong S–Fe(II) tether of the enzyme–substrate interaction. Thus release of the tripeptide from the active site is not likely to be favourable. Consequently, recovery of tripeptide-derived species following protein precipitation, according to the standard procedure, would not furnish sufficient material for 1H NMR analysis. In situ analysis of product(s) formed is not possible due to the paramagnetic nature of the iron(II) in the enzyme active site. Therefore, it appears that substrate 3, or the enzymically-derived 6, is not turned over to give a β-lactam product; this may be due to enzyme inactivation by the fluoride anion released into the active site.
The accumulated evidence presented herein indicates that when ACβFV 3 binds to IPNS under anaerobic conditions, an elimination event occurs resulting in loss of HF and formation of the β,γ-unsaturated tripeptide 6. The results obtained strongly suggest that this process is an enzyme-mediated active site event. It is most likely that the iron of the active site acts as a Lewis acid, coordinating to fluorine, weakening the carbon–fluorine bond and thus initiating the elimination. This role of Lewis acid is a novel activity for the active site iron, and has not been previously observed in any of the iron-containing oxidase enzymes.
Acknowledgments
We would like to thank Dr Robert M. Adlington, Dr Luke A. McNeill and Dr Adam Daruzzaman for help and discussions, Dr Christopher Garner for preparation of the Schöllkopf reagent, and the scientists at the European Synchrotron Research Facility (ESRF), Grenoble for technical assistance. This work was supported by the MRC, BBSRC, EPSRC, U.K., and the Wellcome Trust. A.R.G. thanks the Rhodes Trust for support.
References
- 1.Baldwin J. E., Adlington R. M., Moroney S. E., Field L. D., Ting H.-H. Stepwise ring closure in penicillin biosynthesis. Initial β-lactam formation. J. Chem. Soc. Chem. Commun. 1984:984–986. [Google Scholar]
- 2.Baldwin J. E., Bradley M. Isopenicillin N synthase: mechanistic studies. Chem. Rev. 1990;90:1079–1088. [Google Scholar]
- 3.Baldwin J. E., Shiau C.-Y., Byford M. F., Schofield C. J. Substrate specificity of L-δ-(α-aminoadipoyl)-L-cysteinyl-D-valine synthetase from Cephalosporium acremonium: demonstration of the structure of several unnatural tripeptide products. Biochem. J. 1994;301:367–372. doi: 10.1042/bj3010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roach P. L., Clifton I. J., Hensgens C. M. H., Shibata N., Schofield C. J., Hajdu J., Baldwin J. E. Structure of isopenicillin N synthase complexed with substrate and the mechanism of penicillin formation. Nature (London) 1997;387:827–830. doi: 10.1038/42990. [DOI] [PubMed] [Google Scholar]
- 5.Roach P. L., Clifton I. J., Fülöp V., Harlos K., Barton G. J., Hajdu J., Andersson I., Schofield C. J., Baldwin J. E. Crystal structure of isopenicillin N synthase is the first from a new structural family of enzymes. Nature (London) 1995;375:700–704. doi: 10.1038/375700a0. [DOI] [PubMed] [Google Scholar]
- 6.Ogle J. M., Clifton I. J., Rutledge P. J., Elkins J. M., Burzlaff N. I., Adlington R. M., Roach P. L., Baldwin J. E. Alternative oxidation by isopenicillin N synthase observed by X-ray diffraction. Chem. Biol. 2001;8:1231–1237. doi: 10.1016/s1074-5521(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 7.Burzlaff N. I., Rutledge P. J., Clifton I. J., Hensgens C. M. H., Pickford M., Adlington R. M., Roach P. L., Baldwin J. E. The reaction cycle of isopenicillin N synthase observed by X-ray diffraction. Nature (London) 1999;401:721–724. doi: 10.1038/44400. [DOI] [PubMed] [Google Scholar]
- 8.Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W., Ingolia T. D. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature (London) 1985;318:191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin J. E., Gagnon J., Ting H.-H. N-terminal amino acid sequence and some properties of isopenicillin-N synthetase from Cephalosporium acremonium. FEBS Letters. 1985;188:253–256. doi: 10.1016/0014-5793(85)80382-3. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin J. E., Blackburn J. M., Sutherland J. D., Wright M. C. High-level soluble expression of isopenicillin N synthase isozymes in E coli. Tetrahedron. 1991;47:5991–6002. [Google Scholar]
- 11.Roach P. L., Schofield C. J., Baldwin J. E., Clifton I. J., Hajdu J. Crystallization and preliminary X-ray diffraction studies on recombinant isopenicillin N synthase from Aspergillus nidulans. Prot. Sci. 1995;4:1007–1009. doi: 10.1002/pro.5560040521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roach P. L., Clifton I. J., Hensgens C. M. H., Shibata N., Long A. J., Strange R. W., Hasnain S. S., Schofield C. J., Baldwin J. E., Hajdu J. Anaerobic crystallization of an isopenicillin N synthase Fe(II) substrate complex demonstrated by X-ray studies. Eur. J. Biochem. 1996;242:736–740. doi: 10.1111/j.1432-1033.1996.0736r.x. [DOI] [PubMed] [Google Scholar]
- 13.Rutledge P. J., Burzlaff N. I., Elkins J. M., Pickford M., Baldwin J. E., Roach P. L. A device for high-pressure oxygenation of protein crystals. Anal. Biochem. 2002;308:265–268. doi: 10.1016/s0003-2697(02)00246-4. [DOI] [PubMed] [Google Scholar]
- 14.Long A. J., Clifton I. J., Roach P. L., Baldwin J. E., Schofield C. J., Rutledge P. J. Structural studies on the reaction of isopenicillin N synthase with the substrate analogue δ-(L-α-aminoadipoyl)-L-cysteinyl-D-α-aminobutyrate. Biochem. J. 2003;372:687–693. doi: 10.1042/BJ20021627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkins J. M., Rutledge P. J., Burzlaff N. I., Clifton I. J., Adlington R. M., Roach P. L., Baldwin J. E. Crystallographic studies on the reaction of isopenicillin N synthase with an unsaturated substrate analogue. Org. Biomol. Chem. 2003;1:1455–1460. doi: 10.1039/b212270g. [DOI] [PubMed] [Google Scholar]
- 16.Ogliaro F., Cohen S., Filatov M., Harris N., Shaik S. The high-valent compound of cytochrome P450: the nature of the Fe-S bond and the role of the thiolate ligand as an internal electron donor. Angew. Chem. Int. Ed. 2000;39:3851–3855. doi: 10.1002/1521-3773(20001103)39:21<3851::AID-ANIE3851>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Groves J. T., Gross Z., Stern M. K. Preparation and reactivity of oxoiron(IV) porphyrins. Inorg. Chem. 1994;33:5065–5072. [Google Scholar]
- 18.Penner-Hahn J. E., Eble K. S., McMurry T. J., Renner M., Balch A. L., Groves J. T., Dawson J. H., Hodgson K. O. Structural characterization of horseradish peroxidase using EXAFS spectroscopy. Evidence for Fe=O ligation in compounds I and II. J. Am. Chem. Soc. 1986;108:7819–7825. doi: 10.1021/ja00284a054. [DOI] [PubMed] [Google Scholar]
- 19.Schlichting I., Berendzen J., Chu K., Stock A. M., Maves S. A., Benson D. E., Sweet R. M., Ringe D., Petsko G. A., Sligar S. G. The catalytic pathway of cytochrome P450cam at atomic resolution. Science (Washington, D.C.) 2000;287:1615–1622. doi: 10.1126/science.287.5458.1615. [DOI] [PubMed] [Google Scholar]
- 20.Rohde J.-U., In J.-H., Lim M. H., Berennessel W. W., Bukowski M. R., Stubna A., Munck E., Nam W., Que L. J. Crystallographic and spectroscopic characterization of a nonheme Fe(IV)=O complex. Science (Washington, D.C.) 2003;299:1037–1039. doi: 10.1126/science.299.5609.1037. [DOI] [PubMed] [Google Scholar]
- 21.Solomon E. I. Geometric and electronic structure contributions to function in bioinorganic chemistry: active sites in non-heme iron enzymes. Inorg. Chem. 2000;40:3656–3669. doi: 10.1021/ic010348a. [DOI] [PubMed] [Google Scholar]
- 22.Lange S. J., Que L. J. Oxygen activating nonheme iron enzymes. Curr. Opin. Chem. Biol. 1998;2:159–172. doi: 10.1016/s1367-5931(98)80057-4. [DOI] [PubMed] [Google Scholar]
- 23.Hegg E. L., Que L. J. The 2-His-1-carboxylate facial triad. An emerging structural motif in mononuclear non-heme iron(II) enzymes. Eur. J. Biochem. 1997;250:625–629. doi: 10.1111/j.1432-1033.1997.t01-1-00625.x. [DOI] [PubMed] [Google Scholar]
- 24.Price J. C., Barr E. W., Glass T. E., Krebs C., Bollinger J. M., Jr Evidence for hydrogen abstraction from C1 of taurine by the high-spin Fe(IV) intermediate detected during oxygen activation by taurine:α-ketoglutarate dioxygenase (TauD) J. Am. Chem. Soc. 2003;125:13008–13009. doi: 10.1021/ja037400h. [DOI] [PubMed] [Google Scholar]
- 25.Price J. C., Barr E. W., Tirupati B., Bollinger J. M., Jr, Krebs C. The first direct characterization of a high-valent iron intermediate in the reaction of an α-ketoglutarate-dependent dioxygenase: a high-spin Fe(IV) complex in taurine/α-ketoglutarate dioxygenase (TauD) from Escherichia coli. Biochemistry. 2003;42:7497–7508. doi: 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]
- 26.Mann J. Modern methods for the introduction of fluorine into organic molecules: an approach to compounds with altered chemical and biological activities. Chem. Soc. Rev. 1987;16:381–436. [Google Scholar]
- 27.Kerr J. A. Bond dissociation energies by kinetic methods. Chem. Rev. 1966;66:465–500. [Google Scholar]
- 28.Baghal-Vayjooee M. H., Colussi A. J., Benson S. W. Very low pressure reactor. A new technique for measuring rates and equilibria of radical-molecule reactions at low temperature. Heat of formation of the methyl radical. J. Am. Chem. Soc. 1978;100:3214–3215. [Google Scholar]
- 29.Moroney S. E. Oxford, U.K.: Dyson Perrins Laboratory, University of Oxford; 1985. DPhil thesis. [Google Scholar]
- 30.Leslie A. G. W. Integration of macromolecular diffraction data. Acta Cryst. D. 1999;55:1696–1702. doi: 10.1107/s090744499900846x. [DOI] [PubMed] [Google Scholar]
- 31.Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Cryst. D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 32.Jones T. A., Zou J. Y., Cowan S. W., Kjelgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Cryst. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 33.Groth U., Schöllkopf U. Asymmetric synthesis via heterocyclic intermediates; XIX. On the enantioselective synthesis of β-fluorovaline methyl ester and related α-amino-β-fluorocarboxylic esters. Synthesis. 1983:673–675. [Google Scholar]
- 34.Schöllkopf U., Groth U., Deng C. Enantioselective synthesis of (R)-amino acids using L-valine as chiral agent. Angew. Chem. Int. Ed. 1981;20:798–799. [Google Scholar]
- 35.Schöllkopf U., Nozulak J., Groth U. Asymmetric syntheses via heterocyclic intermediates; XV. Enantioselective synthesis of (R)-(−)-β-hydroxyvaline using L-valine or (S)-O,O-dimethyl-α-methyldopa as chiral auxiliary reagents. Synthesis. 1982:868–870. [Google Scholar]
- 36.Schöllkopf U., Groth U., Gull M.-R., Nozulak J. Asymmetrische synthesen über heterocyclische zwischenstufen, XVIII. Zur enantioselektiven synthese von (2R)-Serin-methylestern oder (2R)-serinen ausgehend vom bislactimether von cyclo-(L-Val-Gly-) Liebigs Ann. Chem. 1983:1133–1151. [Google Scholar]
- 37.Middleton W. J. New fluorinating reagents. Dialkylamino sulphur fluorides. J. Org. Chem. 1975;40:574–578. [Google Scholar]
- 38.Baldwin J. E., Herchen S. R., Johnson B. L., Jung M., Usher J. J., Wan T. Synthesis of δ-(L-α-aminoadipoyl)-L-cysteinyl-D-valine and some 13C- and 15N-labelled isotopomers. J. Chem. Soc. Perkin Trans. I. 1981:2253–2257. [Google Scholar]
- 39.Sheehan J. C., Preston J., Cruickshank P. A. A rapid synthesis of oligopeptide derivatives without isolation of intermediates. J. Am. Chem. Soc. 1965;87:2492–2493. doi: 10.1021/ja01089a034. [DOI] [PubMed] [Google Scholar]
- 40.Bodansky M., Bodansky A. Berlin: Springer–Verlag; 1984. The practice of peptide synthesis. [Google Scholar]
- 41.Baldwin J. E., Adlington R. M., Basak A., Flitsch S. L., Petursson S., Turner N. J., Ting H.-H. Enzymatic synthesis of a new type of penicillin. J. Chem. Soc. Chem. Commun. 1986:975–976. [Google Scholar]
- 42.Petursson S., Baldwin J. E. Synthesis of δ-(L-α-aminoadipoyl)-L-cysteinyl-D-(O-methyl)-D-allothreonine a substrate for isopenicillin-N synthase and its O-methyl-D-threonine epimer. Tetrahedron. 1998;54:6001–6010. [Google Scholar]