Abstract
Homozygous deletion of three nucleotides coding for Ser-171 (S171) of TAL-H (human transaldolase) has been identified in a female patient with liver cirrhosis. Accumulation of sedoheptulose 7-phosphate raised the possibility of TAL (transaldolase) deficiency in this patient. In the present study, we show that the mutant TAL-H gene was effectively transcribed into mRNA, whereas no expression of the TALΔS171 protein or enzyme activity was detected in TALΔS171 fibroblasts or lymphoblasts. Unlike wild-type TAL-H–GST fusion protein (where GST stands for glutathione S-transferase), TALΔS171–GST was solubilized only in the presence of detergents, suggesting that deletion of Ser-171 caused conformational changes. Recombinant TALΔS171 had no enzymic activity. TALΔS171 was effectively translated in vitro using rabbit reticulocyte lysates, indicating that the absence of TAL-H protein in TALΔS171 fibroblasts and lymphoblasts may be attributed primarily to rapid degradation. Treatment with cell-permeable proteasome inhibitors led to the accumulation of TALΔS171 in whole cell lysates and cytosolic extracts of patient lymphoblasts, suggesting that deletion of Ser-171 led to rapid degradation by the proteasome. Although the TALΔS171 protein became readily detectable in proteasome inhibitor-treated cells, it displayed no appreciable enzymic activity. The results suggest that deletion of Ser-171 leads to inactivation and proteasome-mediated degradation of TAL-H. Since TAL-H is a regulator of apoptosis signal processing, complete deficiency of TAL-H may be relevant for the pathogenesis of liver cirrhosis.
Keywords: apoptosis, enzyme activity, inactivation, pentose phosphate pathway, proteasome, transaldolase
Abbreviations: ALLM, N-acetyl-Leu-Leu-Met-CHO; E-64, trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane; EST, (2S,3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester; GST, glutathione S-transferase; IPTG, isopropyl β-D-thiogalactoside; PPP, pentose phosphate pathway; ROI, reactive oxygen intermediate; TAL, transaldolase; TAL-H, human TAL
INTRODUCTION
Metabolism of glucose through the PPP (pentose phosphate pathway) fulfils two unique functions: (i) formation of ribose 5-phosphate for the synthesis of nucleotides, RNA and DNA and (ii) generation of NADPH as a reducing equivalent for biosynthetic reactions. A normal reducing atmosphere, required for cellular integrity, is provided by GSH, which protects cells from damage by ROIs (reactive oxygen intermediates). Regeneration of GSH from its oxidized form, GSSG, depends on NADPH produced by the PPP [1]. The medical importance of PPP was first appreciated when deficiency of glucose-6-phosphate dehydrogenase was found to be associated with haemolytic anaemia [2]. We have demonstrated previously that the level of TAL-H (human transaldolase) expression can control the balance between the two phases of PPP, its overall output as measured by NADPH and glutathione production and, thus, determine susceptibility to cell death in various apoptosis pathways dependent on ROI production [3–5]. Previously, homozygous deletion of three nucleotides coding for Ser-171 (S171) of the TAL-H gene was documented in a 9-year-old girl suffering from liver cirrhosis [6]. Cirrhosis is a consequence of progressive cell death of hepatocytes and biliary epithelial cells [7]. The present study reveals that TAL-H RNA but not protein is present in fibroblasts and lymphoblasts from the patient tested. Whereas the mutant TAL-H gene was effectively transcribed and translated in vitro, recombinant TALΔS171 had no detectable enzymic activity. Treatment with four different proteasome inhibitors elicited the accumulation TALΔS171 in the patient's cells. The results suggest that TALΔS171 is rapidly degraded through the proteasome pathway and causes complete deficiency of TAL-H, which, in turn, may play a key role in pathogenesis of liver cirrhosis.
MATERIALS AND METHODS
Cell culture
Human fibroblasts were maintained in Ham's F-10 medium, supplemented with 20% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B. Fibroblasts were isolated from a skin biopsy obtained from the lateral aspect of the left thigh from a TAL (transaldolase)-deficient patient [6] and age-matched female controls 1 and 2. Human lymphoblasts were cultured in RPMI 1640, supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B. Epstein–Barr virus-transformed TALΔS171 lymphoblasts, and human control lymphoblasts 1 (GM13416; Coriel Cell Repositories, Camden, NJ, U.S.A.) and 2 (JAB), were established as described previously [8]. All the cell lines were maintained in a humidified atmosphere with 5% CO2 at 37 °C. Cell-culture products were purchased from Cellgro (Mediatech, Herndon, VA, U.S.A.).
Northern hybridization analysis
Total cellular RNA was extracted from exponentially growing cells, size-fractionated in a 1% agarose gel, transferred on to a Zetabind nylon membrane (Bio-Rad Laboratories, Hercules, CA, U.S.A.) and hybridized to a [32P]dCTP-labelled 827 bp EcoRI fragment of the full-length TAL-H cDNA, as described previously [9]. After the detection of the TAL-H mRNA signal, the blot was re-probed with a human α-actin-specific probe [10], used as a loading control. The abundance of the TAL-H and β-actin mRNAs was quantified using a 445 SI PhosphorImager with ImageQuant software (Molecular Dynamics, Sunnyvale, CA, U.S.A.).
Western-blot analysis
Cytosolic lysates of lymphoblasts and fibroblasts were prepared in 40 mM triethanolamine (pH 7.6), 10 mM EDTA, 1 mM sodium orthovanadate, 0.1 mM sodium molybdate, 10 mM sodium pyrophosphatase and 50 mM NaF. After three rounds of freeze–thawing, the suspension was pelleted and the supernatant was used in subsequent experiments as a cytosolic lysate. Protein concentrations were determined by using the Bio-Rad Protein Assay (Bio-Rad Laboratories). Protein lysates (10 μg unless otherwise indicated) were separated on 12% SDS/polyacrylamide gel and electroblotted on to nitrocellulose. For whole cell lysates, 2×105 cells were resuspended in 10 μl of a sample buffer and boiled at 95 °C for 5 min before loading. Nitrocellulose strips were immunoblotted with the TAL-H antibody 170 and the β-actin-specific mouse antibody 1501R (Chemicon, Temecula, CA, U.S.A.), as described previously [9]. Automated densitometry was used to quantify the relative levels of protein expression using a Kodak Image Station 440CF with Kodak 1D Image Analysis software (Eastman Kodak Company, Rochester, NY, U.S.A.).
TAL enzyme assays
TAL activity of 20 μg of cytosolic lysates was measured in the presence of 3.2 mM D-fructose 6-phosphate, 0.2 mM erythrose 4-phosphate, 0.1 mM NADH and 10 μg of glycerophosphate dehydrogenase/triosphosphate isomerase in 600 μl of 40 mM triethanolamine (pH 7.6) and 5 mM EDTA, as described previously [3]. Values of enzyme activity, expressed in m-unit/mg of the total cytosolic protein, are the means±S.E.M. for at least four independent measurements.
Site-directed mutagenesis
Wild-type human TAL cDNA (nt 51–1064; GenBank® accession no. L19437) was inserted into pGEX-4T2 vector (clone 1425) as described previously [11]. Mutant TALΔS171 protein-coding cDNA (clone 7172) was created by the deletion of residues 561–563 using the Quik Change Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, U.S.A.). Briefly, 25 ng of the wild-type TAL-H cDNA plasmid (template) was incubated with 125 ng of sense and antisense primers, dNTPs, and subjected to 18 PCR cycles with Pfu Turbo DNA polymerase with denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min and extension at 68 °C for 12 min. DpnI was used to digest the parental supercoiled double-stranded methylated DNA for 1 h at 37 °C. Transformations were performed in Epicurian Coli™ XL1-Blue cells using DpnI-treated DNA. For the in vitro transcription and translation of mutant and wild-type TAL-H cDNAs, cDNA templates were expressed under the control of the T7 promoter in the pCMVTNT vector (Promega, Madison, WI, U.S.A.). Four constructs were generated: the wild-type and mutant TAL-H cDNA sequence with and without an additional Kozak consensus sequence [12] placed immediately upstream of the ATG codon. The coding sequences of wild-type and mutant TAL-H (nt 51–1064; GenBank® accession no. L19437) were amplified by PCR using primers bound by an artificial KpnI site outside the TAL-H sequence (KpnI sense primer, 5′-AATTAAGGTACCATGTCGAGCTCACCCG-3′; and KpnI antisense primer, 5′-TTAATTGGTACCCTACTTTCCATTCTCTGC-3′). One set of primers used with the mutant and wild-type TAL-H cDNA sequences also contained a Kozak consensus sequence, CCACC, between the KpnI site and ATG start codon of the sense primer (KpnI/Kozak sense primer, 5′-AATTAAGGTACCGCCACCATGTCGAGCTCACCCG-3′). Wild-type or mutant template (100 ng each) was incubated with 3 mM MgCl2, 0.2 mM dNTP, 100 ng each of antisense and sense primers and 2.5 units of Taq DNA polymerase (Sigma–Aldrich, St. Louis, MO, U.S.A.) in a 50 μl reaction volume. Then, 30 cycles of PCR were performed with 1 min at 94 °C for denaturing, 1 min at 68 °C for annealing and 1 min at 72 °C for extension. After digestion with KpnI, the PCR products were ligated into the KpnI site of the pCMVTNT vector.
Prokaryotic expression of recombinant protein
Full-length wild-type and mutant TAL-H proteins were expressed as a fusion protein with GST (glutathione S-transferase) encoded by pGEX-2T plasmid vector, as described previously [11]. Optimum stimulation of the expression of the recombinant fusion protein was obtained with 1 mM IPTG (isopropyl β-D-thiogalactoside) after 2 h. TAL-H–GST fusion protein was affinity-purified by the binding of GST to glutathione-coated agarose beads (Sigma). Solubilization was facilitated by sonication and the addition of up to 1.5% (v/v) N-laurylsarcosine, 2% (v/v) Tween 20 and 4% (v/v) Triton X-100. To remove detergents, the bead-bound fusion protein was washed extensively, six times or more, with PBS. TAL was cleaved from GST by 1 NIH unit of thrombin (Sigma) in 0.5 ml of PBS, as described previously [11]. TAL was separated from the agarose bead-bound GST by centrifugation.
In vitro translation
Mutant and wild-type TAL-H cDNAs with or without the leading Kozak consensus sequence [18] were translated in vitro using the TNT Quick coupled transcription–translation system (Promega, Madison, WI, U.S.A.). For generating the [35S]methionine-labelled product, 1 μg of pCMVTNT-based wild-type or mutant TAL-H cDNA was incubated with the TNT Quick master mixture containing nuclease-free rabbit reticulocyte lysate, T7 RNA polymerase, amino acids minus methionine and 20 μCi of Amersham Biosciences Redivue™ L-[35S]methionine. The reaction was carried out at 30 °C for 90 min. Where indicated, 1 μg of firefly luciferase-encoding control plasmid driven by the T7 RNA polymerase promoter (Promega) was added to the reaction mixture. When both the control and TAL-H plasmids were expressed, 0.5 μg of each plasmid was used. Subsequent to translation, 5% of the reaction volume was analysed by SDS/PAGE (12% gel) followed by autoradiography. Expression levels of mutant or wild-type TAL-H and luciferase protein control were assessed using the 445 SI PhosphorImager with ImageQuant software (Molecular Dynamics).
Three-dimensional modelling of TALΔS171 protein
A three-dimensional model has been developed based on the crystal structure of TAL-H determined at 2.45 Å resolution [13] (1 Å≡0.1 nm). Ser-171 was deleted from TAL-H and a three-dimensional model of mutant TALΔS171 protein was built using the homology modelling program MODELLER [14]. The model was refined by energy minimization using the CHARMM (Chemistry at HARvard Macromolecular Mechanics) potential [15] and by 1 ns molecular dynamics simulation in water using GROMACS 2.0 (www.gromacs.org). The resulting structure was subjected to stereochemical analysis by PROCHECK [16].
Proteasome inhibition experiments
Proteasome inhibitors, obtained from Biomol Research Laboratories (Plymouth Meeting, PA, U.S.A.), were dissolved in DMSO to a final stock concentration of 10 mM gliotoxin, 10 mM MG-132, 10 mM clasto-lactacystin β-lactone, 2 mM epoxomycin and 1 mM MG-262. Cells (1×106/ml) were incubated with the indicated proteasome inhibitors for the specified time periods and concentrations. When used as a solvent for proteasome inhibitors, the final concentration of DMSO never exceeded 0.01%. After incubation, the cells were harvested and the TAL-H and β-actin protein expressions were analysed from 12.5 μg of cytosolic lysate by Western blotting. As a reference for normal TAL-H and β-actin protein expressions, untreated control lymphoblasts were run alongside TAL-H-deficient cells.
Cysteine protease inhibitors
Cysteine protease inhibitors E-64 [trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane] and loxistatin or EST [(2S,3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester], the calpain inhibitor ALLM (N-acetyl-Leu-Leu-Met-CHO) and the lysosomal protease inhibitor leupeptin were obtained from Calbiochem (La Jolla, CA, U.S.A.). These protease inhibitors were resuspended in DMSO and used to treat cells at the following maximal concentrations: 1 μM E-64, 10 μM EST, 10 μM ALLM and 10 μM leupeptin. After treatment of the cells for 12 h, protein lysates were prepared as described for proteasome inhibition experiments.
RESULTS
Deletion of the base triplet coding for Ser-171 of TAL-H affects the RNA level but not the protein level of human fibroblast and lymphoblast cells
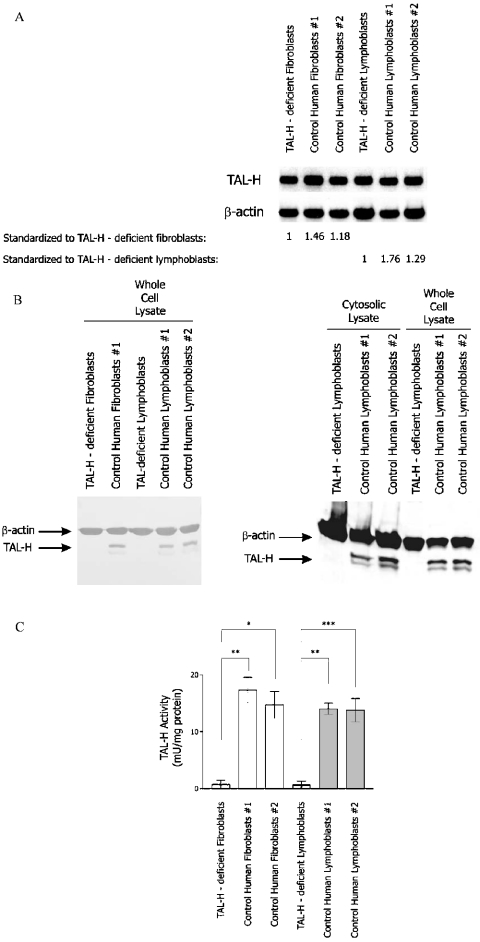
Homozygous deletion of the three nucleotides (corresponding to nt 561–563 of the human TAL cDNA; GenBank® accession no. L19437) coding for Ser-171 of TAL-H has been identified in genomic DNA and cDNA from a 9-year-old girl of Turkish descent [6]. Deletion of Ser-171 was confirmed in cDNAs of lymphoblasts and fibroblasts from the patient (results not shown). The effect of this deletion on steady-state mRNA levels was investigated by Northern-blot analysis. As shown in Figure 1(A), TAL mRNA levels were reduced by 32% in TALΔS171 fibroblasts and by 53% in TALΔS171 lymphoblasts with respect to control fibroblasts and lymphoblasts respectively. However, TAL-H protein levels could not be detected by Western-blot analysis of cytosolic lysates or whole cell extracts from TALΔS171 fibroblasts and lymphoblasts (Figure 1B). Neither fibroblasts nor lymphoblasts of the patient contained detectable TAL enzyme activity (Figure 1C). These findings suggested that the TALΔ561–563 gene could be effectively transcribed; however, the translation or stability of the mutant protein was severely compromised in both TALΔS171 fibroblasts and lymphoblasts.
Figure 1. Measurement of TAL expression by Northern- and Western-blot analyses and enzyme activity assays.
(A) Northern-blot analysis of steady-state TAL mRNA levels in TALΔS171 and control fibroblasts and lymphoblasts. Total RNA (10 μg) from TALΔS171 (TAL-H-deficient) and control fibroblasts and lymphoblasts were separated on 1% agarose gel, transferred on to a nylon membrane and hybridized to TAL cDNA probe 4/1 [9] or human β-actin probe [10], used as an internal control. The relative abundance of TAL transcripts in control cells with respect to TALΔS171 fibroblasts and lymphoblasts set at 1.0 is shown under columns 2 and 3. (B) Western-blot analysis of TAL expression in whole cell extracts (2×105 cells/lane) and cytosolic protein lysates (20 μg/lane) of TALΔS171 and control lymphoblasts. TAL was detected with antibody 170. Actin levels were monitored as loading control. (C) Measurement of TAL enzyme activity in TALΔS171 and control fibroblasts and lymphoblasts. Results are expressed as the means±S.E.M. for five or more independent experiments. *P<0.05, **P<0.01 and ***P<0.001.
Expression of the TALΔS171 protein in prokaryotic and in vitro eukaryotic systems
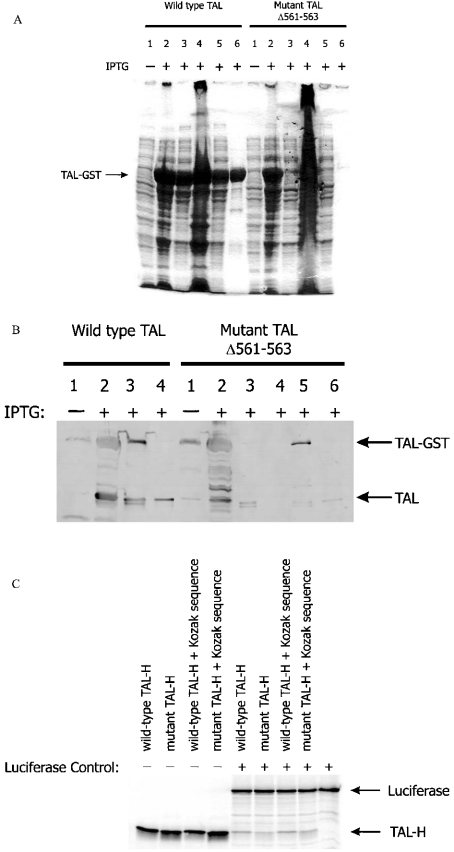
To examine the translation efficiency of TAL cDNA carrying a deletion of residues 561–563, the mutant cDNA (TALΔ561–563) was expressed as a fusion protein with GST, as described previously [11]. Similar to wild-type TAL, TALΔ561–563 was detected as a 66 kDa fusion protein in bacterial whole cell lysates (Figure 2A). In contrast, unlike wild-type TAL, GST–TALΔ561–563 fusion protein failed to solubilize and did not bind to glutathione-coated agarose beads, suggesting conformational changes. Expression of GST–TALΔ561–563 at 20, 25, 30 and 33 °C did not influence solubility. Although the overall yield of mutant TAL was lower when compared with wild-type TAL, solubilization of GST–TALΔ561–563 was achieved, as described previously [17], in the presence of 1.5% N-laurylsarcosine, 2% Tween 20 and 4% Triton X-100. Detergents were removed by washing the bead-bound fusion protein six times with PBS. Subsequently, affinity-purified GST–TALΔ561–563 was cleaved by thrombin (Figure 2B). In comparison with wild-type TAL (activity 14.22±0.47 m-units/μg of protein; P<0.0001), which was purified, in parallel, from detergent-treated supernatant on four independent occasions, TALΔ561–563-encoded TALΔS171 had no enzyme activity (0.013±0.018 m-units/μg of protein).
Figure 2. Production of mutant TAL by prokaryotic expression vectors and in vitro translation.
(A) Prokaryotic expressions of wild-type and mutant TALΔ561–563 cDNAs. The TAL-H protein was expressed as a fusion protein with GST encoded by pGEX-2T plasmid vector [11]. Maximal expression of the recombinant fusion protein was obtained after stimulation with 1 mM IPTG for 2 h. TAL-H–GST fusion protein was affinity-purified through binding of GST to glutathione-coated agarose beads. Protein lysates were analysed on 12% SDS/polyacrylamide gel. Lane 1, whole cell lysates from unstimulated cells; lane 2, whole cell lysates from IPTG-stimulated cells; lane 3, supernatant of IPTG-stimulated cells disrupted by freezing and thawing performed three times; lane 4, pellet of IPTG-stimulated cells disrupted by freezing and thawing performed three times; lane 5, supernatant of IPTG-stimulated cells incubated with GSH-coated agarose beads; lane 6, GSH-coated agarose beads pelleted after exposure to IPTG-stimulated cell supernatant. (B) Western-blot detection of solubilized, affinity-purified and thrombin-cleaved recombinant TAL–GST fusion proteins. IPTG-stimulated cells were disrupted in the absence or presence of 1.5% N-laurylsarcosine, 2% Tween 20 and 4% Triton X-100, and supernatants were incubated with GSH-coated agarose beads. Subsequently, beads were washed six times in 1 ml of PBS, digested overnight with thrombin in 500 μl of PBS and tested for the presence of TAL by Western blotting using antibody 170. Lane 1, whole cell lysate from unstimulated cells; lane 2, whole cell lysate from IPTG-stimulated cells; lane 3, GSH-coated agarose beads exposed to the supernatant of IPTG-stimulated cells disrupted by freezing and thawing performed three times; lane 4, supernatant of thrombin-treated agarose beads previously exposed to the supernatant of IPTG-stimulated cells disrupted by freezing and thawing three times; 5, GSH-coated agarose beads exposed to the supernatant of IPTG-stimulated cells disrupted by freezing and thawing three times in the presence of 1.5% N-laurylsarcosine, 2% Tween 20 and 4% Triton X-100; lane 6, supernatant of thrombin-treated agarose beads previously exposed to the supernatant of IPTG-stimulated cells disrupted by freezing and thawing three times in the presence of 1.5% N-laurylsarcosine, 2% Tween 20 and 4% Triton X-100. (C) In vitro translation of wild-type and mutant TALΔ561–563 RNA by rabbit reticulocyte lysates. For generating the [35S]methionine-labelled product, 1 μg of pCMVTNT-based wild-type or mutant TAL-H cDNA was mixed with nuclease-free rabbit reticulocyte lysate, T7 RNA polymerase, amino acids minus methionine and 20 μCi of L-[35S]methionine and incubated at 30 °C for 90 min. As indicated, firefly luciferase-encoding control plasmid driven by the T7 RNA polymerase promoter was added to the reaction mixture.
To examine translation in a eukaryotic system, rabbit reticulocyte lysates were utilized. To compare the translation efficiency, reactions were also carried out in the presence of a control plasmid encoding the firefly luciferase. As shown in Figure 2(C), both wild-type and mutant TALΔ561–563 cDNAs were effectively translated into the expected 38 kDa protein in vitro. On the basis of repeated studies, inclusion of a Kozak sequence did not significantly influence the translation of wild-type or mutant TAL cDNA. Therefore the absence of detectable TAL in TALΔS171 cells was probably caused by increased degradation.
Three-dimensional modelling of conformational changes in TALΔS171 protein
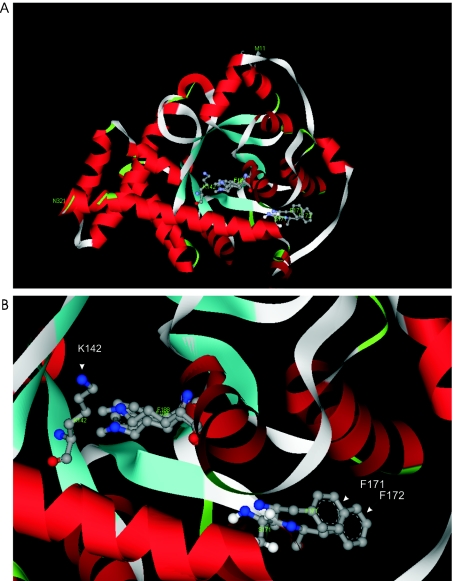
Global structures of the wild-type TAL-H and TALΔS171 were remarkably similar (Figure 3A), as expected after a single residue deletion from a loop region. Ser-171 is in the middle of a relatively large hydrophobic pocket, which is invariant in the TAL subfamily [13], and may play an essential role in substrate binding. Owing to the deletion of Ser-171, Phe-172, which corresponds to Phe-171 in TALΔS171, moved markedly closer, by 3.66 Å, to the centre of the α/β-barrel, towards the catalytic residue Lys-142 (Figure 3B). The new position of Phe-172 is at the centre of the hydrophobic pocket and this may affect the local conformation and self-association properties of the mutant protein.
Figure 3. Conformational changes elicited by the deletion of Ser-171 of TAL.
(A) Overlay of three-dimensional models of TAL-H and TALΔS171 reveals remarkably similar global structures. (B) Phe-171 of TALΔS171 is positioned, by 3.66 Å, closer to the centre of the α/β-barrel when compared with Phe-172 of TAL-H.
Accumulation of TALΔS171 in proteasome inhibitor-treated cells
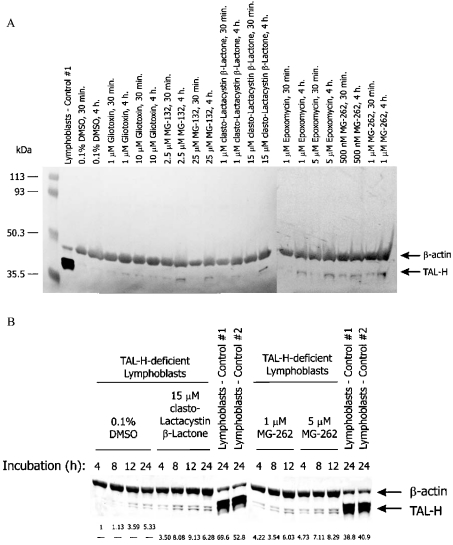
The altered solubility of GST–TALΔS171 fusion protein and three-dimensional modelling of TALΔS171 suggested that deletion of Ser-171 resulted in conformational changes. Misfolded proteins may be rapidly targeted for degradation by the 26 S proteasome in eukaryotic cells. To investigate this possibility, TALΔS171 lymphoblasts were treated with cell-permeable proteasome inhibitors. As shown in Figure 4, clasto-lactacystin β-lactone, epoxomycin, gliotoxin, MG-132 and MG-262 elicited a dose- and time-dependent accumulation of TALΔS171 in whole cell or cytosolic lysates of TAL-deficient cells (Figure 4A). Whereas 0.01% DMSO, used as a solvent for proteasome inhibitors, had no effect, interestingly, 0.1% DMSO led to a 2.17±0.70-fold accumulation of TALΔS171 (P=0.037). DMSO was also found to shield the ΔPhe-508 mutant of the CFTR (cystic fibrosis transmembrane conductance regulator) from degradation by the proteasome [19]. Unlike inhibitors of the proteasome, cysteine protease inhibitors E-64 and EST, the calpain inhibitor ALLM and the lysosomal inhibitor leupeptin did not elicit TAL accumulation in TALΔS171 lymphoblasts (results not shown). Maximal accumulation of TALΔS171 was achieved by a 12 h treatment with clasto-lactacystin β-lactone or MG-262 (Figure 4B). From results of four independent experiments, in comparison with cells treated with 0.1% DMSO alone, the levels of TALΔS171 proteins were increased 4.84±0.86-fold (P=0.009) by 15 μM clasto-lactacystin β-lactone and 4.54±1.03-fold (P=0.022) by 5 μM MG-262. Although TALΔS171 levels of proteasome-treated cells reached 20% of the normal TAL-H levels, these cells did not show significant TAL enzyme activity.
Figure 4. Effects of cell-permeable proteasome inhibitors on TAL protein levels in TALΔS171 lymphoblasts.
(A) Proteasome inhibitors gliotoxin, MG-132, clasto-lactacystin β-lactone, epoxomycin and MG-262 were dissolved in DMSO and added to 106 cells. When used as a solvent for proteasome inhibitors, the final concentration of DMSO was less than 0.01%. After incubation for 30 min or 4 h, protein lysates from whole cell extracts were analysed by Western blotting. TAL was detected with antibody 170 and human β-actin was monitored with the monoclonal antibody 1501R. (B) Effects of the proteasome inhibitors clasto-lactacystin β-lactone and MG-262 on accumulation of TAL-H in TALΔS171 lymphoblasts. Using automated densitometry, TAL-H expression levels of proteasome-treated TALΔS171 lymphoblasts were normalized to internal actin levels and are compared with cells exposed to 0.1% DMSO alone for the same length of time.
DISCUSSION
The present study suggests that deletion of Ser-171 abrogates enzymic activity and leads to rapid degradation of TAL in both fibroblast and lymphoblast cells of the TALΔS171 patient. The steady-state levels of intracellular proteins are generally maintained by a balance of protein synthesis and degradation. Approximately one-half of the coding sequence mutations interfere with gene transcription or translation. The remainder of the mutations does not significantly influence protein synthesis but affects protein folding [20]. Improper protein folding can lead to diseases by three basic mechanisms: (1) aggregation of misfolded protein acquiring resistance to proteolytic cleavage, (2) dominance of mutant protein interfering with the activity of the wild-type product, and (3) loss of function due to rapid degradation of the mutant protein. Unlike wild-type TAL–GST fusion protein, TALΔS171–GST failed to solubilize and bind to glutathione-coated agarose beads in the absence of detergents, suggesting that deletion of Ser-171 caused conformational changes of TAL-H. TALΔ561–563 was effectively transcribed in vivo and was translated in vitro using rabbit reticulocyte lysates, indicating that the absence of mutant TAL-H protein may be attributed primarily to rapid degradation in the patient's cells.
Northern-blot analysis showed normal TALΔ561–563 mRNA levels and Western-blot analysis revealed no TALΔS171 protein in the patient's cells. In contrast, normal levels of protein were produced in vitro, suggesting degradation of TALΔS171 in the patient's cells.
Protein folding in living cells is a complex process involving many interdependent factors. Several disease states are brought about because of irregularities in protein folding. Under normal cellular conditions, ‘quality control’ mechanisms ensure that incorrectly folded or incompletely assembled proteins are degraded. Most of the proteins in mammalian cells are degraded by lysosomal proteases or the proteasome system. Several low-molecular-mass inhibitors of the proteasome were identified previously that can enter cells and selectively inhibit this degradative pathway [21]. All five proteasome inhibitors used in the present study elicited a dose- and time-dependent accumulation of TALΔS171. In contrast, inhibition of cysteine proteases, calpain and lysosomal proteases did not influence the degradation of TALΔS171. After treatment with proteasome inhibitors, TALΔS171 could be detected both in whole cell lysates and cytosolic extracts, suggesting that deletion of Ser-171 led to rapid degradation by the proteasome without affecting the solubility of TAL. Although the mutant protein became readily detectable in proteasome inhibitortreated cells, it displayed no appreciable enzymic activity. Alternatively, the deletion of Ser-171 may interfere with substrate binding and, in the absence of substrate, the protein may adopt a conformation prone to proteasome-mediated degradation. Complete TAL deficiency is consistent with accumulation of sedoheptulose 7-phosphate in a TALΔS171 patient [6]. Parents of the patient carried a heterozygous genotype and showed no evidence of the disease [6]. These findings are suggestive of a recessive inheritance pattern in TAL deficiency.
TAL catalyses the transfer of a three-carbon fragment, corresponding to dihydroxyacetone, from sedoheptulose 7-phosphate and glucose 6-phosphate to glyceraldehyde 3-phosphate and erythrose 4-phosphate respectively, and a variety of other acceptor aldehydes, including non-phosphorylated trioses and tetroses. Absence of TAL enzyme activity is consistent with the accumulation of sedoheptulose 7-phosphate in the patient's cells [6]. Enzymic activity of TAL is regulated in a tissue-specific [22–28] and development-specific manner [29]. TAL-H regulates the mitochondrial transmembrane potential ΔΨm, a key checkpoint in the effector phase of several apoptosis pathways [3–5]; thus, changes in TAL expression may influence cell-type-specific susceptibility to cell death signals. Indeed, with respect to control cells, lymphoblasts and fibroblasts of the TAL-deficient patient exhibit increased susceptibility to H2O2-induced apoptosis (A. Perl, unpublished work). The impact of TAL on apoptosis signalling may be related to the overwhelming influence of TAL-catalysed dihydroxyacetone transfer reactions on the balance between the two branches of PPP [3] that control intracellular NADPH levels and neutralization of ROIs [30]. Clinical manifestations of TAL deficiency are dominated by consequences of liver cirrhosis, which results from increased cell death of hepatocytes and biliary epithelial cells [7]. TAL deficiency has recently been observed in a second patient with liver failure (N. M. Verhoeven, unpublished work). Since TAL-H has been recognized as a regulator of apoptosis signal processing [3–5], complete deficiency of TAL-H may be relevant for pathogenesis of liver cirrhosis.
Acknowledgments
We thank Dr Martha Stipanuk (Cornell University, Ithaca, NY, U.S.A.) for helpful comments on proteasome inhibitors. This work was supported by grant no. RO1 DK 49221 from the National Institutes of Health and by the Central New York Community Foundation.
References
- 1.Mayes P. A. The pentose phosphate pathway and other pathways of hexose metabolism. In: Murray R. K., Granner D. K., Mayes P. A., Rodwell V. W., editors. Harper's Biochemistry. Norwalk, CT: Appleton and Lange; 1993. pp. 201–211. [Google Scholar]
- 2.Cooper R. A., Bunn H. F. Hemolytic anemias. In: Wilson J. D., Braunwald E., Isselbacher K. J., Petersdorf R. G., Martin J. B., Fauci A. S., Root R. K., editors. Harrison's Principles of Internal Medicine. New York: McGraw-Hill; 1991. pp. 1531–1543. [Google Scholar]
- 3.Banki K., Hutter E., Colombo E., Gonchoroff N. J., Perl A. Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. J. Biol. Chem. 1996;271:32994–33001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- 4.Banki K., Hutter E., Gonchoroff N. J., Perl A. Molecular ordering in HIV-induced apoptosis: oxidative stress, activation of caspases, and cell survival are regulated by transaldolase. J. Biol. Chem. 1998;273:11944–11953. doi: 10.1074/jbc.273.19.11944. [DOI] [PubMed] [Google Scholar]
- 5.Banki K., Hutter E., Gonchoroff N., Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J. Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- 6.Verhoeven N. M., Huck J. H., Roos B., Struys E. A., Salomons G. S., Douwes A. C., van der Knaap M. S., Jakobs C. Transaldolase deficiency: liver cirrhosis associated with a new inborn error in the pentose phosphate pathway. Am. J. Hum. Genet. 2001;68:1086–1092. doi: 10.1086/320108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burt A. D. Primary biliary cirrhosis and other ductopenic diseases. Clin. Liver Dis. 2002;6:363–380. doi: 10.1016/s1089-3261(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 8.Chan M. A., Stein L. D., Dosch H. M., Sigal N. H. Heterogeneity of EBV-transformable human B lymphocyte populations. J. Immunol. 1986;136:106–112. [PubMed] [Google Scholar]
- 9.Banki K., Halladay D., Perl A. Cloning and expression of the human gene for transaldolase: a novel highly repetitive element constitutes an integral part of the coding sequence. J. Biol. Chem. 1994;269:2847–2851. [PubMed] [Google Scholar]
- 10.Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human α-, β-, and γ-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol. Cell. Biol. 1983;3:787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banki K., Perl A. Inhibition of the catalytic activity of human transaldolase by antibodies and site-directed mutagenesis. FEBS Lett. 1996;378:161–165. doi: 10.1016/0014-5793(95)01446-2. [DOI] [PubMed] [Google Scholar]
- 12.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorell S., Gergely P., Jr, Banki K., Perl A., Schneider G. The three-dimensional structure of human transaldolase. FEBS Lett. 2000;475:205–208. doi: 10.1016/s0014-5793(00)01658-6. [DOI] [PubMed] [Google Scholar]
- 14.Eswar N., John B., Mirkovic N., Fiser A., Ilyin V. A., Pieper U., Stuart A. C., Marti-Renom M. A., Madhusudhan M. S., Yerkovich B., et al. Tools for comparative protein structure modeling and analysis. Nucleic Acids Res. 2003;31:3375–3380. doi: 10.1093/nar/gkg543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennis S., Kortvelyesi T., Vajda S. Computational mapping identifies the binding sites of organic solvents on proteins. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4290–4295. doi: 10.1073/pnas.062398499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- 17.Frangioni J. V., Neel B. G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 18.Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 2002;299:1–34. doi: 10.1016/S0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bebok Z., Venglarik C. J., Panczel Z., Jilling T., Kirk K. L., Sorscher E. J. Activation of ΔF508 CFTR in an epithelial monolayer. Am. J. Physiol. 1998;275:C599–C607. doi: 10.1152/ajpcell.1998.275.2.C599. [DOI] [PubMed] [Google Scholar]
- 20.Gregersen N., Bross P., Jorgensen M. M., Corydon T. J., Andresen B. S. Defective folding and rapid degradation of mutant proteins is a common disease mechanism in genetic disorders. J. Inherit. Metab. Dis. 2000;23:441–447. doi: 10.1023/a:1005663728291. [DOI] [PubMed] [Google Scholar]
- 21.Lee D. H., Goldberg A. L. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 22.Novello F., McLean P. The pentose phosphate pathway of glucose metabolism. Measurements of the nonoxidative reactions of the cycle. Biochem. J. 1968;107:775–791. doi: 10.1042/bj1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinrich P. C., Morris H. P., Weber G. Behavior of transaldolase (EC 2.2.1.2.) and transketolase (EC 2.2.1.1.) in normal neoplastic, differentiating, and regenerating liver. Cancer Res. 1976;36:3189–3197. [PubMed] [Google Scholar]
- 24.Severin S. E., Stepanova N. G. Interrelationship between glycolysis and the anaerobic part of the pentose phosphate pathway of carbohydrate metabolism in the myocardium. Adv. Enzyme Regul. 1981;19:235–255. doi: 10.1016/0065-2571(81)90018-2. [DOI] [PubMed] [Google Scholar]
- 25.Wood T. New York: Academic Press; 1985. The Pentose Phosphate Pathway. [Google Scholar]
- 26.James H. M., Williams S. G., Bais R., Rofe A. M., Edwards J. B., Conyers R. A. J. The metabolic production of oxalate from xylitol: activities of transketolase, transaldolase, fructokinase and aldolase in liver, kidney, brain, heart, muscle in the rat, mouse, guinea pig, rabbit and human. Int. J. Vitam. Nutr. Res. 1985;28(Suppl.):29–46. [PubMed] [Google Scholar]
- 27.Hothersall J. S., Baquer N. Z., McLean P. Pathways of carbohydrate metabolism in peripheral nervous tissue. I. The contribution of alternative routes of glucose utilization in peripheral nerve and brain. Enzyme. 1982;27:259–267. doi: 10.1159/000459058. [DOI] [PubMed] [Google Scholar]
- 28.Banki K., Colombo E., Sia F., Halladay D., Mattson D., Tatum A., Massa P., Phillips P. E., Perl A. Oligodendrocyte-specific expression and autoantigenicity of transaldolase in multiple sclerosis. J. Exp. Med. 1994;180:1649–1663. doi: 10.1084/jem.180.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baquer N. Z., Hothersall J. S., McLean P., Greenbaum A. L. Aspects of carbohydrate metabolism in developing brain. Dev. Med. Child Neurol. 1977;19:81–104. doi: 10.1111/j.1469-8749.1977.tb08027.x. [DOI] [PubMed] [Google Scholar]
- 30.Ni T. C., Savageau M. A. Application of biochemical systems theory to metabolism in human red blood cells. Signal propagation and accuracy of representation. J. Biol. Chem. 1996;271:7927–7941. doi: 10.1074/jbc.271.14.7927. [DOI] [PubMed] [Google Scholar]