Abstract
Why do animals pursue reward in the face of punishment? Dopamine-releasing neurons that promote reward-seeking behaviour indirectly impair those that encode punishment avoidance, affecting decisions on risk.
The ability to evaluate both risk and reward is necessary for animals to survive in their natural environment. Risk avoidance and reward seeking are influenced by internal state (such as hunger or thirst), environmental context and previous experience, and can have far-reaching effects on mental health1. For instance, motivation for reward seeking is often blunted in depression, but enhanced in drug addiction2,3. Furthermore, increased motivation for reward in disorders such as addiction often corresponds with diminished risk avoidance4. The precise mechanisms that underlie motivational drives have eluded scientists, owing mainly to the sheer number and diversity of neurons in the brain that reinforce reward seeking and punishment avoidance. On page 356, Jovanoski et al. 5 use neurogenetic tools that provide precise control over a mixed population of dopamine-releasing neurons in the brains of fruit flies (Drosophila melanogaster) to clarify the neural mechanisms behind unconstrained reward seeking — the persistent pursuit of reward even in the face of punishment.
A well-characterized region of the fruit-fly brain known as the mushroom body has provided remarkable insight into how memories of experiences are stored, retrieved and updated, as well as why some memories are more enduring than others. This is largely because techniques are now available that allow nearly all of the neurons connected to the mushroom body to be individually manipulated with the temporal precision needed to identify memory circuits and track changes in their activity over time6. Some key principles about the neural encoding of rewarding experiences have emerged from research using the fruit-fly mushroom body as a model7. Reward encoding depends on the presence, timing and intensity of a reward, or on the absence of an expected punishment1,8,9.
Dopamine and related neurotransmitter molecules are required for encoding, extinguishing and updating memories. The dopamine neurons that encode reward are diverse in terms of the genes they express, and can be defined according to the anatomical compartments that they occupy within the mushroom body. Although some dopamine neurons seem to be involved in all types of reward, others are apparently specific to a particular reward or internal state necessary for the expression of reward behaviours.
In fruit flies, dopamine is also required for maladaptive memories, such as those associated with an alcohol reward. Fruit flies develop preferences that last up to seven days for cues previously associated with alcohol, and exhibit a form of unconstrained reward seeking by walking over an electric shock to approach an odour that is predictive of an alcohol reward10. Although alcohol activates a broad population of dopamine neurons associated with reward11, the mechanisms that cause fruit flies to endure electric shocks to attain a reward cue were previously unknown.
Jovanoski et al. used gene-expression patterns to identify a population of dopamine neurons that provide input to the mushroom body. They then artificially activated individual neurons, and were able to establish that these neurons are sufficient to drive unconstrained reward seeking. By associating the artificial activation of this population of dopamine neurons with an odour, the authors showed that fruit flies will tolerate an electric-shock punishment in pursuit of that odour one minute later.
The authors then used genetic tools to identify a subpopulation of these dopamine neurons that, when activated, can artificially instil a shock-resistant reward-seeking behaviour. This subpopulation of dopamine neurons makes connections with discrete regions of the mushroom body that are thought to be necessary for forming short-term associations with sugar and water12–14. The response superseded internal state, because hungry fruit flies pursued an odour associated with artificial activation, rather than satiating their hunger with sugar.
Jovanoski and colleagues also describe a network of opposing reward- and punishment-encoding dopamine neurons responsible for behavioural choice. The reward dopamine neurons indirectly impair the function of the punishment dopamine neurons, and this drives unconstrained reward seeking (Fig. 1). These data resolve how the processing of signals that drive opposing behaviours influences future decisions about risk, and add a fundamental principle through which reward is assessed and drives motivated behaviour.
Figure 1 |. Dopamine-releasing neurons drive the pursuit of reward in the face of punishment.
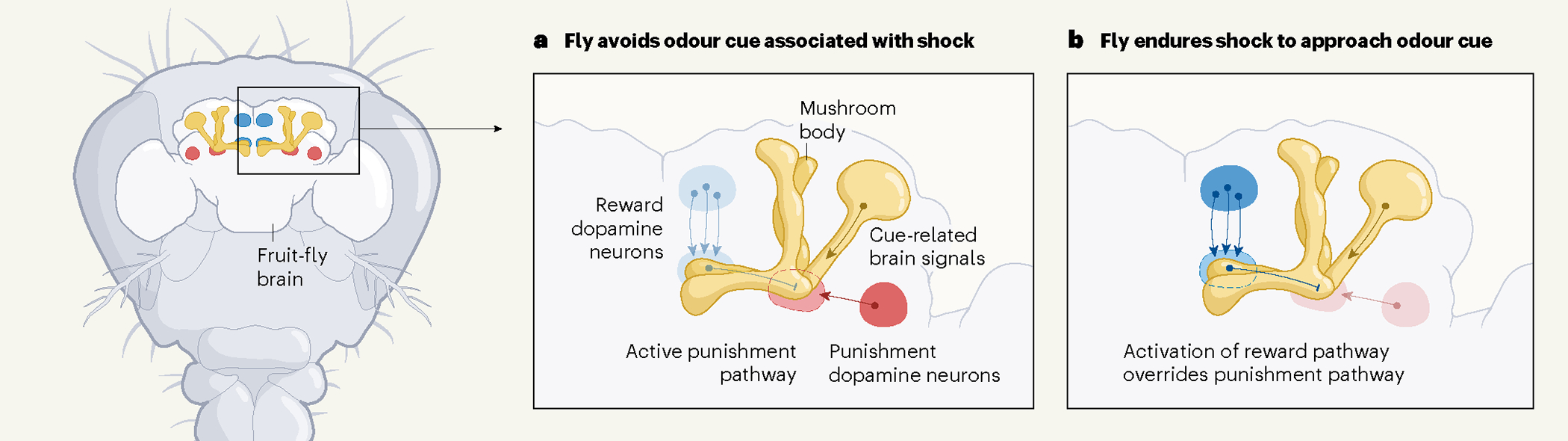
Jovanoski et al.5 show that, in a region of the fruit-fly brain called the mushroom body, distinct subpopulations of neurons that release the neurotransmitter dopamine can reinforce either the seeking of a reward or the avoidance of a punishment. a, Typically, the activity of punishment-encoding neurons (red) overcomes that of reward-encoding neurons (blue), causing fruit flies to avoid an odour cue associated with an electric shock. b, When the odour cue is paired with artificial stimulation of reward-encoding neurons, however, fruit flies will endure an electric shock in pursuit of the odour. Dysregulation of a similar network of opposing dopamine neurons in humans could be responsible for unconstrained reward-seeking behaviour, a feature of addiction.
This work was possible because subpopulations of dopamine neurons within a heterogeneous dopamine reward system could be identified and manipulated, and combining this with approaches that map neural connections showed that these subpopulations receive diverse and highly parallel inputs. The authors’ findings reveal the complexity of reward encoding and the role of functionally interconnected brain compartments in representing multiple reward types that are gated by a variety of motivational states, including thirst and hunger. Future studies might address how long aberrant behavioural choices persist, and whether these mechanisms occur in the face of more intense rewards, such as intoxicating substances.
Given that there are strong parallels between the reward circuitry of fruit flies and that of mammals15, Jovanoski and colleagues’ work provides a fundamental framework for understanding how animals remember rewards and overcome aversive stimuli to seek them. One caveat is that the study uses data averaged from groups of fruit flies, and is therefore not able to address recurrent or compulsive reward-seeking behaviour, which would need to be examined in individual animals. However, a similar internal-state-gated network of opposing dopamine neurons could explain how unconstrained reward seeking occurs in addiction. Further investigation of this network could improve our understanding of depression, addiction and other mental-health disorders in which the balance between risk and reward is disrupted.
Footnotes
The authors declare no competing interests.
Contributor Information
Kristin M. Scaplen, Department of Psychology, Bryant University, Smithfield, Rhode Island 02917, USA.
Karla R. Kaun, Department of Neuroscience, Brown University, Providence, Rhode Island, 02912, USA.
References
- 1.Devineni AV & Scaplen KM Front. Behav. Neurosci. 15, 821680 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eshel N & Roiser JP Biol. Psychiatry 68, 118–124 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Schultz W Neuron 69, 603–617 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Piantadosi PT, Halladay LR, Radke AK & Holmes AJ Neurochem. 157, 1547–1571 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jovanoski KD et al. Nature 623, 356–365 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luan H, Diao F, Scott RL & White BH Front. Neural Circuits 14, 603397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez JS, Brown TM & Kaun KR Oxford Res. Encyclopedia Neurosci. 10.1093/acrefore/9780190264086.013.495 (2023). [DOI] [Google Scholar]
- 8.Cognigni P, Felsenberg J & Waddell S Curr. Opin. Neurobiol. 49, 51–58 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felsenberg J Curr. Opin. Neurobiol. 67, 190–198 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Kaun KR, Azanchi R, Maung Z, Hirsh J & Heberlein U Nature Neurosci. 14, 612–619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scaplen KM et al. eLife 9, e48730 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamagata N et al. Proc. Natl Acad. Sci. USA 112, 578–583 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huetteroth W et al. Curr. Biol. 25, 751–758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin S et al. Nature Neurosci. 17, 1536–1542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaplen KM & Kaun KR J. Neurogenet. 30, 133–148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


