Abstract
Cyclophilin B (CyPB) is a heparin-binding protein first identified as a receptor for cyclosporin A. In previous studies, we reported that CyPB triggers chemotaxis and integrin-mediated adhesion of T-lymphocytes by way of interaction with two types of binding sites. The first site corresponds to a signalling receptor; the second site has been identified as heparan sulphate (HS) and appears crucial to induce cell adhesion. Characterization of the HS-binding unit is critical to understand the requirement of HS in pro-adhesive activity of CyPB. By using a strategy based on gel mobility shift assays with fluorophore-labelled oligosaccharides, we demonstrated that the minimal heparin unit required for efficient binding of CyPB is an octasaccharide. The mutants CyPBKKK− [where KKK− refers to the substitutions K3A(Lys3→Ala)/K4A/K5A] and CyPBΔYFD (where Tyr14-Phe-Asp16 has been deleted) failed to interact with octasaccharides, confirming that the Y14FD16 and K3KK5 clusters are required for CyPB binding. Molecular modelling revealed that both clusters are spatially arranged so that they may act synergistically to form a binding site for the octasaccharide. We then demonstrated that heparin-derived octasaccharides and higher degree of polymerization oligosaccharides inhibited the interaction between CyPB and fluorophore-labelled HS chains purified from T-lymphocytes, and strongly reduced the HS-dependent pro-adhesive activity of CyPB. However, oligosaccharides or heparin were unable to restore adhesion of heparinase-treated T-lymphocytes, indicating that HS has to be present on the cell membrane to support the pro-adhesive activity of CyPB. Altogether, these results demonstrate that the octasaccharide is likely to be the minimal length unit required for efficient binding of CyPB to cell surface HS and consequent HS-dependent cell responses.
Keywords: cell adhesion, cyclophilin B, heparan sulphate, heparin oligosaccharide, mobility shift assay
Abbreviations: ANTS, 8-aminonaphthalene-1,3,6-trisulphonic acid; CyPB, cyclophilin B; dp, degree of polymerization; DPBS, Dulbecco's PBS; FGF-2, fibroblast growth factor-2; HS, heparan sulphate; HSPG, HS proteoglycan; IL-8, interleukin-8
INTRODUCTION
Cyclophilins are highly conserved proteins first characterized as the main binding proteins for cyclosporin A, an immunosuppressive drug widely used in the prevention of graft rejection [1,2]. They were later identified as peptidyl-prolyl cis/trans-isomerases, a family of enzymes that catalyse protein folding [3,4]. Cyclophilins share high sequence similarity in the central core, which contains the catalytic and cyclosporin-binding domains, whereas their C- and N-terminal extensions are structurally unrelated [5]. Cyclophilin B (CyPB) is a secreted isoform, which has been shown to interact with cell surface-binding sites and to trigger chemotaxis and integrin-mediated adhesion of T-lymphocytes to the extracellular matrix [6,7]. We have identified two classes of binding sites, a signalling receptor designated as the type I site, and cell surface heparan sulphate (HS), referred to as the type II site [8]. By using site-directed mutated ligands, we delineated the areas of CyPB involved in the interaction. Binding to the type I site requires the central core of the protein, while binding to the type II site involves the N-terminal specific extension of the protein [9]. These findings may help explain why cyclophilin A and CyPB trigger common cell responses, e.g. chemotaxis, by way of interaction with the same signalling receptor. In contrast, we found that only CyPB is capable of promoting integrin-mediated adhesion of T-lymphocytes to fibronectin [7]. Interaction with the signalling receptor is not sufficient to generate this cellular response, and binding to the type II site appeared absolutely necessary for this unique activity. In support of this idea, we demonstrated that removal of cell surface HS by heparinase I treatment abolished the pro-adhesive activity of CyPB, and mutants of CyPB, modified in their ability to bind to type II sites, were unable to induce cell adhesion [7,10]. These results suggest that CyPB possesses a dual receptor system comprising signalling receptor and HS proteoglycan (HSPG), and both must be present to trigger cell adhesion.
Over the years, hundreds of HS-binding proteins, including growth factors, adhesion proteins, enzymes, cytokines, chemokines, protease inhibitors and virus proteins, have been identified and the number continues to increase. Numerous studies have also converged to reveal that HSPGs are critical regulators of the activities of their ligands [11,12]. Therefore, how HS interacts with these proteins and affects their stability, concentration, conformation and activity is a fundamental question in biology. However, the structural features of HS motifs have been identified in only a few cases [11]. The first example of a defined heparin-binding motif is the sequence required for antithrombin III binding, where the most characteristic feature is the unusual 3-O sulphate group on a 6-O sulphate N-sulphoglucosamine residue [13]. Furthermore, the structural characterization of a HS motif that could bind fibroblast growth factor-2 (FGF-2) has illustrated the importance of contiguous stretches of the disulphated disaccharide N-sulphoglucosamine-iduronate 2-O sulphate for highest binding ability [14]. Another crucial feature of the HS motif is the minimal length for ligand binding and activity. In this way, heparin-derived tetrasaccharides were found sufficient to interact with FGF-2. However, oligosaccharides of degree of polymerization (dp) 10–12 are required for optimizing the proliferative activity of FGF-2 [15]. Another example is the binding domain of the interleukin-8 (IL-8) dimer, described as two hexasaccharide domains separated by less than seven disaccharide units [16]. Specific heparin disaccharides were however sufficient enough to prevent IL-8 binding and exhibit anti-inflammatory properties [17]. These examples clearly demonstrate that the minimal length units required for either binding or biological activity are not strictly related. To explain this discrepancy, several models have been proposed. In the first one, in which the HS-minimal-binding unit would be sufficient to enable activity of the ligand, HS could be visualized as a hand that correctly presents the ligand to its cognate signalling receptor [18–20]. In the HS-dependent dimerization model, two HS-binding units serve for inducing the dimerization of two ligands, and the now homodimeric ligand leads in turn to subsequent dimerization of signalling receptors [21]. Furthermore, two distinct HS-binding motifs could act by bridging the ligand and its receptor [22]. A common feature of these models is the ability of soluble HS to complement the absence of HS on the membrane of target cells, indicating that the ligands could be presented by HSPG on neighbouring cells or in the extracellular matrix [15,19,23]. Conversely, in a co-signalling model, the simultaneous interaction of the ligand with both the HS-binding unit and the receptor is required to enable the clustering of proteoglycan core protein and signalling receptor in the proximity of each other. This clustering facilitates recruitment of co-signalling molecules and interaction with the cytoskeleton by means of the cytosolic tail of HSPG [24,25]. Obviously, these models are not mutually exclusive and are likely to occur simultaneously to modulate cell responses triggered by HS-binding proteins.
In this context, the characterization of the minimal HS unit that binds CyPB is of importance in an attempt to clarify the molecular mechanisms that underline the requirement of HS in the pro-adhesive activity of this factor. However, HS is extremely heterogeneous in sequence and size, and the source of specific HS-binding motifs on T-lymphocytes is limited. Heparin is similar in structure to the sulphated regions of HS, and we demonstrated that the heparin and type II site present on T-lymphocytes shared the same capability to interact with CyPB [8,9]. Therefore, we used in this study heparin-derived oligosaccharides to examine the relationship between the length of these oligosaccharides and their capability to interact with CyPB. The interaction was analysed by using an approach based on the combination of electrophoretic migration of fluorophore-labelled oligosaccharides [26] and gel mobility shift assay [27]. Finally, the biological relevance of HS binding to the activity of CyPB was analysed by testing the ability of heparin-derived oligosaccharides to modulate CyPB-induced adhesion of T-lymphocytes to fibronectin.
EXPERIMENTAL
Materials and cells
Recombinant human CyPB was produced and purified as described [28]. The mutants CyPBKKK− [where KKK− refers to the substitutions K3A(Lys3→Ala)/K4A/K5A] and CyPBΔYFD (where Tyr14-Phe-Asp16 has been deleted) were engineered by site-directed mutagenesis and purified as previously reported [9]. These mutants were checked for their inability to interact with heparin and to promote integrin-mediated adhesion of T-lymphocytes [9,10]. Human fibronectin was a gift from Dr P. Delannoy (University of Lille, France). Heparinase I (EC 4.2.2.7), chondroitinase ABC (EC 4.2.2.4), DNase I (EC 3.1.21.1), neuraminidase (EC 3.2.1.18), Pronase E (EC 3.4.24.4) and antithrombin III were from Sigma (St. Louis, MO, U.S.A.). Recombinant human FGF-2, ANTS (8-aminonaphthalene-1,3,6-trisulphonic acid) and sodium cyanoborohydride were purchased from R & D Systems (Abingdon, Oxon, U.K.), Molecular Probes (Leiden, The Netherlands) and Fluka (Buchs, Switzerland) respectively. Human citrated venous blood samples were obtained from the local blood transfusion centre (Etablissement de Transfusion Sanguine, Lille, France). Isolation of peripheral blood T-lymphocytes and cell heparinase treatment were conducted as described [8]. All other chemicals, except where otherwise mentioned, were purchased from Sigma.
Preparation of heparin-derived oligosaccharides and cell surface HS
Heparin-derived oligosaccharides were obtained as described previously [29]. Briefly, 100 mg of heparin were incubated with 50 units of heparinase I at 30 °C for 30 h. After desalting on a Sephadex G-10 column (Pharmacia Amersham Biotech, Uppsala, Sweden), the digestion mixture was fractionated by filtration on Bio-Gel P-6 (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Pooled fractions corresponding to increasing dp oligosaccharides were eluted by 0.2 M NH4Cl, pH 3.5, desalted and freeze dried. Cell surface HSPGs were purified from peripheral blood T-lymphocytes by anion exchange chromatography on DEAE-Sepharose (Pharmacia) and treated by enzymic digestion of anionic contaminants by sequential treatments with chondroitinase ABC, DNase I and neuraminidase, essentially as described previously [30]. Glycopeptides were then obtained by digestion with Pronase E overnight at 37 °C and subjected to non-reductive β-elimination [31]. Briefly, glycopeptides were incubated in a mixture of aqueous ammonium hydroxide solution and ammonium carbonate at 60 °C for 40 h in the dark. The liberated glycosylamines were converted in reducing oligosaccharides through the addition of boric acid and freeze dried before further analysis.
Fluorescent labelling of heparin-derived oligosaccharides and purified HS
Heparin-derived oligosaccharides and purified cell surface HS were derivatized by reductive amination with ANTS, according to a standard method described in [26]. To each dried sample (up to 50 nmol) was added 5 μl of 0.2 M ANTS in acetic acid/water (3:17, v/v) and 5 μl of 1 M NaCNBH3 in DMSO. The mixture was incubated at 37 °C for 16 h and thereafter dried for 4 h using a centrifugal vacuum evaporator. The fluorescent molecules were then dissolved in electrophoresis binding buffer, containing 20 mM Tris/HCl, 100 mM KCl, 1 mM EDTA and 1 mM dithiothreitol, pH 7.9. In order to check whether ANTS labelling did not lead to some desulphation, the same procedure was applied to heparin and quantitative analysis of total sulphate groups was determined according to the method described in [32]. The sulphate content was estimated at 12.6% (w/w) for untreated heparin compared with 11.8% for treated heparin, indicating that this procedure did not lead to a significant desulphation of heparin (less than 10%).
Gel mobility shift assay
CyPB and ANTS-labelled oligosaccharides were mixed in 40 μl of electrophoresis binding buffer for 30 min at 20 °C. To avoid non-specific interactions, the binding mixtures were complemented with NaCl to a final concentration of 400 mM. The samples were then supplemented with 10 μl of 60% glycerol and subjected to electrophoresis in a 10% (w/v) native polyacrylamide gel (Bio-Rad Laboratories) in 10 mM Tris/1 mM EDTA, pH 7.4. Electrophoresis was routinely carried out at 100 V for 2–3 h at 4 °C in a SE 250 Mighty Small II mini-gel system (10 cm×8 cm) for heparin-derived oligosaccharides and in a SE 600 Standard gel system (10 cm×8 cm) for HS (Hoefer Scientific Instruments, San Francisco, CA, U.S.A.). The electrophoretic buffer used was 40 mM Tris, 40 mM acetic acid and 1 mM EDTA, pH 8.0. A mixture of Bromophenol Blue and Phenol Red was used as electrophoresis markers. At the end of the electrophoresis, images were acquired with the gel Doc 2000 Image analysis apparatus from Bio-Rad Laboratories, equipped with a 365 nm UV transilluminator. Analysis was performed with the supplied software Quantity One. To check the position of free and CyPB-bound oligosaccharides, the gel was either stained with 0.08% (w/v) aqueous Azure A [33] or used for further immunostaining of CyPB. In the latter, gel was incubated overnight in a 10 mM Tris/HCl buffer, pH 7.4, containing 1% SDS, and CyPB was then transferred on to nitrocellulose (Sartorius, Göttingen, Germany) and immunostained with specific rabbit polyclonal antibodies as described previously [34].
Molecular modelling
Molecular modelling was carried out on a personal computer using the WinMGM software, as described previously [9]. The structure of human CyPB was obtained from the Brookhaven National Laboratory protein data bank as file 1CYN. The coordinates of heparin octasaccharide were obtained from the 1HPN file.
Chemotaxis and cell adhesion assays
In vitro chemotaxis was assayed in a micro-chemotaxis chamber containing a 3-μm pore polycarbonate membrane (Corning Costar, Cambridge, MA, U.S.A.), as described previously [7]. In brief, chemo-attractant (600 μl) and cells adjusted to 7.5×106 cells/ml in Dulbecco's PBS (DPBS)/0.5% BSA (100 μl) were respectively added to the bottom and upper chamber. The chamber was then incubated for 1 h at 37 °C, after which the membrane was washed in DPBS, fixed with formaldehyde and stained. The chemotactic index was calculated as the number of cells migrating in response to 15 nM CyPB divided by the number of cells migrating in response to DPBS/BSA alone. Cell adhesion assays were performed in 96-well microtitre plates (Nunc-Polylabo, Strasbourg, France), essentially as described by Allain et al. [7]. Fibronectin was immobilized on to the plates (1 μg/100 μl) in DPBS overnight at 4 °C. Plates were then extensively washed with DPBS and non-specific sites were blocked by the addition of 0.5% of BSA in DPBS for 30 min at 37 °C. For competitive binding assays, peripheral blood T-lymphocytes (10×106 cells/ml in DPBS/BSA) were pre-incubated with 100 nM CyPB in the presence of increasing concentrations of oligosaccharides for 30 min at 37 °C and the mixture was then distributed into the wells (100 μl) for an additional 30 min incubation at 37 °C. In other experiments, heparinase-treated T-lymphocytes (0.75 unit/106 cells, 2 h at 37 °C) were pre-incubated with soluble heparin or derived oligosaccharides, to determine whether these molecules could restore the pro-adhesive activity of the CyPB. In all cases, the plates were then extensively washed with DPBS to remove non-adherent cells and the remaining firmly attached cells were fixed with 3% formaldehyde, pH 7.8, for 20 min at 4 °C, and then stained with 1% Methylene Blue in 100 mM borate buffer, pH 8.2. After extensive washing with borate buffer, cells were lysed with 0.1 M HCl and the coloration, which is proportional to the number of adhered cells, was measured at 590 nm with a microplate Bio-Rad reader Model 550. The number of adherent cells was estimated by using standard curves where absorbance was related to the number of T-lymphocytes. Results were obtained from at least three separate experiments performed in triplicates, and are expressed as the percentage of initially added cells which remained fixed to the adhesive substrate (means±S.E.M.). Statistical significance was determined using the Student's t test, and P values <0.05 were considered as significant.
RESULTS
Evaluation of gel mobility shift assay with labelled oligosaccharides
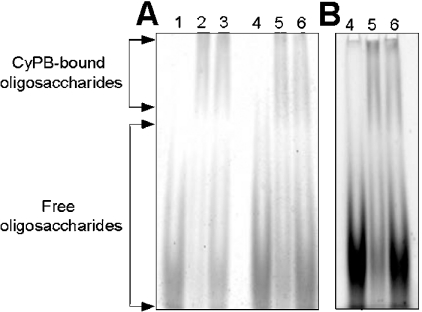
ANTS is a trisulphonated molecule, which confers negative charges on to the derivatized oligosaccharides. To know whether ANTS could interfere either with the electrophoretic mobility of oligosaccharides or with the formation of complex with CyPB, we first compared the separation profiles of unlabelled with ANTS-labelled oligosaccharides. To this end, dp12 oligosaccharides were either unlabelled or labelled with ANTS and then separated by electrophoresis under the same conditions of buffer, voltage and time (Figure 1). Dp12 was used in these experiments as it was expected to contain the minimal length unit for CyPB binding. Following staining with Azure A, the comparison of the profiles revealed that both oligosaccharide types presented the same electrophoretic mobility, indicating that derivatization did not affect the migration of the oligosaccharides (Figure 1A). In the absence of CyPB, dp12 oligosaccharides (10 nmol) were only observed as a broad band at the bottom of the gel. When incubated in the presence of 1 nmol of CyPB, the complex could be visualized as a shifted band at the top of the gel, whereas the excess of unbound oligosaccharides was not slowed. Interestingly, the intensity of the shifted band was unchanged in the presence of either 5 or 10 nmol of oligosaccharides, indicating that a 5:1 ratio of oligosaccharide/CyPB was efficient enough to form the complex (Figure 1A). The fluorescent profile of ANTS-labelled oligosaccharides was then imaged after exposure to a 365 nm UV transilluminator (Figure 1B). As expected, this method of detection allowed the visualization of CyPB/oligosaccharide complex as a shifted band, whereas unbound oligosaccharides appeared as a non-slowed broad band. As a prerequisite for these experiments, we checked that ANTS labelling did not lead to significant desulphation (less than 10%). To rule out the possibility that this low desulphation did not alter the binding capacity of ANTS-labelled oligosaccharides, we compared the migration profiles of well-known heparin-binding proteins, i.e. antithrombin III [13] and FGF-2 [14], in our gel mobility shift assay. Following staining with Azure A, protein–oligosaccharide complexes appeared as shifted bands for both proteins. As expected, no difference could be observed in the electrophoretic profiles obtained with either unlabelled or ANTS-labelled oligosaccharides, confirming that low desulphation did not affect the interaction between proteins and heparin-derived oligosaccharides (results not shown). Taken together, these preliminary data validate the use of gel mobility shift assay with ANTS-labelled oligosaccharides for studying interaction with CyPB.
Figure 1. Comparison of gel mobility shift assay with unlabelled versus ANTS-labelled oligosaccharides.
dp12 oligosaccharides were either unlabelled (lanes 1, 2 and 3) or derivatized with ANTS (lanes 4, 5 and 6) and next subjected to electrophoresis in the absence or presence of CyPB. Lane 1, 10 nmol of unlabelled oligosaccharides; lane 2, 5 nmol of unlabelled oligosaccharides plus 1 nmol of CyPB; lane 3, 10 nmol of unlabelled oligosaccharides plus 1 nmol of CyPB; lane 4, 10 nmol of ANTS-labelled oligosaccharides; lane 5, 5 nmol of ANTS-labelled oligosaccharides plus 1 nmol of CyPB; lane 6, 10 nmol of ANTS-labelled oligosaccharides plus 1 nmol of CyPB. (A) The electrophoretic profile was revealed by staining with aqueous Azure A. (B) Fluorophore-assisted detection of the migration of ANTS-labelled dp12 oligosaccharides (exposure to a UV transilluminator, 0.24 s). A representative gel of three separate experiments is shown.
Determination of the minimal length of heparin-derived oligosaccharides required for CyPB binding
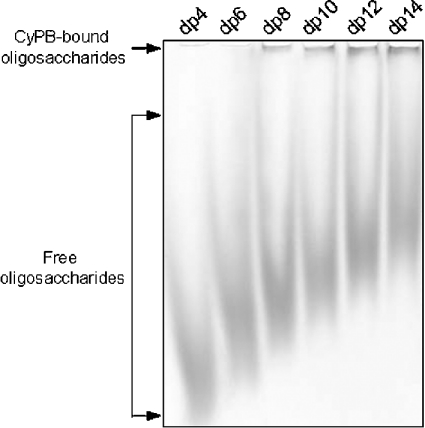
To determine the minimal length of heparin unit required for CyPB binding, heparin-derived oligosaccharides from dp4 to dp14 were labelled with ANTS and used in mobility shift assay. The fluorescent oligosaccharides (1 nmol per sample) were incubated with 0.2 nmol of CyPB and separated by electrophoresis (Figure 2). As described above, the interaction between CyPB and ANTS-labelled oligosaccharides was visualized as shifted bands. By comparison with the profiles shown in Figure 1, it can be noted that CyPB-bound dp12 oligosaccharides appeared as a tight band at the top of the gel that was related to the 5-fold lower amounts of CyPB used in the assay conducted with ANTS-labelled oligosaccharides. Interestingly, tetra- and hexasaccharides were not significantly shifted in the presence of CyPB, indicating that both have low efficiency to form a complex with CyPB. In contrast, significant amounts of octasaccharides and larger dp oligosaccharides could be strongly shifted by CyPB. Following densitometry analysis, it was also noticed that increasing the size of oligosaccharides from dp8 to dp14 did not significantly result in more binding (results not shown). These results indicate that the minimal and efficient length of heparin unit for CyPB binding is an octasaccharide.
Figure 2. Analysis of the minimal length unit required for CyPB binding.
ANTS-labelled oligosaccharides from dp4 to dp14 (1 nmol of each dp oligosaccharide per lane) were incubated with 0.2 nmol of CyPB and subjected to mobility shift assay. The fluorescent profile of the migration of ANTS-labelled oligosaccharides was imaged after exposure to a UV transilluminator for 0.60 s. A representative gel of five separate experiments is shown.
Interactions of CyPBΔYFD and CyPBKKK− with heparin-derived oligosaccharides
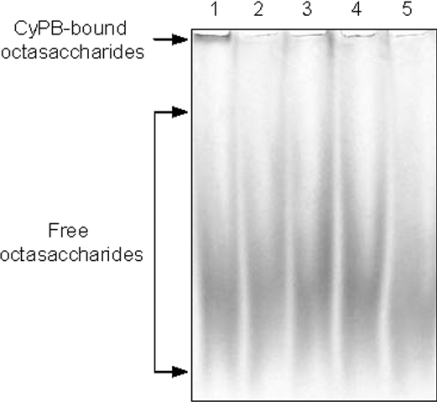
To support the hypothesis that Y14FD16 and K3KK5 tripeptides are required for the interaction with heparin, the mutants CyPBΔYFD and CyPBKKK− were analysed for their ability to form a complex with ANTS-labelled dp8 oligosaccharides (Figure 3). In contrast with wild-type CyPB, both mutants were unable to form a complex with the fluorescent octasaccharides, confirming that both tripeptides are involved in the interaction with heparin. We then tested the capability of unlabelled octasaccharides (10 nmol) or heparin (40 μg) to inhibit the formation of the complex between CyPB and ANTS-labelled octasaccharides. As expected, both competitors strongly reduced the formation of the fluorescent complex. These results indicate that ANTS-labelled and unlabelled octasaccharides share the same binding properties, and ruled out the possibility that the binding of CyPB to the fluorescent octasaccharides is due to interaction between ANTS and either Y14FD16 or K3KK5 sequences.
Figure 3. Analysis of the interaction between heparin-derived octasaccharides and CyPB.
ANTS-labelled octasaccharides (1 nmol per lane) were incubated with 0.2 nmol of CyPB (lane 1), CyPBΔYFD (lane 2) or CyPBKKK− (lane 3), and next subjected to gel mobility shift assay. In other experiments, ANTS-labelled dp8 (1 nmol) and CyPB (0.2 nmol) were co-incubated in the presence of 10 nmol of unlabelled octasaccharides (lane 4) or 40 μg of heparin (lane 5). The fluorescent profile of the migration of ANTS-labelled octasaccharides was imaged after exposure to a UV transilluminator for 0.60 s. A representative gel of three separate experiments is shown.
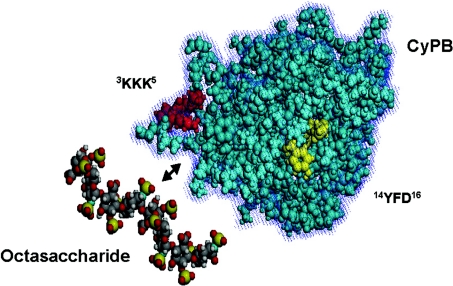
Molecular modelling was then used to examine the relative positions of the two potential binding tripeptides in CyPB. The model presented in Figure 4 clearly demonstrates that K3KK5 and Y14FD16 sequences are spatially juxtaposed in the three-dimensional structure of CyPB so that they may act to form a cradle-like binding site for heparin-derived oligosaccharides. Moreover, we found that the area of CyPB delimited by both clusters can be overlaid by a heparin-derived octasaccharide, supporting the finding that dp8 oligosaccharide represents the minimal length unit required to form a stable complex with CyPB. The K3KK5 cluster is probably involved in ionic interactions with sulphate groups of heparin-derived oligosaccharides, explaining why its replacement had a strong inhibitory effect on the formation of the complex. The Y14FD16 peptide does not contain any basic amino acid, ruling out the possibility that it is involved in ionic interactions with sulphate groups. However, this cluster seems to be of crucial importance, since its deletion dramatically reduced the formation of the complex. Therefore, the Y14FD16 cluster may be involved in stabilizing the interactions between CyPB and dp8 oligosaccharide, suggesting that it probably accounts for the specificity of the heparin-binding motif of CyPB.
Figure 4. Three-dimensional representation of CyPB and heparin octasaccharide.
The model was visualized with the WinMGM program using the co-ordinates files 1CYN for CyPB and 1HPN for the octasaccharide. The K3KK5 and Y14FD16 clusters, which interact with heparin octasaccharide, are indicated.
Effect of heparin oligosaccharides on HS-dependent binding and activity of CyPB
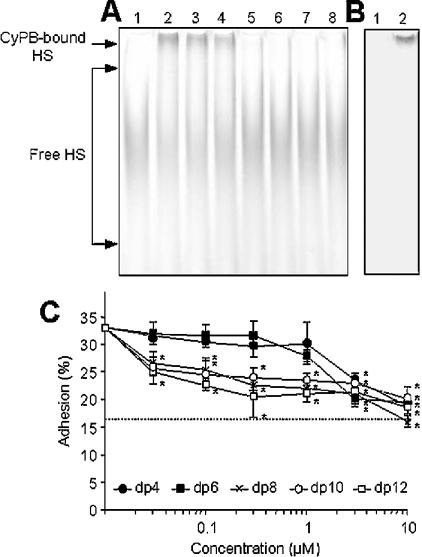
To analyse the ability of heparin-derived oligosaccharides to interfere with HS-dependent binding of CyPB, cell surface HS chains were isolated from T-lymphocytes and thereafter subjected to ANTS labelling. HS was poorly expressed at the membrane of T cells and no more than 60 μg of HS chains were purified from a starting pool of 7×108 T-lymphocytes. In the absence of CyPB, the electrophoretic profile of ANTS-labelled HS (equivalent to 4 μg of purified HS per sample) showed a broad separation, indicating that HS presents a large heterogeneity in terms of molecular species. When incubated in the presence of 0.2 nmol of CyPB, a shifted band appeared at the top of the gel (Figure 5A, lanes 1 and 2). However, most of the fluorescent HS chains were not retarded, suggesting that CyPB interacted with minor species of cell HS. To confirm that interaction between ANTS-labelled HS and CyPB was only related to the shifted band, lanes 1 and 2 (Figure 5A) were immunostained with specific antibodies to CyPB. As expected, CyPB was only detected at the top of the membrane, indicating that the fluorescent shifted band was CyPB-bound HS (Figure 5B). The addition of heparin-derived octasaccharides and higher dp oligosaccharides inhibited the formation of the complex between CyPB and ANTS-labelled HS. In contrast, smaller oligosaccharides appeared as poor competitors in the mobility shift assay. Indeed, no more than 1 nmol of octasaccharides and higher dp oligosaccharides were efficient enough to abolish the interaction between CyPB and ANTS-labelled HS, whereas a similar amount of tetra- or hexasaccharides reduced the interaction by no more than 30% (Figure 5A).
Figure 5. Effect of heparin-derived oligosaccharides on CyPB binding to cell surface HS and related activity.
(A) Purified HS chains from peripheral blood T-lymphocytes were derivatized with ANTS and next subjected to electrophoresis (4 μg per lane) in the absence (lane 1) or presence (lane 2) of 0.2 nmol of CyPB. From lanes 3–8, ANTS-labelled HS (4 μg per lane) was mixed with 1 nmol of heparin-derived oligosaccharides varying from dp4 to dp14 and then subjected to CyPB mobility shift assay. The fluorescent profile of the migration of ANTS-labelled HS was imaged after exposure to a UV transilluminator for 1.44 s. A representative gel of three separate experiments is shown. (B) Immunodetection of CyPB. Lanes 1 and 2 were transferred on to nitrocellulose and the protein was immunostained with rabbit polyclonal antibodies to CyPB (1/1000). (C) Inhibitory effect of heparin-derived oligosaccharides on CyPB-enhanced adhesion of T-lymphocytes to fibronectin. Cells were stimulated with 100 nM CyPB and then allowed to adhere on to fibronectin-coated plates in the absence (control) or presence of increasing concentrations of oligosaccharides varying from dp4 to dp12. The broken line corresponds to cell adhesion on fibronectin obtained in the absence of CyPB. Results are expressed as percentages of initially added cells (1×106 cells per well) remaining associated to the coated well. The results are expressed as the means±S.E.M. of triplicates from at least three separate experiments. Asterisks indicate statistically significant inhibition compared with control (P<0.05).
We then analysed the capability of heparin-derived oligosaccharides to interfere with HS-dependent pro-adhesive activity of CyPB (Figure 5C). In the absence of CyPB, cell adhesion to fibronectin was estimated as 16.2±3.8% of initially added T-lymphocytes. As expected, the addition of 100 nM CyPB led to a 2-fold increase in cell adhesion (32.8±4.2%, n=4).
Incubation with increasing concentrations of octasaccharides or higher dp oligosaccharides strongly reduced the HS-dependent pro-adhesive activity of CyPB. The inhibitory effects of octasaccharides and higher dp oligosaccharides were found to be significant from 100 nM and 30 nM respectively (P<0.05). However, dp10 and dp12 oligosaccharides were no more efficient than octasaccharides for reducing cell adhesion at higher concentrations. The addition of oligosaccharides at 300 nM, which corresponds to a 3:1 ratio of oligosaccharide/CyPB, led to a 1.5-fold decrease in cell adhesion (22.6±0.5%, 24±1.8%, 21.2±3.5% for dp8, dp10 and dp12 respectively, compared with 32.8±4.2% for control values, P<0.05), indicating that increasing dp had no more inhibitory effect on the pro-adhesive activity of CyPB. In contrast, tetra- and hexasaccharides appeared less efficient at inhibiting the activity of CyPB. A significant decrease in cell adhesion to fibronectin could be only observed from 3 μM (P<0.05). Altogether, these results indicate that octasaccharide is likely to be the minimal length unit required for functional binding of CyPB to cell surface HS.
Finally, we tested the possibility that heparin-derived oligosaccharides could substitute for the absence of cell surface HS. We first demonstrated that heparinase treatment did not alter the ability of CyPB to trigger chemotaxis of T-lymphocytes. At the concentration of 15 nM CyPB, the chemotactic index was estimated as 2.6±0.3 for untreated cells versus 2.4±0.2 for heparinase-treated cells (n=3). Conversely, CyPB was unable to enhance the adhesion of heparinase-treated T cells to fibronectin (Table 1), confirming that cell surface HS is required for pro-adhesive activity. Unfortunately, the addition of 500 nM octasaccharides (Table 1) or higher dp oligosaccharides (results not shown) was inefficient at restoring the ability of CyPB to induce adhesion of T-lymphocytes to fibronectin. To know whether oligosaccharides larger than dp14 would be required to present CyPB to the signalling receptor, we reproduced our experiments with heparin (Table 1). Soluble heparin at 5 μg/ml did not modify the ability of CyPB to enhance adhesion of untreated T-cells, whereas the pro-adhesive activity of the protein was potentially reduced at 50 μg/ml. However, the addition of soluble heparin at either 5 or 50 μg/ml did not restore the enhanced adhesion of heparinase-treated T-lymphocytes, indicating that HS has to be anchored at the cell membrane to support the pro-adhesive activity of CyPB.
Table 1. Effect of heparinase treatment on CyPB-induced cell adhesion.
Peripheral blood T-lymphocytes were either untreated or treated with heparinase I (0.75 unit/106 cells, 2 h at 37 °C) and then used for analysis of cell adhesion to fibronectin. Cells were stimulated with 100 nM CyPB and then allowed to adhere on to fibronectin-coated plates in absence or presence of various concentrations of competitors. Results are expressed as percentages of initially added cells (1×106 cells per well) remaining associated to the coated well. The results are expressed as the means±S.E.M. for 3 separate experiments. Asterisks indicate statistically significant inhibition compared with control (P<0.05).
| Untreated T-cells | Heparinase-treated T-cells | |||
|---|---|---|---|---|
| Inhibitor | No CyPB | CyPB (100 nM) | No CyPB | CyPB (100 nM) |
| Control | 16±4 | 33±4 | 17±4 | 16±3 |
| dp8 (500 nM) | 16±3 | 22±2* | 16.5±4 | 17.3±2.5 |
| Heparin (5 μg/ml) | 18±3 | 34±3 | 18±2 | 19.5±3 |
| Heparin (50 μg/ml) | 19±2 | 25±3* | 18.5±2 | 18±3 |
DISCUSSION
CyPB was eluted from a heparin-Sepharose column at 0.6 M NaCl, indicating that it is strongly retained on immobilized heparin [8]. In contrast, the mutants CyPBKKK− and CyPBΔYFD were more rapidly eluted from the column. The K3KK5 sequence appeared to be of more crucial importance, because its replacement dramatically reduced the avidity of CyPBKKK− for heparin. Indeed, CyPBKKK− was eluted at 0.1 M NaCl, whereas 0.3 M NaCl was required to disrupt the interaction between CyPBΔYFD and heparin. We concluded from these experiments that the K3KK5 cluster is involved in ionic interactions with heparin, and the Y14FD16 tripeptide possibly participates in stabilizing the complex [9]. This conclusion was, however, based on the incapability of CyPB mutants to remain bound to heparin in the presence of increasing ionic strength. The present results further support our hypothesis on the role of both Y14FD16 and K3KK5 clusters in the interaction with heparin. We demonstrated that heparin-derived octasaccharide is the minimal length unit capable of forming a stable complex with CyPB. Moreover, we found that neither CyPBΔYFD nor CyPBKKK− were capable of forming a complex with octasaccharides in our mobility shift assay, indicating that one cluster was not sufficient to compensate for the absence of the other one. Interestingly, Y14FD16 and K3KK5 tripeptides are spatially arranged in the three-dimensional structure of CyPB so that they may act synergistically and form a binding site for octasaccharide. This observation suggests that involvement of one cluster independently of the other is not efficient enough to form a stable complex between CyPB and smaller heparin-derived oligosaccharides than dp8. The YFD sequence was already described as a HS-binding sequence in type IV collagen. This peptide is located in the discontinuity of the triple helix of collagen and appeared crucial for promoting cell adhesion [35]. Taken together, these results indicate that YFD cluster probably acts synergistically with KKK tripeptide to form a highly specific binding region responsible for tight interactions with heparin motif.
We then demonstrated that octasaccharide is likely to be also the minimal length unit required for CyPB binding to cell surface HS of T-lymphocytes. Indeed, heparin-derived octasaccharides and higher dp oligosaccharides inhibited the formation of a complex between CyPB and ANTS-labelled HS purified from T-lymphocytes. In contrast, smaller oligosaccharides appeared as poor competitors in our mobility shift assay. Moreover, we showed that the addition of octasaccharides strongly reduced the HS-dependent pro-adhesive activity of CyPB. Interestingly, higher dp oligosaccharides were no more efficient than octasaccharides, indicating that increasing dp had no effect on the capability of heparin-derived oligosaccharides to alter the functional interaction between CyPB and cell surface HS. In previous work, we demonstrated that CyPBΔYFD and CyPBKKK− were both deprived of their ability to induce T-cell adhesion to fibronectin, indicating that removal of one cluster was efficient enough to alter the HS-dependent responses triggered by CyPB [10]. Our results further support the hypothesis on the role of K3KK5 and Y14FD16 clusters in forming a cradle-like binding site required for efficient interaction with octasaccharides and consequent HS-dependent cell responses. Conversely, we found that dp4 and dp6 oligosaccharides had a low inhibitory effect that could be observed only at high concentrations of inhibitors. We reported that the mutant CyPBΔYFD was deprived of pro-adhesive activity, but retained its capability to interact with heparin under physiological conditions, since its elution from a heparin-Sepharose column required the presence of 0.3 M NaCl [9]. These observations indicate that the K3KK5 cluster is efficient enough to allow ionic interactions between CyPBΔYFD and anionic molecules, whereas it is unable to support the pro-adhesive activity in the absence of Y14FD16. Therefore, a possibility remained that small heparin-derived dp4 and dp6 oligosaccharides interacted with K3KK5 sequence of CyPB independently of the Y14FD16 cluster in our adhesion assay. These ionic interactions were likely to occur at high concentrations of dp4 and dp6 oligosacharides, which could lead to alteration in the efficient binding of CyPB to HS motifs and consequent inhibition of HS-dependent cell responses.
HS was originally proposed to modulate cell responses by way of conformational changes in the three-dimensional structure of their biologically active ligands, which appeared related to highest efficiency in the interaction with the signalling receptor [18,19]. We previously reported that CyPBΔYFD and CyPBKKK− shared with wild-type CyPB the same binding parameters for type I sites [9] and retained their ability to induce chemotaxis of T-lymphocytes [7]. Heparinase treatment of responsive cells did not alter the capability of CyPB to interact with type I binding [8] and to induce chemotaxis, as described in the present work. Moreover, heparin-derived octasaccharides were unable to restore the pro-adhesive activity of CyPB on heparinase-treated T-lymphocytes. These results suggest that the role of HS is not restricted to a modification in the structure of CyPB, which would be required to present the ligand under a biologically active form to its cognate signalling receptor. However, recent studies have reported that minimal heparin binding units were not efficient enough to restore the activity of biologically active factors on heparinase-treated cells. For example, high dp oligosaccharides are required to promote the biological activity of hepatocyte growth factor which may be explained by the role of HS in forming a bridge between the growth factor and its cognate signalling receptor Met [23]. Several chemokines and growth factors are biologically active as a dimeric form that could be facilitated by interaction with HS [16,20]. Monomeric FGF-2 could bind to and activate its cognate signalling receptor, but the proliferative response of this growth factor was demonstrated to be dependent on the presence of HS and consequent persistence of signalling events [15]. In these models, heparin or free HS could replace the function of cell surface HS, indicating that HSPG present on neighbouring cells or in the extracellular matrix may promote the biological response of growth factors or chemokines. In our assay, we found, however, that the pro-adhesive activity of CyPB was suppressed when T-lymphocytes were treated with heparinase I, and this HS-dependent activity could not be restored by incubation with soluble heparin. These results suggest that the role of HS is not only restricted to presenting CyPB as a dimeric form or allowing sustained interaction between the protein and its signalling receptor. The finding that HS has to be present on the membrane of responsive cells is not unprecedented and suggests that the core protein of HSPG is also required for promoting cell responses. In this way, syndecan-2 is involved in the binding of granulocyte/macrophage colony-stimulating factor to osteoblasts and appears to act as a functional co-receptor for the modulation of mitogenic activity and signalling of this cytokine [36]. Syndecan-4, but not other HSPGs, was reported to promote FGF-2 binding to endothelial cells and to participate in signalling events related to angiogenesis [37]. In both cases, simultaneous binding of the ligand to HS moieties of HSPG and cognate signalling receptor was proposed to allow clustering of both molecules in the proximity of each other which led to the appearance of additional signalling events. On the assumption that HS-dependent binding of CyPB could be related to a clustering of HSPG and signalling receptor, the appearance of complementary signalling events could explain the activation of integrins and subsequent adhesion of T-lymphocytes to fibronectin. Further experiments are now required to identify the nature of the core protein of HSPG involved in pro-adhesive activity of CyPB and to validate our hypothesis on the role of this HSPG in the appearance of co-signalling events.
HS is a linear polysaccharide covalently attached to the core protein of proteoglycan, initially synthesized as a non-sulphated chain by sequential addition of D-glucuronic acid alternating with N-acetyl-D-glucosamine. This is followed by various modification steps including N-deacetylation and N-sulphation of glucosamine, epimerization of D-glucuronic acid to L-iduronic acid, 2-O sulphation of uronic acid and 6-O and 3-O sulphation of glucosamine. Different enzymes catalyse all steps, and the process is selective and incomplete as the position and the number of modifications in a chain lead to extensive sequence diversity. This diversity is thought to play crucial roles in diverse biological processes, such as proliferation, differentiation, homoeostasis and viral pathogenesis by generating various HS-binding motifs required for specific interactions with extracellular mediators [11]. Information on the structural features of the binding units of HS is vital to understand the rules of HS–protein interactions and may help to design HS mimics that can target protein in human diseases. In this context, the characterization of the detailed structure of the oligosaccharide that could bind CyPB is of importance to clarify the molecular basis of HS-dependent proadhesive activity of CyPB. An approach based on gel mobility shift assays with fluorophore-labelled oligosaccharides has allowed us to determine the minimal length unit involved in CyPB binding. By using the same approach, an octasaccharide library generated by modifications in vitro may contribute to the identification of the structural features required for specific binding of CyPB. This will allow the determination of the critical modifications in the HS-binding unit and the definition of the relationship between modification enzymes, functional groups in HS motifs and biological activity of CyPB. Furthermore, several studies have suggested the participation of CyPB in some pathological disorders and virus infection [34,38–41]. Therefore, the determination of the detailed structure of the HS-binding motif of CyPB will be particularly relevant to establish a strategy based on the generation of HS-derived molecules with the same structural features, and to use these oligosaccharides as a drug to block CyPB-mediated effects in these pathologies.
Acknowledgments
This investigation was supported by the Centre National de la Recherche Scientifique and by the Université des Sciences et Technologies de Lille, France. We would like to thank V. Fuentes and K. Krone for critical reading of the manuscript and C. Robbe for technical advice in the preparation of fluorescent molecules.
References
- 1.Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 3.Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature (London) 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature (London) 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 5.Galat A. Variations of sequences and amino acid compositions of proteins that sustain their biological functions: an analysis of the cyclophilin family of proteins. Arch. Biochem. Biophys. 1999;371:149–162. doi: 10.1006/abbi.1999.1434. [DOI] [PubMed] [Google Scholar]
- 6.Allain F., Denys A., Spik G. Characterization of surface binding sites for cyclophilin B on a human tumor T-cell line. J. Biol. Chem. 1994;269:16537–16540. [PubMed] [Google Scholar]
- 7.Allain F., Vanpouille C., Carpentier M., Slomianny M.-C., Durieux S., Spik G. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2714–2719. doi: 10.1073/pnas.052284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denys A., Allain F., Carpentier M., Spik G. Involvement of two classes of binding sites in the interactions of cyclophilin B with peripheral blood T-lymphocytes. Biochem. J. 1998;336:689–697. doi: 10.1042/bj3360689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpentier M., Allain F., Haendler B., Denys A., Mariller C., Benaïssa M., Spik G. Two distinct regions of cyclophilin B are involved in the recognition of a functional receptor and of glycosaminoglycans on T lymphocytes. J. Biol. Chem. 1999;274:10990–10998. doi: 10.1074/jbc.274.16.10990. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier M., Allain F., Slomianny M.-C., Durieux S., Vanpouille C., Haendler B., Spik G. Receptor type I and type II binding regions and the peptidyl-prolyl isomerase site of cyclophilin B are required for enhancement of T lymphocyte adhesion to fibronectin. Biochemistry. 2002;41:5222–5229. doi: 10.1021/bi015951j. [DOI] [PubMed] [Google Scholar]
- 11.Capila I., Linhardt R. J. Heparin–protein interactions. Angew. Chem. Int. Ed. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Delehedde M., Allain F., Payne S. J., Borgo R., Vanpouille C., Fernig D. G., Deudon E. Proteoglycans in inflammation. Curr. Med. Chem. 2002;1:89–102. [Google Scholar]
- 13.Lindahl U., Backstrom G., Hook M., Thunberg L., Fransson L. A., Linker A. Structure of the antithrombin-binding site in heparin. Proc. Natl. Acad. Sci. U.S.A. 1979;76:3198–3202. doi: 10.1073/pnas.76.7.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbull J. E., Fernig D. G., Ke Y. Q., Wilkinson M. C., Gallagher J. T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J. Biol. Chem. 1992;267:10337–10341. [PubMed] [Google Scholar]
- 15.Delehedde M., Lyon M., Gallagher J. T., Rudland P. S., Fernig D. G. Fibroblast growth factor-2 binds to small heparin-derived oligosaccharides and stimulates a sustained phosphorylation of p42/44 mitogen-activated protein kinase and proliferation of rat mammary fibroblasts. Biochem. J. 2002;366:235–244. doi: 10.1042/BJ20011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spillmann D., Witt D., Lindahl U. Defining the interleukin-8-binding domain of heparan sulfate. J. Biol. Chem. 1998;273:15487–15493. doi: 10.1074/jbc.273.25.15487. [DOI] [PubMed] [Google Scholar]
- 17.Kuschert G. S., Hoogewerf A. J., Proudfoot A. E., Chung C. V., Cooke R. M., Hubbard R. E., Wells T. N., Sanderson P. N. Identification of a glycosaminoglycan binding surface on human interleukin-8. Biochemistry. 1998;37:11193–11201. doi: 10.1021/bi972867o. [DOI] [PubMed] [Google Scholar]
- 18.Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y., Adams D. H., Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol. Today. 1993;14:111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- 20.Kuschert G. S., Coulin F., Power C. A., Proudfoot A. E., Hubbard R. E., Hoogewerf A. J., Wells T. N. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 21.Schlessinger J., Plotnikov A. N., Ibrahimi O. A., Eliseenkova A. V., Yeh B. K., Yayon A., Linhardt R. J., Mohammadi M. Crystal structure of a ternary FGF–FGFR–heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell. 2000;6:743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini L., Burke D. F., von Delft F., Mulloy B., Blundell T. L. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature (London) 2000;407:1029–1034. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 23.Delehedde M., Lyon M., Vidyasagar R., McDonnell T. J., Fernig D. G. Hepatocyte growth factor/scatter factor binds to small heparin-derived oligosaccharides and stimulates the proliferation of human HaCaT keratinocytes. J. Biol. Chem. 2002;277:12456–12462. doi: 10.1074/jbc.M111345200. [DOI] [PubMed] [Google Scholar]
- 24.Rapraeger A. C., Ott V. L. Molecular interactions of the syndecan core proteins. Curr. Opin. Cell Biol. 1998;10:620–628. doi: 10.1016/s0955-0674(98)80038-0. [DOI] [PubMed] [Google Scholar]
- 25.Ilangumaran S., Borisch B., Hoessli D. C. Signal transduction via CD44: role of plasma membrane microdomains. Leuk. Lymphoma. 1999;35:455–469. doi: 10.1080/10428199909169610. [DOI] [PubMed] [Google Scholar]
- 26.Jackson P. The analysis of fluorophore-labelled glycans by high-resolution polyacrylamide gel electrophoresis. Anal. Biochem. 1994;216:243–252. doi: 10.1006/abio.1994.1038. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z. L., Zhang L., Beeler D. L., Kuberan B., Rosenberg R. D. A new strategy for defining critical functional groups on heparan sulfate. FASEB J. 2002;16:539–545. doi: 10.1096/fj.01-0807com. [DOI] [PubMed] [Google Scholar]
- 28.Spik G., Haendler B., Delmas O., Mariller C., Chamoux M., Maes P., Tartar A., Montreuil J., Stedman K., Kocher H. P., et al. A novel secreted cyclophilin-like protein (SCYLP) J. Biol. Chem. 1991;266:10735–10738. [PubMed] [Google Scholar]
- 29.Chai W., Luo J., Lim C. K., Lawson A. M. Characterization of heparin oligosaccharide mixtures as ammonium salts using electrospray mass spectrometry. Anal. Chem. 1998;70:2060–2066. doi: 10.1021/ac9712761. [DOI] [PubMed] [Google Scholar]
- 30.Rahmoune H., Chen H. L., Gallagher J. T., Rudland P. S., Fernig D. G. Interaction of heparan sulphate from mammary cells with acidic fibroblast growth factor (FGF) and basic FGF. Regulation of the activity of basic FGF by high and low affinity binding sites in heparin sulphate. J. Biol. Chem. 1998;273:7303–7310. doi: 10.1074/jbc.273.13.7303. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y., Mechref Y., Novotny M. V. Microscale nonreductive release of O-linked glycans for subsequent analysis through MALDI mass spectrometry and capillary electrophoresis. Anal. Chem. 2001;73:6063–6069. doi: 10.1021/ac015534c. [DOI] [PubMed] [Google Scholar]
- 32.Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal. Biochem. 1971;41:471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- 33.Lyon M., Gallagher J. T. A general method for the detection and mapping of submicrogram quantities of glycosaminoglycan oligosaccharides on polyacrylamide gels by sequential staining with Azure A and ammoniacal silver. Anal. Biochem. 1990;185:63–70. doi: 10.1016/0003-2697(90)90255-8. [DOI] [PubMed] [Google Scholar]
- 34.De Ceuninck F., Allain F., Caliez A., Spik G., Vanhoutte P. M. High binding capacity of cyclophilin B to chondrocyte heparan sulfate proteoglycans and its release from the cell surface by matrix metalloproteinases: possible role as a proinflammatory mediator in arthritis. Arthritis Rheum. 2003;48:2197–2206. doi: 10.1002/art.11099. [DOI] [PubMed] [Google Scholar]
- 35.Koliakos G. G., Kouzi-Koliakos K., Furcht L. T., Reger L. A., Tsilibary E. C. The binding of heparin to type IV collagen: domain specificity with identification of peptide sequences from the alpha 1(IV) and alpha 2(IV) which preferentially bind heparin. J. Biol. Chem. 1989;264:2313–2323. [PubMed] [Google Scholar]
- 36.Modrowski D., Baslé M., Lomri A., Marie P. J. Syndecan-2 is involved in the mitogenic activity and signaling of granulocyte-macrophage colony-stimulating factor in osteoblasts. J. Biol. Chem. 2000;275:9178–9285. doi: 10.1074/jbc.275.13.9178. [DOI] [PubMed] [Google Scholar]
- 37.Volk R., Schwartz J. J., Li J., Rosenberg R. D., Simons M. The role of syndecan cytoplasmic domain in basic fibroblast growth factor-dependent signal transduction. J. Biol. Chem. 1999;274:24417–24424. doi: 10.1074/jbc.274.34.24417. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Cuadrado S., Bustos C., Ruiz-Ortega M., Ortiz A., Guijarro C., Plaza J. J., Egido J. Expression of leucocyte chemoattractants by interstitial renal fibroblasts: up-regulation by drugs associated with interstitial fibrosis. Clin. Exp. Immunol. 1996;106:518–522. doi: 10.1046/j.1365-2249.1996.d01-864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tegeder I., Schumacher A., John S., Geiger H., Geisslinger G., Bang H., Brune K. Elevated serum cyclophilin levels in patients with severe sepsis. J. Clinical Immunol. 1997;17:380–386. doi: 10.1023/a:1027364207544. [DOI] [PubMed] [Google Scholar]
- 40.Endrich M. M., Gehring H. The V3 loop of human immunodeficiency virus type-1 envelope protein is a high-affinity ligand for immunophilins present in human blood. Eur. J. Biochem. 1998;252:441–446. doi: 10.1046/j.1432-1327.1998.2520441.x. [DOI] [PubMed] [Google Scholar]
- 41.Jin Z. G., Melaragno M. G., Liao D. F., Yan C., Haendeler J., Suh Y. A., Lambeth J. D., Berk B. C. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ. Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]