Abstract
BACKGROUND:
The benefits of dietary macronutrients for weight management depend on the integrity of gut hormones. The role of food temperature in the release of satiety hormones and satiety needs elucidation. We aimed to determine the impact of different food temperatures with varying macronutrient compositions on satiety-related gut hormones glucagon-like peptide-1 (GLP-1) and cholecystokinin (CCK) and find the correlation of satiety hormones with appetite scores and remainder-day food (energy) intake.
MATERIALS AND METHODS:
Thirteen healthy participants (eight males and five females) aged 25–35 years with body mass index 18.5–24.9 kg/m2 with no medical illnesses or eating disorders consumed three compositions of meals (high carbohydrate, high fat, and high protein meals) each at three temperatures (cold, warm, and hot) in a randomized, double-blinded, controlled crossover design. Plasma concentrations of peptide hormones were determined at 0, 30, and 240 minutes by enzyme-linked immunosorbent assay, and 24-hours food recall was used for remainder-day food intake (remainder energy). Data were analyzed using SPSS version 27.0. The change in plasma levels of gut hormones with time was assessed using Friedman test; Kruskal-Wallis test was employed to compare GLP-1 and CCK hormonal levels across nine meals.
RESULTS:
A comparison of the three meals at the three temperatures (total of nine groups), showed that the GLP-1 and CCK plasma concentrations were significantly different (P < 0.001). GLP-1 and CCK responses increased more after hot meals than cold meals. Overall, high-fat meals had more effective gut hormone secretions. The area under the curve was increased for GLP-1 in high-fat meals and for CCK in hot meals. The peptide hormones (GLP-1 and CCK) were positively correlated with satiety scores and inversely with remainder food intake.
CONCLUSION:
The temperature of food was found to be an effective stimulus for the regulation of CCK and GLP-1 secretion. Hot food temperature increased satiety hormones (CCK and GLP-1), independent of food macronutrient composition.
Keywords: Food, temperature, high-fat diet, high-protein diet, hormones, nutrients, satiety response
Introduction
Obesity has become a global epidemic, and nutritional strategies are emerging as the best line of treatment for it.[1] Appetite control is the regulation of food intake by two processes: satiation is meal inhibition, while satiety is the feeling of fullness that prevents hunger between meals.[2] Gut–brain communication plays a vital role in satiety. Satiety is influenced by gut hormones released in response to macronutrients, gut hormones also act on hypothalamic anorexigenic or orexigenic nuclei.[3] Cholecystokinin (CCK) hormone delays stomach emptying by inhibiting the vagus nerve, leading to satiation, while glucagon-like peptide-1 (GLP-1) regulates stomach emptying, acid secretion, and ileal brake.[3,4] GLP-1 acts as an incretin hormone, helping with satiety control by providing negative feedback to the stomach and regulating postprandial glucose.[4]
Diets with different compositions of macronutrients are related to various levels of anorexic and orexigenic hormones.[1,2,3,4] Responses to GLP-1 and PYY are significantly increased after meals high in carbohydrates (CHOs).[5] However, fatty[6] and protein-rich[7] foods have been linked to higher CCK in earlier studies. Food temperature has been recognized as a key factor in understanding GLP-1 release, particularly in the Asian population.[8] Cold food items are anticipated to be less satiating compared to hot consumables.[9] Food temperatures, cold and hot, have been shown to impede gastric emptying.[10] The differential responses of plasma GLP-1 and CCK in healthy participants to palatable meals with different macronutrient compositions and dietary temperatures are currently unknown. Literature available on food temperature and its influence on satiety is scarce. This study focused on evaluating the short-term effects of three macronutrient meals (high protein, high fat, and high CHO) at three dietary temperatures (cold, warm, and hot) on the responses of CCK and GLP-1. Moreover, to find out the relationship between satiety hormones and appetite scores and later remainder energy (food) intake in normal, healthy adults.
Materials and Methods
A randomized crossover study design was conducted from November 2022 to October 2023. Out of the 25 participants screened, the study recruited 15 healthy, nonsmoking volunteers aged 25–35 years, with a normal body mass index (BMI) (range of 18.5–24.9 kg/m2). However, two participants dropped out (one because of illness unrelated to the research and the other for personal reasons). Thirteen participants (eight men and five women) completed the study. OpenEpi Info software (Centers for Disease Control, Atlanta, Georgia, United States) was used to calculate the sample size by keeping the power at 80% and the confidence interval at 95%; the minimum sample size calculated for GLP-1 was 10, and for CCK, 14.[11] The participants did not have food allergies (diet history) or dietary restrictions, excluded by the three-factor eating questionnaire revised 18-item version.[12] Ethical approval was obtained from the Institutional Ethical Review Board vide Letter No. IERB/2022/9303-7 dated 12/10/2022, and written informed consent was taken from all participants in the study.
For the randomization of participants, research randomizer software was used.[13] Each participant had a total of nine visits. The participants were served three test meals (high protein, high CHO, and high fat), each at three different temperatures (cold, warm, or hot), for a total of nine different meals. There was an interval of 1 week between the visits to minimize any carry-over effects. Each session day was identical in all respects except for the type of test meal provided. The participants were instructed to have an early dinner (500–700 calories), sufficient sleep (8 h), and avoid any strenuous exercise. On each test day at 8:00 a.m., participants reported to the research laboratory after 10–12 h of fasting. Before the test meal was provided (still fasting), a blood sample was taken and the Visual Analog Scale (VAS) all four questions’ appetite ratings were completed. After the baseline (fasting) measurements, participants were provided with a test meal (each serving 300 g) and a 250-mL bottle of water, served in a laboratory room (at 25°C room temperature) and consumed orally within 30 min with no researchers around or other distractions. The test meal was weighed before and after eating by the researchers with a digital kitchen machine (true sine series TS-200, China, 6 kg–0.01 g). Every 30 min for 4 h, participants’ appetite profiles (hunger, satiety, fullness, and desire to eat) were evaluated using VAS.[14] For hormone analysis, blood samples were taken at 0 min (fasting), 30 min, and 240 min. At the end of the study period (4 hours), participants left the research laboratory. To estimate remainder energy intake, a 24-h recall of foods consumed (lunch, snacks, or dinner) was submitted by the participants the next day,[15] and was estimated by two nutritionists using WinDiets Analysis software [Win Diets 2010 version (Robert Gordon University, Aberdeen, UK)].[16]
For hormone analysis, chilled tubes with sodium ethylenediaminetetraacetic acid were filled with venous blood samples. Using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Bioassay Technology BT Laboratory, UK), human active GLP-1 (pmole/L) and CCK (ng/L) concentrations were determined.[11] For CCK cat no. E1357Hu and GLP-1 cat no. E002Hu kits and an ELISA plate reader (BioTek), 450 (±10) mm wavelength were used. The sandwich ELISA technique was used for the quantitative detection of both CCK and GLP-1. The CCK inter- and intra-assay coefficients of variation were <10% and <3.9%, while for GLP-1, they were <10% and <2.6%. The volume, palatability, smell, texture, and appearance of the three ad libitum test meals were equalized. The high-CHO meal (65% CHO, 10% fat, 25% protein) consisted of rice and chicken, prepared as a combination of boiled basmati rice, potatoes, tomato-based sauce, and boneless chicken in olive oil. A high-protein meal (60% protein, 30% fat, 10% CHO) of chicken steak was prepared with boiled eggs, sauteed vegetables (carrot, potatoes, and onion), and chicken breasts. A high-fat meal (60% fat, 10% CHO, and 30% protein) consisted of paratha roll with boneless chicken, whole wheat roti, olive oil, and mayonnaise. A nutritionist’s help was needed in the assessment of calories (500 kcal) in the preparation of each test meal. The test meals were served at three temperatures: cold (20°C–25°C), warm (40°C–60°C), and hot (60°C–65°C).[17] The temperature of test meals was kept constant by serving it on chafing dishes (Electric Single Hot Plate, 1000 Watts, Yellow-SK-100A Egypt). With the help of a food thermometer (digital kitchen food thermometer TP300 China), the meal temperature was checked before, during, and after the meal intake.
Data were analyzed using the Statistical Package for the Social Sciences software version 27.0 (IBM SPSS Statistics for Windows, Version 27.0; Armonk, NY, USA: IBM Corp.). To evaluate the change in plasma levels of gut hormones with time, the repeated-measures Freidman analysis of variance (ANOVA) was used. To compare GLP-1 and CCK hormonal levels across nine meals, the Kruskal–Wallis H test was used, followed by the Mann–Whitney post hoc for significant values. Using the trapezoidal rule, the total area under the curve (AUC) for GLP-1 and CCK hormones was calculated from 0 to 240 min. To adjust for differences in before-meal hormonal values, adjusted (for fasting) values for postmeal times were generated, and analysis was repeated. Spearman’s correlation was used to find the relationship between satiety hormones, appetite scores, and remainder-day energy intake. P ≤ 0.05 was considered statistically significant.
Results
The participants had a mean (± standard deviation) age of 29 (± 2.8) years, a BMI of 22.53 (2.96) kg/m2, blood pressure systolic 107.31 (4.39) mmHg, and a diastolic 75 (5.40) mmHg. The GLP-1 responses of the study participants (n = 13) in all three experimental studies of test meals (high CHO, high protein, and high fat) at three temperatures (cold, warm, and hot) at 0, 30, and 240-min time points were recorded and summarized in Table 1. The GLP-1 by time interaction was nonsignificant in the three test meals at cold, warm, and hot (P > 0.05; Freidman ANOVA). Comparisons between the three macronutrient groups at three temperatures for GLP-1 values were highly significant (Kruskal–Wallis H, ANOVA, P ≤ 0.001). GLP-1 plasma levels increased from baseline to 240 min following hot high fat, while there was a delayed rise (at 240 min) in hot high CHO and hot high protein. The cold meals (high CHO and high protein) showed blunted responses of GLP-1 (from 0 to 240 min). Overall, high-fat meals had the highest plasma levels of GLP-1 responses than other meal groups (protein and CHO). The warm meals had fluctuations in GLP-1 (either at 30 or at 240 min). The post hoc analysis also revealed that plasma levels of GLP-1 were significantly higher for high fat, high protein, and high CHO at hot temperatures than for pair-wise meals and also higher at cold temperatures for high fat and high CHO compared to pair-wise meals at 240 and 30 minutes respectively (P ≤ 0.05).
Table 1.
Changes in plasma levels of glucagon-like peptide-1 in three meals (high carbohydrate, high protein, and high fat) at three temperatures (cold, warm, and hot)
| Time (min) | High carbohydrate (n=13) | High protein (n=13) | High fat (n=13) | P-valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Cold Mean±SD | Warm Mean±SD | Hot Mean±SD | Cold Mean±SD | Warm Mean±SD | Hot Mean±SD | Cold Mean±SD | Warm Mean±SD | Hot Mean±SD | ||
| 0 | 15.72±3.32 | 12.41±3.06 | 14.67±4.21 | 14.86±3.81 | 9.08±3.16 | 13.08±2.89 | 16.47±3.99 | 12.28±2.61 | 14.06±4.93 | <0.001 |
| 30 | 15.33±4.01 | 13.38±4.36 | 13.75±2.98 | 12.92±4.08 | 8.34±2.14 | 12.93±2.92 | 15.66±3.25 | 12.58±2.58 | 15.40±4.49 | <0.001 |
| 240 | 14.18±3.54 | 12.23±3.55 | 15.02±3.84 | 11.93±3.03 | 8.65±3.57 | 13.20±3.99 | 16.67±2.61 | 12.30±2.30 | 15.42±4.50 | <0.001 |
| P-valuea | 0.11 | 0.11 | 2.38 | 0.58 | 0.73 | 0.12 | 0.13 | 0.58 | 0.37 | |
aFriedman test, bKruskal-Wallis test, SD=Standard deviation
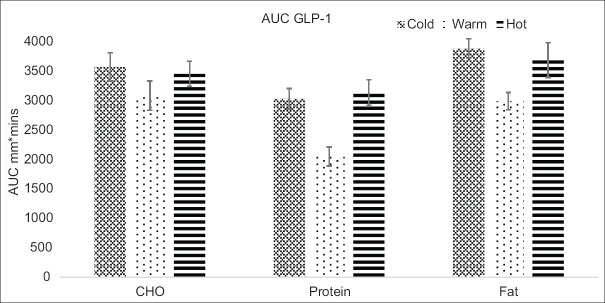
The total AUC (0–240 min) for GLP-1 showed significant differences between the three meals at three temperatures (P < 0.001), as shown in Figure 1. The mean AUC values for GLP-1 of high-fat meals were higher at three temperatures (cold, warm, and hot) compared to high protein and high CHO meals. The AUC was the lowest in warm protein. The AUC of GLP-1 post hoc also revealed that hot meals and cold meals (fat and CHO) were significantly higher than for pair-wise meals (P ≤ 0.05).
Figure 1.
The mean values (± standard error) of the area under the curve (AUC) for glucagon-like polypeptide-1 (GLP-1) levels. CHO = Carbohydrate, AUC = Area under the curve, GLP-1 = Glucagon-like polypeptide-1
The CCK responses in all three meals (CHO protein and fat) at three temperatures at 0, 30, and 240 min are summarized in Table 2. The plasma CCK concentrations were not statistically significant within meals (repeated measures of the Friedman ANOVA, P > 0.05), except for the cold protein meal (P = 0.01). Analysis with the Kruskal–Wallis H ANOVA showed plasma levels of CCK were significantly different in the three meals at three temperatures (P ≤ 0.001). From baseline to 240 min, CCK showed an increase in the hot meals (high fat and high protein), while in hot CHO at 240 min only. However, CCK responses increased following cold high CHO (0–240 min) as exceptional findings. The CCK was the highest in hot fat and hot CHO meals and the lowest in warm protein meals. The adjusted Kruskal–Wallis H ANOVA was repeated for 30- and 240-min values of CCK and GLP-1. The P values remained unchanged. The post hoc test showed that plasma concentrations of CCK were significantly higher for hot meals (high fat, high protein, and high CHO) compared to pair-wise meals (P ≤ 0.05).
Table 2.
Changes in plasma levels of cholecystokinin in three meals (high carbohydrate, high protein, and high fat) at three temperatures (cold, warm, and hot)
| Time (min) | High carbohydrate (n=13) | High protein (n=13) | High fat (n=13) | P-value b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Cold Mean±SD | Warm Mean±SD | Hot Mean±SD | Cold Mean±SD | Warm Mean±SD | Hot Mean±SD | Cold Mean±SD | Warm Mean±SD | Hot Mean±SD | ||
| 0 | 8.08±3.28 | 10.13±3.27 | 11.20±3.00 | 10.65±1.73 | 6.19±1.41 | 9.57±3.15 | 8.85±2.31 | 9.56±1.51 | 10.67±3.02 | <0.001 |
| 30 | 8.76±3.97 | 10.46±2.51 | 10.71±2.27 | 9.68±1.85 | 5.78±1.18 | 10.0±2.1 | 8.41±2.51 | 9.19±2.46 | 10.79±2.95 | <0.001 |
| 240 | 8.81±4.54 | 9.50±1.66 | 11.98±2.59 | 8.51±1.550 | 6.15±1.64 | 10.10±2.2 | 9.27±2.49 | 10.02±2.29 | 10.91±3.20 | <0.001 |
| P-valuea | 0.73 | 0.80 | 0.20 | 0.01 | 0.58 | 0.36 | 0.58 | 0.23 | 0.43 | |
aFriedman test, bKruskal-Wallis test, SD=Standard deviation
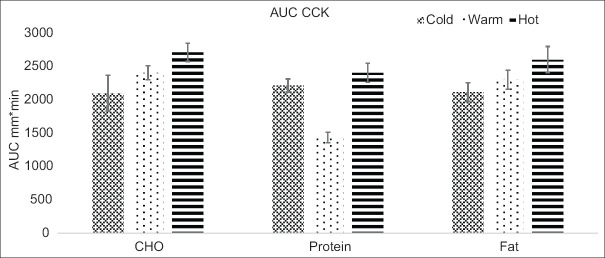
The total AUC (0–240 min) for CCK showed significant differences between the three meals at three temperatures (P = 0.001), as shown in Figure 2. The total AUC mean values for CCK of hot meals were higher for high fat, high protein, and high CHO meals compared to cold meals. The AUC of CCK post hoc also showed that hot meals were significantly higher than for pair-wise meals (P ≤ 0.05).
Figure 2.
Mean values (± standard error) of the area under the curve (AUC) for cholecystokinin (CCK). CHO = Carbohydrate, AUC = Area under the curve
Correlation coefficients of postprandial changes in CCK and GLP-1, appetite profiles, and remainder energy intake following three meals at three temperatures are shown in Table 3. Changes in plasma levels of CCK were positively correlated with changes in hunger sensation and desire to eat at 240 min after hot CHO meals (r = 0.716; P = 0.006; r = 0.613; P = 0.02, respectively). There was a significant positive correlation of GLP-1 with satiety feeling at 30 min after warm protein meals (r = 0.549; P = 0.05) and with the desire to eat at 240 min after a hot CHO (r = 0.618; P = 0.02). There were significant negative correlations between GLP-1 and remainder energy intake at 30 min and 240 min following hot CHO (r = −0.737; P = 0.00, r = −0.578; P = 0.03, respectively). Changes in levels of CCK were correlated inversely with remainder energy intake at 30 and 240 min (r = −0.633; P = 0.02, r = −0.637; P = 0.01, respectively) following hot protein meals. There was a positive correlation between gut hormones (CCK and GLP-1) and satiety scores, while there was a negative correlation with remainder (food) energy intake following hot meals (CHO and protein).
Table 3.
Correlation coefficients of satiety hormones (cholecystokinin and glucagon-like polypeptide-1) with appetite scores and remainder energy intake
| Satiety hormones | Hunger | Satiety | Desire to eat | Remainder energy intake | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| r | P-value | r | P-value | r | P-value | r | P-value | |
| CCK (cold protein) at 30 min | 0.572 | 0.04 | ||||||
| CCK (hot protein) at 30 min | −0.633 | 0.02 | ||||||
| CCK (hot protein) at 240 min | −0.637 | 0.01 | ||||||
| CCK (hot CHO) at 240 min | 0.716 | 0.006 | 0.613 | 0.02 | ||||
| GLP-1 (warm protein) at 30 min | 0.549 | 0.05 | ||||||
| GLP-1 (hot CHO) at 30 min | −0.737 | 0.00 | ||||||
| GLP-1 (hot CHO) at 240 min | 0.618 | 0.02 | −0.578 | 0.03 | ||||
r=Spearman correlation coefficients, CHO=Carbohydrate, CCK=Cholecystokinin, GLP-1=Glucagon-like peptide-1
Discussion
We focused on resolving several discrepancies in the literature concerning the contributions of macronutrients and their dietary temperatures required for the appraisal of the underlying gut hormone mechanisms, satiety, and remainder energy intake. The study is the first to examine postprandial gut hormone responses in young, healthy adults to three meal compositions (fat, protein, and CHOs) at three dietary temperatures (cold, warm, and hot); the study also aimed to find the correlation between gut hormones and satiety perceptions and later energy intake.
Primarily, we found that different compositions (protein, fat, and CHO) served at three temperatures (cold, warm, and hot) affected GLP-1 and CCK plasma levels. Postprandial increases in satiety hormones were seen, especially following the hot meal groups (high fat and high protein). The high-fat meal was the most effective in eliciting both GLP-1 and CCK responses. The novel finding of the short-term study was that hot meals and high-fat meals caused differences in gut hormone secretions but had a weak relationship with appetite scores and remainder energy (food) intake. Therefore, short-term hormonal changes caused by hot meals early in the day had less impact on later energy intake. Our study found that high-fat and hot meals led to a greater AUC for GLP-1 and CCK in satiety hormone responses. Several mechanisms could have been responsible for these differences.
Chaudhri et al.,[3] have shown that dietary protein and fat are more effective stimulators of CCK release than CHO. In addition, Parvaresh et al.,[5] reported that compared to a protein or fat-rich meal, a high-CHO meal had a shorter anorexigenic response with an attenuated release of GLP-1. A similar review by Moris et al., showed that CCK was expressed to a lesser extent under a CHO-rich meal.[18] Our study showed that the release of GLP-1 and CCK was primarily influenced by fat-rich meals and hot meals. These findings were further underscored by significant correlations between satiety hormones and hunger and desire to eat following hot meals. Suzuki et al., reported that elevated plasma concentrations of GLP-1 and CCK may be attributable to metabolic factors such as increased satiety and reduced hunger.[19]
The dietary temperature was one of the crucial factors in determining the results and improving the anorexigenic hormonal responses in our study. This is consistent with studies reporting that hot foods (44°C–55°C)[8] increase GLP-1 secretion, while cold foods are less satisfying.[9] Contrary to our study, Rolls et et al., showed that varied temperatures (60°C–62°C vs. 1°C) of the preloads did not affect satiety or food intake.[20] Exposure to food temperature has the most significant impact on the mouth, esophagus, and stomach in the gastrointestinal tract.[21] Mishima et al., were of the view that food temperature at 60°C increased gastric emptying.[22] The temperature of ingested food acts synergistically with postprandial GLP-1 and CCK plasma levels in delaying gastric emptying.[3,4] These findings justify our result of increased plasma GLP-1 and CCK release following hot meal groups.
An interesting explanation by Moris et al.,[18] is that enteroendocrine or neuropod cells convert sensory stimuli like temperature and nutrients into electrochemical signals, regulating satiety and gut–brain axis through rapid transmission by the vagus nerve. Recent studies on gut physiology can provide insight into the impact of food temperature and its significance.[23,24] Our results indicated that a fat-rich meal out of the three meals effectively coincided with elevated GLP-1 and CCK levels. Although Blundell et al., suggested that high-fat diets had a weak effect on satiety as compared to CHO and proteins.[25] Giezenaar et al., also reported, contrary to our study, that high-protein meals were associated with increased responses of GLP-1 and CCK compared to fat and CHO.[11] Our results agreed with Maffeis et al., that fat-rich diets had a positive response to peptide hormones and satiety.[26] A longer gastric transition time with a fat-rich meal might be associated with gastric emptying delay, a potent response to peptide hormones and satiety.[27]
The Spearman correlation test was utilized to analyze the correlation between hormonal responses, satiety scores, and remainder (food) energy intake. Postprandial plasma levels of GLP-1 and CCK showed a weak correlation with appetite scores and a negative correlation with remainder energy (food) intake following hot CHO and protein meals in normal-weight participants. Despite the significant response of postprandial anorexic hormone (GLP-1 and CCK) levels, the peptide hormones did not truly interpret the subjective satiety feelings. A similar pattern of disparity between appetite measurements and hormonal appetite regulators was seen in some other studies.[28] Subjective appetite ratings may simply reflect a person’s interpretation of their feelings and motivations.[28] Similarly, despite changes in satiety hormones, the remainder energy (food) intake for the rest of the day was affected by a hedonistic appetite. As explained by Campos et al., food intake for pleasure and reward may ignore homeostatic needs even without physiological hunger.[29] Our research findings contributed to the current literature on the satiety hormonal response to food temperature after short-term meal challenges.
The limitations of our study were that all the subjects were of normal weight, and the observations could not be applied to obese individuals. Second, the 24-h dietary recall method is susceptible to errors in memory and recall bias.
Conclusion
Hot food temperature increased the satiety hormones CCK and GLP-1, independent of food macronutrient composition. High-fat foods also cause higher secretions of CCK and GLP-1 compared to proteins and carbs. Food temperature was found to be an effective stimulus for regulating CCK and GLP-1 secretion. Further long-term dietary studies are needed in this field to explore food temperature and macronutrient effects on appetite regulators.
Financial support and sponsorship
This project was partially funded by the Office of Research, Innovation, and Commercialization (ORIC) of Khyber Medical University, Peshawar, Pakistan.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chambers L, McCrickerd K, Yeomans MR. Optimizing foods for satiety. Trends Food Sci Technol. 2015;41:149–60. [Google Scholar]
- 2.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, et al. Appetite control: Methodological aspects of the evaluation of foods. Obes Rev. 2010;11:251–70. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187–209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu VB, Gribble FM, Reimann F. Nutrient-induced cellular mechanisms of gut hormone secretion. Nutrients. 2021;13:883. doi: 10.3390/nu13030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parvaresh Rizi E, Loh TP, Baig S, Chhay V, Huang S, Caleb Quek J, et al. A high carbohydrate, but not fat or protein meal attenuates postprandial ghrelin, PYY and GLP-1 responses in Chinese men. PLoS One. 2018;13:e0191609. doi: 10.1371/journal.pone.0191609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons C, Finlayson G, Caudwell P, Webb DL, Hellström PM, Näslund E, et al. Postprandial profiles of CCK after high fat and high carbohydrate meals and the relationship to satiety in humans. Peptides. 2016;77:3–8. doi: 10.1016/j.peptides.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91:2913–9. doi: 10.1210/jc.2006-0609. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, Zhang P, Ding B, Cao X, Zhong Y, Lee KO, et al. Response of blood glucose and GLP-1 to different food temperature in normal subject and patients with type 2 diabetes. Nutr Diabetes. 2022;12:28. doi: 10.1038/s41387-022-00208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baskentli S, Block L, Morrin M. The serving temperature effect: Food temperature, expected satiety, and complementary food purchases. Appetite. 2021;160:105069. doi: 10.1016/j.appet.2020.105069. [DOI] [PubMed] [Google Scholar]
- 10.Sun WM, Houghton LA, Read NW, Grundy DG, Johnson AG. Effect of meal temperature on gastric emptying of liquids in man. Gut. 1988;29:302–5. doi: 10.1136/gut.29.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giezenaar C, Lange K, Hausken T, Jones KL, Horowitz M, Chapman I, et al. Acute effects of substitution, and addition, of carbohydrates and fat to protein on gastric emptying, blood glucose, gut hormones, appetite, and energy intake. Nutrients. 2018;10:1451. doi: 10.3390/nu10101451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anglé S, Engblom J, Eriksson T, Kautiainen S, Saha MT, Lindfors P, et al. Three factor eating questionnaire-R18 as a measure of cognitive restraint, uncontrolled eating and emotional eating in a sample of young Finnish females. Int J Behav Nutr Phys Act. 2009;6:41. doi: 10.1186/1479-5868-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randomizer.org. Randomizer; 2023. [[Last accessed on 2023 Dec 15]]. Available from: https://www.randomizer.org/
- 14.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 15.Gibson RS, Charrondiere UR, Bell W. Measurement errors in dietary assessment using self-reported 24-hour recalls in low-income countries and strategies for their prevention. Adv Nutr. 2017;8:980–91. doi: 10.3945/an.117.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Robert Gordon University. WInDiets Research Software. Version 2010. Aberdeen, UK: The Robert Gordon University; 2010. [Google Scholar]
- 17.Pramudya RC, Seo HS. Influences of product temperature on emotional responses to, and sensory attributes of, coffee and green tea beverages. Front Psychol. 2017;8:2264. doi: 10.3389/fpsyg.2017.02264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moris JM, Heinold C, Blades A, Koh Y. Nutrient-based appetite regulation. J Obes Metab Syndr. 2022;31:161–8. doi: 10.7570/jomes22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57:359–72. doi: 10.1507/endocrj.k10e-077. [DOI] [PubMed] [Google Scholar]
- 20.Rolls BJ, Fedoroff IC, Guthrie JF, Laster LJ. Effects of temperature and mode of presentation of juice on hunger, thirst and food intake in humans. Appetite. 1990;15:199–208. doi: 10.1016/0195-6663(90)90020-9. [DOI] [PubMed] [Google Scholar]
- 21.López Gastón AR. Cold and warm thermoregulation by oropharynx area and esophagus. Acta Gastroenterol Latinoam. 2000;30:159–64. [PubMed] [Google Scholar]
- 22.Mishima Y, Amano Y, Takahashi Y, Mishima Y, Moriyama N, Miyake T, et al. Gastric emptying of liquid and solid meals at various temperatures: Effect of meal temperature for gastric emptying. J Gastroenterol. 2009;44:412–8. doi: 10.1007/s00535-009-0022-1. [DOI] [PubMed] [Google Scholar]
- 23.Kaelberer MM, Rupprecht LE, Liu WW, Weng P, Bohórquez DV. Neuropod cells: The emerging biology of gut-brain sensory transduction. Annu Rev Neurosci. 2020;43:337–53. doi: 10.1146/annurev-neuro-091619-022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361:eaat5236. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blundell JE, Burley VJ, Cotton JR, Lawton CL. Dietary fat and the control of energy intake: Evaluating the effects of fat on meal size and postmeal satiety. Am J Clin Nutr. 1993;57:772S–7S. doi: 10.1093/ajcn/57.5.772S. [DOI] [PubMed] [Google Scholar]
- 26.Maffeis C, Surano MG, Cordioli S, Gasperotti S, Corradi M, Pinelli L. A high-fat versus a moderate-fat meal in obese boys: Nutrient balance, appetite, and gastrointestinal hormone changes. Obesity (Silver Spring) 2010;18:449–55. doi: 10.1038/oby.2009.271. [DOI] [PubMed] [Google Scholar]
- 27.Little TJ, Horowitz M, Feinle-Bisset C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: Implications for the pathophysiology of obesity. Am J Clin Nutr. 2007;86:531–41. doi: 10.1093/ajcn/86.3.531. [DOI] [PubMed] [Google Scholar]
- 28.Aukan MI, Coutinho S, Pedersen SA, Simpson MR, Martins C. Differences in gastrointestinal hormones and appetite ratings between individuals with and without obesity-A systematic review and meta-analysis. Obes Rev. 2023;24:e13531. doi: 10.1111/obr.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos A, Port JD, Acosta A. Integrative hedonic and homeostatic food intake regulation by the central nervous system: Insights from neuroimaging. Nutrients. 2021;13:2793. doi: 10.3390/brainsci12040431. [DOI] [PMC free article] [PubMed] [Google Scholar]