Abstract
Mb (myoglobin) plus H2O2 catalyses the oxidation of various substrates via a peroxidase-like activity. A Y103F (Tyr103→Phe) variant of human Mb has been constructed to assess the effect of exchanging an electron-rich oxidizable amino acid on the peroxidase activity of human Mb. Steady-state analyses of reaction mixtures containing Y103F Mb, purified linoleic acid and H2O2 revealed a lower total yield of lipid oxidation products than mixtures containing the wild-type protein, consistent with the reported decrease in the rate constant for reaction of Y103F Mb with H2O2 [Witting, Mauk and Lay (2002) Biochemistry 41, 11495–11503]. Irrespective of the Mb employed, lipid oxidation yielded 9(R/S)-HODE [9(R,S)-hydroxy-10E,12Z-octadecadienoic acid] in preference to 13(R/S)-HODE [13(R,S)-hydroxy-9Z,11E-octadecadienoic acid], while 9- and 13-keto-octadecadienoic acid were formed in trace amounts. However, lipid oxidation by the Y103F variant of Mb proceeded with a lower Vmax value and an increased Km value relative to the wild-type control. Consistent with the increased Km, the product distribution from reactions with Y103F Mb showed decreased selectivity compared with the wild-type protein, as judged by the decreased yield of 9(S)-relative to 9(R)-HODE. Together, these data verify that Tyr103 plays a significant role in substrate binding and orientation in the haem pocket of human Mb. Also, the midpoint potential for the Fe(III)/(II) one-electron reduction was shifted slightly, but significantly, to a higher potential, confirming the importance of Tyr103 to the hydrogen-bonding network involving residues that line the haem crevice of human Mb.
Keywords: lipid peroxidation, myoglobin, peroxidase activity, ischaemia reperfusion injury
Abbreviations: C18:2,n−6, linoleic (9-Z,12Z-octadecadienoic) acid; DTPA, diethylenetriaminepenta-acetic acid; globin˙, protein radicals; 9(R/S)-HODE, racemic 9-hydroxy-10E,12Z-octadecadienoic acid; 13(R/S)-HODE, racemic 13-hydroxy-9Z,11E-octadecadienoic acid; 9(S)-HODE, 9(S)-hydroxy-10E,12Z-octadecadienoic acid; 13(R)-HODE, 13(R)-hydroxy-9Z,11E-octadecadienoic acid; HPODE, hydroperoxyoctadecadienoic acid; iR, decrease in potential due to the current (i) multiplied by the internal resistance (R); Mb, myoglobin; metMb, metmyoglobin; OTTLE, optically transparent thin-layer electrode; oxoODE, unresolved mixture of 9-oxo-octadeca-10E,12Z-dieneoic acid and 13-oxo-octadeca-9Z,11E-dieneoic acid; [Ru(NH3)6]Cl3, hexa-ammineruthenium(III) chloride; SHE, standard hydrogen scale; Y103F, Tyr103→Phe variant of human myoglobin
INTRODUCTION
Mb (myoglobin) is a cytoplasmic haem protein found in skeletal, cardiac [1] and smooth muscle cells [2]. The primary function of Mb is that of a reversible carrier of molecular oxygen, yet in the presence of excess H2O2, or low-molecular-mass alkyl peroxide, Mb can act as a pro-oxidant, initiating the oxidation of a range of substrates through a peroxidase-like catalytic cycle [3]. For example, ferric or metmyoglobin (metMb) has a limited ability to reduce H2O2 to water with the concomitant formation of ferryl [Fe(IV)=O] haem and a porphyrin radical cation (Mb-compound I). Ferryl Mb and Mb-compound I are capable of oxidizing lipids [4,5] and proteins [6].
The reaction of metMb with H2O2 also yields protein radicals (globin˙) [7] through heterolytic cleavage of the peroxide [8]. These globin˙ radicals have been assigned to be localized to tyrosine (Tyr103, Tyr146 [9,10] and Tyr151 [9] for sperm whale Mb), tryptophan (Trp14 [11]) and cysteine (Cys110 [12] for human Mb) residues as judged by EPR spectroscopy in combination with site-directed mutagenesis.
Two independent mechanisms for Mb-mediated oxidation of low-molecular-mass fatty acid substrates have been identified. The first involves oxidation of the substrate within the haem crevice through reaction with ferryl Mb, similar to the mechanism for cytochrome P450-mediated oxidation through transfer of the activated ferryl oxygen [13,14]. This Mb-dependent enzymic process results in substrate oxidation with retention of regio- and stereo-chemistry, and is supportive of the notion that substrate binding in the haem crevice precedes bisallylic hydrogen abstraction and the subsequent addition of molecular oxygen [14]. The second mechanism has been termed co-oxidation [13] and results from the reaction of Mb-derived globin˙ with the substrate to yield a radical product that incorporates molecular oxygen in a process that exhibits unrestricted degrees of freedom. Product(s) of co-oxidation show significantly decreased stereo-selectivity when compared with enzymic peroxidase-mediated lipid oxidation.
The limited ability of Mb to initiate lipid and protein oxidation in the presence of peroxide has implications for tissues that are rich in the protein, such as myocardial muscle. The potential for Mb to promote oxidative damage in vivo [15], linked with the protein's ability to catalyse membrane fatty acid peroxidation, is proposed to play a pathological role in myocardial ischaemia reperfusion damage [16,17]. Furthermore, Mb-mediated oxidation of polyunsaturated fatty acid occurs with high degrees of regio- and stereo-selectivity [14] to yield products with the potential to act as potent cell signalling molecules by analogy with oxidation products produced by lipoxygenase enzymes [18–20]. Stereospecific lipid oxidation is a direct consequence of specific binding of the lipid substrate to the catalytic centre of the protein followed by oxygen transfer from the activated ferryl species [14].
In the present study, we established that elimination of Tyr103 slightly alters the redox properties of recombinant human Mb, and that this affects linoleic acid oxidation probably through varying the hydrogen-bonding network in the haem cavity of the protein. Further support is provided for our earlier conclusions that substitution of phenylalanine for Tyr103 (Y103F) influences the rate and both the regio- and stereo-chemical outcomes of Mb-mediated oxidation reactions [21].
EXPERIMENTAL
Materials
Trypsin (Type III, 10200 units/mg of protein), urea, EDTA, TEMPO (2,2,6,6-tetramethylpiperidine-N-oxyl), DTPA (diethylenetriaminepenta-acetic acid) and C18:2,n−6 [linoleic (9-Z,12Z-octadecadienoic) acid], sodium borohydride, tryptone, [Ru(NH3)6]Cl3 [hexa-ammineruthenium(III) chloride] and yeast extract were obtained from Sigma (Sydney, Australia). 13(R/S)-HODE (racemic 13-hydroxy-9Z,11E-octadecadienoic acid), 9(R/S)-HODE (racemic 9-hydroxy-10E,12Z-octadecadienoic acid), 13(R)-HODE [13(R)-hydroxy-9Z,11E-octadecadienoic acid], 9(S)-HODE [9(S)-hydroxy-10E,12Z-octadecadienoic acid], 9-oxoODE (9-oxo-10E,12Z-octadecadienoic acid) and 13-oxoODE (13-oxo-9Z,11E-octadecadienoic acid) were sourced commercially (Cayman Chemicals, Ann Arbor, MI, U.S.A.). [1-14C]-linoleic acid (specific activity 55 mCi/mmol) was obtained from Amersham Biosciences (Little Chalfont, Bucks., U.K.). Dithiothreitol and NaCl were obtained from Fisher Scientific (Fair Lawn, NJ, U.S.A.). H2O2 was sourced from Bio-Rad (Richmond, CA, U.S.A.). Pre-swollen anion-exchange DEAE-cellulose (DE52) was obtained from Whatman (Millipore, North Ryde, NSW, Australia). Buffers were prepared from distilled water that was purified further by passage through a Barnstead Nanopure system. Buffers were stored over Chelex-100® (Bio-Rad) at 4 °C for 24 h to remove contaminating transition metals as verified by the ascorbate autoxidation assay [22]. Solvents and all other chemicals employed were of the highest quality available.
Preparation of recombinant wild-type and variant human Mb
DNA manipulations were performed using procedures described in [23]. Site-directed mutagenesis was performed with the pBlue-script II KS (±) vector (Stratagene, La Jolla, CA, U.S.A.). DNA was amplified by PCR with a high-fidelity PfuTurbo® DNA polymerase (Stratagene). Point mutations in the Mb sequence were confirmed by DNA sequence analysis before protein expression in bacteria. Once the sequence was confirmed, the BamHI/HindIII fragment from the amplified DNA, which also contained the mutant Mb coding, was ligated to the BamHI/HindIII fragment from the vector pMb3 [24] to yield the final expression vector. The circular plasmid was then transformed to the appropriate cell line for protein expression as described [21].
When required, proteins were concentrated by ultrafiltration (Centriprep-10 concentrators; Millipore). Importantly, ferric proteins from each preparation showed ASoret/A280nm ratio >5, consistent with the high purity of the isolated proteins (results not shown). All proteins were stored at −80 °C and thawed on ice immediately before use.
Oxidation of C18:2,n−6
Reaction mixtures contained Mb and purified C18:2,n−6 dispersed in 100 mM phosphate buffer (pH 7.4) and at the final concentrations indicated in the Figure legend(s). Reaction mixtures were initially maintained at 4 °C before initiation of lipid (per)oxidation. Commercial C18:2,n−6 was routinely purified by reversed-phase HPLC (see below) immediately before use. Purified C18:2,n−6 was devoid of detectable lipid oxidation products [limit of detection 10 pmol, as judged by measurement of authentic 9(R/S)- or 13(R/S)-HODE monitored at A234]. The analytically pure fatty acid was resuspended in ethanol to yield a stock solution for use in subsequent oxidation studies. Stock solutions of purified C18:2,n−6 were thoroughly degassed with argon, then employed in the various oxidation studies within 30 min of isolation. Added ethanol in the final reaction mixtures (≤5% v/v) had no effect on Mb-mediated C18:2,n−6 oxidation as judged by monitoring the extent of lipid oxidation directly in reaction mixtures by photometry at A234 (results not shown).
Lipid peroxidation was initiated in the various reaction mixtures by the addition of H2O2 at a fixed H2O2/Mb ratio of approx. 5 mol/mol and incubating the mixture at 37 °C as described previously [14]. Mb shows limited peroxidase ability [25] and H2O2/Mb >10 mol/mol can result in haem degradation, as judged by bleaching of the Soret and the release of haem (characteristic broad absorbance with maxima at A395). Therefore reaction conditions were selected to yield optimal peroxidase activity without introducing complications through Mb protein degradation. DTPA (200 μM) was included in all reaction mixtures before H2O2 addition in order to minimize the possibility of Fenton-type chemistry that would yield a non-specific product distribution of lipid-oxidation products. After 15 min of incubation, reaction mixtures were extracted into chloroform, methanol and water (5:1:1, by vol.). The combined chloroform phase was evaporated to dryness and the residue was resuspended in methanol and treated with sodium borohydride to reduce all HPODEs (hydroperoxyoctadecadienoic acids) to the corresponding HODEs (hydroxyoctadecadienoic acids) [26]. Finally, the methanol fraction containing the HODE products was extracted into chloroform and water (1:5:2, by vol.), and the dried residue was resuspended in hexane/isopropyl alcohol (4:1, v/v) for subsequent HPLC analyses. This process did not result in significant loss of fatty acid, as judged by recovery studies using reactions mixtures spiked with [1-14C]linoleic acid; recovery after sample work-up was 97±2% (mean±S.D., n=6; results not shown).
HPLC analyses
Lipids present in the extract were first separated by reversed-phase HPLC on a LC18-DB column (25×0.46 cm, 5 μM) eluted with methanol/water/ethanoic acid (90:10:0.1, by vol.; 1 ml/min) as described in [26]. Under these conditions, authentic 9(R/S)- and 13(R/S)-HODE eluted between 4–7 min as an unresolved mixture and authentic C18:2,n−6 eluted at 11–13 min, as determined by monitoring A234 and A210 respectively. The mixture of 9(R/S)- and 13(R/S)-HODE was collected, re-extracted [26] and separated by normal-phase HPLC on a LC-Si column (25×0.46 cm, 5 μM) eluted with hexane/isopropyl alcohol/ethanoic acid (100:2:0.1, by vol.; 1 ml/min) [27]. Under these conditions, 13(R/S)- and 9(R/S)-HODE enantiomers eluted at 10–12 min and 17–19 min respectively (A234 was monitored), while 9(R/S)- and 13(R/S)-oxoODE (oxoODE) eluted as an unresolved mixture between 6 and 8 min (A270 was monitored). Concentrations of HODE and oxoODE products were determined by peak area comparison with corresponding authentic standards.
Finally, the absolute stereochemistry of the fatty acid oxidation products was determined by resolving the mixture of 9(R/S)- and 13(R/S)-HODE enantiomers with a chiral column (Chiralcel OD-H; Diacel Chemical Industries), eluted with hexane/isopropyl alcohol/ethanoic acid (95:5:0.1, by vol.; 1 ml/min) and the A234 was monitored [26,27]. Retention times for the various enantiomers were 13(R)-HODE, 8.3 min; 13(S)-HODE, 10.1 min; 9(R)-HODE, 10.6 min and 9(S)-HODE, 12.1 min.
Peroxidase activity
Steady-state parameters for the reactions of wild-type and Y103F variant human Mb (final concentration 50 μM) with H2O2 (250 μM) and C18:2,n−6 (0–9.5 mM) were determined by monitoring the accumulation of total oxidation products [i.e., the sum of 9(R/S)- and 13(R/S)-HODE, and 9(R/S)- and 13(R/S)-oxoODE] measured in the various reaction mixtures after 15 min of incubation at 37 °C. Overall, reaction conditions were selected to maximize the yield of HODE products without significant accumulation of oxoODE. The latter are formed through a process that involves degradation of the lipid hydroperoxide precursors to the corresponding HODEs via a homolytic mechanism involving alkoxyl radical formation [28,29]. Formation of oxoODE was not previously reported in the analogous reactions with native and recombinant Mb from sperm whale [14].
At least six different concentrations of C18:2,n−6, including two concentrations greater than the calculated Km value, were employed to construct the Michaelis–Menten plot for each protein tested. Consistent with a previous report [14], concentrations of C18:2,n−6 >2 mM dispersed in phosphate buffer were slightly turbid and, therefore, likely to represent nominal concentrations of added substrate. The Km and Vmax values were obtained by fitting to the Michaelis–Menten equation as described previously [21]. Despite miscibility problems with higher concentrations of C18:2,n−6, the linearity of the plots (R2≥0.83) verified the methods employed (results not shown).
Spectroelectrochemical measurement
The midpoint potential for the Fe(III)/Fe(II) redox couple of wild-type and Y103F variant Mb (final concentration 500 μM in 100 mM phosphate buffer, pH 7.4, 20 °C) was determined by potentiometric titration with an OTTLE (optically transparent thin-layer electrode) cell with platinum mesh working and auxiliary electrodes and a commercial Ag/AgCl reference electrode (BASi, West Lafayette, IN, U.S.A.). Electrolysis was performed in situ with a Princeton Instruments PAR-173A potentiostat/galvanostat and electronic spectra were collected with a Hewlett-Packard diode array detector (HP-8452A) suitable to accommodate the OTTLE cell. A black cloth was used to cover the OTTLE cell during spectral accumulation to decrease the effects of stray light. In all cases, internal resistance (R) in the aqueous solutions employed was small and, therefore, iR compensation was not applied. Commercial [Ru(NH3)6]Cl3 (final concentration, 40 μM) was added to the protein to act as a mediator for the electrochemical reduction process [30].
Solution potentials were determined with respect to the Ag/AgCl reference electrode and converted into the SHE (standard hydrogen scale) as described previously [31]. Transformed data were then plotted according to the Nernst equation with Prism software (GraphPad, San Diego, CA, U.S.A.), yielding linear regression correlations of R2≥0.95 and a slope near unity corresponding to a reversible one-electron reduction process [32].
Electronic absorption spectra
Electronic spectra were obtained with a Cary 3E UV/visible spectrophotometer. Spectra of the wild-type Mb and the Y103F variant Mb prepared in phosphate buffer (100 mM, pH 7.4) showed Soret (λmax, 409 nm) and visible bands (λmax, 505–508 and 630–633 nm) similar to those of native Mb [33]. Where required, Mb solutions were quantified using the reported molar absorption coefficient ε409 ∼153×103 M−1·cm−1 [34].
Statistical analyses
Statistical analyses were performed using Prism software. Student's t-test was employed to compare paired data sets with Welch's correction for unequal variance where appropriate. Statistical significance was accepted at the 95% confidence level (P<0.05).
RESULTS
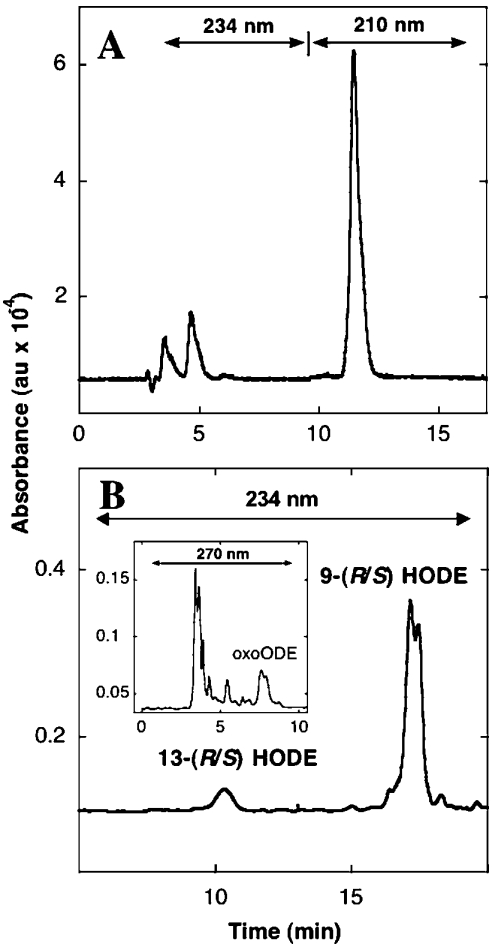
In the presence of H2O2, wild-type human Mb oxidized C18:2,n−6 to a mixture of products detected by reversed-phase HPLC (Figure 1A), consistent with the oxidation of C18:2,n−6 by other mammalian Mb [14]. Resolution of the conjugated diene-containing total oxidation product by normal-phase HPLC (Figure 1B) indicated a clear regiospecific product distribution with 9(R/S)-HODE accumulating in preference to 13(R/S)-HODE. An unresolved mixture of 9- and 13-oxoODE (Figure 1B, inset) was also produced during the oxidation reaction. However, in direct support of the study design, these ketones accumulated in only trace amounts relative to the HODE end-products.
Figure 1. Reversed- and normal-phase chromatograms showing oxidation products of C18:2,n−6 produced in the presence of added Mb and H2O2.
(A) Freshly purified C18:2,n−6 (final concentration 500 μM in 100 mM phosphate buffer, pH 7.4) yields a mixture of oxidized products, after incubation with Mb (50 μM) and H2O2 (250 μM) for 15 min at 37 °C. The mixture of oxidation products elutes as an unresolved peak between 4 and 7 min after reversed-phase HPLC. The peak eluting at 11–13 min corresponds to residual C18:2,n−6 in the reaction mixture. Separation of the oxidized lipids collected from (A) with normal-phase HPLC (B) yields 9(R/S)-HODE, 13(R/S)-HODE (ε234, 23000 mol−1·cm−1). Inset in (B) shows 9- and 13-oxoODE that eluted as an unresolved mixture labelled oxoODE (ε270, 19600 mol−1·cm−1). Retention times for authentic 9- and 13-oxoODE were 7.2 and 8 min respectively. HPLC conditions were as outlined in the Experimental section. Data are representative of at least three independent (HPLC) analyses with different Mb preparations. DTPA (200 μM) was included in all reaction mixtures before H2O2 addition. au, arbitrary units.
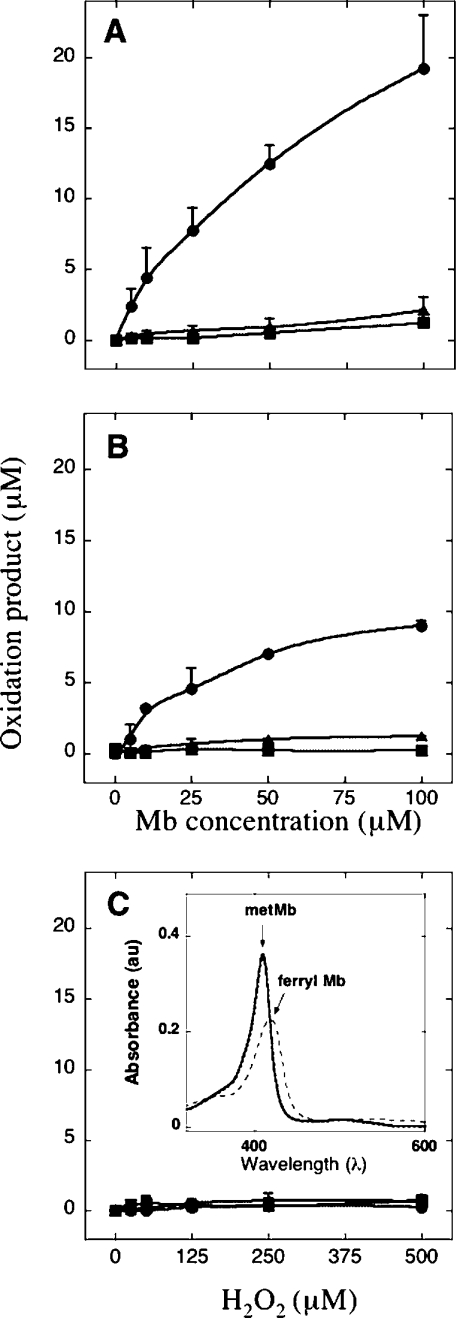
Independent of whether wild-type or Y103F variant Mb was employed, oxidation of C18:2,n−6 stimulated by Mb and added H2O2 (final H2O2/Mb, approx. 5 mol/mol) proceeded in a dose-dependent fashion with increasing lipid oxidation measured with increasing protein content (Figure 2). The efficiency of lipid oxidation was consistently greater in reactions containing wild-type Mb than in those containing Y103F Mb, as judged by comparing the extent of total lipid oxidation for any given reaction condition (i.e. the sum of HODE and oxoODE) (cf. Figures 2A and 2B). Carefully matched control reactions with H2O2 added to C18:2,n−6 in the presence of DTPA did not yield significant concentrations of HODE or oxoODE (Figure 2C). Independent of the Mb type employed, addition of purified C18:2,n−6 to the protein did not yield a ferryl species; however, ferryl Mb was generated immediately after the addition of H2O2 to initiate C18:2,n−6 oxidation (Figure 2C, inset).
Figure 2. Dose-dependent oxidation of C18:2,n−6 by added Mb and H2O2.
Increasing yield of 9(R/S)-HODE (•), 13(R/S)-HODE (▴) (measured at 234 nm) and total oxoODE (▪) (measured at 270 nm) was determined in mixtures containing C18:2,n−6 (1 mM) and an increasing dose of wild-type Mb (A), Y103F Mb (B) each treated with H2O2 (Mb/H2O2 ratio, 5 mol/mol) or corresponding concentrations of H2O2 alone (C). All reaction mixtures were incubated for 15 min at 37 °C, worked-up as outlined in the Experimental section and analysed for oxidation products with HPLC. Results are means±S.D. of three independent analyses. Where error bars are not shown, the symbol is larger than the error. Inset in (C) shows the electronic absorbance spectrum for wild-type Mb (2.5 μM, solid line) immediately after addition of purified C18:2,n−6 (100 μM, dotted line) and addition of H2O2 (12.5 μM, broken line) to the mixture. Note, solid and dotted lines overlap with a small decrease in peak intensity detected at A409. A shift in Soret maxima (A409 to A419), indicated by the arrows, together with a concomitant decrease in peak intensity, is consistent with the formation of ferryl Mb upon addition of H2O2. DTPA (200 μM) was included in all reaction mixtures. au, arbitrary units.
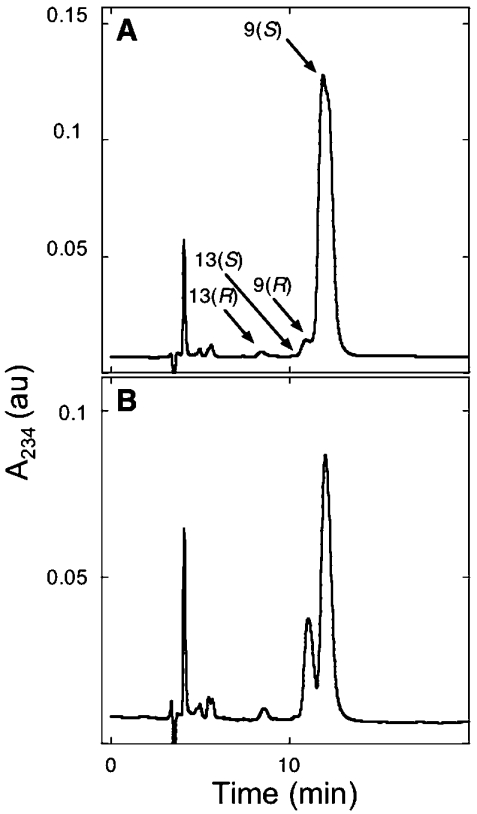
Next, a range of steady-state parameters for the process of Mb-mediated fatty acid oxidation was investigated (Table 1). Based on the dose-dependency studies (Figure 2), reaction mixtures containing Mb and H2O2 at 50 and 250 μM respectively, were considered to be suitable to explore the extent of lipid oxidation with increasing concentrations of added C18:2,n−6. Under these conditions, both wild-type and variant human Mb oxidized added C18:2,n−6 to yield fatty acid oxidation products in a positional (regio-) and stereo-specific fashion after 15 min of incubation at 37 °C (Figure 3). Overall, 9-HODEs were formed in preference to 13-HODEs, and 9(S)-HODE accumulated in excess over 9(R)-HODE (Figure 3). However, by comparison with wild-type Mb, reactions containing Y103F Mb consistently produced relatively more 9(R)-HODE (cf. Figures 3A and 3B, and Table 1).
Table 1. Kinetic parameters for the reactions of wild-type and Y103F variant human Mb with H2O2 and added C18:2,n−6.
Reactions were performed in 100 mM phosphate buffer (pH 7.4) maintained at 37 °C with Mb (final concentration, 50 μM), H2O2 (250 μM) and 18:2 (0–9.5 mM). Where possible, kinetic data are reported as the mean±S.D. of n≥3 independent analyses. Enantiomers of 9- and 13-HODE were separated by normal-phase chiral HPLC as described in the Experimental section. Product distributions for 9- and 13-HODEs are presented as an average obtained over all conditions studied.
| Protein | Km (mM) | Vmax (pmol/min−1·nmol Mb protein−1) | Yield 9(S):9(R) | Yield 13(S):13(R) | Source |
|---|---|---|---|---|---|
| Wild-type sperm whale Mb | 6±1 | 224±30 | 81:16 | − | [14] |
| Wild-type human Mb | 4.3±0.8 | 238±10 | 94:6 | 18:82 | Present study |
| Y103F Mb | 8.6±0.3* | 148±19* | 71:29* | 17:83 | Present study |
| H2O2 alone | − | − | 49:51* | 25:75 | Present study |
* Significantly different from corresponding value for wild-type Mb (P<0.05).
Figure 3. Resolution of 9(R/S)- and 13(R/S)-HODE regio-isomers isolated from oxidation reactions containing C18:2,n−6, Mb and H2O2.
The 9(R/S)- and 13(R/S)-HODE products isolated from reaction between (1 mM) C18:2,n−6 and (50 μM) wild-type (A) or Y103F (B) Mb and added H2O2 (250 μM) were resolved with normal-phase chiral chromatography with the absorbance monitored at 234 nm. HPLC conditions were as outlined in the Experimental section. Note that 9- and 13-oxoODE products were not observed under these conditions. Results are representative of at least three independent (HPLC) analyses with different Mb preparations. DTPA (200 μM) was included in all reaction mixtures before H2O2 addition. au, arbitrary units.
Reaction of Mb and 13(S)-hydroperoxy-9,11-octadecadienoic acid [13(S)-HPODE] can yield ferryl Mb and lipid alkoxyl radicals through degradation of the HPODE [29]. Lipid alkoxyl radicals formed by this process may then act as chain-initiating species that promote lipid (per)oxidation in the presence of excess C18:2,n−6 [5,35]. However, lipid oxidation initiated by a nonenzymic, free radical mechanism would not be expected to yield a stereospecific product distribution favouring one HPODE enantiomer, which is the case for ferryl Mb-mediated (enzymic) lipid oxidation [14]. Alternatively, in the absence of a suitable substrate (such as a bisallylic hydrogen-containing fatty acid), the alkoxyl radical may decompose to yield oxoODE [28] or epoxides and alcohols [36] that may result in the depletion of conjugated diene [29].
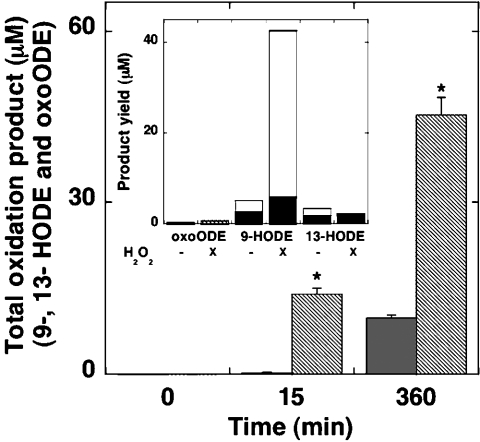
To rule out the possibility that the reaction of human Mb with HPODE contributes to significant C18:2,n−6 lipid (per)oxidation, we performed oxidation studies with mixtures of wild-type Mb and freshly purified C18:2,n−6 in the absence of added H2O2 (Figure 4). Mixtures of Mb and excess C18:2,n−6 did not yield detectable oxidation products after 15 min of incubation at 37 °C, whereas measurable lipid oxidation was detected after 360 min. However, the concentration of total oxidation products remained significantly lower than that measured in the presence of Mb and H2O2 at either 15 or 360 min (Figure 4). Furthermore, and in direct contrast with Mb-mediated C18:2,n−6 oxidation in the presence of H2O2, the distribution of HODE products exhibited decreased regio- and stereo-specificity, while concentrations of oxoODE were similar (Figure 4, inset). Thus Mb-mediated fatty acid oxidation in the absence of added peroxide yielded a greater proportion of 13(R/S)-HODE, while the distribution of 9-HODE enantiomers was near identical (Figure 4, inset). Taken together, these data strongly support the notion that Mb-mediated ‘enzymic’ oxidation of C18:2,n−6 in the presence of added H2O2 is a dominant pathway that yields regio- and stereo-specific HPODE products. In contrast, degradation of 9(R/S)- or 13(R/S)-HPODE to yield oxoODE probably only occurs at times when the concentration of unoxidized fatty acid becomes limiting relative to the concentration of HPODE.
Figure 4. Oxidation of C18:2,n−6 by added Mb and H2O2 occurs at a greater rate and with greater product specificity than with added Mb in the absence of peroxide.
C18:2,n−6 (1 mM) was mixed with wild-type Mb (50 μM) and treated with (hatched bars) or without (grey bars) H2O2 (250 μM) and the combined total yield of 9- and 13-HODE and oxoODE products was determined, after 15 or 360 min of incubation at 37 °C, using HPLC, as described in the legend to Figure 1. Results are means±S.D. from at least three independent analyses. *Significantly different (P<0.05) from yield of total oxidation products determined after 360 min of incubation. The inset shows the mean accumulation of oxoODE and stereoisomers of 9- (white bar) and 13-HODE (black bar) with and without 250 μM H2O2 (as indicated) after 360 min. HODE enantiomers were resolved by normal-phase chiral HPLC as described in the legend to Figure 3. Error bars (≤5.8% of the maximal value) are not shown for reasons of clarity. DTPA (200 μM) was included in all reaction mixtures.
Previous EPR spectroscopic studies using spin-trapping agents have indicated that hydroxyl radicals are not produced from reactions of H2O2 with human [12] or equine [37] Mb, ruling out the possibility that this oxidant initiates lipid oxidation. Furthermore, DTPA has been included in all reaction mixtures to eliminate possible hydroxyl radical production by Fenton chemistry. As enantiomeric 13-HODEs were not detected in purified C18:2,n−6 (results not shown), it was concluded that the accumulation of 13-HODEs involved a reaction of C18:2,n−6 with wild-type or mutant Mb plus peroxide. Interestingly, the extent of accumulation of 13(R)-HODE was generally greater than 13(S)-HODE (e.g. Figures 3A and 3B, and Table 1) and independent of the protein/fatty acid ratio (results not shown), or the presence or absence of protein (Table 1).
Double-reciprocal plots of the steady-state concentrations of total oxidation products (HODE plus oxoODE), measured after 15 min of incubation, against the corresponding nominal C18:2,n−6 concentrations afforded linear relationships for the wild-type and Y103F variant Mb (results not shown). Values of Km and Vmax for wild-type human Mb were similar (Table 1) to those reported for recombinant sperm whale Mb [14]. However, by comparison, Km and Vmax were decreased and increased respectively for Y103F Mb, probably reflecting a lower rate constant for the reaction of this variant Mb with H2O2 [21], and a decreased binding strength of the C18:2,n−6 in the haem crevice.
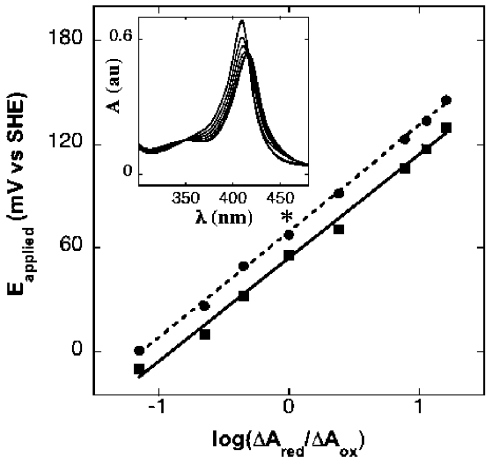
The midpoint reduction potential determined here for wild-type human Mb (58±3 mV compared with SHE) is similar to that reported for wild-type horse heart Mb determined spectroelectrochemically [30] or with cyclic voltammetry [38] (Figure 5 and inset). In contrast, the midpoint reduction potential determined for the Y103F variant of Mb was shifted by a small, but significant, value to a higher potential (67±2 mV) than the corresponding wild-type Mb (Figure 5).
Figure 5. Determination of midpoint reduction potentials of wild-type and Y103F Mb.
Reduction potentials were determined by spectroelectrochemical thin-layer absorbance spectroscopy as described in the Experimental section. Nernst plots of data determined for the applied potential-dependent changes in A431 were fitted by linear regression to yield the midpoint reduction potential for the wild-type (▪) and Y103F variant (•) Mb as described [30]. Results are means±S.D. of three independent spectral accumulations at the same applied potential. Where error bars are not shown, the symbol is larger than the error. *Significantly different to the corresponding midpoint potential for wild-type Mb (P<0.05). The inset shows representative electronic absorption spectra measured between 350 and 450 nm for solutions of wild-type Mb (0.5 mM protein in 100 mM phosphate buffer, pH 7.4, 20 °C) obtained at applied potentials ranging 0 to −280 mV (compared with SHE). A limited number of spectra are shown for clarity. au, arbitrary units.
DISCUSSION
Our previous kinetic analyses of the reaction of human wild-type and Y103F variant Mbs with H2O2 indicated a decreased absolute rate constant for the heterolytic cleavage of H2O2 by the protein lacking Tyr103 [21]. This decreased peroxidase activity was manifested in decreased Vmax and increased Km values determined for thioanisole sulphoxidation. Consistent with these data, the present study shows that Y103F Mb oxidizes C18:2,n−6 in the presence of added H2O2 with decreased efficacy compared with the wild-type protein. This conclusion is supported by decreases in Vmax value and the extent of lipid oxidation initiated by the Y103F Mb compared with wild-type Mb (Figure 2). In addition, the increased Km value obtained for reactions between Y103F Mb and C18:2,n−6 indicates a weaker substrate binding that is consistent with the observed decreased stereospecificity for 9-HODE accumulation in reactions containing the Y103F variant human Mb. In contrast, fatty acid oxidation in the absence of added H2O2 does not generate a detectable ferryl Mb species (Figure 3, inset); oxidation products accumulate relatively slowly and, where present, with decreased regio- and stereo-specificity. Overall, Mb-mediated C18:2,n−6 oxidation in the presence of H2O2 yields ferryl Mb as the primary oxidant responsible for initiating lipid oxidation and binding of the polyunsaturated fatty acid in the haem cavity before oxidation affords a specific enzymic product distribution consistent with other mammalian Mb [14].
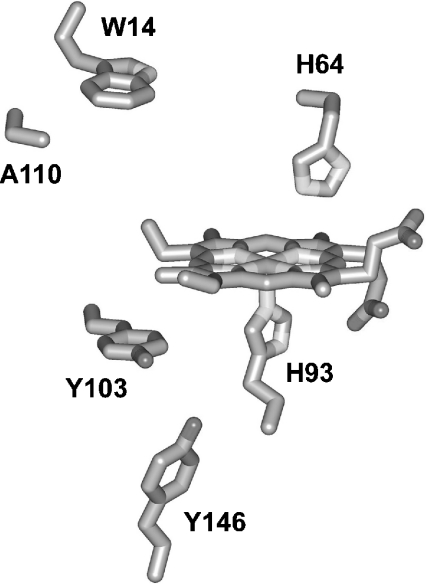
Our data also indicate that Tyr103 is important in regulating the physical properties of human Mb. Such changes in physical properties may be interpreted at a structural level. As the three-dimensional structure of wild-type human Mb has not been reported, distances between elements within this protein may be estimated from the X-ray crystal structure of the Lys45→Arg/Cys110→Ala variant of human Mb [24] (Figure 6). Tyr103 is the tyrosine residue closest to the haem group, as is the case for sperm whale [39] and horse [40] Mb, with the shortest distance between the phenyl carbon and the haem edge being approx. 3.4 Å(1 Å=0.1 nm). Tyr103 is also near the proximal His93, with the closest atom-to-atom separation being approx. 3.5 Å. Importantly, no significant change in the α-helicity or thermal melting points was evident between the Y103F variant and wild-type protein as determined by CD spectroscopy [33]. Furthermore, the axial symmetry of the haem-iron assessed by low-temperature EPR was not altered by introduction of the point mutation [21]. Taken together, these data indicate that the point mutation (Y103F) does not significantly alter Mb tertiary structure. Therefore the decreased peroxidase activity of the Y103F variant Mb, noted in the present study and elsewhere [21,33], are probably independent of structural factors that affect the haem crevice.
Figure 6. Structural elements of the haem pocket of the Lys45→Arg/Cys110→Ala variant of human Mb.
Proximal and distal histidine residues of the protein are shown below and above the plane of the haem prosthetic group respectively. Other critical residues are included to show proximity to the haem group. Note that wild-type human Mb contains Cys110 in place of Ala110. The data shown are derived from the crystal structure determined at 2.8 Å resolution [24]. Amino acids are indicated by their one-letter codes.
One possible explanation for the decreased peroxidase activity is that the point mutation affects hydrogen bonding within the haem crevice. Indeed, the reported decrease in the pKa value for the acid–base transition of Y103F Mb [33] is supportive of the notion that the point mutation introduces a subtle change to the haem pocket that alters either the dielectric field or the strength of the hydrogen bonding within the haem crevice. This in turn affects the binding strength of the aqua ligand to the ferric iron centre and hence, the pKa for the variant Mb. Alterations in hydrogen bonding that affect the donor power of His93 coincidentally modulate midpoint reduction potentials [41]. Thus a high donor power, which also yields a higher pKa value, results in a low reduction potential [42]. Conversely, low donor power decreases the pKa value and increases the midpoint reduction potential, which is the case for Y103F variant Mb. Furthermore, the decrease in stereospecificity of C18:2,n−6 oxidation catalysed by Y103F Mb in the presence of added H2O2 is fully consistent with an alteration to hydrogen bonding, within the active site, that results in weaker substrate binding and hence a greater degree of freedom to the substrate. Together, these data support the idea that the close proximity to the haem edge allows Tyr103 to play an active role in the binding and orientation of exogenous substrates {e.g. C18:2,n−6 and thioanisole (shown in the present study and elsewhere [21])} and ligands (e.g. water [33]) within the haem pocket of Mb.
For peroxidases, the reduction potential for the Fe(IV)/(III) redox equilibrium probably reflects catalytic activity [34]. However, the inherent instability of the Mb compound I ([21] and references therein) has precluded the measurement of the midpoint potential for the Fe(IV)/(III) redox process in Mb. Therefore the Fe(III)/(II) redox potential reported here must be considered as a surrogate measure of how Mb redox behaviour is affected by changes in the hydrogen-bonding network surrounding the haem crevice. Notably, the midpoint potential values for the Fe(III)/(II) reduction in wild-type and variant human Mbs are approx. 200 mV lower than that measured for true peroxidases such as horseradish peroxidase [43]. However, any direct correlation between the Fe(III)/(II) redox potential and peroxidase activity is probably not meaningful.
As indicated in the Introduction, Mb exhibits a quasi-lipoxy-genase activity that may have pathophysiological implications. The pro-oxidative activity of Mb is potentiated by calcium [44] that is present in relatively high abundance in the extracellular space. Activation of extracellular Mb toward pro-oxidation may play a role in oxidative stress associated with post-ischaemia reperfusion damage to Mb-rich tissues, such as the heart. As shown in the present study, the product distribution from Mb-mediated oxidation of C18:2,n−6 favours the accumulation of 9(R/S)- over 13(R/S)-HODE, with 9(S)-HODE being the major product. In contrast, the reverse product distribution is obtained with C18:2,n−6 oxidized by soybean or recombinant human lipoxygenase [26,27], or non-enzymically by free radicals in the presence of vitamin E [45,46]. Therefore careful analyses of the regio- and stereo-isomeric distribution of C18:2,n−6 oxidation products in samples from heart or skeletal muscle tissues exposed to ischaemia reperfusion may provide a useful diagnostic tool to ascertain whether or not there is a role for Mb-derived oxidants in the pathogenesis of ischaemia reperfusion damage.
In summary, the present studies on the peroxidase activity of human Y103F Mb provides further evidence linking decreased peroxidase activity of the variant to a change in the hydrogen-bonding network within the haem crevice. We show that replacing Tyr103 in human Mb with phenylalanine leads to alterations to the physical properties of the protein, including the midpoint reduction potential, although this represents only a minor contribution to the energetics of oxidation with changes in selectivity and other kinetic parameters probably dominated by changes in substrate interactions within the haem pocket. An extensive hydrogen-bonding network involving Leu89, Ser92, His93 and His97 on the proximal side of the Mb haem pocket are implicated in stabilizing the proximal geometry of the protein [47]. Our functional studies investigating changes in kinetic and physical properties of the Y103F variant of human Mb strongly suggest that Tyr103, a residue conserved in the sequence of all mammalian Mb, is also involved in the hydrogen-bonding network on the proximal side of the haem crevice. Overall, site-directed mutagenesis has provided a powerful tool to investigate the role of specific amino acid residues on physical properties of proteins.
Acknowledgments
We thank Dr Aviva Levina (Sydney University) for excellent assistance with spectroelectrochemistry and Professor Grant Mauk (University of British Columbia, Vancouver, Canada) for many thoughtful discussions. This research was supported by the Australian Research Council (Australian Research Fellowship DP034325 awarded to P.K.W. and Professorial Fellowship DP0208409 awarded to P.A.L.) and the National Health and Medical Research Council of Australia (Senior Principal Research Fellowship 151602 awarded to R.S.).
References
- 1.Wittenberg B. A., Wittenberg J. B. Transport of oxygen in muscle. Annu. Rev. Physiol. 1989;51:857–878. doi: 10.1146/annurev.ph.51.030189.004233. [DOI] [PubMed] [Google Scholar]
- 2.Qiu Y., Sutton L., Riggs A. F. Identification of myoglobin in human smooth muscle. J. Biol. Chem. 1998;273:23426–23432. doi: 10.1074/jbc.273.36.23426. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Raven E., Mauk A. G. Myoglobin. In: Sykes A. G., editor. Advances in Inorganic Chemistry: Heme–Fe Proteins. vol. 51. London: Academic Press; 2000. pp. 1–49. [Google Scholar]
- 4.Hogg N., Rice-Evans C., Darley-Usmar V., Wilson M. T., Paganga G., Bourne L. The role of lipid hydroperoxides in the myoglobin-dependent oxidation of LDL. Arch. Biochem. Biophys. 1994;314:39–44. doi: 10.1006/abbi.1994.1409. [DOI] [PubMed] [Google Scholar]
- 5.Witting P. K., Willhite C. A., Davies M. J., Stocker R. Lipid oxidation in human low-density lipoprotein induced by metmyoglobin/H2O2: involvement of α-tocopheroxyl and phosphatidylcholine alkoxyl radicals. Chem. Res. Toxicol. 1999;12:1173–1181. doi: 10.1021/tx9900472. [DOI] [PubMed] [Google Scholar]
- 6.Irwin J. A., Ostdal H., Davies M. J. Myoglobin-induced oxidative damage: evidence for radical transfer from oxidized myoglobin to other proteins and antioxidants. Arch. Biochem. Biophys. 1999;362:94–104. doi: 10.1006/abbi.1998.0987. [DOI] [PubMed] [Google Scholar]
- 7.George P., Irvine D. H. Free radical production in oxidation–reduction reactions of peroxidase, catalase and metmyoglobin. Br. J. Radiol. 1954;27:131–137. doi: 10.1259/0007-1285-27-314-131. [DOI] [PubMed] [Google Scholar]
- 8.Allentoff A. J., Bolton J. L., Wilkis A., Thompson J. A., Ortiz de Montellano P. R. Heterolytic versus homolytic peroxide bond cleavage by sperm whale myoglobin and myoglobin mutants. J. Am. Chem. Soc. 1992;114:9744–9749. [Google Scholar]
- 9.Tew D., Ortiz de Montellano P. R. The myoglobin protein radical. Coupling of Tyr-103 to Tyr-151 in the H2O2-mediated cross-linking of sperm whale myoglobin. J. Biol. Chem. 1988;263:17880–17886. [PubMed] [Google Scholar]
- 10.Davies M. J. Identification of a globin free radical in equine myoglobin treated with peroxides. Biochim. Biophys. Acta. 1991;1077:86–90. doi: 10.1016/0167-4838(91)90529-9. [DOI] [PubMed] [Google Scholar]
- 11.DeGray J. A., Gunther M. R., Tschirret-Guth R., Ortiz de Montellano P. R., Mason R. P. Peroxidation of a specific tryptophan of metmyoglobin by hydrogen peroxide. J. Biol. Chem. 1997;272:2359–2362. doi: 10.1074/jbc.272.4.2359. [DOI] [PubMed] [Google Scholar]
- 12.Witting P. K., Douglas D. J., Mauk A. G. Reaction of human myoglobin and H2O2: involvement of a thiyl radical produced at cysteine 110. J. Biol. Chem. 2000;275:20391–20398. doi: 10.1074/jbc.M000373200. [DOI] [PubMed] [Google Scholar]
- 13.Catalano C. E., Ortiz de Montellano P. R. Oxene transfer, electron abstraction, and co-oxidation in the epoxidation of stilbene and 7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by hemoglobin. Biochemistry. 1987;26:8373–8380. doi: 10.1021/bi00399a052. [DOI] [PubMed] [Google Scholar]
- 14.Rao S. I., Wilks A., Hamberg M., Ortiz de Montellano P. R. The lipoxygenase activity of myoglobin: oxidation of linoleic acid by the ferryl oxygen rather than protein radical. J. Biol. Chem. 1994;269:7210–7216. [PubMed] [Google Scholar]
- 15.Witting P. K., Mauk A. G., Douglas D. J., Stocker R. Reaction of human myoglobin and peroxynitrite: characterizing biomarkers for myoglobin-derived oxidative stress. Biochem. Biophys. Res. Commun. 2001;286:352–356. doi: 10.1006/bbrc.2001.5397. [DOI] [PubMed] [Google Scholar]
- 16.Kanner J., Harel S. Initiation of membranal lipid peroxidation by activated metmyoglobin and methemoglobin. Arch. Biochem. Biophys. 1985;237:314–321. doi: 10.1016/0003-9861(85)90282-6. [DOI] [PubMed] [Google Scholar]
- 17.Galaris D., Eddy L., Arduini A., Cadenas E., Hochstein P. Mechanisms of reoxygenation injury in myocardial infarction: implications of a myoglobin redox cycle. Biochem. Biophys. Res. Commun. 1989;160:1162–1168. doi: 10.1016/s0006-291x(89)80125-1. [DOI] [PubMed] [Google Scholar]
- 18.Hatley M. E., Srinivasan S., Reilly K. B., Bolick D. T., Hedrick C. C. Increased production of 12/15 lipoxygenase eicosanoids accelerates monocyte/endothelial interactions in diabetic db/db mice. J. Biol. Chem. 2003;278:25369–25375. doi: 10.1074/jbc.M301175200. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn H., Belkner J., Zaiss S., Fahrenklemper T., Wohlfeil S. Involvement of 15-lipoxygenase in early stages of atherogenesis. J. Exp. Med. 1994;179:1903–1911. doi: 10.1084/jem.179.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baer A. N., Costello P. B., Green F. A. Stereospecificity of the hydroxyeicosatetraenoic and hydroxyoctadecadienoic acids produced by cultured bovine endothelial cells. Biochim. Biophys. Acta. 1991;1085:45–52. doi: 10.1016/0005-2760(91)90230-f. [DOI] [PubMed] [Google Scholar]
- 21.Witting P. K., Mauk A. G., Lay P. A. Role of tyrosine-103 in myoglobin peroxidase activity: kinetic and steady-state studies on the reaction of wild-type and variant recombinant human myoglobins with H2O2. Biochemistry. 2002;41:11495–11503. doi: 10.1021/bi025835w. [DOI] [PubMed] [Google Scholar]
- 22.Buettner G. R. Use of ascorbate as test for catalytic metals in simple buffers. Methods Enzymol. 1990;186:125–127. doi: 10.1016/0076-6879(90)86100-a. [DOI] [PubMed] [Google Scholar]
- 23.Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. Methods Enzymol. 1987;154:329–350. doi: 10.1016/0076-6879(87)54083-6. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard S. R., Hendrickson W. A., Lambright D. G., Boxer S. G. X-ray crystal structure of a recombinant human myoglobin mutant at 2.8 Å resolution. J. Mol. Biol. 1990;213:215–218. doi: 10.1016/S0022-2836(05)80181-0. [DOI] [PubMed] [Google Scholar]
- 25.Gunther M. R., Tschirret-Guth R. A., Witkowska H. E., Fann Y. C., Barr D. P. Ortiz#x00A0;De#x00A0;Montellano P. R., Mason R. P. Site-specific spin trapping of tyrosine radicals in the oxidation of metmyoglobin by hydrogen peroxide. Biochem. J. 1998;330:1293–1299. doi: 10.1042/bj3301293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upston J. M., Neuzil J., Witting P. K., Alleva R., Stocker R. Oxidation of free fatty acids in low density lipoprotein by 15-lipoxygenase stimulates nonenzymic, α-tocopherol-mediated peroxidation of cholesteryl esters. J. Biol. Chem. 1997;272:30067–30074. doi: 10.1074/jbc.272.48.30067. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn H., Belkner J., Suzuki H., Yamamoto S. Oxidative modification of human lipoproteins by lipoxygenases of different positional specificities. J. Lipid Res. 1994;35:1749–1759. [PubMed] [Google Scholar]
- 28.Mashima R., Tilley L., Siomos M.-A., Papalexis V., Raftery M. J., Stocker R. Plasmodium falciparum histidine-rich protein-2 (PfHRP2) modulates the redox activity of ferri-protoporphyrin IX (FePPIX) J. Biol. Chem. 2002;277:14514–14520. doi: 10.1074/jbc.M109386200. [DOI] [PubMed] [Google Scholar]
- 29.Reeder B. J., Wilson M. T. Mechanism of the reaction of myoglobin with the lipid hydroperoxide hydroperoxyoctadecadienoic acid. Biochem J. 1998;330:1317–1323. doi: 10.1042/bj3301317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildebrand D. P., Lim K. T., Rosell F. I., Twitchett M. B., Wan L., Mauk A. G. Spectroscopic and functional studies of a novel quadruple myoglobin variant with increased peroxidase activity. J. Inorg. Biochem. 1998;70:11–16. doi: 10.1016/s0162-0134(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 31.Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation–reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- 32.Bard A. J., Faulkner F. R. New York: J. Wiley & Sons Publishers; 1980. Electrochemical Methods, Fundamentals and Applications; p. 195. [Google Scholar]
- 33.Witting P. K., Mauk A. G. Reaction of human myoglobin and H2O2: electron transfer between tyrosine 103 phenoxyl radical and cysteine 110 yields a protein-thiyl radical. J. Biol. Chem. 2001;276:16540–16547. doi: 10.1074/jbc.M011707200. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda-Saito M., Hori H., Andersson L. A., Prince R. C., Pickering I. J., George G. N., Sanders C. R., 2nd, Lutz R. S., McKelvey E. J., Mattera R. Coordination structure of the ferric heme iron in engineered distal histidine myoglobin mutants. J. Biol. Chem. 1992;267:22843–22852. [PubMed] [Google Scholar]
- 35.Galaris D., Sevanian A., Cadenas E., Hochstein P. Ferrylmyoglobin-catalyzed linoleic acid peroxidation. Arch. Biochem. Biophys. 1990;281:163–169. doi: 10.1016/0003-9861(90)90427-z. [DOI] [PubMed] [Google Scholar]
- 36.Nui Q. J., Mendenhall G. D. Yields of singlet molecular oxygen from peroxyl radical termination. J. Am. Chem. Soc. 1992;114:165–172. [Google Scholar]
- 37.Turner J. J., Rice-Evans C. A., Davies M. J., Newman E. S. The formation of free radicals by cardiac myocytes under oxidative stress and the effects of electron-donating drugs. Biochem. J. 1991;277:833–837. doi: 10.1042/bj2770833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Dyke B. R., Saltman P., Armstrong F. A. Control of myoglobin electron-transfer rates by the distal (nonbound) histidine residue. J. Am. Chem. Soc. 1996;118:3490–3492. [Google Scholar]
- 39.Takano T. Structure of myoglobin refined at 2.0 Å resolution: I. Crystallographic refinement of metmyoglobin obtained from sperm whale. J. Mol. Biol. 1977;110:537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- 40.Evans S. V., Brayer G. D. High-resolution study of the three-dimensional structure of horse heart metmyoglobin. J. Mol. Biol. 1990;213:885–897. doi: 10.1016/S0022-2836(05)80270-0. [DOI] [PubMed] [Google Scholar]
- 41.Moore G. R., Pettigrew G. W., Rogers N. K. Factors influencing redox potentials of electron transfer proteins. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4998–4999. doi: 10.1073/pnas.83.14.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore G. R., Williams R. J. P. Structural basis for the variation in redox potential of cytochromes. FEBS Lett. 1977;79:229–232. doi: 10.1016/0014-5793(77)80793-x. [DOI] [PubMed] [Google Scholar]
- 43.Farhangrazi Z. S., Fossett M. E., Powers L. S., Ellis W. R., Jr Variable-temperature spectroelectrochemical study of horseradish peroxidase. Biochemistry. 1995;34:2866–2871. doi: 10.1021/bi00009a017. [DOI] [PubMed] [Google Scholar]
- 44.Iwase H., Takatori T., Sakurada K., Nagao M., Niijima H., Matsuda Y., Kobayashi M. Calcium is required for quasi-lipoxygenase activity of hemoproteins. Free Radical Biol. Med. 1998;25:943–952. doi: 10.1016/s0891-5849(98)00150-6. [DOI] [PubMed] [Google Scholar]
- 45.Porter N. A., Weber B. A., Smith K. J. Autoxidation of polyunsatured fatty acids. Factors controlling the stereochemistry of product hydroperoxides. J. Am. Chem. Soc. 1980;102:5597–5601. [Google Scholar]
- 46.Upston J. M., Terentis A. C., Morris K., Keaney J. F., Jr, Stocker R. Oxidized lipid accumulates in the presence of α-tocopherol in atherosclerosis. Biochem. J. 2002;363:753–760. doi: 10.1042/0264-6021:3630753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson E. S., Friedman J. M., Chien E. Y., Sligar S. G. Functional implications of the proximal hydrogen-bonding network in myoglobin: a resonance Raman and kinetic study of Leu89, Ser92, His97, and F-helix swap mutants. Biochemistry. 1998;37:12301–12319. doi: 10.1021/bi980752u. [DOI] [PubMed] [Google Scholar]