Abstract
Tomato (Lycopersicon esculantum) ASR1 (abscisic acid stress ripening protein), a small plant-specific protein whose cellular mode of action defies deduction based on its sequence or homology analyses, is one of numerous plant gene products with unknown biological roles that become over-expressed under water- and salt-stress conditions. Steady-state cellular levels of tomato ASR1 mRNA and protein are transiently increased following exposure of plants to poly(ethylene glycol), NaCl or abscisic acid. Western blot and indirect immunofluorescence analysis with anti-ASR1 antibodies demonstrated that ASR1 is present both in the cytoplasmic and nuclear subcellular compartments; approx. one-third of the total ASR1 protein could be detected in the nucleus. Nuclear ASR1 is a chromatin-bound protein, and can be extracted with 1 M NaCl, but not with 0.5% Triton X-100. ASR1, overexpressed in Escherichia coli and purified to homogeneity, possesses zinc-dependent DNA-binding activity. Competitive-binding experiments and SELEX (systematic evolution of ligands by exponential enrichment) analysis suggest that ASR1 binds at a preferred DNA sequence.
Keywords: abiotic stress, DNA-binding protein, salinity, salt stress, water stress, zinc-dependent DNA binding
Abbreviations: ASR1, abscisic acid stress ripening; DAPI, 4,6-diamidino-2-phenylindole; Ni-NTA, Ni2+-nitrilotriacetate; SELEX, systematic evolution of ligands by exponential enrichment; TBS, Tris-buffered saline
INTRODUCTION
Exposure of plants to salinity or drought conditions results in many physiological, biochemical and molecular changes, including massive changes in the profile of gene expression (reviewed in [1–3]). A large number of genes for which expression is modulated by water stress have been identified in a variety of plant species [1,2,4]. The biological role of some of these cloned genes can be inferred from sequence similarity to previously studied proteins of both plant and non-plant origin. However, about half of these genes encode proteins whose roles cannot be predicted from their primary amino acid sequences [5,6]. Thus the biological activity of the majority of these proteins remains to be determined [1,2,4].
Stress-regulated proteins can be classified into two groups: proteins that take part in signal transduction and proteins that directly play a role in plant survival under stress conditions. Proteins of the first group include transcription factors, RNA-binding proteins, protein kinases and phosphatases [3,7,8]. The second group contains proteins whose activity enables the plant to survive stress conditions and includes proteins involved in ion homoeostasis [9], water channels [10], enzymes involved in scavenging of activated oxygen species [11], enzymes involved in the synthesis of osmolytes and compatible solutes [12,13] and protective proteins [2].
Understanding the molecular details of plant stress response depends on clarifying the biological activity of individual proteins and their interaction with other cellular components. Such knowledge will assist in development of transgenic plants with improved salinity and drought tolerance.
Tomato (Lycopersicon esculantum) Asr1 (abscisic acid stress ripening) cDNA was first cloned by differential screening [14] and shown to encode a highly-charged 13-kDa protein. In roots and shoots of hydroponically grown tomato seedlings low levels of ASR1 mRNA and protein are detected [15]. The addition of NaCl, poly(ethylene glycol) or abscisic acid to the growth medium resulted in a transient elevation of the steady-state levels of both ASR1 mRNA and ASR1 protein [15]. Asr1 expression is also developmentally regulated; levels of ASR1 mRNA are increased during fruit ripening [14]. ASR1 is a plant-specific protein; no homologous non-plant proteins can be detected using FASTA- or BLAST-based searches of existing databases. In tomato, Asr1 belongs to a small gene family [16,17], with homologues cloned from gymnosperm [18], monocot [19,20] and dicot [21–23].
In the present study we determined that ASR1 is localized in both cytoplasmic and nuclear chromatin compartments, and have studied the DNA-binding activity in vitro of Escherichia coli-expressed ASR1. Using a purified protein, we demonstrated that ASR1 confers DNA-sequence-specific zinc-dependent DNA-binding activity.
EXPERIMENTAL
Plant materials
Tomato (L. esculantum Mill., cv. Ailsa Craig) plants were grown hydroponically in Hoagland's solution as described previously [15].
Cellular fractionation
Leaves and roots were homogenized by 5 pulses (Polytron homogenizer, Kinematica, Luzern, Switzerland; 15 s pulses at full speed) in ice-cold buffer (25 mM Hepes, pH 7.5, 0.5 M sucrose, 10 mM MgCl2, 10 mM β-mercaptoethanol, 1 mM ZnCl2 and 1 mM PMSF) at a tissue/buffer ratio of 1:5 (w/v) (modified from [24]). The homogenate was filtered through 4 layers of Miracloth (Calbiochem, San Diego, CA, U.S.A.). For nuclei isolation, the homogenate was centrifuged for 15 min at 530 g. Chloroplasts in the top of the pellet were dislodged and removed and the bottom of the pellet was resuspended in 8 ml of homogenization buffer, layered on to 4 ml of 70% (v/v) Percoll containing 25 mM Hepes, pH 7.5, 0.5 M sucrose, 10 mM MgCl2, 10 mM β-mercaptoethanol and 1 mM ZnCl2, then centrifuged for 30 min at 2100 g (Hettich 1624 swing-out rotor; Hettich, Tuttlingen, Germany). Nuclei were pelleted while chloroplasts were concentrated at the sucrose–Percoll interphase. The nuclear pellets were resuspended in 1 ml of homogenization buffer, centrifuged at 530 g for 15 min, and resuspended in homogenization buffer. For the estimation of cellular distribution of ASR1, filtered homogenates were loaded directly on to the Percoll layer and centrifuged as described above. Acid soluble proteins were prepared by the addition of 0.25 vol. of 1 M H2SO4. The mixture was incubated on ice for 10 min, and centrifuged at 4 °C for 15 min at 10000 g. Supernatants were diluted with 0.25 vol. of 100% (w/v) trichloroacetic acid, followed by incubation on ice for 10 min, then centrifugation at 4 °C for 15 min at 10000 g. Pellets were washed with chilled acetone, and solubilized in double-strength sample buffer [25].
Extraction of nuclear proteins
A suspension of isolated nuclei in homogenization buffer was mixed with equal volumes of water, 1% (w/v) Triton X-100 or 2 M NaCl. The mixtures were incubated for 30 min on ice with occasional mixing, and centrifuged at 10000 g for 15 min at 4 °C. Supernatants were saved and pellets were resuspended in an equal volume of homogenization buffer. Acid soluble proteins were extracted as described above.
Protein electrophoresis
Denaturating SDS/PAGE [15% (w/v) gel] were run in high Tris concentrations [26], and were either stained with Coomassie Blue or electroblotted on to nitrocellulose (0.45 μm; Schleicher and Schuell, Dassel, Germany). Western-blot analysis membranes were stained with Ponceau S. Assay of DNA-binding on membranes was performed without pre-staining of protein electroblots.
Expression and purification of ASR1 protein
An NdeI restriction site was introduced by G→T site-directed mutagenesis of the base upstream of the translation start ATG codon. The resulting ASR1-encoding cDNA was subcloned into the NdeI–EcoRI sites of pRSET B vector (Invitrogen). This construct resulted in the expression of a full-length non-tagged ASR1 protein, and was used to transform E. coli BL21 cells. Logarithmically growing E. coli cells were induced to express the ASR1 protein with 0.4 mM IPTG (isopropyl β-D-thiogalactoside). After 3 h of induced expression, cells were washed twice in 20 mM Tris/HCl, pH 7, resuspended in the same buffer containing 1 mM PMSF and sonicated. Insoluble material was removed by centrifugation at 12000 g for 15 min at 4 °C. ASR1 did not accumulate in inclusion bodies, and was recovered in the supernatant which was further clarified by centrifugation for 15 min at 100000 g then loaded on to a heparin–agarose column (Econo-Pac, Bio-Rad Laboratories, Hercules, CA, U.S.A., or HiTrap, Amersham-Pharmacia, Uppsala, Sweden) pre-equilibrated in the resuspension buffer. The column was washed with 10 vol. of 20 mM Tris/HCl, pH 7. ASR1 was eluted with an NaCl gradient (100–150 mM). ASR1-containing fractions were pooled, NaCl was added to a final concentration of 0.3 M and mixed with Ni-NTA (Ni2+-nitrilotriacetate)–agarose resin (Qiagen, Hilden, Germany). The suspension was incubated on ice with agitation for 2 h, and poured into a column which was washed with 10 vol. of buffer containing 50 mM sodium Pi, pH 7, 0.3 M NaCl, and 10% (w/v) glycerol, 10 vol. of the same buffer, but at pH 6, then repeated at pH 5. ASR1 was eluted in the same buffer at pH 4, aliquoted and stored at −70 °C. About 0.5 mg of ASR1 could be purified from 1 litre of bacterial culture.
Immunological assays
Affinity-purified anti-ASR1 antibodies were prepared by adsorption of the previously described anti-ASR1 serum [15] to ASR1 protein blotted on to nitrocellulose membranes, and cut into strips. The membrane strips were washed three times in Tris-buffered saline, and the anti-ASR1 antibodies were eluted in 0.2 M glycine, pH 2.5. The solution was immediately neutralized with Tris base, and glycerol was added to yield 50% (w/v) in the final volume. The affinity-purified IgG were stored at −20 °C. Binding of the antibodies in Western-blot analysis was visualized using peroxidase-conjugated goat anti-rabbit IgG (Amersham Phramacia) or phosphatase-conjugated-protein A (Sigma, St. Louis, MO, U.S.A.) and chemiluminscence [27] or colour [28] assays. Polyclonal rabbit anti-human α-tubulin antibody (sc-5546) was obtained from Santa Cruz Biotechnology, monoclonal mouse anti-human histone H3 antibody (05-499) was obtained from Upstate, and peroxidase-conjugated goat anti-mouse IgG from Promega.
Indirect immunofluorescence
All incubations and washes were performed with 50 mM Pipes buffer, pH 7. Seedling tissues were fixed for 30 min in 4% (w/v) formaldehyde in 50 mM Pipes buffer, pH 7. The fixed leaves were washed and stored overnight at 4 °C. The tissue was washed three times, and incubated for 1 h at 22–25 °C in a cell wall-degrading enzyme mix [1% (w/v) driselase (Sigma), 1% (w/v) cellulysin and 0.1% (w/v) macerozyme (Calbiochem)] in Pipes buffer. The tissues were washed four times, rinsed with de-ionized water, and squashed on to poly(L-lysine)-coated glass slides. Tissue membranes were permeabilized on to the slides by extraction with TBS (Tris-buffered saline; 10 mM Tris/HCl, pH 8/150 mM NaCl) containing 0.1% (w/v) Nonidet P40, and were washed in TBS then incubated overnight at 4 °C with anti-ASR1 antibodies in TBS containing 1% (w/v) BSA and 0.02% (w/v) sodium azide (TBS/BSA buffer). The slides were washed six times in TBS, and incubated for 1 h at 22–25 °C with TBS/BSA buffer containing FITC-labelled goat anti-rabbit IgG (Sigma) and 1 mg/ml DAPI (4,6-diamidino-2-phenylindole). Finally, the slides were washed six times with TBS, mounted and viewed by fluorescence or confocal microscopy.
DNA-binding assays
Purified ASR1 was mixed with the radiolabelled DNA probe in a buffer containing 20 mM Hepes, pH 7.5, 0–25 mM NaCl, 0.8 mM ZnCl2 and 10% (w/v) glycerol. The mixtures were incubated at 22–25 °C for 30 min. Binding was analysed by one of the following three methods.
Mobility shift assay
Glycerol [10% (w/v)] containing Bromophenol Blue and Xylene Cyanol FF were added to the reaction mixtures, which were then loaded on to 5% (w/v) polyacrylamide gels cast and electrophoresed in the same buffer. The gels were dried and exposed to X-ray film.
Trapping of DNA–protein complex on to nitrocellulose
Water-wetted nitrocellulose (0.2 μm; Schleicher and Schuell, Dassel, Germany) was placed into a slot-blot apparatus (Hoeffer). The wells were washed with the same buffer used in each of the binding assays. The reaction mixtures were filtered through the nitrocellulose, and the wells were washed again with the same buffer. The filters were exposed to X-ray film while the radioactivity retained in each well was determined using a beta-counter (Beckman).
South-Western-blot analysis
Purified ASR1, resolved by SDS/PAGE, was electroblotted on to nitrocellulose (0.2 μm; Schleicher and Schuell). The membrane was sequentially washed with deionized water and 20 mM Hepes, pH 7. The membrane was incubated for 30 min at 22–25 °C with binding mixture containing the radiolabelled DNA probe. The filters were then washed three times with 20 mM Hepes, pH 7.5, and exposed to X-ray film. The DNA probes used were the multicloning sequence of pBluescript (227 bp) amplified by PCR using universal forward and reverse primers, and GGAAACAGCTATGACCATGAAGCTTN15GGATCCACTAGTTCTAGA. DNA was radiolabelled by PCR amplification in presence of [α-32P]dCTP.
The preferred ASR1 DNA-binding sequence
The preferred ASR1 DNA-binding site was determined in vitro using SELEX (systematic evolution of ligands by exponential enrichment) [29]. Oligonucleotides used were CGAGGTCGACGGTATCGN15GGATCCACTAGTTCTAGA and GGAAACAGCTATGACCATGAAGCTTN15GGATCCACTAGTTCTAGA. Radiolabelled DNA probes were prepared by PCR using primer pairs CGAGGTCGACGGTATCG and TCTAGAACTAGTGGATCC, and GGAAACAGCTATGACCA and TCTAGAACTAGTGGATCC respectively in a reaction mix containing [α-32P]dCTP. Protein-bound DNA was separated from free DNA by native electrophoresis on polyacrylamide gels, or by binding on Ni-NTA–agarose beads. The DNA sequence was determined after eight cycles of binding. Consensus was determined using DIALIGN 2 [30], followed by minor manual improvement of the results.
RESULTS
Subcellular localization of ASR1
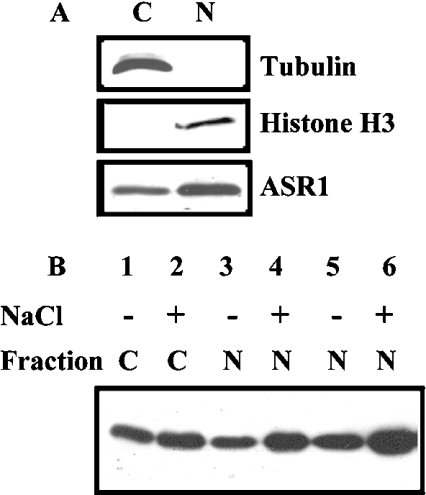
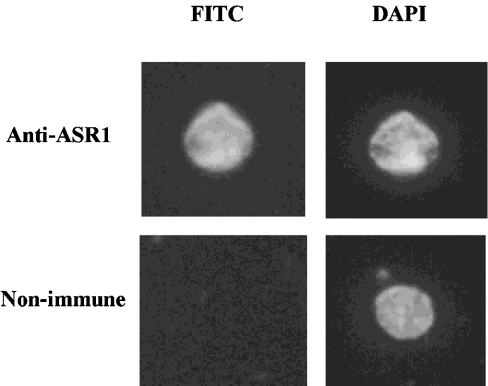
Subcellular fractionation and Western-blot analyses were used to determine the localization of ASR1 protein. ASR1 is present in both the 530 g pellet and 100000 g supernatant, but not in 10000 g (or even 100000 g) pellet(s). The 530 g pellet mainly contains nuclei and chloroplasts. In order to get nuclear fraction without chloroplasts, homogenate was centrifuged over 70% (w/v) Percoll layer (Figure 1A). On the basis of protein content, ASR1 was more enriched in the nucleus than cytoplasm (Figure 1A). ASR1 distribution between nuclear and cytoplasmic fractions was determined in both non-stressed and salt-stressed plants (Figure 1B). Approx. one-third of ASR1 was recovered in the nuclear fraction. Furthermore, plant salt-stress did not appear to affect the subcellular distribution, but rather increased the overall levels of ASR1 protein recovered in both fractions. ASR1 detected in the nuclear fraction was not the result of cytoplasmic contamination, as concluded from immunological detection of tubulin and histone H3, markers for cytoplasm and nucleus compartments respectively (Figure 1A). Nuclear localization of ASR1 was confirmed using indirect immunofluorescence of isolated nuclei (Figure 2). ASR1 staining appeared evenly distributed in the nuclei, as determined by confocal microscopy.
Figure 1. Subcellular distribution of ASR1.
Hydroponically grown tomato plants were transferred to fresh Hoagland's mineral solution, with or without 150 mM NaCl. Plants were harvested after 48 h. Leaves were homogenized and fractionated as described in the Experimental section. (A) Samples containing 15 μg of cytoplasmic (C) or nuclear (N) proteins were analysed by Western blotting using the indicated antibodies. (B) Quantification of subcellular distribution of ASR1. The pellet containing the nuclear fraction was resuspended in isolation buffer in the same volume as the initial homogenate. The presumed cytoplasmic (C) or nuclear (N) fractions (at the following loading equivalents: 1×, lanes 1 and 2; 3×, lanes 3 and 4; 6×, lanes 5 and 6), were resolved by SDS/PAGE, electroblotted, stained and probed with anti-ASR1 serum.
Figure 2. Indirect immunostaining of tomato nuclei.
Isolated nuclei from salt-stressed plants were incubated with affinity purified anti-ASR1 or non-immune serum. Nuclei were counter-stained with DAPI. Magnification, ×1000.
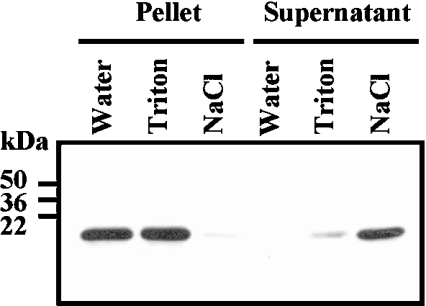
Whether ASR1 is chromatin bound, distributed in the nucleoplasm or accumulated in the nuclear envelope, was determined with isolated tomato nuclei extracted with Triton X-100. As shown in Figure 3, ASR1 was not solubilized by the detergent, but was retained in the chromatin fraction. On the other hand, 1 M NaCl, which was capable of extracting chromatin-bound proteins, also released ASR1, suggesting that ASR1 is chromatin bound.
Figure 3. ASR1 is chromatin bound.
Isolated nuclei were extracted with water, 0.5% (w/v) Triton X-100 or 1 M NaCl, as described in the Experimental section. Equivalent loading of pellet and supernatant acid soluble proteins were resolved by SDS/PAGE, blotted on to nitrocellulose and analysed using anti-ASR1 serum.
ASR1 protein has DNA-binding activity
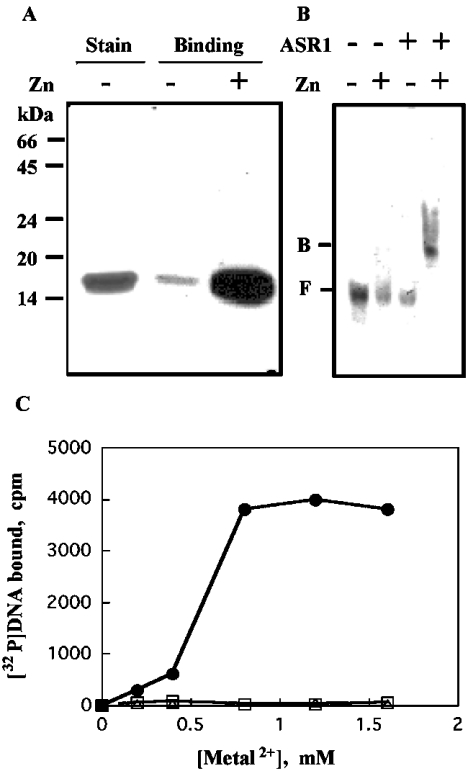
To determine if chromatin-bound ASR1 associates with DNA directly or via interaction with other chromatin-bound protein(s), DNA-binding activity of purified ASR1 was assayed. Full-length ASR1 was expressed in E. coli. The expressed ASR1 was soluble, and could be purified to homogeneity (see the Experimental section). Heparin is widely used for the purification of nucleic acid-binding proteins, whereas Ni-NTA–agarose is used for the purification of His6-tagged recombinant proteins [31]. Since ASR1 has 18 histidine residues, five of which are in one sequence (His6-His10), Ni-NTA–agarose was effective in purifying the native full-length ASR1 protein. Purified ASR1 (Figure 4A, lane 1) was assayed for DNA-binding activity by South-Western (Figure 4A, lanes 2 and 3) and mobility shift (Figure 4B) assays, and by trapping of the protein-bound DNA on to nitrocellulose membrane (Figure 4C). ASR1 bound DNA in a zinc-dependent manner. DNA binding was also dependent on the pH, the nature of the buffer and the ionic strength. Optimal binding activity was observed in Hepes buffer at pH 8. Far less binding was observed in Tris or Tricine buffers, probably due to sequestering of zinc ions by these buffers [32]. Moreover, binding activity was observed at low ionic strengths, but not at NaCl concentrations greater than 75 mM. On this basis, a buffer consisting of 20 mM Hepes, pH 8, 25 mM NaCl and 0.8 mM ZnCl2 was chosen to further assess the binding characteristics of ASR1 to DNA. Zinc could not be replaced by other bivalent cations, such as calcium or magnesium (Figure 4C), although nickel and manganese ions could induce DNA binding at concentrations of 10 mM or greater (results not shown).
Figure 4. ASR1 possesses a zinc-dependent DNA-binding activity.
ASR1 was expressed in E. coli and purified to homogeneity. Purified ASR1 was assayed for DNA-binding using radiolabelled DNA, as described in the Experimental section. (A) For South-Western-blot analysis, membranes containing ASR1 were incubated with the radiolabelled 50-bp DNA probe and exposed to X-ray film (see the Experimental section). Stain, Coomassie Blue staining of purified ASR1. (B) Mobility shift assays: binding mixtures (containing the radiolabelled 50-bp DNA probe) were loaded on to 5% (w/v) polyacrylamide gels exposed to X-ray film (see the Experimental section). F, free; B, bound. (C) For filtration through nitrocellulose, reaction mixtures containing in addition radiolabelled 227-bp pBluescript polylinker DNA probe and various bivalent metal ions were incubated and filtered through 0.22 μm nitrocellulose membranes using a slot-blot manifold (see the Experimental section). Membrane-retained radioactivity was determined for ZnCl2 (•), MgCl2 (▵) and CaCl2 (□).
ASR1 is a sequence-specific DNA-binding protein
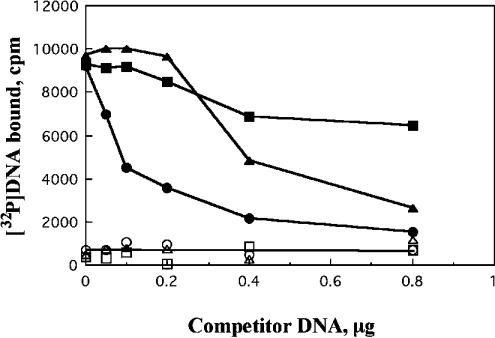
We found that ASR1 has binding sequence preferences on DNA. DNA-binding competition studies were performed, using non-labelled DNA preparations as the competitor DNA. Preferential DNA competition efficiency was pBluescript>poly(dI-dC)·poly(dI-dC)>bacteriophage λ DNA (Figure 5).
Figure 5. ASR1 binds selective DNA sequences.
Mixtures containing radiolabelled DNA, ASR1, ZnCl2 and the indicated amounts of non-labelled DNA were incubated then filtered through nitrocellulose membranes. Membrane-retained radioactivity was determined as described in Figure 4(C). ▪ and □, bacteriphage λ DNA; ▴ and ▵, poly(dI-dC)·poly(dI-dC); • and ○, pBluescript. Open symbols, controls without ASR1; closed symbols, with ASR1.
The difference in the competing potency for DNA binding of the DNA molecule used suggested that ASR1 is a sequence-specific DNA-binding protein. The SELEX procedure was used to determine the preferred DNA-binding site of ASR1 in vitro. This method is used for the in vitro determination of preferred DNA sequences of DNA-binding proteins [31]. For the first SELEX assay we used the 50 bp oligonucleotide mixture CGAGGTCGACGGTATCGN15GGATCCACTAGTTCTAGA as the starting population. Consensus was determined using DIALIGN 2 [30], followed by minor manual improvement of the results. The selected sequences contained a stretch of cytosines (2–6) next to the right border of the random sequence (Figure 6A). This result was unexpected, since the preferred sequence is expected to be at a random location within the variable sequence. These results suggested that the right border served as a nucleation centre, and that the binding site comprises the selected nucleotides, as well as the 5′ of the left border (Figure 6A, underlined). A second oligonucleotide mixture bait, GGAAACAGCTATGACCATGAAGCTTN15GGATCCACTAGTTCTAGA was prepared. This oligonucleotide contained a different left linker which does not act as nucleation centre. The consensus binding sequence C2–3(C/G)A was determined in the second SELEX assay (Figure 6B). The selected sequences in both assays are in agreement with each other.
Figure 6. SELEX analysis for ASR1 DNA binding.
Eight binding cycles of SELEX were performed with purified ASR1 and the double-stranded oligonucleotide CGAGGTCGACGGTATCGN15GGATCCACTAGTTCTAGA (A) or GGAAACAGCTATGACCATGAAGCTTN15GGATCCACTAGTTCTAGA (B). Nucleotide sequences of individual inserts which were subcloned into plasmid are shown. The 5′ of the left border sequence is underlined. Consensus was determined using DIALIGN 2 [30], with minor manual adjustments. The core of the consensus sequence is indicated in bold.
DISCUSSION
Several signal transduction pathways have been implicated in the activation of salt- and water-stress-induced genes [3,7,8,33]. Cellular levels of both ASR1, a small (13 kDa) highly-charged protein, as well as its mRNA, are transiently increased following exposure to salt and water stresses [15]. The amino acid composition of ASR1 is unusual; 62 of the 115 residues (54%) are charged (23 acidic, 21 basic and 18 histidine residues). The protein has been also shown to be regulated by an abscisic acid-dependent pathway [15]. ASR1 homologues have only been found in the plant kingdom, and thus its biological role defies suggestion merely on the basis of sequence homology comparisons with nonplant proteins. Moreover, ASR1 does not seem to contain any protein signatures that might suggest a biological function.
Subcellular localization of ASR1 indicated that approx. one-third is nuclear, with the majority dispersed in the cytoplasm (Figure 1). Many nuclear proteins are also found in the cytoplasm [34]. Translocation of proteins between the two compartments might be used to regulate the activity of such proteins, or one protein might display a distinct role in each compartment [35]. Multiple nucleoplasmic transport pathways have been suggested [34]. Molecules with masses up to 40–60 kDa have been shown capable of diffusing passively through nuclear pores along their concentration gradient, whereas others are actively transported [34] by utilizing so-called nuclear localization signals (short amino acid sequences rich in basic residues) that are recognized by receptor molecules which dock with their cargo at the nuclear pore [34,36]. Although ASR1 can easily pass through the nuclear pore due to its small size, it contains the putative nuclear localization signal sequence KKDAKKEEKKKLR (residue in bold type are suggested to be functioning as the signal) close to its C-terminus (residues 92–105).
Induction of plant nuclear proteins by abiotic stresses has also been described [37–42]. Some of these abiotic stress proteins have also been found in both nuclear and cytosolic fractions [35,39,42].
ASR1 is a chromatin-bound protein (Figure 3), suggesting that it is associated either directly with DNA or indirectly via protein–protein interaction with other chromatin-bound protein(s). We found that ASR1 has a zinc-dependent DNA-binding activity (Figure 4) which is distinguishable from non-specific ion-exchange-like interactions. The calculated iso-electric point of ASR1 is 7.3, since optimal zinc-dependent DNA-binding activity occurred at pH 7.5, but not at lower pH values where the protein acquires an excessive positive charge. It is noteworthy that general ion-exchange type of binding would not be expected to demonstrate a particular metal ion or buffer specificity.
The ability of non-labelled DNA to compete for binding with a labelled DNA probe was seen to vary between different DNA molecules (Figure 5), implying that ASR1 has a preferential binding sequence on DNA. The results of the SELEX binding analysis (Figure 6) also support the suggestion that ASR1 is more selective towards the sequence C2–3(C/G)A.
There are five histidine residues close to the N-terminus of ASR1. Such a linear stretch of histidine residues is known to display high affinity for metal ions such as nickel or zinc [43], allowing for the common usage of Ni-NTA–agarose columns to purify these metal-containing proteins on the basis of them also carrying histidine clusters [44]. Native ASR1 has high affinity towards metals and is effectively purified to homogeneity on Ni-NTA–agarose (Figure 4A). Although we observed that zinc binding to ASR1 enhances its DNA-binding activity (Figure 4). The zinc finger, a common motif in DNA-binding proteins [45], is not found in ASR1. Sequence analysis of ASR1 show that ASR1 does not contain any other known zinc-binding motifs described previously [46]. Thus the locus and stoichiometry of zinc ligands on ASR1 remains to be discovered, together with the protein's potential role as a chromatin protective protein or in signal pathway in plants under variously induced stressful environments.
Acknowledgments
This research was supported by a research grant from The Israel Science Foundation awarded to D.B.-Z. We thank Dr Peter S. Coleman for critical reading of the manuscript.
References
- 1.Bray E. A. Plant responses to water deficit. Trends Plant Sci. 1997;2:48–54. [Google Scholar]
- 2.Hasegawa P. M., Bressan R. A., Zhu J. K., Bonhert H. J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 3.Shinozaki K., Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 2000;3:217–223. [PubMed] [Google Scholar]
- 4.Ingram J., Bartels D. The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:377–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- 5.Aubourg S., Rouze P. Genome annotation. Plant Physiol. Biochem. 2001;39:181–193. [Google Scholar]
- 6.Bohnert H. J., Ayoubi P., Borchert C., Bressan R. A., Burnap R. L., Cushman J. C., Cushman M. A., Deyholos M., Fischer R., Galbraith D. W., et al. A genomics approach towards salt stress tolerance. Plant Physiol. Biochem. 2001;39:295–311. [Google Scholar]
- 7.Rock C. D. Pathways to abscisic acid-regulated gene expression. New Phytol. 2000;148:357–396. doi: 10.1046/j.1469-8137.2000.00769.x. [DOI] [PubMed] [Google Scholar]
- 8.Xiong L., Zhu J. K. Abiotic stress signal transduction in plants: Molecular and genetic perspectives. Physiol. Plantarum. 2001;112:152–166. doi: 10.1034/j.1399-3054.2001.1120202.x. [DOI] [PubMed] [Google Scholar]
- 9.Blumwald E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000;12:431–434. doi: 10.1016/s0955-0674(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 10.Johansson I., Karlsson M., Johanson U., Larsson C., Kjellbom P. The role of aquaporins in cellular and whole plant water balance, Biochim. Biophys. Acta. 2000;1465:324–342. doi: 10.1016/s0005-2736(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 11.Bohnert H. J., Sheveleva E. Plant stress adaptations – making metabolism move. Curr. Opin. Plant Biol. 1998;1:267–274. doi: 10.1016/s1369-5266(98)80115-5. [DOI] [PubMed] [Google Scholar]
- 12.Hare P. D., Cress W. A., Van Staden J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998;21:535–553. [Google Scholar]
- 13.Bohnert H. J., Shen B. Transformation and compatible solutes. Sci. Hortic. 1999;78:237–260. [Google Scholar]
- 14.Iusem N. D., Bartholomew D. M., Hitz W. D., Scolnik P. A. Tomato (Lycopersicon esculantumI) transcript induced by water deficit and ripening. Plant Physiol. 1993;102:1353–1354. doi: 10.1104/pp.102.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amitai-Zeigerson H., Scolnik P. A., Bar-Zvi D. Tomato Asr1 mRNA and protein are transiently expressed following salt stress, osmotic stress and treatment with abscisic acid. Plant Sci. 1995;110:205–213. [Google Scholar]
- 16.Amitai-Zeigerson H., Scolnik P. A., Bar-Zvi D. Genomic nucleotide sequence of tomato Asr2, a second member of the stress/ripening-induced Asr1 gene family. Plant Physiol. 1994;106:1699–1700. doi: 10.1104/pp.106.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi M., Lijavetzky D., Bernacchi D., Hopp H. E., Iusem N. Asr genes belong to a gene family comprising at least three closely linked loci on chromosome 4 in tomato. Mol. Gen. Genet. 1996;252:489–492. doi: 10.1007/BF02173015. [DOI] [PubMed] [Google Scholar]
- 18.Padmanabhan V., Dias D. M. A. L., Newton R. J. Expression analysis of a gene family in loblolly pine (Pinus taeda L.) induced by water deficit stress. Plant Mol. Biol. 1997;35:801–807. doi: 10.1023/a:1005897921567. [DOI] [PubMed] [Google Scholar]
- 19.Wang C. S., Liau Y. E., Huang J. C., Wu T. D., Su C. C., Lin C. H. Characterization of a desiccation-related protein in lily pollen during development and stress. Plant Cell Physiol. 1998;39:1307–1314. doi: 10.1093/oxfordjournals.pcp.a029335. [DOI] [PubMed] [Google Scholar]
- 20.Vaidyanathan R., Kuruvilla S., Thomas G. Characterization and expression pattern of an abscisic acid and osmotic stress responsive gene from rice. Plant Sci. 1999;140:21–30. [Google Scholar]
- 21.Silhavy D., Hutvagner G., Barta E., Banfalvi Z. Isolation and characterization of a water-stress inducible cDNA clone from Solanum chacoense. Plant Mol. Biol. 1995;27:587–595. doi: 10.1007/BF00019324. [DOI] [PubMed] [Google Scholar]
- 22.Canel C., Bailey-Serres J. N., Roose M. L. Pummelo fruit transcript homologous to ripening-induced gene. Plant Physiol. 1995;108:1323–1324. doi: 10.1104/pp.108.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mbeguie A., Mbeguie D., Gomez R. M., Fils-Lycaon B. Molecular cloning and nucleotide sequence of an abscisic acid-, stress-, ripening-induced ASR-like protein from apricot fruit. Gene expression during fruit ripening. Plant Physiol. 1997;115:1288. [Google Scholar]
- 24.Tavladoraki P., Giuliano G. Isolation of nuclei from plant tissues. In: Negrutiu I., Gharti-Chherti G., editors. A Laboratory Guide for Cellular and Molecular Plant Biology. Basel: Birkhauser Verlag; 1991. pp. 181–183. [Google Scholar]
- 25.Leammli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using high-molarity Tris buffer system without urea. Anal. Biochem. 1986;155:83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 27.Schneppenheim R., Budde U., Dahlmann N., Rautenberg P. Luminography – a new highly sensitive visualization method for electrophoresis. Electrophoresis. 1991;12:367–372. doi: 10.1002/elps.1150120508. [DOI] [PubMed] [Google Scholar]
- 28.Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal. Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 29.Mauhin V., Lutz Y., Dennefeld C., Alberga A. Definition of the DNA-binding site repertoire for the Drosophila transcription factor SNAIL. Nucleic Acids Res. 1993;21:3951–3957. doi: 10.1093/nar/21.17.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgenstern B. DIALIGN 2: improvement of the segment-to-segment approach to multiple sequence alignment. Bioinformatics. 1999;15:211–218. doi: 10.1093/bioinformatics/15.3.211. [DOI] [PubMed] [Google Scholar]
- 31.Labrou N., Clonis Y. D. The affinity technology in downstream processing. J. Biotechnol. 1994;36:95–119. doi: 10.1016/0168-1656(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 32.Dawson R. M. C., Elliott D. C., Elliott W. H., Jones K. M. 3rd edition. Oxford: Oxford University Press; 1986. Data for Biochemical Research. [Google Scholar]
- 33.Neill S. J., Burnett E. C. Regulation of gene expression during water deficit stress. Plant Growth Regul. 1999;29:23–33. [Google Scholar]
- 34.Yoneda Y. Nucleoplasmic protein traffic and its significance to cell function. Genes Cells. 2000;5:777–787. doi: 10.1046/j.1365-2443.2000.00366.x. [DOI] [PubMed] [Google Scholar]
- 35.Houde M., Daniel C., Lachapelle M., Allard F., Laliberte S., Sarhan F. Immunolocalization of freezing-tolerance-associated proteins in the cytoplasm and nucleoplasm of wheat crown tissues. Plant J. 1995;8:583–593. doi: 10.1046/j.1365-313x.1995.8040583.x. [DOI] [PubMed] [Google Scholar]
- 36.Jans D. A., Xiao C.-Y., Lam E. H. C. BioEssays. 2000;22:532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 37.Ascenzi R., Gantt J. S. Molecular genetic analysis of the drought-inducible linker histone variant in Arabidopsis thaliana. Plant Mol. Biol. 1999;41:159–169. doi: 10.1023/a:1006302330879. [DOI] [PubMed] [Google Scholar]
- 38.Castillo J., Rodrigo I., Marquez J. A., Zuniga A., Franco L. A pea nuclear protein that is induced by dehydration belongs to the vicilin superfamily. Eur. J. Biochem. 2000;267:2156–2165. doi: 10.1046/j.1432-1327.2000.01229.x. [DOI] [PubMed] [Google Scholar]
- 39.Godoy J. A., Lunar R., Torres-Schumann S., Moreno J., Rodrigo R. M., Pintor-Toro J. A. Expression, tissue distribution and subcellular localization of dehydrin TAS14 in salt-stresses tomato plants. Plant Mol. Biol. 1994;26:1921–1934. doi: 10.1007/BF00019503. [DOI] [PubMed] [Google Scholar]
- 40.Kovacs I., Ayaydin F., Oberschall A., Ipacs I., Bottka S., Pongor S., Dudits D., Toth E. C. Immunolocalization of a novel annexin-like protein encoded by a stress and abscisic acid responsive gene in alfalfa. Plant J. 1998;15:185–197. doi: 10.1046/j.1365-313x.1998.00194.x. [DOI] [PubMed] [Google Scholar]
- 41.Munnik T., Ligterink W., Meskiene I., Calderini O., Beyerly J., Musgrave A., Hirt H. Distinct osmo-sensing protein kinase pathways are involved in signalling moderate and severe hyper-osmotic stress. Plant J. 1999;20:381–388. doi: 10.1046/j.1365-313x.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 42.Scippa G. S., Griffith A., Chiatante D., Bray E. A. The H1 histone variant of tomato, H1-S, is targeted to the nucleus and accumulates in chromatin in response to water-deficit stress. Planta. 2000;211:173–181. doi: 10.1007/s004250000278. [DOI] [PubMed] [Google Scholar]
- 43.Christianson D. W. Structural biology of zinc. Adv. Protein Chem. 1991;42:281–355. doi: 10.1016/s0065-3233(08)60538-0. [DOI] [PubMed] [Google Scholar]
- 44.Crowe J., Dobeli H., Gentz R., Hochuli E., Stuber D., Henco K. 6×His-Ni-NTA chromatography as a superior technique in recombinant protein expression/purification. Methods Mol. Biol. 1994;31:371–387. doi: 10.1385/0-89603-258-2:371. [DOI] [PubMed] [Google Scholar]
- 45.Laity J. H., Lee B. M., Wright P. E. Zinc finger proteins: new insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001;11:39–46. doi: 10.1016/s0959-440x(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 46.Vallee B. L., Auld D. S. Functional zinc-binding motifs in enzymes and DNA-binding proteins. Faraday Discuss. 1992;93:47–65. doi: 10.1039/fd9929300047. [DOI] [PubMed] [Google Scholar]