Abstract
Oral and maxillofacial surgery is a specialized surgical field devoted to diagnosing and managing conditions affecting the oral cavity, jaws, face and related structures. In recent years, the integration of 3D printing technology has revolutionized this field, offering a range of innovative surgical devices such as patient-specific implants, surgical guides, splints, bone models and regenerative scaffolds. In this comprehensive review, we primarily focus on examining the utility of 3D-printed surgical devices in the context of oral and maxillofacial surgery and evaluating their efficiency. Initially, we provide an insightful overview of commonly utilized 3D-printed surgical devices, discussing their innovations and clinical applications. Recognizing the pivotal role of materials, we give consideration to suitable biomaterials and printing technology of each device, while also introducing the emerging fields of regenerative scaffolds and bioprinting. Furthermore, we delve into the transformative impact of 3D-printed surgical devices within specific subdivisions of oral and maxillofacial surgery, placing particular emphasis on their rejuvenating effects in bone reconstruction, orthognathic surgery, temporomandibular joint treatment and other applications. Additionally, we elucidate how the integration of 3D printing technology has reshaped clinical workflows and influenced treatment outcomes in oral and maxillofacial surgery, providing updates on advancements in ensuring accuracy and cost-effectiveness in 3D printing-based procedures.
Keywords: 3D printing, oral and maxillofacial surgery, patient-specific implants, surgical guides, splints
Graphical Abstract
Introduction
Oral and maxillofacial surgery is a speciality devoted to the diagnosis and management of conditions affecting the oral cavity, jaws, face and related structures. While this field offers considerable advancements and benefits, it presents several challenges that surgeons must navigate, including notable anatomical diversity among individuals, surgical complexity and technological limitations [1, 2]. 3D printing, also known as additive manufacturing, presents as an innovative technology that enables the production of three-dimensional objects from digital designs [3]. Departing from conventional manufacturing methods, which often involve subtractive processes such as cutting or drilling materials [4], 3D printing constructs objects layer by layer. This approach allows for the fabrication of intricate and complex geometries that would be difficult or unfeasible to achieve using traditional methods [5, 6].
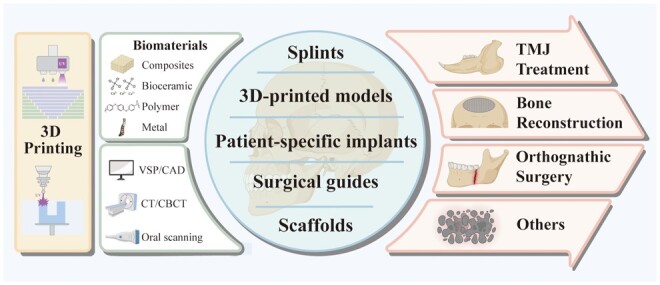
A pivotal advantage of 3D printing lies in its ability to create intricate structures and customized items with exceptional precision [7, 8]. This capability empowers designers and surgeons with unprecedented flexibility to optimize designs and create patient-specific implants (PSIs) and complementary surgical tools tailored to possess high functionality [9, 10]. Consequently, the applications of 3D-printed PSIs, surgical guides, splints regenerative 3D models and regenerative scaffolds have extended across diverse domains, including bone reconstruction in oral and maxillofacial regions (OMFs), orthognathic surgery and temporomandibular joint (TMJ) treatment and other applications (Figure 1). By conducting digital design on patient-derived anatomical data, obtained via techniques such as computed tomography (CT) scans or intraoral scanning, surgeons can develop implants, surgical guides and other implements precisely aligned with the patient’s anatomy. This precision ensures the accuracy of surgical interventions, improves patient outcomes and reduces complication rates [11]. Moreover, by generating 3D-printed replicas of the patient’s anatomical structure, surgeons gain better insights into pathological anatomy and can simulate surgical plans, enabling preoperative analysis, assessment of diverse treatment methodologies and anticipation of potential challenges or complications. This preoperative preparation enhances efficiency and efficacy in surgical planning, ultimately leading to improved patient care [12, 13].
Figure 1.
Schematic of 3D printing and 3D-printed surgical devices in oral and maxillofacial surgery. This figure was created using BioRender (https://biorender.com/).
Additionally, 3D printing demonstrates the potential to optimize production procedures by eliminating the need for manual fabrication or adjustments of implants and surgical guides. This increased efficiency translates to shorter timeframes, shorter surgical waiting times and augmented overall operational efficiency within the operating room [14]. As a result, 3D printing has become an indispensable tool for innovation and problem-solving across a wide range of challenges encountered in oral and maxillofacial surgery. Therefore, this review primarily focuses on examining the utility of 3D-printed surgical devices in the context of oral and maxillofacial surgery and evaluating their efficiency. Initially, we provide an overview of commonly used 3D-printed surgical devices, discussing their innovation, clinical applications and the materials and technologies involved. Additionally, we delve into the rejuvenating impact of 3D-printed surgical devices within various oral and maxillofacial surgery subdivisions, with particular emphasis on bone reconstruction, orthognathic surgery and TMJ treatment. We discuss how 3D printing has reshaped clinical workflow and treatment outcomes in oral and maxillofacial surgery while updating the progress in enhancing accuracy in 3D printing-based procedures.
Common surgical devices and suited materials established for oral and maxillofacial surgery
We created a table that provides detailed information on commonly utilized surgical devices, including data sources, software, materials, printers, applications and key features (see Table 1). Furthermore, we developed a timeline of applications of 3D printing and 3D-printed surgical devices in oral and maxillofacial surgery to visually illustrate significant milestones and innovations within the scope of our review (Figure 2). Additionally, we conducted database searches and generated statistical graphics to analyze the patent status and paper publications related to 3D-printed surgical devices in oral and maxillofacial surgery over the past 15 years (Figure 3). See methodology in Supplementary data.
Table 1.
Commonly used surgical devices in oral and maxillofacial surgery with detailed information
| 3D-printed devices | Data source | Materials | Design software | 3D printer | Printing technology | Applications | Key features | Ref |
|---|---|---|---|---|---|---|---|---|
| Surgical guides | CBCT & intraoral scan | Ti6Al4V ELI | Magics | Metalsys15 | SLM | Orthognathic surgery: Le Fort 1 osteotomy and BSSO |
|
[15] |
| CT | Polyamide | 3-Matic and Magics | NM | NM | Mandible and donor osteotomy guides |
|
[11] | |
| CT | Resin | 3-Matic | Objet Eden260VS | PolyJet | Cutting and repositioning templates and final splint for maxilla repositioning in bimaxillary orthognathic surgery | Accuracy: the mean linear distance between the planned and actual postoperative position is 1.17 ± 0.66 mm. | [16] | |
| CT | MED610 resin or NextDent SG | 3-Matic 13.0 | NM | NM | Patient-specific fibula malleolus cap for harvesting |
|
[17] | |
| Patient-specific implant | CT/MRI | PEEK | Mimics | Intamsys FUNMAT HT | FFF | Reconstruction of zygomatic deformities |
|
[18] |
| CBCT | Glass-ceramic (BGS-7) | 3-Matic | NM | NM | Reconstruction of zygomatic bone defects |
|
[19] | |
| CT | PC-ISO | Mimics | Stratasys FDM®Fortus 900mc | FDM | Orbital fractures restoration |
|
[20] | |
| CT | Titanium MG1 | CAD | AM250 3D metal printer | Laser fusion | Custom-made bone-anchored titanium prosthesis for reconstruction after total bilateral maxillectomy |
|
[21] | |
| NM | PCL/β-TCP mixture | 3-Matic | Multi-head deposition system | NM | PCL/β-TCP scaffolds for mandibular reconstruction |
|
[22] | |
| Splints | Cast scanning | Formlabs Clear Resin | Dolphin | Form 2 | SLA | Single-jaw orthognathic surgery |
|
[23] |
| CT and cast scanning | Resin | Geomagic Freeform | ProJet 7000 | SLA | Orthognathic surgery |
|
[24] | |
| Resin models scanning | Resin | Geomagic Studio 2013 | NOVA3D Bene3 | SLA | Orthognathic surgery |
|
[25, 26] |
NM, not mentioned.
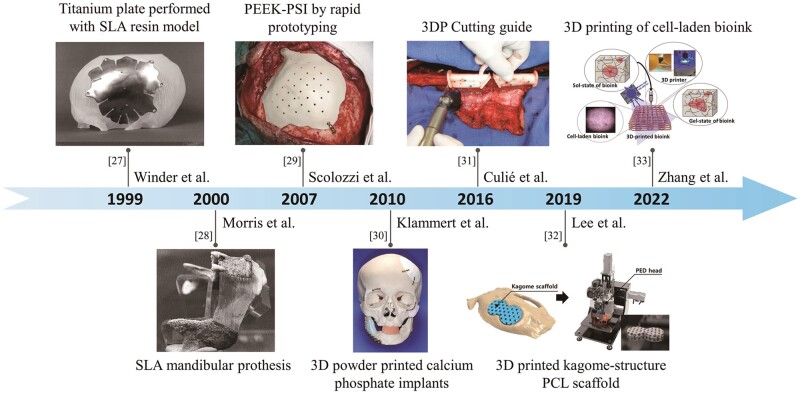
Figure 2.
Timeline of applications of 3D printing and 3D printed surgical devices in oral and maxillofacial surgery. Custom titanium plate performed with stereolithography resin model for cranioplasty in 1999 [27] (from Ref. [27] licensed under Taylor & Francis license). Stereolithography appearance (SLA) mandibular prosthesis fitted within the mandibular defect site in 2000 [28] (from Ref. [28] licensed under John Wiley & Sons license). Polyetheretherketone (PEEK)-PSI by rapid prototyping for orbito-fronto-temporal reconstruction in 2007 [29] (from Ref. [29] licensed under Wolters Kluwer license). 3D powder printed calcium phosphate (CP) implants for craniofacial defects reconstruction in 2010 [30] (from Ref. [30] licensed under Elsevier license). 3DP cutting guide for fibular free-flap harvesting in 2016 [31] (from Ref. [31] licensed under Elsevier license). 3D-printed kagome-structure scaffold for bone regeneration in 2019 [32] (from Ref. [32] licensed under Elsevier license). 3D-printed cell-laden light-curable chitosan scaffold for bone tissue regeneration in 2022 [33] (from Ref. [33] licensed under Elsevier license).
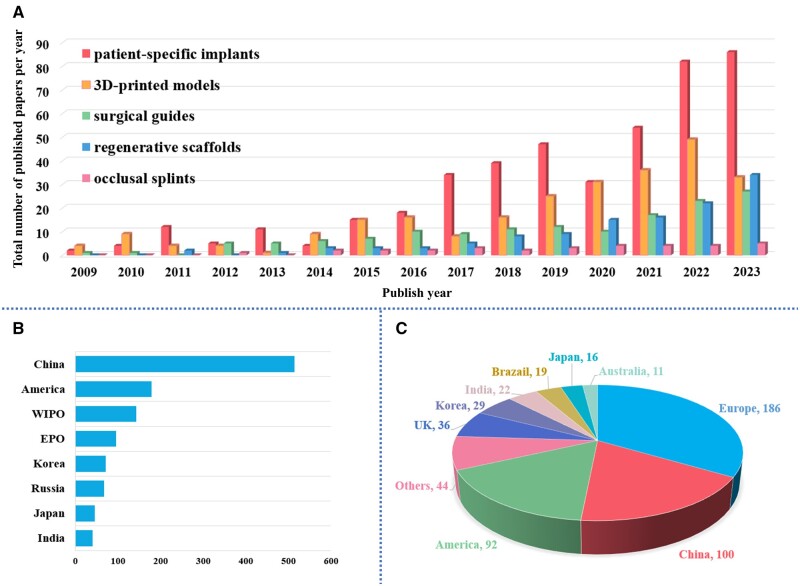
Figure 3.
Statistic graphics of papers and patent status of 3D-printed surgical devices in oral and maxillofacial surgery in recent 15 years. (A) Publication status of papers on 3D-printed surgical devices in oral and maxillofacial surgery in the past 15 years. (B) The patent application status in each area of the world. (C) The total number of Investigator-Initiated Clinical Trial/Research (IIT/IIR) and Industry-Sponsored Clinical Trial (IST) in each area of the world.
Patient-specific cutting and positioning guides
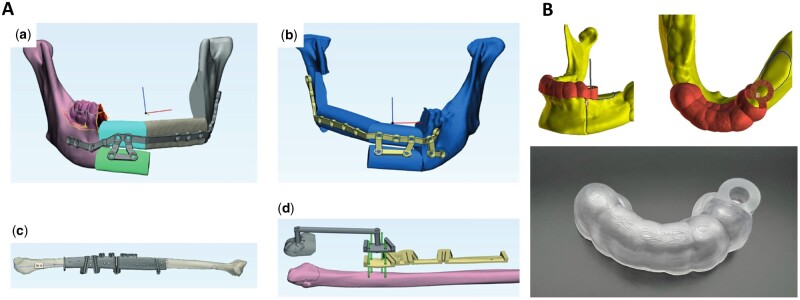
Throughout the excision of damaged areas, the integration of customized cutting guides can significantly assist in precisely determining the location and orientation of osteotomies, while repositioning guides can establish the optimal placement of bone grafts for prosthesis rehabilitation [11, 34]. These guides have demonstrated efficacy in enhancing accuracy, reducing operative time, facilitating complex reconstructions and aiding intraoperative decision-making in various procedures such as orthognathic osteotomies [15, 16], necrotic/tumorous bone resection [11], donor bone harvesting [11, 17, 35], bony segments repositioning [36], implants fitting [37] and bone biopsies [38]. For instance, Pu et al. [17] introduced a novel fibula malleolus cap for fibula flap harvesting to address challenges encountered in reducing the extent of deviations in terms of locations and angles of distal fibula osteotomies (Figure 4A).
Figure 4.
3D Printed surgical guides for precise cutting and positioning. (A) Conventional method vs. malleolus cap method for fibula flap harvest: (a) virtual planning in the control group; (b) virtual planning in the study group; (c) single fibula harvest guide in the control group; (d) two combinational guides with segmentation guide, fibula cap, and screw holes in the study group [17] (from Ref. [17] licensed under Creative Commons Attribution 4.0 license). (B) A virtual and 3D-printed surgical guide [39] (from Ref. [39] licensed under Elsevier license).
Splints
During a two-jaw surgery, intermediate splints (ISs) are used to align the maxilla with the mandible, followed by final splints (FSs) to relocate the mandible to acquire the final occlusion. Conventional plaster model-based surgery is associated with inherent inaccuracies originating from steps such as impression-taking, model pouring, trimming, mounting, face-bow transfer and manual splint fabrication. In contrast, virtual surgical planning and 3D printing have offered a more efficient workflow with digital splints, theoretically offering greater accuracy by eliminating these sources of deviation [40]. Nevertheless, printed splints might not always fit well within dentitions due to potential systematic errors that can occur during digital scanning and 3D printing procedures. Two studies innovatively proposed to fabricate splints from positively offset dental models, particularly focusing on interproximal areas that may not be clearly captured by dentition scanning, which could enhance the fit of splints [25, 41]. According to Wang et al. [26], it is recommended that both 2 or 3-mm occlusal coverage depth (OCD) for ISs and FSs can be considered due to their precision relative to clinical acceptability. After carefully conducted design of digital splints, resin materials are 3D-printed using commercial printers, such as ProJet 7000 [24], Objet Eden260VS [16], NOVA3D Bene3 [25], D20II [42] and others, utilizing stereolithography (SLA) or digital light processing (DLP) technology.
Patient-specific implants
Standard-sized implants and autogenous bone grafts are considered as the gold standard and employed in conventional treatments to restore bone defects. However, these methods necessitate customization to fit the shape of the defects, which can be labor-intensive and time-consuming [43]. This challenge is particularly pronounced in oral and maxillofacial regions, where complex anatomical structures like the orbital floor pose difficulties in obtaining an exact 3D shape. Even minor discrepancies between the implant and the bone defect can lead to implant instability or failure. To address these challenges, 3D printing has emerged as a promising alternative for fabricating customized implants that precisely match the original structures in a shorter timeframe [44].
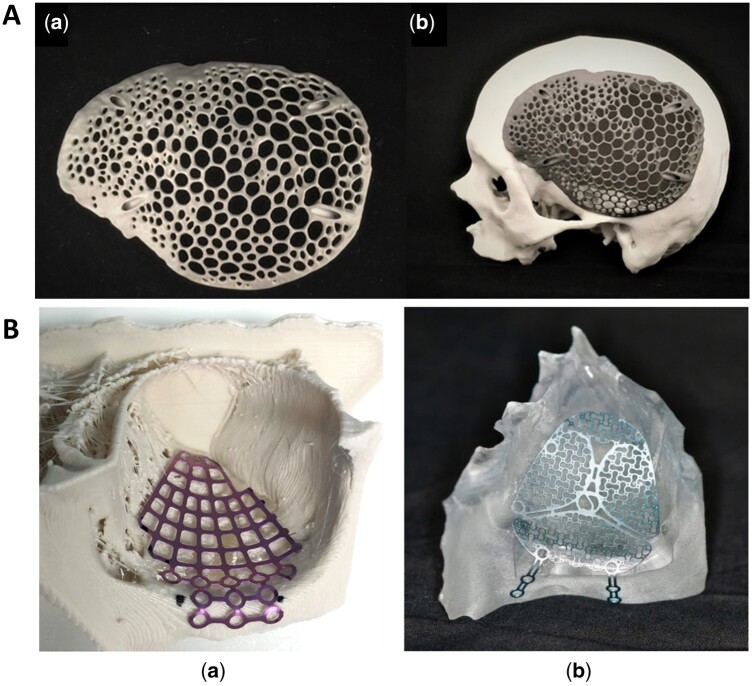
Notably, 3D-printed titanium implants have garnered significant attention, particularly for reconstructing large-sized load-bearing areas. Titanium implants offer exceptional high strength-to-weight ratio, rigidness, biocompatibility, anti-infection, corrosion resistance, and nonmagnetic properties [36, 45]. However, they are often costly and heavier than the original anatomy, leading to subsidence resulting from stress shielding effects and disparities in elasticity modulus. [43]. To address these issues, researchers have explored the use of biocompatible polymer materials with reduced weight [20, 46]. Others have focused on optimizing the internal configuration of titanium implants using 3D printing methods such as selective laser melting (SLM) [47] and electron beam melting (EBM) [48]. These methods allow for the design of desired internal configurations, maintaining porosity and pore continuity to reduce implant weight and enhance osteointegration. Li et al. [49] designed Ti–6Al–4V plate with a honeycomb structure via SLM. After 6 months, the bone tissue has integrated with and enveloped the honeycomb scaffold, indicating significate now bone formation and effective integration with the scaffold and showing its potential clinical application. Furthermore, 3D printing enables the fabrication of biomimetic implants with complex internal structures resembling natural bones. Sharma et al. [47] designed and printed a biomimetic customized titanium cranial implant for cranioplasty, incorporating an interconnected strut macrostructure that mimics bone trabeculae, utilizing the voronoi diagram as a basis for its construction. This voronoi design, weighing only 30 g, needed less material and fabrication time while exhibiting excellent protective strength (Figure 5A). Additionally, surface modification techniques have been explored to enhance implant-bone fusion [48, 50]. Major et al. [51] proposed PSI with a metallic core (TiAlNb7 alloy) and a bioactive coating (Polylactide (PLA) granulate with β-tricalcium phosphate (β-TCP) addition) to stimulate bone growth.
Figure 5.
3D-printed PSI for defective bone reconstruction. (A) (a) Voronoi pattern cranial implant by SLM; (b) a skull biomodel demonstrating accurate fit (from Ref. [47] licensed under Creative Commons Attribution 4.0 license). (B) Orbital pre-bent plates made from a 3D printing anatomical model: (a) matrix MIDFACE mesh plate on a polylactic acid (PLA) model; (b) medartis modus midface OPS 1.5 plate on a clear MED610 model (from Ref. [52] licensed under Creative Commons Attribution 4.0 license).
To address these challenges, biodegradable/bioabsorbable polymer biomaterials, such as polycaprolactone (PCL), polylactic acid (PLA), polyetheretherketone (PEEK), polyvinyl chloride (PVC) and others have gained popularity due to their rigidity, lightweight nature and biocompatibility [52–55]. Polyaryletherketone polymers, such as PEEK, are particularly suitable for use as bone substitutes due to their high strength, superior chemical resistance, light weight, radiolucency, reduction of CT artifacts and similar elastic modulus to human bones [53, 56–58]. Bioceramics, such as β-TCP or hydroxyapatite (HA), have been confirmed to promote osteoblastic differentiation of human mesenchymal stem cells, promoting osteogenesis and improving bone-implant contact ratio [52, 59, 60]. Bioceramics are frequently employed as bone void fillers in reconstructive surgery. However, their mechanical brittleness and the inability to directly fabricate implants limit their application as personalized implants [61]. However, integrating bioceramics into biodegradable polymeric matrices to develop composite bone-promoting implants enhances their bioactivity and may endow osteoinductivity to the composite biomaterials [22, 62–65]. Guillaume et al. [2] proposed a novel HA-filled poly(trimethylene carbonate) (PTMC) implant for orbital floor repair, designed to provide temporary support to the orbital content and degrade over time while stimulating neo-bone formation. Additionally, Lee et al. [19] conducted a clinical trial to assess the efficacy and stability of the 3D-printed CaOSiO2-P2O5-B2O3 glass-ceramics (BGS-7) implants. Cone beam computed tomography (CBCT) analysis obtained after 6 months revealed 100% bone fusion and an average fusion rate of 76.97%. No osteolysis was observed around implants, and the mean displacement distance was 0.4149 mm for all 10 implants. The satisfaction score on the visual analog scale was 9, and no adverse events were observed in any of the cases.
3D model
Oral and maxillofacial surgeries present unique challenges including delicate anatomical structures and limited operative field exposure. Surgical simulation is thus becoming essential in medical training, offering a controlled setting for refining surgical skills [66, 67]. While cadaveric bone has conventionally been established as the gold standard for simulation, its limited availability and high costs pose significant obstacles to widespread adoption [1, 68]. Consequently, 3D-printed bone models have emerged as a cost-effective and readily accessible alternative, providing highly realistic training resources [69].
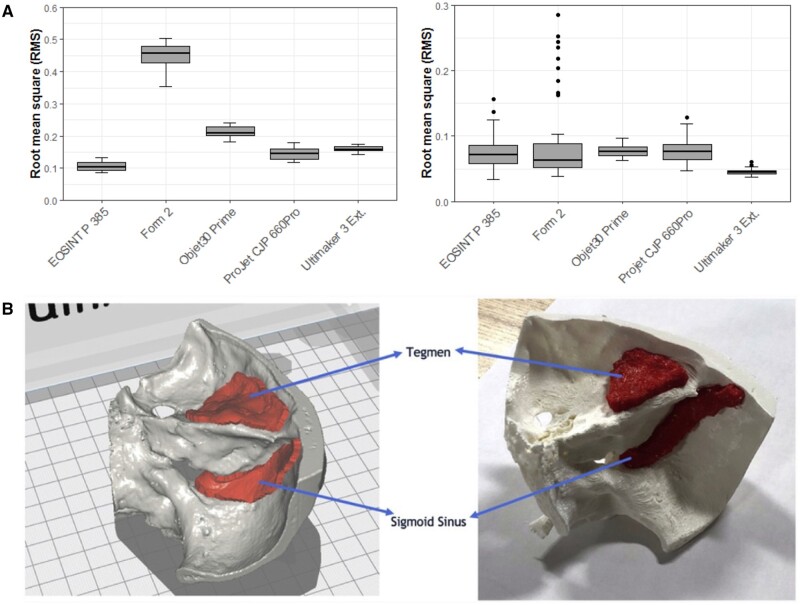
Achieving satisfying accuracy in 3D-printed bone models is of paramount importance and involves meticulous attention to various factors throughout the manufacturing process [70, 71]. Recent advancements have demonstrated that all 3D printing technologies can produce surgical models with satisfying accuracy in all three dimensions. However, certain technologies, such as material jetting (MJ) and powder bed fusion (PBF), exhibit superior accuracy compared to others like binder jetting (BJ) [72–74]. Studies comparing different printing technologies have revealed variations in dimensional accuracy but generally clinically insignificant differences (Figure 6A) [75]. Additionally, 3D-printed bone models offer the unique advantage of replicating pathological conditions, which could significantly enhance the educational experience for trainees in oral and maxillofacial surgery.
Figure 6.
3D printed bone models. (A) Box plot demonstrating trueness root mean square (RMS) (mm) values and precision RMS (mm) values of models fabricated by various printers [75] (from Ref. [75] licensed under Creative Commons Attribution 4.0 license). (B) Computer-aided design (CAD) design of temporal bone model and the ultimate 3D-printed temporal bone model [76] (from Ref. [76] licensed under Elsevier license).
Haptic feedback, crucial for simulating tactile sensations during surgery, relies on the mechanical features of the building biomaterials [77]. Tensile strength and elastic modulus are essential parameters for achieving bone-like tactile sensations [78]. Although standardized quantitative tests for haptic feedback are lacking, a certain number of studies have proposed 3D-printed models to provide satisfactory feedback. Different printers and materials exhibit unique characteristics, influencing anatomy replication and surgical simulation. For instance, in a study by Shujaat et al. [79], the ProJet CJP 660Pro printer utilizing a gypsum-like material achieved the highest scores in stimulating osteotomy. However, it received lower scores for drilling holes, screw insertion and removal. Conversely, several studies have indicated that the haptic feedback of models generated from nylon-like materials was not favorable [80].
Cost remains a critical factor in the widespread adoption of 3D-printed bone models for surgical training. In-house production involves additional expenses, including software, printers, materials and operator training [74]. Material extrusion (ME) printers are often cited as cost-effective options, albeit with compromises in accuracy [81]. However, recent developments, such as a cost-effective protocol for fabricating temporal bone models using desktop printers and PLA filament, demonstrate the potential to minimize costs significantly (Figure 6B) [76]. The resulting model demonstrated anatomical accuracy (XYZ accuracy = 12.5, 12.5, 5 μm) and provided appropriate tactile feedback during surgical drilling. The total material cost for fabricating the model was approximately $1.50, significantly lower than the cost of the cadaveric temporal bone or other 3D-printed models. Additionally, printing time varies considerably depending on the technology and materials chosen, further impacting overall expenses [82]. In a study by Msallem et al. [75], different printing technologies, including FFF, SLA, selective laser sintering (SLS), MJ and binder jetting (BJ) were used to print anatomical mandibular models. The minimal fabrication time for each model, approximately 48 min, was achieved using SLS technology with the EOSINT P 385 (EOS GmbH, Krailling, Germany) 3D printer.
Regenerative scaffolds and bioprinting
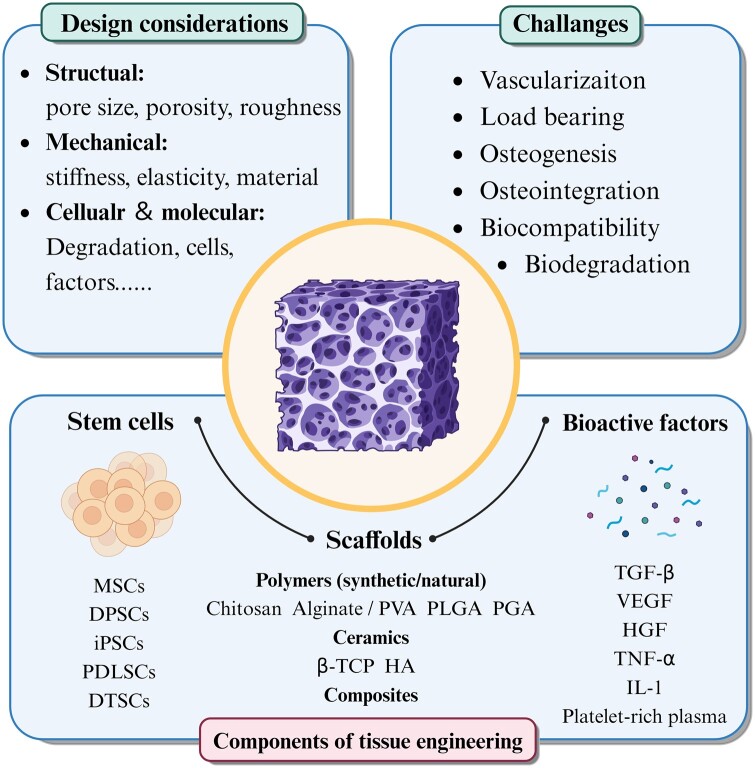
Traditional reconstruction strategies, such as free tissue transfer, autografts, allografts and rigid fixation, are frequently associated with limitations, including bone nonunion, donor site morbidity, restricted availability, potential disease transmission and immunogenic rejection [83–85]. Bone tissue engineering (BTE) presents a compelling alternative, offering the potential for more effective, patient-tailored solutions that promote natural tissue regeneration and functional restoration (Figure 7) [86–88]. Central to the success of BTE strategies is the concept of regenerative scaffolds [89]. These 3D frameworks serve as temporary templates, providing structural support and guidance for tissue regeneration, and the design and composition of scaffolds significantly influence their ability to mimic the native tissue environment and foster optimal healing outcomes [90, 91].
Figure 7.
Schematic of regenerative scaffolds for tissue engineering purpose. Regenerative scaffolds generally consist of three main components including, scaffolds matrix (to support cell behaviors and tissue deposition), bioactive factors (to induce stem cell proliferation and differentiation and promote tissue regeneration and vascularization) and stem cells. Generally, we have to pay attention to several aspects when designing and developing scaffold, including mechanical, structural, cellular and molecular properties to optimize its regenerative potential. However, researchers encounter several significant challenges in tissue engineering such as osteogenesis, biodegradation and vascularization.
Among the various techniques available for scaffold fabrication, 3D printing has emerged as a potent tool with transformative potential, offering several extra advantages, including personalized design, high repeatability, low production costs and rapid prototyping (Table 2) [52, 100, 101]. The ability to tailor the scaffold’s architecture at a micron- and nano-scale level allows for the development of complicated architectures that greatly resemble native tissue microenvironments [102]. For example, Lee et al. [32] 3D printed a kagome-structure and PCL-made scaffold with excellent mechanical strength and enhanced osteoconductivity, which is a promising alternative for bone regeneration in complex and large defects. Additionally, 3D printing enables the incorporation of bioactive agents, growth factors, small molecules and multiple agent combinations directly into the scaffold matrix, further enhancing its regenerative potential [64]. For instance, the employment of growth factors, such as bone morphogenic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF), can significantly improve osteogenesis and vascularization [96, 98]. Lee et al. [97] applied a coating of bone demineralized and decellularized extracellular matrix (bdECM) onto pre-existing 3D-printed polycaprolactone/tricalcium phosphate (PCL/TCP) structures to augment their osteoinductivity and osteoconductivity. Moreover, the incorporation of cells into these scaffolds enhances their regenerative potential and accelerates tissue healing processes, such as adipose-derived mesenchymal stem cells (ADSCs) and bone marrow-derived mesenchymal stem cells (BMSCs) [103]. Furthermore, the scalability and reproducibility of 3D printing technology facilitate its integration into clinical practice, offering clinicians a versatile tool for addressing diverse patient needs [104, 105].
Table 2.
Regenerative scaffolds in oral and maxillofacial regions
| Composition | Bioactive component | 3D printing technology/equipment | Measurement | Feature | Ref |
|---|---|---|---|---|---|
| PCL | — | PED/Lab-made precision 3D printer | In vitro and in vivo (New Zealand white rabbits) |
|
[32] |
| Gelatin/genipin | — | 3DF/3D Bioplotter (EnvisionTEC, Gladbeck, Germany) | In vitro | Supported the viability, attachment, and chondrogenic differentiation of hBMSC | [92] |
| PLA | BMP-2 coating | FDM/3DXP—One | In vitro and in vivo (mature female minipigs) |
|
[93] |
| Titanium | — | SLM/concept laser (Concept Laser GmbH, Germany) | In vivo (beagles and three patients) |
|
[94] |
| Mg/nCSi | — | DLP/facility supplied by 10 Dimensions Technology Co., China | In vitro and in vivo (male New Zealand white rabbits) |
|
[95] |
| Silk/HAP | BMP2, VEGF and NGF | Paste extrusion/lab made | In vitro | Synergistic effects of BMP-2, VEGF and NGF in inducing osteoblastic differentiation in vitro for tissue engineering. | [96] |
| PCL/LAP | — | 3DF/3D Bioplotter (Envisiontec, Germany) | In vitro and in vivo (Sprague Dawley rats) |
|
[64] |
| PLGA/TCP | rhBMP-2 | MJP/Project 3510 HD Plus (3D System, USA) | In vitro and in vivo (male rhesus monkeys) |
|
[86] |
| PCL/TCP | bdECM and ADSCs | Fused deposition/NM | In vivo (beagles) | Additional ADSC injection improved mandibular ossification in and around scaffold. | [97] |
| PCL/TCP scaffold + Me-HA/Me-Gel hydrogel | Resveratrol (RSV) and Strontium ranelate (SrRn) | 3DF/3D-Bioplotter® (Manufacturer Series, EnvisionTEC; Dearborn, MI) | In vitro and in vivo (Sprague Dawley rats) |
|
[98] |
| Ti3C2MXene/BCP/SA | BBR | Bio-Architect®-Pro 3D printer (Regenovo Biotechnology Co. Ltd, China) | In vitro and In vivo (New Zealand white rabbits) |
|
[99] |
Ceramics and polymers represent two prominent classes of materials utilized in the fabrication of 3D-printed scaffolds for oral and maxillofacial reconstruction [106]. Ceramic-based scaffolds, such as hydroxyapatite and TCP, exhibit excellent osteoconductivity and biocompatibility, making them ideal candidates for promoting bone regeneration [107]. Polymers, on the other hand, offer versatility in scaffold design and mechanical properties, allowing for tailored solutions that accommodate specific patient needs [32, 108, 109]. Furthermore, the integration of bioresorbable polymers facilitates the gradual replacement of the scaffold with regenerated tissue over time, promoting long-term functional outcomes [110]. Composite scaffolds offer a solution by combining the strengths of individual materials while mitigating their respective weaknesses [111]. This synergistic approach allows for the design of scaffolds with enhanced mechanical properties, enhanced bioactivity and improved degradation kinetics, thereby overcoming many of the challenges associated with single material-based scaffolds [112]. For example, the incorporation of bioactive ceramics, such as calcium phosphates (CaP) and hydroxyapatite (HA), into a polymer matrix can enhance osteoconductivity and promote bone regeneration [96, 113]. Jeong et al. [52] fabricated a PCL/β-TCP scaffold for complex zygomatico-maxillary reconstruction.
Zhang et al. [98] fabricated scaffolds through 3D printing, comprising a mixture of polycaprolactone/β-tricalcium phosphate (PCL/TCP) and a hydrogel-based bioink encapsulating resveratrol and strontium ranelate. The resulting 3D-printed scaffolds, incorporating small molecules within the hydrogel, notably enhanced bone formation upon implantation within critical-sized mandibular bone defects in rat models. Bioactive metallic materials have been extensively employed in bone tissue engineering (BTE), and incorporating them into the synthetic scaffold presents a simple method to improve the osteogenesis and mechanical characteristics of composite scaffolds [114]. For instance, Wang et al. [115] developed PCL/Zn scaffolds with improved mechanical properties, cytocompatibility and osteogenic effect.
While still in its initial stages, bioprinting enables the printing of living tissues or organs based on cell-laden bioinks [116, 117]. These bioinks are formulated to maintain cell viability, structural integrity and functionality throughout the entire printing procedure [118]. A critical challenge lies in enabling the fabrication of finely structured three-dimensional objects while preserving the high viability and functionality of the loaded cells [119]. A wide range of bioprinting technologies has been explored for biomedical applications, with extrusion-based as one of the most promising strategies [120].
Defect reconstruction in OMFs with great esthetic outcomes
Severe bone defects in the oral-maxillofacial region resulting from trauma and tumors not only significantly impact patients’ physiological and mental well-being but also present substantial challenges for reconstruction. According to one recent epidemiology study carried out in China, mandible fractures (31.97%) were the most common, followed by zygoma fractures (25.3%) [121]. Orbital fractures, common midface injuries, can lead to severe functional impairment [14]. The complex, anatomical region and limited intraoperative visibility pose challenges for orbital floor reconstruction. Titanium meshes have emerged as practical solutions for orbital reconstruction, and pre-bending these meshes according to 3D-printed anatomical models can achieve more exact reconstruction in terms of orbital volume [122–124]. Compared to freehand surgery, 3D virtual surgical planning plus 3D printing (VSP)-assisted surgery procedure results in significantly smaller alterations in orbit height and volume during maxillary reconstruction [125]. In the VSP group, esthetic evaluation employing color-gradient maps reveals a smoother and more symmetric curve in the post-operative appearance. Another study [126] examined the effectiveness of intraoperative bending of titanium mesh compared to pre-bent ‘hybrid’ patient-specific titanium mesh (Figure 5B). The utilization of pre-formed plates according to 3D-printed anatomical models yielded a non-significant absolute volume difference in the intervention group, while a significant difference in volume was noted in the conventional group. Moreover, surgery time was significantly reduced. An alternative methodology involves mirroring intact anatomy on the opposite side to replace the fractured orbit, thereby generating PSIs [20, 126]. Typically, the workflow for reconstructing PSIs (orbital, zygomatic, etc.) consists of five stages: (i) CT data acquisition: obtaining patient CT images. (ii) 3D model generation: creating a 3D model via computer programs (i.e. mimics) from the obtained CT images. (iii) Implant design: constructing of PSIs using the mirror reconstruction method, which involves defining a midsagittal plane as the cutting plane and mirroring the defect-free half to the defective half to maintain symmetry, followed by converting the designed implants into standard triangle language (STL) file format [18, 20]. (iv) Material selection: choosing suitable materials, such as titanium, PEEK or Ti6Al4V for specific implant construction [127, 128]. (v) 3D printing of implants.
Surgical reconstruction following total maxillectomy poses significant challenges, with conventional palatal obturator prostheses (PAPs) often failing to achieve satisfactory anatomical and functional outcomes. Gueutier et al. [21] introduced a reconstruction approach following total bilateral maxillectomy, which involves the implantation of a pre-developed titanium implant obtained through 3D printing. This approach yielded successful functional and anatomical outcomes without signs of rejection or infection during a follow-up period of 6–12 months, offering a new therapeutic option when free flaps are contraindicated. The vascularized fibula flap is now regarded as the gold standard in routine mandibular reconstruction. 3D/VSP has shown the potential to reduce the occurrence of radiographic non-union and complications in mandibular free fibula flap (FFF) reconstruction procedures [129]. Pu et al. [17] proposed a novel fibula malleolus cap to counteract these challenges during fibula flap harvesting, which significantly reduced deviations in the locations and angles of distal fibula osteotomies (Figure 4A). The utilization of the malleolus cap during simultaneous dental implant placement into flaps improved accuracy in terms of implant platform locations, apex locations, and angles [35, 130]. Compared to conventional osteotomy tools, 3D-printed guides offer universality, reusability and cost-efficiency. In cases of long-term segmental mandibular defects, mandibular deviation, malocclusion and departure from the design of the mandibular movement often occur. Dental rehabilitation and mandibular reconstruction are extensive projects requiring collaboration and subject to various influences. Li et al. [131] demonstrated that the integration of 3D virtual surgical planning (VSP), 3D-printed surgical guides, vascularized flap, immediate dental implants and occlusal reconstruction could offer patients enhanced appearance and occlusal reconstruction, while also reducing the necessity for multiple surgeries. Another study [132] investigated the accuracy and its influencing factors in mandibular reconstruction utilizing VSP, 3D-printed osteotomy guides and pre-bent reconstruction plates (VSP/3D-printed-guide/plate). it revealed that decreased accuracy was strongly associated with the increasing number of donor-bone segments and the length of donor-bone. These results further confirm VSP/3D-printed-guide/plate as a dependable and precise method for mandibular reconstruction.
Complete restoration of mandibular defects typically necessitates both mandibular reconstruction and dental implant placement, conducted in two sequential surgeries [133]. However, Miljanovic et al. [39] proposed a potential solution to streamline this process. They introduced a solution involving prepositioned dental implants, thereby reducing the need for two separate surgeries (Figure 4B). In this approach, a designed mandibular implant is placed before mandibular reconstruction with a 3D-printed guide. The strength and stability of the surgical guide were via finite element analysis (FEA), demonstrating its ability to withstand the forces encountered during surgery [134].
Accuracy management in orthognathic surgery
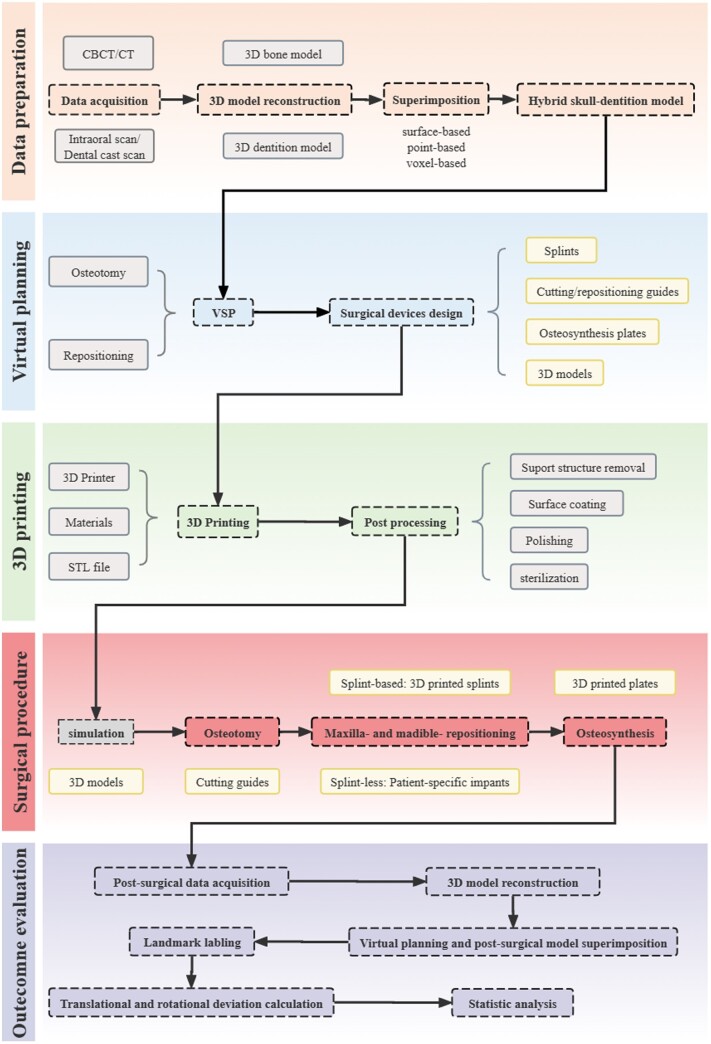
Orthognathic surgery is a procedure aimed at enhancing facial aesthetics and correcting jaw bone deformities resulting from diseases, injuries or genetic factors [135]. Technological advancements have significantly affected the evolution of orthognathic surgery over the past decades, driven by the need for accurate positioning and osteosynthesis of bone segments. Virtual surgical planning enables preoperative digital design and simulation, while 3D printing facilitates accurately transferring virtual plans to the operating room [25, 136]. We have outlined the complete workflow of orthognathic surgery (Figure 8). Despite the critical importance of accurately delivering the preoperative plan to the operating room in digital orthognathic surgery, there remains no universally agreed-upon methodology or statistical method for evaluating deviations. Existing methods include calculating translational and rotational deviation between manually set landmarks on the simulation and post-surgical digital models. Some software solutions available on the market offer automated superimposition of preoperative plans and postoperative images, the establishment of x, y and z axes, and subsequent calculation of translational (linear) and rotational (angular) discrepancies, such as Geomagic [15] and IPS Case Designer [137].
Figure 8.
Workflow of orthognathic surgery integrated with 3D printing and virtual surgical planning (VSP). General workflow encompasses steps including data preparation, virtual planning 3D printing, surgical procedures and outcome evaluation.
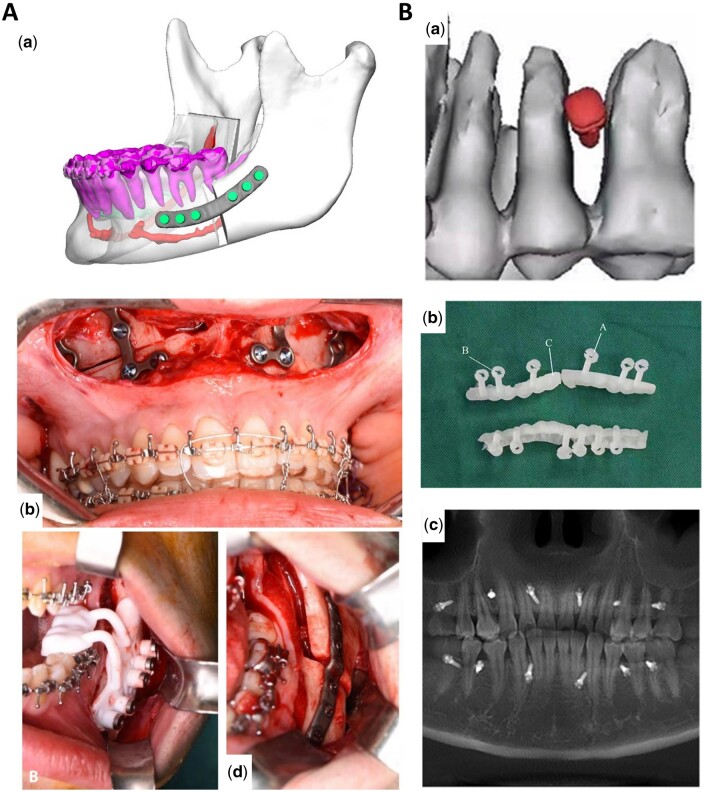
Digital splints already exhibit superior accuracy, yet splints-based surgery modalities possess intrinsic errors due to the translation and rotation of the TMJ in the supine and anesthetized patient. Therefore, waferless patient-specific cutting guides and plate systems have garnered increasing interest among researchers [138, 139]. Numerous studies have demonstrated that patient-specific cutting guides and plates showed greater accuracy in repositioning maxilla compared to standard computer-aided design/computer-aided manufacturing (CAD/CAM) fabricated occlusal splints [137, 140–144]. Recently, Diaconu et al. [145] conducted a stratified meta-analysis including 115 single-piece Le Fort I studies utilizing PSIs, revealing that PSIs were statistically more accurate than conventional CAD/CAM splint or wafer-based osteosynthesis. Though the results are promising, studies evaluating the accuracy of PSI in orthognathic surgery are quite monotonous, as they concentrated on maxilla single-piece Le Fort osteotomies, making it challenging to conclude their potential in multisegmented maxillary osteotomies, mandibular repositioning and other areas. In mandibular orthognathic surgery, the utilization of 3D-printed PSI is less reported, as obtaining a stable reference for fixation on the lateral mandibular aspect poses significant challenges [146–148]. Compared to the maxilla, mandible repositioning deviations in PSI-aided surgery are spotted greater, but all below the 2-mm threshold [149]. These results may be attributed to inadequate hole positioning accuracy, the impact of attached masticatory muscles, and potential pterygoid-masseteric spasm [146, 150]. Although the maxilla-first approach is more common in two-jaw surgery, the argument between the maxilla-first and mandible-first approach has been lasting for decades [151]. Badiali et al. [147, 148] suggested that the mandibular PSI-guided mandible-first procedure accurately transfers the virtual plan to the patient (Figure 9A). This integrated procedure successfully replicated the virtually planned maxilla-mandibular positioning, allowing for precise and flexible intraoperative vertical correction. Importantly, this approach did not significantly impact frontal symmetry, achieving satisfactory aesthetic outcomes. Intraoperatively, manipulating multiple segments to place them into the PSI is technically challenging. This is due to the need to immobilize relatively small bone segments into the PSI plate while achieving movement of the individual segments in multiple planes, which may result in mutual interference between the bones [77, 150, 153, 154]. To address this challenge, Wang et al. [155] proposed the advantage of using PSI add-on wafers, which can improve the accuracy of surgical procedures based on virtual plans while facilitating intraoperative manipulation.
Figure 9.
3D printed plates and screws in orthognathic surgery. (A) (a) Patient-specific plates CAD design; (b) intraoperative view of free hand bent plates; (c) 3D printing positioning guide; (d) CAD/CAM plate [147] (from Ref. [147] licensed under Creative Commons Attribution 4.0 license). (B) (a) Virtual placement of intermaxillary fixation screws; (b) digital guide model; (c) the tomographic image of IMFS installed with a digital guide [152] (from Ref. [152] licensed under Elsevier license).
While preliminary research has confirmed the accuracy of patient-specific osteosynthesis, there remains a gap in our understanding regarding the stability of bony segments when applying this approach, with only one study evaluating 3D printing-based patient-specific osteosynthesis [15, 156]. This study revealed that 3D-printed patient-specific Ti6Al4V ELI plates processed by SLM, together with an osteotomy guide, exhibited superior accuracy in both translations and rotations compared to direct bending of the ready-made plates [15]. In addition, they measured the stability of the patient-specific plates and osteotomy guides over a 6-month period, which showed a significant improvement in stability. During the follow-up period, no major complications such as tooth loss, nerve damage, malocclusion or postoperative recurrence and infection were noted except in one patient. Intermaxillary fixation screws (IMFS) are commonly used to achieve temporary fixation of the maxilla-mandible in patients with maxillomandibular fractures or those undergoing orthognathic surgery. Cui et al. [152] designed and manufactured a 3D-printed guide with reduced the incidence of damage to the periodontal ligament (PDL) and tooth roots, thereby improving the success rate of IMFS implantation (Figure 9B).
Temporomandibular joint treatment
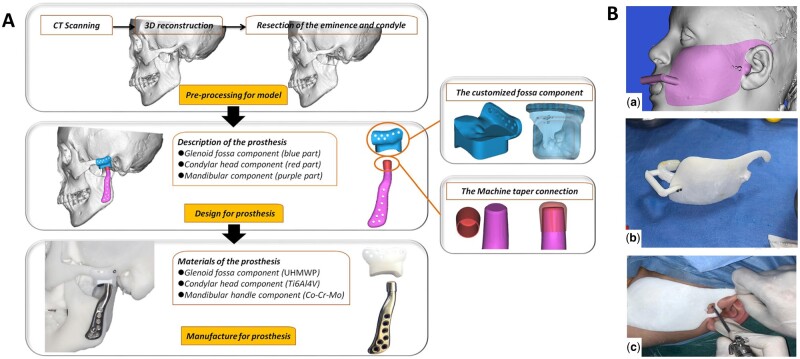
Severe TMJ diseases, including TMJ ankylosis, end-stage TMJ osteoarthritis, comminuted condylar fractures and tumors, often necessitate the removal of the affected joint structures and subsequent reconstruction to restore both the anatomical integrity and functional capabilities [157–159]. Total alloplastic TMJ prosthesis is recognized as a reconstructive method for joint defects, and 3D printing plays a pivotal role in the manufacturing process of these complex prostheses for total TMJ replacement [160, 161]. 3D printing enables customizing TMJ prostheses according to the patient’s unique anatomical requirements [50].
Several commercially available TMJ prostheses have been developed for clinical use, however, these prostheses do not always accurately match the unique TMJ anatomy of Chinese patients. This highlights the pressing need for research and development of TMJ prostheses designed specifically for the Chinese population. Recognizing this need, Zheng et al. [162] devised a novel customized TMJ prosthesis utilizing 3D printing technology (Figure 10A). The prosthesis consists of three components: the fossa component, fabricated from ultrahigh-molecular-weight polyethylene; the condylar head component, manufactured from cobalt-chromium-molybdenum alloy; and the mandibular component, fabricated from titanium alloy. Post-surgical outcomes were evaluated, and no complications were reported. After TMJ prosthesis implantation, patients showed significant improvement in pain level, jaw function, diet and maximum intermaxillary opening. During functional activities, the TMJ joint undergoes complex movements involving rotation and translation in three-dimensional space [164]. Designing a TMJ prosthesis that accurately replicates such complex kinematics presents a significant challenge. Addressing this issue, Cheng et al. [165] developed a 3D-printed porous condylar prosthesis using Ti-6Al-4V alloy, which possessed lower weight and a similar modulus of elasticity close to that of cortical bone. Finite element analyses (FEAs) confirmed the superior mechanical behavior of this prosthesis. The resection of the condyle often results in the detachment of the lateral pterygoid muscle, which is crucial for mandibular laterotrusion and protrusion movements. To address this concern, Zou et al. [166] introduced a 3D-printed titanium (Ti) alloy TMJ prosthesis, exhibiting the potential for muscle attachment and ingrowth, and potentially improving mandibular movement. Furthermore, they demonstrated the benefits of a 3D-printed porous tantalum (Ta) scaffold for facilitating muscle attachment, further augmenting the potential for clinical application [167]. The utilization of 3D-printed guides during condyle reconstruction has also proven advantageous in preventing post-surgical deviations in the condylar position [168, 169].
Figure 10.
3D-printed new TMJ prosthesis with three different components. (A) The processing of the new TMJ prosthesis with three main components [162] (from Ref. [162] licensed under Creative Commons Attribution 4.0 license). (B) (a) Virtual design of the injection guide; (b) 3D-printed patient-specific guide; (c) guide fitting to the soft tissue of patients [163] (from Ref. [163] licensed under Elsevier license).
The TMJ constitutes a complex structure where interacting bones are covered with cartilage and separated by a small disc, facilitating smooth movement. However, the regenerative capacity of the TMJ disc is limited, posing a persistent challenge for its replacement [170, 171]. Hydrogels are characterized by a three-dimensional porous network structure containing a large amount of water, which provides lubricating properties for joints. However, its mechanical properties, such as low strength and toughness, hinder its application as an artificial weight-bearing TMJ disc [116, 172, 173]. To address this limitation, Jiang et al. [174] synthesized a novel TMJ disc by combining polyvinyl alcohol (PVA) hydrogel with a 3D-printed polycaprolactone (PCL) framework.
PCL implants were manufactured using a layer-by-layer deposition technique, while hydrogels are cross-linked by a simple cyclic freeze-thaw method. The mechanical strength of the resulting PVA + PCL artificial intervertebral discs has comparable mechanical strength to natural intervertebral discs. Experiments with the artificial disc in goats with TMJ disc defects demonstrated that it was able to maintain joint stability and protect the condylar cartilage and bone from damage. The PCL implants were fabricated using a layer-by-layer deposition technique, with the hydrogel crosslinked through a cyclic freeze-thaw method. Compared to the natural disc, the resulting PVA + PCL artificial disc exhibited comparable mechanical robustness. Experimental implementation of the artificial disc in goats demonstrated its ability to preserve the stability of joints and protect the condylar structures from damage. Combining 3D-printed scaffolds and mesenchymal stem cells, tissue engineering presents another promising alternative for TMJ repair [175, 176]. This approach involves fabricating scaffolds with precise dimensions and structures using 3D printing technology and incorporating mesenchymal stem cells to promote tissue regeneration. The combination of these innovative techniques holds great potential for overcoming the limitations of current treatment options and enhancing the prospects for successful TMJ repair [177].
Some therapies for TMJ disorders involve injecting substances into specific compartments of the joint, such as the superior or inferior compartments. To minimize the risk of complications, such as bleeding, hematoma and intracranial perforation, researchers have explored using 3D-printed surgical guides for precise needle insertion (Figure 10B) [163, 178]. These guides aim to ensure accurate placement and reduce the likelihood of adverse events. The findings of these studies have demonstrated minimal angle deviation, indicating the potential of 3D-printed guides to enhance the safety and precision of needle insertion procedures. Furthermore, in cases of post-traumatic TMJ ankylosis, a tissue graft is inserted to restore joint function during inter-positional arthroplasty, 3D-printed splints have shown promising utility in occlusal stabilization and maintaining the desired gap between the graft and adjacent structures [179].
Other applications
There is a growing trend in using 3D-printed models in medical education, especially in anatomy teaching and surgical training [180–182]. A series of randomized control trials and meta-analysis have reported that compared with traditional educational materials such as 2D CT images and illustrations, 3D-printed skull models are inexpensive, accurate and fast to produce teaching material in morphology education [183–186]. For instance, the cost of printing each model in ABS using a FDM printer is generally less than $10. [187]. Moreover, 3D-printed models can provide surgeons with a tangible platform providing visual and haptic perceptibility for meticulous dissection and reconstruction exercises, allowing them to familiarize themselves with the complexities of specific pathologies before entering the operating room [73, 188].
Recently, it has been gradually evident that ultrasound imaging can also serve as base data to generate 3D models using surface-rendered sonographic views. As a result, researchers have dedicated their great effort to generating 3D models from prenatal surface-rendered views and demonstrated their potential in parental education of fetal malformations [189–191]. For instance, Nicot et al. [189, 190] produced ABS models of cleft lip fetus using a low-cost 3D printer and a surface-rendered oropalatal (SROP) sonographic view. Those models necessitate 60 70 g of ABS and a printing time of about 6∼7 h, costing only France $2 [189–191]. Such 3D models could be extended to replicate other facial malformation replications and enhance parents’ comprehension of the deformities of their unborn children.
Suspicious radiological images of jaw bone often necessitate histopathological examination to confirm a final diagnosis. The employment of guided biopsy using 3D-printed guides could minimize the chance of devitalization of the neighboring teeth and nerves [192, 193]. Valdec et al. [194] designed and 3D-printed a tooth-supported drilling template trephine biopsy, demonstrating high accuracy and great predictability sampling, while possessing minimal invasiveness. Compared to free-handed biopsies, guided procedures showed significantly lower mean deviation between the biopsy axes [38].
Some patients may experience reoccurrence after surgical resection, making localized anti-tumor therapy crucial for inhibiting local recurrence [195, 196]. With the development of biomaterials and drug delivery systems, 3D porous scaffolds capable of controlled anti-tumor drug release have garnered increasing interest in cancer treatment [197–199]. Local chemotherapy using chemo drug-loaded scaffolds can significantly reduce the side effects of chemo drugs on non-tumorous tissues and organs [200, 201]. More recently, stimuli-responsive scaffolds that enable on-demand drug release in the presence of external stimuli have gained increasing interest [202, 203]. For instance, near-infrared (NIR)-responsive drug-loaded scaffolds are among the most extensively studied systems for cancer therapy, as they can achieve NIR-induced drug release and thermal effect simultaneously, exerting synergistic chemo-photothermal anti-cancer effects [204]. Dutta et al. [205] 3D-printed hydrogel scaffolds with NIR-controlled release of antitumor drugs, effectively killing osteosarcoma cells.
Conclusion and future outlook
The integration of 3D printing technology has significantly transformed oral and maxillofacial surgery by enabling customization and personalization through the creation of 3D-printed surgical devices including PSIs, surgical guides, splints and 3D models. Furthermore, the convergence between 3D printing and virtual surgical planning has revolutionized surgical workflows, leading to improved accuracy, reduced surgical time, and decreased costs. Consequently, these advancements have greatly benefited procedures such as bone reconstruction, orthognathic surgery, and TMJ treatment, while also enhancing patient communication and education. Overall, 3D printing and its associated surgical devices have streamlined workflow and elevated precision across various applications within oral and maxillofacial surgery.
However, there are still some research limitations of 3D printing during oral and maxillofacial surgical operations. Firstly, the diversity of clinically available building materials is limited and properties haven’t been optimized enough. Take titanium for example, osseointegration and stress shielding are the two biggest concerns needing improvement. Optimization of internal structures and proper surface modifications are needed in further research, together with new building material exploitation. Besides, confirmation of long-term implantation stability of biomaterials is absent in most studies, leading to possible worries. Additionally, the cost-effectiveness of 3D printing has to be carefully managed for broader clinical applications, which is greatly impacted by technology and its associated materials. Besides, the initial investment required for acquiring 3D printers and the ongoing expenses for materials can be significant and there may be a learning curve for surgeons and healthcare professionals to become proficient in utilizing 3D printing technology effectively. Moreover, the regulatory landscape surrounding 3D-printed medical devices is still evolving. Standardized evaluation methodology to ensure the safety and efficacy of 3D-printed implants and other surgical tools hasn’t been cautiously discussed and established so far, which is the most crucial aspect regrading 3D printing applications. Regulatory authorities must establish guidelines and standards for producing and using 3D-printed medical devices to ensure patient safety.
Encouragingly, there are several exciting future directions to further exploit the potential of 3D printing in oral and maxillofacial surgery. With the development in regenerative medicine, 3D printing of bioactive scaffolds holds the potential to generate live tissues similar to the original ones. These scaffolds can be 3D-printed with biocompatible materials and designed to mimic the structure and properties of natural tissues. By incorporating factors that promote tissue growth and vascularization, these scaffolds can enhance the body’s ability to regenerate damaged or missing tissue following surgery. Bioprinting creates functional tissue structures by printing living cells laden with biocompatible biomaterials. In oral and maxillofacial surgery, bioprinting holds promise for the fabrication of functional tissues such as bone, cartilage, or mucosa. Drug delivery systems can be incorporated into implants or scaffolds to deliver therapeutic agents directly to the surgical site. This can include antimicrobial agents to prevent infection, growth factors to promote tissue regeneration, or analgesics to manage post-operative pain. By delivering drugs locally to the site of surgery, these systems can improve therapeutic outcomes while minimizing systemic side effects.
In conclusion, 3D printing and generated 3D-printed surgical devices have revolutionized the field of oral and maxillofacial surgery by offering customized and precise solutions for PSIs, surgical guides, and models. It enhances surgical accuracy, improves patient outcomes, and streamlines the production process. However, challenges such as cost and regulatory considerations must be addressed for the broader adoption of 3D printing in oral and maxillofacial surgery. By addressing those limitations and diving into promising research directions, 3D printing holds great promise for further advancements in innovative techniques, improved patient outcomes, and enhanced overall experiences in oral and maxillofacial surgery.
Supplementary Material
Contributor Information
Xiaoxiao Wang, State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases & Department of Orthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan 610041, China; Department of Biotherapy, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
Min Mu, Department of Biotherapy, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
Jiazhen Yan, School of Mechanical Engineering, Sichuan University, Chengdu, Sichuan 610065, China.
Bo Han, School of Pharmacy, Shihezi University, and Key Laboratory of Xinjiang Phytomedicine Resource and Utilization, Ministry of Education, Shihezi, 832002, China, Shihezi 832002, China.
Rui Ye, State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases & Department of Orthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan 610041, China.
Gang Guo, Department of Biotherapy, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
Funding
This work was financially supported by National Natural Sciences Foundation of China (Nos. 81400522, 31971308 and U1903211), Science and Technology Planning Project of Shihezi University (2023AB047), and Sichuan Science and Technology Program (2022YFS0007).
Supplementary data
Supplementary data are available at Regenerative Biomaterials online.
Conflicts of interest statement. None declared.
References
- 1. Guntaka PK, Harris JA, Niedziela CJ, Bass M, Afshar S.. The landscape of international oral and maxillofacial surgery collaborations from 1996 to 2020: a scoping review of the published literature. Int J Oral Maxillofac Surg 2022;51:1362–9. [DOI] [PubMed] [Google Scholar]
- 2. Guillaume O, Geven MA, Varjas V, Varga P, Gehweiler D, Stadelmann VA, Smidt T, Zeiter S, Sprecher C, Bos RRM, Grijpma DW, Alini M, Yuan H, Richards GR, Tang T, Qin L, Yuxiao L, Jiang P, Eglin D.. Orbital floor repair using patient specific osteoinductive implant made by stereolithography. Biomaterials 2020;233:119721. [DOI] [PubMed] [Google Scholar]
- 3. Wang S, Zhao S, Yu J, Gu Z, Zhang Y.. Advances in translational 3D printing for cartilage, bone, and osteochondral tissue engineering. Small 2022;18:e2201869. [DOI] [PubMed] [Google Scholar]
- 4. Hatt LP, Wirth S, Ristaniemi A, Ciric DJ, Thompson K, Eglin D, Stoddart MJ, Armiento AR.. Micro-porous PLGA/β-TCP/TPU scaffolds prepared by solvent-based 3D printing for bone tissue engineering purposes. Regen Biomater 2023;10:rbad084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Yuan H, Chandrakar A, Moroni L, Habibovic P.. 3D porous Ti6Al4V-beta-tricalcium phosphate scaffolds directly fabricated by additive manufacturing. Acta Biomater 2021;126:496–510. [DOI] [PubMed] [Google Scholar]
- 6. Wang J, Tang Y, Cao Q, Wu Y, Wang Y, Yuan B, Li X, Zhou Y, Chen X, Zhu X, Tu C, Zhang X.. Fabrication and biological evaluation of 3D-printed calcium phosphate ceramic scaffolds with distinct macroporous geometries through digital light processing technology. Regen Biomater 2022;9:rbac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raja N, Park H, Gal CW, Sung A, Choi Y-J, Yun H.. Support-less ceramic 3D printing of bioceramic structures using a hydrogel bath. Biofabrication 2023;15:035006. [DOI] [PubMed] [Google Scholar]
- 8. Nakano H, Suzuki K, Inoue K, Nakajima Y, Mishima K, Ueno T, Demura N.. Application of the homologous modeling technique for precision medicine in the field of oral and maxillofacial surgery. J Pers Med 2022;12:1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Willson K, Atala A.. Medical 3D printing: tools and techniques, today and tomorrow. Annu Rev Chem Biomol Eng 2022;13:481–99. [DOI] [PubMed] [Google Scholar]
- 10. Cao Y, Sun L, Liu Z, Shen Z, Jia W, Hou P, Sang S.. 3D printed-electrospun PCL/hydroxyapatite/MWCNTs scaffolds for the repair of subchondral bone. Regen Biomater 2023;10:rbac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Annino DJ, Hansen EE, Sethi RK, Horne S, Rettig EM, Uppaluri R, Goguen LA.. Accuracy and outcomes of virtual surgical planning and 3D-printed guides for osseous free flap reconstruction of mandibular osteoradionecrosis. Oral Oncol 2022;135:106239. [DOI] [PubMed] [Google Scholar]
- 12. Ma L, Xiao D, Kim D, Lian C, Kuang T, Liu Q, Deng H, Yang E, Liebschner MAK, Gateno J, Xia JJ, Yap P-T.. Simulation of postoperative facial appearances via geometric deep learning for efficient orthognathic surgical planning. IEEE Trans Med Imaging 2023;42:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang Y-H, Lee B, Chuy JA, Goldschmidt SL.. 3D printing for surgical planning of canine oral and maxillofacial surgeries. 3D Print Med 2022;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dvoracek LA, Lee JY, Unadkat JV, Lee YH, Thakrar D, Losee JE, Goldstein JA.. Low-cost, three-dimensionally-printed, anatomical models for optimization of orbital wall reconstruction. Plast Reconstr Surg 2021;147:162–6. [DOI] [PubMed] [Google Scholar]
- 15. Kim S-H, Lee S-M, Park J-H, Yang S, Kim J-W.. Effectiveness of individualized 3D titanium-printed orthognathic osteotomy guides and custom plates. BMC Oral Health 2023;23:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen H, Bi R, Hu Z, Chen J, Jiang N, Wu G, Li Y, Luo E, Zhu S.. Comparison of three different types of splints and templates for maxilla repositioning in bimaxillary orthognathic surgery: a randomized controlled trial. Int J Oral Maxillofac Surg 2021;50:635–42. [DOI] [PubMed] [Google Scholar]
- 17. Pu JJ, Choi WS, Yeung WK, Yang W-F, Zhu W-Y, Su Y-X.. A comparative study on a novel fibula malleolus cap to increase the accuracy of oncologic jaw reconstruction. Front Oncol 2021;11:743389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moiduddin K, Mian SH, Umer U, Alkhalefah H, Ahmed F, Hashmi FH.. Design, analysis, and 3D printing of a patient-specific polyetheretherketone implant for the reconstruction of zygomatic deformities. Polymers (Basel) 2023;15:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee U-L, Lim J-Y, Park S-N, Choi B-H, Kang H, Choi W-C.. A clinical trial to evaluate the efficacy and safety of 3D printed bioceramic implants for the reconstruction of zygomatic bone defects. Materials 2020;13:4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. A M, Mohan AA, Reddy MH.. Manufacturing of customized implants for orbital fractures using 3D printing. Bioprinting 2021;21:e00118. [Google Scholar]
- 21. Gueutier A, Kün-Darbois J-D, Laccourreye L, Breheret R.. Anatomical and functional rehabilitation after total bilateral maxillectomy using a custom-made bone-anchored titanium prosthesis. Int J Oral Maxillofac Surg 2020;49:392–6. [DOI] [PubMed] [Google Scholar]
- 22. Lee S, Choi D, Shim J-H, Nam W.. Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction. Sci Rep 2020;10:4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mascarenhas W, Makhoul N.. Efficient in-house 3D printing of an orthognathic splint for single-jaw cases. Int J Oral Maxillofac Surg 2021;50:1075–7. [DOI] [PubMed] [Google Scholar]
- 24. Lee S-J, Yoo J-Y, Woo S-Y, Yang HJ, Kim J, Huh K-H, Lee S-S, Heo M-S, Hwang SJ, Yi W-J.. A complete digital workflow for planning, simulation, and evaluation in orthognathic surgery. J Clin Med 2021;10:4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang P, Wang Y, Xu H, Huang Y, Shi Y, Chen S, Bai D, Xue C.. Effect of offset on the precision of 3D-printed orthognathic surgical splints. Clin Oral Investig 2023;27:5141–51. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Wang P, Xiang X, Xu H, Tang Y, Zhou Y, Bai D, Xue C.. Effect of occlusal coverage depths on the precision of 3D-printed orthognathic surgical splints. BMC Oral Health 2022;22:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winder J, Cooke R, Gray J, Fannin T, Fegan T.. Medical rapid prototyping and 3D CT in the manufacture of custom made cranial titanium plates. J Med Eng Technol 1999;23:26–8. [DOI] [PubMed] [Google Scholar]
- 28. Morris CL, Barber RF, Day R.. Orofacial prosthesis design and fabrication using stereolithography. Aust Dent J 2000;45:250–3. [DOI] [PubMed] [Google Scholar]
- 29. Scolozzi P, Martinez A, Jaques B.. Complex orbito-fronto-temporal reconstruction using computer-designed PEEK implant. J Craniofac Surg 2007;18:224–8. [DOI] [PubMed] [Google Scholar]
- 30. Klammert U, Gbureck U, Vorndran E, Rödiger J, Meyer-Marcotty P, Kübler AC.. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J Cranio-Maxillo-Fac Surg: Off Publ Eur Assoc Cranio-Maxillo-Fac Surg 2010;38:565–70. [DOI] [PubMed] [Google Scholar]
- 31. Culié D, Dassonville O, Poissonnet G, Riss J-C, Fernandez J, Bozec A.. Virtual planning and guided surgery in fibular free-flap mandibular reconstruction: a 29-case series. Eur Ann Otorhinolaryngol Head Neck Dis 2016;133:175–8. [DOI] [PubMed] [Google Scholar]
- 32. Lee S-H, Lee K-G, Hwang J-H, Cho YS, Lee K-S, Jeong H-J, Park S-H, Park Y, Cho Y-S, Lee B-K.. Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater Sci Eng C Mater Biol Appl 2019;98:949–59. [DOI] [PubMed] [Google Scholar]
- 33. Chang HK, Yang DH, Ha MY, Kim HJ, Kim CH, Kim SH, Choi JW, Chun HJ.. 3D printing of cell-laden visible light curable glycol chitosan bioink for bone tissue engineering. Carbohydr Polym 2022;287:119328. [DOI] [PubMed] [Google Scholar]
- 34. Vosselman N, Glas HH, Merema BJ, Kraeima J, Reintsema H, Raghoebar GM, Witjes MJH, de Visscher SAHJ.. Three-dimensional guided zygomatic implant placement after maxillectomy. J Pers Med 2022;12:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meyer S, Hirsch J-M, Leiggener CS, Msallem B, Sigron GR, Kunz C, Thieringer FM.. Fibula graft cutting devices: are 3D-printed cutting guides more precise than a universal, reusable osteotomy jig? J Clin Med 2020;9:4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palka L, Konstantinovic V, Pruszynski P, Jamroziak K.. Analysis using the finite element method of a novel modular system of additively manufactured osteofixation plates for mandibular fractures—a preclinical study. Biomed Signal Process Control 2021;65:102342. [Google Scholar]
- 37. van Baar GJC, Leeuwrik L, Lodders JN, Liberton NPTJ, Karagozoglu KH, Forouzanfar T, Leusink FKJ.. A novel treatment concept for advanced stage mandibular osteoradionecrosis combining isodose curve visualization and nerve preservation: a prospective pilot study. Front Oncol 2021;11:630123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Postl L, Mücke T, Hunger S, Wuersching SN, Holberg S, Bissinger O, Burgkart R, Malek M, Krennmair S.. Biopsies of osseous jaw lesions using 3D-printed surgical guides: a clinical study. Eur J Med Res 2022;27:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miljanovic D, Seyedmahmoudian M, Horan B, Stojcevski A.. Novel and accurate 3D-printed surgical guide for mandibular reconstruction with integrated dental implants. Comput Biol Med 2022;151:106327. [DOI] [PubMed] [Google Scholar]
- 40. Ho C-T, Lin H-H, Lo L-J.. Intraoral scanning and setting up the digital final occlusion in three-dimensional planning of orthognathic surgery: its comparison with the dental model approach. Plast Reconstr Surg 2019;143:1027e–36e. [DOI] [PubMed] [Google Scholar]
- 41. Ye N, Wu T, Dong T, Yuan L, Fang B, Xia L.. Precision of 3D-printed splints with different dental model offsets. Am J Orthod Dentofac Orthop 2019;155:733–8. [DOI] [PubMed] [Google Scholar]
- 42. Reymus M, Fabritius R, Keßler A, Hickel R, Edelhoff D, Stawarczyk B.. Fracture load of 3D-printed fixed dental prostheses compared with milled and conventionally fabricated ones: the impact of resin material, build direction, post-curing, and artificial aging—an in vitro study. Clin Oral Investig 2020;24:701–10. [DOI] [PubMed] [Google Scholar]
- 43. de Arruda JAA, Silva LV de O, Kato C de NA de O, Schuch LF, Batista AC, Costa NL, Tarquinio SBC, Rivero ERC, Carrard VC, Martins MD, Sobral APV, Mesquita RA.. A multicenter study of malignant oral and maxillofacial lesions in children and adolescents. Oral Oncol 2017;75:39–45. [DOI] [PubMed] [Google Scholar]
- 44. Pabst A, Goetze E, Thiem DGE, Bartella AK, Seifert L, Beiglboeck FM, Kröplin J, Hoffmann J, Zeller A-N.. 3D printing in oral and maxillofacial surgery: a nationwide survey among university and non-university hospitals and private practices in Germany. Clin Oral Investig 2022;26:911–9. [DOI] [PubMed] [Google Scholar]
- 45. Goodson AMC, Parmar S, Ganesh S, Zakai D, Shafi A, Wicks C, O'Connor R, Yeung E, Khalid F, Tahim A, Gowrishankar S, Hills A, Williams EM.. Printed titanium implants in UK craniomaxillofacial surgery. part II: perceived performance (outcomes, logistics, and costs). Br J Oral Maxillofac Surg 2021;59:320–8. [DOI] [PubMed] [Google Scholar]
- 46. Pereira I, Pereira JE, Maltez L, Rodrigues A, Rodrigues C, Oliveira M, Silva DM, Caseiro AR, Prada J, Maurício AC, Santos JD, Gama M.. Regeneration of critical-sized defects, in a goat model, using a dextrin-based hydrogel associated with granular synthetic bone substitute. Regen Biomater 2021;8:rbaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharma N, Ostas D, Rotar H, Brantner P, Thieringer FM.. Design and additive manufacturing of a biomimetic customized cranial implant based on Voronoi diagram. Front Physiol 2021;12:647923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee U-L, Yun S, Lee H, Cao H-L, Woo S-H, Jeong Y-H, Jung T-G, Kim CM, Choung P-H.. Osseointegration of 3D-printed titanium implants with surface and structure modifications. Dent Mater 2022;38:1648–60. [DOI] [PubMed] [Google Scholar]
- 49. Li Z, Lu Y, Liu Q, Ni S, Zhou M, Zheng S, Lin J, Sun H.. Applicative assessment of a selective laser melting 3D-printed Ti–6Al–4 V plate with a honeycomb structure in the reconstruction of a mandibular defect of a beagle dog. ACS Biomater Sci Eng 2023;9:6472–80. [DOI] [PubMed] [Google Scholar]
- 50. Chen C, Huang B, Liu Y, Liu F, Lee I-S.. Functional engineering strategies of 3D printed implants for hard tissue replacement. Regen Biomater 2023;10:rbac094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Major R, Kowalczyk P, Surmiak M, Łojszczyk I, Podgórski R, Trzaskowska P, Ciach T, Russmueller G, Kasperkiewicz K, Major Ł, Jabłoński R, Kropiwnicki J, Lackner JM.. Patient specific implants for jawbone reconstruction after tumor resection. Colloids Surf B Biointerfaces 2020;193:111056. [DOI] [PubMed] [Google Scholar]
- 52. Jeong W-S, Kim Y-C, Min J-C, Park H-J, Lee E-J, Shim J-H, Choi J-W.. Clinical application of 3D-printed patient-specific polycaprolactone/beta tricalcium phosphate scaffold for complex zygomatico-maxillary defects. Polymers (Basel) 2022;14:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luo C, Liu Y, Peng B, Chen M, Liu Z, Li Z, Kuang H, Gong B, Li Z, Sun H.. PEEK for oral applications: recent advances in mechanical and adhesive properties. Polymers (Basel) 2023;15:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tan X, Wang Z, Yang X, Yu P, Sun M, Zhao Y, Yu H.. Enhancing cell adhesive and antibacterial activities of glass-fibre-reinforced polyetherketoneketone through mg and ag PIII. Regen Biomater 2023;10:rbad066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ren Y, Huang L, Wang Y, Mei L, Fan R, He M, Wang C, Tong A, Chen H, Guo G.. Stereocomplexed electrospun nanofibers containing poly (lactic acid) modified quaternized chitosan for wound healing. Carbohydr Polym 2020;247:116754. [DOI] [PubMed] [Google Scholar]
- 56. Oladapo BI, Zahedi SA, Ismail SO, Omigbodun FT.. 3D printing of PEEK and its composite to increase biointerfaces as a biomedical material: a review. Colloids Surf B Biointerfaces 2021;203:111726. [DOI] [PubMed] [Google Scholar]
- 57. Lommen J, Schorn L, Sproll C, Haussmann J, Kübler NR, Budach W, Rana M, Tamaskovics B.. Reduction of CT artifacts using polyetheretherketone (PEEK), polyetherketoneketone (PEKK), polyphenylsulfone (PPSU), and polyethylene (PE) reconstruction plates in oral oncology. J Oral Maxillofac Surg 2022;80:1272–83. [DOI] [PubMed] [Google Scholar]
- 58. Zheng Z, Liu P, Zhang X, Jingguo Xin N, Yongjie Wang N, Zou X, Mei X, Zhang S, Zhang S.. Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants. Mater Today Bio 2022;16:100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo R, Zhang R, Liu S, Yang Y, Dong W, Wang M, Mi H, Liu M, Sun J, Zhang X, Su Y, Liu Y, Huang D, Li R.. Biomimetic, biodegradable and osteoinductive treated dentin matrix/α-calcium sulphate hemihydrate composite material for bone tissue engineering. Regen Biomater 2023;10:rbad061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qiang H, Hou C, Zhang Y, Luo X, Li J, Meng C, Liu K, Lv Z, Chen X, Liu F.. CaP-coated Zn-Mn-li alloys regulate osseointegration via influencing macrophage polarization in the osteogenic environment. Regen Biomater 2023;10:rbad051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ammarullah MI, Santoso G, Sugiharto S, Supriyono T, Wibowo DB, Kurdi O, Tauviqirrahman M, Jamari J.. Minimizing risk of failure from ceramic-on-ceramic total hip prosthesis by selecting ceramic materials based on tresca stress. Sustainability 2022;14:13413. [Google Scholar]
- 62. Uddin M, Dhanasekaran PS, Asmatulu R.. Mechanical properties of highly porous PEEK bionanocomposites incorporated with carbon and hydroxyapatite nanoparticles for scaffold applications. Prog Biomater 2019;8:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kumar A, Mir SM, Aldulijan I, Mahajan A, Anwar A, Leon CH, Terracciano A, Zhao X, Su T-L, Kalyon DM, Kumbar SG, Yu X.. Load-bearing biodegradable PCL-PGA-beta TCP scaffolds for bone tissue regeneration. J Biomed Mater Res B Appl Biomater 2021;109:193–200. [DOI] [PubMed] [Google Scholar]
- 64. Xu X, Xiao L, Xu Y, Zhuo J, Yang X, Li L, Xiao N, Tao J, Zhong Q, Li Y, Chen Y, Du Z, Luo K.. Vascularized bone regeneration accelerated by 3D-printed nanosilicate-functionalized polycaprolactone scaffold. Regen Biomater 2021;8:rbab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fan R, Deng X, Zhou L, Gao X, Fan M, Wang Y, Guo G.. Injectable thermosensitive hydrogel composite with surface-functionalized calcium phosphate as raw materials. Int J Nanomed 2014;9:615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bertin H, Huon J-F, Praud M, Fauvel F, Salagnac J-M, Perrin J-P, Mercier J-M, Corre P.. Bilateral sagittal split osteotomy training on mandibular 3-dimensional printed models for maxillofacial surgical residents. Br J Oral Maxillofac Surg 2020;58:953–8. [DOI] [PubMed] [Google Scholar]
- 67. Cho K-H, Papay FA, Yanof J, West K, Bassiri Gharb B, Rampazzo A, Gastman B, Schwarz GS.. Mixed reality and 3D printed models for planning and execution of face transplantation. Ann Surg 2021;274:e1238–e1246. [DOI] [PubMed] [Google Scholar]
- 68. Shu J, Luo H, Zhang Y, Liu Z.. 3D printing experimental validation of the finite element analysis of the maxillofacial model. Front Bioeng Biotechnol 2021;9:694140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nica DF, Gabor AG, Duma V-F, Tudericiu VG, Tudor A, Sinescu C.. Sinus lift and implant insertion on 3D-printed polymeric maxillary models: ex vivo training for in vivo surgical procedures. J Clin Med 2021;10:4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Reymus M, Fotiadou C, Hickel R, Diegritz C.. 3D-printed model for hands-on training in dental traumatology. Int Endod J 2018;51:1313–9. [DOI] [PubMed] [Google Scholar]
- 71. Im C-H, Park J-M, Kim J-H, Kang Y-J, Kim J-H.. Assessment of compatibility between various intraoral scanners and 3D printers through an accuracy analysis of 3D printed models. Materials 2020;13:4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meglioli M, Naveau A, Macaluso GM, Catros S.. 3D printed bone models in oral and cranio-maxillofacial surgery: a systematic review. 3D Print Med 2020;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wanibuchi M, Noshiro S, Sugino T, Akiyama Y, Mikami T, Iihoshi S, Miyata K, Komatsu K, Mikuni N.. Training for skull base surgery with a colored temporal bone model created by three-dimensional printing technology. World Neurosurg 2016;91:66–72. [DOI] [PubMed] [Google Scholar]
- 74. Favier V, Zemiti N, Caravaca Mora O, Subsol G, Captier G, Lebrun R, Crampette L, Mondain M, Gilles B.. Geometric and mechanical evaluation of 3D-printing materials for skull base anatomical education and endoscopic surgery simulation—a first step to create reliable customized simulators. PLoS One 2017;12:e0189486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Msallem B, Sharma N, Cao S, Halbeisen FS, Zeilhofer H-F, Thieringer FM.. Evaluation of the dimensional accuracy of 3D-printed anatomical mandibular models using FFF, SLA, SLS, MJ, and BJ printing technology. J Clin Med 2020;9:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gadaleta DJ, Huang D, Rankin N, Hsue V, Sakkal M, Bovenzi C, Huntley CT, Willcox T, Pelosi S, Pugliese R, Ku B.. 3D printed temporal bone as a tool for otologic surgery simulation. Am J Otolaryngol 2020;41:102273. [DOI] [PubMed] [Google Scholar]
- 77. Rios O, Lerhe B, Chamorey E, Savoldelli C.. Accuracy of segmented le fort I osteotomy with virtual planning in orthognathic surgery using patient-specific implants: a case series. JCM 2022;11:5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang X, Shujaat S, Shaheen E, Jacobs R.. Quality and haptic feedback of three-dimensionally printed models for simulating dental implant surgery. J Prosthet Dent 2022;131:660–7. [DOI] [PubMed] [Google Scholar]
- 79. Shujaat S, da Costa Senior O, Shaheen E, Politis C, Jacobs R.. Visual and haptic perceptibility of 3D printed skeletal models in orthognathic surgery. J Dent 2021;109:103660. [DOI] [PubMed] [Google Scholar]
- 80. Haffner M, Quinn A, Hsieh T-Y, Strong EB, Steele T.. Optimization of 3D print material for the recreation of patient-specific temporal bone models. Ann Otol Rhinol Laryngol 2018;127:338–43. [DOI] [PubMed] [Google Scholar]
- 81. Legocki AT, Duffy-Peter A, Scott AR.. Benefits and limitations of entry-level 3-dimensional printing of maxillofacial skeletal models. JAMA Otolaryngol Head Neck Surg 2017;143:389–94. [DOI] [PubMed] [Google Scholar]
- 82. Liu X-Y, Chen C, Xu H-H, Zhang Y, Zhong L, Hu N, Jia X-L, Wang Y-W, Zhong K-H, Liu C, Zhu X, Ming D, Li X-H.. Integrated printed BDNF/collagen/chitosan scaffolds with low temperature extrusion 3D printer accelerated neural regeneration after spinal cord injury. Regen Biomater 2021;8:rbab063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kämmerer PW, Al-Nawas B.. Bone reconstruction of extensive maxillomandibular defects in adults. Periodontol 2000 2023;93:340–57. [DOI] [PubMed] [Google Scholar]
- 84. Gong Z, Zhang S, Li P, Liu J, Xu Y.. Femoral artery-nourished anteromedial thigh flap: a new perspective in oral and maxillofacial defect reconstruction. Oral Oncol 2021;117:105295. [DOI] [PubMed] [Google Scholar]
- 85. Matsuda Y, Okui T, Karino M, Aoi N, Okuma S, Hayashida K, Sakamoto T, Kanno T.. Postoperative oral dysfunction following oral cancer resection and reconstruction: a preliminary cross-sectional study. Oral Oncol 2021;121:105468. [DOI] [PubMed] [Google Scholar]
- 86. Cao S-S, Li S-Y, Geng Y-M, Kapat K, Liu S-B, Perera FH, Li Q, Terheyden H, Wu G, Che Y-J, Miranda P, Zhou M.. Prefabricated 3D-printed tissue-engineered bone for mandibular reconstruction: a preclinical translational study in primate. ACS Biomater Sci Eng 2021;7:5727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Visscher DO, Farré-Guasch E, Helder MN, Gibbs S, Forouzanfar T, van Zuijlen PP, Wolff J.. Advances in bioprinting technologies for craniofacial reconstruction. Trends Biotechnol 2016;34:700–10. [DOI] [PubMed] [Google Scholar]
- 88. Chuan D, Fan R, Wang Y, Ren Y, Wang C, Du Y, Zhou L, Yu J, Gu Y, Chen H, Guo G.. Stereocomplex poly(lactic acid)-based composite nanofiber membranes with highly dispersed hydroxyapatite for potential bone tissue engineering. Compos Sci Technol 2020;192:108107. [Google Scholar]
- 89. Wang Y, Guo G, Chen H, Gao X, Fan R, Zhang D, Zhou L.. Preparation and characterization of polylactide/poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) hybrid fibers for potential application in bone tissue engineering. Int J Nanomed 2014;9:1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hayashi K, Yanagisawa T, Kishida R, Ishikawa K.. Effects of scaffold shape on bone regeneration: tiny shape differences affect the entire system. ACS Nano 2022;16:11755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang Z, Wang Y, Yan J, Zhang K, Lin F, Xiang L, Deng L, Guan Z, Cui W, Zhang H.. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev 2021;174:504–34. [DOI] [PubMed] [Google Scholar]
- 92. Helgeland E, Mohamed-Ahmed S, Shanbhag S, Pedersen TO, Rosén A, Mustafa K, Rashad A.. 3D printed gelatin-genipin scaffolds for temporomandibular joint cartilage regeneration. Biomed Phys Eng Express 2021;7:055025. [DOI] [PubMed] [Google Scholar]
- 93. Bouyer M, Garot C, Machillot P, Vollaire J, Fitzpatrick V, Morand S, Boutonnat J, Josserand V, Bettega G, Picart C.. 3D-printed scaffold combined to 2D osteoinductive coatings to repair a critical-size mandibular bone defect. Mater Today Bio 2021;11:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li Y, Liu H, Wang C, Yan R, Xiang L, Mu X, Zheng L, Liu C, Hu M.. 3D printing titanium grid scaffold facilitates osteogenesis in mandibular segmental defects. NPJ Regen Med 2023;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wei Y, Wang Z, Lei L, Han J, Zhong S, Yang X, Gou Z, Chen L.. Appreciable biosafety, biocompatibility and osteogenic capability of 3D printed nonstoichiometric wollastonite scaffolds favorable for clinical translation. J Orthop Transl 2024;45:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fitzpatrick V, Martín-Moldes Z, Deck A, Torres-Sanchez R, Valat A, Cairns D, Li C, Kaplan DL.. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials 2021;276:120995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lee JS, Park TH, Ryu JY, Kim DK, Oh EJ, Kim HM, Shim J-H, Yun W-S, Huh JB, Moon SH, Kang SS, Chung HY.. Osteogenesis of 3D-printed PCL/TCP/bdECM scaffold using adipose-derived stem cells aggregates; an experimental study in the canine mandible. Int J Mol Sci 2021;22:5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang W, Shi W, Wu S, Kuss M, Jiang X, Untrauer JB, Reid SP, Duan B.. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication 2020;12:035020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tan Y, Sun H, Lan Y, Khan HM, Zhang H, Zhang L, Zhang F, Cui Y, Zhang L, Huang D, Chen X, Zhou C, Sun J, Zhou X.. Study on 3D printed MXene-berberine-integrated scaffold for photo-activated antibacterial activity and bone regeneration. J Mater Chem B 2024;12:2158–79. [DOI] [PubMed] [Google Scholar]
- 100. Subbiah R, Hipfinger C, Tahayeri A, Athirasala A, Horsophonphong S, Thrivikraman G, França CM, Cunha DA, Mansoorifar A, Zahariev A, Jones JM, Coelho PG, Witek L, Xie H, Guldberg RE, Bertassoni LE.. 3D printing of microgel-loaded modular microcages as instructive scaffolds for tissue engineering. Adv Mater 2020;32:e2001736. [DOI] [PubMed] [Google Scholar]
- 101. Lee SS, Kleger N, Kuhn GA, Greutert H, Du X, Smit T, Studart AR, Ferguson SJ.. A 3D-printed assemblable bespoke scaffold as a versatile microcryogel carrier for site-specific regenerative medicine. Adv Mater 2023;35:e2302008. [DOI] [PubMed] [Google Scholar]
- 102. Li H, Fan R, Zou B, Yan J, Shi Q, Guo G.. Roles of MXenes in biomedical applications: recent developments and prospects. J Nanobiotechnol 2023;21:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang F, Cai X, Shen Y, Meng L.. Cell–scaffold interactions in tissue engineering for oral and craniofacial reconstruction. Bioact Mater 2023;23:16–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Placone JK, Engler AJ.. Recent advances in extrusion-based 3D printing for biomedical applications. Adv Healthc Mater 2018;7:e1701161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Grijalva Garces D, Strauß S, Gretzinger S, Schmieg B, Jüngst T, Groll J, Meinel L, Schmidt I, Hartmann H, Schenke-Layland K, Brandt N, Selzer M, Zimmermann S, Koltay P, Southan A, Tovar GEM, Schmidt S, Weber A, Ahlfeld T, Gelinsky M, Scheibel T, Detsch R, Boccaccini AR, Naolou T, Lee-Thedieck C, Willems C, Groth T, Allgeier S, Köhler B, Friedrich T, Briesen H, Buchholz J, Paulus D, von Gladiss A, Hubbuch J.. On the reproducibility of extrusion-based bioprinting: round robin study on standardization in the field. Biofabrication 2023;16:015002. [DOI] [PubMed] [Google Scholar]
- 106. Feng B, Zhang M, Qin C, Zhai D, Wang Y, Zhou Y, Chang J, Zhu Y, Wu C.. 3D printing of conch-like scaffolds for guiding cell migration and directional bone growth. Bioact Mater 2023;22:127–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang X, Liu Y, Zhang M, Zhai D, Wang Y, Zhuang H, Ma B, Qu Y, Yu X, Ma J, Ma H, Yao Q, Wu C.. 3D printing of black bioceramic scaffolds with micro/nanostructure for bone tumor-induced tissue therapy. Adv Healthc Mater 2021;10:e2101181. [DOI] [PubMed] [Google Scholar]
- 108. Xue J, Qin C, Wu C.. 3D printing of cell-delivery scaffolds for tissue regeneration. Regen Biomater 2023;10:rbad032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ling Z, Zhao J, Song S, Xiao S, Wang P, An Z, Fu Z, Shao J, Zhang Z, Fu W, Song S.. Chitin nanocrystal-assisted 3D bioprinting of gelatin methacrylate scaffolds. Regen Biomater 2023;10:rbad058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pasini C, Pandini S, Re F, Ferroni M, Borsani E, Russo D, Sartore L.. New poly(lactic acid)-hydrogel core-shell scaffolds highly support MSCs’ viability, proliferation and osteogenic differentiation. Polymers (Basel) 2023;15:4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Siebert L, Luna-Cerón E, García-Rivera LE, Oh J, Jang J, Rosas-Gómez DA, Pérez-Gómez MD, Maschkowitz G, Fickenscher H, Oceguera-Cuevas D, Holguín-León CG, Byambaa B, Hussain MA, Enciso-Martinez E, Cho M, Lee Y, Sobahi N, Hasan A, Orgill DP, Mishra YK, Adelung R, Lee E, Shin SR.. Light-controlled growth factors release on tetrapodal ZnO-incorporated 3D-printed hydrogels for developing smart wound scaffold. Adv Funct Mater 2021;31:2007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ma H, Yang C, Ma Z, Wei X, Younis MR, Wang H, Li W, Wang Z, Wang W, Luo Y, Huang P, Wang J.. Multiscale hierarchical architecture-based bioactive scaffolds for versatile tissue engineering. Adv Healthc Mater 2022;11:e2102837. [DOI] [PubMed] [Google Scholar]
- 113. Li F, Yan Y, Wang Y, Fan Y, Zou H, Liu H, Luo R, Li R, Liu H.. A bifunctional MXene-modified scaffold for photothermal therapy and maxillofacial tissue regeneration. Regen Biomater 2021;8:rbab057. [Google Scholar]
- 114. Zhao X, Wang S, Wang F, Zhu Y, Gu R, Yang F, Xu Y, Xia D, Liu Y.. 3D-printed Mg-1Ca/polycaprolactone composite scaffolds with promoted bone regeneration. J Magnesium Alloys 2024;12:966–79. [Google Scholar]
- 115. Wang S, Gu R, Wang F, Zhao X, Yang F, Xu Y, Yan F, Zhu Y, Xia D, Liu Y.. 3D-printed PCL/Zn scaffolds for bone regeneration with a dose-dependent effect on osteogenesis and osteoclastogenesis. Mater Today Bio 2022;13:100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hu S, Yi Y, Ye C, Liu J, Wang J.. Advances in 3D printing techniques for cartilage regeneration of temporomandibular joint disc and mandibular condyle. Ijb 2023;9:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hogan KJ, Öztatlı H, Perez MR, Si S, Umurhan R, Jui E, Wang Z, Jiang EY, Han SR, Diba M, Jane Grande-Allen K, Garipcan B, Mikos AG.. Development of photoreactive demineralized bone matrix 3D printing colloidal inks for bone tissue engineering. Regen Biomater 2023;10:rbad090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zandrini T, Florczak S, Levato R, Ovsianikov A.. Breaking the resolution limits of 3D bioprinting: future opportunities and present challenges. Trends Biotechnol 2023;41:604–14. [DOI] [PubMed] [Google Scholar]
- 119. Cai Y, Chang SY, Gan SW, Ma S, Lu WF, Yen C-C.. Nanocomposite bioinks for 3D bioprinting. Acta Biomater 2022;151:45–69. [DOI] [PubMed] [Google Scholar]
- 120. Mohabatpour F, Duan X, Yazdanpanah Z, Tabil XL, Lobanova L, Zhu N, Papagerakis S, Chen X, Papagerakis P.. Bioprinting of alginate-carboxymethyl chitosan scaffolds for enamel tissue engineering in vitro. Biofabrication 2022;15:015022. [DOI] [PubMed] [Google Scholar]
- 121. Wusiman P, Maimaitituerxun B, Saimaiti A, Moming A, Guli,. Epidemiology and pattern of oral and maxillofacial trauma. J Craniofacial Surg 2020;31:e517–e520. [DOI] [PubMed] [Google Scholar]
- 122. Le Clerc N, Baudouin R, Carlevan M, Khoueir N, Verillaud B, Herman P.. 3D titanium implant for orbital reconstruction after maxillectomy. J Plast Reconstr Aesthet Surg 2020;73:732–9. [DOI] [PubMed] [Google Scholar]
- 123. Weadock WJ, Heisel CJ, Kahana A, Kim J.. Use of 3D printed models to create molds for shaping implants for surgical repair of orbital fractures. Acad Radiol 2020;27:536–42. [DOI] [PubMed] [Google Scholar]
- 124. Yu X, Li G, Zheng Y, Gao J, Fu Y, Wang Q, Huang L, Pan X, Ding J.. ‘Invisible’ orthodontics by polymeric ‘clear’ aligners molded on 3D-printed personalized dental models. Regen Biomater 2022;9:rbac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang Y, Qu X, Jiang J, Sun J, Zhang C, He Y.. Aesthetical and accuracy outcomes of reconstruction of maxillary defect by 3D virtual surgical planning. Front Oncol 2021;11:718946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sigron GR, Rüedi N, Chammartin F, Meyer S, Msallem B, Kunz C, Thieringer FM.. Three-dimensional analysis of isolated orbital floor fractures pre- and post-reconstruction with standard titanium meshes and “hybrid” patient-specific implants. J Clin Med 2020;9:1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Naujokat H, Gökkaya AI, Açil Y, Loger K, Klüter T, Fuchs S, Wiltfang J.. In vivo biocompatibility evaluation of 3D-printed nickel-titanium fabricated by selective laser melting. J Mater Sci Mater Med 2022;33:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Xia D, Yang F, Zheng Y, Liu Y, Zhou Y.. Research status of biodegradable metals designed for oral and maxillofacial applications: a review. Bioact Mater 2021;6:4186–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. May MM, Howe BM, O'Byrne TJ, Alexander AE, Morris JM, Moore EJ, Kasperbauer JL, Janus JR, Van Abel KM, Dickens HJ, Price DL.. Short and long-term outcomes of three-dimensional printed surgical guides and virtual surgical planning versus conventional methods for fibula free flap reconstruction of the mandible: decreased nonunion and complication rates. Head Neck 2021;43:2342–52. [DOI] [PubMed] [Google Scholar]
- 130. Zhang S, Sun X, Kang C, Yang M, Zhao Y, Wang C.. Study on repairing canine mandibular defect with porous Mg–Sr alloy combined with Mg–Sr alloy membrane. Regen Biomater 2020;7:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Li J, Li X, Ma K, Sun J, Bai N, Liu Y.. Rehabilitation of long-term mandibular defects by whole-process digital fibula flap combining with implants: a case report. J Prosthodont 2023;32:187–95. [DOI] [PubMed] [Google Scholar]
- 132. Annino DJ, Sethi RK, Hansen EE, Horne S, Dey T, Rettig EM, Uppaluri R, Kass JI, Goguen LA.. Virtual planning and 3D‐printed guides for mandibular reconstruction: factors impacting accuracy. Laryngoscope Investig Otolaryngol 2022;7:1798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhang J, Wang J, You J, Qin X, Chen H, Hu X, Zhao Y, Xia Y.. Surface demineralized freeze-dried bone allograft followed by reimplantation in a failed mandibular dental implant. Regen Biomater 2024;11:rbad102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lisiak-Myszke M, Marciniak D, Bieliński M, Sobczak H, Garbacewicz Ł, Drogoszewska B.. Application of finite element analysis in oral and maxillofacial surgery-a literature review. Materials 2020;13:3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pourtaheri N, Peck CJ, Gowda A, Parsaei Y, Allam O, Patel VK, Park E, Yu J, Lopez J, Steinbacher DM.. Perceived age and personality profiling after orthognathic surgery. Plast Reconstr Surg 2022;150:146–54. [DOI] [PubMed] [Google Scholar]
- 136. Apostolakis D, Michelinakis G, Kamposiora P, Papavasiliou G.. The current state of computer assisted orthognathic surgery: a narrative review. J Dent 2022;119:104052. [DOI] [PubMed] [Google Scholar]
- 137. Kuehle R, Scheurer M, Bouffleur F, Fuchs J, Engel M, Hoffmann J, Freudlsperger C.. Accuracy of patient-specific implants in virtually planned segmental le fort I osteotomies. JCM 2023;12:6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Karanxha L, Rossi D, Hamanaka R, Giannì AB, Baj A, Moon W, Del Fabbro M, Romano M.. Accuracy of splint vs splintless technique for virtually planned orthognathic surgery: a voxel-based three-dimensional analysis. J Cranio-Maxillofac Surg 2021;49:1–8. [DOI] [PubMed] [Google Scholar]
- 139. Kraeima J, Schepers RH, Spijkervet FKL, Maal TJJ, Baan F, Witjes MJH, Jansma J.. Splintless surgery using patient-specific osteosynthesis in le fort I osteotomies: a randomized controlled multi-centre trial. Int J Oral Maxillofac Surg 2020;49:454–60. [DOI] [PubMed] [Google Scholar]
- 140. Lo L-J, Niu L-S, Liao C-H, Lin H-H.. A novel CAD/CAM composite occlusal splint for intraoperative verification in single-splint two-jaw orthognathic surgery. Biomed J 2021;44:353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hanafy M, Akoush Y, Abou-ElFetouh A, Mounir RM.. Precision of orthognathic digital plan transfer using patient-specific cutting guides and osteosynthesis versus mixed analogue-digitally planned surgery: a randomized controlled clinical trial. Int J Oral Maxillofac Surg 2020;49:62–8. [DOI] [PubMed] [Google Scholar]
- 142. Sánchez-Jáuregui E, Baranda- Manterola E, Ranz- Colio Á, Bueno de Vicente Á, Acero- Sanz J.. Custom made cutting guides and osteosynthesis plates versus CAD/CAM occlusal splints in positioning and fixation of the maxilla in orthognathic surgery: a prospective randomized study. J Cranio-Maxillofac Surg 2022;50:609–14. [DOI] [PubMed] [Google Scholar]
- 143. Aly P, Mohsen C.. Comparison of the accuracy of three-dimensional printed casts, digital, and conventional casts: an in vitro study. Eur J Dent 2020;14:189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Li B, Wei H, Jiang T, Qian Y, Zhang T, Yu H, Zhang L, Wang X.. Randomized clinical trial of the accuracy of patient-specific implants versus CAD/CAM splints in orthognathic surgery. Plast Reconstr Surg 2021;148:1101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Diaconu A, Holte MB, Berg-Beckhoff G, Pinholt EM.. Three-dimensional accuracy and stability of personalized implants in orthognathic surgery: a systematic review and a meta-analysis. JPM 2023;13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Imai H, Yamashita Y, Takasu H, Fujita K, Ono T, Hirota M, Mitsudo K.. Accuracy and influencing factors of maxillary and mandibular repositioning using pre-bent locking plates: a prospective study. Br J Oral Maxillofac Surg 2023;61:659–65. [DOI] [PubMed] [Google Scholar]
- 147. Badiali G, Bevini M, Lunari O, Lovero E, Ruggiero F, Bolognesi F, Feraboli L, Bianchi A, Marchetti C.. PSI-guided mandible-first orthognathic surgery: maxillo-mandibular position accuracy and vertical dimension adjustability. J Pers Med 2021;11:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Badiali G, Bevini M, Ruggiero F, Cercenelli L, Lovero E, De Simone E, Rucci P, Bianchi A, Marchetti C.. Validation of a patient-specific system for mandible-first bimaxillary surgery: ramus and implant positioning precision assessment and guide design comparison. Sci Rep 2020;10:13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Abel AR, Ho K, Neugarten JM.. What is the accuracy of bimaxillary orthognathic surgery using occlusally-based guides and patient-specific fixation in both jaws? a cohort study and discussion of surgical techniques. J Oral Maxillofac Surg 2022;80:1912–26. [DOI] [PubMed] [Google Scholar]
- 150. Harding J, Hartsfield JK, Mian AS, Allan BP, Naoum S, Lee RJH, Goonewardene MS.. Accuracy of mandibular proximal segment position using virtual surgical planning and custom osteosynthesis plates. Int J Oral Maxillofac Surg 2022;51:219–25. [DOI] [PubMed] [Google Scholar]
- 151. Borikanphanitphaisan T, Lin C-H, Chen Y-A, Ko EW-C.. Accuracy of mandible-first versus maxilla-first approach and of thick versus thin splints for skeletal position after two-jaw orthognathic surgery. Plast Reconstr Surg 2021;147:421–31. [DOI] [PubMed] [Google Scholar]
- 152. Cui M-X, Xiao L-C, Yue J, Xue L-F, Xiao W-L.. Effect of a digital guide on the positional accuracy of intermaxillary fixation screw implantation in orthognathic surgery. J Plast Reconstr Aesthet Surg 2022;75:e15–e22. [DOI] [PubMed] [Google Scholar]
- 153. Holte MB, Diaconu A, Ingerslev J, Thorn JJ, Pinholt EM.. Virtual surgical analysis: long-term cone beam computed tomography stability assessment of segmental bimaxillary surgery. Int J Oral Maxillofac Surg 2022;51:1188–96. [DOI] [PubMed] [Google Scholar]
- 154. da Costa Senior O, Vaes L, Mulier D, Jacobs R, Politis C, Shaheen E.. Three dimensional assessment of segmented le fort I osteotomy planning and follow-up: a validation study. J Dent 2021;111:103707. [DOI] [PubMed] [Google Scholar]
- 155. Wang R, Choi WS.. Wafer as an adjunct to plating patient-specific implants for the multi-segment maxilla: a useful tool. Int J Oral Maxillofac Surg 2022;51:1055–8. [DOI] [PubMed] [Google Scholar]
- 156. H van der W, Kraeima J, Spijkervet FKL, Schepers RH, Jansma J.. Postoperative skeletal stability at the one-year follow-up after splintless le fort I osteotomy using patient-specific osteosynthesis versus conventional osteosynthesis: a randomized controlled trial. Int J Oral Maxillofac Surg 2023;52:679–85. [DOI] [PubMed] [Google Scholar]
- 157. Ohrbach R, Greene C.. Temporomandibular disorders: priorities for research and care. J Dent Res 2022;101:742–3. [DOI] [PubMed] [Google Scholar]
- 158. Olliver SJ, Broadbent JM, Thomson WM, Farella M.. Occlusal features and TMJ clicking: a 30-year evaluation from a cohort study. J Dent Res 2020;99:1245–51. [DOI] [PubMed] [Google Scholar]
- 159. Wu Y, Cisewski SE, Coombs MC, Brown MH, Wei F, She X, Kern MJ, Gonzalez YM, Gallo LM, Colombo V, Iwasaki LR, Nickel JC, Yao H.. Effect of sustained joint loading on TMJ disc nutrient environment. J Dent Res 2019;98:888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. De Meurechy NKG, Zaror CE, Mommaerts MY.. Total temporomandibular joint replacement: stick to stock or optimization by customization? Craniomaxillofac Trauma Reconstr 2020;13:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Oldhoff MGE, Mirzaali MJ, Tümer N, Zhou J, Zadpoor AA.. Comparison in clinical performance of surgical guides for mandibular surgery and temporomandibular joint implants fabricated by additive manufacturing techniques. J Mech Behav Biomed Mater 2021;119:104512. [DOI] [PubMed] [Google Scholar]
- 162. Zheng J, Chen X, Jiang W, Zhang S, Chen M, Yang C.. An innovative total temporomandibular joint prosthesis with customized design and 3D printing additive fabrication: a prospective clinical study. J Transl Med 2019;17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Mahmoud K, Galal N, Ali S, Gibaly A, Elbehairy MS, Mounir M.. Computer-guided arthrocentesis using patient-specific guides: a novel protocol for treatment of internal derangement of the temporomandibular joint. J Oral Maxillofac Surg 2020;78:372.e1-372–e7. [DOI] [PubMed] [Google Scholar]
- 164. Nickel JC, Iwasaki LR, Gonzalez YM, Gallo LM, Yao H.. Mechanobehavior and ontogenesis of the temporomandibular joint. J Dent Res 2018;97:1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Cheng K-J, Liu Y-F, Wang JH, Wang R, Xia J, Xu X, Jiang X-F, Dong X-T.. 3D-printed porous condylar prosthesis for temporomandibular joint replacement: design and biomechanical analysis. THC 2022;30:1017–30. [DOI] [PubMed] [Google Scholar]
- 166. Zou L, Zhong Y, Xiong Y, He D, Li X, Lu C, Zhu H.. A novel design of temporomandibular joint prosthesis for lateral pterygoid muscle attachment: a preliminary study. Front Bioeng Biotechnol 2020;8:630983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zou L, Zhong Y, Li X, Yang X, He D.. 3D-printed porous tantalum scaffold improves muscle attachment via integrin-β1-activated AKT/MAPK signaling pathway. ACS Biomater Sci Eng 2023;9:889–99. [DOI] [PubMed] [Google Scholar]
- 168. Hurrell MJL, Singh J, Leinkram D, Clark JR.. Patient specific implant with high condylar neck osteotomy for temporomandibular joint preservation in segmental mandibulectomy. Oral Oncol 2022;134:106084. [DOI] [PubMed] [Google Scholar]
- 169. Tang Q, Li Y, Yu T, Chen X, Zhou Z, Huang W, Liang F.. Association between condylar position changes and functional outcomes after condylar reconstruction by free fibular flap. Clin Oral Investig 2021;25:95–103. [DOI] [PubMed] [Google Scholar]
- 170. Zhou Y, Al-Naggar IMA, Chen P-J, Gasek NS, Wang K, Mehta S, Kuchel GA, Yadav S, Xu M.. Senolytics alleviate the degenerative disorders of temporomandibular joint in old age. Aging Cell 2021;20:e13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Li Y, Zhou M, Zheng W, Yang J, Jiang N.. Scaffold-based tissue engineering strategies for soft–hard interface regeneration. Regen Biomater 2023;10:rbac091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wang Y, Wang Q, Luo S, Chen Z, Zheng X, Kankala RK, Chen A, Wang S.. 3D bioprinting of conductive hydrogel for enhanced myogenic differentiation. Regen Biomater 2021;8:rbab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Chen H, Fei F, Li X, Nie Z, Zhou D, Liu L, Zhang J, Zhang H, Fei Z, Xu T.. A facile, versatile hydrogel bioink for 3D bioprinting benefits long-term subaqueous fidelity, cell viability and proliferation. Regen Biomater 2021;8:rbab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Jiang N, Yang Y, Zhang L, Jiang Y, Wang M, Zhu S.. 3D-printed polycaprolactone reinforced hydrogel as an artificial TMJ disc. J Dent Res 2021;100:839–46. [DOI] [PubMed] [Google Scholar]
- 175. Moura C, Trindade D, Vieira M, Francisco L, Ângelo DF, Alves N.. Multi-material implants for temporomandibular joint disc repair: tailored additive manufacturing production. Front Bioeng Biotechnol 2020;8:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Helgeland E, Rashad A, Campodoni E, Goksøyr Ø, Pedersen TØ, Sandri M, Rosén A, Mustafa K.. Dual-crosslinked 3D printed gelatin scaffolds with potential for temporomandibular joint cartilage regeneration. Biomed Mater 2021;16:035026. [DOI] [PubMed] [Google Scholar]
- 177. Peng B-Y, Singh AK, Tsai C-Y, Chan C-H, Deng Y-H, Wu C-M, Chou Y-R, Tsao W, Wu C-Y, Deng W-P.. Platelet-derived biomaterial with hyaluronic acid alleviates temporal-mandibular joint osteoarthritis: clinical trial from dish to human. J Biomed Sci 2023;30:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Custódio ALN, Cameron A, Bakr M, Little C, Chrcanovic BR, Reher P.. Positioning accuracy assessment of minimally invasive percutaneous injection techniques for the treatment of temporomandibular disorders. Dento Maxillo Facial Radiol 2021;50:20200313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Zhu F, Zhi Y, Xu X, Wu J, Si J, Shi J, Xu B.. Interpositional arthroplasty of post-traumatic temporomandibular joint ankylosis: a modified method. J Cranio-Maxillo-Fac Surg: Off Publ Eur Assoc Cranio-Maxillo-Fac Surg 2021;49:373–80. [DOI] [PubMed] [Google Scholar]
- 180. Yang M-Y, Tseng H-C, Liu C-H, Tsai S-Y, Chen J-H, Chu Y-H, Li S-T, Lee J-J, Liao W-C.. Effects of the individual three-dimensional printed craniofacial bones with a quick response code on the skull spatial knowledge of undergraduate medical students. Anat Sci Educ 2023;16:858–69. [DOI] [PubMed] [Google Scholar]
- 181. AlAli AB, Griffin MF, Calonge WM, Butler PE.. Evaluating the use of cleft lip and palate 3D-printed models as a teaching aid. J Surg Educ 2018;75:200–8. [DOI] [PubMed] [Google Scholar]
- 182. Rama M, Schlegel L, Wisner D, Pugliese R, Ramesh S, Penne R, Watson A.. Using three-dimensional printed models for trainee orbital fracture education. BMC Med Educ 2023;23:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Al-Badri N, Touzet-Roumazeille S, Nuytten A, Ferri J, Charkaluk M-L, Nicot R.. Three-dimensional printing models improve long-term retention in medical education of pathoanatomy: a randomized controlled study. Clin Anat 2022;35:609–15. [DOI] [PubMed] [Google Scholar]
- 184. Mogali SR, Chandrasekaran R, Radzi S, Peh ZK, Tan GJS, Rajalingam P, Yee Yeong W.. Investigating the effectiveness of three-dimensionally printed anatomical models compared with plastinated human specimens in learning cardiac and neck anatomy: a randomized crossover study. Anat Sci Educ 2022;15:1007–17. [DOI] [PubMed] [Google Scholar]
- 185. Chen S, Pan Z, Wu Y, Gu Z, Li M, Liang Z, Zhu H, Yao Y, Shui W, Shen Z, Zhao J, Pan H.. The role of three-dimensional printed models of skull in anatomy education: a randomized controlled trail. Sci Rep 2017;7:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ardila CM, González-Arroyave D, Zuluaga-Gómez M.. Efficacy of three-dimensional models for medical education: a systematic scoping review of randomized clinical trials. Heliyon 2023;9:e13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Nicot R, Druelle C, Schlund M, Roland-Billecart T, Gwénaël R, Ferri J, Gosset D.. Use of 3D printed models in student education of craniofacial traumas. Dent Traumatol 2019;35:296–9. [DOI] [PubMed] [Google Scholar]
- 188. McMillan A, Kocharyan A, Dekker SE, Kikano EG, Garg A, Huang VW, Moon N, Cooke M, Mowry SE.. Comparison of materials used for 3D-printing temporal bone models to simulate surgical dissection. Ann Otol Rhinol Laryngol 2020;129:1168–73. [DOI] [PubMed] [Google Scholar]
- 189. Nicot R, Couly G, Ferri J, Levaillant J-M.. Three-dimensional printed haptic model from a prenatal surface-rendered oropalatal sonographic view: a new tool in the surgical planning of cleft lip/palate. Int J Oral Maxillofac Surg 2018;47:44–7. [DOI] [PubMed] [Google Scholar]
- 190. Nicot R, Druelle C, Hurteloup E, Levaillant J-M.. Prenatal craniofacial abnormalities: from ultrasonography to three-dimensional printed models. Ultrasound Obst Gyn 2019;54:835–6. [DOI] [PubMed] [Google Scholar]
- 191. Schlund M, Levaillant J-M, Nicot R.. Three-dimensional printing of prenatal ultrasonographic diagnosis of cleft lip and palate: presenting the needed ‘know-how’ and discussing its use in parental education. Cleft Palate Craniofac J 2020;57:1041–4. [DOI] [PubMed] [Google Scholar]
- 192. De Ketele A, Meeus J, Shaheen E, Verstraete L, Politis C.. The usefulness of cutting guides for resection or biopsy of mandibular lesions: a technical note and case report. J Stomatol Oral Maxillofac Surg 2023;124:101272. [DOI] [PubMed] [Google Scholar]
- 193. Lotz M, Schumacher C, Stadlinger B, Ikenberg K, Rücker M, Valdec S.. Accuracy of guided biopsy of the jawbone in a clinical setting: a retrospective analysis. J Cranio-Maxillofac Surg 2021;49:556–61. [DOI] [PubMed] [Google Scholar]
- 194. Valdec S, Schiefersteiner M, Rücker M, Stadlinger B.. Guided biopsy of osseous pathologies in the jaw bone using a 3D-printed, tooth-supported drilling template. Int J Oral Maxillofac Surg 2019;48:1028–31. [DOI] [PubMed] [Google Scholar]
- 195. Chamoli A, Gosavi AS, Shirwadkar UP, Wangdale KV, Behera SK, Kurrey NK, Kalia K, Mandoli A.. Overview of oral cavity squamous cell carcinoma: risk factors, mechanisms, and diagnostics. Oral Oncol 2021;121:105451. [DOI] [PubMed] [Google Scholar]
- 196. Liu W, Shen X, Shi L, He Y.. Whether early-stage (n0 neck) oral cancer patients received primary tumor resection without neck dissection benefit from immune checkpoint therapy. Int J Surg 2023;109:3715–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Yang C, Blum NT, Lin J, Qu J, Huang P.. Biomaterial scaffold-based local drug delivery systems for cancer immunotherapy. Sci Bull (Beijing) 2020;65:1489–504. [DOI] [PubMed] [Google Scholar]
- 198. Wang C, Yu Y, Irfan M, Xu B, Li J, Zhang L, Qin Z, Yu C, Liu H, Su X.. Rational design of DNA framework-based hybrid nanomaterials for anticancer drug delivery. Small 2020;16:e2002578. [DOI] [PubMed] [Google Scholar]
- 199. Zielińska A, Karczewski J, Eder P, Kolanowski T, Szalata M, Wielgus K, Szalata M, Kim D, Shin SR, Słomski R, Souto EB.. Scaffolds for drug delivery and tissue engineering: the role of genetics. J Control Release 2023;359:207–23. [DOI] [PubMed] [Google Scholar]
- 200. Wang H, Najibi AJ, Sobral MC, Seo BR, Lee JY, Wu D, Li AW, Verbeke CS, Mooney DJ.. Biomaterial-based scaffold for in situ chemo-immunotherapy to treat poorly immunogenic tumors. Nat Commun 2020;11:5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Xu Y, Wang S, Xiong J, Zheng P, Zhang H, Chen S, Ma Q, Shen J, Velkov T, Dai C, Jiang H.. Fe3O4-incorporated metal-organic framework for chemo/ferroptosis synergistic anti-tumor via the enhanced chemodynamic therapy. Adv Healthc Mater 2024;13:e2303839. [DOI] [PubMed] [Google Scholar]
- 202. Hu J, Chen S, Yang Y, Li L, Cheng X, Cheng Y, Huang Q.. A smart hydrogel with anti-biofilm and anti-virulence activities to treat pseudomonas aeruginosa infections. Adv Healthc Mater 2022;11:e2200299. [DOI] [PubMed] [Google Scholar]
- 203. El-Husseiny HM, Mady EA, Hamabe L, Abugomaa A, Shimada K, Yoshida T, Tanaka T, Yokoi A, Elbadawy M, Tanaka R.. Smart/stimuli-responsive hydrogels: cutting-edge platforms for tissue engineering and other biomedical applications. Mater Today Bio 2022;13:100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Liu C, Wang Z, Wei X, Chen B, Luo Y.. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater 2021;131:314–25. [DOI] [PubMed] [Google Scholar]
- 205. Dutta SD, Ganguly K, Hexiu J, Randhawa A, Moniruzzaman M, Lim K-T.. A 3D bioprinted nanoengineered hydrogel with photoactivated drug delivery for tumor apoptosis and simultaneous bone regeneration via macrophage immunomodulation. Macromol Biosci 2023;23:e2300096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.