Abstract
Early events play a decisive role in virus multiplication. We have shown previously that activation of MAPK/ERK1/2 (mitogen-activated protein kinase/extracellular-signal-regulated kinase 1/2) and protein kinase A are pivotal for vaccinia virus (VV) multiplication [de Magalhães, Andrade, Silva, Sousa, Ropert, Ferreira, Kroon, Gazzinelli and Bonjardim (2001) J. Biol. Chem. 276, 38353–38360]. In the present study, we show that VV infection provoked a sustained activation of both ERK1/2 and RSK2 (ribosomal S6 kinase 2). Our results also provide evidence that this pattern of kinase activation depends on virus multiplication and ongoing protein synthesis and is maintained independently of virus DNA synthesis. It is noteworthy that the VGF (VV growth factor), although involved, is not essential for prolonged ERK1/2 activation. Furthermore, our findings suggest that the VV-stimulated ERK1/2 activation also seems to require actin dynamics, microtubule polymerization and tyrosine kinase phosphorylation. The VV-stimulated pathway MEK/ERK1/2/RSK2 (where MEK stands for MAPK/ERK kinase) leads to phosphorylation of the ternary complex factor Elk-1 and expression of the early growth response (egr-1) gene, which kinetically paralleled the kinase activation. The recruitment of this pathway is biologically relevant, since its disruption caused a profound effect on viral thymidine kinase gene expression, viral DNA replication and VV multiplication. This pattern of sustained kinase activation after VV infection is unique. In addition, by connecting upstream signals generated at the cytoskeleton and by tyrosine kinase, the MEK/ERK1/2/RSK2 cascade seems to play a decisive role not only at early stages of the infection, i.e. post-penetration, but is also crucial to define the fate of virus progeny.
Keywords: egr-1, extracellular-signal-regulated kinase 1/2 (ERK1/2), MAPK/ERK kinase (MEK), signal transduction, vaccinia virus (VV), VV growth factor (VGF)
Abbreviations: Ara C, cytosine arabinoside; BAD, Bcl-2/Bcl-XL-antagonist, causing cell death; CHX, cycloheximide; egr-1, early growth response gene-1; ERK1/2, extracellular-signal-regulated kinase 1/2; FBS, foetal bovine serum; GEN, genistein; hpi, hour post-infection; IMV, intracellular mature virus; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; MOI, multiplicity of infection; PKA, protein kinase A; PTX, pertussis toxin; RSK, ribosomal S6 kinase; TK, thymidine kinase; VV, vaccinia virus; VGF, VV growth factor; VGF−, mutant VV; WR, wild-type VV
INTRODUCTION
The orthopoxvirus vaccinia virus (VV) is the prototype member of the Poxviridae family of viruses, which replicates in the cytoplasm of infected cells. Its linear double-stranded DNA genome encodes more than 260 gene products and many of them are associated with virus–host interaction [1,2]. Among them are the homologues of cytokine and chemokine receptors, which enable the virus to modulate host defences and, consequently, to evade the immune and inflammatory responses [3–5]. Furthermore, VV is also capable of secreting VGF (VV growth factor), a polypeptide with structural and functional similarities to the EGF (epidermal growth factor) and TGF-α (transforming growth factor-α) [6,7], and has been associated with cell proliferative response [8]. The secretion of growth factor homologues by other poxviruses seems to be a common theme employed by this family of viruses to stimulate mitogenesis, emphasizing the crucial roles played by these proteins at the early stages of poxvirus multiplication [9].
MAPK/ERK1/2 (mitogen-activated protein kinase/extracellular-signal-regulated kinase 1/2) plays a vital role in the transmission not only of mitogenic, but also survival signals in response to a variety of extracellular stimuli [10]. Since cell survival is vital for virus multiplication, it comes with no surprise that many viruses activate/manipulate this pathway. Thus viruses such as hepatitis B virus [11], herpes virus [12], HIV [13], influenza virus [14], coxsackievirus [15] and VV [16], among others, activate the MAPK/ERK1/2 pathway at the early stages of infection. The virus-induced change in gene expression after ERK activation is mediated by the phosphorylation of downstream transcription factors such as FOS, JUN, ATF1/2, cAMP-response-element-binding protein, Elk-1, among others, which in turn bind to the 5′ regulatory sequence of target genes to promote their transcription [16,17].
The present study was undertaken to investigate the role played by ERK1/2 during VV infection. Since we have shown previously that activation of ERK1/2 was required to deliver mitogenic signals at the early stages of infection [16], we now extend these investigations and provide evidence that these kinases are also required for the whole virus multiplication cycle. By integrating upstream signals generated by diverse pathways at MEK/ERK/RSK2 (where MEK stands for MAPK/ERK kinase and RSK for ribosomal S6 kinase) signalling molecules, VV seems to modulate the most adequate intracellular environment to generate its progeny.
EXPERIMENTAL
Cells and reagents
A31 cells (a clone derived from mouse Balb/c 3T3) were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% (v/v) heat-inactivated FBS (foetal bovine serum; Cultilab, Campinas, São Paulo, Brazil) and antibiotics, in 5% CO2 at 37 °C. Cells were starved after reaching 80–90% of confluence when the medium was changed to 1% FBS and incubated for 12–24 h. Viral TK (thymidine kinase) message was investigated by using a specific probe as described in [18]. The anti-phospho-ERK1/2 and -Elk-1 antibodies were purchased from Cell Signaling Technology (Beverly, MA, U.S.A.) and the anti-phospho-RSK2 and anti-egr-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The inhibitors used throughout the experiments were purchased either from Calbiochem (La Jolla, CA, U.S.A.) or from Sigma (São Paulo, Brazil).
Virus and virus infection
WR (wild-type VV) strain and VGF− (mutant VV) (vSC20) [19] were propagated into Vero cells and highly purified by sucrose-gradient sedimentation as described in [20]. There are two infective forms of VV, the IMV (intracellular mature virus) and the EEV (extracellular enveloped virus), which use distinct mechanisms to enter the target cells. Whereas the IMV form requires a signalling-dependent mechanism for entry, the EEV seems to be independent [21]. It is believed that the IMV remains inside the cells that are released after cell lysis. The experiments presented in this study were performed with the IMV form of VV.
VV was UV-inactivated after exposure of the virus stock for 5 min to an UV lamp producing irradiation predominantly at 365 nm. Then the UV-irradiated virus was tested for virus infectivity. Virus that was no longer capable of forming plaques compared with the non-irradiated virus was assumed to be UV-inactivated.
VV infections of A31 cells were performed when the cultures reached 80–90% of confluence. Cells were infected in the absence of FBS at the indicated MOI (multiplicity of infection) and for the times shown. Cells were incubated with the indicated inhibitor for 30 min before VV infection and throughout the infection.
Unless otherwise stated, following concentration of inhibitors were used: PD98059 (50 μM), GEN (genistein; 100 μM), SB203580 (10 μM), H89 (20 μM), CHX (cycloheximide; 100 μg/ml), cytochalasin D (0.1–2.0 μM), nocodazole (10–30 μM), PTX (pertussis toxin; 500 μg/ml) and Ara C (cytosine arabinoside; 40 μg/ml). The doses of inhibitors used throughout the experiments were established based on experimental observations, i.e. they did not cause any harm to the cells, as was verified at the end of the experiments by Trypan Blue dye exclusion.
Assays of virus infectivity
Cells were cultured as above at a density of 1.5×106 cells/well on a six-well culture dish and then VV-infected. Infections were performed at an MOI of 10.0 for 10 h to ensure that 90% of the cells were infected, as was verified microscopically by the cytopathic effect at the end of the experiment (Table 1). The same was also observed when the experiments were performed with the MOI of 1.0, 30–36 hpi (hour post-infection). The time course of virus growth with time points varying from 6 up to 48 hpi (Figure 9) was performed as described above. Infections were terminated after 6, 12, 24, 36 and 48 h; virus was then collected and assayed for infectivity. Cells were preincubated for 30 min with PD98059 (50 or 100 μM) or GEN (50 or 100 μM) as indicated before virus infection for an additional 10 h (Table 1) or for 6– 48 h as shown in Figure 9. Cultures were then washed with cold PBS, followed by three freeze–thaw cycles. Viruses were collected from the supernatant of centrifuged frozen–thawed cells and then assayed for infectivity as described in [20]. Each experiment was performed in triplicate and the results represent the average values. The results were confirmed by at least three independent experiments with identical results.
Table 1. Effect of MEK and tyrosine kinase inhibition on VV multiplication.
Cells were incubated either with PD98059 or with GEN at the indicated concentrations, before virus infection. Infections were performed at an MOI of 10.0 either with WR or VGF− virus. The course of the infection was microscopically monitored until the cytopathic effect reached ≈90% of the culture. Viruses were then collected and assayed for infectivity.
| No. | Treatment | Virus yield (%) | Inhibition (%) |
|---|---|---|---|
| 1 | None | 100* | 0 |
| 2 | VV-WR-PD (50 μM) | 34 | 66 |
| 3 | VV-WR-PD (100 μM) | 18 | 82 |
| 4 | VV-VGF−-PD (50 μM) | 36 | 64† |
| 5 | VV-VGF−-PD (100 μM) | 16 | 84† |
| 6 | VV-WR-GEN (50 μM) | 13 | 87 |
| 7 | VV-WR-GEN (100 μM) | 9 | 91 |
* Untreated virus control was taken as 100% (=6.0×106 p.f.u./ml; VV-WR).
† Untreated virus control was taken as 100% (=9.0×105 p.f.u./ml; VV-VGF).
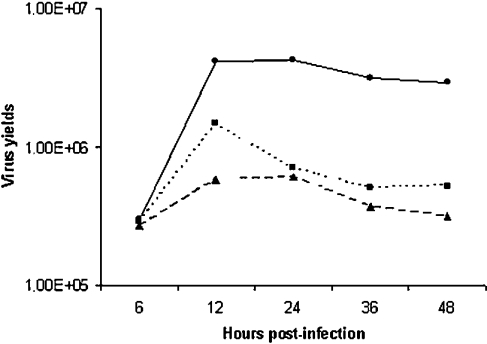
Figure 9. PD98059 and GEN inhibit VV growth.
A time course of VV growth was performed either in the absence or in the presence of PD98059 or GEN (50 μM). Cells were infected with VV at MOI of 10 and 6, 12, 24, 36 or 48 hpi, virus was collected and assayed for infectivity. Results are representative of at least two independent experiments. –•–, VV; --▴--, Gen + VV; ···▪···, PD98059 + VV.
RNA isolation and Northern blotting
A31 cells (5×106) were cultured and starved as above. Then the cells were incubated with PD98059 (50 μM) before virus infection at an MOI of 10.0 for the times shown. Total RNA was isolated as described elsewhere [22], and 15 μg of RNA/sample was loaded, electrophoresed on a 1.2% denaturing agarose–formaldehyde gel, transferred on to nylon membrane (Amersham Biosciences, São Paulo, Brazil) and UV cross-linked for 2 min. The membrane was then probed with viral TK probe labelled with [α-32P]dCTP (Amersham Biosciences) to a specific activity of 1–5×108 c.p.m./μg of DNA by using a multiprime DNA-labelling system. Hybridization and washing procedures were performed as described in [22]. The membrane was then stripped off the probe and re-probed with 18 S rRNA, labelled with [γ-32P]ATP by using phage T4 polynucleotide kinase from Promega (Madison, WI, U.S.A.), which was used as an internal control for RNA loading.
Dot-blot assays
A31 cells (5×106) were cultured and starved as above and then were infected with VV at an MOI of 10.0 for 3, 5, 7 or 9 h, either in the presence or absence of PD98059 (50 μM). After the infections, the cells were scraped from the dishes and collected by centrifugation as described in [23]. In brief, after centrifugation, the cells were washed with cold PBS and resuspended in 0.3 ml of loading buffer [10×SSC (1.5 M NaCl/150 mM sodium citrate, pH 7.0.), 1 M ammonium acetate]. Then the cells were frozen and thawed three times followed by the addition of 0.45 ml of loading buffer. Each sample (25 μl) was applied under vacuum to Hybond-N membranes (Amersham Biosciences) using a HYBRI-DOT Manifold apparatus (BRL, Life Technologies, Rockville, MD, U.S.A.). The DNA was denatured for 10 min with 500 mM NaOH, plus 1.5 M NaCl, and the membrane was washed twice with 10×SSC in situ. DNA was then cross-linked by exposing the membrane to UV light for 5 min. Hybridization conditions were as described above for Northern blot.
Viral DNA isolation and labelling
Viral DNA was isolated from purified virus stocks by treatment with proteinase K, SDS and 2-mercaptoethanol, followed by phenol/chloroform extraction as described previously [24]. DNA was labelled with [α-32P]dCTP by using the Rediprime kit (Amersham Biosciences), according to the manufacturer's instructions. Labelled DNA was then denatured and used to probe the dot-blotted samples.
Densitometric analysis
The phosphorylated ERK1/2 was quantified by using a densitometer (phosphoimager, Typhoon 9210; Amersham Biosciences) and normalized to the levels of total ERK1/2 in the same sample. The changes in protein phosphorylation with respect to control values were estimated. The results were expressed in terms of the ratio P-ERK/total ERK (arbitrary units).
Lysate preparation
Cells were grown and starved as above and were then infected with VV at MOI of 10.0, unless stated otherwise, for the indicated times. Cells were preincubated with the specific inhibitor as indicated for 30 min, and throughout the infection, and then VV-infected. Cells were then washed twice and lysed in lysis buffer [20 mM Tris-acetate, pH 7.0/0.27 M sucrose/1 mM EDTA/1 mM EGTA/1% Triton X-100/10 mM α-glycerophosphate/50 mM NaF/5 mM sodium pyrophosphate/4 μg/ml leupeptin/1 mM sodium orthovanadate/1 mM benzamidine/0.1% (v/v) 2-mercaptoethanol]. Lysates were scraped and collected into Eppendorf tubes and then centrifuged at 13000 g for 20 min at 4 °C.
Electrophoresis and immunoblotting
Cell lysates (30 μg/sample) were separated by SDS/PAGE (10% gel) and then transferred on to nitrocellulose membranes as described in [16]. Membranes were blocked overnight at 4 °C with PBS containing 5% (w/v) non-fat milk and 0.1% Tween 20. The membranes were washed three times with PBS, containing 0.1% Tween 20, and then incubated with specific rabbit or goat polyclonal antibody (1:1000 or 1:2500) in PBS containing 5% (w/v) BSA and 0.1% Tween 20. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary anti-rabbit or anti-goat antibody (1:3000). Immunoreactive bands were visualized by using ECL® detection system according to the manufacturer's instructions (Amersham Biosciences).
RESULTS
Prolonged kinase activation after VV infection
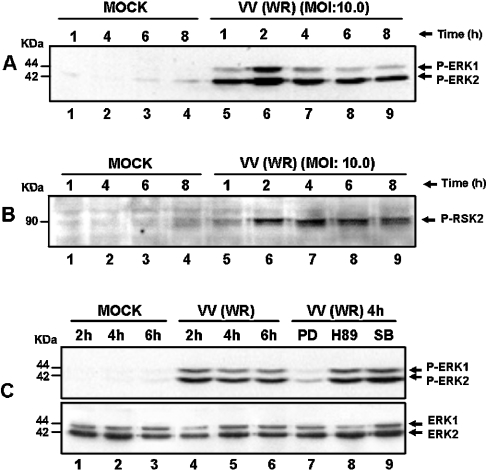
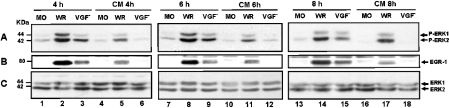
We have shown previously that, soon after VV infection, there was no temporal difference in terms of MAPK/ERK1/2 activation irrespective of the MOI used, i.e. 1.0, 5.0 or 25.0 [16]. Nonetheless, under these conditions, a remarkable quantitative MOI-dependent effect was clearly seen. This observation prompts us to investigate whether ERK1/2 was also recruited after VV infection at later stages of virus multiplication. Interestingly, VV caused a prolonged and yet sustained co-activation of distinct protein kinases. Figure 1(A) shows that ERK1/2 was phosphorylated after virus infection at an MOI of 10.0 from 1.0 up to 8.0 hpi, an event that was prolonged until 10.0 hpi (results not shown). It may be noted that ERK1/2 activation depends on the MOI and was verified up to 30.0 hpi at an MOI of 1.0 (results not shown). Furthermore, the latest ERK1/2 activation verified with each MOI was paralleled quantitatively by the observed cytopathic effect. The activation of these MAPKs was followed by stimulation of the downstream kinase RSK2 with kinetics that paralleled with those of ERK1/2 (Figure 1B). We also show that MEK is specifically recruited to activate ERK1/2, since PD98059 abrogated ERK activation, whereas the PKA (protein kinase A) (H89) and p38MAPK (SB203580) inhibitors did not (Figure 1C, upper panel). We have also observed that MEK is equally necessary for RSK2 activation (see below; and Figure 3B, middle panel).
Figure 1. Sustained kinase activation after VV infection.
Cells were serum-starved and then infected with VV at the MOI and times indicated. Western-blot analysis was performed with cell lysates, and 30 μg of protein/lane were fractionated by SDS/PAGE, transferred on to nitrocellulose and then probed with the specific anti-phospho-antibody. Cells were either mock- or VV-infected. (A, B) ERK1/2- and RSK2-activation respectively. (C) Upper panel: PD98059 caused a specific inhibition of ERK1/2 phosphorylation. Cells were either left untreated (lanes 1–6), or preincubated for 30 min with protein kinase inhibitors: 50 μM MEK (PD98059), lane 7; 20 μM PKA (H89), lane 8 or 10 μM p38MAPK (SB203580), lane 9; then mock-infected (lanes 1–3) or VV-infected (WR) at an MOI of 10.0 as indicated. Lower panel: the same blot was re-probed with total ERK as an internal control for protein loading. The position of the phosphoproteins or the molecular-mass standard (kDa) is indicated on the right or on the left respectively. The results were consistently repeated in at least two independent experiments.
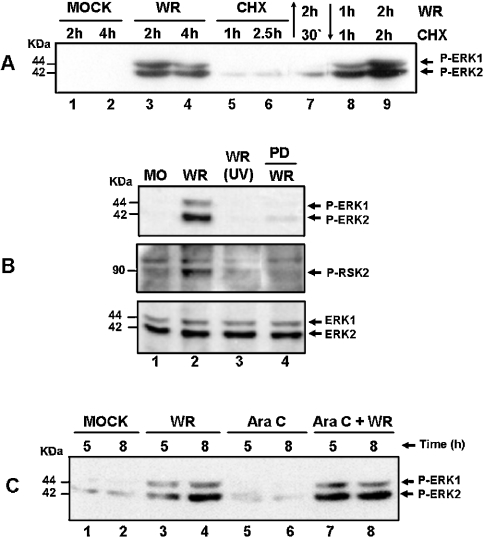
Figure 3. VV multiplication and ongoing protein synthesis is a prerequisite for ERK/RSK activation.
Western-blot analysis was performed as described in Figure 1. Cells were either mock-infected (MO) or VV-infected (WR) at an MOI of 10.0, for the indicated times. (A) Effect of CHX (100 μg/ml) on ERK1/2 activation. Lanes: 1 and 2, mock-infected; 3 and 4, VV-infected; 5 and 6, incubated with CHX alone; 7, incubated with CHX for 30 min. Before VV infection for 2 h, lanes 8 and 9 were infected with VV for 1 and 2 h and then treated with CHX for 1 and 2 h respectively. (B) Top and middle panels: ERK1/2 and RSK2 activations respectively rely on virus multiplication. Cells were infected with the WR, or with UV-inactivated virus (UV), or preincubated with PD98059 (50 μM) before VV infection for 4 h as shown and then probed with anti-phospho-ERK or anti-phospho-RSK2. Bottom panel: the same blot as above probed with anti-total ERK1/2 for loading control. (C) Prolonged ERK activation occurs independent of virus DNA synthesis. Lanes 1 and 2, mock-infected; lanes 3 and 4, VV-infected; lanes 5 and 6, incubated with Ara C (40 μg/ml) alone; lanes 7 and 8, incubated with Ara C for 30 min before virus infection. The results were consistently reproduced in at least two independent experiments.
VGF is partially required for ERK/RSK activation
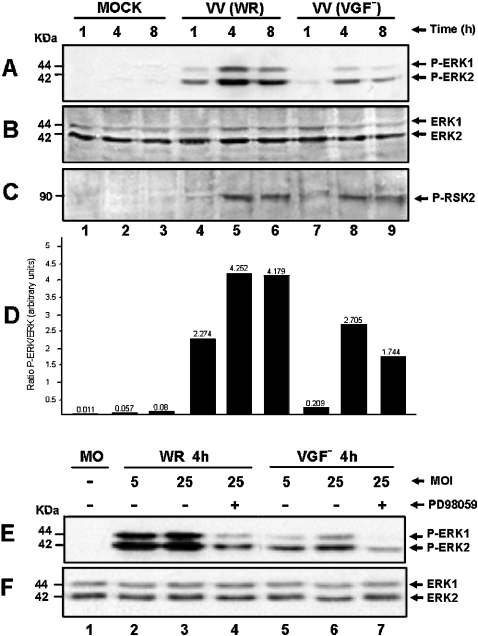
To investigate whether VGF plays a role in the prolonged ERK/RSK activation, cells were either infected with WR or VGF− at an MOI of 10.0 for the indicated times. Interestingly, although it appears that VGF might also be involved in the sustained activation of ERK1/2, although not fundamentally necessary, it seems, nonetheless, that this level of kinase activation is sufficient for RSK2 activation (Figures 2A and 2C, lanes 7–9).
Figure 2. VGF is partially required for MAPK activation.
Western-blot analysis was performed as described in Figure 1. Cells were VV-infected at an MOI of 10.0 for the times shown. (A) Cells were either mock-infected (lanes 1–3) or infected with WR (lanes 4–6) or with VGF− virus (lanes 7–9), blotted and then probed with anti-phospho-ERK1/2. (B) Same as in (A), except that the blot was probed with anti-ERK1/2 for loading control. (C) Same as in (A), except that the blot was probed with anti-phospho-RSK2. Blots are representative of at least two independent experiments with identical results. (D) Represents the quantification of two independent experiments taken from Figure 2, showing the ratio between phospho-ERK1/2 (A) and total ERK1/2 (B). Blots were quantified by phosphoimager. (E) Cells were either mock-infected (lane 1) or infected for 4 h with the WR (lanes 2–4) at an MOI of 5.0 (lane 2) or 25.0 (lanes 3 and 4). Lanes 5–7: infected with the VGF− at an MOI of 5.0 (lane 5) or 25.0 (lanes 6–7), blotted and then probed with anti-phospho-ERK1/2. (F) Same as in (E), except that the blot was probed with total ERK1/2 as a control of protein loading.
To determine whether the level of kinase activation after VGF− infection is of biological relevance, additional experiments were performed. Cells were infected at an MOI of 5.0 or 25.0 and then they were either left untreated or pretreated with the MEK inhibitor PD98059. Figure 2(E) shows that ERK1/2 was phosphorylated in an MOI-dependent manner. The highest level of kinase activation seems to be achieved with the MOI of 25.0 (lanes 3 and 6). To confirm that ERK1/2 activation is relevant to the biology of the mutant virus, we then infected A31 cells with VGF− at an MOI of 10.0. The course of infection, either in the presence or absence of PD98059, was then microscopically monitored until the cytopathic effect was observed in ≈90% of the cells. Virus was then collected and assayed for infectivity. The results are presented in Table 1, which shows that there is a dose-dependent decrease in VGF− virus yield in the presence of PD98059, as was also observed for the wild-type virus. However, the mutant is not as capable as the WR in activating ERK1/2 (Figure 2A), which may explain, at least partially, why the mutant's multiplication is lower.
Sustained activation of ERK/RSK relies on de novo protein synthesis
We then investigated whether ongoing protein synthesis exerted any effect on VV-stimulated ERK1/2 activation. Cells were pre-incubated with CHX and then VV-infected. Figure 3(A) shows that, in this circumstance, the virus is no longer capable of activating the kinases (lane 7). However, ERK1/2 gets activated if virus infection preceded the incubation with CHX (lanes 8–9). Furthermore, our results also provide evidence that virus multiplication is an essential requirement for ERK1/2 activation (Figure 3B, upper panel), since the UV-inactivated virus is no longer capable of activating the kinases (lane 3). The same was equally true for RSK2 (Figure 3B, middle panel). The next experiment was designed to evaluate whether ERK activation occurred independent of VV DNA synthesis. The infection was performed in the presence of Ara C, an inhibitor of poxviral DNA synthesis. Our findings showed that kinase phosphorylation was maintained independent of virus DNA replication (Figure 3C, lanes 7–8), emphasizing a specific role for an early virus gene(s), but not intermediate or late genes, in the process.
VGF is necessary for both full ERK1/2 activation and EGR-1 (early growth response-1) expression
Consistent with the idea of the requirement of ongoing protein synthesis for VV-stimulated ERK1/2 activation, the next experiment was designed to demonstrate that VGF, although not fundamental, is necessary for full ERK activation. It has been known that hyperplasia, associated with VV infection, is a consequence of VGF secretion by an infected cell [8]. Thus cells were infected either with WR or VGF−, and then their supernatants were taken 4, 6 and 8 hpi (conditioned medium, CM) and used to stimulate starved cells for an additional 4, 6 or 8 h respectively. Figure 4 shows that VGF is an absolute requirement for full ERK activation (Figure 4A), since its absence at the supernatants of VGF−-infected cells correlates with their inability to phosphorylate ERK1/2. Moreover, it is also necessary for the expression of the VV-stimulated egr-1 (Figure 4B).
Figure 4. VGF is necessary for both full ERK1/2 activation and EGR-1 expression.
Western-blot analysis was performed as described in Figure 1. Infections were performed at an MOI of 10.0. Cells were mock-infected (lanes 1, 7 and 13), or infected with WR (lanes 2, 8 and 14), or infected with VGF− (lanes 3, 9 and 15), for 4, 6 or 8 h respectively. Alternatively, the supernatants from WR- or VGF−-infected cells were taken after 4, 6 and 8 hpi, collected (conditioned medium, CM) and used to stimulate starved cells for an additional 4, 6 or 8 h respectively. Blots were then probed with (A) anti-phospho-ERK1/2, (B) anti-EGR-1, (C) same blot as in (A) except that the blot was probed with anti-total ERK1/2 for protein loading. Molecular-mass standards (kDa) are indicated on the left. Arrows on the right indicate the investigated proteins. The experiments were repeated at least twice with identical results.
ERK activation leads to Elk-1 phosphorylation
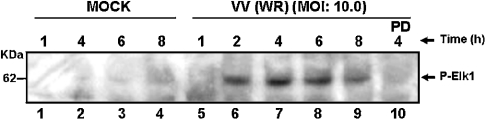
It may be noted that sustained ERK1/2 activation after VV infection leads to prolonged phosphorylation of the transcription factor Elk-1 (Figure 5), with kinetics that paralleled those observed for ERK1/2. Elk-1 phosphorylation was specifically impaired by preincubation with the MEK inhibitor (lane 10).
Figure 5. Sustained MAPK activation leads to Elk-1 phosphorylation.
Western-blot analysis of VV-infected cells as described in Figure 1. Cells were either mock-infected (lanes 1–4) or infected with WR at an MOI of 10.0 for 1, 2, 4, 6 or 8 h (lanes 5–9). Blots were then probed with anti-phospho-Elk-1. Preincubation with PD98059 (50 μM) blocked the VV-stimulated Elk-1 phosphorylation (lane 10). The blot is representative of two independent experiments with identical results.
VV stimulates an upstream tyrosine-kinase-sensitive pathway
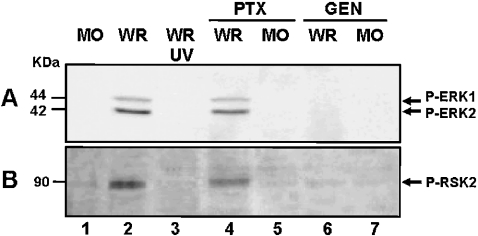
It is known that tyrosine phosphorylation also plays a crucial role on poxvirus entry [21,25]. Thus the next experiment was designed to evaluate whether this upstream tyrosine-kinase-sensitive pathway was connected to the downstream ERK1/2 activation after VV infection. We demonstrated that tyrosine phosphorylation is, in fact, another upstream and yet essential event not only for VV-activated ERK1/2 but also for RSK2 activation (Figures 6A and 6B, lanes 6 and 7). These effects, however, do not seem to be shared by the G-protein-coupled receptor, since preincubation with PTX had no impact on VV-stimulated ERK/RSK activation (Figures 6A and 6B, lanes 4 and 5). In addition, our results also demonstrated that this tyrosine-sensitive pathway is of biological relevance as shown in Table 1.
Figure 6. VV stimulates a tyrosine-kinase-sensitive pathway.
(A, B) Effects of G-protein-coupled receptors and tyrosine kinase inhibitors on VV-stimulated ERK1/2 and RSK2 activation respectively. Western-blot analysis was performed as described above. Cells were mock-infected (lane 1), or were incubated either with 500 μg/ml PTX (lanes 4–5) or with 100 μM GEN (lanes 6–7) for 30 min before VV infection at an MOI of 10.0 for 2 h. As a control for kinase activation, cells were either infected with VV (WR) or with UV-inactivated VV (UV) (lanes 2 and 3) respectively. Blots are representative of at least two independent experiments with identical results.
VV-induced actin polymerization is fundamental for both ERK and RSK activation
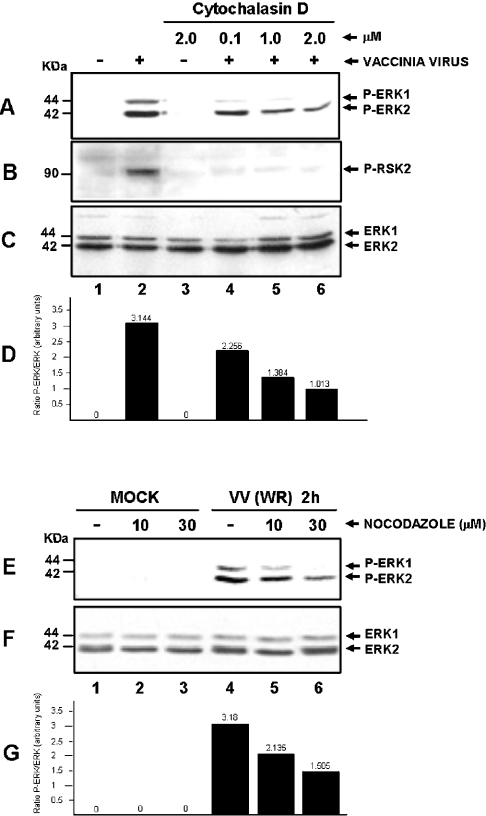
Since actin polymerization is required to facilitate both the spreading [26] and virus entry [21], we examined whether actin played a decisive role in the activation of ERK and RSK by VV. To that end, cells were preincubated for 30 min with the indicated cytochalasin D concentration, a known agent that prevents actin assembly [27]. VV infection was performed at an MOI of 10.0 for 2 h. Figure 7(A) shows that VV-stimulated ERK1/2 activation was significantly reduced after cytochalasin D treatment in a dose-dependent manner (lanes 4–6), whereas RSK2 activation was completely abolished (Figure 7B). Our results also demonstrate that when the infection was performed in the presence of nocodazole, a microtubule-depolymerizing drug [28], a remarkable dose-dependent lowering in ERK activation was also observed (Figure 7E, lanes 5 and 6). Figures 7(D) and 7(G) show the quantification of the results presented in Figures 7(A) and 7(E) and normalized to the levels of total ERK1/2 from Figures 7(C) and 7(F) respectively.
Figure 7. VV-induced actin polymerization is necessary for both ERK and RSK activations.
Western-blot analysis of MO-infected (lanes 1 and 3) or VV-infected cells at an MOI of 10.0 for 2 h (lanes 2 and 4–6), performed with different concentrations of cytochalasin D as shown. Activation of (A) ERK1/2 or (B) RSK2. (C) Blot (A) was re-probed with total ERK for loading control. (E) Effect of nocodazole on VV-stimulated ERK1/2 activation. Cells were either left untreated (lanes 1 and 4) or incubated with the indicated nocodazole concentration before Mock infection (lanes 1–3) or VV infection (lanes 4 –6) and then probed with anti-phospho-ERK1/2. The results were confirmed by at least two independent experiments. (F) Blot (E) was re-probed with anti-total ERK for loading control. (D, G) Quantification of two independent experiments taken from Figure 7, showing the ratio between phospho-ERK1/2 and total ERK1/2 (A/C) or (E/F) respectively. Blots were quantified by phosphoimager.
ERK inhibition is associated with both delayed/prolonged VV early-gene expression and viral DNA replication
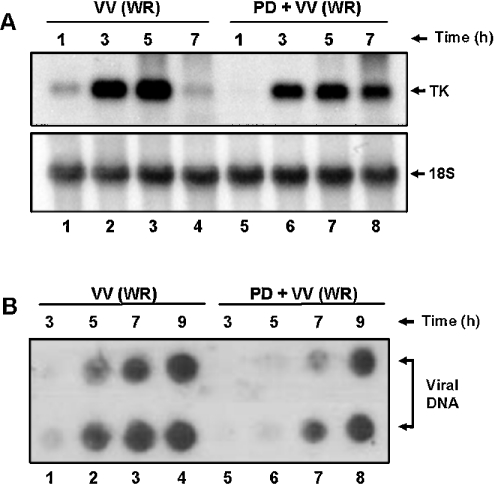
It is known that prolonged VV early-gene expression is associated with inhibition of virus DNA replication [29]. Our results show that the steady-state levels of viral TK mRNA were verified from 1 up to 5 hpi (lanes 1– 4). In contrast, TK expression was delayed and prolonged in the presence of an MEK inhibitor, which was still observable at 7 hpi (Figure 8A, lanes 5–8). Consistent with the delay observed above, Figure 8(B) demonstrates that the inhibition of ERK1/2 activation was paralleled by a simultaneous decrease in the viral DNA synthesis. Although, in the absence of PD98059, viral DNA was increasingly detected from 5 to 9 hpi (lanes 1–4), simultaneous incubation with the inhibitor provoked a significant delay in the detection of viral DNA (lanes 5–8).
Figure 8. A delayed/prolonged expression of viral TK gene and viral DNA replication after ERK blockade.
(A) Northern-blot assay performed as described in the Experimental section. Upper panel: cells were left untreated (lanes 1–4) or incubated with 50 μM PD for 30 min (lanes 5–8), before VV (WR) infection at an MOI of 10.0 for the times shown and then hybridized with labelled TK probe. Lower panel: blot was stripped of the probe and re-probed with 18 S rRNA as a control for RNA loading. (B) Dot-blot assay performed in duplicates as described in the Experimental section. Cells were either left untreated (lanes 1– 4) or treated with PD98059 (50 μM) (lanes 5–8) for 30 min before and during VV infection at an MOI of 10.0. Infections were performed for 3, 5, 7 or 9 h. Samples were prepared, transferred under vacuum to nylon membrane and then probed with labelled VV DNA.
MEK/ERK/RSK2/Elk-1/EGR-1 pathway is required for VV multiplication
To examine the biological consequences of the MEK/ERK/RSK2/Elk-1/EGR-1 pathway after VV infection, cells were incubated either with PD98059 or GEN before VV infection at an MOI of 10.0 for 10 h. The virus was then collected and assayed for infectivity. Table 1 shows that, under this experimental condition, there is a significant dose-dependent decrease in virus yields, demonstrating that this pathway is required not only for the early events during VV infection, but is also crucial, since the virus generates its progeny. Furthermore, to rule out the possibility that the inhibitors delay instead of inhibiting virus multiplication, a time course of virus growth was determined. Figure 9 shows that, in the absence of the inhibitor, virus yields reached a plateau approx. 12 hpi, whereas a decrease of ∼1 log in virus titre was observed after 12–24 hpi and reached a plateau thereafter, when the infection was performed in the presence of GEN or PD98059 respectively.
DISCUSSION
Activation of the MAPK/ERK1/2 is a common theme in response to growth factor, cytokines and hormones. After ligand binding to their cognate cell-surface receptor, the extracellular stimulus is transmitted to the nucleus to change the expression of diverse genes associated with a particular biological response (reviewed in [30]). Since these kinases are associated with a variety of cellular responses, among them, proliferation, survival, differentiation and apoptosis [10], and based on the co-evolution of viruses and their hosts, it is not surprising that these microorganisms can also recruit/manipulate them to create a more adequate intracellular environment to generate their progenies [31].
We have shown previously that the MAPK/ERK1/2 played a vital role in the early steps of VV infection [16]. Our results provided evidence that their activation took place at times when most of the viruses were not yet internalized and that the activation, at least kinetically, followed virus entry. In the present study, we demonstrated that ERK activation after VV infection played an equally fundamental role in other stages of the infection. It is noteworthy that VV-stimulated ERK1/2 activation was prolonged and yet sustained, i.e. it was detectable from the early stages of the infection, up to times when the cytopathic effect was clearly visualized in at least 90% of the culture. Thus sustained kinase activation was consistently the same 10 hpi at an MOI of 10.0 (Figure 1A) or 30–36 hpi at MOI of 1.0 (results not shown).
ERK1/2 activation also seems to be an essential requirement to regulate signals associated with diverse biological functions during infection with other viruses. Thus early events in HIV-1 infection are dependent on the MAPK/ERK pathway, which improves virus infectivity [32]. MAPK is also required for Visna virus replication and virus-induced neuropathology [33]. On the other hand, ERK activation by the herpes simplex virus exerts an anti-apoptotic effect on hippocampal neurons [17], among others.
The reason why VV stimulates and sustains the activation of both ERK1/2 remains to be further investigated. However, we hypothesize that a virus gene product either directly, as we demonstrated for VGF gene (Figures 2A and 4A), or indirectly, may be involved in the process, because (i) a UV-irradiated virus does not activate the kinases and (ii) for de novo protein synthesis it is absolutely required (see below and Figure 3). Whether the virus inactivates a phosphatase, such as protein phosphatase PP2A [34] or MAPK phosphatase MKP [35], or even another mechanism responsible for deactivating the kinases is, therefore, an interesting issue that remains to be pursued further.
The family of p90 RSK comprises three isoforms RSK1–3, which interact with and are activated by ERK [36] and inhibition of the ERK pathway prevents their activation [37]. Remarkable also was the observation that ERK activation after VV infection was paralleled by the activation of the downstream kinase RSK2 (Figure 1B), an event that is equally verified whether the infection is performed either with WR or VGF− (Figure 2C). It is probable that the level of ERK1/2 activation elicited by the VGF− (Figure 2A), although weaker than the wild-type, is sufficient to activate its substrate RSK2 to the same extent (Figure 2C). On the basis of this observation, we do not believe that the differential ERK activation by the viruses is just a consequence of delayed VGF− multiplication. Although both ERK and RSK activations after VGF− infection were smaller than those observed for the wild-type virus, it seems, nonetheless, that their activation is biologically relevant because preincubation with PD98059 resulted in a decrease in virus yields (Table 1).
Consistent with the theory of transmission of survival signals mediated by the ERKs after VV infection, is the observation of prolonged and sustained activation of RSK2. RSK family members have been implicated in cell survival by phosphorylating the proapoptotic protein BAD (Bcl-2/Bcl-XL-antagonist, causing cell death) [38]. BAD in turn is kept in the cytoplasm where it is no longer capable of antagonizing the pro-survival function of Bcl-XL [39]. Since both phosphorylation of BAD [40] and VV-stimulated RSK2 activation (Figure 3B) are sensitive to MEK inhibition, this may explain the biological consequences of VV-sustained activation of both ERK and RSK2. Thus our findings, although indirectly, reinforce the growing body of evidence associated with the anti-apoptotic mechanisms triggered after VV infection [41,42].
Our results also support the idea that sustained ERK activation after VV infection relies on both de novo protein synthesis (Figure 3A) and viral gene expression (Figure 3B). The same observations occur with RSK2 activation too (Figure 3B, middle panel). In addition, the maintenance of ERK activity occurred independent of virus DNA synthesis (Figure 3C), highlighting the role played by an early virus gene(s), such as VGF (Figure 2A), on MAPK activation.
It is largely accepted that the viral protein VGF plays a decisive role in both viral spread and virulence [7,19]. In vitro studies conducted with purified VGF protein have also demonstrated that it can elicit mitogenic response [9]. However, our in vivo experiments showed that VGF is not a prerequisite to transmit the VV-stimulated mitogenic signal [16]. In accordance with these results, the present study demonstrated that VGF appears to be an additional requirement for full ERK1/2 activation (Figure 4A). Either the virus-infected cells or supernatants from cultures infected with either the WR or the VGF− showed that VGF is only necessary for maximal ERK activation, although this level of kinase activation seems to be sufficient to stimulate RSK2.
The zinc finger transcription factor EGR-1 has been associated with diverse biological functions varying from proliferation/differentiation to apoptosis [43]. By modulating the expression of target genes, EGR-1 couples extracellular stimuli with long-term responses. Consistent with a sequential ERK/RSK activation, the expression of EGR-1, a downstream target of these kinases [44], was also verified (Figure 4B). Again, there was a remarkable parallel between prolonged kinase activation after VV infection and EGR-1 expression. The observation that VGF is needed not only for full ERK activation (Figure 4A), but also for maximal EGR-1 expression as well is significant (Figure 4B).
A global analysis of cellular gene expression after VV infection in HeLa cells has been published recently [45]. In this study, however, the authors were not able to detect egr-1 mRNA among the VV-stimulated genes. The reason(s) why they failed to do so remains a matter of speculation. We have also investigated the gene's expression during VV infection in HeLa cells and found that, kinetically, the pattern of mRNA accumulation is the same as that observed in the A31 cell line, although not as robust as that verified with this line (P. N. G. Silva and C. A. Bonjardim, unpublished work).
The pathway that couples the VV-stimulated proliferation/survival signals with the expression of EGR-1 seems to be mediated by the ternary complex factor Elk-1 [46]. Our assumption is based on the observation that both VV-stimulated Elk-1 phosphorylation (Figure 5) and EGR-1 expression (Figure 4B) share the same upstream kinases MEK/ERK/RSK, since MEK inhibition abrogated equally Elk-1 phosphorylation (Figure 5) and EGR-1 expression (P. N. G. Silva and C. A. Bonjardim, unpublished work).
The upstream molecules that deliver the signals to ERK/RSK2 after VV infection, however, remain to be investigated further. It has been published that Mixoma virus, another poxvirus, recruits both the p21-activated kinase (PAK-1) and Raf-1 to transduce signals that are vital for virus multiplication [26]. Since PAK-1 and Raf-1 might be potential upstream molecules to transmit signals resulting in MEK/ERK activation, this issue requires further investigation.
In accordance with the previously reported involvement of tyrosine kinase with both VV [22] and Mixoma [26] virus entry, our results went one step further and connected the signals generated by this pathway to the downstream activation of ERK1/2 and RSK2 (Figures 6A and 6B). It appears, nonetheless, that G-protein-coupled receptors are not involved in the process, at least the PTX-sensitive isoforms (Figures 6A and 6B). Moreover, our results also demonstrated that this tyrosine-kinase-sensitive pathway is of biological relevance, since its inhibition caused a significant reduction in virus yields (Table 1).
Our findings also established a link between the need for the remodelling of cytoskeleton during the early stages of VV infection [22] and VV-stimulated ERK/RSK activation (Figures 7A and 7B).
Although the reason why RSK2 is no longer activated after treatment with cytochalasin (cf. Figure 2C with Figure 7B) is an issue that remains to be investigated further, there are some reports in the literature demonstrating that either ERK activation [47] or nuclear translocation [48] is compromised after cytochalasin exposure. Our results also connected the microtubule network with the VV-stimulated ERK activation (Figure 7E). Our assumption is based on the following: (i) it is known that the organization of the microtubule may be involved in the early post-entry stages of VV multiplication [49]; (ii) microtubules are also required for early VV mRNA synthesis and stability [50]; and (iii) de novo protein synthesis is needed for ERK activation (Figure 3A).
Interesting also was the finding that the MEK/ERK pathway plays a critical role in the expression of the viral early gene TK (Figure 8A). These results are in accordance with our previous results concerning the involvement of these kinases with virus multiplication [16]. TK expression was delayed and prolonged in the presence of the MEK inhibitor, rather than being repressed, consistent with the results reported by others [30]. In line with these results was also the observation that VV-stimulated ERK1/2 activation leads to viral DNA replication (Figure 8B), since the same delay verified with TK expression after PD98059 treatment was paralleled by viral genome replication.
Taken together, the results from Figures 8(A) and 8(B) suggest that PD98059 might be causing a delay in virus multiplication. However, on the basis of the results presented in Figure 9, we demonstrated that both inhibitors PD98059 and GEN do really inhibit instead of delaying virus growth, since virus multiplication in the presence of the inhibitor is approx. 1 log lower than that found in the absence of the inhibitor, i.e. when comparing the growth curves as they reached a plateau (24–48 hpi).
Our results strongly suggest that the signalling pathway involving MEK/ERK/RSK2/Elk-1/EGR-1 after VV infection is not only necessary for the transmission and maintenance of proliferation/survival signals, but also has a profound effect on virus multiplication as well (Table 1), emphasizing the biological consequences of this pathway.
These findings also corroborated our previous results demonstrating the fundamental roles played by the MEK/ERK/TCF/SRF/AP-1/c-fos pathway (where TCF stands for ternary complex factor, SRF for serum response factor and AP-1 for activation protein 1, usually a dimmer of FOS/JUN) in both the transmission of early mitogenic signals triggered after VV infection and the generation of virus progeny [16]. Since we have previously shown that PKA was recruited to transmit the early mitogenic signals leading to ATF1 phosphorylation, we have also investigated whether ATF1 was required at the early-to-mid stages of VV multiplication. Although preliminary, our results showed that ATF1 is also required in other steps, other than the early stage, during VV multiplication (results not shown), consistent with the involvement of PKA at these stages of virus multiplication [16].
Altogether our findings suggest that events initiated at early steps during VV infection have a profound impact on the whole VV multiplication cycle. Thus, the remodelling of the cytoskeleton, associated with the simultaneous requirement of a yet undefined tyrosine-kinase pathway and leading to ERK/RSK activation, begins to define what culminates from the end-stage of virus multiplication, i.e. the generation of virus progeny.
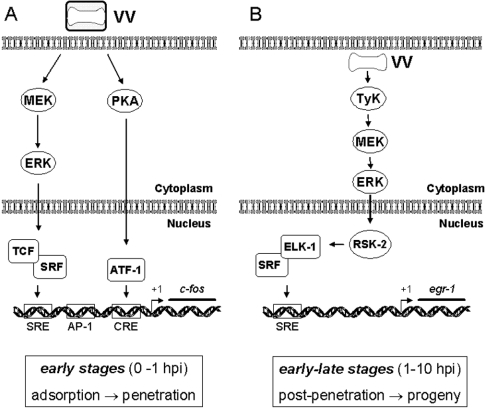
On the basis of the results presented in this paper and our previous results [16], we propose a model for the signalling events that seems to be required not only for the transmission of diverse signals, but also for VV multiplication (Figure 10; see the legend for details).
Figure 10. Signalling pathways triggered after VV infection.
(A) Early events after VV infection, up to 1 hpi, rely on the activation of MEK/ERK. Both kinase activation and transient c-fos expression are verified very early during virus–host interaction and are independent of the viral protein VGF. A structural component of the virus seems to be sufficient for c-fos stimulation, since no virus gene expression is required. PKA is also recruited to trigger the early signals. (B) Events that occur between 1 hpi until late stages of VV multiplication. Sustained ERK1/2 and RSK2 activation lead to Elk-1 phosphorylation, which in turn binds, possibly in association with SRF, to the cis-acting element SRE found at the 5′-untranslated region of EGR-1 (P. N. G. Silva and C. A. Bonjardim, unpublished work) to promote a sustained egr-1 expression. In connection with this pathway, upstream events are also required, which include tyrosine phosphorylation and actin dynamics. Viral progeny results from an orchestrated programme, which is initiated soon after virus–host interaction and requires, at least partially, sustained MEK/ERK/RSK2 activation.
To our knowledge this pattern of sustained kinase activation after VV infection is unique. There are several reports in the literature concerning MAPK/ERK activation after virus infection resulting in either transient [15] or biphasic ERK activation [14]. Sustained ERK activation has also been observed after stable transfection of plasmid bearing virus gene [11,51], but not in the context of virus infection. However, none of them have, so far, demonstrated the decisive role played by these kinases during the whole virus infection cycle. Thus by integrating diverse upstream signals at the MEK/ERK/RSK pathway, combined with the different associated biological outcomes, VV manages to achieve the most adequate intracellular environment to generate its progeny.
Acknowledgments
We are grateful to A. S. Lopes, H. M. V. Gama, M. A. Souza, J. R. dos Santos and D. Lemos for their secretarial/technical assistance. We also thank B. M. Silva for the artwork of Figure 10. Dr H. A. Armelin and Dr M. C. Sogayar (Department of Biochemistry, University of São Paulo, Brazil), kindly provided the A31 cell line. WR was obtained from Dr C. Jungwirth (Universität Würzburg, Würzburg, Germany) and VGF− (vSC20) was a gift from Dr B. Moss (National Institute of Allergy and Infectious Diseases, Bethesda, MD, U.S.A.). PTX was a gift from Dr M. M. Teixeira (Department of Biochemistry and Immunology, Federal University of Minas Gerais). This work was supported by grants from Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). A.A.A. and A.C.T.C.P. are a recipient of a predoctoral fellowship from CNPq, A.C.T.C.P. and P.N.G.S. from CAPES (Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior) and L.P.S. from the Brazilian Ministry of Culture, Science and Technology. C.A.B., E.G.K., R.T.G., P.C.P.F. and C.R. are recipients of research fellowships from CNPq.
References
- 1.Moss B. Poxviridae viruses and their replication. In: Fields B. N., Knipe D. M., Howley P. M., editors. Fields Virology. 3rd edn. vol. 2. Publishers, NY: Lippincott-Raven; 1996. pp. 2637–2672. [Google Scholar]
- 2.Symons J. A., Alcami A., Smith G. L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell (Cambridge, Mass.) 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 3.Upton C., Mossman K., McFadden G. Encoding of a homolog of the IFN-γ receptor by myxoma virus. Science. 1992;258:1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- 4.Bowie A., Kiss-Toth E., Symons J. A., Smith G. L., Dower S. K., O'Neill L. A. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10162–10167. doi: 10.1073/pnas.160027697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcami A. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 2003;3:36–50. doi: 10.1038/nri980. [DOI] [PubMed] [Google Scholar]
- 6.Twardzik D. R., Brown J. P., Ranchalis J. E., Todaro G. J., Moss B. Vaccinia virus-infected cells release a novel polypeptide functionally related to transforming and epidermal growth factors. Proc. Natl. Acad. Sci. U.S.A. 1985;82:5300–5304. doi: 10.1073/pnas.82.16.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown J. P., Twardzik D. R., Marquardt H., Todaro G. J. Vaccinia virus encodes a polypeptide homologous to epidermal growth factor and transforming growth factor. Nature (London) 1985;313:491–492. doi: 10.1038/313491a0. [DOI] [PubMed] [Google Scholar]
- 8.Buller R. M., Chakrabarti S., Moss B., Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988;164:182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- 9.Tzahar E., Moyer J. D., Waterman H., Barbacci E. G., Bao J., Levkowitz G., Shelly M., Strano S., Pinkas-Kramarski R., Pierce J. H., et al. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO J. 1998;17:5948–5963. doi: 10.1093/emboj/17.20.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sears R. C., Nevins J. R. Signaling networks that link cell proliferation and cell fate. J. Biol. Chem. 2002;277:11617–11620. doi: 10.1074/jbc.R100063200. [DOI] [PubMed] [Google Scholar]
- 11.Lee S., Tarn C., Wang W. H., Chen S., Hullinger R. L., Andrisani O. M. Hepatitis B virus X protein differentially regulates cell cycle progression in X-transforming versus nontransforming hepatocyte (AML12) cell lines. J. Biol. Chem. 2002;277:8730–8740. doi: 10.1074/jbc.M108025200. [DOI] [PubMed] [Google Scholar]
- 12.Perkins D., Pereira E. F., Gober M., Yarowsky P. J., Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) functions as a dominant regulator of apoptosis in hippocampal neurons involving activation of the ERK survival pathway and upregulation of the antiapoptotic protein Bad-1. J. Virol. 2002;76:1435–1449. doi: 10.1128/JVI.76.3.1435-1449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X., Gabuzda D. Mitogen-activated protein kinase phosphorylates and regulates the HIV-1 Vif protein. J. Biol. Chem. 1998;273:29879–29887. doi: 10.1074/jbc.273.45.29879. [DOI] [PubMed] [Google Scholar]
- 14.Pleschka S., Wolff T., Ehrhardt C., Hobom G., Planz O., Rapp U. R., Ludwig S. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 2001;3:301–305. doi: 10.1038/35060098. [DOI] [PubMed] [Google Scholar]
- 15.Opavsky M. A., Martino T., Rabinovitch M., Penninger J., Richardson C., Petric M., Trinidad C., Butcher L., Chan J., Liu P. P. Enhanced ERK-1/2 activation in mice susceptible to coxsackievirus-induced myocarditis. J. Clin. Invest. 2002;109:1561–1569. doi: 10.1172/JCI13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Magalhães J. C., Andrade A. A., Silva P. N., Sousa L. P., Ropert C., Ferreira P. C., Kroon E. G., Gazzinelli R. T., Bonjardim C. A. A mitogenic signal triggered at an early stage of vaccinia virus infection: implication of MEK/ERK and protein kinase A in virus multiplication. J. Biol. Chem. 2001;276:38353–38360. doi: 10.1074/jbc.M100183200. [DOI] [PubMed] [Google Scholar]
- 17.Perkins D., Pereira E. F., Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 2003;77:1292–1305. doi: 10.1128/JVI.77.2.1292-1305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Fonseca F. G., Trindade G. S., Silva R. L., Bonjardim C. A., Ferreira P. C., Kroon E. G. Characterization of a vaccinia-like virus isolated in a Brazilian forest. J. Gen. Virol. 2002;83:223–228. doi: 10.1099/0022-1317-83-1-223. [DOI] [PubMed] [Google Scholar]
- 19.Buller R. M., Chakrabarti S., Cooper J. A., Twardzik D. R., Moss B. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J. Virol. 1988;62:866–874. doi: 10.1128/jvi.62.3.866-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joklik W. R. The purification of four strains of poxvirus. Virology. 1962;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- 21.Locker J. K., Kuehn A., Schleich S., Rutter G., Hohenberg H., Wepf R., Griffiths G. Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell. 2000;11:2497–2511. doi: 10.1091/mbc.11.7.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franco G. R., de Carvalho A. F., Kroon E. G., Lovagie S., Werenne J., Golgher R. R., Ferreira P. C., Bonjardim C. A. Biological activities of a human amniotic membrane interferon. Placenta. 1999;20:189–196. doi: 10.1053/plac.1998.0364. [DOI] [PubMed] [Google Scholar]
- 23.Rempel R. E., Anderson M. K., Evans E., Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J. Virol. 1990;64:574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marques J. T., Trindade G. D., Da Fonseca F. G., Dos Santos J. R., Bonjardim C. A., Ferreira P. C., Kroon E. G. Characterization of ATI, TK and IFN-α/βR genes in the genome of the BeAn 58058 virus, a naturally attenuated wild orthopoxvirus. Virus Genes. 2001;23:291–301. doi: 10.1023/a:1012521322845. [DOI] [PubMed] [Google Scholar]
- 25.Johnston J. B., Barrett J. W., Chang W., Chung C. S., Zeng W., Masters J., Mann M., Wang F., Cao J., McFadden G. Role of the serine-threonine kinase PAK-1 in myxoma virus replication. J. Virol. 2003;77:5877–5888. doi: 10.1128/JVI.77.10.5877-5888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frischknecht F., Moreau V., Rottger S., Gonfloni S., Reckmann I., Superti-Furga G., Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature (London) 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 27.Cooper J. A. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storrie B., White J., Rottger S., Stelzer E. H., Suganuma T., Nilsson T. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldick C. J., Jr, Moss B. Characterization and temporal regulation of mRNAs encoded by vaccinia virus intermediate-stage genes. J. Virol. 1993;67:3515–3527. doi: 10.1128/jvi.67.6.3515-3527.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazzalin C. A., Mahadevan L. C. MAPK-regulated transcription: a continuously variable gene switch? Nat. Rev. Mol. Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- 31.Greber U. F. Signalling in viral entry. Cell. Mol. Life Sci. 2002;59:608–626. doi: 10.1007/s00018-002-8453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacque J. M., Mann A., Enslen H., Sharova N., Brichacek B., Davis R. J., Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber S. A., Bruett L., Douglass B. R., Herbst D. S., Zink M. C., Clements J. E. Visna virus-induced activation of MAPK is required for virus replication and correlates with virus-induced neuropathology. J. Virol. 2002;76:817–828. doi: 10.1128/JVI.76.2.817-828.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alessi D. R., Gomez N., Moorhead G., Lewis T., Keyse S. M., Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr. Biol. 1995;5:283–295. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- 35.Sun H., Charles C. H., Lau L. F., Tonks N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell (Cambridge, Mass.) 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 36.Smith J. A., Poteet-Smith C. E., Malarkey K., Sturgill T. W. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- 37.Lazar D. F., Wiese R. J., Brady M. J., Mastick C. C., Waters S. B., Yamauchi K. Pessin J. E., Cuatrecasas P., Saltiel A. R. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J. Biol. Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- 38.Bonni A., Brunet A., West A. E., Datta S. R., Takasu M. A., Greenberg M. E. Cell survival promoted by the Ras-MAPK signalling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 39.Zha J., Harada H., Yang E., Jockel J., Korsmeyer S. J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell (Cambridge, Mass.) 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 40.Scheid M. P., Schubert K. M., Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J. Biol. Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 41.Kibler K. V., Shors T., Perkins K. B., Zeman C. C., Banaszak M. P., Biesterfeldt J., Langland J. O., Jacobs B. L. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J. Virol. 1997;71:1992–2003. doi: 10.1128/jvi.71.3.1992-2003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia M. A., Guerra S., Gil J., Jimenez V., Esteban M. Anti-apoptotic and oncogenic properties of the dsRNA-binding protein of vaccinia virus, E3L. Oncogene. 2002;21:8379–8387. doi: 10.1038/sj.onc.1206036. [DOI] [PubMed] [Google Scholar]
- 43.Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. , et al. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell (Cambridge, Mass.) 1988;53:37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- 44.Gineitis D., Treisman R. Differential usage of signal transduction pathways defines two types of serum response factor target gene. J. Biol. Chem. 2001;276:24531–24539. doi: 10.1074/jbc.M102678200. [DOI] [PubMed] [Google Scholar]
- 45.Guerra S., Lopez-Fernandez L. A., Pascual-Montano A., Munoz M., Harshman K., Esteban M. Cellular gene expression survey of vaccinia virus infection of human HeLa cells. J. Virol. 2003;77:6493–6506. doi: 10.1128/JVI.77.11.6493-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janknecht R., Ernst W. H., Pingoud V., Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidson L., Pawson A. J., Millar R. P., Maudsley S. Cytoskeletal reorganization dependence of signalling by the gonadotropin-releasing hormone receptor. J. Biol. Chem. 2004;279:1980–1993. doi: 10.1074/jbc.M309827200. [DOI] [PubMed] [Google Scholar]
- 48.Kawamura S., Miyamoto S., Brown J. H. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J. Biol. Chem. 2003;278:31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 49.Ploubidou A., Moreau V., Ashman K., Reckmann I., Gonzalez C., Way M. Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 2000;19:3932–3944. doi: 10.1093/emboj/19.15.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mallardo M., Schleich S., Krijnse Locker J. Microtubule-dependent organization of vaccinia virus core-derived early mRNAs into distinct cytoplasmic structures. Mol. Biol. Cell. 2001;12:3875–3891. doi: 10.1091/mbc.12.12.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giambartolomei S., Covone F., Levrero M., Balsano C. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the Hepatitis C virus (HCV) core protein. Oncogene. 2001;20:2606–2610. doi: 10.1038/sj.onc.1204372. [DOI] [PubMed] [Google Scholar]