Abstract
MST1 (mammalian Sterile20-like 1) and MST2 are closely related Class II GC (protein Ser/Thr) kinases that initiate apoptosis when transiently overexpressed in mammalian cells. In the present study, we show that recombinant MST1/2 undergo a robust autoactivation in vitro, mediated by an intramolecular autophosphorylation of a single site [MST1(Thr183)/MST2(Thr180)] on the activation loop of an MST dimer. Endogenous full-length MST1 is activated by a variety of stressful stimuli, accompanied by the secondary appearance of a 36 kDa Thr183-phosphorylated, caspase-cleaved catalytic fragment. Recombinant MST1 exhibits only 2–5% activation during transient expression; endogenous MST1 in the cycling HeLa or KB cells has a similar low fractional activation, but 2 h incubation with okadaic acid (1 μM) results in 100% activation. Endogenous MST1 immunoprecipitated from KB cells is specifically associated with substoichiometric amounts of the growth inhibitory polypeptides RASSF1A and NORE1A (novel Ras effector 1A; a Ras-GTP-binding protein). Co-expression of RASSF1A, RASSF1C, NORE1A and NORE1B with MST1 markedly suppresses MST1(Thr183) phosphorylation in vivo and abolishes the ability of MST1 to undergo Mg-ATP-mediated autoactivation in vitro; direct addition of purified NORE1A in vitro also inhibits MST1 activation. In contrast, co-transfection of MST1 with NORE1A modified by the addition of a C-terminal CAAX motif results in a substantial increase in MST1(Thr183) phosphorylation, as does fusion of a myristoylation motif directly on to the MST1 N-terminus. Moreover, MST1 polypeptides, bound via wild-type NORE1A to Ras(G12V) (where G12V stands for Gly12→Val), exhibit higher Thr183 phosphorylation compared with MST1 bound to NORE1A alone. Nevertheless, serum stimulation of KB cells does not detectably increase the activation state of endogenous MST1 or MST2 despite promoting the recruitment of the endogenous NORE1–MST1 complex to endogenous Ras. We propose that the NORE1/RASSF1 polypeptides, in addition to their role in maintaining the low activity of MST1 in vivo, direct MST1 to sites of activation and perhaps co-localization with endogenous substrates.
Keywords: MST1, MST2, NORE1, Ras, RASSF1, tumour suppressor
Abbreviations: IFN, interferon; LATS, large tumour suppressor; MBP, myelin basic protein; MST, mammalian Sterile20-like; NORE1, novel Ras effector 1; PAK, p21-activated kinase; RASSF, Ras association domain family protein; TNFα, tumour necrosis factor α
INTRODUCTION
MST1 (mammalian Sterile20-like 1) [1], also known as Krs-2 [2], is a 487-amino-acid Class II GC (protein Ser/Thr) kinase [3] that contains an N-terminal catalytic domain in the Ste20 class, followed by a non-catalytic tail containing, successively, an autoinhibitory segment and a coiled-coil domain that mediates dimerization [4]. First isolated by PCR on the basis of its homology with Ste20 [1], MST1 and its close paralogue, MST2 (78% identity), were independently identified by ‘in-gel’ kinase assays as a 61 kDa protein kinase activated in mammalian cells by severe stress (0.25 M sodium arsenite or 55 °C), okadaic acid or staurosporine [2]; MST1/2 probably correspond to the 63 kDa protein kinase in chick embryo fibroblasts that become activated 24 h after transformation by vSrc [5]. Recombinant MST1, recovered after transient expression, exhibits a spontaneous kinase activity, which is increased approx. 10-fold by deletion of MST amino acids 331–394 [4]. Notably, MST1 contains a caspase-3-recognition motif (DEMD326), and several reports [6–8] have identified the generation of a catalytically active 36 kDa fragment of MST during apoptosis; MST1 also has a site (TMTD349) whose cleavage by caspase 6/7 yields an active 41 kDa fragment [9,10]. In addition, overexpression of MST1 alone is sufficient to initiate apoptosis in a variety of cell backgrounds through pathways that involve activation of SAPK (stress-activated protein kinase)/Jnk (c-Jun N-terminal kinase) [6], p53 [11] and perhaps other effectors. Full-length MST1 is predominantly cytosolic, but it cycles rapidly and continuously through the nucleus [11,12], where it may associate with the nuclear protein DAP-4 (death-associated protein-4) [11]. The catalytically active 36 kDa fragment generated during apoptosis accumulates in the nucleus [12] and has been reported to catalyse the phosphorylation of histone 2B at Ser14, which is associated with chromosome condensation during apoptosis [13].
Loss-of-function mutations in Drosophila MST (called hippo or hpo) were identified in screenings for the genes that modify the growth and proliferation of retinal cells [14–16]. MST-mutant retina exhibits a failure to arrest proliferation appropriately during development, attributed to an increased expression of cyclin E, as well as the failure of developmental apoptosis, which is perhaps related to decreased degradation of the caspase inhibitor DIAP (Drosophila inhibitor of apoptosis). This phenotype, which can be rescued by overexpression of human MST2, resembles that previously observed with loss-of-function mutants in genes encoding the protein kinase LATS (large tumour suppressor) [17,18] as well as the non-catalytic WW domain protein Salvador [19] (also called Shar-pei [20]). MST/hippo binds to the Salvador C-terminal coiled-coil segment and phosphorylates Salvador as well as LATS, the latter in a Salvador-dependent manner [18–21]. Thus MST functions in an antiproliferative and proapoptotic pathway in Drosophila development together with Salvador and LATS.
The regulation of endogenous MST1/2 as well as their physiological functions in mammalian cells are still poorly understood. We recently reported [22] that MST1/2 [but not other GC kinase subfamilies, e.g. germinal centre kinase or SOK1 (Sterile20-like oxidant stress response kinase-1)/YSK1 (yeast Sps1/Sterile20-related kinase-1)] bind to the protein NORE (novel Ras effector), a putative Ras-GTP effector [23] and growth suppressor [24,43]. NORE1 (isoforms A and B [25]) is the founding member of a small gene family that includes the tumour suppressor RASSF1 (Ras association domain family protein 1) (isoforms A–G) [26], RASSF2 (isoforms A–C) [27], RASSF3 (isoforms A–C), RASSF4 (ADO37; isoforms A–D), RASSF5/NORE1 (isoforms A and B) and RASSF6 (isoforms A and B). Mouse NORE1A is a 413-amino-acid polypeptide that contains an N-terminal proline-rich domain (amino acids 17–118), a central zinc finger (amino acids 118–165), a Ras-binding domain of the RalGDS/AF6 variety (RA domain; amino acids 267–358) [28] and a conserved C-terminal tail (amino acids 359–413). The C-terminal 300 amino acids of NORE is approx. 50% identical with the tumour suppressor RASSF1A, and this segment of both polypeptides, encompassing the zinc finger, RA domain and C-terminal tail, is approx. 40% identical with a central segment of the 615-amino-acid Caenorhabditis elegans protein T24F1.3. Notably, all three polypeptides bind co-expressed MST1 via their conserved C-terminal tails [22]. Thus GST (glutathione S-transferase)–NORE(358–413) is sufficient to bind FLAG–MST1 and, reciprocally, GST–MST(456–487) is sufficient to bind FLAG–NORE. We showed previously that MST immunoprecipitated from KB cells co-precipitates endogenous NORE [22]. Moreover, a two-hybrid screening of a human lung cDNA library using an MST bait yielded multiple copies of NORE, RASSF1, RASSF2 and RASSF3. Thus these NORE/RASSF family members are probably physiological binding partners of MST. NORE binds specifically to the GTP-liganded form of Ras, and the ability of overexpressed Ki-Ras(G12V) (where G12V stands for Gly12→Val) to induce apoptosis is strongly inhibited by expression of the segments of NORE or MST1 that interact with each other, i.e. GST–NORE(358–413) or GST–MST1(456–487) [22]. These findings indicate that the NORE–MST complex is critical to the mechanism of V12Ki-Ras-induced apoptosis; however, the function of the NORE/MST and putative RASSF1–MST complexes in normal cell physiology and the regulatory significance of the interaction between MST and the NORE/RASSF polypeptides are not known.
In the present study, we explore the mechanism of MST1 activation and the effects of NORE and RASSF1 on this process. We demonstrate that MST1/2 are activated by an intramolecular autophosphorylation catalysed within an MST dimer. A phospho-specific antibody directed to the site of activating phosphorylation [MST1(Thr183)/MST2(Thr180)] allows semi-quantitative estimation of MST1/2 activation in vivo using cell extracts; in addition, MST assay conditions are defined that enable quantitative estimation of the extent of MST1 activation. Using these methods, we show that the activation state of both endogenous and recombinant MST1 in cycling mammalian cells is 2–5% of the maximal activity. Immunoprecipitates of endogenous MST1 contain substoichiometric amounts of endogenous NORE and RASSF1A. Moreover, co-expressed NORE and RASSF1 suppress MST1 activity in vivo and the ability of MST1 to autoactivate in vitro. Thus endogenous NORE1A and RASSF1A, which are bound to MST1 in vivo, are potent inhibitors of MST1 activation. Nevertheless, when NORE1A and MST1 are co-expressed with Ras, the MST1 polypeptides bound via NORE1A to Ras(G12V) exhibit a much higher level of activation compared with MST1 polypeptides complexed with NORE1A alone, without, however, inducing an overall activation of MST1. We infer that NORE1A (and probably RASSF1 polypeptides) participate in the activation of endogenous MST1/2, in response to Ras-like small GTPases and other inputs yet to be identified, and serve to localize and restrict activated MST polypeptides to their site(s) of action.
EXPERIMENTAL
Materials
Wild-type and mutant variants of MST1, MST2, NORE1, RASSF1A and RASSF1C cDNAs were described in [22]. Anti-FLAG antibody was obtained from Sigma. Calyculin was purchased from Gibco (Invitrogen). TNFα (tumour necrosis factor α), IFNα (interferon α), IFNβ, IFNγ, okadaic acid, sodium arsenite, etoposide, cisplatin, cyclophosphamide, geldanamycin, tunicamycin, forskolin and sphingomyelinase were obtained from Calbiochem (San Diego, CA, U.S.A.).
Preparation of anti-phospho-MST polyclonal antibody
A polyclonal anti-phosphopeptide antibody to a synthetic phosphopeptide DTMAKRNT(PO4)VIGTPF, corresponding to MST1 (amino acids 176–189) and MST2 (amino acids 173–186), was coupled with KLH (keyhole-limpet haemocyanin) and used to immunize rabbits. The immune serum was first adsorbed on to columns containing the unphosphorylated peptide; phosphopeptide-specific antibodies were isolated by adsorption of the flow-through on the immobilized phosphopeptide, followed by elution at low pH.
Cell culture and transfection
HEK-293 cells were maintained in Dulbecco's modified Eagle's medium containing 10% foetal bovine serum. KB cells were cultured in a minimum essential medium (Eagle) with 2 mM L-glutamine and Earle's balanced salt solution adjusted to contain 1.5 g/l sodium bicarbonate, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate and 10% foetal bovine serum. Cells were cultured in the presence of 100 units/ml penicillin and 100 μg/ml streptomycin (Gibco) at 37 °C in a humidified atmosphere containing 5% CO2. Cells were seeded on plates 24 h before transfection and then transfected with LIPOFECTAMINE™ (Invitrogen) according to the manufacturer's instructions.
Western blotting and immunoprecipitation
Cells were washed twice with PBS 24 h after transfection. Then, the cells were either extracted into SDS sample buffer and used for immunoblotting or extracted into a cold lysis buffer [30 mM Hepes, pH 7.4/1% Triton X-100/20 mM KCl/1 mM NaF/20 mM β-glycerophosphate/1 mM EDTA/1 mM Na3VO4/1 mM dithiothreitol/1 mM PMSF/0.1 μM calyculin/the protease inhibitor mixture Complete (Roche Molecular Biochemicals)]. Cell lysates were cleared by centrifugation at 10000 g for 20 min. For immunoprecipitation of the endogenous MST1, the lysate was incubated with 1 μg of anti-KRS-2 antibody (Zymed Laboratories, San Francisco, CA, U.S.A.) for 2 h at 4 °C with rocking, followed by the addition of Protein A/G Plus–agarose (Santa Cruz Biotechnology) or Dynabeads Protein A (Dynal Biotech ASA, Oslo, Norway); the incubation was continued for another 1 h. For immunoprecipitation of FLAG-tagged proteins, cell lysate was incubated with anti-FLAG–agarose beads (Sigma) for 2 h. Immunoprecipitates were washed six times with cold lysis buffer, and the targeted proteins were eluted with the peptide antigen: 0.25 mg/ml MST1 peptide in lysis buffer or 0.1 mg/ml FLAG peptide (Sigma) in lysis buffer. SDS cell lysates or eluates were resolved by SDS/PAGE and transferred on to a PVDF membrane (Amersham Biosciences). The membrane was blocked in TBS-T buffer (20 mM Tris/HCl, pH 7.5/150 mM NaCl/0.05% Tween 20) containing 5% (w/v) non-fat milk at room temperature (22 °C) for 1 h and then incubated with the primary antibody overnight at 4 °C, followed by an incubation for 1 h at room temperature with horseradish peroxidase-conjugated anti-IgG (Jackson ImmunoResearch Laboratories). Peroxidase activity was detected on X-ray films using an enhanced chemiluminescence detection system (SuperSignal; Pierce).
In vitro kinase assay
In vitro activation of endogenous MST1 or FLAG-tagged MST of various forms was accomplished by incubation with 10 mM MgCl2 and 100 μM ATP in kinase buffer [50 mM Hepes, pH 7.4/10% (w/v) glycerol/100 mM NaCl/1 mM NaF/20 mM β-glycerophosphate/1 mM EDTA/1 mM EGTA/1 mM Na3VO4/1 mM dithiothreitol] at 30 °C. At the indicated intervals, aliquots were removed and diluted 10-fold into kinase buffer at 30 °C containing 1 mg/ml MBP (myelin basic protein) (Sigma) and 1 μCi of [γ-32P]ATP; after 2 min, the reactions were terminated by adding SDS gel buffer. The mixtures were separated by SDS/PAGE (15% gel) and analysed by liquid-scintillation counting of the excised band of 32P-MBP.
RESULTS
Overexpression of recombinant wild-type MST1 is sufficient to initiate apoptosis in a variety of cell backgrounds, whereas the MST1 ATP site mutant, K59R, lacks this ability. Although the spontaneous kinase activity exhibited by MST1 extracted after transient expression in HEK-293 or COS-7 cells can be attributed, in part, to the co-precipitation of small amounts of the caspase-cleaved catalytic fragment, which has a much higher specific activity compared with full-length MST1, a significant kinase activity is also exhibited by the caspase-resistant MST1 mutant (D326N/D349N) [9,10]. These findings indicate that uncleaved MST1 acquires catalytic activity in vivo before its activation of caspase.
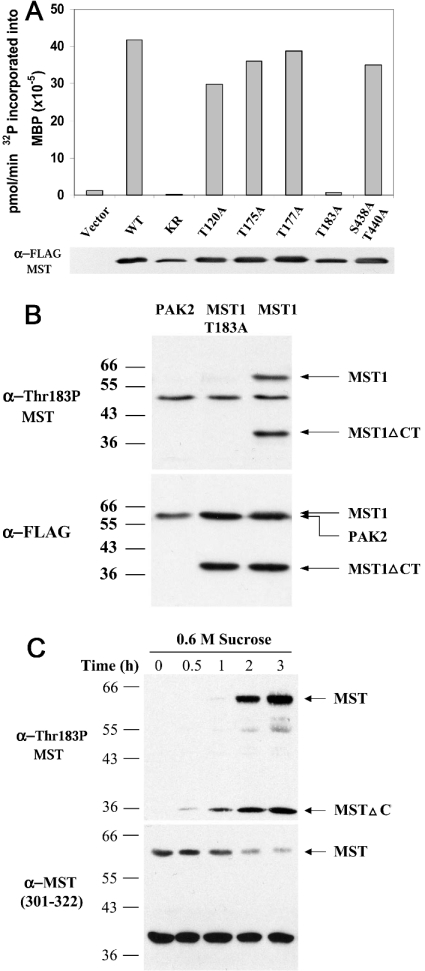
The ability of okadaic acid and other phosphatase inhibitors to stimulate the activity of the 60 kDa form of MST1, as measured by ‘in-gel’ kinase assay, as well as the inhibition of MST1 kinase activity by phosphatase treatment in vitro, indicates that MST1 phosphorylation promotes MST activation. Lian et al. [29] found that an anti-phosphopeptide antibody directed to the site activating phosphorylation on the activation loop of α-PAK (α-p21-activated kinase) and γ-PAK was cross-reactive with MST1/2; using this antibody, they observed an increased immunoreactivity of the full-length MST1 polypeptide in polymorphonuclear cells subjected to hyperosmolarity by the addition of 0.25 M sucrose. This suggested that the phosphorylation at the corresponding threonine residue on the MST activation loop [i.e. MST1(Thr183) and MST2(Thr180)] was also important for the activation of MST. In fact, mutation of MST1 Thr183 to alanine reduces MST1 activity by 98%, which is comparable with the inhibition caused by the mutation K59R at the MST1 ATP-binding site (Figure 1A). In contrast, the conversion of Thr175 or Thr177 on the MST activation loop into alanine had no significant effect on the MST1 activity assayed in vitro, supporting the view that the phosphorylation at Thr183 is uniquely important for MST1 activation (Figure 1A). We therefore prepared a polyclonal anti-phosphopeptide antibody directed to a synthetic phosphopeptide DTMAKRNT(PO4)VIGTPF, corresponding to MST1 (amino acids 176–189) and MST2 (amino acids 173–186). After affinity purification, this antibody was immunoreactive with transiently expressed FLAG–MST1 (and FLAG–MST2; results not shown), but not with FLAG–MST1(T183A) or with FLAG–PAKα (Figure 1B). Notably, two bands of recombinant phospho-MST1 were detected, corresponding to full-length MST1 and a 36 kDa caspase-cleaved product. The anti-MST(T183P) antibody was also reactive with endogenous MST1; thus, the addition of 0.6 M sucrose resulted in a slow increase in P-MST (phospho-MST) immunoreactivity at both 60 and 36 kDa, reflecting the activation of endogenous MST1 as well as its cleavage, presumably consequent to caspase 3 activation. Note that despite the strong T183P immunoblot signal at 36 kDa in response to sucrose, a corresponding MST1 (or MST2; results not shown) polypeptide signal was not evident. This indicates that the specific activity of the caspase-cleaved 36 kDa MST1 fragment is much higher when compared with full-length MST1, perhaps reflecting a preferential cleavage of the activated MST1 polypeptide. A similar but less robust time-dependent activation of MST was seen after the addition of sodium arsenite (results not shown). Using the ability of this anti-phosphopeptide antibody to detect activated forms of MST, we surveyed a variety of agents for their ability to activate MST in HeLa cells. In addition to the effects shown in Figure 1(C) and slight activation in response to severe heat shock (55 °C for 10 min) and staurosporine, this survey was entirely negative. MST was not activated within 1 h by the addition of the cytokines TNFα, IFNα, IFNβ, IFNγ and interleukin-1β or by the addition or withdrawal of serum or by epidermal growth factor addition. Negative results were also seen with a variety of protein synthesis inhibitors, DNA-damaging agents (etoposide, cisplatinum, UV irradiation, cyclophosphamide), protein denaturants (geldanamycin, tunicamycin), forskolin or sphingomyelinase. Thus rapid and/or sustained activation of endogenous MST1/2 in vivo was observed only in response to selected proapoptotic stimuli.
Figure 1. MST1 Thr183 is a phosphorylation site necessary for MST1 kinase activity.
(A) The effect of site-specific mutations on the activity of MST1. The kinase activity of FLAG-tagged MST1 variants was assayed after transient expression in HEK-293 cells. WT, wild-type MST1; KR, K59R. FLAG–MST1 polypeptides were purified from the cell lysates by adsorption on FLAG–agarose, then eluted with FLAG peptide and equal amounts of each MST1 polypeptide was assayed at 30 °C for MBP kinase activity (10 mM Mg2+/40 μM ATP for 15 min). After SDS/PAGE, 32P incorporation into MBP was determined; an anti-FLAG immunoblot of the MST1 polypeptides assayed is also shown. (B) Specificity of an anti-MST1(T183P) phosphopeptide antibody (α-Thr183P MST). cDNAs encoding FLAG-tagged PAK2, MST1(T183A) and MST1 were expressed in COS-7 cells. After 48 h, cells were extracted into a buffer containing Triton X-100; aliquots were resolved by SDS/PAGE and subjected to immunoblotting using anti-MST1(T183P) (upper panel) and anti-FLAG antibodies (α-FLAG; lower panel). The anti-MST1(T183P) antibody reacts with full-length MST1 and the caspase-cleaved MST1 catalytic fragment (MST1ΔCT), but not with MST1(T183A) or PAK2. (C) Endogenous MST1-Thr183 phosphorylation in intact cells. HeLa cells were treated with 0.6 M sucrose for 3 h (lanes 1–5). At the indicated time points, cells were extracted in Triton X-100 buffer; aliquots were resolved by SDS/PAGE and subjected to immunoblotting using anti-MST1(T183P) antibody (upper panel) and an anti-MST1 (301–322) antibody [α-MST (301–322); lower panel].
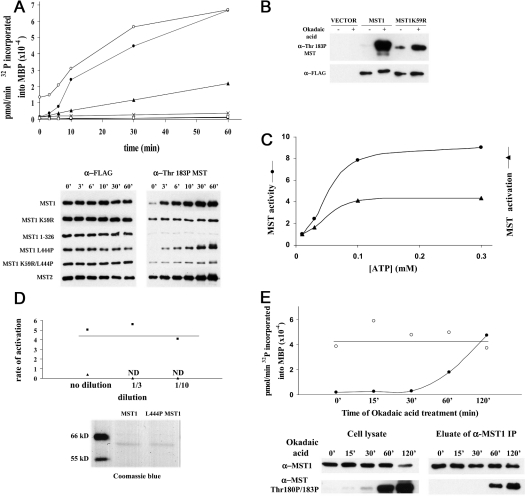
Next, we sought to explore the biochemical basis for MST1 activation. Since we aimed to quantify the MST1 activation state in vivo, it was necessary to define the conditions that enable MST1 to undergo autoactivation in vitro, so as to establish conditions that allow MST1 assay in vitro without engendering significant additional activation during the assay itself. Previous work had shown that MST1 exists as a dimer mediated through its coiled-coil domain, and the activity of the wild-type enzyme can be increased in vivo by truncation or by treatment of cells with okadaic acid [2,4]. FLAG–MST1 was purified on FLAG antibody beads and eluted with FLAG peptide; the enzyme was then incubated with Mg2+ (10 mM) and ATP (100 μM) at 30 °C; at the indicated intervals (Figure 2A), aliquots were diluted 10-fold and assayed by phosphorylation of MBP for 2 min at 10 μM ATP. As seen in Figure 2(A), there is a time-dependent increase in the apparent MST kinase activity that varies in multiple experiments between 20- and 50-fold. MST1 activation in vitro is accompanied by a progressive increase in phosphorylation at Thr183, a modification that is probably the cause of activation. It is noteworthy that MST1(K59R), despite exhibiting detectable T183P when examined directly after extraction, exhibits little further increase during incubation in vitro with Mg-ATP, indicating that Thr183 phosphorylation is probably catalysed by the MST1 catalytic domain itself, rather than by a co-precipitating kinase acting on MST1. This conclusion is supported further by a much smaller increase in Thr183 phosphorylation in MST1(K59R) during treatment in vivo with okadaic acid when compared with wild-type MST1 (Figure 2B). The EC50 for the ATP-dependent activation of MST1 is approx. 40–50 μM; once activated, the EC50 for Mg-ATP in the phosphorylation of MBP is also approx. 40–50 μM (Figure 2C). The rate of MST1 autoactivation is similar whether the kinase is immobilized on FLAG–agarose or eluted into solution, suggesting that activation is not diffusion-limited, but proceeds via an intramolecular phosphorylation within the MST1 dimer (results not shown). This conclusion is strongly supported by the behaviour of the dimer-deficient variants MST1(L444P) and MST1(1–326); when incubated with Mg-ATP (0.1 mM) at similar low polypeptide concentrations, no ATP-dependent activation or Thr183 phosphorylation of MST(1–326) was detected (Figure 2A), and for MST1(L444P) the activities were very much less than the wild-type MST1 (Figures 2A and 2D); the modest rate of Thr183 phosphorylation and autoactivation evident with MST(L444P), about one-tenth of that for the wild-type MST1, is rapidly lost with dilution, whereas the rate of MST1 autoactivation is unchanged over a 10-fold dilution (Figure 2D). We infer that the modest autoactivation in vitro seen with MST1- (L444P) reflects intermolecular phosphorylation by the MST1(L444P) monomers.
Figure 2. Autophosphorylation and autoactivation of MST1 in vitro and in vivo.
(A) Autophosphorylation and autoactivation in vitro of wild-type MST1/2 and various MST1 mutants. FLAG-tagged MST variants (•, MST1; ▪, MST1(K59R); ×, MST1(1–326); ▴, MST1(L444P); □, MST1(K59R/L444P); ○, MST2), transiently expressed in HEK-293 cells, were extracted into Triton X-100 buffer, purified on FLAG–agarose beads and eluted with FLAG peptide. The enzyme was mixed with 10 mM Mg2+ and 100 μM ATP at 30 °C; at the indicated intervals, aliquots were incubated with MBP and 10 μM [γ-32P]ATP for 2 min (upper panel). After SDS/PAGE, 32P incorporation into MBP (upper panel) was determined and immunoblotting was performed using anti-FLAG (bottom left) and anti-MST1(T183P) (bottom right). (B) MST1(Thr183) phosphorylation requires MST1 catalytic activity. FLAG-tagged MST1 and kinase-dead MST1(K59R), transiently expressed in HEK-293 cells, were extracted into SDS with or without the addition of 1 μM okadaic acid 1 h before harvesting. Immunoblots of MST1(T183P) (upper panel) and FLAG (lower panel) are shown. (C) MST1 activation does not alter the affinity of MST1 for Mg-ATP. The EC50 for the Mg-ATP-dependent activation of MST1 (▴) is compared with the EC50 for the MST1-catalysed phosphorylation of MBP by the fully activated MST1 kinase (•), each performed as in (A). Both processes exhibit an EC50 of approx. 40–50 μM. (D) The rate of MST1 autoactivation in vitro is independent of dilution. Similar concentrations of wild-type MST1 (▪) and the dimer-deficient MST1(L444P) polypeptide (▴), as shown by Coomassie Blue staining (lower panel), were incubated undiluted or after 3- and 10-fold dilutions with Mg (10 mM) and ATP (100 μM) at 30 °C; aliquots were removed at intervals and assayed for kinase activity by phosphorylation of MBP for 2 min at 10 μM ATP, as in (A). The rate of increase in kinase activity was multiplied by the dilution to give the rate of activation (pmol of 32P transferred to MBP/min). ND, no activation detected. (E) The effect of okadaic acid on the activation state of endogenous MST1. HeLa cells were incubated with 1 μM okadaic acid for the time periods indicated. Cell lysates were prepared using Triton X-100 buffer. Endogenous MST1 was immunoprecipitated and eluted with the MST1(301–322) peptide. Both cell lysates (lower left) and eluates (lower right) were immunoblotted for T180P/T183P MST. At each time point, aliquots of the MST1 eluates were incubated at 30 °C with 10 mM Mg2+ alone or 10 mM Mg2+ and 100 μM ATP. After 1 h, MST1 kinase (upper panel) was assayed by MBP phosphorylation at 10 μM [γ-32P]ATP for 2 min. The upper horizontal line indicates the consensus ‘100% activation’.
From these features, it appears that, for the measurement of MST1 kinase activity, 10 μM ATP at 30 °C for 2 min after a prior incubation for 60 min at 30 °C with or without 0.1 mM ATP/10 mM Mg2+ are conditions suitable to allow an accurate estimation of the fractional activation in vivo of MST1. Using these conditions, we examined the effect of okadaic acid on the activation state of MST1; incubation of cells with 1 μM okadaic acid results in a time-dependent increase in the fractional activation of immunoprecipitated endogenous MST1, which reaches nearly 100% after 120 min (Figure 2E). Thus it appears that both endogenous and recombinant MST1 exhibit a fractional activation in non-synchronized cells of approx. 2–5% of maximal.
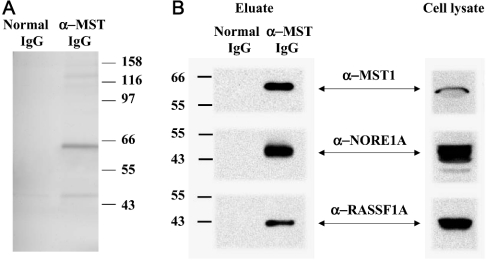
Despite the low fractional activation of the recombinant enzyme, the excess catalytic activity produced by recombinant MST1 is sufficient to produce a dose-dependent increase in apoptosis. The evident proapoptotic potency of active MST1, together with the low extent of fractional activation of endogenous MST, point to the existence of potent inhibitory influences acting on MST1 in vivo. The ability of okadaic acid to promote MST activation indicates that basal MST1 autophosphorylation is taking place continuously, but is effectively negated by cellular protein phosphatases. The persistently low fractional activation of recombinant MST1 despite substantial overexpression suggests that MST1-phosphatase activity and the abundance of MST1 inhibitors far exceed the abundance/autophosphorylating activity of endogenous MST1 under basal conditions. MST activation will require a reduction in this inhibitory input or a positive ‘activating’ input, or both. Seeking proteins associated with endogenous MST1 extracted from proliferating KB cells, we prepared immunoprecipitates using anti-MST1 IgG or non-immune IgG, and eluted each immunoprecipitate with the synthetic peptide antigen used for generating the anti-MST1 antibody; a silver stain of these eluates, separated by SDS/PAGE and representative of three such experiments, is shown in Figure 3(A). In each experiment, the most abundant polypeptide band specific to the anti-MST1 eluate is a 56 kDa band corresponding to MST1 itself. No other band approaches within approx. 20% of the abundance of MST1. Thus there is no evidence for a stoichiometric, tightly bound MST1-associated polypeptide. These eluates were also probed for the presence of endogenous NORE1A and RASSF1A (Figure 3B). Notably, both NORE1A and RASSF1A were detected by immunoblotting the eluate of the anti-MST1 immunoprecipitate, but were absent from the eluate of the non-immune IgG. This demonstrates that a portion of MST1 in KB cells is bound to both NORE1A (as shown previously [22]) and RASSF1A (NORE1B is not expressed in KB cells, and RASSF1C is not detected by the antibody employed).
Figure 3. MST1 endogenous to KB cells is associated with substoichiometric amounts of NORE1A and RASSF1A.
(A) Proliferating KB cells were extracted into a buffer containing Triton X-100 and immunoprecipitates were prepared using anti-MST1 IgG or non-immune IgG. After washing with the extraction buffer, both immunoprecipitates were eluted with the synthetic MST1 peptide (311–322). The eluates were separated by SDS/PAGE and the gel was stained with silver. The results are representative of three experiments; the faint bands at 47 and 125 kDa are usually equally abundant in the normal IgG eluate. (B) Aliquots of KB cell lysates (right) and the eluates of the normal IgG and anti-MST1 immunoprecipitates (left) were resolved by SDS/PAGE and subjected to immunoblotting using anti-MST1, anti-NORE1A and anti-RASSF1A antibodies.
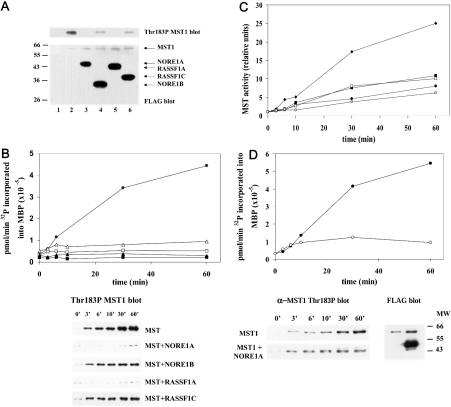
Therefore we then sought to determine the effects of NORE1 and RASSF1 isoforms on MST1 activity in vivo and on MST1 activation in vitro. FLAG–MST1 was co-expressed with an excess of FLAG-tagged versions of NORE1A, NORE1B, RASSF1A and RASSF1C; cells were extracted directly into either SDS or Triton X-100 and aliquots were subjected to immunoblotting for the FLAG epitope and for MST1(T183P). As shown in Figure 4(A), each of the FLAG-tagged NORE/RASSF1 polypeptides is expressed in excess of FLAG–MST1; under these conditions, each of these polypeptides strongly suppresses MST1(T183P) immunoreactivity; however, the NORE1A and RASSF1A isoforms appear to be more potent than the shorter isoforms NORE1B and RASSF1C. Nevertheless, each is capable of suppressing MST1 autoactivation in vivo, at least when present in excess of the MST1. We aimed to determine whether this inhibition persists in vitro by comparing the ability of recombinant MST1, either expressed alone or co-expressed with an excess of the NORE/RASSF1 polypeptides, to catalyse ATP-dependent activation in vitro. It is noteworthy that MST1 co-expressed with any of the NORE/RASSF1 isoforms is incapable of catalysing T183P autophosphorylation/autoactivation in vitro (Figure 4B). Moreover, MST1 activation in vitro is also suppressed by co-expression with mutant NORE1A polypeptides (Figure 4C), which still bind MST1 despite lacking the N-terminal proline-rich and zinc finger segments (NORE1A 250–413) or the ability to bind Ras-GTP (NORE LKKF304 to Ala4) or both. Inhibition of MST1 autoactivation is also evident when purified recombinant FLAG–NORE is added to purified, recombinant MST1 in vitro (Figure 4D). Thus the binding of NORE to MST1 appears to be sufficient to inhibit MST1 autoactivation.
Figure 4. NORE1 and RASSF1 isoforms suppress MST1 activation.
(A) HEK-293 cells were transfected with empty FLAG vector (lane 1) or with FLAG–MST1 (lanes 2–6) alone or together with an excess of each of the FLAG-tagged versions of NORE1A (lane 3), NORE1B (lane 4), RASSF1A (lane 5) and RASSF1C (lane 6). Cells were extracted into SDS sample buffer. Aliquots separated by SDS/PAGE were immunoblotted using anti-FLAG (bottom) and anti-MST(T183P) antibody. (B) NORE1 and RASSF1 suppress MST1 autoactivation in vitro. FLAG-tagged MST1, expressed alone (•) or together with NORE1A (▪), NORE1B (□), RASSF1A (▴) or RASSF1C (Δ), was purified using FLAG–agarose beads and then eluted with FLAG peptide and incubated with 10 mM Mg2+ and 100 μM ATP at 30 °C. At the indicated intervals, aliquots were diluted 10-fold and incubated for 2 min with MBP and 10 μM [γ-32P]ATP; 32P incorporation into MBP is shown in the upper panel. Other aliquots were resolved by SDS/PAGE and probed for MST1(T183P) (lower panel). (C) Mutant NORE1A polypeptides suppress MST1 autoactivation in vitro. FLAG-tagged MST1, expressed alone (♦) or together with NORE1A wild-type (▪), full-length NORE1A(LKKF304) (•), NORE1A(250–413) (□) or NORE1A(250–413/LKKF304) (○), was purified on FLAG–agarose, eluted with FLAG peptide and incubated with 10 mM Mg2+ and 100 μM ATP at 30 °C and then assayed for the activation of MBP kinase as in (B). (D) Addition of NORE1A to MST1 in vitro inhibits MST autoactivation. FLAG-tagged MST1 and NORE1A were expressed separately in HEK-293 cells, purified on FLAG–agarose beads and eluted with FLAG peptide. Portions of both eluates, where the amount of NORE1A exceeds by severalfold the amount of MST1 polypeptide, was incubated for 1 h at 4 °C either alone or with an excess of NORE1A (bottom right). The mixture was then warmed to 30 °C and Mg2+ (10 mM) or ATP (100 μM) was added. At the indicated intervals, aliquots were diluted 10-fold and incubated with MBP and 10 μM [γ-32P]ATP (upper panel), separated by SDS/PAGE for MBP kinase assay (upper panel) and immunoblotting using anti-FLAG and anti-MST1(T183P) (lower left).
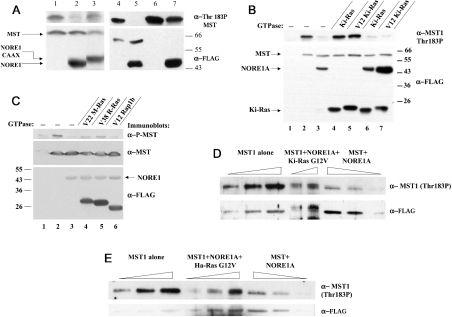
We showed previously that membrane recruitment of MST1, either by the addition of the N-terminal cSrc myristoylation motif or by co-expression with a NORE modified by the addition of the Ki-Ras4B C-terminal polybasic and CAAX motif, substantially augments MST1 proapoptotic efficacy [22]. In the present study, we show that MST1 co-expression with NORE-CAAX (Figure 5A, cf. lanes 2 and 3) or MST1 myristoylation, despite diminishing MST1 polypeptide expression (especially myristoylation; Figure 5A, cf. lanes 4 and 5 with lanes 6 and 7 respectively), result in a marked increase in MST1 Thr183 phosphorylation (Figure 5A). Co-expression with an excess of wild-type or mutant active Ha-Ras(G12V) or Ki-Ras(G12V), either alone or together with wild-type NORE1A, does not significantly stimulate the overall Thr183 phosphorylation on co-expressed MST1 or overcome the inhibition by co-expressed NORE1A (Figure 5B). Co-expression of NORE1A and MST1 with several other activated small GTPases of the Ras subfamily shown previously to bind NORE [30] only slightly overcomes the inhibition of MST1(Thr183) phosphorylation engendered by co-expressed NORE1A (Figure 5C). Nevertheless, the efficiency of formation of the Ras(G12V)–NORE1–MST1 ternary complex is low; we showed previously [22] that even when the three polypeptides were expressed at optimal ratios, MST1 abundance in the Ras(G12V) immunoprecipitate was not more than 20% when compared with an aliquot of the cell extract containing an amount of NORE1 equal to that in the Ras(G12V) immunoprecipitate. Since MST1 associates with Ras(G12V) only through NORE1 [22], we examined the phosphorylation state of MST1 co-purified with Ras(G12V) when compared with an equal amount of MST1 bound to NORE1A alone (Figures 5D and 5E). It is evident that the Thr183 phosphorylation of MST1 associated with either Ki-Ras(G12V) (Figure 5D) or Ha-Ras(G12V) (Figure 5E) is substantially greater than that of MST1 in complex with NORE1A alone. Moreover, MST bound to Ki-Ras(G12V) is markedly up-shifted (Figure 5D), indicating the occurrence of substantial MST1 phosphorylation in addition to that at Thr183. Nevertheless, consistent with the inability of Ras(G12V) to promote the overall activation of MST1 (Figure 5B), serum stimulation of KB cells, despite promoting the association of an endogenous NORE–MST1 complex with endogenous cRas [22], does not increase the overall MST1(Thr183) phosphorylation (results not shown). Although the association of the NORE1A–MST1 complex with Ras-GTP is accompanied by an apparent activation of MST1, the activated form of MST1 appears to be physically restricted to the Ras–NORE1A complex.
Figure 5. MST1 kinase activity is stimulated by membrane recruitment and when bound to Ras-like GTPases.
(A) Membrane recruitment activates MST1. HEK-293 cells were transfected with FLAG-tagged cDNAs of MST1 (lanes 1–5), MST1 and NORE1A (lanes 2 and 5), MST1 and NORE1A-CAAX (lane 3), myristoylated MST1 (Myr-MST1) (lane 6), Myr-MST1 and NORE1A (lane 7). After 48 h, cells were extracted directly into SDS sample buffer. Aliquots were separated by SDS/PAGE and immunoblotted using anti-FLAG (bottom) and anti-MST1(T183P) (top). (B) HEK-293 cells were transfected with empty pCVM5 FLAG vector (lane 1) or with FLAG-tagged cDNAs of MST1 (lanes 2–7) alone (lane 2) or with NORE1A (lane 3); wild-type Ki-Ras (lane 4); mutant active Ki-Ras (G12V) (lane 5); NORE1A plus wild-type Ki-Ras (lane 6); NORE1A plus mutant active Ki-Ras (G12V) (lane 7). After 48 h, cells were extracted directly into SDS sample buffer. Aliquots were separated by SDS/PAGE and immunoblotted using anti-FLAG (bottom) and anti-MST1(T183P) (upper). (C) HEK-293 cells were transfected with empty pCVM5 FLAG vector (lane 1) or with FLAG-tagged cDNAs of MST1 (lanes 2–6) alone (lane 2) or with NORE1A (lane 3); NORE1A plus (Val22)M-Ras (lane 4); NORE1A plus (Val38)R-Ras (lane 5); NORE1A plus (Val12)Rap1b (lane 6). SDS extracts were prepared, separated by SDS/PAGE and immunoblotted using anti-FLAG-epitope (bottom), anti-MST1 (lower; FLAG–MST1 was below detection) and anti-MST1(T183P). (D) HEK-293 cells were transfected with pCVM5 FLAG–MST1 alone (lanes 1–3) or with an excess of FLAG–NORE1A (lanes 6–8) or with excess FLAG–NORE1A and GST–Ki-Ras(G12V) (lanes 4 and 5). The GST–Ki-Ras(G12V) polypeptides were affinity-purified on GSH-Sepharose and subjected to SDS/PAGE (lanes 4, 5) along with aliquots of the extracts from cells expressing MST1 alone (lanes 1–3) or MST1 plus NORE1A (lanes 6–8). The abundance of FLAG–MST1 (lower panel) and the level of MST1(T183P) (upper panel) were estimated by immunonblotting. The experiment shown is representative of four replicates with equivalent results. (E) Experimental details are the same as in Figure (D), except that GST–Ha-Ras(G12V) was employed in place of Ki-Ras(G12V); this experiment was performed twice with equivalent results.
DISCUSSION
MST1 is activated by intramolecular transphosphorylation
During the final stages of the present study, Glantschnig et al. [31] and Deng et al. [32] identified MST(Thr183) and MST2(Thr180) respectively to be the sites of phosphorylation critical for kinase activation, consistent with the results shown in Figure 1(A). The present study builds upon these observations by designing and validating quantitative and semi-quantitative (by anti-P-MST immunoblotting) methods for the measurement of the fractional activation of recombinant and endogenous MST kinases. In addition, we explore the interaction and regulation of MST1 by the NORE/RASSF1 polypeptides. As seen in Figure 2(A), recombinant MST1 is capable of 20–50-fold autoactivation in vitro, mediated by an autophosphorylation that occurs within an MST1 oligomer, presumably a dimer. The rate of activation in vitro of wild-type MST1 is unaffected over a 10-fold dilution. Introduction of a point mutation (L444P) into the MST1 coiled-coil segment, previously shown to interfere with oligomerization [4,22], diminishes the rate of autoactivation by >90%, and renders it sensitive to dilution, presumably reflecting conversion into a primarily bimolecular reaction. MST1 activation in vitro is attributable to phosphorylation at Thr183 within the ‘activation loop’, which is probably catalysed in trans within the dimer. Conversion of Thr183 into Ala reduces MST1 activity by >98%, almost as severely as does mutation at the ATP-binding site (K59R). A phospho-specific antibody directed to the sequence surrounding P-Thr183 (which is identical in MST2) thus provides an accurate semi-quantitative monitor of the extent of MST1 activation. Notably, Thr183 is not the only site of autophosphorylation on MST1 evident during autoactivation in vitro or after transient expression, as reported previously [31,32]; our MS analysis uncovered phosphorylation at MST1 Thr177, Thr120 and perhaps Ser438 or Thr440 (results not shown); others have observed autophosphorylation at Ser327 [9]. Nevertheless, as seen in Figure 1(A), mutation at several sites in the activation loop other than Thr183 has no effect on the activity of recombinant MST1. We did generate a phosphopeptide antibody specific for MST1 P-Thr177, which gave results for endogenous MST1, under all the circumstances examined, essentially identical with those observed for the P-Thr183-specific antibody. Consequently, it appears that Thr177 is a site of physiological MST1 autophosphorylation in vivo as well as in vitro, but without a detectable effect on MST1 activity. The possibility that MST1 autoactivation in vitro is catalysed by a co-purifying protein kinase is largely eliminated by the finding that MST1(K59R) shows no change in T183P immunoreactivity during incubation in vitro with Mg2+-ATP. Moreover, phosphorylation at Thr183 in vivo in response to okadaic acid treatment is very much higher in wild-type MST1 than in MST1(K59R); it is probable that the basal and moderate okadaic acid-stimulated Thr183 phosphorylation in MST1(K59R) in vivo is attributable to heterodimerization of a fraction of the recombinant polypeptide with endogenous wild-type MST1/2. Autoactivation/Thr183 phosphorylation in vitro does not alter the apparent affinity of MST1 for Mg2+-ATP; half-maximal activation and activity of the fully activated kinase are both achieved near 40–50 μM ATP; this contrasts with the situation for protein kinase A and the insulin receptor kinase, which exhibits a substantial increase in affinity for ATP after activation, but is consistent with the behaviour of cdk2 (cyclin-dependent kinase) and several protein tyrosine kinases (v-Fps, Tie2; reviewed in [33]). Thus MST1 Thr183 phosphorylation must greatly enhance phosphoryl transfer or protein substrate binding (or both).
Our ability to assay MST1 kinase activity in vitro without engendering significant activation during the assay has enabled the designing of a quantitative assay for the extent of MST1 activation in vivo. As expected, the fractional activation of endogenous MST1 in cycling cells is very low, perhaps 2%, similar to that seen with transiently overexpressed recombinant MST1. Interestingly, transiently expressed MST2 shows a distinctly higher initial fractional activity (Figure 2A); however, the lack of an antibody suitable for immunoprecipitation precludes a direct comparison with endogenous MST2. Little or no endogenous MST1(P-Thr183)/MST2(P-Thr180) immunoreactivity is evident in cycling cells; however, incubation with okadaic acid at a concentration (1 μM) sufficient to inhibit both protein phosphatases 1 and 2A results in the appearance of detectable P-Thr183/P-Thr180 immunoreactivity within 30 min, with a rapid increase during the next 90 min. Minor but measurable MST1 fractional activation is observed at 30 min, and complete activation in vivo is evident at 120 min. These results (Figure 2E) demonstrate the utility of the P-Thr183 immunoblotting to monitor MST1/2 activation in vivo. We were surprised to find that overexpression of MST1 by itself has little or no effect on the fractional activation of the kinase. Given its low fractional activation, MST1 must be a potent proapoptotic effector inasmuch as modest overexpression of wild-type MST1 is sufficient to initiate apoptosis. The mechanisms and targets through which MST1 initiates apoptosis are largely unknown; however, the proapoptotic efficacy of MST1 has been attributed, in part, to the caspase 3-mediated cleavage of MST1 after Asp326, releasing the autoinhibitory domain, and yielding an active catalytic fragment. Our results indicate, however, that cleavage after Asp326 in the absence of prior Thr183 phosphorylation is probably not sufficient to generate an active catalytic fragment. A recombinant MST1(1–326) polypeptide exhibits very little catalytic activity after transient expression [even less than the dimer-deficient MST1(L444P)] and shows a greatly diminished ability to catalyse autophosphorylation/autoactivation in vitro (Figure 2A). The importance of a dimeric MST1 polypeptide for the generation of a highly competent kinase domain leads us to infer that the proapoptotic stimuli that result in the production of an active MST1-Thr183-phosphorylated 36 kDa catalytic fragment (as seen in Figure 1C in response to hyperosmolar stress) must engender the activation of full-length MST1 before its cleavage. The enhanced proapoptotic efficacy of recombinant MST1(1–326) when compared with full-length MST1 may be partly due to the unrestricted access of the former to the nucleus. Interestingly, Deng et al. [32] have reported that caspase 3 cleavage also decreases greatly the susceptibility of MST2 to deactivation in vitro by protein phosphatase.
MST regulation by the NORE1/RASSF1 polypeptides
The substantial proapoptotic potency of ‘inappropriately’ expressed recombinant MST1 suggests that the activity of endogenous MST1 must be rigorously constrained. Clearly, the activity of endogenous protein phosphatases represents one crucial constraint; however, MST1 overexpression has little impact on fractional activation, indicating that MST1 dephosphorylation in vivo is very efficient and some activating input is necessary to overcome the negating action of ambient phosphatase activity. Results of the present study indicate that the MST1 partners NORE and RASSF1 probably provide a second mechanism for the regulation of MST1 activity. Although endogenous MST1 is not associated with a single predominant cellular partner (Figure 3A), a fraction of endogenous MST1 is constitutively and specifically bound to the NORE1A and RASSF1A polypeptides (Figure 3B). From our previous demonstration [22] that MST1/2 bind to the homologous C-termini of these two polypeptides and our recovery of many isoforms of NORE, RASSF1, RASSF2 and RASSF3 (all of which contain this C-terminal segment) using an MST1 bait in a two-hybrid screen, it is probable that a fraction of endogenous MST1/2 is bound to whichever of these proteins is expressed. Results of the present study indicate that NORE1/RASSF1 polypeptides play a dual role in the regulation of MST1/2 kinases. One role of this interaction is to provide a further constraint on the extent of MST1/2 activation. We co-expressed MST1 with an excess of these polypeptides simply to ensure that most of the recombinant MST1 would be bound to the co-expressed NORE/RASSF1 polypeptides. Under such conditions, both isoforms of NORE and RASSF1 are capable of suppressing MST1 autoactivation in vivo and in vitro. This is accomplished in vitro through the direct binding of these NORE/RASSF1 polypeptides to MST1. In the intact cell, however, the longer isoforms NORE1A and RASSF1A are more potent inhibitors when compared with the respective shorter isoforms NORE1B and RASSF1C, suggesting that additional inhibitory mechanisms may be operative in vivo. There is considerable evidence indicating that RASSF1A is a tumour suppressor, whereas the shorter RASSF1C isoform lacks this function [26,34,35]; RASSF1A has been shown to retard cell-cycle progression in G1 and inhibit cyclin D1 accumulation, an effect not observed with RASSF1C [36]. RASSF1A has also been shown to associate both with microtubules during interphase and with spindles and centrosomes during mitosis; RASSF1A can stabilize microtubules against depolymerization in response to cold or nocodazole [37]. Recent evidence indicates that NORE1 may also be a growth inhibitor in mammalian cells [24]. NORE overexpression inhibits growth in a subset of tumour cell lines; this is accomplished independent of its ability to bind RasGTP [43]. The NORE1B isoform (identified as RAPL), in addition to its ability to bind RasGTP, has been observed to bind activated Rap1 in stimulated T cells and to regulate lymphocyte adhesion by associating with the integrin LFA-1 (lymphocyte function-associated antigen-1) and relocating at the immunological synapse [38]. NORE overexpression does not promote apoptosis in mammalian cells [22], but such a response has been observed with overexpression of RASSF1C [39]. Whether and how any of these responses elicited by the NORE and RASSF1 polypeptides relate to their ability to bind MST1/2 is not known.
Overexpression of MST1 in mammalian cells is proapoptotic, and genetic evidence in Drosophila indicates that the MST1/2 homologue hippo functions both as a negative regulator of cell proliferation and as a proapoptotic effector during development [14–16,21]. We have observed that the abundance of MST1/2 in cultured mammalian cells increases during M phase (results not shown), suggesting the persistence of a mitotic function for MST1 and/or MST2. Conversely, since homologues of NORE/RASSF1 are not evident in the Drosophila genome, an assessment of their functional relationship to MST1/2 in that background is precluded. Nevertheless, considering that the loss of RASSF1A expression in mammalian cells as well as the loss of hippo expression in Drosophila each results in increased cellular proliferation, how best to rationalize the present observation that RASSF1A and NORE1 act to inhibit MST1 activation? Two general scenarios appear plausible; in one, the NORE/RASSF polypeptides may serve primarily as substrates and effectors of MST1, so that loss of either gives an overlapping phenotype. Results of the present study do not address this possibility, which remains viable. They do support an alternative (or additional) mechanism wherein the MST–NORE (and MST–RASSF1) complexes represent reservoirs of inactive MST awaiting activating inputs; i.e. in addition to suppressing the basal activity of MST1, NORE/RASSF may direct the kinase to specific cellular sites and/or co-localize it with upstream activators and/or substrates. In the present study, we show that localization of recombinant MST1 at the membrane greatly augments MST1(Thr183) phosphorylation (Figure 5A), consistent with the previous finding that such a localization significantly increased MST1 proapoptotic efficacy [22]. Moreover, the MST1 polypeptides that associate through NORE1 with Ras-GTP exhibit higher Thr183 phosphorylation than those complexed with NORE1 alone (Figures 5D and 5E), indicating that Ras-GTP can promote MST1 activation in vivo, either directly or, probably, by its recruitment of MST1 to the membrane. This apparent Ras-GTP activation of MST1 differs markedly from the role of Ras-GTP in the regulation of Raf kinases in several respects; not only does c-Raf1 binds directly to Ras-GTP [40], but this interaction serves to initiate a complex sequence of Raf modifications mediated by other proteins, which results in a stable activation independent of the continued association with Ras-GTP; these modifications include protein phosphatase 2A-catalysed dephosphorylation, Raf phosphorylation by other protein kinases and reconfiguration of 14-3-3 binding [40–42]. In contrast, MST activation is achieved entirely by transmolecular autophosphorylation within the MST dimer (Figures 2A, 2B and 2D), and the ‘activated’ MST1 polypeptides appear to be confined to those bound to Ras-GTP (Figures 5B–5E). Although we have shown previously that NORE1A, which is in a constitutive complex with MST1([22] and Figure 3B), is recruited to endogenous Ras in response to serum treatment of KB cells [23], this recruitment is not accompanied by a stable overall activation of MST ([22] and results not shown); we presume that cellular protein phosphatases mediate a rapid deactivation of MST1 once it dissociates from Ras-GTP. This behaviour implies that the actions of Ras-GTP that are mediated by MST1 are effected while MST1 is associated with Ras at the membrane. In this manner, the Ras-GTP–NORE1 complex provides not only an activating signal, but also serves to localize MST1 action, a function analogous perhaps to that attributed to the Drosophila WW domain protein Salvador [19] (also called Shar-pei [20]), which serves as a ‘scaffold’ enabling the effective interaction of hippo with its target LATS.
In summary, we propose that the NORE/RASSF1 polypeptides, by binding the MST1/2 kinases through their homologous SARAH motifs (Salvador/RASSF/Hippo motifs), provide an array of adaptor proteins that help to maintain the MST1/2 kinases in an inhibited state, but have the ability to couple them with diverse activating inputs at various cellular sites and concomitantly specify the sites of MST1/2 action. Nevertheless, the MST1/2 kinases have adaptors of other structural classes, and the NORE1/RASSF1 polypeptides probably participate in signalling pathways that do not involve the MST kinases [43]. Further progress in defining the functional role of the NORE/RASSF1 polypeptides in MST1 action in vivo is limited at present by the lack of information about the physiological states, apart from certain forms of stress-induced apoptosis, that result in MST1 activation, and the physiological outputs that require MST1/2. Therefore defining other physiological circumstances that alter MST1 activity in vivo, as well as MST substrates, are high priorities.
Acknowledgments
This work was supported by NIH grants DK17776 and CA073818 and institutional funds. A.K. was supported by a postdoctoral fellowship from the Leukemia and Lymphoma Society. We thank J. Prendable for assistance in preparing the paper, and an anonymous reviewer for constructive comments and the suggestion to examine MST1 specifically bound to Ras-GTP.
References
- 1.Creasy C. L., Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J. Biol. Chem. 1995;270:21695–21700. doi: 10.1074/jbc.270.37.21695. [DOI] [PubMed] [Google Scholar]
- 2.Taylor L. K., Wang W.-C. R., Erikson R. L. Newly identified stress-responsive protein kinases, Krs-1 and Krs-2. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10099–10104. doi: 10.1073/pnas.93.19.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan T., Watanabe N. M., Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 4.Creasy C. L., Ambrose D., Chernoff J. The Ste20-like protein kinase MST, dimerizes and contains an inhibitory domain. J. Biol. Chem. 1996;271:21049–21053. doi: 10.1074/jbc.271.35.21049. [DOI] [PubMed] [Google Scholar]
- 5.Wang H.-C., Erikson R. L. Activation of protein serine/threonine kinases p42, p63, and p87 in Rous sarcoma virus-transformed cells: signal transduction/transformation-dependent MBP kinases. Mol. Cell. Biol. 1992;3:1329–1337. doi: 10.1091/mbc.3.12.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graves J. D., Gotoh Y., Draves K. E., Ambrose D., Han D. K., Wright M., Chernoff J., Clark E. A., Krebs E. G. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like MST1. EMBO J. 1998;17:2224–2234. doi: 10.1093/emboj/17.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee K. K., Murakawa M., Nishida E., Tsubuki S., Kawashima S.-I., Safkamaki K., Yonehara S. Proteolytic activation of MST/Krs, STE20-related protein kinase by caspase during apoptosis. Oncogene. 1998;16:3029–3037. doi: 10.1038/sj.onc.1201840. [DOI] [PubMed] [Google Scholar]
- 8.Reszka A. A., Halasy-Nagy J. M., Masarachia P. J., Rodan G. A. Bisphosphonates act directly on the osteoclast to induce caspase cleavage of Mst1 kinase during apoptosis. A link between inhibition of the mevalonate pathway and regulation of an apoptosis-promoting kinase. J. Biol. Chem. 1999;274:34967–34973. doi: 10.1074/jbc.274.49.34967. [DOI] [PubMed] [Google Scholar]
- 9.Graves J. D., Draves K. E., Gotoh Y., Krebs E. G., Clark E. A. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J. Biol. Chem. 2001;276:14909–14915. doi: 10.1074/jbc.M010905200. [DOI] [PubMed] [Google Scholar]
- 10.Lee K.-K., Ohyama T., Yajima N., Tsubuki S., Yonehara S. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J. Biol. Chem. 2001;276:19276–19285. doi: 10.1074/jbc.M005109200. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y., Khokhlatchev A., Figeys D., Avruch J. Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J. Biol. Chem. 2002;277:47991–48001. doi: 10.1074/jbc.M202630200. [DOI] [PubMed] [Google Scholar]
- 12.Ura S., Masuyama N., Graves J. D., Gotoh Y. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10148–10153. doi: 10.1073/pnas.181161698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung W. L., Cheung W. L., Ajiro K., Samejima K., Kloc M., Cheung P., Mizzen C. A., Beeser A., Etkin L. D., Chernoff J., et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase 1. Cell (Cambridge, Mass.) 2003;113:507–517. doi: 10.1016/s0092-8674(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 14.Harvey K. F., Pfleger C. M., Hariharan I. K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell (Cambridge, Mass.) 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 15.Wu S., Huang J., Dong J., Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell (Cambridge, Mass.) 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 16.Ryan S., Udan M., Kango-Singh R. N., Chunyao T., Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 17.Xu T., Wang W., Zhang S., Stewart R. A., Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 18.Turenchalk G. S., St John M. A., Tao W., Xu T. The role of lats in cell cycle regulation and tumorigenesis. Biochim. Biophys. Acta. 1999;1424:M9–M16. doi: 10.1016/s0304-419x(99)00021-9. [DOI] [PubMed] [Google Scholar]
- 19.Tapon T., Harvey K. F., Bell D. W., Wahrer D. C. R., Schiripo T. A., Haber D. A., Hariharan I. K. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell (Cambridge, Mass.) 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 20.Kango-Singh M., Nolo R., Tao C., Verstreken P., Hiesinger P. R., Bellen H. J., Halder G. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129:5719–5730. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- 21.Pantalacci S., Tapon N., Léopold L. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 22.Khokhlatchev A., Rabizadeh S., Xavier R., Nedwidek M., Chen T., Zhang X.-F., Seed B., Avruch J. Identification of a novel Ras-regulated proapoptotic pathway. Curr. Biol. 2002;12:253–265. doi: 10.1016/s0960-9822(02)00683-8. [DOI] [PubMed] [Google Scholar]
- 23.Vavvas D., Avruch J., Zhang X.-F. Identification of NORE1 as a potential Ras effector. J. Biol. Chem. 1998;273:5439–5442. doi: 10.1074/jbc.273.10.5439. [DOI] [PubMed] [Google Scholar]
- 24.Vos M. D., Martinez A., Ellis C. A., Vallecorsa T., Clark G. J. The pro-apoptotic Ras effector Nore1 may serve as a Ras-regulated tumor suppressor in the lung. J. Biol. Chem. 2003;278:21938–21943. doi: 10.1074/jbc.M211019200. [DOI] [PubMed] [Google Scholar]
- 25.Tommasi S., Dammann R., Jin S. G., Zhang X.-F., Avruch J., Pfeifer G. P. RASSF3 and NORE1: identification and cloning of two human homologues of the putative tumor suppressor gene RASSF1. Oncogene. 2002;21:2713–2720. doi: 10.1038/sj.onc.1205365. [DOI] [PubMed] [Google Scholar]
- 26.Dammann R., Li C., Yoon J.-H., Chin P. L., Bates S., Pfeifer D. P. Epigenetic inactivation of a Ras association domain family protein from the lung tumour suppressor locus 3p21.3. Nat. Genet. 2000;25:315–319. doi: 10.1038/77083. [DOI] [PubMed] [Google Scholar]
- 27.Vos M. D., Ellis C. A., Elam C., Ülkü A. S., Taylor B. J., Clark G. J. RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. J. Biol. Chem. 2003;278:28045–28051. doi: 10.1074/jbc.M300554200. [DOI] [PubMed] [Google Scholar]
- 28.Ponting C. P., Benjamin D. R. A novel family of Ras-binding domains. Trends Biochem. Sci. 1996;21:422–425. doi: 10.1016/s0968-0004(96)30038-8. [DOI] [PubMed] [Google Scholar]
- 29.Lian J. P., Toker A., Badwey J. A. Phosphorylation of the activation loop of γ p21-activated kinase(γ-Pak) and related kinases (MSTs) in normal and stressed neutrophils. J. Immunol. 2001;166:6349–6357. doi: 10.4049/jimmunol.166.10.6349. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz-Vega S., Khokhlatchev S., Nedwidek M., Zhang X.-F., Dammann R., Pfeifer G. F., Avruch J. The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the Ras-GTP binding protein Nore1. Oncogene. 2002;21:1381–1390. doi: 10.1038/sj.onc.1205192. [DOI] [PubMed] [Google Scholar]
- 31.Glantschnig H., Rodan G. A., Reszka A. A. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J. Biol. Chem. 2002;277:42987–42996. doi: 10.1074/jbc.M208538200. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y., Pang A., Wang J. H. Regulation of mammalian STE20-like kinase 2 (MST2) by protein phosphorylation/dephosphorylation and proteolysis. J. Biol. Chem. 2003;278:11760–11767. doi: 10.1074/jbc.M211085200. [DOI] [PubMed] [Google Scholar]
- 33.Adams J. A. Activation loop phosphorylation and catalysis in protein kinases: is there functional evidence for the autoinhibitor model? Biochemistry. 2003;43:601–607. doi: 10.1021/bi020617o. [DOI] [PubMed] [Google Scholar]
- 34.Burbee D. G., Forgacs E., Zochbauer-Muller S., Shivakumar L., Fong K., Gao B., Randle D., Kondo M., Virmani A., Bader S., et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J. Natl. Cancer Inst. 2001;93:691–699. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreijerink K., Braga E., Kuzmin I., Geil L., Duh F. M., Angeloni D., Zbar B., Lerman M. I., Stanbridge E. J., Minna J. D., et al. The candidate tumor suppressor gene, RASSF1A, from human chromosome 3p21.3 is involved in kidney tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7504–7509. doi: 10.1073/pnas.131216298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shivakumar L., Minna J., Sakamaki T., Pestell T. R., White M. A. The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation. Mol. Cell. Biol. 2002;22:4309–4318. doi: 10.1128/MCB.22.12.4309-4318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L., Tommasi S., Lee D.-H., Dammann R., Pfeifer G. P. Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene. 2003;22:8125–8136. doi: 10.1038/sj.onc.1206984. [DOI] [PubMed] [Google Scholar]
- 38.Katagiri K., Maeda A., Shimonaka M., Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 39.Vos M. D., Ellis C. A., Bell A., Birrer M. J., Clark G. J. Ras uses the novel tumor suppressor RASSF1 as an effector to mediate apoptosis. J. Biol. Chem. 2000;275:35669–35672. doi: 10.1074/jbc.C000463200. [DOI] [PubMed] [Google Scholar]
- 40.Avruch J., Khokhlatchev A., Kyriakis J. M., Luo Z., Tzivion G., Vavvas D., Zhang X. F. Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog. Horm. Res. 2001;56:127–155. doi: 10.1210/rp.56.1.127. [DOI] [PubMed] [Google Scholar]
- 41.Yoder J. H., Chong H., Guan K. L., Han M. Modulation of KSR activity in Caenorhabditis elegans by Zn ions, PAR-1 kinase and PP2A phosphatase. EMBO J. 2004;23:111–119. doi: 10.1038/sj.emboj.7600025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raabe T., Rapp U. R. Ras signaling: PP2A puts Ksr and Raf in the right place. Curr. Biol. 2003;13:R635–R637. doi: 10.1016/s0960-9822(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 43.Aoyama Y., Avruch J., Zhang X. F. Nore1 inhibits tumor cell growth independent of Ras or the MST1/2 kinases. Oncogene. 2004;23:3426–3433. doi: 10.1038/sj.onc.1207486. [DOI] [PubMed] [Google Scholar]