Abstract
DRM (detergent-resistant membranes), which are resistant to solublization by non-ionic detergents, have been demonstrated to be involved in many key cell functions such as signal transduction, endocytosis and cholesterol trafficking. Covalent modification of proteins by fatty acylation has been proposed to be an important protein-targeting mechanism for DRM association. However, little is known concerning the effects of LCSFA (long-chain saturated fatty acids) on protein composition of DRM in human cancer cells. In the present study, we found that, in Hs578T human breast cancer cells, the major protein increased in DRM in response to the LCSFA stearate (C18:0) was annexin II. Our results demonstrated that annexin II accumulated in DRM specifically in response to physiological concentrations of stearate and palmitate (C16:0), but not long-chain unsaturated fatty acids, in a time- and concentration-dependent manner. This process was reversible and dependent on cholesterol and intracellular calcium. Although calcium was necessary for this translocation, it was not sufficient to induce the annexin II translocation to DRM. We also demonstrate that stearate induced the acylation of caveolin but not that of annexin II. Association of annexin II with caveolin, although not necessarily direct, specifically occurs in DRM in response to stearate. Finally, bromostearate, a stearate analogue that effectively blocks protein acylation, does not induce annexin II translocation to DRM. We conclude that exogenously added LCSFA strongly induces the translocation of annexin II to DRM in Hs578T human breast cancer cells at least partially by association with acylated caveolin.
Keywords: annexin II, caveolin, detergent-resistant membrane, fatty acid, stearate
Abbreviations: BAPTA, bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid; DMEM, Dulbecco's modified Eagle's medium; DRM, detergent-resistant membranes; DSM, detergent-soluble membranes; FAF, fatty acid free; FBS, foetal bovine serum; GPI, glycosylphosphatidylinositol; LCSFA, long-chain saturated fatty acid(s); LCUFA, long-chain unsaturated fatty acids; MALDI–TOF, matrix-assisted laser-desorption ionization–time-of-flight; MβCD, methyl-β-cyclodextrin; NEFA, non-esterified fatty acid
INTRODUCTION
Lipid-rich plasma membrane rafts are cholesterol and glycosphingolipid-enriched membrane microdomains, which include caveolae (vesicular organelles) and numerous signalling proteins. Rafts and other closely related membrane microdomains are resistant to solublization at 4 °C by non-ionic detergents and, therefore, can be isolated as what has been termed DRM (detergent-resistant membranes) [1,2]. Rafts have been demonstrated to be involved in many key cell functions such as signal transduction, endocytosis and cholesterol trafficking [3]. One interesting property of lipid rafts is that they can include or exclude proteins to variable extents. Protein acylation has been proposed to be an important protein targeting mechanism for raft association. In addition, recent fluorescence resonance energy transfer studies in living cells have confirmed that lipid modifications are important for protein localization either into or out of plasma membrane lipid rafts [4]. Covalent protein modification by fatty acylation has been shown to occur on a wide variety of cellular signalling proteins. Acylated proteins known to be associated with rafts include src family tyrosine kinases, endothelial nitric oxide synthase, Gα subunits of heterotrimeric G-proteins and caveolin [5–8].
Both lipid rafts and caveolae have been implicated in the development of cancer. Specifically, caveolin-1, a major structural component of caveolae, has been demonstrated to be increased in primary and metastatic human prostate cancer [9] as well as high-grade bladder cancer [10]. Lipid rafts in caveolin negative human prostate cancer cells have been shown to be important mediators of akt-regulated survival [11]. On the other hand, increased caveolin-1 expression in ovarian cancer cell lines [12] and MCF-7 human breast cancer cells [13] decreased cell growth. Our goal was to determine changes in DRM protein in response to exogenously added LCSFA (long-chain saturated fatty acids) in human breast cancer cells.
In Hs578T human breast cancer cells, the major protein accumulating in DRM in response to exogenously added LCSFA was annexin II. We have characterized a specific acylation-dependent process whereby LCSFA induce the translocation of annexin II to DRM most likely via association with caveolin.
EXPERIMENTAL
Materials
Hs578T breast cancer cell line was from A.T.C.C. (Manassas, VA, U.S.A.). DMEM (Dulbecco's modified Eagle's medium), FBS (foetal bovine serum), trypsin-EDTA, penicillin/streptomycin, Protein A–agarose and Protein-G–agarose were from Gibco Life Technologies (Carlsbad, CA, U.S.A.). Micro BCA™ Protein Assay Reagent kit was from Pierce (Rockford, IL, U.S.A.). Anti-annexin II and anti-caveolin were from Transduction Laboratories (San Diego, CA, U.S.A.). Stearic acid, MβCD (methyl-β-cyclodextrin), n-octyl β-D-glucopyranoside, FAF (fatty acid free)–BSA, mouse IgG1, goat IgG, insulin and protease inhibitor cocktail [104 mM 4-(2-aminoethyl)benzenesulphonyl fluoride/1.5 mM pepstatin A/1.4 mM E-64/4 mM bestatin/2 mM leupeptin/0.08 mM aprotinin] were from Sigma (St Louis, MO, U.S.A.). Horseradish peroxidase-conjugated IgGs, ECL® Western Blotting Detection Reagents, Amplify and Hyperfilm™ MP were supplied by Amersham Biosciences (Piscataway, NJ, U.S.A.). PVDF membrane was from Millipore (Bedford, MA, U.S.A.). [9,10-3H]Stearic acid was from American Radiolabeled Chemicals (St Louis, MO, U.S.A.). NEFA (non-esterified fatty acid) C assay kit was from Wako Chemicals GmbH (Neuss, Germany).
Buffers
Buffer A consisted of 50 mM Hepes, 150 mM NaCl, 1.0 mM sodium orthovanadate, 1.0% Triton X-100 and 10% (v/v) glycerol with cocktail protease inhibitors (1 μl/ml buffer, pH 7.4). Buffer B was buffer A without 1.0% Triton X-100, but with 50 mM n-octyl β-D-glucopyranoside and 0.5% SDS (pH 7.4). Buffer C (5×sample buffer) was 0.3 M Tris (pH 6.8), 2% (w/v) SDS, 50% glycerol and 0.125% (w/v) Bromophenol Blue. Buffer D consisted of 25 mM Tris, 140 mM sodium chloride and 2.7 mM potassium chloride (pH 8.0).
Cell culture, DSM (detergent-soluble membranes) and DRM
Hs578T breast cancer cells were maintained in 10% (v/v) FBS in DMEM with 100 units/ml penicillin G sodium and 100 μg/ml streptomycin sulphate plus 10 μg/ml insulin. The cultured cells were starved for 48 h in DMEM with 0.5% inactivated FBS before use, treated under different conditions and washed three times with ice-cold PBS. DSM and DRM were prepared by adding 1 ml of buffer A, scraping cells into an Eppendorf tube, rotating for 1 h at 4 °C, and centrifuging at 12000 g for 15 min at 4 °C. Trypan Blue staining indicated no decrease in cell viability of starved versus non-starved cells. Cells were determined to be responsive to epidermal growth factor after starvation, and FACS analysis of cells using propidium iodide and FITC-labelled annexin V indicated no difference in living, dead or apoptotic cells in control versus stearate-treated cells after starvation (results not shown). The supernatant contained DSM, and DRM and were prepared from the pellet by further dissolving with buffer B by pipetting.
Protein acylation assay
Cells were cultured in a 10 cm Petri dish to 60–70% confluence, and starved for 2 days. [9,10-3H]Stearic acid (2 mCi) was dried by a gentle stream of nitrogen gas and solubilized into a 1% FAF–BSA in starvation medium by vortex-mixing for 5 min (>90% recovered in aqueous solution). Cells were incubated in this solution at 37 °C for 6 h. Cells were washed with PBS three times, and DSM and DRM were prepared. After being fixed in propan-2-ol/water/acetic acid (5:13:2, by vol.) for 30 min, the gel was soaked in Amplify with agitation for 30 min. After drying, the gel was exposed to radiography film for 30–40 days at −80 °C.
Loading fatty acid to BSA
Fatty acids were added to aqueous solutions via BSA by the method of Spector and Hoak [14]. Briefly, fatty acids were dissolved in hexane, mixed with celite and dried under a stream of nitrogen gas. The celite was then added, mixed with 1% FAF–BSA in starvation buffer, and the celite was filtered out. The pH solution was adjusted to 7.4 and the addition, filtration and pH adjusting were repeated two more times. BSA is a physiologic carrier of fatty acids and was used to avoid the introduction of organic solvents to solutions coming into contact with cells. Fatty acid concentration was determined using an enzymic-colorimetric assay (NEFA C assay kit).
MS
MS of protein was performed according to the strategy described previously [15]. Briefly, the protein band was excised from a one-dimensional polyacrylamide gel. After reduction and alkylation, proteins were digested in the gel with an excess of sequencing-grade trypsin. Mass spectra were recorded on a PerSeptive Biosystems DE-Pro MALDI–TOF-MS (matrix-assisted laser-desorption ionization–time-of-flight mass spectrometry system; Applied Biosystems, Foster City, CA, U.S.A.) at the University of Alabama at Birmingham, Mass Spectrometry Facility. The matrix used for MALDI–TOF-MS was α-cyano-4-hydroxycinnamate (Sigma). Results were used to search the NCBI (National Center for Biotechnology Information) database using MASCOT search engine from Matrixscience.com.
Immunoprecipitation
Hs578T human breast cancer cells, 60% confluent 10 cm dishes, were starved for 2 days and samples were prepared as indicated. Samples were precleared by incubation with 20 μl of Protein A or Protein-G–agarose and 20 μg of IgG for 1 h at 4 °C and then incubated with Protein A or Protein-G–agarose and 5 μg of antibody overnight at 4 °C. Precipitated immunocomplexes were then washed three times with buffer A, boiled in buffer C, resolved by SDS/PAGE and immunoblotted.
SDS/PAGE and immunoblotting
Proteins were separated by 10% SDS/PAGE by the method of Laemmli [16]. The separated proteins were then stained with Coomassie Blue solution (Pierce) or transferred on to PVDF membrane. The PVDF membrane was blocked with buffer D, containing 5% non-fat dry milk for 1 h at room temperature (22–24 °C). The primary antibodies were diluted in buffer D that contained 5% dry milk and incubated with PVDF for 2 h at room temperature or 4 °C overnight. PVDF membranes were washed with buffer D. The secondary antibodies (all conjugated with horseradish peroxidase) were diluted in buffer D and incubated with PVDF membranes for 1 h at room temperature. The PVDF membranes were then washed and the bands were visualized by chemiluminescence (ECL® Western Blotting Detection Reagents) according to the manufacturer's instructions.
Statistics and densitometry
Densitometry was performed using Scion Image (Scion, Frederick, MD, U.S.A.). Descriptive statistics were expressed as means±S.E.M. as indicated. A paired Student's t test was used to compare treatment groups and a P<0.05 was considered significant.
RESULTS
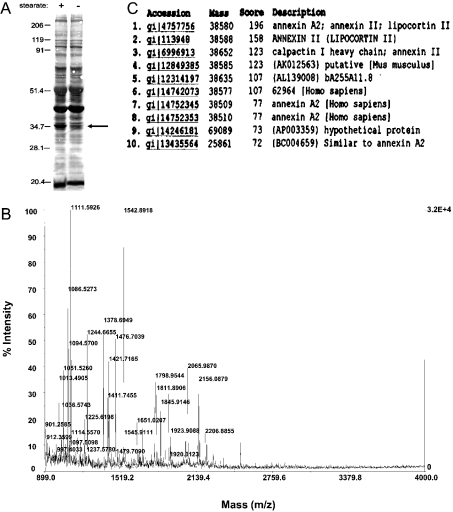
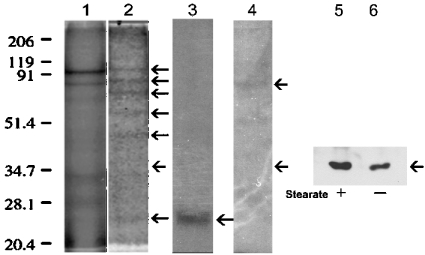
The addition of certain dietary fatty acids to growth media can result in these fatty acids being attached to cellular proteins [5]. In addition, changing the acylation domains of certain proteins appears to be sufficient to regulate their partitioning into DRM [4]. However, little is known concerning the effects of LCSFA on DRM protein composition in human cancer cells. After treatment of human Hs578T breast cancer cells for 6 h with stearate, we observed a band in raft containing DRM at approx. 36 kDa, which significantly increased compared with controls (Figure 1A). MS was used to identify this protein. Figure 1(B) shows the MALDI-MS spectrum of the tryptic digest peptides from the 36 kDa protein excised from the gel in Figure 1(A). The peptide masses in the MS fingerprint matched the predicted annexin II peptide masses (Figures 1B and 1C). We confirmed these results using immunoblots (Figure 2A).
Figure 1. SDS/PAGE of DRM with or without stearate treatment and identification of the 36 kDa protein by MS.
(A) Human breast cancer cells (Hs578T) were incubated with or without 50 μM stearate for 6 h, lysed and DRM and DSM were prepared as described in the Experimental section. Proteins were resolved by SDS/PAGE and stained with Coomassie Blue. Molecular-mass standards are indicated in kDa. The arrow indicates the band (approx. 36 kDa), which was excised for enzymic digestion by trypsin and subsequent MS analysis. (B) The gel slice from SDS/polyacrylamide was excised and subjected to in-gel digestion by trypsin. After digestion, a portion of the supernatant was removed and analysed by high-accuracy peptide mass mapping using MALDI–TOF-MS analysis. The band excised from the gel contained a single identifiable component. (C) The peptide masses obtained by MALDI–TOF-MS were used to search the NCBI database using MASCOT search engine from Matrixscience.com. The two peptide masses with the highest scores (accession numbers 4757756 and 113948) indicated that the 36 kDa protein was annexin II.
Figure 2. Stearate-induced movement of annexin II to DRM is concentration- and time-dependent, reversible and specific.
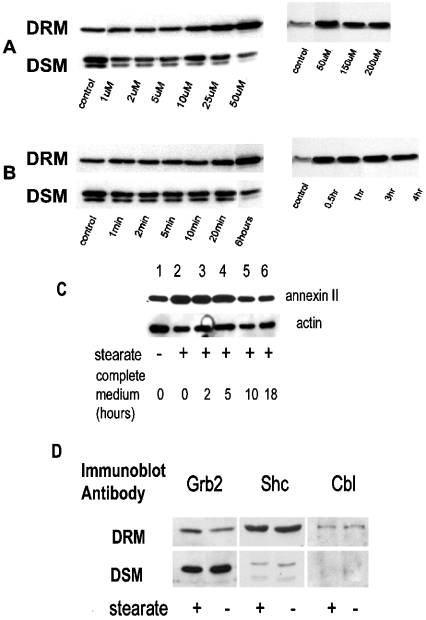
Hs578T cells were incubated with stearate at different concentrations as indicated in (A) for 6 h and lysed. DRM and DSM were prepared, resolved by SDS/PAGE and immunoblotted for annexin II as described in the Experimental section. The left and right panels in both (A, B) are two separate experiments. In (B), cells were incubated with 50 μM stearate for the indicated times. In (C), cells were treated with 50 μM stearate for 6 h; then the stearate was removed and cells were incubated with complete media for the times indicated and DRM were prepared as described in the Experimental section. Immunoblot was developed for annexin II and actin as indicated (representative of three separate experiments). Densitometry results of the three experiments demonstrate that the stearate-treated samples at 0 and 2–5 h are increased versus controls (P=0.0217 and P=0.0439 respectively), whereas the 10 h and 18–24 h samples were not different from the controls (P=0.6366 and P=0.8837 respectively). In (D), Hs578T cells were serum-starved 48 h before use and treated with or without 50 μM stearate for 6 h. DSM and DRM were prepared, and proteins were resolved by SDS/PAGE, transferred on to PVDF and immunoblotted as indicated (representative of two separate experiments). Although there appears to be an increase in Grb2 in the DRM in the presence of stearate in comparison with the control, this was not a consistent observation.
To determine the dynamics of stearate-induced annexin II translocation, we first investigated the concentration of fatty acid and time requirements. Figure 2(A) indicates that the movement of annexin II is stearate-concentration-dependent and begins between 10 and 25 μM stearate and is maximum at ∼50 μM. Interestingly, annexin II appears as two bands in DSM and a single band in DRM. Treating cells with 50 μM stearate demonstrated that at least a 20 min incubation was required for annexin II to translocate, and was maximum at ∼30 min (Figure 2B). In addition, annexin II build-up in DRM in response to stearate is reversible, with levels reaching baseline between 5 and 10 h after stearate removal (Figure 2C). Since annexin II has been proposed to function as a scaffolding protein, we were interested to determine whether other proteins moved along with annexin II. We found that Grb2, Shc and Cbl did not move in parallel with annexin II, indicating a degree of specificity in this process (Figure 2D).
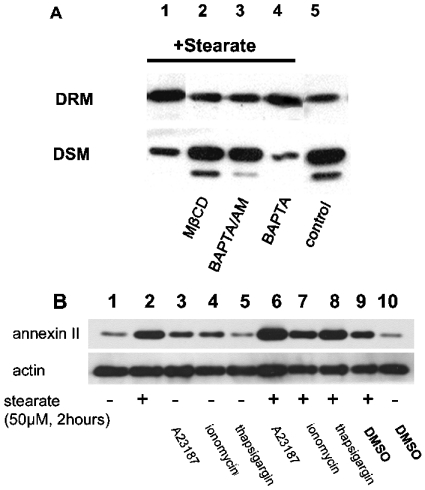
Cholesterol is critical for the formation of DRM [17,18]. We predicted that cholesterol would be an important regulator of annexin II translocation by stearate. To examine the potential role of cholesterol in the process, we used the cholesterol-depleting drug MβCD. Figure 3(A) indicated that when cholesterol was depleted with MβCD, annexin II translocation was blocked.
Figure 3. Movement of annexin II to DRM is dependent on cholesterol and intracellular calcium.
Cells were treated as described below and DRM and DSM were resolved by SDS/PAGE and immunoblotted for annexin II. In (A), cells were incubated with 50 μM stearate for 6 h (lane 1) and 1% MβCD was used 30 min before addition of 50 μM stearate (lane 2). Cells were incubated with membrane-permeable Ca2+ chelator BAPTA/AM (50 μM) or membrane-impermeable Ca2+ chelator BAPTA (50 μM) for 20 min followed by stearate (50 μM) for 6 h (lanes 3 and 4 respectively). In lane 5, cells were not treated with stearate (control; representative of two separate experiments). In (B), cells were incubated with 50 μM stearate for 2 h with or without 0.1 μM A23187 or 0.1 μM ionomycin or 2 μM thapsigargin as indicated. Since DMSO was used as a vehicle for the calcium agents, 0.08% DMSO was added to cells for 2 h as indicated. DRM were resolved by SDS/PAGE and immunoblotted for annexin II and actin as indicated (representative of three separate experiments). Densitometry results of three experiments indicate that stearate significantly increased annexin II in DRM compared with controls (lane 1 versus lane 2) (P=0.043, n=3). Additionally, stearate treatment with calcium agents also increased annexin II in DRM compared with the calcium agents alone (lane 3 versus lane 6, P=0.0024; lane 4 versus lane 7; P=0.0393; and lane 5 versus lane 8, P=0.0085).
The annexins are known to bind to biological membranes in a calcium-dependent manner. Babiychuk and Draeger's results demonstrated that annexin II translocated to detergent-resistant sites in the smooth-muscle cell plasmalemma in a calcium-dependent manner [19]. We also found that stearate-dependent translocation of annexin II was dependent on intracellular calcium concentration. Results in Figure 3(A) revealed that annexin II was not translocated in the presence of an intracellular calcium chelator glycine BAPTA/AM [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis(acetoxymethyl ester)], but was translocated in the presence of an extracellular calcium chelator BAPTA. Calcium had no significant effect on annexin II translocation to DRM in Hs578T human breast cancer cells (Figure 3B).
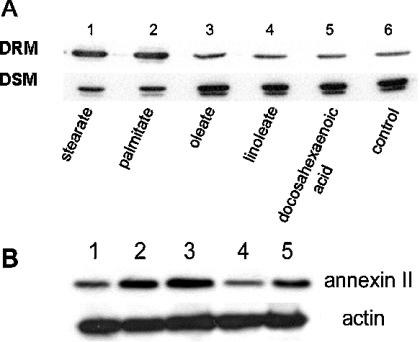
LCUFAs (long-chain unsaturated fatty acids) are also known to acylate proteins [5]. We determined the effect of individual LCUFAs on the translocation of annexin II and compared them with LCSFA. Specifically, we compared saturated [stearate (C18:0) and palmitate (C16:0)] with unsaturated [oleate (C18:1), linoleate (C18:2) and docosahexanoic acid (C22:6)] fatty acids. Figure 4 indicates that unsaturated fatty acids have no effect on the translocation of annexin II, whereas both LCSFA, stearate and palmitate, stimulated this process.
Figure 4. Annexin II translocation is specific for saturated fatty acids.
Hs578T cells were treated as described below and DRM and DSM were prepared and resolved by SDS/PAGE and immunoblotted for annexin II as described in the Experimental section. In (A), cells were incubated for 6 h with 50 μM LCSFA (lanes 1 and 2), stearate (C18:0) and palmitate (C16:0) or LCUFA (lanes 3–5), oleate (C18:1), linoleate (C18:2) and 25 μM docosahexaenoic acid (C22:6) as indicated. Control cells were not treated with stearate (lane 6) (representative of two separate experiments). In (B), cells were starved for 48 h and then treated with 50 μM stearate for 6 h (lane 2), 50 μM bromostearate for 6 h (lane 4). Non-stearate-treated cells were used for control (lane 1). As bromostearate was dissolved in DMSO, stearate plus DMSO is also shown for comparison (lane 3) and DMSO alone (lane 5). Immunoblots from DRM of the above stated conditions were developed for annexin II and actin as indicated (representative of three separate experiments). Densitometry results of the three experiments indicate that stearate–DMSO (lane 3) is increased versus bromostearate (lane 4) and DMSO (lane 5; P=0.0200 and P=0.0300 respectively). DMSO appears to have an effect on annexin II translocation; however, it is not statistically different from bromostearate (lanes 4 and 5) in our limited set of three experiments; P=0.2552.
A growing number of viral and cellular proteins have been shown to be modified by covalent attachment of fatty acids. Liang et al. [5] showed that fatty acylation of Src family kinases is essential for localization to the plasma membrane and to DRM. In addition, McCabe and Berthiaume's results indicated that N-terminally acylated proteins, such as Yes, GAP-43 (growth-associated protein 43), Gα1, Gα0 and Fyn, were enriched in DRM [20]. In those proteins, loss of acylation resulted in the loss of translocation to lipid rafts. They also showed that cholesterol plays a key role in the proper localization of fatty acylated proteins. On the basis of these results and our observation that annexin II translocation was specifically induced by saturated fatty acids (Figure 4A), and not induced by bromostearate (Figure 4B), a nonmetabolizable analogue of stearate, it is likely that protein acylation by stearate played a role in the translocation of annexin II.
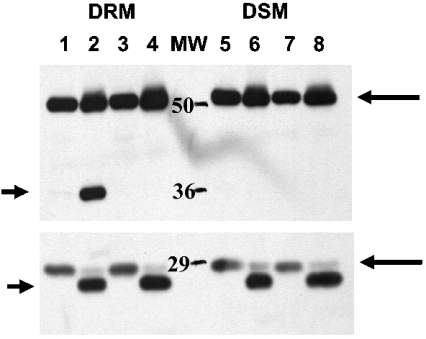
Our results indicated that proteins were acylated by stearate treatment in both DRM and DSM (Figure 5). Interestingly, although several proteins were clearly acylated in DRM, there was no acylated protein visible at 36 kDa, indicating that annexin II does not appear to be acylated directly by stearate treatment. This was confirmed by annexin II immunoprecipitation (Figure 5). Since several DRM proteins were acylated by stearate treatment, it is possible that protein acylation other than annexin II may be involved with annexin II translocation. Studies by Uittenbogaard and Smart [8] have demonstrated that acylation of caveolins at Cys143 and Cys156, but not at Cys133, was necessary for the formation of a caveolin–cholesterol chaperone complex. They also found that acylation of caveolin at Cys133, but not at Cys143 and Cys156, was necessary for the association of annexin II with caveolin [21]. Our results indicate that a protein of approximately the same molecular mass (∼25 kDa) as caveolin was acylated by stearate in DRM (Figure 5). Immunoprecipitation confirmed that caveolin was acylated by stearate treatment (Figure 5). Interestingly, there did not appear to be any other acylated proteins associated with caveolin. However, at least one non-caveolin-acylated protein was associated with annexin II. These data suggest that annexin II translocation to DRM involves the acylation of caveolin by stearate. To determine whether annexin II and caveolin associate in DRM, we used caveolin antibody to immunoprecipitate caveolin in DRM and DSM with and without stearate treatment. Annexin II was co-immunoprecipitated with caveolin in DRM but not DSM and only when cells were treated with stearate (Figure 6). Epidermal growth factor receptor was not detected in caveolin immunoprecipitates (results not shown).
Figure 5. Caveolin but not annexin II is acylated by stearate.
Cells were treated with 32 μM (final concentration) of [9,10-3H]stearic acid for 6 h. The DRM and DSM were resolved by SDS/PAGE, fixed and autofluography was performed as described in the Experimental section. Immunoprecipitation was performed as described in the Experimental section. Lane 1, DSM; 2, DRM; 3, DRM caveolin-immunoprecipitated; 4, DRM annexin II-immunoprecipitated. Arrows in lane 2 indicate the proteins in DRM acylated by stearate except the arrow at ∼35 kDa, which indicates the molecular mass of annexin II (also in lane 4). Arrow in lane 3 indicates caveolin. Upper arrow in lane 4 indicates acylated protein associating with annexin II in DRM. Lanes 5 and 6 are immunoblots that indicate annexin II is immunoprecipitated from DRM. Immunoprecipitation performed as described above from DRM of cells treated with 32 μM stearate-treated cells for 6 h as indicated (immunoprecipitation antibody: polyclonal anti-annexin II antibody from Santa Cruz Biotechnology; immunoblot monoclonal anti-annexin II antibody from Transduction Laboratories).
Figure 6. Annexin II association with caveolin is stearate/acylation-dependent.
Cells in 10 cm plates were serum-starved for 48 h and treated with or without 50 μM stearate for 6 h, and DRM and DSM were prepared. Caveolin and caveolin-associated proteins were immunoprecipitated with caveolin IgG (5 μg/sample) as indicated (immunoprecipitation antibody) and resolved by SDS/PAGE. The proteins were transferred on to PVDF and immunoblotted for annexin II and caveolin. In lanes 1, 3, 5 and 7, non-immune mouse IgG was used for immunoprecipitation as a negative control. Arrows on the right indicate the antibody heavy and light chains, whereas the arrows on the left indicate annexin II (upper) and caveolin (lower). The experiment shown is representative of three separate experiments.
DISCUSSION
Protein acylation by LCSFA is an important process in regulating protein location within the cell. Specifically, LCSFA have been shown to increase selectively protein content in rafts [4]. DRM are relatively non-polar areas of the plasma membrane, which include rafts and caveolae. These microdomains are composed of cholesterol, sphingolipids and proteins. Details of the regulation of protein trafficking in and out of DRM are not well understood. Our results indicate that certain LCSFA directly mediate a major, reversible translocation of annexin II from DSM to DRM probably via an acylation, calcium and cholesterol-dependent process.
Annexins are ubiquitous calcium and membrane-binding proteins. The name annexin originates from the ability to annex or bind to phospholipids. Annexin II is one of the 13 annexins described to date.
Proteins associate with DRM by at least three different mechanisms: GPI (glycosylphosphatidylinositol) anchors [22], acylation and/or direct interaction with cholesterol [23,24] and via domains concentrated with hydrophobic residues [25]. Although there is no evidence about annexin II directly interacting with cholesterol or GPI anchors, a number of studies show that annexin II can associate with rafts or raft-like structures rich in cholesterol and GPI-anchored proteins. In mammary epithelial cells, as well as in baby hamster kidney and smooth-muscle cells, annexin II is significantly enriched in the raft fraction in the presence of calcium, and in baby hamster kidney cells, membrane-bound annexin II can be released specifically after cholesterol sequestration [19,26,27]. We demonstrate two immunoreactive bands of annexin II in DSM, but only one band in DRM. The DRM band is the upper band in DSM. What accounts for the different bands is unclear. However, annexin II can undergo post-translational modifications that could account for the two bands, such as tyrosine phosphorylation [28] and partial proteolysis [29]. One study demonstrated that annexin II isoforms have distinct N-termini resulting from different cDNA [30]. Alternatively, the two bands could be the result of splice variants.
Studies have indicated that annexin II can be associated with DRM in a calcium-dependent manner and may promote the calcium-dependent association of lipid raft microdomains [19]. Our results support these studies in that calcium is important in increasing annexin II association with DRM. However, increased intracellular calcium did not have a significant effect on annexin II translocation to DRM. Also, LCSFA have been demonstrated to increase sequestration of intracellular calcium rather than increase release of intracellular calcium [31]. These results are consistent with calcium being necessary but not sufficient for LCSFA-induced annexin II translocation.
Acylated proteins have been demonstrated to partition into DRM [32]. Consistent with this premise, our results indicate several acylated proteins in response to stearate treatment in DRM as well as DSM. These results support LCSFA protein acylation as a targeting mechanism for localization of protein to DRM.
Importantly, we demonstrate that this targeting mechanism is a reversible process and it occurs at physiological concentrations of NEFAs (non-esterified fatty acids or free fatty acids). In humans, circulating NEFA concentrations vary considerably depending on age, state of fasting and certain diseases. Nevertheless, for non-obese, fasting, normal subjects NEFAs range from 0.41 to 0.68 mM [33]. In obesity and Type II diabetes, fasting NEFA levels are approx. 0.85±0.29 mM (means±S.D.) [33]. Palmitate is ∼20–25% and stearate is ∼10% of human circulating NEFAs [33]. Thus at an NEFA concentration of 0.50 mM, stearate concentration would be ∼50 μM and palmitate would be ∼100 μM. Both these NEFA concentrations are sufficient to cause annexin II translocation to DRM.
Although protein acylation by stearate has been demonstrated, a breakdown of stearate to palmitate and subsequent acylation by palmitate cannot be ruled out. Interestingly, annexin II does not appear to be directly acylated. In contrast with our results other studies have indicated that addition of LCUFA to T-lymphocytes resulted in a significant enrichment of DRM with LCUFA and appeared to displace proteins from rafts [34]. They concluded that displacement of acylated proteins from rafts by LCUFA was largely due to changes in lipid composition of membrane rafts rather than altered protein acylation. Thus an alternative explanation for our observations is that LCSFA increase the ordered state of DRM microdomains and thereby favour the incorporation of acylated proteins. On the other hand, protein acylation and especially palmitoylation, as well as raft localization, have been demonstrated to be inhibited by bromopalmitate [35]. Since we observed the strongest effects on annexin II translocation with stearate, we utilized bromostearate, a non-metabolizable analogue of stearate. Bromostearate did not induce annexin II translocation and argues that protein acylation is probably required.
Other acylated proteins may act as chaperones targeting annexin II to DRM indirectly. The fact that plasma membrane cholesterol depletion inhibits annexin II translocation indicates that conventional DRM are involved since cholesterol is important in caveolae formation. Caveolin is a cholesterol-binding protein, which is an abundant component of caveolae. Caveolin appears to function in modulating signal transduction and is an important regulator in cholesterol trafficking. As mentioned previously, acylation of caveolin plays an important role in cholesterol transport [8]. In the present study, we demonstrated that annexin II was associated with caveolin in an LCSFA acylation-dependent manner indicating that it translocates specifically to the caveolae in DRM. One possible explanation is that annexin II interacts directly with caveolin. Many peptides that interact with caveolin share two properties: (i) a preponderance of aromatic amino acids in a short stretch and (ii) a characteristic spacing between these aromatic residues [36]. Annexin II does not appear to have a classical caveolin-binding domain; however, it does have areas that contain a preponderance of aromatic amino acids. Specifically, the 12-amino-acid stretch from 307 to 318 and the 10-amino-acid stretch from 229 to 238 contain five and four aromatic amino acids respectively. These may be potential interaction sites for annexin II and caveolin.
While the interaction of annexin II and caveolin appears to be an acylation-dependent phenomenon, it should be noted that caveolin was acylated in DSM as well as DRM (results not shown); however, annexin II only associated with caveolin in the DRM. This may indicate different acylation sites in DRM caveolin versus DSM caveolin. Alternatively, another acylated protein or a specific lipid such as a phospholipid, glycerolipid or sphingolipid, for example, may be required to mediate the annexin II DRM translocation. The increased partitioning of this lipid into DRMs would correspond to the increase in the attachment of fatty acids with saturated side chains (e.g. stearate) to the glycerolipid or sphingolipid backbone of that lipid. This increase in the saturation would then increase its partitioning into the liquid-ordered phase of the membrane and recruit annexin II to DRMs in the process. This process would be expected to be inhibited by bromostearate incubation, since bromostearate needs to be converted into bromostearoyl-CoA for it to be active. Bromostearoyl-CoA, like bromopalmitoyl-CoA, is a potent inhibitor of several enzymes that synthesize lipids as well as protein fatty acyl transferase. Thus the inhibition of annexin II translocation by bromostearate is consistent with the inhibition of both protein acylation and stearate incorporation into lipids. Another potential explanation for annexin II translocation is that LCSFA may further enrich DRM, creating an environment which favours the translocation of acylated proteins and annexin II. Such a paradigm has been described by Stulnig et al. [34] for LCUFA and has been discussed (see above).
Annexin II has been proposed to have many functions. Extracellularly, it has been linked to cell–cell adhesion, extracellular matrix interaction, cholesterol transport and plasminogen activation. Intracellularly, it has been suggested to be a docking protein, which affects multiple cell functions including membrane-cytoskeletal linkage, exocytosis, endocytosis and trafficking. Regarding a specific role for annexin II in DRM, it has been shown to stabilize membrane microdomain structure by promoting the calcium-dependent association of rafts in smooth-muscle cells [29]. In addition, annexin II associated with rafts has been proposed to be involved in rearrangements of the actin cytoskeleton [37]. In vitro and in vivo studies indicate that LCSFA inhibit breast cancer cell proliferation [38,39]. Couet et al. [36] showed that caveolin may function as a general kinase inhibitor and caveolin binding may play a negative regulatory role in signal transduction. Whether there is a role for LCSFA-induced translocation of annexin II in cancer progression remains to be determined. In addition, it will be important to determine whether LCSFA-induced annexin II translocation occurs in other cancer cell types as well as in vascular endothelial and smooth-muscle cells.
In summary, we have demonstrated that physiological concentrations of LCSFA specifically induce a major translocation of annexin II from DSM to DRM in association with caveolin, in a reversible, calcium and cholesterol-dependent manner in human breast cancer cells. This change in location of annexin II likely involves acylation and can be mediated by stearate as well as palmitate.
References
- 1.Brown D. A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 2.Brown D. A., London E. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 3.Sowa G., Pypaert M., Sessa W. C. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 5.Liang X., Nazarian A., Erdjument-Bromage H., Bornmann W., Tempst P., Resh M. D. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J. Biol. Chem. 2001;276:30987–30994. doi: 10.1074/jbc.M104018200. [DOI] [PubMed] [Google Scholar]
- 6.Moffett S., Brown D. A., Linder M. E. Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 7.Shaul P. W., Smart E. J., Robinson L. J., German Z., Yuhanna I. S., Ying Y., Anderson R. G. W., Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 8.Uittenbogaard A., Smart E. J. Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation, and rapid transport of cholesterol to caveolae. J. Biol. Chem. 2000;275:25595–25599. doi: 10.1074/jbc.M003401200. [DOI] [PubMed] [Google Scholar]
- 9.Mouraviev V., Li L., Tahir S. A., Yang G., Timme T. M., Goltsov A., Ren C., Satoh T., Wheeler T. M., Ittmann M. M., et al. The role of caveolin-1 in androgen insensitive prostate cancer. J. Urol. 2002;168:1589–1596. doi: 10.1016/S0022-5347(05)64526-0. [DOI] [PubMed] [Google Scholar]
- 10.Rajjayabun P. H., Gard S., Durkan G. C., Charlton R., Robinson M. C., Mellon J. K. Caveolin-1 expression is associated with high-grade bladder cancer. Urology. 2001;58:811–814. doi: 10.1016/s0090-4295(01)01337-1. [DOI] [PubMed] [Google Scholar]
- 11.Zhuang L., Lin J., Lu M. L., Solomon K. R., Freeman M. R. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]
- 12.Bagnoli M., Tomassetti A., Figini M., Flati S., Dolo V., Canevari S., Miotti S. Downmodulation of caveolin-1 expression in human ovarian carcinoma is directly related to α-folate receptor overexpression. Oncogene. 2000;19:4754–4763. doi: 10.1038/sj.onc.1203839. [DOI] [PubMed] [Google Scholar]
- 13.Lee S. W., Reimer C. L., Oh P., Campbell D. B., Schnitzer J. E. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- 14.Spector A. A., Hoak J. C. An improved method for the addition of long chain fatty acid to protein solution. Anal. Biochem. 1969;32:297–302. doi: 10.1016/0003-2697(69)90089-x. [DOI] [PubMed] [Google Scholar]
- 15.Pandey A., Podtelejnikov A. V., Blagoev B., Bustelo X. R., Mann M., Lodish H. F. Analysis of receptor signaling pathways by mass spectrometry: identification of vav-2 as a substrate of the epidermal and platelet-derived growth factor receptors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:179–184. doi: 10.1073/pnas.97.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U. K. Cleavage of structural proteins during assembly of the head of the bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Rietveld A. G., Simons K. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochem. Biophys. Acta. 1998;376:467–479. doi: 10.1016/s0304-4157(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 18.Simons K., Ikonen E. Functional rafts in cell membranes. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 19.Babiychuk E. B., Draeger A. Annexins in cell membrane dynamics: Ca2+-regulated association of lipid microdomains. J. Biol. Chem. 2000;150:1113–1124. doi: 10.1083/jcb.150.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCabe J. B., Berthiaume L. G. N-terminal protein acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae. Mol. Biol. Cell. 2001;12:3601–3617. doi: 10.1091/mbc.12.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uittenbogaard A., Everson W. V., Matveev S. V., Smart E. J. Cholesteryl ester is transported from caveolae to internal membranes as part of a caveolin–annexin II lipid–protein complex. J. Biol. Chem. 2002;277:4925–4931. doi: 10.1074/jbc.M109278200. [DOI] [PubMed] [Google Scholar]
- 22.Mayor S., Rothberg K., Maxfield F. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- 23.Parton R. G. Caveolae and caveolins. Curr. Opin. Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 24.Shenoy-Scaria A. M., Dietzen D. J., Kwong J., Link D. C., Lublin D. M. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J. Cell. Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheiffele P., Roth M. G., Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harder T., Gerke V. The annexin II(2)p11 (2) complex is the major component of the Triton X-100 insoluble low-density fraction prepared from MDCK cells in the presence of Ca(2+) Biochem. Biophys. Acta. 1994;1223:375–382. doi: 10.1016/0167-4889(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 27.Oliferenko S., Paiha K., Harder T., Gerke V., Schwarzler C., Schwarz H., Beug H., Günthert U., Huber L. Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J. Cell. Biol. 1999;146:843–854. doi: 10.1083/jcb.146.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erikson R. L., Erikson E. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell (Cambridge, Mass.) 1980;21:829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- 29.Babiychuk E. B., Monastyrskaya K., Burkhard F. C., Wray S., Draeger A. Modulating signaling events in smooth muscle: cleavage of annexin II abolishes its binding to lipid rafts. FASEB J. 2002;16:1177–1184. doi: 10.1096/fj.02-0070com. [DOI] [PubMed] [Google Scholar]
- 30.Izant J. G., Bryson L. J. Xenopus annexin II (calpactin I) heavy chain has a distinct amino terminus. J. Biol. Chem. 1991;266:18560–18566. [PubMed] [Google Scholar]
- 31.Watras J., Messineo F. C., Herbette L. G. Mechanisms of fatty acid effects on sarcoplasmic reticulum. I. Calcium–fatty acid interaction. J. Biol. Chem. 1984;259:1319–1324. [PubMed] [Google Scholar]
- 32.Melkonian K. A., Ostermeyer A. G., Chen J. Z., Roth M. G., Brown D. A. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. J. Biol. Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 33.Hunnicutt J. W., Hardy R. W., Williford J., McDonald J. M. Saturated fatty acid-induced insulin resistance in rat adipocytes. Diabetes. 1994;43:540–545. doi: 10.2337/diab.43.4.540. [DOI] [PubMed] [Google Scholar]
- 34.Stulnig T. M., Huber J., Leitinger N., Imre E.-M., Angelisova P., Nowotny P., Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J. Biol. Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 35.Webb Y., Hermida-Matsumoto L., Resh M. D. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 36.Couet J., Li S., Okamoto T., Sherer P. E., Lisanti M. P. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 37.Zobiack N., Rescher U., Laarmann S., Michgehl S., Schmidt M. A., Gerke V. Cell-surface attachment of pedestal-forming enteropathogenic E. coli induces a clustering of raft components and a recruitment of annexin 2. J. Cell Sci. 2002;115:91–98. doi: 10.1242/jcs.115.1.91. [DOI] [PubMed] [Google Scholar]
- 38.Welsch C. W. Relationship between dietary fat and experimental mammary tumorigenesis: a review and critique. Cancer Res. 1992;52(Suppl.):2040s–2048s. [PubMed] [Google Scholar]
- 39.Wickramasinghe N. S. M. D., Jo H., McDonald J. M., Hardy R. W. Stearate inhibition of breast cancer cell proliferation. Am. J. Pathol. 1996;148:987–995. [PMC free article] [PubMed] [Google Scholar]