Abstract
Despite the importance of glutamatergic signalling in the co-ordination of hormone secretion, the identity of the enzyme for the production of glutamate in β-cells is still unresolved. We have found that the endocrine pancreas co-expresses two isoforms of GA (glutaminase), denoted as kidney-type (KGA) and liver-type (LGA), with a complementary cellular pattern of expression. Whereas KGA was mainly present in α-cells, LGA was very abundant in β-cells. This spatial segregation may have important functional implications, facilitating a differential regulation of glutamate production in insulin- and glucagon-secreting cells.
Keywords: glutamate, glutaminase, insulin, islets of Langerhans, isoform, pancreas
Abbreviations: GA, glutaminase; KGA, kidney-type glutaminase; LGA, liver-type glutaminase
INTRODUCTION
Increasing evidence supports the idea that glutamate functions both as an intercellular transmitter [1] and as an intracellular messenger [2] in the islets of Langerhans. Nevertheless, the activation of glutamate receptors does not appear to correlate consistently with either insulin or glucagon secretion [1]. Thus the modes of glutamate-mediated signalling in the islets appear to be complicated. Understanding the mechanisms by which this transmitter plays its roles in the endocrine pancreas requires not only a detailed knowledge of the implicated receptors but is also essential to determine how glutamate signal production is regulated. When and how is glutamate produced in the pancreas are unresolved key questions.
Glutamine is an abundant amino acid found in plasma and has no transmitter activity by itself; hence it is an excellent candidate as the precursor of the transmitter glutamate. Phosphate-activated glutaminase (GA; EC 3.5.1.2), an enzyme that catalyses the conversion of glutamine to glutamate, has been considered as a major enzyme for the production of neurotransmitter glutamate in the central nervous system [3]. In 1995, Seino and co-workers [4] performed a study on the expression of the enzyme GA in pancreas. They concluded that this enzyme was present in endocrine cells at the periphery of the islets but it was absent from β-cells, suggesting that glutamate might be released as a transmitter from α-cells but not from the β-cells in the islets [4]. More recently, it has been postulated that glutamate is produced and stored together with insulin in secretory granules of the β-cells [2]. However, since no GA has been identified in this cell type, there is an open debate about the enzyme producing that glutamate [5,6].
In mammals, there are two non-allelic genes encoding for GA, known as kidney (KGA) and liver (LGA) types [7]. For many years, the expression of LGA was believed to be restricted to the liver of postnatal animals [8]. However, we have recently reported the co-expression of both KGA and LGA mRNAs in pancreas [7]. In the present study, we confirm the presence of both isoforms at the protein level in the endocrine pancreas. More interestingly, we report a spatial segregation between KGA and LGA within the islets. Whereas KGA is mainly present in the glucagon-containing α-cells, LGA is very abundant in the insulin-containing β-cells. We discuss the potential implications of these findings in the context of the intercellular glutamatergic communication, and its role in the regulation of hormone secretion.
EXPERIMENTAL
Animals
Male Sprague–Dawley rats (240–260 g) and New Zealand White rabbits were used in these studies. All animal experiments were performed in accordance with the National Institutes of Health Guide for the care and use of laboratory animals and approved by the committee set up to study animal use for research at Málaga University. All efforts were made to minimize the number of animals used and their suffering.
Antibodies against KGA and LGA
The generation and purification of anti-KGA and anti-LGA have been described previously [9,10]. Briefly, a 596 bp fragment, encoding the last 119 amino acid residues of the human KGA protein, was cloned into the pQE-31 vector to express in Escherichia coli a His-tagged recombinant protein that was purified by immobilized metal-ion affinity chromatography. The purified protein was used as the antigen to raise rabbit polyclonal antibodies. On the other hand, the whole coding sequence of human LGA was expressed in E. coli as a recombinant protein using the vector pET-3c. The recombinant LGA protein, present in the inclusion bodies, was purified by preparative SDS/PAGE. The protein band was briefly stained with Coomassie Brilliant Blue, cut and used for hyperimmunization of New Zealand White rabbits. For purification of the antibodies, 5–10 mg of the recombinant proteins were coupled with a CNBr-activated Sepharose 6MB gel according to the manufacturer's instructions (Amersham Biosciences) and used for affinity purification of their respective antisera. A second polyclonal antibody against LGA was raised. For this purpose, a peptide of 17 residues (SHCGRGGWGHPSRSPLY) coupled with keyhole limpet haemocyanin was used for the immunization of New Zealand White rabbits. This anti-peptide antibody was directed against the unique N-terminus of the full-length form of LGA originally found in breast tumour cells [11]. Since this 17-mer sequence is not present in the truncated LGA isoform expressed in rat liver, the anti-peptide serum was used to differentiate between the truncated and the full-length LGA forms.
Isolation and analyses of islets of Langerhans
Rat pancreas was inflated with 10 ml of collagenase solution (0.4 mg/ml, collagenase type XI; Sigma). The distended pancreas was excised and the digestion performed in a silicone-treated scintillation vial at 37 °C under vigorous shaking. Digested tissue was rinsed 3 or 4 times with Hanks balanced salt solution (HBSS) and the islets were purified on a discontinuous Ficoll (Amersham Biosciences) gradient consisting of 25, 23, 20 and 11% Ficoll in HBSS. For immunoblot analyses of KGA and LGA expressions, isolated islets were solubilized with Laemmli's sample buffer and then subjected to SDS/PAGE (10% gel). The protein was transferred on to nitrocellulose membranes and incubated overnight at 4 °C in the presence of the indicated antibody. After incubation with the secondary antibody (goat anti-rabbit conjugated to horseradish peroxidase) and washes, the detection was performed using an enhanced chemiluminescence method (ECL®; Amersham Biosciences).
Double-immunofluorescence labelling
Rats were deeply anaesthetized and perfused transcardially with PBS followed by ice-cold fixative solution (4% paraform-aldehyde/75 mM lysine/10 mM sodium metaperiodate). Pancreas was excised, post-fixed overnight at 4 °C with the same fixative solution and embedded in paraffin. After blocking the endogenous peroxidase activity, avidin, biotin and biotin-binding proteins 10-μm-thick sections were used for the immunofluorescence experiments. Double labelling was made possible by using primary antibodies raised in different species (rabbit, goat and mouse) in conjuction with fluorocrome-conjugated species-specific secondary antibodies: goat anti-rabbit coupled with Alexa 488, goat anti-mouse coupled with Alexa 568 or donkey anti-goat coupled with Alexa 568 (Molecular Probes). Primary antibodies were raised against glucagon (mouse monoclonal antibody, diluted 1:6000; Sigma), insulin (mouse monoclonal antibody, diluted 1:6000, Sigma), somatostatin (goat polyclonal antibody, diluted 1:1000, Santa Cruz Biotechnology), KGA (affinity-purified rabbit polyclonal antibody, diluted 1:500) and LGA (affinity-purified rabbit polyclonal antibody, diluted 1:10). Control experiments, which were used to determine the level of non-specific staining, included incubating sections with pre-immune serum instead of primary antibody, omission of primary antibody and/or blocking the primary antibody by preincubation with the corresponding antigen before incubation of the antibody with the tissue. Under these conditions, no specific staining was observed. Preparations were examined under a confocal laser microscope (Leica TCS-NT).
RESULTS
To assess the specificity of our affinity-purified KGA and LGA antisera, we used them to probe crude extracts from rat brain and liver. Although brain and liver have been reported to contain both the GA isoforms [9,12], because the KGA/LGA ratio is very high in brain and very low in liver, the crude extracts from liver can be used as negative and positive controls for KGA and LGA respectively. Analogously, brain crude extracts were used as negative and positive controls for LGA and KGA respectively. Whereas the anti-KGA antibody detected a very intense band in brain samples, the anti-LGA antibody failed to recognize that band in the same sample, excluding the possibility of cross-reaction with KGA (Figure 1). In contrast, a discrete immunoreactive band was observed in rat liver extracts when probed with anti-LGA but not with anti-KGA (Figure 1). To test the proposition that in the endocrine pancreas both GA genes are expressed at the level of protein, we immunoblotted isolated islets with our isoform-specific antibodies. A clear immunoreactive band was observed in both cases, strongly suggesting the co-expression of KGA and LGA proteins in this endocrine organ (Figure 1). To test whether our anti-GA antibodies cross-react with insulin, glucagon or any other small polypeptide, we fractionated pancreatic samples using a Tricine–SDS/PAGE system [13]. Polypeptides were transferred on to nitrocellulose membranes that were probed with anti-GA antibodies. Neither anti-KGA nor anti-LGA antibodies reacted with small polypeptides (results not shown).
Figure 1. LGA and KGA proteins are detected in pancreatic islets.
Representative Western blots showing the presence of both GA isoforms (LGA and KGA) in islets isolated from rat pancreas. To assess potential cross-reactivity and to illustrate the isoform specificity of our purified anti-GA antibodies, rat brain and liver samples were immunoblotted as described in the text.
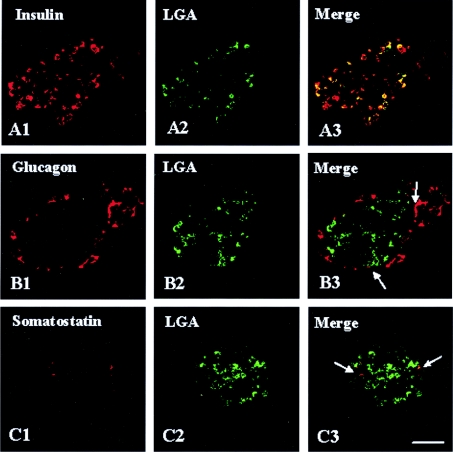
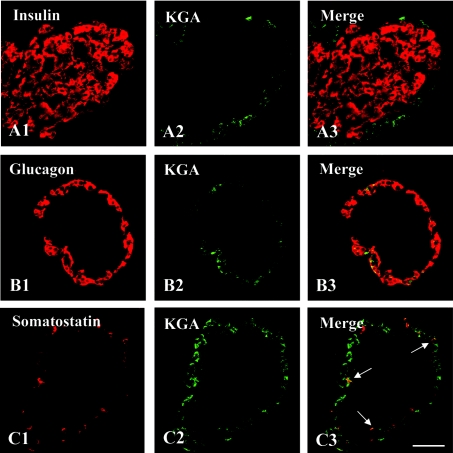
When the presence of the LGA protein in the pancreas was confirmed, further studies were undertaken to identify chemically the islet cells exhibiting LGA immunoreactivity. To this end, double-immunofluorescence labelling and laser-scanning confocal microscopy studies were performed using antibodies against insulin, glucagon or somatostatin as β-, α- and δ-cell markers respectively, together with our isoform-specific anti-LGA (Figure 2) or anti-KGA (Figure 3) polyclonal antibodies. The results clearly indicated that essentially all insulin-containing cells abundantly expressed LGA (Figure 2A3). Some somatostatin-positive cells also exhibited LGA immunoreactivity (Figure 2C3), whereas this GA isoform was absent from most of the glucagon-containing cells (Figure 2B3). In contrast, KGA immunoreactivity was completely absent from β-cells (Figure 3A3), whereas it was clearly detected in α- and δ-cells (Figures 3B3 and 3C3 respectively).
Figure 2. LGA is abundantly expressed in β-cells from rat pancreas.
Double-immunofluorescence labelling with anti-LGA (green) and endocrine cell markers: insulin, glucagon or somatostatin (red). In confocal overlay images, yellow represents co-localization of the two antigens. (A3) LGA and insulin, (B3) LGA and glucagon and (C3) LGA and somatostatin. Note that, whereas most of the insulin-containing cells exhibited a strong LGA immunoreactivity, only a few α- and δ-cells showed a weak, albeit detectable (arrows), immunolabelling. Scale bar, 57 μm.
Figure 3. KGA is not detected in β-cells from rat pancreas.
Double-immunofluorescence labelling with anti-KGA (green) and endocrine cell markers: insulin, glucagon or somatostatin (red). In confocal overlay images, yellow represents co-localization of the two antigens. (A3) KGA and insulin, (B3) KGA and glucagon and (C3) KGA and somatostatin. KGA was present in α- and δ-cells (B3 and C3 respectively) but was not detected in insulin-containing cells (A3). Scale bar, 57 μm.
Two LGA forms have been described so far. One was found in rat liver [14] and the other was originally found in human breast tumour cells [11]. Both enzymes share a considerable degree of identity (94%) although the latter extended over 67 residues at the N-terminal end. To address which form of LGA was expressed in the endocrine pancreas, we have exploited an anti-LGA antibody directed against a peptide that is absent from the shorter LGA form found in rat liver. The immunostaining pattern obtained using this anti-peptide antibody was indistinguishable from the one observed when the antibody used was that against the recombinant LGA protein (results not shown).
DISCUSSION
Both enzymic forms, KGA and LGA, display different kinetic, immunological and molecular characteristics. One of the more remarkable kinetic differences between both isoforms is that the enzyme from liver is not inhibited by the end-product glutamate, whereas KGA exhibits a strong susceptibility to glutamate inhibition [15]. Since Krebs noted that the GA activity of liver differed from that of other tissues, LGA was believed to be restricted to the liver, whereas KGA was supposed to be present in all other tissues with GA activity [8]. Recently, we have reported the presence of LGA mRNA and protein in the brain of different mammalian species [9]. We also found LGA mRNA in samples from human pancreas [7]. In the present study, we have further extended our initial finding. The occurrence of LGA protein in rat pancreas has been proved by immunocytochemistry and Western-blot analyses. Thus it can be concluded that, besides liver, there are at least two other organs, brain and pancreas, expressing the LGA isoform.
Erecinska and co-workers [16] reported in 1992 that pancreatic islets possessed a powerful GA activity with properties resembling those of the KGA isoenzyme; for instance, the activity measured in pancreas was inhibited by glutamate. These authors also concluded that the high GA activity observed in the islets may be of importance for optimal insulin secretion. However, 3 years later, Seino and co-workers [4] failed to detect KGA immunoreactivity in insulin-containing cells, whereas an intense KGA immunoreactivity was detected at the periphery of the islets. In the present study, we have confirmed and extended this observation, identifying KGA-positive cells at the periphery of the islets as glucagon- and somatostatin-containing cells (Figure 3).
Despite the absence of KGA in β-cells, growing evidence suggests a fundamental role for intracellular glutamate in the regulation of insulin secretion, although the precise mechanisms are a matter of debate. Thus some authors have suggested that glutamate, generated by cataplerosis from glucose-derived α-ketoglutarate, may act downstream of mitochondrial processes, participating in the coupling of glucose catabolism with insulin secretion [2,5]. In contrast, other authors favour the concept that the anaplerotic glutamate to α-ketoglutarate conversion would stimulate insulin secretion [17–19]. Nevertheless, the incorporation of glutamate into the insulin-containing secretory granules in β-cells is considered to be a key event in the granule priming process [20,21]. Once priming has been completed, glutamate will be co-released with insulin under the suitable stimulus. In addition, in β-cells, glutamate also acts as a precursor of the signalling amino acid γ-aminobutyric acid that is stored in this cell type [22]. Since KGA was not found in β-cells [4], Wollheim and co-workers proposed that the enzyme glutamate dehydrogenase (GDH) acts in the reverse direction to catalyse the production of glutamate [2,5]. However, their suggestion that net flux through GDH is towards the synthesis rather than the oxidation of glutamate has been contradicted by other reports [6,19]. Therefore a significant finding obtained in the present study is that insulin-containing cells express LGA. Furthermore, the use of an anti-peptide antibody directed against the unique 17-mer sequence of the longer form of LGA allowed us to conclude that most of the LGA protein being expressed by β-cells corresponds to the longer version of LGA. However, we cannot rule out the possibility of the co-existence of both forms as e.g. in rat brain (L. Olalla, A. Gutiérrez and J. C. Aledo, unpublished work). In any event, this is the first report of the expression of the longer form of LGA in rat, suggesting that both forms may be the result of alternative splicing processes, rather than gene variations among species.
Within the islets, KGA and LGA showed a striking complementary pattern of cellular expression, with KGA being present in many, but not all, α-cells, whereas LGA was abundantly expressed by most β-cells (Figures 2 and 3). Also since β-cells account for approx. 60–70% of the endocrine cell population, whereas α-cells represent approx. 15–20%, we conclude that LGA is the major GA protein in pancreas. Since GA activity assayed in islet extracts is susceptible to glutamate inhibition [16], one may feel tempted to speculate that LGA in pancreas may exhibit susceptibility to glutamate inhibition, a kinetic characteristic believed to belong only to KGA [8]. However, an inherent limitation of the immunocytochemical technique is that it reveals the amount of enzyme protein, rather than the level of enzyme activity. Thus before any further interpretation of the current results can be made, it is essential to know the relative activities of KGA and LGA.
Insulin and glucagon are two of the main hormones involved in glycemia homoeostasis. Their secretion from the endocrine pancreas must be closely co-ordinated. To achieve this co-ordination under a wide range of physiological conditions, there is a very complex cross-talk between α- and β-cells. In this sense, the glutamatergic signalling system has been proved to be present in islets of Langerhans where it can alter hormone secretion [1]. Despite the unquestionable importance of glutamate as a transmitter, its precise physiological roles in islet function and its cellular mechanisms are not clear. How is glutamate production regulated in the islets is a critical issue that remains unresolved. Our finding that KGA and LGA show specific cell-type segregation may represent the biochemical basis to achieve a higher flexibility in the regulation of glutamate production within the islets. Since KGA and LGA enzymes are encoded by two separated genes [7], each one possessing a unique promoter sequence that has been cloned [14,23], it will be very interesting to determine the elements conferring the tissue-specific expression observed in pancreatic islets.
Acknowledgments
We thank Dr Antonio Peñafiel for assistance with laser scanning confocal microscopy. We are grateful to Dr Javier Márquez for his comments on the paper. This work was supported by grant FIS PI030214 from the Spanish Fondo de Investigación Sanitaria.
References
- 1.Moriyama Y., Hayashi M. Glutamate-mediated signalling in the islets of Langerhans: a thread entangled. Trends Pharmacol. Sci. 2003;24:511–517. doi: 10.1016/j.tips.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Maechler P., Wollheim C. B. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature (London) 1999;402:685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 3.Nicklas W. J., Zeevalk G., Hyndman A. Interactions between neurons and glia in glutamate/glutamine compartmentation. Biochem. Soc. Trans. 1987;15:208–210. doi: 10.1042/bst0150208. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki N., Kuromi H., Gonoi T., Okamoto Y., Ishida H., Seino Y., Kaneko T., Iwanaga T., Seino S. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J. 1995;9:686–691. [PubMed] [Google Scholar]
- 5.Hoy M., Maechler P., Efanov A. M., Wollheim C. B., Berggren P. O., Gromada J. Increase in cellular glutamate levels stimulates exocytosis in pancreatic β-cells. FEBS Lett. 2002;531:199–203. doi: 10.1016/s0014-5793(02)03500-7. [DOI] [PubMed] [Google Scholar]
- 6.Li C., Najafi H., Daikhin Y., Nissim I. B., Collins H. W., Yudkoff M., Matschinsky F. M., Stanley C. A. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J. Biol. Chem. 2003;278:2853–2858. doi: 10.1074/jbc.M210577200. [DOI] [PubMed] [Google Scholar]
- 7.Aledo J. C., Gómez-Fabre P. M., Olalla L., Márquez J. Identification of two human glutaminase loci and tissue-specific expression of the two related genes. Mamm. Genome. 2000;11:1107–1110. doi: 10.1007/s003350010190. [DOI] [PubMed] [Google Scholar]
- 8.Curthoys N. P., Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu. Rev. Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- 9.Olalla L., Gutiérrez A., Campos J. A., Khan Z. U., Alonso F. J., Segura J. A., Márquez J., Aledo J. C. Nuclear localization of L-type glutaminase in mammalian brain. J. Biol. Chem. 2002;277:38939–38944. doi: 10.1074/jbc.C200373200. [DOI] [PubMed] [Google Scholar]
- 10.Campos J. A., Aledo J. C., Segura J. A., Alonso F. J., Gómez-Fabre P. M., Núñez de Castro I., Márquez J. Expression of recombinant human L-glutaminase in Escherichia coli: polyclonal antibodies production and immunological analysis of mouse tissues. Biochim. Biophys. Acta. 2003;1648:17–23. doi: 10.1016/s1570-9639(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 11.Gómez-Fabre P. M., Aledo J. C., Castillo-Olivares A., Alonso F. J., Núñez de Castro I., Márquez J. Molecular cloning, sequencing and expression studies of the human breast cancer cell glutaminase. Biochem. J. 2000;345:365–375. [PMC free article] [PubMed] [Google Scholar]
- 12.Lohmann R., Souba W. W., Bode B. P. Rat liver endothelial cell glutamine transporter and glutaminase expression contrast with parenchymal cells. Am. J. Physiol. 1999;276:G743–G750. doi: 10.1152/ajpgi.1999.276.3.G743. [DOI] [PubMed] [Google Scholar]
- 13.Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 14.Chung-Bok M.-I., Vincent N., Jhala U., Watford M. Rat hepatic glutaminase: identification of the full coding sequence and characterization of a functional promoter. Biochem. J. 1997;324:193–200. doi: 10.1042/bj3240193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro R. A., Morehouse R. F., Curthoys N. P. Inhibition by glutamate of phosphate-dependent glutaminase of rat kidney. Biochem. J. 1982;207:561–566. doi: 10.1042/bj2070561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalik M., Nelson J., Erecinska M. Glutamate production in islets of Langerhans: properties of phosphate-activated glutaminase. Metabolism. 1992;41:1319–1326. doi: 10.1016/0026-0495(92)90102-g. [DOI] [PubMed] [Google Scholar]
- 17.Fahien L. A., MacDonald M. J., Kmiotek E. H., Mertz R. J., Fahien C. M. Regulation of insulin release by factors that also modify glutamate dehydrogenase. J. Biol. Chem. 1988;263:13610–13614. [PubMed] [Google Scholar]
- 18.MacDonald M. J., Fahien L. A. Glutamate is not a messenger in insulin secretion. J. Biol. Chem. 2000;275:34025–34027. doi: 10.1074/jbc.C000411200. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand G., Ishiyama N., Nenquin M., Ravier M. A., Henquin J. C. The elevation of glutamate content and the amplification of insulin secretion in glucose-stimulated pancreatic islets are not causally related. J. Biol. Chem. 2002;277:32883–32891. doi: 10.1074/jbc.M205326200. [DOI] [PubMed] [Google Scholar]
- 20.Rorsman P., Reström E. Glutamate primes the pump. Nature (London) 1999;402:595–596. doi: 10.1038/45110. [DOI] [PubMed] [Google Scholar]
- 21.Eto K., Yamashita T., Hirose K., Tsubamoto Y., Ainscow E. K., Rutter G. A., Kimura S., Noda M., Iino M., Kadowaki T. Glucose metabolism and glutamate analog acutely alkalinize pH of insulin secretory vesicles of pancreatic β-cells. Am. J. Physiol. 2003;285:E262–E271. doi: 10.1152/ajpendo.00542.2002. [DOI] [PubMed] [Google Scholar]
- 22.Winnock F., Ling Z., De Proft R., Dejonghe S., Schuit F., Gorus F., Pipeleers D. Correlation between GABA release from rat islet β-cells and their metabolic state. Am. J. Physiol. 2002;282:E937–E942. doi: 10.1152/ajpendo.00071.2001. [DOI] [PubMed] [Google Scholar]
- 23.Taylor L., Liu X., Newsome W., Shapiro R. A., Srinivasan M., Curthoys N. P. Isolation and characterization of the promoter region of the rat kidney-type glutaminase gene. Biochim. Biophys. Acta. 2001;1518:132–136. doi: 10.1016/s0167-4781(01)00183-x. [DOI] [PubMed] [Google Scholar]