Abstract
Previous studies have shown that adenophostin A is a potent initiator of the activation of SOCs (store-operated Ca2+ channels) in rat hepatocytes, and have suggested that, of the two subtypes of Ins(1,4,5)P3 receptor predominantly present in rat hepatocytes [Ins(1,4,5)P3R1 (type I receptor) and Ins(1,4,5)P3R2 (type II receptor)], Ins(1,4,5)P3R1s are required for SOC activation. We compared the abilities of Ins(1,4,6)P3 [with higher apparent affinity for Ins(1,4,5)P3R1] and Ins(1,3,6)P3 and Ins(1,2,4,5)P4 [with higher apparent affinities for Ins(1,4,5)P3R2] to activate SOCs. The Ins(1,4,5)P3 analogues were microinjected into single cells together with fura 2, and dose–response curves for the activation of Ca2+ inflow and Ca2+ release from intracellular stores obtained for each analogue. The concentration of Ins(1,4,6)P3 which gave half-maximal stimulation of Ca2+ inflow was substantially lower than that which gave half-maximal stimulation of Ca2+ release. By contrast, for Ins(1,3,6)P3 and Ins(1,2,4,5)P3, the concentration which gave half-maximal stimulation of Ca2+ inflow was substantially higher than that which gave half-maximal stimulation of Ca2+ release. The distribution of Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 in rat hepatocytes cultured under the same conditions as those employed for the measurement of Ca2+ inflow and release was determined by immunofluorescence. Ins(1,4,5)-P3R1s were found predominantly at the cell periphery, whereas Ins(1,4,5)P3R2s were found at the cell periphery, the cell interior and nucleus. It is concluded that the idea that a small region of the endoplasmic reticulum enriched in Ins(1,4,5)P3R1 is required for the activation of SOCs is consistent with the present results for hepatocytes.
Keywords: endoplasmic reticulum, inositol trisphosphate receptor, store-operated Ca2+ channel
Abbreviations: [Ca2+]cyt, cytoplasmic Ca2+ concentration; Ca2+o, extracellular Ca2+; DiOC6(3), 3,3′-dihexyloxacarbocyanine iodide; ER, endoplasmic reticulum; Ins(1,4,5)P3R, Ins(1,4,5)P3 receptor; SOC, store-operated Ca2+ channel
INTRODUCTION
SOCs (store-operated Ca2+ channels) in the plasma membrane are required for regulation of the [Ca2+]cyt (cytoplasmic free Ca2+ concentration) and re-filling the ER (endoplasmic reticulum) Ca2+ stores in hepatocytes and in other non-excitable cells, and in some excitable cells [1,2]. Several studies have shown that SOCs are required for the maintenance of agonist-induced oscillations in [Ca2+]cyt [3,4], and there is evidence that SOCs are more effective in re-filling the ER than non-selective cation channels [5]. The activation of SOCs is initiated by a decrease in the concentration of Ca2+ in the ER induced by the action of Ins(1,4,5)P3 at Ins(1,4,5)P3Rs [Ins(1,4,5)P3 receptors] and by Ca2+ at ryanodine receptors [1,2]. There is some evidence which suggests there may be a direct interaction (conformational coupling) between some Ins(1,4,5)P3Rs and some putative SOCs or other types of plasma membrane Ca2+ channels (reviewed in [1,2]). Evidence that the activation of SOCs involves, or requires, a specific region of the ER close to the plasma membrane in the vicinity of SOCs [6–9] and/or continuity of the whole ER [10,11] has been reported. While there have been numerous experiments designed to determine the relationship between the degree to which Ca2+ in the ER is decreased and the activation of SOCs, and the location of this decrease in ER Ca2+ [12,13], no clear answers have so far been obtained.
Rat hepatocytes in situ are polarized epithelial cells with clearly defined canalicular, basal and basolateral membrane regions (reviewed in [14]). Freshly isolated rat hepatocytes lose much of this polarity, but after culture for a few hours begin to regain some polarity [15]. Normal rat hepatocytes possess predominantly Ins(1,4,5)P3R1 (type I) and Ins(1,4,5)P3R2 (type II), with very little Ins(1,4,5)P3R3 (type III) [16,17]. Ins(1,4,5)P3R2s predominate, and may be responsible for the generation of [Ca2+]cyt waves emanating from the canalicular region [17]. There is evidence that Ins(1,4,5)P3R1s are associated with a subregion of the ER which is close to the plasma membrane and attached to the peripheral F-actin [18,19].
The results of previous studies with rat hepatocytes, which employed adenophostin A, an Ins(1,4,5)P3 analogue with a very high affinity for Ins(1,4,5)P3R, Glc(2,3′,4′)P3 (2-hydroxyethyl-α-D-glucopyranoside 2,3′,4′-trisphosphate), and an antibody against Ins(1,4,5)P3R1, which inhibits Ins(1,4,5)P3R function, suggested that a subregion of the ER and the Ins(1,4,5)P3R1 are both required for the activation of SOCs [9]. Adenophostin A has been used to investigate the role of Ins(1,4,5)P3R and the ER in the mechanism of activation of SOCs in several other cell types [7,20], leading, in some studies, to the conclusion that a small subregion of the ER is required for SOC activation [7].
The aim of the present study was to test further the roles of Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 in the activation of SOCs in rat hepatocytes. This has been done using Ins(1,4,5)P3 analogues with different apparent affinities for Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 [21] to initiate intracellular Ca2+ release and Ca2+ inflow. The intracellular locations of Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 under the same primary cell culture conditions as those employed for the measurement of Ca2+ inflow and release were also determined by immunofluorescence. The results provide evidence which indicates that a region of the ER (or another intracellular Ca2+ store) which is enriched in Ins(1,4,5)P3R1 is involved in the activation of SOCs.
MATERIALS AND METHODS
Materials
D-myo-Ins(1,3,6)P3 [22], D-myo-Ins(1,4,6)P3 [23], and D-myo-Ins(1,2,4,5)P4 [24] were synthesized as described previously. D-myo-2-Deoxy-Ins(1,4,5)P3 (A. M. Riley and B. V. L. Potter, unpublished work) was synthesized from D-3,6-di-O-benzyl-4,5-O-(2,3-dimethoxybutane-2,3-diyl)-myo-inositol [25]. All ligands were prepared as their tri-ethylammonium salts and were homogeneous by routine spectroscopic methods. The freeze-dried form of each Ins(1,4,5)P3 analogue was reconstituted in water, washed through 0.5 ml of Chelex-100 resin (to replace the tri-ethylammonium cation with Na+) and again freeze-dried. Stock solutions were prepared by dissolving the freeze-dried Ins(1,4,5)P3 analogue in 125 mM KCl. Monoclonal antibodies KM1112 and KM1083 specific for Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 respectively [26] were kindly provided by Professor K. Mikoshiba, University of Tokyo, Tokyo, Japan. Horseradish-peroxidase-conjugated goat anti-mouse IgG was from Sigma. Nitrocellulose and PVDF membranes and ECL® (enhanced chemiluminescence) detection reagents were provided by Amersham Biosciences. SaOS-2 human osteosarcoma cells (A.T.C.C., Rockville, MD, U.S.A.) were kindly provided by Dr T. J. McCann, Babraham Institute, Cambridge, U.K., and L15 mouse fibroblasts [27] by Professor K. Mikoshiba. SaOS-2 cells [28] and L15 mouse fibroblasts [29] were cultured as described previously.
Isolation of hepatocytes and measurement of [Ca2+]cyt
The isolation of hepatocytes from Hooded Wistar rats, attachment of hepatocytes to collagen-coated coverslips, the microinjection of fura 2 and Ins(1,4,5)P3 analogues and measurement of the fluorescence of single hepatocytes loaded with fura 2 (using a ratiometric technique) were carried out as described previously [9]. Changes in Ca2+ concentration are expressed as changes in the fluorescence ratio (F340nm/F380nm). The dilution factor for the microinjection of agents to hepatocytes was determined to be 1:75 [9] and was used to estimate the intracellular concentrations of the Ins(1,4,5)P3 analogues microinjected into hepatocytes.
For estimates of the amounts of Ca2+ released from intracellular stores and rates of Ca2+ inflow, hepatocytes attached to collagen-coated coverslips were microinjected with fura 2 together with a given Ins(1,4,5)P3 analogue, incubated for 10 min (to allow the cells to re-seal [9]), transferred to a medium containing no added Ca2+, and the fluorescence ratios were measured. Rates of Ca2+ inflow in single hepatocytes were estimated following the addition of Ca2+o (extracellular Ca2+) to cells incubated in the absence of added Ca2+o (the ‘Ca2+ add-back’ protocol) [9] from measurement of the initial rate of the Ca2+-induced increase in [Ca2+]cyt (expressed as change in fluorescence ratio units per min). Since the amount of Ca2+ which accumulates in the cytoplasmic space in a Ca2+ add-back protocol depends on both the rate of inflow across the plasma membrane and the rate of removal from the cytoplasmic space by transport into intracellular stores and the extracellular space, the initial rate of increase in [Ca2+]cyt is a more accurate reflection of the rate of Ca2+ inflow through SOCs than the value of the subsequent plateau in [Ca2+]cyt (underlying assumptions discussed in [30]).
To estimate the amount of Ca2+ released from intracellular stores by a given Ins(1,4,5)P3 analogue, vasopressin (40 nM) was added to cells loaded with the Ins(1,4,5)P3 analogue plus fura 2, or with fura 2 alone. The amount of Ca2+ released by the Ins(1,4,5)P3 analogue was calculated as the difference between the vasopressin-induced release of Ca2+ measured in the absence and presence of the Ins(1,4,5)P3 analogue. It has previously been shown, using EGTA to chelate extracellular Ca2+, that the rapid increase in [Ca2+]cyt induced by vasopressin in the presence of extracellular Ca2+ reflects the release of Ca2+ from intracellular stores with little contribution from Ca2+ inflow across the plasma membrane [9]. This was confirmed in the present series of experiments by measuring vasopressin-induced Ca2+ release (the height of the vasopressin-induced peak of [Ca2+]cyt) in the presence of Ca2+o (1.5 mM) and in the presence of both Ca2+o and EGTA (2 mM). Experiments were performed with cells microinjected with fura 2 plus Ins(1,4,6)P3 (10 μM), Ins(1,2,4,5)P4 (70 μM) and Ins(1,3,6)P3 (110 μM). For each analogue, there was no significant difference in the amount of Ca2+ released in response to vasopressin (40 nM) measured in the presence and absence of EGTA. The amounts (fluorescence ratio units) of Ca2+ release induced by vasopressin were 0.28±0.02 and 0.36±0.04 (+EGTA), 0.52±0.05 and 0.51±0.05 (+EGTA), and 0.58±0.04 and 0.53±0.03 (+EGTA) (means±S.E.M., n=8–15) for cells containing Ins(1,4,6)P3, Ins(1,2,4,5)P4, and Ins(1,3,6)P3 respectively.
Western blot analysis
Tissue preparation, protein assays, SDS gels, and semi-dry blotting were performed essentially as described previously [29]. SDS-minigels (6%) were run using the Bio-Rad Immunoblot assay kit. Standard homogenization, Western blotting and transfer buffers were used throughout, and either nitrocellulose or PVDF membranes were used for immunoblotting. Bands of Ins(1,4,5)-P3R1 and Ins(1,4,5)P3R2 were detected using mouse monoclonal anti-Ins(1,4,5)P3R1 KM1112 or mouse monoclonal anti-Ins-(1,4,5)P3R2 KM1083 [26], goat anti-mouse IgG conjugated to horseradish peroxidase as secondary antibody and ECL® detection. ‘SeeBlue’ pre-stained standards (4 –250 kDa, Novex) were used as molecular-mass markers. On 6% SDS-minigels, the myosin standard (250 kDa) ran very close to the Ins(1,4,5)P3R1 and Ins(1,4,5)-P3R2.
Immunolocalization of Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2
Rat hepatocytes grown on collagen-coated coverslips were rinsed with PBS and fixed with 4% (w/v) paraformaldehyde in PBS for 30 min, rinsed with PBS, then permeabilized with 0.1% (v/v) Triton X-100 in PBS for 10 min at room temperature. Cells were again rinsed with PBS, blocked with 10% (w/v) foetal bovine serum in PBS for 1 h at room temperature, washed with PBS containing 0.05% (v/v) Tween 20 (PBS-T), and incubated overnight at 4 °C with either mouse monoclonal anti-Ins(1,4,5)P3R1 KM1112 or mouse monoclonal anti-Ins(1,4,5)P3R2 KM1083 [26]. The cells were then washed with PBS-T and incubated for 1 h at room temperature in the dark with the secondary antibody, Cy3-conjugated anti-mouse IgG, at 1:1000 dilution in PBS-T containing 1% (v/v) serum. After washing with PBS-T, then four times in PBS, the coverslips were mounted on glass slides and viewed using a Bio-Rad MRC-1000 confocal microscope, krypton–argon laser, and Chroma 31002 (excitation 515–550 nm, emission 575–615 nm) filters, and 60× oil objective. The location of the ER in fixed freshly isolated rat hepatocytes in primary culture was determined using DiOC6(3) (3,3′-dihexyloxacarbocyanine iodide) and confocal microscopy as described previously [9].
RESULTS
Effects of Ins(1,4,6)P3, Ins(1,3,6)P3 and Ins(1,2,4,5)P4 on Ca2+ inflow and release
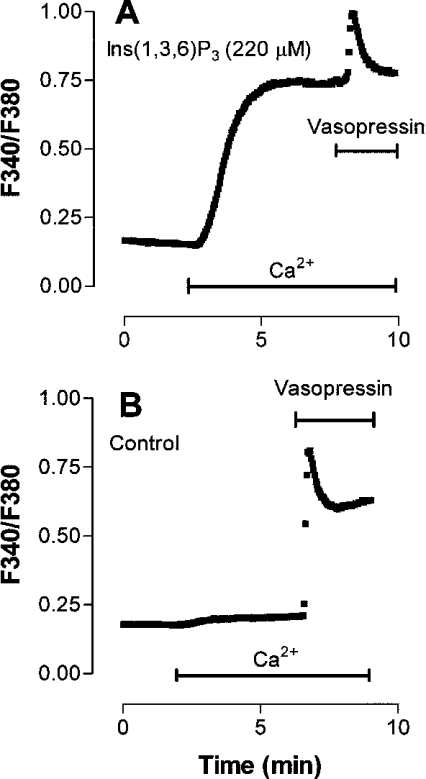
The strategy employed was to use Ins(1,4,5)P3 analogues with different affinities for Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 as probes for the involvement of each of these receptor subtypes in the activation of SOCs. The analogues employed were D-myo-Ins(1,4,6)P3 [with a higher affinity for Ins(1,4,5)P3R1] and D-myo-Ins(1,3,6)P3 and Ins(1,2,4,5)P4 [with a higher affinity for Ins(1,4,5)P3R2] [21]. The abilities of D-myo-Ins(1,4,6)P3, Ins-(1,3,6)P3 and Ins(1,2,4,5)P4 to activate Ca2+ release and Ca2+ inflow in rat hepatocytes were investigated by microinjecting each analogue into hepatocytes together with fura 2. The cells were incubated in the absence of added Ca2+o, then Ca2+o was added (to allow an estimate of the rate of Ca2+ inflow) followed by vasopressin (to release Ca2+ remaining in the intracellular stores). It has previously been shown that each of these analogues is resistant to metabolism by 5′-phosphatase and 3′-kinase activities [31–34]. The results obtained for a high intracellular concentration (estimated to be 220 μM) of Ins(1,3,6)P3 are shown in Figure 1(A), and those for a control cell, microinjected with fura 2 alone, in Figure 1(B). The amount of Ca2+ released from the ER by the Ins(1,4,5)P3 analogue was estimated by determining the difference between the Ca2+ released by vasopressin in the control cell and that released by vasopressin in the cell loaded with the Ins(1,4,5)P3 analogue. (The rationale for this procedure is discussed in more detail in the Materials and methods section.)
Figure 1. Effects of Ins(1,3,6)P3 on Ca2+ inflow and the vasopressin-induced increase in [Ca2+]cyt in single rat hepatocytes.
Single hepatocytes were injected with fura 2 plus 220 μM Ins(1,3,6)P3 (estimated intracellular concentration) (A) or fura 2 alone (B) 10 min before beginning the measurement of fluorescence. Additions of Ca2+ (1.5 mM) and vasopressin (40 nM) were made as indicated by the horizontal bars. Each trace is representative of those obtained for 21–97 individual cells from two to seven separate hepatocyte preparations.
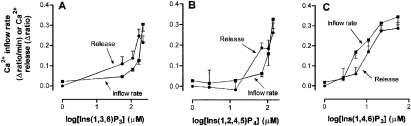
The dose–response curve for the effect of Ins(1,3,6)P3 on Ca2+ release and Ca2+ inflow is shown in Figure 2(A). This shows that the concentration of Ins(1,3,6)P3 which gave half-maximal stimulation of Ca2+ inflow is substantially higher than that which gave half-maximal stimulation of Ca2+ release. A similar dose–response pattern, but with less pronounced differences, was observed with Ins(1,2,4,5)P4 (Figure 2B). D-2-Deoxy-Ins(1,4,5)P3 {with higher affinity for Ins(1,4,5)P3R2 than Ins(1,4,5)P3R1 [21]} was also tested. However, the microinjection of D-2-deoxy-Ins(1,4,5)P3 had no effect on either Ca2+ release or Ca2+ inflow, most likely due to its rapid metabolism in liver cells [35].
Figure 2. Comparison of concentration–response curves for the effects of Ins(1,3,6)P3, Ins(1,2,4,5)P4 and Ins(1,4,6)P3 on the release of Ca2+ from intracellular stores and on Ca2+ inflow.
Ins(1,3,6)P3 (A), Ins(1,2,4,5)P4 (B) or Ins(1,4,6)P3 (C) was co-injected into single hepatocytes with fura 2 10 min before beginning the measurement of fluorescence, and Ca2+ and vasopressin were added to the incubation medium as described in Figure 1. Rates of Ca2+ inflow (▪) and amounts of Ca2+ release (•) were estimated as described in the Materials and methods section. Each data point is the mean±S.E.M. of the values obtained from 17–97 individual hepatocytes from one to seven separate hepatocyte preparations.
In contrast with the results obtained with Ins(1,3,6)P3 and Ins(1,2,4,5)P4, when the experiment was conducted with Ins-(1,4,6)P3 (Figure 2C), the concentration of Ins(1,4,6)P3 which gave half-maximal stimulation of Ca2+ inflow was found to be lower than that which gave half-maximal stimulation of Ca2+ release. Thus relative to the ability to induce Ca2+ release, Ins(1,4,6)P3 is more effective than Ins(1,3,6)P3 in inducing Ca2+ inflow.
Intracellular distribution of Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 under conditions employed for the measurement of Ca2+ inflow
The intracellular locations of Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 were determined using antibodies specific for these proteins and immunofluorescence. The specificity of the antibodies for Ins-(1,4,5)P3R1 and Ins(1,4,5)P3R2 was confirmed by Western blot analysis using extracts of L15 mouse fibroblasts [27], which express Ins(1,4,5)P3R1, but do not express significant levels of Ins(1,4,5)P3R2 [29], and extracts of SaOS-2 human osteoblasts, which predominantly express Ins(1,4,5)P3R2 and Ins(1,4,5)P3R3 [28]. A single band corresponding to the expected size of the Ins(1,4,5)P3R was observed in each case (results not shown). In extracts of rat liver, each antibody also gave a single band corresponding to the expected size of the Ins(1,4,5)P3R1 or Ins-(1,4,5)P3R2 (results not shown).
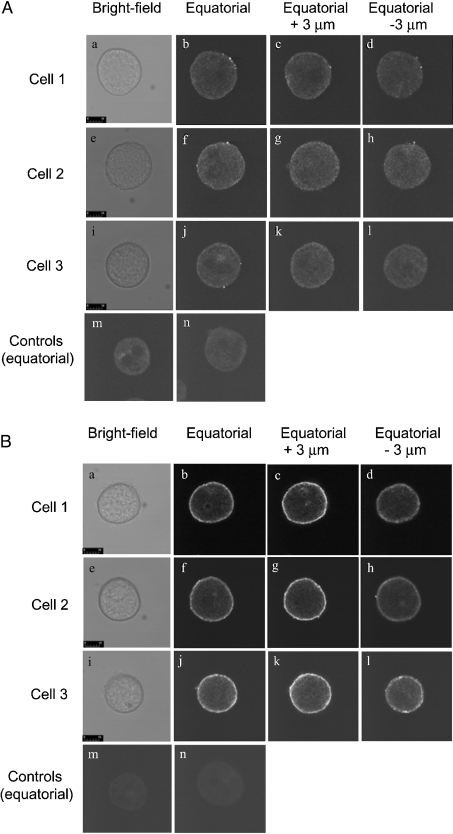
In immunofluorescence experiments with hepatocytes cultured for 2 h under conditions similar to those employed for the measurement of Ca2+ inflow and release, Ins(1,4,5)P3R1 was principally located in a band at the cell periphery, with little in the interior of the cytoplasmic space, and none in the nucleus (Figure 3A). Ins(1,4,5)P3R2 was also located in a clearly defined band at the cell periphery with some expression also in the cytoplasmic space (Figure 3B). In some experiments, labelling of the nucleus by anti-Ins(1,4,5)P3R2 was seen (results not shown). Panels b, f and j of each of Figures 3(A) and 3(B) show equatorial sections from three representative cells. Images were also obtained at 3 μm above (panels c, g, k) and below (panels d, h, l) the equatorial plane (the diameter of the cultured cells in the z axis was 10–20 μm). More intense staining was observed for the images obtained 3 μm above the equatorial plane (closer to the coverslip to which the cells were attached).
Figure 3. Localization by immunofluorescence of the type I (A) and type II (B) Ins(1,4,5)P3Rs in rat hepatocytes grown for 2 h in primary culture.
The culture of rat hepatocytes, permeabilization and cell fixation, staining with anti-Ins(1,4,5)P3 antibody, and confocal microscopy were performed as described in the Materials and methods section. (A) Cells stained with mouse monoclonal anti-Ins(1,4,5)P3R1 antibody KM1112 and anti-mouse antibody conjugated to Cy3. (B) Cells stained with mouse monoclonal anti-Ins(1,4,5)P3R2 antibody KM1083 and anti-mouse antibody conjugated to Cy3. The results shown are those obtained for one of three experiments employing three separate rat hepatocyte preparations which each gave similar results. The scale bar represents 10 μm. Panels a–d, e–h and i–l each represent a different single cell, showing a bright-field image, an equatorial image, 3 μm above equatorial image (z plane) and 3 μm below equatorial respectively. Panels m and n are controls in which primary antibody has been omitted. All images in (A) and (B) were obtained with the same confocal gain setting.
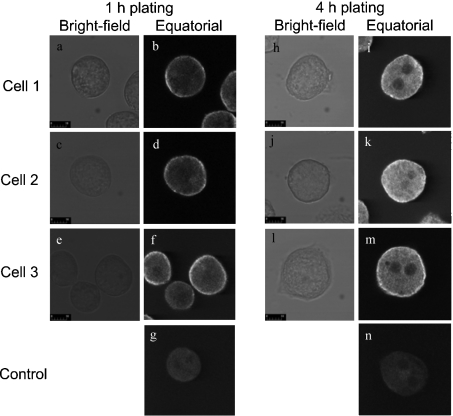
Freshly isolated hepatocytes exhibit little polarity, but when attached to a collagen-coated glass surface begin to regain polarity (assessed, for example, by formation of the cortical actin cytoskeleton) after about 4 h in culture [15,36]. To determine whether re-polarization affects the intracellular distribution of Ins(1,4,5)-P3R, the distribution of Ins(1,4,5)P3R2 was determined at 1 and 4 h after the initiation of cell culture. Ins(1,4,5)P3R2 was studied because it gave a more intense immunofluorescence signal than that generated by Ins(1,4,5)P3R1. Images obtained in the equatorial plane are shown in Figure 4. There was a significant increase in total immunofluorescence due to Ins(1,4,5)P3R2 at 4 h, possibly due to increased synthesis and/or decreased degradation of Ins-(1,4,5)P3R, although there was no change in the intracellular distribution of Ins(1,4,5)P3R2 (Figure 4).
Figure 4. Localization by immunofluorescence of the Ins(1,4,5)P3R2 in rat hepatocytes grown for 1 and 4 h in primary culture.
The culture of rat hepatocytes, permeabilization and cell fixation, staining with anti-Ins(1,4,5)P3 antibody, and confocal microscopy were performed as described in the Materials and methods section. Cells were stained with mouse monoclonal anti-Ins(1,4,5)P3R2 antibody KM1083 and anti-mouse antibody conjugated to Cy3. The results shown are those obtained for one of three experiments employing three separate rat hepatocyte preparations which each gave similar results. The scale bar represents 10 μm. Panels a–f represent different cells showing the bright-field images (a, c and e) and immunofluorescence (equatorial sections) (b, d and f) for cells cultured at 1 h. Panels h–m represent different cells showing the bright-field images (h, j and l) and immunofluorescence (equatorial sections) (i, k and m) for cells cultured at 4 h. Panels g and n are controls in which the primary antibody has been omitted.
The distribution of the ER in hepatocytes cultured under conditions similar to those employed for the measurement of Ca2+ inflow was also determined using DiOC6(3). The results indicate that the ER is distributed throughout the cytoplasmic space and, at the periphery of the cell, extends to the plasma membrane (results not shown; and [15]).
DISCUSSION
The most interesting aspect of the present study is the observation that the relationship between the dose–response curves for Ca2+ inflow and Ca2+ release for each of Ins(1,3,6)P3 and Ins(1,2,4,5)P4 differs from that for Ins(1,4,6)P3. Thus Ins(1,4,6)P3, which, on the basis of studies conducted with Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 expressed in insect Sf9 cells [21], has a higher affinity for Ins(1,4,5)P3R1 than Ins(1,4,5)P3R2, activated Ca2+ inflow at lower concentrations than those which induced significant Ca2+ release. In contrast, Ins(1,3,6)P3 and Ins(1,2,4,5)P4, each of which has a higher affinity for Ins(1,4,5)P3R2 than Ins(1,4,5)P3R1 [21], induced Ca2+ release at lower concentrations than those which activated substantial Ca2+ inflow. These results suggest that, in intact hepatocytes, the binding of Ins(1,4,5)P3 to Ins(1,4,5)P3R1s is more effective in activating SOCs than the binding of Ins(1,4,5)P3 to Ins(1,4,5)P3R2s.
It is recognized that there are a number of assumptions made in the interpretation of the dose–response curves for the Ins(1,4,5)-P3 analogues reported in the present paper. First, the measured difference in apparent affinity of a given analogue for Ins(1,4,5)-P3R1 and Ins(1,4,5)P3R2 is relatively small [21]. Secondly, it is assumed that Ins(1,3,6)P3 and Ins(1,4,6)P4 are resistant to metabolism [33,34] and diffuse throughout the cytoplasmic space [37], so that the cytoplasmic concentration of the Ins(1,4,5)P3 analogue remains approximately constant during the period over which Ca2+ inflow and Ca2+ release are measured. Thirdly, it was not possible to raise the intracellular concentrations of Ins(1,3,6)P3 and Ins(1,2,4,5)P4 to saturate the Ins(1,4,5)P3R. Fourthly, the amount of Ca2+ released was estimated using a somewhat indirect strategy. While these assumptions appear valid, some caution should be exercised in interpreting the dose–response curves. Nevertheless, the difference in the patterns of the dose–response curves for analogues with different affinities for Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 is quite striking.
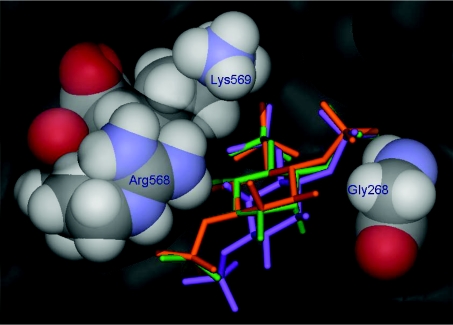
Previously, the differences in affinities of Ins(1,3,6)P3, Ins-(1,4,6)P3 and Ins(1,4,5)P3 for Ins(1,4,5)P3Rs have been rationalized by comparing their molecular structures in diagrams that align the phosphate groups of the three molecules [21,33,38]. Molecular docking experiments using the recently published X-ray crystal structure [39] of the Ins(1,4,5)P3R1 binding domain can be used to extend this model by suggesting how the relative affinities of Ins(1,4,6)P3 and Ins(1,3,6)P3 for Ins(1,4,5)P3R1 may be related to their abilities to mimic Ins(1,4,5)P3 in the context of the Ins(1,4,5)P3R1-binding site (Figure 5). An equivalent crystal structure for an Ins(1,4,5)P3R2 domain is not yet available, but it is interesting to note that the residues of Ins(1,4,5)P3R1 that interact with Ins(1,4,5)P3 are all conserved in Ins(1,4,5)P3R2, with the exception that Gly268 in Ins(1,4,5)P3R1 is replaced by leucine in the Ins(1,4,5)P3R2 sequence in both the mouse [40] and rat [41] (reviewed in [42]) Ins(1,4,5)P3R2 sequences. It is possible that the replacement of glycine with the sterically bulky and hydrophobic leucine residue at this position could influence the local environment at the Ins(1,4,5)P3R2-binding site, perhaps with consequences for the relative affinities of Ins(1,4,5)P3, Ins(1,4,6)P3, Ins(1,3,6)P3 and also Ins(1,2,4,5)P4.
Figure 5. Structure of the Ins(1,4,5)P3R1-binding site based on the X-ray crystal structure of the mouse Ins(1,4,5)P3R1 core binding domain.
The crystallographically observed position of bound Ins(1,4,5)P3 [39] is shown in orange. Molecular docking experiments suggest that Ins(1,4,6)P3 (green) may be a relatively effective mimic of Ins(1,4,5)P3 at the Ins(1,4,5)P3R1-binding site because it can bind in an orientation that allows its phosphate groups to mimic the three phosphate groups of Ins(1,4,5)P3, while its axial 2-hydroxy group is accepted by an open region close to Gly268. However, Ins(1,3,6)P3 (purple) may be prevented from adopting a similar binding mode due to unfavourable steric interactions of its axial 2-hydroxy group with Arg568 and Lys569. Molecular-docking experiments were carried out using the X-ray crystal structure of the Ins(1,4,5)P3-binding core of mouse type 1 InsP3R in complex with Ins(1,4,5)P3 (PDB code 1N4K [39]) according to methods previously described [43]. For clarity, six molecules of water included in the docking experiments, and the hydrogen atoms of Ins(1,4,5)P3, Ins(1,4,6)P3 and Ins(1,3,6)P3 are not shown.
Both Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 were found to be predominantly located at the periphery of the cell in rat hepatocytes cultured under conditions similar to those employed for the measurement of Ca2+ inflow (single cells attached to collagen-coated glass slides and cultured for 2 h). At this stage of culture, the cells exhibit little polarity [15,36]. Some co-location of Ins-(1,4,5)P3R1 and Ins(1,4,5)P3R2 at the periphery of the cell may be due to the formation of Ins(1,4,5)P3R1–Ins(1,4,5)P3R2 heterotetramers [44]. It is interesting to note that while Ins(1,4,5)P3R1 and Ins(1,4,5)P3R2 were both found concentrated near the plasma membrane in hepatocytes cultured for 2 h, the ER was found distributed throughout the cytoplasmic space. This suggests that, for cells in this in vitro condition, the majority of the ER has a very low density of Ins(1,4,5)P3R. Previous histochemical studies with freshly isolated hepatocytes (unpolarized), hepatocyte couplets (partially polarized) and hepatocytes in situ (completely polarized) have shown that Ins(1,4,5)P3R1 is located principally near the plasma membrane, while Ins(1,4,5)P3R2 is located at the plasma membrane and bile canaliculus [17–19]. Moreover, Ins(1,4,5)P3R2 located around the bile canaliculus appears to be responsible for the initiation of Ca2+ waves [17]. Studies employing subcellular fractionation and electron microscopy have provided clear evidence that, in liver cells, some Ins(1,4,5)P3R1s are located in regions of the ER that are closely associated with the plasma membrane through F-actin [18,19]. The present immunofluorescence results showing Ins(1,4,5)P3R1 at the cell periphery are consistent with these results. Thus the role of Ins-(1,4,5)P3R1 in apparently selectively activating Ca2+ inflow is associated with the location of Ins(1,4,5)P3R1 close to the plasma membrane.
Taken together, the present results with Ins(1,4,5)P3 analogues selective for either Ins(1,4,5)P3R1 or Ins(1,4,5)P3R2, the previous results using an anti-Ins(1,4,5)P3R1 antibody to inhibit Ins-(1,4,5)P3 function and adenophostin A to activate Ca2+ inflow [9], and the evidence that some Ins(1,4,5)P3R1s are closely associated with the plasma membrane [18,19], suggest that, in hepatocytes, the activation of SOCs requires the release of Ca2+ from a small region of the ER close to the plasma membrane and enriched in Ins(1,4,5)P3R1. The requirement for Ins(1,4,5)P3R1 for the activation of SOCs in hepatocytes may simply reflect enrichment of those putative subregions of the ER involved in the activation of SOCs with Ins(1,4,5)P3R1. It is hypothesized in the present paper that the role of Ins(1,4,5)P3R1 is solely to mediate release of Ca2+ from the ER. At this stage, there is no evidence for direct interaction between the Ins(1,4,5)P3R1 protein and the SOC protein in hepatocytes. In vivo, where the activation of SOCs is initiated by Ins(1,4,5)P3 generated by the hormone-induced activation of phospholipase C, additional factors will likely also influence the relative ability of Ins(1,4,5)P3 to activate SOCs and release Ca2+from intracellular stores. These include the rate of metabolism of Ins(1,4,5)P3 [6], the effects of Ca2+ on the affinity of Ins(1,4,5)P3 for Ins(1,4,5)P3Rs [45] and the locations of hormone receptors and phospholipase C in relation to that of Ins(1,4,5)P3Rs [46,47].
Acknowledgments
We acknowledge financial support from the Wellcome Trust (Programme Grant 060554 to B.V.L.P.) and NHMRC Grant 102125 (to G.J.B. and R.B.G.). The provision of monoclonal antibodies KM1112 against Ins(1,4,5)P3R1 and KM1083 against Ins(1,4,5)P3R2 by Professor K. Mikoshiba, University of Tokyo, Tokyo, Japan, is gratefully acknowledged. We also thank Dr S. J. Mills for gifts of synthetic Ins(1,4,6)P3 and Ins(1,2,4,5)P4, and acknowledge Dr Jenny Hiscock for conducting the confocal microscopy, and Ms Lee-Anne Addis for preparation of the typescript.
References
- 1.Venkatachalam K., van Rossum D. B., Patterson R. L., Ma H. T., Gill D. L. The cellular and molecular basis of store-operated calcium entry. Nat. Cell Biol. 2002;4:E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 2.Clapham D. E. TRP channels as cellular sensors. Nature (London) 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 3.Dolmetsch R. E., Xu K., Lewis R. S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature (London) 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 4.Gregory R. B., Barritt G. J. Evidence that Ca2+-release-activated Ca2+ channels in rat hepatocytes are required for the maintenance of hormone-induced Ca2+ oscillations. Biochem. J. 2003;369:1–7. doi: 10.1042/BJ20021671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregory R. B., Sykiotis D., Barritt G. J. Evidence that store-operated Ca2+ channels are more effective than intracellular messenger-activated non-selective cation channels in refilling rat hepatocyte intracellular Ca2+ stores. Cell Calcium. 2003;35:241–251. doi: 10.1016/s0143-4160(03)00106-4. [DOI] [PubMed] [Google Scholar]
- 6.Parekh A. B., Fleig A., Penner R. The store-operated calcium current ICRAC: nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 7.Bird G. S., Takahashi M., Tanzawa K., Putney J. W., Jr Adenophostin A induces spatially restricted calcium signalling in Xenopus laevis oocytes. J. Biol. Chem. 1999;274:20643–20649. doi: 10.1074/jbc.274.29.20643. [DOI] [PubMed] [Google Scholar]
- 8.Turner H., Fleig A., Stokes A., Kinet J.-P., Penner R. Discrimination of intracellular calcium store sub-compartments using TRPV1 release channel activity. Biochem. J. 2003;371:341–350. doi: 10.1042/BJ20021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory R. B., Wilcox R. A., Berven L. A., van Straten N. C. R., van der Marel G. A., van Boom J. H., Barritt G. J. Evidence for the involvement of a small subregion of the endoplasmic reticulum in the inositol trisphosphate receptor-induced activation of Ca2+ inflow in rat hepatocytes. Biochem. J. 1999;341:401–408. [PMC free article] [PubMed] [Google Scholar]
- 10.Mogami H., Tepikin A. V., Petersen O. H. Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J. 1998;17:435–442. doi: 10.1093/emboj/17.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park M. K., Petersen O. H., Tepikin A. V. The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakowski D., Parekh A. B. Sarcoplasmic/endoplasmic-reticulum-Ca2+-ATPase-mediated Ca2+ reuptake, and not Ins(1,4,5)P3 receptor inactivation, prevents the activation of macroscopic Ca2+ release-activated Ca2+ current in the presence of physiological Ca2+ buffer in rat basophilic leukaemia-1 cells. Biochem. J. 2001;353:561–567. doi: 10.1042/0264-6021:3530561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofer A. M., Fasolato C., Pozzan T. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+] J. Cell Biol. 1998;140:325–334. doi: 10.1083/jcb.140.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barritt G. J. Calcium signalling in liver cells. In: Pochet R., Donato R., Haiech J., Heizmann C., Gerke V., editors. Calcium: The Molecular Basis of Calcium Action in Biology and Medicine. Dordrecht: Kluwer Academic Publishers; 2001. pp. 73–94. [Google Scholar]
- 15.Wang Y.-J., Gregory R. B., Barritt G. J. Regulation of F-actin and endoplasmic reticulum organization by the trimeric G-protein Gi2 in rat hepatocytes: implication for the activation of store-operated Ca2+ inflow. J. Biol. Chem. 2000;275:22229–22237. doi: 10.1074/jbc.M001563200. [DOI] [PubMed] [Google Scholar]
- 16.Wojcikiewicz R. J. H. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 17.Hirata K., Pusl T., O'Neill A. F., Dranoff J. A., Nathanson M. H. The Type II inositol 1,4,5-trisphosphate receptor can trigger Ca2+ waves in rat hepatocytes. Gastroenterology. 2002;122:1088–1100. doi: 10.1053/gast.2002.32363. [DOI] [PubMed] [Google Scholar]
- 18.Rossier M. F., Bird G. S., Putney J. W., Jr Subcellular distribution of the calcium-storing inositol 1,4,5-trisphosphate-sensitive organelle in rat liver. Possible linkage to the plasma membrane through the actin microfilaments. Biochem. J. 1991;274:643–650. doi: 10.1042/bj2740643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lievremont J.-P., Hill A.-M., Tran D., Coquil J.-F., Stelly N., Mauger J.-P. Intracellular calcium stores and inositol 1,4,5-trisphosphate receptor in rat liver cells. Biochem. J. 1996;314:189–197. doi: 10.1042/bj3140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parekh A. B., Riley A. M., Potter B. V. L. Adenophostin A and ribophostin, but not inositol 1,4,5-trisphosphate or manno-adenophostin, activate the Ca2+ release-activated Ca2+ current, ICRAC, in weak intracellular Ca2+ buffer. Biochem. J. 2002;361:133–141. doi: 10.1042/0264-6021:3610133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nerou E. P., Riley A. M., Potter B. V. L., Taylor C. W. Selective recognition of inositol phosphates by subtypes of the inositol trisphosphate receptor. Biochem. J. 2001;355:59–69. doi: 10.1042/0264-6021:3550059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley A. M., Payne R., Potter B. V. L. Unambiguous total synthesis of the enantiomers of myo-inositol 1,3,4,-trisphosphate: 1 L-myo-inositol 1,3,4-trisphosphate mobilises intracellular Ca2+ in Limulus photoreceptors. J. Med. Chem. 1994;37:3918–3927. doi: 10.1021/jm00049a011. [DOI] [PubMed] [Google Scholar]
- 23.Mills S. J., Potter B. V. L. Synthesis of D- and L-myo-inositol 1,4,6-trisphosphate, regioisomers of a ubiquitous second messenger. J. Org. Chem. 1996;61:8980–8987. doi: 10.1021/jo961280x. [DOI] [PubMed] [Google Scholar]
- 24.Mills S. J., Potter B. V. L. Synthesis of the enantiomers of myo-inositol 1,2,4,5-tetrakisphosphate, a regioisomer of myo-inositol 1,3,4,5-tetrakisphosphate. J. Chem. Soc. Perkin Trans. 1997;1:1279–1286. [Google Scholar]
- 25.Riley A. M., Correa V., Mahon M. F., Taylor C. W., Potter B. V. L. Bicylic analogues of D-myo-inositol 1,4,5-trisphosphate based upon adenophostin A: synthesis and biological activity. J. Med. Chem. 2001;44:2108–2117. doi: 10.1021/jm0005499. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama T., Furuya A., Monkawa T., Yamamoto-Hino M., Satoh S., Ohmori K., Miyawaki A., Hanai N., Mikoshiba K., Hasegawa M. Monoclonal antibodies distinctively recognizing the subtypes of inositol 1,4,5-trisphosphate receptor: application to the studies on inflammatory cells. FEBS Lett. 1994;354:149–154. doi: 10.1016/0014-5793(94)01099-4. [DOI] [PubMed] [Google Scholar]
- 27.Miyawaki A., Furuichi T., Maeda N., Mikoshiba K. Expressed cerebellar-type inositol 1,4,5-trisphosphate receptor, P400, has calcium release activity in a fibroblast L cell line. Neuron. 1990;5:11–18. doi: 10.1016/0896-6273(90)90029-f. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox R. A., Forsythe I. D., McCann T. J. Microinjection of myo-inositol(1,4,5)trisphosphate and other calcium-mobilizing agents into intact adherent cells. Methods Mol. Biol. 1999;114:193–208. doi: 10.1385/1-59259-250-3:193. [DOI] [PubMed] [Google Scholar]
- 29.Mackrill J. J., Wilcox R. A., Miyawaki K., Nahorski S. R., Challiss R. A. Stable overexpression of the type 1 inositol 1,4,5-trisphosphate receptor in L fibroblasts: subcellular distribution and functional consequences. Biochem. J. 1996;318:871–878. doi: 10.1042/bj3180871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes B. P., Auld A. M., Barritt G. J. Effect of extracellular Ca2+ on plasma membrane Ca2+ inflow and cytoplasmic free Ca2+ in isolated hepatocytes. Biochim. Biophys. Acta. 1987;928:208–216. doi: 10.1016/0167-4889(87)90123-6. [DOI] [PubMed] [Google Scholar]
- 31.Hirata M., Narumoto N., Watanabe Y., Kanematsu T., Koga T., Ozaki S. DL-myo-inositol 1,2,4,5-tetrakisphosphate, a potent analogue of D-myo-inositol 1,4,5-trisphosphate. Mol. Pharmacol. 1994;45:271–276. [PubMed] [Google Scholar]
- 32.Wilcox R. A., Safrany S. T., Lampe D., Mills S. J., Nahorski S. R., Potter B. V. Modification at C2 of myo-inositol 1,4,5-trisphosphates and tetrakisphosphates with potent biological activities. Eur. J. Biochem. 1994;223:115–124. doi: 10.1111/j.1432-1033.1994.tb18972.x. [DOI] [PubMed] [Google Scholar]
- 33.Hirata M., Watanabe Y., Yoshida M., Koga T., Ozaki S. Roles for hydroxyl groups of D-myo-inositol 1,4,5-trisphosphate in the recognition by its receptor and metabolic enzymes. J. Biol. Chem. 1993;268:19260–19266. [PubMed] [Google Scholar]
- 34.Murphy C. T., Bullock A. J., Lindley C. J., Mills S. J., Riley A. M., Potter B. V. L., Westwick J. Enantiomers of myo-inositol-1,3,4-trisphosphate and myo-inositol-1,4,6-trisphosphate: stereospecific recognition by cerebellar and platelet myo-inositol-1,4,5-trisphosphate receptors. Mol. Pharmacol. 1996;50:1223–1230. [PubMed] [Google Scholar]
- 35.Hirata M., Watanabe Y., Ishimatsu T., Ikebe T., Kimura Y., Yamaguchi K., Ozaki S., Koga T. Synthetic inositol trisphosphate analogs and their effects on phosphatase, kinase, and the release of Ca2+ J. Biol. Chem. 1989;264:20303–20308. [PubMed] [Google Scholar]
- 36.Thibault N., Claude J. R., Ballet F. Actin filament alteration as a potential marker for cholestasis: a study in isolated rat hepatocyte couplets. Toxicology. 1992;73:269–279. doi: 10.1016/0300-483x(92)90069-q. [DOI] [PubMed] [Google Scholar]
- 37.Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 38.Hirata M., Takeuchi H., Riley A. M., Mills S. J., Watanabe Y., Potter B. V. L. Inositol 1,4,5-trisphosphate receptor subtypes differentially recognize regioisomers of D-myo-inositol 1,4,5-trisphosphate. Biochem. J. 1997;328:93–98. doi: 10.1042/bj3280093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosanac I., Alattia J.-R., Mal T. K., Chan J., Talarico S., Tong F. K., Tong K. I., Yoshikawa F., Furuichi T., Iwai M., et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature (London) 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 40.Ross C. A., Danoff S. K., Schell M. J., Snyder S. H., Ullrich A. Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudhof T. C., Newton C. L., Archer B. T., III, Ushkaryov Y. A., Mignery G. A. Structure of a novel InsP3 receptor. EMBO J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor C. W., Genazzani A. A., Morris S. A. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg H. J., Riley A. M., Laude A. J., Taylor C. W., Potter B. V. L. Synthesis and Ca2+ mobilising activity of purine-modified mimics of adenophostin A: a model for the adenophostin-Ins(1,4,5)P3 receptor interaction. J. Med. Chem. 2003;46:4860–4871. doi: 10.1021/jm030883f. [DOI] [PubMed] [Google Scholar]
- 44.Onoue H., Tanaka H., Tanaka K., Doira N., Ito Y. Heterooligomer of Type 1 and Type 2 inositol 1,4,5-trisphosphate receptor expressed in rat liver membrane fraction exists as tetrameric complex. Biochem. Biophys. Res. Commun. 2000;267:928–933. doi: 10.1006/bbrc.1999.2065. [DOI] [PubMed] [Google Scholar]
- 45.Broad L. M., Armstrong D. L., Putney J. W., Jr Role of the inositol 1,4,5-trisphosphate receptor in Ca2+ feedback inhibition of calcium release-activated calcium current (ICRAC) J. Biol. Chem. 1999;274:32881–32888. doi: 10.1074/jbc.274.46.32881. [DOI] [PubMed] [Google Scholar]
- 46.Paradiso A. M., Mason S. J., Lazarowski E. R., Boucher R. C. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature (London) 1995;377:643–646. doi: 10.1038/377643a0. [DOI] [PubMed] [Google Scholar]
- 47.Short A. D., Winston G. P., Taylor C. W. Different receptors use inositoltrisphosphate to mobilize Ca2+ from different intracellular pools. Biochem. J. 2000;351:683–686. [PMC free article] [PubMed] [Google Scholar]