Abstract
The innate immune system has the capacity to recognize a wide range of pathogens based on conserved PAMPs (pathogen-associated molecular patterns). In the case of bacterial LPS (lipopolysaccharide) recognition, the best studied PAMP, it has been shown that the innate immune system employs at least three cell-surface receptors: CD14, TLR4 (Toll-like receptor 4) and MD-2 protein. CD14 binds LPS from Enterobacteriaceae and then transfers it to MD-2, leading to TLR4 aggregation and signal transduction. LPS analogues such as lipid IVa seem to act as LPS antagonists in human cells, but exhibit LPS mimetic activity in mouse cells. Although TLR4 has been shown to be involved in this species-specific discrimination, the mechanism by which this is achieved has not been elucidated. The questions that remain are how the innate immune system can discriminate between LPS from different bacteria as well as different LPS analogues, and whether or not the structure of LPS affects its interaction with the CD14–TLR4–MD-2 cluster. Is it possible that the ‘shape’ of LPS induces the formation of different receptor clusters, and thus a different immune response? In the present study, we demonstrate using biochemical as well as fluorescence-imaging techniques that different LPS analogues trigger the recruitment of different receptors within microdomains. The composition of each receptor cluster as well as the number of TLR4 molecules that are recruited within the cluster seem to determine whether an immune response will be induced or inhibited.
Keywords: innate recognition, lipopolysaccharide (LPS), lipopolysaccharide-activation cluster Toll-like receptor 4 (TLR4)
Abbreviations: CHO, Chinese-hamster ovary; CXCR4, chemokine receptor 4; FRAP, fluorescence recovery after photobleaching; FRET, fluorescence resonance energy transfer; GDF5, growth-differentiation factor 5; GM-1, monosialoganglioside; HRP, horseradish peroxidase; hsp, heat-shock protein; IκB, inhibitory κB; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; mAb, monoclonal antibody; MAPK, mitogen-activated protein kinase; MEB, membrane-extraction buffer; MHC, major histocompatibility complex; MNC, mononuclear cell; NF-κB, nuclear factor κB; PBS-T, PBS with Tween 20; pLA, penta-acyl lipid A; SAPK, stress-activated protein kinase; TLR, Toll-like receptor; TNF-α, tumour necrosis factor α
INTRODUCTION
The function of the innate immune system is thought to be the recognition of invading pathogens, the activation of inflammation to control the pathogen and the subsequent activation of the acquired immune response. The TLR (Toll-like receptor) family of proteins is an integral part of the human innate immune system [1,2]. TLRs are expressed on immune cells and are able to distinguish a great variety of microbial ligands, such as cell wall components such as LPS (lipopolysaccharide) from Gram-negative bacteria and lipoteichoic acid from Gram-positive bacteria [3].
Exposure of mammals to LPS leads to immediate cell activation and release of pro-inflammatory cytokines. This activation can, in most cases, lead to overproduction of cytokines that are harmful and, in most cases, deadly for the host. At least three cell-surface molecules have been recognized as components of the mammalian signalling receptor for LPS: CD14 [4], TLR4 [5–7] and MD-2 [8]. CD14 has been shown to bind LPS and TLR4 to function as the mammalian signal transducer for bacterial LPS [5–7]. Activation of TLR4 by LPS is absolutely dependent upon the presence of MD-2, a secreted glycoprotein that contributes to ligand recognition by the LPS receptor [9–12].
Even though the components of the mammalian LPS ‘sensing machinery’ were for the most part identified, our understanding of the initial events that take place as the ligand (LPS) engages its receptor(s) were still unclear. The study by Visintin et al. [13] shed new light on those initial events, demonstrating that LPS binds to MD-2, which in turn binds to TLR4 and induces aggregation and signal transduction. The possibility that additional receptor components such as hsps (heat-shock proteins) [14,15], CXCR4 (chemokine receptor 4) [15] or CD55 [16] have been suggested to be part of this activation cluster, possibly acting as additional LPS-transfer molecules.
Although we now know that LPS recognition is a complex biological cascade, that involves the formation of activation clusters containing TLR4–MD-2 and possibly other molecules as well, important questions still remain: do different types of LPS structures induce the formation of this cluster? It has been shown that several LPS from non-enterobateria, such as lipid A from Rhodobacter sphaeroides (RSLA) and Rhodobacter capsulatus, as well as precursors and analogues of toxic lipid A from Escherichia coli LPS, such as the tetra-acyldisaccharide lipid A precursor designated lipid IVA, and penta-acyl lipid A (pLA) can act as LPS antagonists because of their cylindrical shape [17–20]. In contrast, in mouse or hamster macrophages, RSLA and lipid IVA are LPS mimetics [21], thus exhibiting an uncommon species-specific pharmacology. Does the structure of LPS affect its interaction with the TLR4–MD-2 complex? Do cylindrical LPS molecules induce the formation of clusters, and what is the composition of these clusters? Does the formation of different clusters affect the immune response against a particular ligand?
In the present study, we have chosen to elucidate the mechanism behind TLR4-mediated species-specificity by investigating whether LPS analogues such as pLA, or tetra-acyl lipid A (406) can stimulate the formation of activation clusters identical with that of LPS. By determining the composition of the different activation clusters in response to several LPS analogues, we should be able to unravel the mechanism behind the LPS species-specificity.
Using FRET (fluorescence resonance energy transfer), we provide evidence that the TLR4–MD-2 complex is part of the LPS-activation cluster that we had identified previously [15]. Stimulation by LPS analogues such as pLA and 406 induces the formation of an activation cluster that comprises TLR4–MD-2, and several proteins such as CD55, hsp70 and hsp90, and GDF5 (growth-differentiation factor 5). In contrast with the activation cluster formed by LPS, the cluster formed after pLA stimulation seems not to involve CXCR4 or CD81. Furthermore, upon stimulation with pLA or 406, TLR4 clustering was not induced, and the TLR4–MD-2 complex seems to be recruited to a lesser extent into lipid rafts. This results in an inhibition in NF-κB (nuclear factor κB) activation, suggesting that TLR4–MD-2 recruitment and clustering is crucial for triggering this signalling cascade.
Our results indicate that LPS activates multiple signalling cascades through the co-ordinated clustering of multiple receptors. In response to LPS analogues such as pLA or 406, there is a different combinational association of receptors, less recruitment of TLR4–MD-2 within lipid rafts that leads to activation of the MAPK (mitogen-activated protein kinase) and SAPK (stress-activated protein kinase) pathway, but not the NF-κB, and thus a ‘tailored’ response to that particular bacterial antigen.
Overall, our data support the hypothesis that the innate immune system can respond to diverse bacterial products by forming different receptor clusters. The structure of LPS seems to be crucial for the formation of these clusters and the subsequent signal transduction. There seems to be a core group of receptors (TLRs) that are involved in the innate recognition of bacteria, and depending on the different ligands other molecules are recruited into the cluster. The composition of the receptor cluster will eventually determine the immune response against the particular pathogen.
MATERIALS AND METHODS
LPS and partial structures
LPS from E. coli deep rough mutant Re (strain F515) was extracted according to the phenol/chloroform/petrol ether procedure [22]. pLA was extracted from a mutant of strain F515, which produced only a penta-acyl, but no hexa-acyl, lipid A. This lipid A (pLA) was isolated from the parent LPS by acetate buffer treatment; after isolation, the resulting lipid A was purified and converted into the triethylamine form. The known chemical structure of LPS and pLA was checked by modern MS analysis [23]. Synthetic monophosphoryl lipid A analogue compound 505 corresponding to the 1-phosphoryl hexa-acyl lipid A was synthesized as described previously [24].
Materials
Hybridoma cells secreting 26ic (anti-CD14) and W6/32 secreting MHC (major histocompatibility complex) class I specific mAb (monoclonal antibody) were obtained from the American Type Culture Collection (A.T.C.C., Manassas, VA, U.S.A.). Hsp70-specific rabbit polyclonal serum was obtained from Dako (Cambridge, U.K.). Hsp90-specific rabbit polyclonal serum was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). TLR4-specific mAb, HTA-125 was purchased from HyCult Biotechnology (Uden, The Netherlands), MyD88 polyclonal antibody was purchased from eBioscience (San Diego, CA, U.S.A.), MD-2 polyclonal antibody was purchased from Imgenex (San Diego, CA, U.S.A.), CD81 was purchased from Becton Dickinson (Cowley, Oxford, U.K.). Cy3 and Cy5 labelling kits were purchased from Amersham Biosciences (Little Chalfont, Bucks., U.K.). Goat polyclonal serum directed against GDF5 and rabbit polyclonal serum against CXCR4, as well as Hsc70 (heat-shock cognate protein)-specific mouse mAb was purchased from Autogen Bioclear (Calne, Wilts., U.K.). Rat mAb against human hsp90α was obtained from Bioquote (York, U.K.). FITC–CD25 was obtained from Serotec (Kidlington, Oxford, U.K.).
Cell lines
CHO (Chinese-hamster ovary) cells transfected with hCD14 and hTLR4 cDNA in a reporter background was kindly provided by Professor D. Golenbock (University of Massachusetts Medical School, Worcester, MA, U.S.A.). CHO cells were maintained in Ham's F12 from Invitrogen (Paisley, Scotland, U.K.) supplemented with 10% (v/v) foetal calf serum and 500 mg/ml gentamicin sulphate (G418, Sigma). Cells were grown in 80 cm3 tissue culture flasks (Nunc). Trypsin/EDTA (0.05% Trypsin/0.53 mM EDTA) was used for passaging the cells.
Isolation of human monocytes
Monocytes were isolated from human A+ buffy coats. Adherent cell monolayers [(1–2)×105 monocytes/well] were cultured in 24-well plates in serum-free medium (Gibco, Paisley, Scotland, U.K.) supplemented with 0.01% L-glutamine and 40 μg of gentamicin/ml.
Cell labelling for FRET
Human monocytes on microchamber culture slides (LabTek; Gibco), were labelled with 100 μl of a mixture of donor-conjugated antibody (Cy3) and acceptor-conjugated antibody (Cy5). The cells were rinsed twice in PBS/0.02% (w/v) BSA, before fixation with 4% (w/v) formaldehyde for 15 min. The cells were fixed in order to prevent potential re-organization of the proteins during the course of the experiment.
Confocal imaging
Cells were imaged on a Carl Zeiss LSM510 confocal microscope (with an Axiovert 200 fluorescent microscope) using a 1.4 NA 63× Zeiss objective. The images were analysed using LSM 2.5 image analysis software (Carl Zeiss). Cy3 and Cy5 were detected using the appropriate filter sets. Using typical exposure times for image acquisition (<5 s), no fluorescence was observed from a Cy3-labelled specimen using the Cy5 filters, nor was Cy5 fluorescence detected using the Cy3 filter sets.
FRET measurements
FRET is a non-invasive imaging technique used to determine molecular proximity. FRET can occur over 1–10 nm distances, and effectively increases the resolution of light microscopy to the molecular level. It involves non-radiative transfer of energy from the excited state of a donor molecule to an appropriate acceptor [25]. The rate of energy transfer is inversely proportional to the sixth power of the distance, between donor and acceptor [26,27]. The efficiency of energy transfer (E) is defined with respect to r and R0, the characteristic Förster distance by:
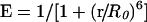 |
(1) |
In the present study, FRET was measured using a method as previously described [26,27]. Briefly, samples were labelled with donor- and acceptor-conjugated antibodies, and energy transfer was detected as an increase in donor fluorescence (dequenching) after complete photobleaching of the acceptor molecule.
Cells labelled only with the 26ic-Cy3 probe were used in order to determine the minimum time required to bleach Cy3. Cy3 was bleached by continuous excitation with an arc lamp using a Cy3 filter set for 5 min. Under these conditions, Cy5 was not bleached.
FRET images were calculated from the increase in donor fluorescence after acceptor photobleaching by:
 |
(2) |
The scaling factor of 10000 was used in order to expand E to the scale of the 12-bit images.
Isolation of lipid rafts
Human monocytes (1×108) were lysed in 500 μl of MEB (membrane-extraction buffer; 150 mM NaCl, 20 mM Mes, pH 6.5) containing 1% (v/v) Triton X-100 and protease inhibitors (500 μM PMSF and 5 mM iodo-acetamide) for 1 h on ice. The cells were mixed with an equal volume of 90% sucrose in MEB and placed at the bottom of a centrifuge tube. The sample was overlaid with 5.5 ml of 30% sucrose and 4.5 ml of 5% sucrose in MEB and centrifuged at 28000 rev./min in a Beckman Coulter SW40 rotor for 16 h. Fractions (1 ml) were gently removed from the top of the gradient and n-octylglucoside was added to each fraction (60 μM final) to solubilize rafts. For isolation of cellular membranes following LPS stimulation, human monocytes were stimulated with either 100 ng/ml LPS in 5% HPS (human pooled serum), or 100 ng/ml 505 lipid A, or 100 ng/ml of pLA for 30 min at 37 °C, before solubilization in MEB.
Western blotting
Equal portions of each fraction were analysed by SDS/PAGE and transferred on to a nitrocellulose filter (Schleicher & Schuell, Dassel, Germany) or Immobilon P membranes (Millipore) for 1 h at 220 mA in the presence of transfer buffer (20 mM Tris acetate, 0.1% SDS, 20% isopropanol, pH 8.3). After transfer, the membrane was blocked for 1 h in blocking solution [5% low fat dried milk dissolved in PBS-T (PBS with Tween 20)] and washed with PBS-T (three rinses, a 15 min wash and two 10 min washes). Membranes were probed with the appropriate dilution of primary antibody for 1 h followed by washing with PBS-T. Membranes were incubated with HRP (horseradish peroxidase) conjugated to either swine anti-rabbit Ig (1:4000), donkey anti-goat Ig or rabbit anti-mouse Ig for 1 h. After extensive washing with PBS-T, the antigen was visualized using the ECL® (enhanced chemiluminescence) procedure (Amersham Biosciences) according to the manufacturer's instructions.
Fluorescent probes
Cholera toxin was purchased from List Biological Laboratories (Campbell, CA, U.S.A.) and conjugated to Cy5. Antibodies against the molecules of interest were conjugated with Cy3 using the labelling kits from Amersham Biosciences.
FRAP (fluorescence recovery after photobleaching) measurements
FRAP measurements were performed as described previously [28–30]. Briefly, slides containing labelled cells were placed on to a temperature-controlled microscope stage (Model TS-4; Physitemp, Clifton, NJ, U.S.A.), and were allowed to equilibrate to the desired temperature for FRAP measurements. After equilibration, the beam of an argon ion laser (Innova 100-10) was focused on to the desired area on the cell. The laser beam was of Gaussian cross-sectional intensity distribution, with a half-width at 1/e2 height of the laser beam at its point of focus equal to 1.24 μm spot radius. FRAP measurements were recorded and analysed as described previously [29].
Cytokine assays
For stimulation of human MNCs (mononuclear cells) by LPS, MNCs were isolated from heparinized blood of healthy donors as described previously [31]. The cells were resuspended in serum-free medium and their number was equilibrated at 5×106 cells/ml. For stimulation, 200 μl of MNCs (1×106 cells) were transferred into each well of a 96-well culture plate. The lipids were incubated for 30 min at 37 °C, serially diluted in serum-free RPMI 1640 medium, and added to the cultures at 20 μl per well. The cultures were incubated for 4 h at 37 °C under 5% CO2. Supernatants were collected after centrifugation of the culture plates for 10 min at 400 g and were stored at −20 °C until determination of TNF-α (tumour necrosis factor α) content. Immunological determination of TNF-α was carried out in a sandwich ELISA using an mAb against TNF-α (clone 6b from Intex AG, Muttent, Switzerland) and was described in detail previously [32].
RESULTS
TNF-α secretion in response to LPS and different LPS analogues
Before investigating whether or not LPS analogues induce the formation of TLR4–MD-2 receptor clusters, we investigated cytokine production in response to the different LPS test analogues. Human monocytes were stimulated with increasing concentrations of LPS, 1-monosphosphoryl lipid A (505), pLA, and 406.
We measured TNF-α production in response to various concentrations of either LPS or different LPS analogues, ranging from 10 pg/ml to 10 μg/ml (Figure 1). We found that LPS and 505 triggered similar cytokine production, particularly at higher concentrations. As expected, pLA and 406 did not stimulate any significant cytokine production up to a concentration of 1 μg/ml. It was decided that, for all experiments, 100 ng/ml of the different compounds would be used in order to stimulate cells.
Figure 1. TNF-α secretion in response to LPS and different LPS analogues.
Human MNCs were stimulated with various concentrations of either LPS (▪), 1-monophosphoryl lipid A (505) (•), pLA (▴) or tetra-acyl lipid A (406) (□). The cells were stimulated for 4 h at 37 °C under 5% CO2. TNF-α content was determined using a sandwich ELISA. Results are means±S.D. for a number of independent experiments.
TLR4 involvement in the LPS-activation cluster
Since it has recently been demonstrated that TLR4–MD-2 clustering is crucial for LPS-induced signalling [13], we sought to investigate in the present study whether or not there are additional receptor components within these clusters.
Previous studies had suggested the association of several molecules, such as CD11b/CD18, CD55, CD81, hsp70, hsp90, GDF5 and CXCR4, in response to LPS [15,33]. Thus in the present study, we used FRET, a non-invasive biophysical method, in order to investigate their molecular associations with the TLR4–MD-2 clusters.
We measured FRET in terms of dequenching of donor fluorescence after complete photobleaching of the acceptor fluorophore. We tested the energy-transfer efficiency in our system using a positive control, i.e. energy transfer between mAbs to different epitopes on CD14 molecules, showing that the maximum energy-transfer efficiency (E%) was 38±1.5. A negative control between Cy3–CD14 and Cy5–W6/32 (a mAb specific for MHC class I) was also used, which revealed no significant energy transfer (4±1.0). We proceeded to measure FRET between TLR4 and the receptor molecules that have been reported to form LPS-induced clusters before LPS stimulation. TLR4 was found not to associate with these receptor molecules before LPS stimulation (Figure 2A).
Figure 2. TLR4–MD-2 heterotypic associations in response to different LPS structures.
Human monocytes were stimulated with the different LPS structures (100 ng/ml) for 10 min. Energy transfer between TLR4 (Cy3) (A) or MD-2 (Cy3) (B) and the different receptors was measured from the increase in donor (Cy3) fluorescence after acceptor (Cy5) photobleaching. Results are percentages of energy transfer±S.D. calculated from three independent experiments.
In order to determine whether or not TLR4 associated with these molecules following LPS stimulation, human monocytes were isolated from the peripheral blood of healthy donors, and were stimulated for 10 min at 37 °C with 100 ng/ml LPS before fixation and labelling with the fluorescent probes. Energy transfer between TLR4–Cy3 and the various Cy5-labelled molecules was measured. TLR4 was found to associate with CD11b/CD18, CD55, CD81, hsp70, hsp90, GDF5 and CXCR4 after LPS stimulation. Control experiments using the method described by Kenworthy and Edidin [26] ruled out the possibility that the FRET observed was due to randomly distributed molecules (results not shown).
Similar results were obtained when we tested the association of MD-2 with these receptor molecules, suggesting that the TLR4–MD-2 complex forms clusters with these molecules (Figure 2B).
Formation of activation clusters following 505 lipid A stimulation
As a control, we tested whether or not the same receptors were recruited after stimulation by synthetic monophosphoryl 505 lipid A. Monophosphoryl 505 lipid A (100 ng/ml) seems to be triggering approximately the same TNF-α production as LPS (Figure 1). Human monocytes were stimulated with 100 ng/ml of 505 lipid A for 10 min at 37 °C before fixation and labelling with fluorescent probes. Energy transfer was measured between TLR4–Cy3 or MD-2–Cy3 and various Cy5-labelled receptor molecules. The synthetic 505 lipid A was found to induce the formation of activation clusters identical with those formed in response to LPS (Figure 2).
Formation of activation clusters following pLA stimulation
In order to investigate whether or not different LPS structures are able to induce TLR4–MD-2 clustering and formation of receptor clusters, we proceeded to test whether pLA or tetra-acyl lipid A (406), which have a cylindrical shape [19], were able to form the same activation clusters as LPS. pLA has been shown previously to have an antagonistic activity to that of LPS [19], whereas compound 406 is totally inactive (Figure 1).
Human monocytes were stimulated with either 100 ng/ml pLA or compound 406 for 10 min at 37 °C before fixation and labelling with fluorescent probes. Energy transfer was measured between the different receptor pairs. TLR4–MD-2 were found to associate with hsp70, hsp90 and CD55 in response to the LPS analogues, but CD11b/CD18, CD81 or CXCR4 were shown not to be in close proximity (Figure 2), thus suggesting that different LPS structures induce the formation of different receptor clusters.
Recruitment of TLR4–MD-2 in lipid rafts following pLA stimulation
It has been shown previously that regions of the plasma membrane known as lipid rafts, or microdomains facilitate LPS-induced cell activation [34,35]. TLR4 has been shown to be recruited within these microdomains following LPS stimulation, and this clustering is crucial for LPS-induced cytokine production [34]. In order to test whether or not stimulation by different ligands affects the recruitment of TLR4 within lipid rafts, plasma microdomains were isolated from a human monocytic cell line, Mono-Mac 6, on the basis of their insolubility in Triton X-100 and low buoyant density in sucrose gradients [36]. Mono-Mac 6 cells were treated with 1% (v/v) Triton X-100 in buffer for 1 h on ice and then subjected to sucrose density-gradient centrifugation as described in the Materials and methods section.
GM-1 monosialoganglioside, a raft-associated lipid, was detected using HRP-conjugated cholera toxin. We found that GM-1 migrated near the top of the sucrose gradient (fractions 2–5), indicating that this procedure was effective in separating membrane rafts from the rest of the cellular membrane (Figure 3A).
Figure 3. TLR4 is present in lipid rafts following pLA stimulation.
Human monocytes were either not stimulated (A–C, H), or stimulated with 100 ng/ml LPS in 5% HPS (human pooled serum) (D, E, I) or 100 ng/ml pLA (F, G) for 30 min before solubilization with 1% (v/v) Triton X-100 buffer for 1 h on ice, and then subjected to sucrose density-gradient centrifugation. Fractions were collected from the top of the gradient, 1% n-octylglucoside was added to each fraction, and equivalent portions of each fraction were analysed by SDS/PAGE and immunoblotting. The lipid raft marker was detected using HRP-conjugated cholera toxin (A), the nitrocellulose membranes were also probed with either TLR4-specific mAb (B, D, F), MD-2-specific mAb (C, E, G) or transferrin-receptor-specific mAb (H, I). The relative positions of the raft and non-raft (soluble) fractions are indicated.
TLR4 and MD-2 were found not to be present in lipid raft fractions before stimulation (Figures 3B and 3C). In order to test whether or not the TLR4–MD-2 complex was present in microdomains after pLA stimulation, we immunoblotted the nitrocellulose membranes with HTA125, a TLR4-specific mAb or an MD-2 polyclonal antibody, followed by the addition of HRP-conjugated rabbit anti-mouse or swine-anti-rabbit Ig. We found that TLR4–MD-2 was present in the lipid raft fractions of the gradient (Figures 3F and 3G), although to a lesser extent when compared with their recruitment after LPS stimulation (Figures 3D and 3E). In addition, Western blotting using an antibody against the transferrin receptor, a known non-raft receptor molecule, revealed that the transferrin receptor is not recruited within lipid rafts following LPS stimulation (Figure 3I), thus demonstrating the specificity of the recruitment. Experiments employing HRP-conjugated rabbit anti-mouse Ig in the absence of a primary antibody revealed no protein bands, thus demonstrating the specificity of the antibodies.
Quantification of the extent of TLR4–MD-2 recruitment in lipid rafts following pLA stimulation was performed using FRET (Table 1). TLR4 molecules were labelled with Cy3–HTA125, MD-2 was also labelled with Cy3–MD-2 polyclonal antibody, and GM-1, a raft-associated lipid, was labelled with Cy5–cholera toxin. FRET measurements confirmed the presence of TLR4–MD-2 in lipid rafts after LPS stimulation. Similar to the biochemical studies, FRET measurements demonstrated further that there was less energy transfer between GM-1 and TLR4–MD-2 after pLA stimulation, suggesting that there is less recruitment of TLR4–MD-2 molecules within lipid rafts following stimulation by LPS antagonists.
Table 1. TLR4 association with lipid rafts.
Energy-transfer between different pairs was detected from the increase in donor fluorescence after acceptor photobleaching. Results are means±S.D. for a number of independent experiments.
| Stimulation | Donor (Cy3) | Acceptor (Cy5) | E±ΔE (%) |
|---|---|---|---|
| − | TLR4 | GM-1 ganglioside | 4±0.5 |
| LPS | TLR4 | GM-1 ganglioside | 31±0.5 |
| 505 lipid A | TLR4 | GM-1 ganglioside | 29±1.5 |
| pLA | TLR4 | GM-1 ganglioside | 15±0.5 |
| 406 | TLR4 | GM-1 ganglioside | 12±2.5 |
FRAP measurements of TLR4 following LPS and pLA stimulation
It is thought that microdomains on the plasma membrane arise through the confinement of diffusible membrane proteins [37]. In order to determine the lateral diffusion of TLR4 on the plasma membrane before and after stimulation by different ligands, we used the FRAP technique. We had previously employed this approach in order to observe the lateral diffusion of CD14 before and after stimulation by bacterial products [30]. At 22 °C, the diffusion coefficient of TLR4 before LPS stimulation was found to be (2.95±0.3)×10−9 cm2/s with 65±10% fluorescence recovery. Upon stimulation with 100 ng/ml LPS, TLR4 was found to have a diffusion coefficient of (2.10±0.2)×10−9 cm2/s with 49±12% fluorescence recovery, almost identical with that obtained before LPS stimulation. Similarly, upon stimulation with 100 ng/ml pLA, the diffusion coefficient of TLR4 was (2.51±0.4)×10−9 cm2/s with 61±8% fluorescence recovery.
At physiological temperature (37 °C), the confinement of TLR4 within microdomains was more apparent. Before LPS stimulation, TLR4 was found to have a diffusion coefficient of (2.01±0.1)×10−9 cm2/s with 62±8% fluorescence recovery (Figure 4A), whereas upon LPS stimulation, the diffusion coefficient of TLR4 was (9.73±0.3)×10−10 cm2/s with 58±6% fluorescence recovery (Figure 4B), suggesting that upon LPS stimulation, the diffusion of TLR4 was restricted. In contrast, upon stimulation by pLA, TLR4 was found to have a diffusion coefficient of (3.11±0.3)×10−9 cm2/s with 70±10% fluorescence recovery (Figure 4C), showing a slight increase in diffusion coefficient and suggesting no apparent confinement within microdomains (Table 2). Indeed, the increase in diffusion coefficient suggests that pLA stimulation caused a release of previously confined TLR4.
Figure 4. Lateral diffusion of TLR4 before and after stimulation by different LPS analogues.
FRAP curve of Oregon Green–TLR4 before stimulation (A) and after stimulation by either 100 ng/ml of LPS (B) or 100 ng/ml of pLA (C). The diffusion coefficient of TLR4 was the mean±S.D. of individual curves (n=10).
Table 2. Lateral diffusion of TLR4.
Results are means±S.D. of several determinations (n=10).
| Receptor | Stimulation | Temperature | Diffusion coefficient (cm2/s) | Percentage recovery |
|---|---|---|---|---|
| TLR4 | − | 22 °C | (2.95±0.3)×10−9 | 65±10 |
| TLR4 | LPS | 22 °C | (2.10±0.2)×10−9 | 49±12 |
| TLR4 | pLA | 22 °C | (2.51±0.4)×10−9 | 61±8 |
| αvβ1 | − | 22 °C | (1.80±0.4)×10−9 | 15±4 |
| αvβ1 | LPS | 22 °C | (2.2±0.1)×10−9 | 12±5 |
| αvβ1 | pLA | 22 °C | (2.3±0.6)×10−9 | 12±3 |
| TLR4 | − | 37 °C | (2.01±0.1)×10−9 | 62±8 |
| TLR4 | LPS | 37 °C | (9.73±0.3)×10−10 | 57±6 |
| TLR4 | pLA | 37 °C | (3.11±0.3)×10−9 | 70±10 |
As a control, we determined the lateral diffusion of αvβ1, an integrin that is not associated with LPS recognition. At 22 °C, the diffusion coefficient of αvβ1 before LPS stimulation was found to be (1.80±0.4)×10−9 cm2/s with 15±4% fluorescence recovery. Upon stimulation with 100 ng/ml LPS, αvβ1 was found to have a diffusion coefficient of (2.2±0.1)×10−9 cm2/s with 12±5% fluorescence recovery, almost identical with that obtained before LPS stimulation. Similarly, upon stimulation with 100 ng/ml pLA, the diffusion coefficient of αvβ1 was (2.3±0.6)×10−9 cm2/s with 12±3% fluorescence recovery. As expected, the lateral diffusion and fluorescence recovery of αvβ1 remained unaffected before and after stimulation by LPS or pLA, thus suggesting that the TLR4 mobility observed in response to these ligands is specific.
LPS analogues activate MAPK and SAPK signalling cascades
In order to determine the significance of the formation of the different activation clusters in response to different LPS analogues, we investigated which signalling cascades are triggering in each event. It has been shown that LPS can activate signalling cascades, such as the MAPKs, in addition to NF-κB [38,39], thus we investigated the activation of NF-κB, as well as on the phosphorylation of p42 MAPK, p38 MAPK and JNK (c-Jun N-terminal kinase)/SAPK in response to stimulation by LPS or LPS analogues.
We therefore immunoblotted nitrocellulose membranes with antibodies specific for total IκB (inhibitory κB), p42 MAPK, p38 and JNK/SAPK, as well as with phosphospecific antibodies for IκB, p42 MAPK, p38 MAPK and JNK/SAPK (Figure 5). These antibodies only recognize the activated form of the molecule. We found that we could detect activated IκB, p38 MAPK and JNK/SAPK in response to LPS (Figure 5). The phosphorylation was shown to be dose-dependent. Similar results were obtained in response to 505 stimulation.
Figure 5. Signalling in response to LPS and LPS analogues.
Human monocytes were stimulated with the indicated amounts of LPS and pLA for 10 min. Subsequently, whole lysates were analysed by SDS/PAGE and transferred on to nitrocellulose. The nitrocellulose membranes were probed with phosphospecific antibodies against activated IκB, p42 MAPK, JNK/SAPK and p38 MAPK, followed by incubation of HRP-conjugated antibodies (upper panels in each pair). The amount of total protein content in the lysates was detected by immunoblotting with the indicated antibodies against total protein levels (lower panels in each pair).
Upon stimulation with pLA or 406, we could not detect any phosphorylation of IκB, probably due to the fact that TLR4 was recruited to a lesser extent within lipid rafts. Surprisingly, we could still detect p38 MAPK, as well as JNK/SAPK phosphorylation, although to a lesser extent when compared with those in response to LPS (Figure 5), thus suggesting that even without recruitment of TLR4 within the lipid raft, there still was activation of the MAPK signalling cascades, possibly through the other receptors that were recruited.
Functional significance of TLR4–MD-2 association with lipid rafts
Although we have demonstrated previously that TLR4 recruitment within lipid rafts is crucial for LPS-induced cytokine secretion, we proceeded to demonstrate further the functional significance of TLR4–MD-2 recruitment within lipid rafts by evaluating the ability of LPS to stimulate cells that had been treated with raft-disrupting agents. The CHO/CD14/TLR4 LPS-reporter cell line was used, which up-regulates surface expression of CD25. CD25 expression was measured before (Figure 6, dark grey bars) and after (Figure 6, black bars) LPS stimulation. CD25 was found to be specifically up-regulated in response to LPS. In contrast, if the cells were pre-treated with a raft-disrupting drug before LPS stimulation, CD25 surface expression was inhibited (Figure 6, light grey bars), thus suggesting that lipid raft integrity is crucial for TLR4-mediated LPS-induced activation. Control experiments using CHO/CD14/TLR4 cells stimulated with pLA demonstrated further the inability of pLA to trigger cytokine secretion (Figure 6, white bars).
Figure 6. Disrupting lipid raft integrity inhibits LPS-mediated cellular activation.
The CHO parental cell line or CHO/CD14/TLR4 reporter cell line was either not stimulated, or stimulated with 100 ng/ml LPS or 100 ng/ml pLA in the absence of nystatin. LPS stimulation was also performed in the presence of 60 μg/ml nystatin. The induction of CD25 expression was detected with FITC–CD25. Fluorescence was detected using a FACSCalibur (Becton Dickinson), counting 10000 cells per sample.
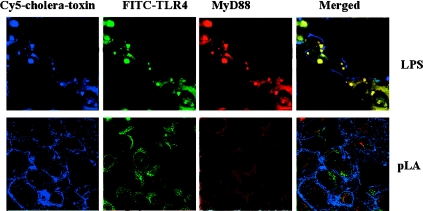
In addition, we investigated the recruitment of MyD88 in lipid rafts following stimulation by bacterial products using triple-labelling and confocal microscopy. MyD88 has been shown to bind to the intracellular signalling domain of TLRs, inducing downstream signalling processes [40], and is required for full responses to LPS. It was demonstrated that in cells stimulated with LPS, TLR4 was found to form clusters and to co-localize with the lipid raft marker, as well as MyD88. The MyD88 intracellular pool was observed to translocate to the cytoplasmic side of the plasma membrane (Figure 7, upper panels). In contrast, after stimulation with pLA, TLR4 did not form clusters, and did not co-localize with either the lipid raft marker or MyD88 (Figure 7, lower panels), thus suggesting that in response to LPS analogues, TLR4 is not recruited within lipid rafts and does not aggregate, and thus there is no significant signalling via the MyD88 pathway.
Figure 7. MyD88 translocates to the plasma membrane after LPS stimulation.
Human monocytes were stimulated for 10 min with either LPS (upper panels) or pLA (lower panels). Lipid rafts were visualized using Cy5–cholera toxin (blue), MyD88 was visualized using a MyD88-specific antibody conjugated to TRITC (tetramethylrhodamine β-isothiocyanate; red), TLR4 was visualized using FITC–TLR4 (green).
DISCUSSION
In the present study, we have sought to answer whether or not the structure or ‘shape’ of LPS affects its interaction with the CD14–TLR4–MD-2 cluster. We stimulated human monocytes with LPS, 505 lipid A and LPS antagonists, pLA and 406, and determined receptor associations using FRET. Following LPS stimulation, a cluster of receptors comprising TLR4, CD11b/CD18, CD55, CD81, hsp70, hsp90, GDF5 and CXCR4 was formed. Similarly, after stimulation by 505 lipid A, the same activation cluster was formed, suggesting that the 1-phosphoryl lipid A moiety of LPS is sufficient in order to trigger the recruitment of these receptors. This correlates with the observation that 505 lipid A is endotoxically highly active [41]. In contrast, when human monocytes were stimulated by pLA, which is endotoxically inactive at the concentration used, a different activation cluster was formed. pLA stimulation triggered the recruitment of hsp70, hsp90, CD55 and, to a lesser extent, TLR4. CD11b/CD18, CD81, GDF5 and CXCR4 were not recruited within the cluster of receptors. It is worth noting that in response to LPS antagonists, there was no recruitment of CD11b/CD18 or the tetraspan CD81. Since it has been shown that membrane organization is accomplished through two-dimensional networks made up by proteins of the tetraspan family [42], it is possible that recruitment of CD81 might play an important role in the aggregation of these receptor complexes.
In addition, when we investigated which signalling cascades are triggered in response to pLA stimulation, we found that IκB was not phosphorylated, since TLR4 is recruited to the lipid raft to a much lesser extent. The failure to recruit TLR4 within the lipid raft led to the inhibition of NF-κB activation, thus demonstrating the importance of TLR4 in triggering this signalling cascade. Surprisingly, we found that, although TLR4 was not recruited, there was some activation of p42 MAPK, p38 MAPK and JNK/SAPK in response to pLA, thus suggesting that additional components of the pLA-induced cluster participate in signalling. Furthermore it was shown that TLR4–MD-2 recruitment within lipid rafts after LPS stimulation correlated with the translocation of MyD88 to the cytoplasmic side of the plasma membrane. In the case where cells were stimulated by an LPS antagonist, only a small pool of TLR4 molecules was recruited within lipid rafts in order to associate with the receptor cluster, and thus no significant translocation of MyD88 was observed. The composition of the receptor cluster as well as the stoichiometry of receptors within it seem to play a major role in what type of signal will be transduced in response to the particular pathogen. It seems that a certain number of TLR4 molecules must associate with the cluster in order to trigger signalling. In the case of LPS antagonists, not enough TLR4 molecules are recruited and thus we do not get the NF-κB activation.
Similar results were obtained when we observed in real time the movement of TLR4 before and after LPS stimulation. It is believed that microdomains on the plasma membrane arise through the confinement of diffusible membrane proteins [37]. Thus we looked at the diffusion of TLR4 before and after stimulation with LPS and pLA, and whether or not it was showing signs of confinement. At physiological temperature (37 °C), we found that upon LPS stimulation, TLR4 was diffusing much more slowly, suggesting restriction in its lateral diffusion and thus confinement within microdomains. In contrast, stimulation by pLA, which is a proven LPS antagonist, induced an increase when compared with the diffusion of TLR4 before stimulation. This suggests that stimulation by pLA causes the release of a proportion of previously confined TLR4 molecules. This data is in agreement with our biochemical and FRET analysis that revealed that only a small proportion of the TLR4 pool is associated with lipid rafts following stimulation by LPS antagonists.
Overall, our data suggest that the structure or ‘shape’ of LPS induces the formation of different receptor clusters (Figure 8). These data are in agreement with our previous hypothesis [15,43,44] and demonstrate that different combinational associations of receptors are recruited depending on the type of LPS with which the cells are being challenged. Clustering of different combinations of receptors into membrane microdomains, such as lipid rafts, seems to act as a mechanism for recognizing and regulating cell signalling in response to a broad range of microbial pathogens. The recruitment of different receptors within these specialized sites of the plasma membrane seems to be triggered by different pathogens. It has been shown recently that not only do these rafts play a major role in clustering receptors that are important for recognition and signalling of bacterial pathogens, but also that these sites are important for the subsequent LPS internalization [45]. Thus lipid rafts seem to provide a dynamic micro-environment where ligand binding promotes the recruitment and clustering of specific receptors. The composition of the receptor cluster as well as the stoichiometry of the receptors involved will determine the signalling that is triggered and will eventually define the specific cellular response against a particular pathogen.
Figure 8. The shape of LPS determines receptor-cluster formation.
Formation of different activation clusters in response to different LPS shapes. (a) E. coli LPS (adopting a conical shape) induces a strong pro-inflammatory signal through the formation of an activation cluster containing a variety of receptors such as CD14, multiple TLR4 molecules, hsps, CXCR4 and integrins. (b) pLA adopting a less conical shape induces the formation of a different activation cluster containing hsps, CD55 and fewer TLR4 molecules, thus does not induce a pro-inflammatory response.
Acknowledgments
This work was supported by the Wellcome Trust and the Deutsche Forschungsgemeinschaft (SFB367, project B8). We thank Doug Golenbock for providing us with his CHO reporter cell line.
References
- 1.Medzhitov R., Janeway C. A. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 2.Akira S. Toll-like receptors and innate immunity. Adv. Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 4.Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding-protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A., He X. L., Smirnova I., Liu M. Y., VanHuffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., et al. Defective LPS signaling in C3H/Hej and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi S. T., Lariviere L., Leveque G., Clermont S., Moore K. J., Gros P., Malo D. Endotoxin-tolerant mice have mutations in toll-like receptor 4 (TLR4) J. Exp. Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow J. C., Young D. W., Golenbock D., Christ W. J., Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 8.Nagai Y., Akashi S., Nagafuku M., Ogata M., Iwakura Y., Akira S., Kitamura T., Kosugi A., Kimoto M., Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 9.Akashi S., Shimazu R., Ogata H., Nagai Y., Takeda K., Kimoto M., Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signalling via the Toll-like receptor 4–MD-2 complex on mouse peritoneal macrophages. J. Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 10.Hajjar A. M., Ernst R. K., Tsai J. H., Wilson C. B., Miller S. I. Human Toll-like receptor 4 recognises host-specific LPS modifications. Nat. Immunol. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 11.Viriyakosol S., Tobias P. S., Kitchens R. L., Kirkland T. N. MD-2 binds to bacterial lipopolysaccharide. J. Biol. Chem. 2001;276:38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 12.da Silva Correia J., Soldau K., Christen U., Tobias P., Ulevitch R. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex: transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 13.Visintin A., Latz E., Monks B. G., Espevik T., Golenbock D. T. Lysines 128 and 132 enable lipopolysaccharide binding to MD2, leading to Toll-like receptor 4-aggregation and signal transduction. J. Biol. Chem. 2003;278:48313–48320. doi: 10.1074/jbc.M306802200. [DOI] [PubMed] [Google Scholar]
- 14.Byrd C. A., Bornmann W., Erdjument-Bromage H., Tempst P., Pavletich N., Rosen N., Nathan C. F., Ding A. Heat shock 90 mediates macrophage activation by Taxol and bacterial lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5645–5650. doi: 10.1073/pnas.96.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Triantafilou K., Triantafilou M., Dedrick R. L. A CD14-independent LPS receptor cluster. Nat. Immunol. 2001;4:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 16.Heine H., El-Samalouti V. T., Notzel C., Pfeiffer A., Lentschat A., Kusumoto S., Schmitz G., Hamann L., Ulmer A. J. CD55/decay accelerating factor is part of the lipopolysaccharide-induced receptor complex. Eur. J. Immunol. 2003;33:1399–1408. doi: 10.1002/eji.200323381. [DOI] [PubMed] [Google Scholar]
- 17.Rose J. R., Christ W. J., Bristol J. R., Kawata T., Rossignol D. P. Agonistic and antagonistic activities of bacterially derived Rhodobacter sphaeroides lipid A: comparison with activities of synthetic material of the proposed structure and analogs. Infect. Immun. 1995;63:833–839. doi: 10.1128/iai.63.3.833-839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loppnow H., Libby P., Freudenberg M. A., Kraus J. H., Weckesser J., Mayer H. Cytokine induction by lipopolysaccharide (LPS) corresponds to the lethal toxicity and is inhibited by non-toxic Rhodobacter capsulatus LPS. Infect. Immun. 1990;58:3743–3750. doi: 10.1128/iai.58.11.3743-3750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seydel U., Oikawa M., Fukase K., Kusumoto S., Bradenburg K. Intrinsic conformation of lipid A is responsible for agonistic and antagonistic activity. Eur. J. Biochem. 2000;267:3032–3039. doi: 10.1046/j.1432-1033.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 20.Schromm A., Bradenburg K., Loppnow H., Moran A. P., Koch M. H. J., Rietschel E., Seydel U. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur. J. Biochem. 2000;267:2008–2013. doi: 10.1046/j.1432-1327.2000.01204.x. [DOI] [PubMed] [Google Scholar]
- 21.Delude R. L., Savedra R., Zhao H. L., Thieringer R., Yamamoto S., Fenton M. J., Golenbock D. T. CD14 enhances cellular-responses to endotoxin without imparting ligand-specific recognition. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9286–9292. doi: 10.1073/pnas.92.20.9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galanos C., Luderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur. J. Biochem. 1969;9:245–250. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 23.Lindner B. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of lipopolysaccharides. In: Holst O., editor. Bacterial Toxins, Methods and Protocols. Totowa: Humana Press; 2002. [Google Scholar]
- 24.Liu W. C., Oikawa M., Kukase K., Suda Y., Kusumoto S. A divergent synthesis of lipid A and its chemically stable unnatural analogues. Bull. Chem. Soc. Jpn. 1999;72:1377–1382. [Google Scholar]
- 25.Wu P., Brand L. Resonance energy transfer: methods and applications. Anal. Biochem. 1994;218:1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]
- 26.Kenworthy A. K., Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 Å using imaging fluorescence resonance energy transfer. J. Cell. Biol. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenworthy A. K., Edidin M. Imaging fluorescence resonance energy transfer as probe of membrane organisation and molecular associations of GPI-anchored proteins. In: Gelb M. H., editor. Methods in Molecular Biology. Totowa: Humana Press; 1998. pp. 37–49. [DOI] [PubMed] [Google Scholar]
- 28.Ladha S., Mackie A., Clark D. Cheek cell membrane fluidity measured by fluorescence recovery after photobleaching and steady state anisotropy. J. Membr. Biol. 1994;142:223–229. doi: 10.1007/BF00234944. [DOI] [PubMed] [Google Scholar]
- 29.Ladha S., Mackie A., Harvey L., Clark D., Lea E., Brullemans M., Duclohier H. Lateral diffusion in planar lipid bilayers: a FRAP investigation of its modulation by lipid composition, cholesterol or alamethicin content and divalent cations. Biophys. J. 1996;71:1364–1373. doi: 10.1016/S0006-3495(96)79339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triantafilou K., Triantafilou M., Ladha S., Mackie A., Fernandez N., Dedrick R. L., Cherry R. J. Fluorescence recovery after photobleaching reveals that lipopolysaccharide rapidly transfers from CD14 to heat shock proteins 70 and 90 on the cell membrane. J. Cell Sci. 2001;114:2535–2545. doi: 10.1242/jcs.114.13.2535. [DOI] [PubMed] [Google Scholar]
- 31.Brandenburg K., Matsuura M., Heine H., Muller M., Kiso M., Ishida H., Koch M. H., Seydel U. Biophysical characterization of triacyl monosaccharide lipid partial structures in relation to bioactivity. Biophys. J. 2002;83:322–333. doi: 10.1016/S0006-3495(02)75172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurgens G., Muller M., Garidel P., Koch M. H., Nakakubo H., Blume A., Brandenburg K. Investigation into the interaction of recombinant human serum albumin with Re-lipopolysaccharide. J. Endotoxin Res. 2002;8:115–126. doi: 10.1179/096805102125000263. [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer A., Bottcher A., Orso E., Kapinsky M., Nagy P., Bodnar A., Spreitzer I., Liebisch G., Drobnik W., Gempel K., et al. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 2001;31:3153–3164. doi: 10.1002/1521-4141(200111)31:11<3153::aid-immu3153>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Triantafilou M., Miyake K., Golenbock D., Triantafilou K. Mediators of the innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 35.Wang P. Y., Kitchens R., Munford R. S. Bacterial lipopolysaccharide binds to CD14 in low density domains of the monocyte-macrophage plasma membrane. J. Inflammation. 1996;47:126–137. [PubMed] [Google Scholar]
- 36.Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 37.Edidin M. Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Cell Biol. 2001;11:492–496. doi: 10.1016/s0962-8924(01)02139-0. [DOI] [PubMed] [Google Scholar]
- 38.Hambleton J., Weinstein S. L., Lem L., DeFranco A. L. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstein S. L., Gold M. R., DeFranco A. L. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc. Natl. Acad. Sci. U.S.A. 1990;88:4148–4152. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C. A. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 41.Takada H., Kotani S. Structural requirements of lipid A for endotoxicity and other biological activities. Crit. Rev. Microbiol. 1989;16:477–480. doi: 10.3109/10408418909104475. [DOI] [PubMed] [Google Scholar]
- 42.Rubinstein E., Le Naour F., Lagaudriere-Gesbert C., Billard M., Conjeaud H., Boucheix C. CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. Eur. J. Immunol. 1996;26:2657–2665. doi: 10.1002/eji.1830261117. [DOI] [PubMed] [Google Scholar]
- 43.Triantafilou M., Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;22:295–298. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 44.Triantafilou M., Brandenburg K., Gutsmann T., Seydel U., Triantafilou K. Innate recognition of bacteria: engagement of multiple receptors. Crit. Rev. Immunol. 2002;22:251–268. [PubMed] [Google Scholar]
- 45.Latz E., Visintin A., Lien E., Fitzgerald K., Monks B., Kurt-Jones E., Golenbock D. T., Espevik T. LPS rapidly transfers to and from the Golgi apparatus with the TLR4/MD-2/CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]