Summary
The evolutionarily conserved HIRA/Hir histone chaperone complex and ASF1a/Asf1 co-chaperone cooperate to deposit histone (H3/H4)2 tetramers on DNA for replication-independent chromatin assembly. The molecular architecture of the HIRA/Hir complex and its mode of histone deposition have remained unknown. Here we report the cryo-EM structure of the S. cerevisiae Hir complex with Asf1/H3/H4 at 2.9–6.8 Å resolution. We find that the Hir complex forms an arc-shaped dimer with a Hir1/Hir2/Hir3/Hpc2 stoichiometry of 2/4/2/4. The core of the complex containing two Hir1/Hir2/Hir2 trimers and N-terminal segments of Hir3 forms a central cavity containing two copies of Hpc2, with one engaged by Asf1/H3/H4, in suitable position to accommodate a histone (H3-H4)2 tetramer, while C-terminal segments of Hir3 harbor nucleic acid binding activity to wrap DNA around the Hpc2-assisted histone tetramer. The structure suggests a model for how the Hir/Asf1 complex promotes formation of histone tetramers and their subsequent deposition onto DNA.
Keywords: histone chaperone, chromatin, nucleosome, transcription, cryo-electron microscopy, cross-linking mass spectrometry
eTOC blub
Kim et al. reveals the molecular framework of the HIRA/Hir histone chaperone complex, unveiling its essential function in replication-independent chromatin assembly. It provides key molecular insights into histone deposition assisted by Asf1.
Graphical Abstract

Introduction
The negatively charged DNA within the nucleus of eukaryotic cells is packaged into a chromatin structure comprised of nucleosome building blocks each containing about 150 base pairs of DNA wrapped around an octamer of two copies each of the core histones H2A, H2B, H3 and H41. Nucleosome assembly occurs in an ordered fashion where the deposition of an (H3/H4)2 tetramer is followed by the deposition of two H2A/H2B dimers2. While the majority of nucleosomes comprise canonical histones (H3.1/H3.2, H4, H2A, and H2B), some contain histone variants (H2AZ, CENPA, and H3.3), harboring amino acid substitutions, that mark specific chromatin regions for distinct DNA templated activities such as centromere formation, DNA replication, DNA repair or transcriptional up or down regulation3,4.
Histone trafficking and nucleosome assembly require the coordinated activity of histone chaperone proteins and their associated protein complexes2,5,6. Distinct histone chaperones and co-chaperone complexes are generally dedicated to binding and deposition of specific histone complexes, such as the canonical H3/H4 dimers and (H3/H4)2 tetramers, or H2A/H2B dimers6,7. Some have been reported to have broader chaperoning activity; for example, the NAP1 histone chaperone functions in deposition of both H2A/H2B dimers and (H3/H4)2 tetramers8,9. The histone chaperone anti-silencing function 1 (ASF1a/ASF1b) exclusively chaperones H3/H4 dimers containing either canonical H3.1 or variant H3.3 sequences10–14. The trimeric chromatin assembly factor (CAF-1) histone chaperone complex interacts indiscriminately with ASF1a/b to facilitate replication-dependent or DNA repair-dependent (H3.1/H4)2 histone deposition, while the trimeric histone cell cycle regulator (HIRA) histone chaperone complex preferentially interacts with ASF1a to facilitate replication independent (H3.3/H4)2 histone deposition5,15–23.
The HIRA histone chaperone complex is conserved from yeast to metazoans24. While initially reported as the tetrameric Hir1/Hir2/Hir3/Hpc2 complex in the yeast S. cerevisiae25,26; to date, the trimeric human HIRA/UBN1/CABIN1 has been more extensively dissected 18,27–31. In metazoans, the HIRA complex is composed of HIRA, Ubinuclein 1 (UBN1) and Calcineurin-binding protein 1 (CABIN1) and transiently associates with ASF1a for the deposition of H3.3 into chromatin at regions of active transcription, developmentally regulated genes and sites of DNA damage or repair19,32–36.
Biochemical, biophysical, and X-ray crystallographic studies have identified regions within the constituent proteins of the HIRA complex that contribute to H3.3-specific histone deposition. The HIRA ‘scaffold protein’ contains an N-terminal WD40 domain that binds UBN137, a C-terminal trimerization domain that binds CABIN129,38 and a central B-domain sequence that binds ASF1a18. UBN1 mediates H3.3/H4-specific binding through a Hpc2-related domain (HRD) and interaction with HIRA through a preceding domain N-terminal to the HRD (NHRD)30,37. CABIN1, the largest subunit of the complex, is predicted to have tetratricopeptide repeats (TPR) of antiparallel α-helical pairs, but its functional roles are less well established27,38.
The budding yeast, S. cerevisiae, has only one form of histone H3, more closely related to metazoan H3.3 than the canonical metazoan H3.1/H3.239. Yeast H3 is deposited by the Hir (Hir1/Hir2/Hir3/Hpc2) complex in a replication-independent manner or by the CAF-1 (Cac1/Cac2/Cac3) complex during DNA replication40–42. Hir1 and Hir2, yeast homologs of HIRA, encompass structural features of HIRA, including an N-terminal WD40 domain, and a C-terminal trimerization domain (Figure S1), while the B-domain sequence is present only in Hir118,27. Hpc2 is the homolog of UBN1, retaining the two conserved HRD and the NHRD domains; although these domains reside at the C-terminal end of the protein, while in human UBN1 they are positioned near the N-terminus43,44. Hir3 is the homolog of CABIN1 and maintains the TPR repeats38. Quantitative mass spectrometry experiments have suggested that the Hir complex is composed of Hir1, Hir2, Hir3, and Hpc2 in a molar ratio of 1:2:1:244.
X-ray crystallographic studies involving segments of HIRA have revealed the mode of H3.3/H4-specific interaction30, HIRA trimerization through its C-terminal trimerization domain29 and ASF1a recruitment18. However, a more comprehensive understanding of how the intact HIRA histone chaperone complex coordinates histone deposition has resisted characterization. Here we have determined the structure of the S. cerevisiae Hir complex with Asf1/H3/H4 at 2.9–6.8 Å resolution by single-particle cryo-EM and crosslinking mass spectrometry (XL-MS) analyses. The Hir complex forms an arc-shaped roughly 2-fold symmetric dimer, containing a Hir1/Hir2/Hir3/Hpc2 stoichiometry of 2/4/2/4, with the core of the complex held together by extensive interactions between the Hir1/2 trimerization domains and the two Hir3 subunits. The structure reveals two features of functional significance: a central “cavity”, in which two copies of the Hpc2 HRD from each sub-complex within the dimer are positioned in a suitable conformation for assembly of a histone (H3/H4)2 tetramer, and a Hir3 “Tail”, which may support double-stranded DNA positioning with respect to the cavity, for tetrasome formation, as further supported by genetic analysis. Critically, despite the near 2-fold symmetric scaffold, our structure reveals only one copy of Asf1/H3/H4 engaged in the central cavity through binding to the HRD, with another presumed Asf1/H3/H4 complex poised outside of the cavity for subsequent deposition of a histone (H3/H4)2 tetramer on DNA.
Results
The Hir complex with Asf1/H3/H4 can be assembled for cryo-EM analysis
Affinity purification of the Hir complex by means of a tandem affinity purification (TAP) tag on S. cerevisiae Hir2, followed by chromatography on a Q column, yielded a single homogeneous peak of the Hir complex (Figure S2A). Bottom-up proteomics mass spectrometry confirmed the previously described four subunits of the Hir complex, Hir1–3 and Hpc2 29, with a trace amount of histones. Mass photometry experiments showed one single peak corresponding to a total molecular weight of ~1.24 MDa (Figure S2B), attesting to the homogeneity of the complex. Whereas the Hir complex itself was only stable at high ionic strength, addition of Asf1/H3/H4, expressed and purified in recombinant form from bacteria, significantly increased the solubility of the Hir complex, revealing a single peak of the complex in a glycerol gradient at low ionic strength (Figure S2C,D). To confirm the binding of Asf1/H3/H4 and determine the stoichiometry of the complex, peak glycerol gradient fractions were subjected to intensity-based absolute quantification (iBAQ) (Figure S2E). The resulting stoichiometry agrees well with values obtained by previous normalized peptide-spectrum-match based quantification44. The molar ratio of Hpc2/Asf1/H3/H4 indicates that approximately only one Hpc2 out of four is bound by Asf1/H3/H4. Concentrated peak glycerol gradient fractions were vitrified in ice, disclosing fields of monodispersed particles (Figure S3A). The particles often appeared arc-shaped and associated with large, apparently mobile, flanking regions beneath the arc.
A total ~4.7 million particles were collected and 639,629 particles converged to a map of the entire Hir complex at an average nominal resolution of 2.96 Å (Figure S3B) with the size consistent with the measured molecular weight by mass photometry. The Hir complex adopts an arc-shaped roughly 2-fold symmetric dimer with one well ordered (Copy A) and the other less ordered (Copy B) (Figures 1 and S3B). The central core region comprises two copies of the Hir1/Hir2/Hir2 heterotrimer core (Figure 1A and highlighted in right panel of 1B) and two copies of the N-terminal half of the Hir3 TPR repeats (Map 1: EMD-40006, PDB:8GHL). The central core region is well resolved to a resolution of ~2.9 Å (Figures S3B and S4). The rest of map was more poorly resolved, likely reflecting greater mobility relative to the central core, and thus subjected to focused classification. This included a region containing the C-terminal half of the Hir3 TPR repeats, the Hir2 WD40 domain, and Hpc2, which was refined to 6.8 Å (Figure S3C) (Map 2: EMD-40029, PDB:8GHA). A third region of the structure, containing the Hir1 WD40 domain and Asf1/H3/H4 was refined to a lower resolution of ~12 Å (Figure S5) (Map 3: EMD-40030, PDB:8GHM). Maps 2 and 3 were of sufficient quality to assign an initial sequence register using AlphaFold45 and build models in a manner consistent with extensive crosslinking mass spectrometry data (Figures 2 and S3D,E). The resulting model accounts for ~81% of the mass of the ~1.24 MDa complex, whereas the remaining ~19%, absent from our model, contains two copies of the Hir2 WD40 domain and all residues N-terminal to the NHRD/HRD for all four copies of the Hpc2 (Composite Map: EMD-40037, PDB:8GHN; Movie S1, S2; data summarized in Table S1).
Figure 1. Overall cryo-EM structure of the Hir complex.

(A) Domain architecture of each subunit of the Hir complex.
(B) Overall side view (left) and top view (right) of the Hir complex. The Hir complex adopts an arc-shaped roughly 2-fold symmetric dimer (copy A and copy B on the left and right, respectively). Each copy consists of one copy each of Hir1 (orange) and Hir3 (green), and two copies each of Hir2 (dark purple and light purple) and Hpc2 (indigo blue and cyan). In the right panel, two copies of the heterotrimer core are highlighted.
(C and D) Overall composite density map without Asf1/H3/H4 (C) and schematic with Asf1/H3/H4 (D) in the same orientations as in (B).
(E-H) Structure and location of Hir3 (E), Hir1 (F), Hir21 (G), and Hir22 (H), of the Hir complex in copy A.
Figure 2. Crosslinking mass spectrometry (XL-MS) of the Hir complex.
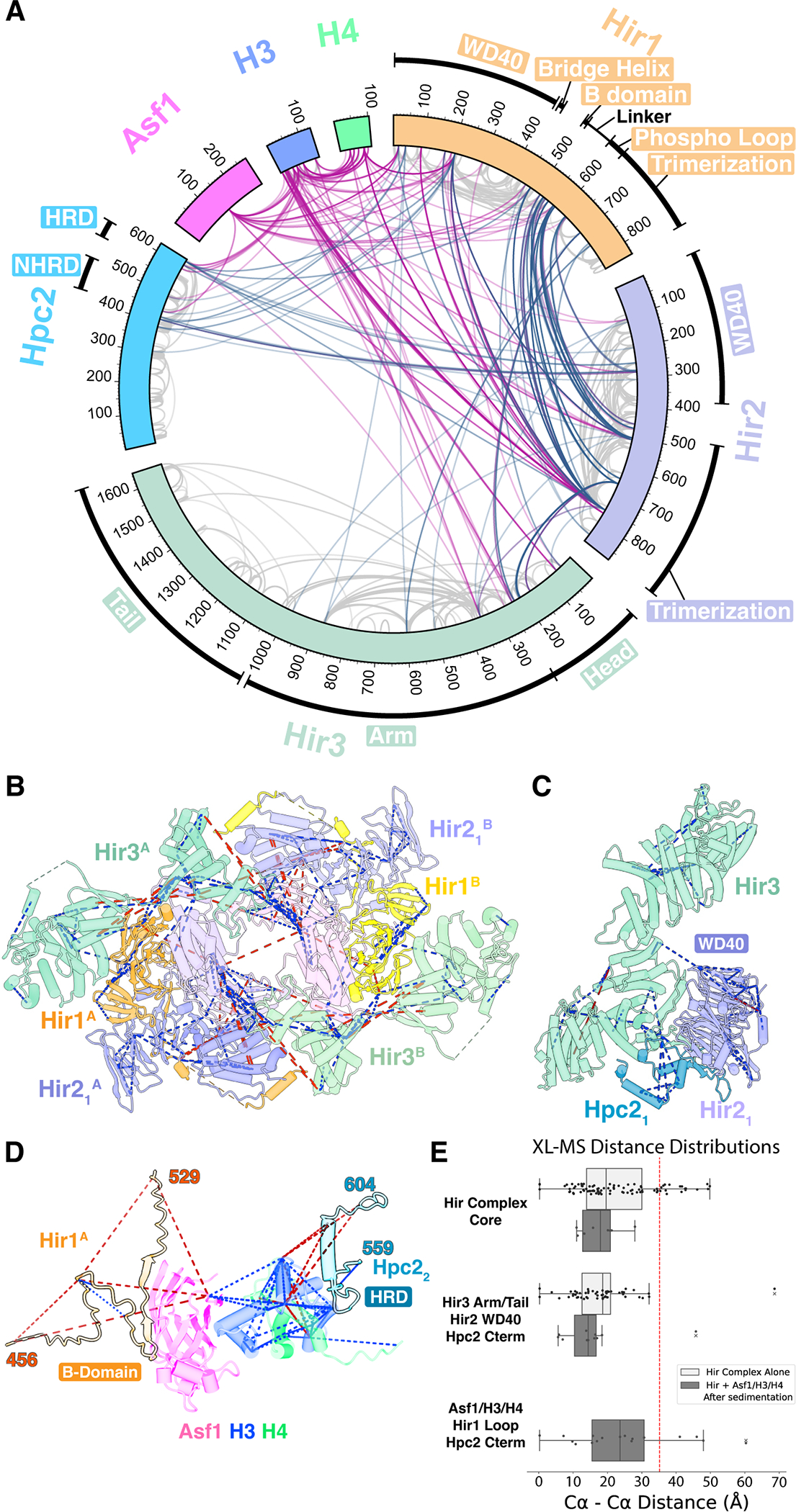
(A) Circular plot of unique cross-linked residue pairs for the Hir complex alone (steel blue) and the Hir complex-Asf1/H3/H4 (violet red). Intra-subunit unique residue pairs are indicated in gray.
(B-D) Cross-links are mapped on the central core (B), Hir3 (Tail)-Hir2(WD40)-Hpc2 (C), and Asf1/H3/H4 (blue, green, magenta) complexed with the Hir1 B-domain (light orange) and the Hpc2 HRD domain (indigo blue) (D). Blue and red dashed lines indicate cross-links with Cα-Cα distances of less than 35 Å and greater than 35 Å, respectively.
(E) Distance distribution plots for cross-links mapped in (B), (C), and (D) with vertical dotted line indicating a distance cutoff of 35 Å. Corresponding Cα–Cα distances were calculated to copy A and copy B for each XL, and only a shorter Cα–Cα distance was used to validate our cryo-EM model. Hir complex alone and Hir complex-Asf1/H3/H4 datasets are separately plotted.
Overall structure of the Hir complex reveals an arc-shaped roughly 2-fold symmetric dimer
A more detailed view of the entire arc-shaped dimer of the Hir complex, reveals that each copy is composed of Hir1, Hir2, Hir3, and Hpc2 in a 1:2:1:2 stoichiometry (Figure 1A–D and Movie S3). Within each copy, one Hir1 and two Hir2 (referred to as Hir21 and Hir22) form a heterotrimer through their C-terminal trimerization domains and together (copies A and B) form a dimer of trimers within the entire complex (Figure 1A and highlighted in right panel of 1B). The two Hir3 molecules (copies A and B) form arcs on the peripheries of the two Hir1/Hir2 trimers and are composed of three distinct domains (Figure 1A,E): an N-terminal “Head” domain that bridges between the two copies of the Hir1-Hir21-Hir22 heterotrimer core in the central core, while the “Arm” and “Tail” domains curve away from the central core with an outer dimension spanning ~25 nm. In each copy, the WD40 domains of Hir1 and Hir21 are located adjacent to the heterotrimer core and associated with Hpc2 (Figure 1F,G), forming a central “cavity” that could accommodate Asf1/H3/H4 (Movie S4).
Crosslinking mass spectrometry of the Hir complex validates the cryo-EM structure
The cryo-EM structure was further validated by XL-MS of Hir complex alone and with Asf1/H3/H4 (Data S1, S2). A total of 493 unique site-specific crosslinks were identified from the Hir complex without Asf1/H3/H4 (Figure 2A). Crosslinks corresponded to structures of the central core (Figure 2B) and Hir3-Hir2 (WD40)-Hpc2 (Figure 2C) with a violation rate (Cα–Cα distances longer than 35 Å) of 17.34 % and 2.89 %, respectively (Figure 2E). There was no significant change in the general crosslinking pattern of the Hir complex upon the addition of Asf1/H3/H4 (Figures 2A and S6). H3 and Asf1 made primary contacts with the Hpc2 HRD and the Hir1 B-domain with 4 and 3 crosslinks observed, respectively (Figure 2D). Crosslinks also corresponded to a homology model of yeast Asf1/H3/H4 bound to the Hpc2 HRD (residues 559–604) and the Hir1 B-domain (residues 456–529), derived from a combination of previous crystal structures of their human homologs18,30 (Figure 2D). In addition, the main body of H3 formed five crosslinks with residues of the Hir1 WD40 domain and two with the Hir1 phosphorylated loop46, which is localized primarily on the interior surface of the central cavity and supports the positioning of Asf1/H3/H4 within the Hir complex cavity (see below, Figure 7B).
Figure 7. Asymmetric binding of Asf1/H3/H4 to the central cavity and proposed model of histone deposition by the Hir complex.

(A) Cutaway views of the Hir complex, seen from bottom showing the central cavity surrounded by two copies of Hir1 (orange and yellow). Also shown is low-resolution density (gray) corresponding to one copy of Asf1/H3/H4 (magenta, blue, and green) and two copies of Hir1 WD40 domains revealed by focused refinement. Unmodeled densities (dashed circles) bridging between the Hir1 trimerization domains and Asf1/H3/H4 are due to the Hir1 acidic stretches (residues 570–620) in copy A and copy B.
(B) Zoomed view of Asf1/H3/H4 flanked by the Hir1 WD40 domains of copy A (orange) and copy B (yellow). Rotated by 90° relative to the lower panel of (A). Histone H3 (blue) is tethered to the Hir1 WD40 domain of copy B (yellow) through the Hpc2 HRD (indigo blue), while Asf1 (magenta) is tethered to the Hir1 bridge helix (residues 421–435) of copy A (orange) through the B-domain and a 40-residue linker. Unique cross-linked residue pairs identified by XL-MS are indicated by red dashed lines.
(C) Table summarizing the data from surface plasmon resonance measurements of interactions between histone substrates (analytes) and immobilized Hir complex (surface ligand). For yAsf1FL/hH3.3FL/hH4FL and yAsf1FL/hH3.1FL/hH4FL, binding curves were fit using a Heterogeneous Ligand model. For yeastAsf1 alone, a 1:1 binding model was used.
(D) SPR kinetic curves between histone substrates and immobilized Hir complex. See also Figure S16.
(E) A proposed model of histone deposition by the Hir complex. The DNA-binding patch is indicated by a blue dashed oval.
The linker regions of Hir1/Hir21/Hir22 follow distinct paths and contribute to stability of the 2:1 Hir2-Hir1 assembly.
The structure of the Hir1-Hir21-Hir22 heterotrimer core resembles the previous crystal structure of the human HIRA trimer counterpart29 (Figures S7A–C): β-strand subdomains in C-terminal domains of Hir1, Hir21 and Hir22 form a heterotrimer, while two copies of the C-terminal α-helical subdomain of Hir2 (not present in Hir1) stabilize the inter-subunit interaction (Figure S7). The heterotrimer core adopts a nearly exact three-fold symmetry with structures of each trimerization domain virtually identical to each other, except for the absence of the Hir1 α-helical subdomain. N-terminal to the trimerization domain is a linker region connecting to the WD40 domain, which follows distinct paths for each subunit. The path of the linker in Hir1 extends from the last structured C-terminal residue (Gly407) of the Hir1 WD40 domain to a “bridge” helix on the periphery of the Hir21 trimerization domain, follows a groove on the surface of the Hir21 trimerization domain towards the central cavity, and finally ends at the central core (Figures 3A,B and S7B). Two segments, one including the B-domain (residues 492–501) and the other including the phosphorylated loop46 (residues 571–619), respectively, are not observed at high resolution, but extended to bind the histone substrate in the cavity at low resolution (see below, Figure 7). For Hir2, ~72 residues (residues 433–504) and ~32 residues (residues 473–504) in Hir21 and Hir22 follow distinct paths and converge at the center of the heterotrimer and make extensive contacts with Hir1 including its linker region (Figure S7D). In support of this, the C-terminal half of the Hir1 linker region (residues ~501–570) forms extensive cross-links with the equivalent Hir2 linker region (residues ~434–496) (Figure 2A), and hir1 mutants containing deletion of ~30–40 residues of this liker region showed a strong cryptic transcription phenotype (see below, Fig. S15). This asymmetric interface including the linker regions likely contributes to stability of the 2:1 Hir2-Hir1 heterotrimer and/or the entire higher-order dimer.
Figure 3. Structure of the central core of the Hir complex at 2.96 Å resolution.

(A) Top view of the central core of the Hir complex. Boxed areas are enlarged in (C), (D), and (E), respectively.
(B) Same as (A) but viewed from side. Boxed area is enlarged in (F).
(C-F) Enlarged views of inter-subunit interactions of Hir22 (copy B)-Hir3 (copy B) (C), Hir1 (copy B)-Hir3 (copy B) (D), Hir22 (copy A)-Hir21(copy A)-Hir3 (copy B) (E), and Hir1 (copy A)-Hir3 (copy B) (F). Side chains involved in the inter-subunit interactions are shown. Conserved residues are labeled in bold.
The trimerization domains form a 2:1 Hir2-Hir1 heterotrimer.
In an effort to further explore the nature of the 2:1 Hir2-Hir1 stoichiometry critical in assembly of the higher-order structure, Hir1 (residues 599–840, termed Hir1CTD) and Hir2 (residues 471–875, termed Hir2CTD) were recombinantly expressed individually or co-expressed in and purified from E. coli (Figure S8A). While Hir2CTD was soluble in isolation, Hir1CTD was insoluble unless co-expressed with Hir2CTD (data not shown). Hir2CTD alone or the Hir1CTD / Hir2CTD complex were purified to monodispersity and subjected to sedimentation equilibrium analytical ultracentrifugation revealing that while Hir2CTD forms a dimeric species, Hir1CTD/ Hir2CTD forms a Hir1CTD(1)/ Hir2CTD (2) heterotrimer (Figures S8B and S8C) as observed in the cryo-EM structure and comparable to the homotrimer reported for human HIRA.
The Head module of Hir3 mediates a conserved higher-order dimer interface
Hir3, a representative of the TPR family of curved solenoids, is entirely α-helical and contains 70 antiparallel α-helices that are between 10 and 30 amino acid residues long (see below, Figure 5A,B). The Head module contains three canonical TPR motifs of antiparallel α-helices: α1- α2, α3- α4, and α5- α6, whereas the Arm and Tail modules have extra α-helices and β-hairpins, unlike typical TPR-containing proteins which have a continuous solenoid. The Arm and Tail modules are also both split into two N-terminal and C-terminal subdomains which are flexible relative to each other. In each copy (copy A and copy B), the Head and Arm modules wrap around the Hir1-Hir21-Hir22 heterotrimer core (Figure 3A), with the Head primarily in contact with Hir22 and Arm primarily in contact with Hir1 (Figure 3C,D). This relative positioning is consistent with previous biophysical analysis of CABIN1 that demonstrated the N-terminal ~700 residues are responsible for interacting with HIRA38. At the Hir22-Hir3 (Head) interface, the C-terminal hydrophobic face of a Hir22 helix (residues 749–764), absent from the previously reported crystal structure of the HIRA homotrimer core29, lies along a hydrophobic groove between the TPR repeats α3-α4 and α5-α6 of Hir3 (Figure 3C). At this hydrophobic interface, residues of Hir22 (Leu756, Phe759, and Ala760) and Hir3 (Val116, Val120, and Ile123) are highly conserved. In contrast, the Hir1-Hir3 (Arm) interface is less conserved but confers stability to the N-terminal subdomain of the Arm (N-term Arm) in the central core (Figure 3D). Notably, the Hir3 Head domain inserts between the two copies of the Hir1-Hir21-Hir22 heterotrimers within the core to bridge together the two Hir1-Hir21-Hir22 heterotrimers of the core (Figure 3E). Also contributing to this bridging interaction is the Hir1 linker region that wraps around the trimerization domain of Hir21 to insert a “bridge helix” into a hydrophobic groove between the TPR repeats α1-α2 and α3-α4 of Hir3 (Figure 3B,F).
Figure 5. Structure of Hir3 and conserved basic regions.

(A) Schematic diagram of the secondary structure of yeast Hir3. Canonical TPR motifs (TPR1–8) were identified by TPRpred58. The Head, Arm, and Tail domains are made up of α1-α9, α10-α41, and α42-α70, respectively. The Arm and Tail domains are further divided into N-terminal and C-terminal subdomains, respectively.
(B) Structure of yeast Hir3 with secondary structure annotations. Seen in two side views rotated 180° with respect to each other.
(C) The basic channel and the DNA-binding patch are indicated with electrostatic potential overlaid. Boxed area is enlarged in (D) while oval area is enlarged in (E).
(D) Enlarged view of the basic channel in (C) with side chains of conserved residues (W1280, Y1284, K1288, K1292, Y1328, K1336, and R1340).
(E) Enlarged view of the putative DNA-binding patch in (C) with side chains of conserved residues (K1482, K1483, R1486, R1485, K1558, and K1565).
The Hpc2 HRD associated with the Hir2 WD40 domain is occluded from histone binding, but confers stability to the Hir3 Tail
While the Head and N-term Arm modules of Hir3 were well resolved in the central core, the C-terminal subdomain of the Arm (C-term Arm) and the entire Tail (together with the WD40 region of Hir21 and NHRD/HRD of Hpc21, see below) were more poorly resolved and therefore subjected to focused classification (Figures 4A,B, and S3C–E). The resulting 6.8 Å resolution map (Map 2, EMD-40029) resolved all α-helices of the Hir3 Tail module as cylindrical densities, except for the five C-terminal helices (α66-α70), which are apparently mobile relative to the rest of the Tail module (Movie S2). The map revealed density for the Hir21 WD40 β-propeller comprising seven blades proximal to its trimerization domain in the central core. Between the Hir3 Tail domain and the Hir21 WD40 domain, we observed additional cryo-EM density that we assigned to the C-terminal region of Hpc2 harboring the NHRD and HRD regions (Figures 4B,C). This positioning is consistent with the previously characterized interaction and stabilization of the WD40 region of HIRA with the NHRD region of UBN137. The model is in good agreement with eight observed crosslinks between the Hir2 WD40 domain and Hpc2, one crosslink between the Hir2 WD40 domain and the Hir3 Tail, and two crosslinks between the Hpc2 and the Hir3 Tail (Figure 2C).
Figure 4. Structures of the Hir3(Arm and Tail)-Hir21(WD40)-Hpc2 subcomplex.

(A) Overall side view of the Hir complex, overlaid with focused-refined map for Hir3(C-term Arm and Tail)-Hir21(WD40)-Hpc2.
(B) Enlarged area of the focused-refined map in (B) but rotated by 90° (left) relative to (A). Hir3 Arm and Tail modules are in green. The Hir21 WD40 domain is in light purple. The Hpc2 is in indigo blue.
(C) Enlarged view of the interface between Hir21 WD40 (light purple), Hir3 Tail (light green), Hpc2 NHRD (indigo blue) and Hpc2 HRD (cyan). Viewed roughly in the same orientation as (B).
(D) Secondary structure schematics for (C).
The Hir3(Tail)-Hir2(WD40)-Hpc2 model reveled two non-canonical features of the Hir3 TPR repeats in contact with the Hir2 WD40 domain (Figure 4C): first, α42 (residues 1034–1048) is almost vertical to the other α-helices of the Hir3 TPR repeats and lies on the bottom surface of the WD40 propeller. Second, a β-hairpin (β3-β4; residues 1225–1239) projects away from the main body of the TPR repeats and constitutes a “latch” on the side surface of the WD40 propeller. Two key segments of Hpc2 were identified on the Hir21 WD40 propeller: residues 424–508 and residues 564–604 (Figures 4C, D). Between these segments, the central ~66 residues (residues 509–563) were not observed (Figure 4D). The first segment corresponds almost exactly to the NHRD previously defined by biochemical analysis to interact with the HIRA WD40 domain37: residues 451–455 and residues 473–477 form ß-strands (β2 and β3) along the outer edge of blades 7 and 6 of the Hir21 WD40 propeller, respectively (Figure 4D). A following α-helix (α1; residues 477–485) folds back onto β1 and β2 to stabilize the inter-chain β-sheet formation of blade 7 of the WD40 propeller by forming a hydrophobic core of its own. The second segment corresponds almost exactly to the HRD previously defined by biochemical and structural analyses to interact with histone H3: the histone binding loop (residues 565–577 including a conserved Phe575)30, previously shown to be in direct contact with histone H3 helices, lies along a hydrophobic groove between the antiparallel TPR α-helical pair α43 and α52. Following α-helix 3 (α3; residues 578–589), β-strand 4 (β4; residues 595–598), and the intervening hydrophobic loop (residues 590–596) which contains a conserved GFFV motif (Gly593, Phe594, Phe595, and Val596) are stabilized in an extended form along the Hir3 latch β-hairpin (Figures 4C, D). Importantly, the association of the Hir2-associated Hpc2 HRD region with the Hir3 β-hairpin apparently occludes the histone binding loop from histone engagement. This observation is further supported by the abundant crosslinks formed between Asf1/H3/H4 and the Hir1 WD40 domain, while only a few crosslinks formed between Asf1/H3/H4 and the Hir2 WD40 domain (Figure 2A).
Two conserved basic regions are present on the Hir3 Tail
Using the atomic model of Hir3, we identified two conserved positively-charged regions on the C-terminal subdomain of the Hir3 Tail (C-term Tail) that might be important for histone deposition or recruitment of the complex to its genomic targets. The first is a channel flanked by positively charged surfaces within four helices (α53, α54, α59, α61) on the concave surface of the arc-shaped Hir3 (Figure 5C,D). The channel is covered by the N-terminal subdomain (N-term Tail) and effectively forms a tunnel of 8–12 Å in diameter and about 30 Å in length. Given the size and charge properties of this channel, we hypothesized that it might be used to engage single stranded RNA or an acidic polypeptide. The size of the channel is not sufficient to accommodate double-stranded DNA, which would be too wide (Figure 5C,D). The interior of the channel is composed of very highly conserved Lysine and Arginine residues (Lys1288, Lys1292, Lys1336, and Arg1340), and conserved aromatic residues (Trp1280, Tyr1284, and Tyr1328) (Figure 5D).
Distinct from the basic channel is a conserved patch of positively charged residues on the concave surface of the distal C-terminal end of the Hir3 Tail (Figure 5E). The patch is formed on three helices (α62, α68, α70), comprising six conserved Lysine/Arginine residues (Lys1482, Lys1483, Arg1485, Arg1486, Lys1558, Lys1565 in S. cerevisiae), the last three of which are conserved in humans. This patch is located adjacent to the density attributed to Asf1/H3/H4 in the cavity (see below, Figure 7), and may therefore serve a role in positioning double-stranded DNA near the histones for subsequent histone deposition (see Discussion).
To directly evaluate the nucleic acid binding potential of Hir3 in solution, we utilized an orthologous Hir3 protein from Chaetomium thermophilum (ctHir3), which could be readily overexpressed recombinantly in bacteria in the absence of the other complex subunits. When expressed and purified in isolation, ctHir3 exists as a mixture of monomers and dimers (Figure S9A,B). We employed a fluorescence polarization binding assay to investigate the ability of ctHir3 to bind to a variety of fluorescein-labeled nucleic acid probes and found that ctHir3 binds single stranded RNA and DNA with apparent dissociation constants of 292 nM and 531 nM, respectively (Figure S11E). In the same titration range, ctHir3 shows much weaker binding to double stranded DNA (a dissociation constant could not be calculated). These data suggest that ctHir3 binds preferentially to single stranded nucleic acids in isolation- likely through electrostatic interactions within the basic channel. Double stranded DNA binding capacity of the Hir complex may be strengthened within its higher-order dimeric structure.
To obtain a higher resolution view of the conserved basic surface of ctHir3, we determined the single particle cryo-EM structure. Consistent with mass photometry data, the isolated ctHir3 formed a mixture of monomeric and dimeric particles, although dimeric particles were better resolved and produced a cryo-EM map to an overall resolution of 3.9 Å (Figure S10). Overall, the ctHir3 structure alone adopts a similar elongated shape as ScHir3 with some notable differences. Unlike ScHir3, the Head and N-term Arm modules of ctHir3 are poorly resolved, likely due to the absence of the stabilizing HIRA trimerization domains. Like ScHir3, the ctHir3 C-term Arm and N-terminal Tail modules were well resolved and superimposed well with the ScHir3 Arm domain and N-terminal Tail (Figure S11 and S12), suggesting that other subunits of the complex don’t significantly alter the C-term Arm and N-terminal Tail module structure. The ctHir3 Tail domain was better resolved than the Tail domain of ScHir3 and notably showed comparable channel/patch containing many conserved basic residues also present in ScHir3 (Figure S11). Of note, the ctHir3 C-term Tail shifts ~9 Å compared to that in the ScHir3 to form a narrower basic tunnel (5–6 Å in diameter) (Figure S12A), suggesting its plasticity and flexibility. The basic channel of ctHir3 contained a strong cryo-EM density that could represents a reducing agent Tris(2-ChloroEthyl) Phosphate (TCEP) (Figure S11A, inset), which makes direct contact with numerous basic residues within the channel. It is conceivable the basic channel generates a potent electric field that facilitates the accommodation of multivalent anions such as TCEP and phosphate. ctHir3 contains ~400 additional residues C-terminal to the Tail module that are not conserved in ScHir3 and are poorly resolved in the cryo-EM map.
To further evaluate the nucleic acid binding properties of the basic channel of ctHir3, we mutated two of the conserved basic residues of the basic channel- Lys1358 and Lys1362 (ScLys1288, ScLys1292) to alanine and repeated the fluorescence polarization binding assay (Figure S11E). The mutant ctHir3 eluted similarly to wild type by size exclusion chromatography (Figure S9C), but showed a reduced capacity to bind to ssRNA, ssDNA, and dsDNA probes (Figure S11E). No dissociation constant could be determined for any of these nucleic acid substrates, suggesting that residues Lys1358 and Lys1362 contribute to the electrostatic binding of nucleic acids to ctHir3.
Mutations of the double-stranded DNA-binding patch confer a cryptic transcription phenotype
To evaluate the biological significance of the conserved charged surfaces on the Hir3 Tail module, we used a reporter assay that allows for detection of aberrant transcription initiation from within the coding region of FLO847,48(Figure 6A). In this reporter, the region of FLO8 downstream of the cryptic transcription start site (TATA +1626) was replaced with the HIS3 coding sequence, while the FLO8 promoter was replaced with the GAL1 promoter to regulate full-length FLO8-HIS3 transcription by different carbon sources. HIS3 is out of frame with the FLO8 transcript, such that cryptic transcription initiated from an internal TATA at +1626, by a defective transcription-coupled chromatin assembly by assembly factors such as subunits of the Hir complex, allows growth in the absence of histidine. Using this reporter assay, we characterized two DNA-binding patch mutants (4x and 6x patch mutants) and two basic channel mutants (2x and 7x channel mutants) (Figures 6B and S14). Also, we evaluated complex integrity of these mutants by a pull-down assay by means of a Flag-tag on Hir1 (Figure 6C). The two double-stranded DNA-binding patch mutants (4x and 6x patch mutants) remained intact in the pull-down assay and conferred a strong His+ phenotype upon transcription activation (+2% galactose). By contrast, the 2x channel mutant carrying K1288A and K1292A, defective in single-stranded nucleic acid binding in ctHir3 (Figure S11E), remained intact in the pull-down assay, but conferred no His+ phenotype. The other channel mutant (7x channel mutant) did not show pull-down activity, suggesting a defect in preserving complex integrity (see Discussion). Based on these observations, we conclude that the double stranded DNA-binding patch, but not the basic channel, is required for transcription-coupled chromatin assembly, and thus the two conserved charged surfaces may be functionally distinct.
Figure 6. Cryptic-transcription reporter assay using Hir3 putative nucleic acid binding mutants.

(A) Schematic of cryptic promoter reporter assay. (B) Cryptic transcription phenotypes of Hir3 nucleic acid binding mutant alleles. Mutant alleles were spotted in a 10-fold dilution series on the indicated medium for three days.
A low copy number plasmid pRSII315 carrying hir3 mutant alleles was transformed into the hir3Δ strain. As a control, the empty vector was transformed into the parent strain (Background) or the hir3Δ strain (Knockout). From top to bottom, 1) Background, empty vector; 2) Wildtype, HIR3 wild type; 3) ssDNA/RNA-channel 2x mutant, K1288A, K1292A; 4) ssDNA/RNA-channel 7x mutant, W1280A, Y1284A, K1288A, K1292A, Y1328A, K1336A, R1340A; 5) DNA-patch 4x mutant, K1482A, K1483A, R1485A, R1486A; 6) DNA-patch 6x mutant, K1482A, K1483A, R1485A, R1486A, K1558A, K1565A; 7) Knockout, empty vector. (C) Pull-down assay to test complex stability of hir3 alleles. Same strains as (B) were grown in SC-Leu media, pulled down by a Flag-tag on Hir1, and analyzed by SDS-PAGE.
One Asf1/H3/H4 complex is engaged in the central cavity
After focused refinement of the central cavity including the Hir1 WD40 domain (Map 3, EMD-40030), additional cryo-EM density was observed at 8–12 Å resolution between the two copies of the Hir1(WD40)-Hpc2, which we modeled as one Asf1/H3/H4 complex in a manner consistent with XL-MS (Figures 7A,B and S5). To model this section of the cryo-EM map, we generated an AlphaFold model of the Hir1(WD40)-Hpc2, which was almost identical to the Hir2(WD40)-Hpc2 (Figure 4C) and fit this as a rigid body into the donut-shape density corresponding to the WD40 propeller of Hir1 (Figure 7A). The fitting was validated by the proximity of the last structured C-terminal residue (Gly407) of the WD40 domain to the last N-terminal residue (Lys410) of the Hir1 linker of the central core (Figure S5B). In the resulting model, β3 C-terminal to the histone-binding loop of the Hpc2 HRD is located inside the cavity (Figure 7B). Because they are ~70 Å apart from each other and approximately related by the pseudo-dyad axis of the Hir complex, this is a suitable position to accommodate a histone (H3-H4)2 tetramer. The density we observed in the center of the cavity, however, appeared asymmetric and more consistent with a single copy of Asf1/H3/H4 than with a (H3-H4)2 tetramer (Figures 7A and S5), consistent with our iBAQ analysis (Figure S2E). Consequently, a homology model of Asf1/H3/H4 bound to the Hpc2 histone-binding loop (residues 565–577 from copy B) and the Hir1 B-domain (residues 477–500 from copy A) (Figure 2D) was manually fitted into the density, followed by rigid-body refinement, and was validated by crosslinks (Figure 7B). After the fitting, there are two residual densities attributed to the phosphorylated loops 46 of Hir1 (residues 570–620) extending from the Hir1 trimerization domains to Asf1/H3/H4 (Figures 7A, S5B and Movie S1). There is no density attributable to a second copy of Asf1/H3/H4 in the cavity. In the cavity, there is not sufficient space to accommodate two copies of Asf1/H3/H4. Specifically, if one copy of Asf1/H3/H4 is engaged in the cavity through interactions with the Hpc2 HRD (copy B) and the Hir1 B-domain (copy A) (Figure 7A–B), introducing a second copy would result in a clash with the first copy.
To further evaluate the placement of Asf1/H3/H4 in the Hir complex cavity we probed the binding properties of Asf1 with and without histones to the Hir complex using real-time binding assays with Surface Plasmon Resonance (SPR) (Figures 7C, D). The HIR complex was immobilized on a sensor chip via standard amine coupling, and its interaction with either full-length yeastAsf1 alone (yAsf1FL), yeastAsf1/humanH3.3/humanH4 (yAsf1FL/hH3.3FL/hH4FL), or yeastAsf1/humanH3.1/humanH4 (yAsf1FL/hH3.1FL/hH4FL) was characterized under flow (Figures 7C–D and S16). Asf1 alone displayed a fast association and dissociation curve, with a relatively weak affinity (KD = 132 nM). Addition of 3 μM RNA, but not polyglutamic acid, as a competitor efficiently competed off Asf1 binding (Figure S17), suggesting that Asf1 binds to the Hir complex mainly through interaction between the C-terminal acidic stretch and the Hir3 basic channel. By contrast, the kinetic curve of yAsf1FL/hH3.3FL/hH4FL represented a heterogeneous ligand binding mode comprising two independent bindings: one exhibited slow association and dissociation with a relatively strong affinity (KD2 = 18 nM), while the other demonstrated fast association and dissociation with a relatively weak affinity (KD1 = 196 nM), comparable to Asf1 alone. The strong affinity was significantly reduced (KD2 = 48 nM) when H3.3 was replaced by H3.1. These findings are consistent with that the relatively strong affinity state corresponds to the binding of Asf1/H3/H4 to the cavity including the Hpc2 HRD and the B-domain, while the weaker affinity state corresponds to the binding of another Asf1/H3/H4 to the Hir3 basic channel through the Asf1 C-terminal acidic stretch (Figure 7E). Due to the weaker affinity, the second copy of Asf1/H3/H4 was likely dissociated from the complex during the sedimentation process for cryo-EM, XL-MS, and iBAQ analyses. This asymmetric heterogeneous binding of Asf1/H3/H4 likely explains why one copy of Hir3 (copy A) appears more ordered than the other in our cryo-EM structure (Figure S3) and is directly linked to the orientation of Asf1/H3/H4 within the cavity (Figures 7E and S5). Together, our structural and biochemical data suggest that the Hir complex/Asf1/H3/H4 represents a stable intermediate in the dynamic process of histone deposition, amenable to subsequent deposition of the (H3-H4)2 tetramer on DNA (Figure 7E and Movie S4).
Discussion
Structural studies of the intact HIRA complex have been hampered by difficulties in preparing the complex in a form that is suitable for X-ray crystallographic or cryo-EM analysis. Therefore, structural analysis has been limited to X-ray crystallography of subcomplexes18,29,30, and the mechanism of histone deposition has remained unresolved. We developed a procedure for isolating the Hir complex, the yeast ortholog of the metazoan HIRA complex, in a form amenable to structural studies, and determined the structure of the entire complex bound to an Asf1/H3/H4 trimer at 2.9–6.9 Å resolution by cryo-EM and extensive XL-MS analyses. The structure reveals an arc-shaped roughly 2-fold symmetric dimer of the Hir complex and maps the relative positions of two critical components of functional significance for histone deposition, the Hpc2 HRD and the Hir1 B-domain. Two copies of Hpc2 HRD related by the pseudo-dyad axis of the Hir complex are located on the interior of the central cavity in a configuration compatible with assembly of a histone (H3/H4)2 tetramer, although one HRD in the cavity is blocked from histone binding and thus the complex represents an intermediate in which only one Asf1/H3/H4 is bound. Our observed Hir complex assembly correlates with the biological assembly of the endogenous HIRA complex pulled-down from human cells with tagged H3.3, in which the majority of purified HIRA complex was reported to associate with ASF1a/H3.3/H4 and not an (H3.3/H4)2 tetramer19, although our reporter assay indicates the presence of Asf1-independent pathway for transcription-coupled chromatin assembly (see below). In addition, our cryo-EM map resolved two copies of the phosphorylated loop of Hir1 (residues 570–620), extending from the Hir1 trimerization domains to Asf1/H3/H4 (Figures 7A and S5B). Although deletion of the phosphorylated loop of Hir1 did not have on impact on transcription-coupled chromatin assembly in our reporter assay (Figure S15), it is noted a phosphomimic form of the phosphorylated loop of HIRA has been reported to reduce H3.3 deposition and suppress the activation of muscle genes in myotubes46.
In each copy of the higher-order dimer, the Hir1-Hir21-Hir22 heterotrimer core, with a nearly exact three-fold symmetry, accommodates only one copy of Hir3 primarily through the C-terminal α-helical subdomain of Hir22 (Figure 3C). The conserved hydrophobic Hir1 linker region on the periphery of the Hir21 trimerization domain blocks the equivalent binding site on Hir21, while directly interacting with the Hir3 Head in the other copy at the higher-order dimer interface (Figure 3B,F). The requirement for the Hir1 linker region for the unbalanced stoichiometry and the higher-order dimerization may explain previous analytical ultracentrifugation experiments revealing a HIRA trimerization domain–CABIN1 stoichiometry of 3:2 when the linker region of HIRA is omitted29. Higher-order dimerization of H3/H4-specific chaperone complexes has been observed but is typically driven by homodimerization domains within chaperone subunits, as in HJURP49 and Rtt10650, or is induced by DNA-binding, as in CAF-151. The constitutively dimeric state of the Hir complex mediated by extensive interactions among all subunits emphasizes the importance of maintaining the integrity of the entire endogenous complex in overcoming the limitations of previous structural studies.
The Hir complex also contains two distinct basic regions on the surface of the Hir3 Tail domain (Figure 5C–E). The basic channel is confined between two subdomains of the Tail to form a tunnel characterized by highly conserved Lysine, Arginine, and Tyrosine residues. While AlphaFold calculations predicted ~11 residues (residues 181–191) of the Asf1 acidic stretch stabilized as a turn within the basic channel (Figure S18), the strong electric field could attract other negatively charged molecules including RNA and multivalent anions such as TCEP and phosphate in either a specific or nonspecific manner. We find that RNA (but not TCEP, phosphate, or polyglutamic acid) competes with Asf1 for binding the basic channel at least in vitro (Figure S17). Notably, previous in vivo RNA-protein crosslinking analysis in mouse embryonic stem cells identified several RNA-crosslinked sites on Cabin152; however, whether specific RNAs bind to the channel and how they function in vivo remain to be determined.
Distinct from the basic channel is the putative double-stranded DNA binding patch, which may accommodate ~10–15 bp DNA double helix on the distal C-terminal Tail at the entrance of the central cavity (Figure 5C–E). The presence of the putative DNA-binding patch aligns with a DNA path identified by a computational prediction with RoseTTAFoldNA53 (Figure S13). Strikingly, when the path is extended with B-form DNA towards the cavity, it follows a ~20 nm (~60 bp) path just beneath Asf1/H3/H4 at the pseudo-dyad axis of the Hir complex and reaches the DNA binding patch of the other copy of the dimer. The DNA is free of protein contacts in the middle, thus providing a platform for histone deposition. Although strong double-stranded DNA-binding activity was not detected by our fluorescence polarization binding assays using recombinant ctHir3 (Figure S11E), DNA binding may be promoted within the crowded nuclear landscape or the higher-order dimer of the entire Hir complex. Considering that previous in vitro assays reconstituted from purified factors demonstrate that the Hir complex itself, along with Asf1, is capable of supporting histone deposition activity41, weak DNA binding activity through the DNA-binding patch may be sufficient to support DNA positioned with respect to the cavity and may be suitable for histone handoff from the Hir complex to DNA. DNA binding as a prerequisite for histone deposition by the Caf1 histone chaperone complex has also been proposed51,54–56. Indeed, recent cryo-EM studies on the Caf1 complex demonstrates that DNA-binding nucleates the dimerization of two Caf1/H3/H4 complexes in appropriate position to deposit a (H3/H4)2 heterotetramer onto DNA. In contrast, the Hir complex structure is a preformed dimeric complex and implies a more synergistic relationship within the dimer in which histones and DNA are simply poised for the energetically favorable process of tetrasome formation to occur. In addition, unlike in the Caf1 complex, Asf1 appears to play a more active role in the histone deposition mechanism of the Hir complex, particularly in recruiting Asf1/H3/H4 through its C-terminal acidic stretch. Furthermore, in light of previous in vitro studies demonstrating the enhancement of Asf1 affinity to H3/H4 by its C-terminal stretch 57, the Hir complex may aid in dissociation of Asf1 from the H3/H4 dimer by sequestering the acidic stretch within the basic tunnel away from the cavity (Figure 7E). However, in contrast to the DNA-binding patch mutations exhibiting a robust cryptic transcription phenotype, all the basic channel mutations we examined did not have impact on transcription-coupled chromatin assembly in our reporter assay, except for the 7x mutant, which compromised complex integrity (Figures 6 and S14). This suggests that the requirement for Asf1 can be circumvented for transcription-coupled chromatin assembly, akin to the human system32, as further supported by the lack of discernible phenotypes observed for Hir1 B-domain mutations (Figure S15). Similarly, Asf1-dependent and -independent pathways are known for the Caf1 histone chaperone complex51,54,55.
Notably, a significant portion of the Hir complex plays a strictly scaffolding function to present a site for histone deposition activity within the central cavity. The exterior scaffold is largely two-fold symmetric and relatively well resolved and likely interacts and coordinates with different nuclear factors to deposit histones during different DNA templated events such as transcription and DNA repair. The interior regions framing the cavity- particularly the WD40 regions of Hir1, the C-terminal region of Hir3, and Hpc2- have a less strictly defined orientation. The interior of the cavity is asymmetric and contains only one bound ASF1/H3/H4 complex, with another presumed ASF1/H3/H4 complex waiting outside of the cavity (Figure 7E); this observation may highlight the ability of the complex to perform the dynamic, processive activity of assembling and depositing histone tetramers in the nucleus. We posit that the second copy may be only transiently engaged in the cavity to form a histone (H3/H4)2 tetramer, and the resulting Hpc2-assisted histone tetramer is energetically unfavorable and short lived prior to capture by DNA, which is positioned by the Hir3 Tail domains. Wrapping the DNA around the histone (H3/H4)2 tetramer facilitates dissociation of the HRDs from histones as proposed30, and the resulting (H3-H4)2 tetrasome is released (Figure 7E). While our model of HIRA/Hir-mediated histone deposition is consistent with our data, the molecular details for how double-stranded DNA binds the Hir complex- including a potential role for single-stranded DNA/RNA binding in histone deposition- await further studies.
Limitations of the Study
While the central core of the Hir complex was resolved at high resolution, peripheral regions such as the Hir1 WD40–Hpc2 HRD and the Hir3 C-terminal Tail domain were observed at significantly lower resolution. The lower resolution in these areas can be attributed to their variable contact with the central core. Such variability in the position and orientation at the periphery is likely required for accommodating histone substrates, allowing them to adjust within the cavity upon histone tetramer formation and subsequent DNA deposition, all while maintaining essential interactions. In addition, while we find that RNA or ssDNA binds to the Hir3 basic channel and competes with Asf1 in vitro, how such binding functions in vivo to regulate Hir-mediated histone deposition activity remains to be determined.
STAR Methods
Resource availability
Lead contact
Further information and requests for reagents should be directed to and will be fulfilled by the lead contact, Kenji Murakami (kenjim@pennmedicine.upenn.edu).
Materials Availability
Plasmids and strains generated in this study are available upon request from lead contact with a completed Materials Transfer Agreement.
Data and Code availability
The cryo-EM density maps of the Hir complex bound to Asf1/H3/H4 were deposited in the Electron Microscopy Data Bank (EMDB) (Map 1: EMD-40006, Map 2: EMD-40029, Map 3: EMD-40030, Composite Map: EMD-40037) and associated atomic coordinates were deposited in the Protein Data Bank (PDB IDs: 8GHL, 8GHA, 8GHM, and 8GHN respectively). The cryo-EM density map of ctHir3 was deposited in EMD-B (Map 4: EMD40078) and associated atomic coordinate was deposited in PDB (8GIX). Bottom-up proteomics and crosslinking mass spectrometry raw data, protein databases, and search results were deposited in the Proteomics Identifications Database (PRIDE) Archive (PXD040705). Raw pictures of SDS-PAGE gels, plates and representative micrographs were deposited in Mendeley Data: http://dx.doi.org/10.17632/7fbn428nsp.1
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Bacterial strains
NEB® 5-alpha competent cells were used for molecular cloning, amplification of the recombinant plasmids and mutagenesis. Rosetta 2 (DE3) competent cells were used for bacterial expression of yeast full length H3, H4, and Asf1. BL21-Gold competent cells were used for bacterial expression of yeast N-terminal truncated Hir1 and Hir2, full length of human H3.1, H3.3 and H4 and ctHir3 wildtype and mutant.
Yeast strains
The protease deficient strain CB010 (MATa pep4::HIS3 prb1::LEU2 prc::HISG can1 ade2 trp1 ura3 his3 leu2–3,112 cir-o GAL+ RAF+ SUC+) cells were used for genomic tagging and Hir complex purification. CKY826 (kanMX-GAL1pr-flo8::HIS3 his3Δ200 leu2Δ1 lys2–128∂ trp1Δ63 ura3–52) cells were used for CRISPR-Cas9 system and FY2713 (MATa kanMX-GAL1pr-flo8::HIS3 his3Δ200 leu2Δ1 lys2–128∂ trp1Δ63 ura3–52) cells were used for plasmid system for cryptic transcription reporter assay.
Method details
Plasmid and Strain construction
To create a TAP-tagged Hir2 strain, we used a PCR-mediated strategy for genomic tagging in the protease deficient strain CB010 (MATa pep4::HIS3 prb1::LEU2 prc::HISG can1 ade2 trp1 ura3 his3 leu2–3,112 cir-o GAL+ RAF+ SUC+). The C-terminal Tobacco Etch Virus (TEV)/protein A tagging cassette was amplified from the pCEMM-CTAP(KanMX) plasmid with primers complementary to the C terminus of HIR2. Yeast were transformed using a standard lithium acetate transformation and transformants were plated on synthetic complete media with G418. Positive clones were restreaked on YPAD (yeast extract, peptone, adenine, and glucose) plates with 200ug/mL of G418 and incorporation at the HIR2 locus was verified by genomic PCR. The resulting CB010 Hir2-TAP (MATa pep4::HIS3 prb1::LEU2 prc::HISG can1 ade2 trp1 ura3 his3 leu2–3,112 cir-o HIR2 TAP KanMX) was used for all Hir complex purifications.
The cDNA encoding Hir1(599–840) was PCR amplified from Saccharomyces cerevisiae genomic DNA (Sigma-Aldrich) with primers 5’-CGCGAATTCACGAGCAACAGTATTGAC-3’ (EcoRI site underlined) and 5’-GCGCTCGAGTTATTTATATAACGACCAACA-3’ (XhoI site underlined). The resulting PCR product was ligated into the EcoRI/XhoI sites of custom engineered pRSFduet-1 (Novagen) Escherichia coli expression vector harboring a N-terminal GST tag that was removable by TEV protease cleavage. The cDNA encoding Hir2(471–875) was PCR amplified from Saccharomyces cerevisiae genomic DNA with primers 5’-CGCGGATCCCAGAAAAAAGAGCTACAG-3’ (BamHI site underlined) and 5’-GCGCTCGAGTTAAGATATTATATTCATTTC-3’ (XhoI site underlined). The resulting PCR product was ligated into the BamHI/XhoI sites of a custom engineered pETduet-1 (Novagen) Escherichia coli expression vector harboring an N-terminal 6xHis tag that is removable by TEV protease cleavage.
For ctHir3, a codon optimized gene sequence for recombinant full length ctHir3 (CTHT_0035880) expression in E. coli was synthesized (Bio Basic) and ligated between NotI and PacI sites in a custom-engineered pET-Duet-1 (Novagen) E. coli expression vector carrying an N-terminal 6xHis tag that is removable by TEV protease cleavage. The plasmid encoding ctHir3_K1358AK1362A was generated by site directed mutagenesis using primers synthesized by IDT and In-Fusion Snap Assembly Mix (Takara).
Plasmid construction of native Hir3 expression vector and its nucleic acid binding mutants
The DNA encoding Hir3 with flanking regions from −478 nt to +457 nt was PCR amplified from Saccharomyces cerevisiae genomic DNA with primers 5’-GTCTCTCGAGGACATGATAGAACTGTCATATATCTTAGAG-3’ (XhoI site underlined) and 5’-TCGGCGGCCGCCAATTAAATCATCCAAAGGCAACGTTTTAGT-3’ (NotI site underlined). The resulting PCR product was ligated into the XhoI/NotI sites of pRSII315 Saccharomyces cerevisiae shuttle vector. The following site-directed Hir3 mutants were generated by GenScript: 1) Hir3 RNA-channel 2x mutant (K1288A, K1292A), 2) Hir3 RNA-channel 7x mutant (W1280A, Y1284A, K1288A, K1292A, Y1328A, K1336A, R1340A), 3) Hir3 DNA-patch 4x mutant (K1482A, K1483A, R1485A, R1486A), 4) Hir3 DNA-patch 6x mutant (K1482A, K1483A, R1485A, R1486A, K1558A, K1565A).
Protein purification
Endogenous Hir complex was purified from CB010 Hir2 TAP as follows. Genomically tagged yeast were grown in 100 liters of YPAD media to an OD600 of 15. Cells were harvested, washed in water, and resuspended in lysis buffer (40 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES) pH 7.6, 10% glycerol, 500 mM NaCl) with protease inhibitors prior to freezing. Cells were lysed by bead beating in lysis buffer with 2 mM DTT (Dithiothreitol), 0.1% Tween-20, and protease inhibitors, and lysis was clarified by centrifugation. Following centrifugation, the clarified lysate was loaded onto an IgG sepharose column and washed with 20 column volumes of wash buffer (40 mM HEPES pH 7.6, 2 mM DTT, 300mM Ammonium Sulfate, 10% glycerol, 500 mM NaCl and protease inhibitors) and then equilibrated with 10 column volumes of cleavage buffer (40 mM HEPES pH 7.6, 2 mM DTT, 10% glycerol, 500 mM Potassium Acetate, 0.05% 3-(decyldimethyl-ammonio)-propanesulfonate (3DS)). The Hir complex was cleaved off the IgG Sepharose by treatment with TEV protease for 15 hours and eluted off the column with cleavage buffer. Fractions containing the Hir complex were concentrated by spin concentrator and then loaded onto a HiTrap Q HP (GE Life Science/Cytiva). Hir complex was eluted by a linear salt gradient of buffer A (20 mM HEPES pH 7.6, 2 mM DTT, 250 mM potassium acetate, 10% glycerol, 0.05% 3DS) to buffer B (20 mM HEPES pH 7.6, 2 mM DTT, 1.2 M potassium acetate, 10% glycerol, 0.05% 3DS). Peak fractions containing Hir complex were pooled, concentrated and flash frozen for later use.
Full length yeast H3/H4 were purified recombinantly from E. coli as previously described 59. H3 and His-tagged H4 proteins were overexpressed separately into inclusion bodies in BL21 Rosetta (DE3) (Fisher Scientific). Histone transformed BL21 Rosetta (DE3) cells were grown in LB medium at 37 °C until they reached an OD600 of 0.6 at which point protein expression was induced by addition of isopropyl 1-thio-β-D-galactopyranoside (IPTG) to a final concentration of 0.6 mM for 4 hours at 37 °C. To purify H3 and H4, cells were lysed with sonication in lysis buffer (50 mM Tris pH 7.5, 100 mM NaCl, 1 mM Ethylenediaminetetraacetic Acid (EDTA) and 1 mM BME (2-Mercaptoethanol)). Lysate was clarified with centrifugation for 30 minutes at 15k RPM. The resulting pellet was washed with first 20 mL, then 50 mL of lysis buffer with 1%Triton x-100 (v/v) clarification of the solution between wash steps by centrifugation for 15 minutes at 15k RPM. The final wash step was performed in lysis buffer without detergent, with the resulting pellet representing the insoluble fraction containing H3 and His-tagged H4. H3 insoluble pellets were resuspended in denaturing buffer (20 mM Tris pH 7.5, 1 mM EDTA, 6 M guanidine, 100 mM NaCl, 1 mM DTT) while His-tagged H4 denaturing buffer was supplemented with 20mM imidazole. Inclusion bodies were nutated in denaturing resuspension buffer at 4°C for 2 hours, then insoluble aggregates were pelleted by centrifugation at 30,000×g for 30 minutes. Denatured H3 was purified by ion exchange chromatography by loading the soluble supernatant onto HiTrap SP HP column (GE Healthcare) equilibrated in denaturing chromatography buffer (10 mM Tris, 100 mM NaCl, 1 mM EDTA, 7 M Urea and 1 mM DTT) buffer and eluting over a linear gradient of 100mM NaCl to 1M NaCl in denaturing buffer. Denatured His-tagged H4 was purified by Nickel affinity chromatography by loading the soluble supernatant on a HisTrap column (GE Healthcare) equilibrated in denaturing nickel chromatography buffer (10 mM Tris, 500 mM NaCl, 20 mM imidazole, 1 mM EDTA, 7 M Urea and 1 mM DTT). The column was then washed with denaturing buffer and then eluted from the column with a linear gradient of gradient of 20 mM imidazole to 750 mM imidazole. Eluted peak fractions were acidified with 1% trifluoroacetic acid and further purified by reverse phase chromatography on a Sep-Pak C18 6 cc Vac Cartridge column (Waters Corporation). Purified histones where aliquoted, lyophilized and stored frozen.
Refolding of H3 and H4 was conducted by resuspending the lyophilized H3 and H4 in pH 7.5 unfolding buffer (20 mM Tris, 6 M guanidinium, 1 mM EDTA, 5 mM DTT) at 4°C. Unfolded histones were mixed together at equimolar amounts and then dialyzed against two changes of non-denaturing buffer (20 mM Tris pH 7.5, 1.0 M NaCl, 5 mM DTT and 1 mM DTT) followed by a short dialysis into a low-salt buffer (20 mM Tris pH 7.5, 100 mM NaCl, 5 mM DTT and 1 mM EDTA) directly prior to ion-exchange purification using a HiTrap SP HP column (GE Healthcare). The 6 × Histidine tags on H4 was either left on the protein or removed by TEV protease treatment during the refolding reaction. Following ion exchange the refolded H3/H4 histones were concentrated and stored at 4°C. Full-length humanH3.3/H4 and humanH3.1/H4 were purified recombinantly from E. coli as previously described 30 and refolded using the same methods as for yeast H3/H4.
Full length yeast Asf1 was purified recombinantly from E. coli as follows. Asf1in a custom pET-Duet-1 (Novagen) E. coli expression vector with a TEV cleavable N-terminal 6xHis tag was transformed into BL21 Rosetta (DE3) (Fisher Scientific). Cells were grown in liquid culture until they reached an OD600 of 0.5 at which point protein expression was induced by addition of IPTG to a final concentration of 0.5 mM and grown for overnight at 20 °C. To purify Asf1, cells were resuspended in lysis buffer (20 mM Tris pH 7.5, 500 mM potassium acetate, 20mM imidazole, 0.1% Triton, 2 mM DTT with protease inhibitors) and lysed by sonication. Lysate was clarified by centrifugation at 13k rpm for 30 minutes. Clarified lysate was loaded a HisTrap column (GE Healthcare) equilibrated in nickel chromatography buffer (20 mM Tris pH 7.5, 300 mM potassium acetate, 20 mM imidazole, 2 mM DTT) and then eluted from the column with a linear gradient of 20 mM imidazole to 750 mM imidazole. Eluted peak fractions were pooled and dialyzed overnight against dialysis buffer (20 mM Tris pH 7.5, 150 mM potassium acetate, 2 mM DTT). Dialyzed protein was further purified by ion exchange chromatography by loading onto HiTrap Q HP column (GE Healthcare) equilibrated in ion exchange chromatography buffer (20 mM Tris, 100 mM potassium acetate, 2 mM DTT) and eluting over a linear gradient of 100 mM potassium acetate to 2 M potassium acetate. Peak fractions were pooled and concentrated.
GST-Hir1(599–840) and His-Hir2(471–875) were expressed individually or co-expressed in BL21-Gold (DE3) E. coli cells (Agilent) induced with 0.8 mM IPTG and expressed overnight at 18°C. The co-expressed GST-Hir1(599–840)/His-Hir2(471–875) complex cells were resuspended in 1xPBS supplemented with 5 mM BME and 1 mM PMSF, the individually expressed His-Hir2(471–875) cells were resuspended in 25mM Tris pH 8.0, 500 mM NaCl, 25 mM Imidazole, 5 mM BME, and 1 mM PMSF. Resuspended cells were lysed by sonication and clarified by centrifugation. The GST-Hir1(599–840)/His-Hir2(471–875) complex supernatant was subjected to GST affinity chromatography followed by on-column cleavage through incubation with TEV protease overnight at 4°C before elution into buffer containing 20 mM Tris pH 8.0, 50 mM NaCl, and 5 mM BME. The His-Hir2(471–875) was subjected to Ni IMAC chromatography and eluted with buffer containing 25 mM Tris pH 8.0, 500 mM NaCl, 200 mM Imidazole, and 5 mM BME; TEV protease was added, and the elution pool was dialyzed overnight at 4°C into buffer containing 20 mM Tris pH 8.0, 50 mM NaCl, and 5 mM BME. The cleaved Hir2(471–875) and Hir1(599–840)/Hir2(471–875) complex were then subjected to ion-exchange chromatography using a HiTrapQ HP column (Cytiva). After ion-exchange, proteins were concentrated using a 10kDa MWCO spin concentrator (Millipore) and loaded onto a Superdex 200 10/300 GL column (Cytiva) for size-exclusion chromatography purification in buffer containing 20mM Tris pH 8.0, 300 mM NaCl, and 1 mM Tris (2-carboxyethyl) phosphine (TCEP). Peak fractions were pooled and concentrated, and flash frozen for later use.
His-ctHir3 and His-ctHir3_K1358AK1362A were expressed overnight at 18°C in BL21(DE3) cells (Agilent) cultured in Terrific Broth (RPI) and induced with 0.5 mM IPTG at OD600≅1.1. Cells were harvested by centrifugation and resuspended in buffer containing 20 mM HEPES, pH 8, 500 mM NaCl, 10 mM imidazole, 5 mM BME, and 1 mg/mL PMSF and lysed by sonication. Lysate was clarified by centrifugation and the supernatant was subjected to nickel affinity chromatography to isolate the His-tagged protein. His-ctHir3 was eluted with 200 mM imidazole and dialyzed overnight at 4°C into buffer containing 20 mM HEPES, pH 8, 50 mM NaCl, and 5 mM BME. His-ctHir3 was then subjected to ion-exchange chromatography using a HiTrap Q column (GE Healthcare). Following ion exchange chromatography, protein was concentrated using a 100 kDa MWCO spin concentrator (Millipore) and loaded onto a Superose 6 increase column (GE Healthcare) equilibrated with buffer containing 20 mM HEPES, pH 8, 300 mM NaCl, and 0.5 mM TCEP for Cryo-EM grid preparation. Protein designated for fluorescence polarization assays was loaded onto a Superose 6 increase column (GE Healthcare) equilibrated with buffer containing 20 mM HEPES, pH 7.5, 100 mM NaCl, 5% glycerol, and 0.5 mM TCEP. Peak fractions were pooled and concentrated, and flash frozen for later use.
Protein complex assembly
The trimeric Asf1/H3/H4 was assembled by a modified protocol as previously reported60. In short, refolded H3/H4 tetramers and excess recombinant purified Asf1 were combined and diluted to 250 mM potassium acetate and incubated at 30 °C for 20 minutes. The Asf1-H3/H4 mixture was concentrated with a 10,000 MWCO spin concentrator and purified by size exclusion chromatography using a 30/1000 Superdex 75 filtration column (GE Life Science) equilibrated in running buffer (20 mM HEPES pH 7.6, 250 mM potassium acetate, 10% glycerol, 1 mM DTT). Fractions containing the trimeric Asf1/H3/H4 were pooled, concentrated, and stored at 4°C for later use. The trimeric yeastAsf1/humanH3.3/humanH4 and yeastAsf1/humanH3.1/humanH4 used for SPR were also assembled in the same manner by combining full-length yeast Asf1 and full-length humanH3.3/H4 (or full-length humanH3.1/H4), and purified by size exclusion chromatography.
The Hir complex-Asf1/H3/H4 co-complex was assembled as follows: the purified Hir complex was mixed with Asf1/H3/H4 at a 1:2 molar ratio and incubated on ice for 30 minutes. The sample was directly sedimented at 32k rpm for 16 hours and 30 minutes in a 10% to 40% glycerol gradient (20 mM HEPES pH 7.6, 200 mM potassium acetate, 2 mM magnesium acetate, 2 mM DTT) at 4°C using a Beckman SW60 Ti rotor. Glycerol gradients were prepared using a Gradient Master device (BioComp Instruments). When cross-linking for cryo-EM analysis, 0.125% (v/v) glutaraldehyde (EM grade, Sigma Aldrich) was added to the 40% glycerol solution before gradient preparation. Samples were then fractionated with a Piston Gradient Fractionator (BioComp Instruments). Cross-linking reactions were quenched by the addition of glycine-HCl buffer (pH 7.5) to a final concentration of 40 mM. To locate the peak, un-crosslinked fractions were subjected to TCA precipitation and analyzed by SDS-PAGE. Corresponding crosslinked peak fractions were concentrated and stored at −80°C.
Proteomic mass spectrometry of the Hir complex-Asf1/H3/H4
The un-crosslinked fractions where the complex was located were reduced with 10 mM DTT (US Biological) for 30 min at 30 °C, followed by alkylation with final 50 mM iodoacetamide (Sigma-Aldrich) for 30 min at 30 °C. Each fraction was digested with trypsin (Thermo Fisher Scientific) in 1:10 (w/w) enzyme/protein ratio for overnight at 30°C and acidified in final 1% trifluoroacetic acid (TFA; Thermo Fisher Scientific). All fractions were desalted with Pierce™ C18 Spin Columns, vacuum-dried and resuspended with LC-MS grade water containing 0.1% (v/v) TFA for MS analysis.
Each fraction was analyzed by a Q-Exactive HF mass spectrometer (Thermo Fisher Scientific) coupled to a Dionex Ultimate 3000 UHPLC system (Thermo Fisher Scientific) equipped with an in-house–made 15-cm-long fused silica capillary column (75 μm inner diameter), packed with a reversed-phase ReproSil-Pur C18-AQ 2.4-μm resin (Dr. Maisch GmbH, Ammerbuch, Germany) column. Elution was performed using a gradient from 5 to 38 % B (90 min), followed by 95 % B (5 min), and re-equilibration from 95 to 5 % B (5 min) with a flow rate of 300 nl/min (mobile phase A: water with 0.1 % formic acid; mobile phase B: 80 % acetonitrile with 0.1 % formic acid). Data were acquired in data-dependent tandem MS (MS/MS) mode. Full-scan MS settings were as follows: mass range, 200 to 1600 (mass/charge ratio); resolution, 120,000; MS1 AGC target 3E6; MS1 Maximum IT, 100 ms. MS/MS settings were as follows: resolution, 30,000; AGC target 5E5; MS2 Maximum IT, 100 ms; fragmentation was enforced by higher-energy collisional dissociation with stepped collision energy of 25, 27, 30; loop count, top 20; isolation window, 1.4 m/z; MS2 Minimum AGC target, 800; charge exclusion: unassigned, 1, 8 and > 8; peptide match, preferred; exclude isotope, on; dynamic exclusion, 45 s.
Relative stoichiometry calculation of the Hir complex-Asf1/H3/H4
Each raw data was separately analyzed by MaxQuant 2.4.1361 with the default setting with following modifications: proteomic database from EBI reference proteome of entire S. cerevisiae with contaminants; Min. peptide length, 3; Max. peptide mass [Da], 6000, Min. peptide length for unspecific search, 3; Max. peptide length for unspecific search, 30; Use only unmodified peptides, Oxidation (M), and Acetyl (Protein N-term) for quantification from both unique and razor peptides, and advanced ratio estimation. iBAQ with log fit and charge normalization, advanced site intensities, and Top3 were all enabled. Each protein tables were filtered by subunit accession numbers and each protein iBAQ value was divided by the sum of total subunit iBAQ value. Ratio values were plotted in Python Seaborn boxplot and swarmplot.
Cryo-EM sample preparation and data collection
To prepare cryo-EM grids for the Hir complex bound to Asf1/H3/H4, the glutaraldehyde-fixed sample described above was thawed and dialyzed in EM buffer (20 mM HEPES pH 7.6, 200 mM potassium acetate, 5 mM DTT) for 30 min immediately prior to making grids. Two microliters of the dialyzed sample were then applied to glow-discharged (1 min, easiGlow, Pelco) R2/2 300-mesh Quantifoil holey carbon grids (Electron Microscopy Sciences). The grids were manually blotted for 2 to 3 seconds using Whatman Grade 41 filter paper (Sigma-Aldrich) from the backside of the grid and flash-frozen in liquid ethane with a Leica EM CPC plunger (Leica Microsystems). EM grids were prepared in batches and the freezing conditions were optimized at the Beckman Center for Cryo-Electron Microscopy (the University of Pennsylvania). Optimized grids were imaged using a FEI Titan Krios G3i transmission electron microscope (TEM) operating at 300 kV, equipped with a K3 direct electron detector (Gatan) and a Bioquantum energy quantum filter (Gatan) at a nominal magnification of 64,000x in super-resolution mode (nominal pixel size of 0.68 Å) at a defocus range between −1.0 to −3.0 μm. A total of 25,420 images was collected over the course of 6 days in three datasets. The exposure time was 3.95 seconds, divided into 42 frames, at a nominal dose of 42.3–42.8 e−/Å2.
To prepare ctHir3 cryo-EM grids, 3μL of 0.5 mg/mL His-ctHir3 was applied to glow discharged (easiGlow, Pelco) Quantifoil R0.6/1 300 mesh copper grids, blotted for 2 seconds (blot force=10) under 88% humidity at 4°C and plunged into liquid ethane using an FEI Vitrobot Mark IV. EM grids were prepared in batches and the freezing conditions were optimized by screening at the Beckman Center for Cryo-Electron Microscopy (the University of Pennsylvania). Optimized grids were imaged using a FEI Titan Krios G3i transmission electron microscope (TEM) operating at 300 kV, equipped with a K3 direct electron detector (Gatan) and a Bioquantum energy quantum filter (Gatan) and at a nominal magnification of 105,000x in super-resolution mode (pixel size of 0.42 Å) at a defocus range between −1.0 to −2.6 μm. A total of 4,695 images was collected. The exposure time was 1.9 seconds, divided into 35 frames, at a nominal dose of ~42.6 e−/Å2.
Image processing and 3D reconstruction
Hir complex/Asf1/H3/H4
A combination of software including cryoSPARC v.3.3.262 and Relion 3.1.363 were used to process cryo-EM dataset cooperatively. Dataset 1, 2, and 3 (25,446 movies) were imported in cryoSPARC and patch motion correction and CTF estimation were performed.
Dataset 1 (3,069 images) was used for reference free “blob” picking and two rounds of reference-free 2D classifications to remove particles that lacked clear features. Selected 2D classes were utilized for template picking on the entire dataset and the particles went through 2D classification to filter out junk particles. Ab-initio modeling was performed with the selected particles to produce three classes. The best map went through multiple rounds of non-uniform refinement and heterogenous refinement. The best class containing a total 1,159,488 particles, were exported to Relion for an additional round of 3D classification. The best class containing 639,629 particles yielded a 5.66 Å resolution map after 3D auto-refinement. After multiple rounds of CTF refinement and Bayesian polishing with iterative resolution improvements, 3.61 Å resolution map was obtained and particles were exported to cryoSPARC. The imported map and 639,629 particles went through heterogenous refinement and non-uniform refinement to yield 2.96 Å resolution map (Map 1) containing 495,250 particles.
Hir3(Tail)-Hir2(WD40)-Hpc2 focused classification
The 639,629 particles in the best Relion class were subjected to one round of 3D auto-refinement with relaxing C2 symmetry before focused classification with signal subtraction. The region corresponding to Hir3(Tail)-Hir2(WD40)-Hpc2 was segmented with UCSF Chimera64 Segger65 and a corresponding mask was created in Relion. Particles were subtracted with the mask and went through 3D classification with local angular search. The best class including 44% of particles was refined with 3D auto-refinement to 6.8Å resolution (Map 2).
Asf1/H3/H4 focused classification
The 366,702 particles were subjected to focused classification of Hir1(WD40)-Asf1/H3/H4 after 3D classification with 1.13M particles. Particles were subtracted with a corresponding mask and went through 3D classification without alignment. The high resolution feature included classes with total 43.1% of particles were reverted to original and 3D classification without alignment was performed. The best and major class cointaining 92.8% particles was refined with 3D auto-refinement to a nominal 14.3 Å resolution (3.6Å for Core, 8–12 Å resolution for Asf1/H3/H4, and 12–20Å resolution for Hir3 Arm/Tail) (Map 3).
A composite map of the focused-refined maps and the entire Hir complex map was created in UCSF Chimera64 using vopmax command after normalizing focused refined maps relative to the entire map using vop threshold and vop scale commands.
ctHir3
Data collected on ctHir3 were processed in cryoSPARC (v3.1.1) in the following manner. Movies collected in super-resolution mode on a Gatan K3 were binned during motion corrected then CTF corrected by Patch CTF estimation. Blob picking using an elliptical blob with maximum diameter of 250 angstroms was used to automatically pick particles which were subjected to reference free 2D classification. Visual analysis showed 2D classes for both monomeric and dimeric ctHir3 so a combination of monomeric and dimeric 2D classes were used for template-based picking. Template picked particles were again analyzed by 2D classification, with particles from self-consistent classes being used for ab initio model generation and heterogenous refinement, at which point it was clear that both a monomer and asymmetric dimer form were present. Due to challenges in disambiguating monomer and dimer classes at the 2D level, all template-based particles were subjected to heterogenous refinement using a monomer, dimer and dummy as references. The ctHir3 dimer represented the major class and so was processed by further heterogenous refinement and non-uniform refinement resulting in a 3.92 Å reconstruction derived from 181,856 particles (Map 4).
Model building and refinement
For Map 1 (the central core), an initial model of the central core was obtained by rigid-body fitting of two copies each of AlphaFold models45 of the Hir1-Hir21-Hir22 heterotrimers, the Hir3 Head, and the N-term Arm using UCSF Chimera64. The model was manually adjusted using real-space refinement in Coot66 and subsequently refined with rigid body, local grid search, ADP (B-factors), and morphing refinement in Phenix67.
For Map 2 (Hir3(Tail)-Hir2(WD40)-Hpc2), AlphaFold models of Hir3(N-term Tail)-Hir2(WD40)-Hpc2 and the Hir3 C-term tail were fitted into respective densities in UCSF Chimera, manually adjusted by Coot, and refined with rigid body refinement in Phenix.
For Map 3 (Hir1(WD40)-Hpc2 and Asf1/H3/H4 + B-domain + Hpc2 HRD), AlphaFold models of Hir1(WD40)-Hpc2 and Asf1/H3/H4 + B-domain + Hpc2 HRD were fitted as rigid bodies into respective densities using UCSF Chimera.
For Map 4 (ctHir3 dimer), the top 20 models generated by AlphaFold were examined. The best-fit model was fitted into two copies of the ctHir3 dimer, manually adjusted by Coot, and refined with rigid body refinement in Phenix.
Crosslinking mass spectrometry sample preparation and data collection
One hundred micrograms of purified Hir complex at 0.5 mg/ml in buffer C [40 mM Hepes (pH 7.6), 2 mM DTT, 10% glycerol, 500 mM Potassium Acetate, 0.05 % 3DS] and Hir complex/Asf1/H3/H4 at 0.5 mg/ml in buffer 200 [20 mM Hepes (pH 7.6), 2 mM DTT, 200 mM Magnesium Acetate, 10–40 % glycerol, 200 mM Potassium Acetate] were separately mixed with final 6 mM DSBU (Thermo Fisher Scientific) and incubated on ice for 2 hours. The reaction was quenched by adding 50 mM ammonium bicarbonate for 30 minutes, and the reaction was further stopped by trichloroacetic acid (TCA) precipitation. Crosslinked proteins were precipitated with 20 % (w/v) TCA (Sigma-Aldrich) on ice for 60 min. Proteins were pelleted by centrifugation at 21,000g for 15 min at 4 °C and washed with 10% TCA in 0.1 M tris-HCl and then with acetone (Thermo Fisher Scientific). The solvent was discarded, and the pellet was air-dried and stored at −80 °C for analysis by MS.
Cross-linked proteins were resuspended in 50 μl of resuspension buffer (2.5 % SDS and 50 mM triethylammonium bicarbonate final concentrations) and reduced with 10 mM DTT (US Biological) for 30 min at 30 °C, followed by alkylation with final 50 mM iodoacetamide (Sigma-Aldrich) for 30 min at 30 °C. The proteins were processed using an S-Trap column according to the protocol recommended by the supplier (Protifi, C02-mini) and digested with trypsin (Thermo Fisher Scientific) in 1:10 (w/w) enzyme/protein ratio for overnight at 30°C. Peptides eluted from the column were vacuum-dried and resuspended with 0.1% (v/v) trifluoroacetic acid (TFA; Thermo Fisher Scientific) to fractionate peptides with Pierce™ High pH Reversed-Phase Peptide Fractionation Kit (Thermo Fisher Scientific) according to the recommended protocol by the supplier. All fractions were vacuum-dried and resuspended with LC-MS grade water containing 0.1% (v/v) TFA for MS analysis.
Each fraction was analyzed by a Q-Exactive HF mass spectrometer (Thermo Fisher Scientific) coupled to a Dionex Ultimate 3000 UHPLC system (Thermo Fisher Scientific) equipped with an in-house–made 15-cm-long fused silica capillary column (75 μm inner diameter), packed with a reversed-phase ReproSil-Pur C18-AQ 2.4-μm resin (Dr. Maisch GmbH, Ammerbuch, Germany) column. Elution was performed using a gradient from 5 to 45 % B (90 min), followed by 90 % B (5 min), and re-equilibration from 90 to 5 % B (5 min) with a flow rate of 3–400 nl/min (mobile phase A: water with 0.1 % formic acid; mobile phase B: 80 % acetonitrile with 0.1 % formic acid). Data were acquired in data-dependent tandem MS (MS/MS) mode. Full-scan MS settings were as follows: mass range, 300 to 1800 (mass/charge ratio); resolution, 120,000; MS1 AGC target 1E6; MS1 Maximum IT, 200 ms. MS/MS settings were as follows: resolution, 30,000; AGC target 2E5; MS2 Maximum IT, 300 ms; fragmentation was enforced by higher-energy collisional dissociation with stepped collision energy of 25, 27, 30; loop count, top 12; isolation window, 1.5 m/z; fixed first mass, 130; MS2 Minimum AGC target, 800; charge exclusion: unassigned, 1, 2, 3, 8 and > 8; peptide match, off; exclude isotope, on; dynamic exclusion, 45 s68.
Crosslinked peptide search
Raw files were converted to mzML format with ThermoRawFileParser 1.2.369. Search engine MeroX 2.0.1.470 was used to identify and validate cross-linked peptides. MeroX was run in RISEUP mode, with default crosslinker mass and fragmentation parameters for DSBU with minor tweaks: precursor mass range, 1000 to 10,000 Da; minimum precursor charge, 4; precursor and fragment ion precisions, 5.0 and 10.0 ppm, respectively; maximum number of missed cleavages, 3; carbamidomethylation of cysteine and oxidation of methionine, as fixed and variable modifications, respectively; results were filtered for score (>10) and target-decoy approach FDR (<1 %). All search results from each fraction’s MS acquisition were combined and filtered by recalculated FDR at 1 %. K-K residue pairs were selected for the site-localization when crosslinked peptides contained miscleaved lysine residues and site localization scores were tie. Visualization of the crosslinks were performed with CX-Circos 2.0 alpha71, XiNet72 and ChimeraX 1.573 XMAS 1.1.2.74
Sedimentation Equilibrium Analytical Ultracentrifugation
Proteins purified by size-exclusion chromatography were subjected to analytical ultracentrifugation in buffer containing 20 mM Tris pH 8.0, 300 mM NaCl, and 1 mM TCEP. Analytical ultracentrifugation experiments were conducted with an XL-I analytical ultracentrifuge (Beckman-Coulter) and an AN-60 Ti Rotor with a six-channel charcoal-filled Epon centerpieces and quartz windows. Data were collected at 4°C with detection at 280 nM. Sedimentation equilibrium analyses were conducted using global fits to data acquired at 3 centrifugation speeds and 3 sample concentrations with the single species fitting model in the program SEDPHAT75. All fit RMSDs were less than 0.01. The partial specific volume (υ) and buffer density (ρ) were determined from chemical composition by the program UltraScan III.
Hir2CTD alone or the Hir1CTD / Hir2CTD complex were purified to monodispersity and subjected to sedimentation equilibrium analytical ultracentrifugation at three speeds (2898, 6520, and 11591 ×g) and three concentrations (0.2, 0.4, and 0.6 OD 280 nm) to determine the molecular assembly. Linear comparisons of experimental data with theoretical data generated using the program Heteroanalysis76 indicate that Hir2 is dimeric, while the Hir1CTD/Hir2CTD forms a Hir1CTD(1)/ Hir2CTD (2) heterotrimer (Figure S8B). Single species MW analysis of the three speed/concentration data using the program SEDPHAT77 determined solution molecular weights of Hir2 alone and the Hir1CTD/Hir2CTD complex to be ~91 kDa and ~118 kDa respectively (Figure S8C). These data indicate a Hir2CTD dimer (theoretical MW = 93kDa) and a Hir1CTD(1)/ Hir2CTD(2) heterotrimer (theoretical MW = 120 kDa).
Mass Photometry
All data were collected using a TwoMP mass photometer (Refeyn) calibrated with bovine serum albumin (66 kDa), beta amylase (224 kDa), and thyroglobulin (660 kDa). Movies were acquired for 3,000 frames (60 s) using AcquireMP software and default settings. Final protein concentrations were empirically determined to achieve ~50 binding events per second by diluting with same background buffers. Raw data were analyzed through DiscoverMP software (2022 R1) with histogram mode and mass plot. Automated Gaussian peak detection was applied to estimate the masses, molecule counts and sigma.
Fluorescence Polarization Binding Assay
The ssRNA probe used was synthesized by IDT with a 5-FAM moiety at the 5’ end (5’AAAACAAAUAUGCAUAUUAUCGAGAGG3’). The dsDNA probe was assembled from DNA fragments synthesized by IDT with a 5-FAM moiety on the 5’ end of the sense strand (5’AGCGGCCCCATGCCCGGGCGG3’). The FAM-labeled sense fragment and unlabeled antisense fragment were dissolved in a buffer containing 10 mM Tris, pH 7.5, 10 mM NaCl, and 1 mM EDTA and were mixed at a 1:1 sense: antisense ratio, were incubated at 95 °C for five minutes, then were slowly cooled to room temperature to allow for annealing. The ssDNA probe was synthesized with a 5-FAM moiety at the 5’ end (5’TAGGGAGTAATCCCCTTGGCGG3’). To generate binding curves, increasing concentrations of ctHir3 were titrated into buffer containing 20 mM HEPES pH 7.5, 100 mM NaCl, 0.5 mM TCEP, 2 mg/mL Bovine serum albumin (BSA), and 10nM of nucleic acid probe. All FP data were collected in triplicate or quadruplicate using a TECAN Spark plate reader and fluorescence values were normalized to the background fluorescence of each probe-containing buffer alone. Curves were fit using a one site total binding model with GraphPad Prism™.
Cryptic transcription reporter assay
To create the hir3Δ or hir1Δ strains, we replaced the gene encoding Hir3 or Hir1 with hygromycin using a PCR-mediated strategy in the yeast strain FY2713 (kanMX-GAL1pr-flo8::HIS3 his3Δ200 leu2Δ1 lys2–128∂ trp1Δ63 ura3–52)47. The coding region of HIR3 or HIR1 along with approximately +/− ~200 bp was cloned into the low copy number plasmid pRSII315 and mutations were introduced by site-directed mutagenesis using QuikChange (Agilent). Plasmids harboring the hir3 or hir1 alleles were transformed into the hir3Δ or the hir1Δ strain, grown to OD 1.0, and spotted in a 10-fold dilution series on the indicated medium, followed by incubation at 30°C for three days.
The three hir3 alleles (2x, 7x, and hir3 c-termΔ) were also created using CRISPR-Cas9 single-plasmid pML107 78 essentially as described in 79. The annealed gRNA oligos (CKO3914 (forward) plus CKO3915(reverse)) were cloned into pML107/pCK2408 using SwaI and BclI restriction digestion sites. 400ng of pML107 or pCK3163 (HIR3 sgRNA) were transformed into CKY826 along with 500 ng of desired double strand repair fragment following the yeast high-efficiency transformation protocol described in 80. Gene fragments containing a PAM site mutation resistant to cleavage by pCK3163 carrying Cas9/HIR3 sgRNA or PAM site and 7x RNA mutant were synthesized by Twist. Gene fragments were amplified by CKOCKO3965/3966 to extend homology arms prior to use in repair. 2x RNA hir3 allele was created by mutagenic primer (CKO3971/CKO3972) amplification of PAM site mutant DNA. Transformants were grown on selective SC-Leu plates. Multiple colonies for each rescue DNA were picked and examined for phenotypes on SC-His as well as genotyping by PCR and sequencing.
Surface Plasmon Resonance (SPR)
The binding kinetics of Hir complex with different analytes, yAsf1FL, yAsf1FL/hH3.1FL/hH4FL, and yAsf1FL/hH3.3FL/hH4FL, was monitored quantitatively by using Biacore (Cytiva) T200. The carboxymethyldextran sensor chip (CMD200L, Xantec) was conditioned with 1M NaCl, 50 mM sodium borate (pH 9.0) followed by activation with 100 mM EDC/NHS using HBS as the running buffer. After activation, HIR complex was diluted to 15 ug/mL in 10 mM sodium acetate (pH 5.0) and immobilized to the chip at three different densities on flow cell 2,3, and 4 via amine coupling. Flow cell 1 was the reference cell for measuring the background binding. After 30 min, the remaining activated sites were blocked in 1M ethanolamine (pH 8.0). Running buffer was switched to HBS, 0.05% Tween, 0.3 mM TCEP, and 1mM MgCl2. Each analyte sensorgram was measured under the following cycle: 1) desalted and diluted analyte was injected for 120 sec (yAsf1FL and yAsf1FL/hH3.1/hH4) or 150 sec (yAsf1FL/hH3.3FL/hH4FL) and switched to the running buffer for 300 sec (yAsf1FL and yAsf1FL/hH3.1FL/hH4FL) or 600 sec (yAsf1FL/hH3.3FL/hH4FL) with flow rate 30uL/min, 2) 1M NaCl was injected in the running buffer for 30 sec to regenerate the ligand. For competition assays, yAsf1FL was premixed with 3 μM 20-nt RNA (AGCAUUCAAAGAGGAGAGGU) (Dharmacon) or 3 μM poly-L-glutamic acid of MW 1,500–5,500 (Sigma P1818).
Sensorgrams from flow cell 2, 3, and 4 were subject to background, flow cell 1, subtraction and set of sensorgrams from serial diluted analyte (16, 32, 63, 125, 250, 500, 1000 nM) were separately analyzed in Biacore T200 Evaluation Software 3.2.1. yAsf1FL alone was examined by Kinetic screening with 1:1 binding model to estimate Kon and Koff as well as KD. The estimated KD was validated by steady-state analysis, as yAsf1FL binding exhibited fast-on, fast-off curves. yAsf1/hH3.1/hH4 and yAsf1/hH3.3/hH4 were examined by Kinetic screening with Heterogeneous binding model to estimate two independent Kon, Koff and KD values. Main figure was generated by Seaborn from the exported values and supplementary figures were directly generated from Biacore evaluation software.
Pull down assay of the Hir complex
The hir3Δ strain or FY2713 was Flag tagged on Hir1 by a PCR-mediated strategy using pCK1099 TOPO 3xFLAG NATMX as a template. The 3xFlag tag on Hir1 conferred no cryptic transcription phenotype in the reporter assay (data not shown). A single colony of each transformed yeast with a target plasmid was inoculated in 25 ml SC-Leu media overnight at 30°C at 200 rpm. Overnight cultures were transferred to 2L of SC-Leu media flasks and cells were harvested at O.D. 2. Harvested cells were spun down at 3,000 ×g for 15 minutes at 4°C. Pelleted cells were washed with water, spun down, and flash-frozen by pushing yeast paste through 50 mL syringe into LiN2. Each frozen yeast noodle was cryo-ground in Cryomill (Retsch) with the following parameters: auto precooling, 5 cryo cycles with 2 min of 25 Hz and 30 sec of 5 Hz cooling interval. 1.6 g of cryoground powder of each yeast strain was measured and resuspended with 10 mL of resuspension buffer: 40 mM Hepes pH 7.6, 500 mM KOAc, 10% glycerol, 1 mM DTT, 1% 3-(decyldimethyl-ammonio) propanesulfonate (Sigma), and protease inhibitors. Resuspended lysates were spun down at 20,000 ×g for 10 minutes at 4°C. Clarified lysates were transferred to 50 mL falcon tube with cheese cloth. Protein concentration of each lysate was measured in nanodrop and each lysate was normalized to match lowest concentration. Separately, 50 μl slurry bead volume of EZview anti-Flag agarose (Pierce) was washed three times with 1 mL of resuspension buffer. Each of the normalized clarified lysates was incubated with washed beads for 2 hours at 4°C. Beads were washed four times using 1 mL of resuspension buffer. Washed beads were incubated with 30 μl of 10 mg/ml Flag peptide for 20 minutes at room temperature. Eluates were run on NuPAGE 4–12% BT SDS-PAGE gel (Invitrogen).
Quantification and statistical analysis
In SPR assays, averages and sample standard deviations of Kon, Koff, KD, Rmax and Chi2 were calculated with n=2 in Excel. In iBAQ stoichiometry, in each replicate (n=5), each subunit’s iBAQ value was divided by the sum of all subunit values to obtain each subunit’s relative molar ratio and each ratio was plotted in box-whisker plot, which shows the three quartile values of distribution and whiskers extends to points that lie within 1.5 IQRs of lower and upper quartiles.
Supplementary Material
Data S1 Crosslinking mass spectrometry search result of the Hir complex alone, related to Figure 2. (separate file)
MeroX search results were combined and curated to show only unique site-pairs in the table. All the columns after “unknown 2” column were generated during the postprocessing to generate unique site-pair entries, UCSF ChimeraX XMAS, and CX-Circos input files.
Data S2 Crosslinking mass spectrometry search result of the Hir complex bound to Asf1/H3/H4, related to Figure 2. (separate file)
Same as Data S1, but with Asf1/H3/H4.
Movie S1 Composite map of the Hir complex bound to Asf1/H3/H4, related to Figure 1 and 7.
The composite map was generated by combining Maps1–3. Models and color codes are as in Figure 1. The Movie was created by Chimera 64.
Movie S2 Slab views of composite map of the Hir complex bound to Asf1/H3/H4, related to Figure 1, 3, 4, 5, and 7.
Slab views to show the model fitting. Models and color codes are as in Figure 1. The Movie was created by Chimera 64.
Movie S3 Sequential ribbon display of the Hir complex, related to Figure 1, 3, 4, 5, and 7.
Models and color codes are as in Figure 1. The Movie was created by Chimera64.
Movie S4 Overall view of Hir complex and proposed model of recruitment of Asf1/H3/H4, related to Figure 1, 3, 4, 5, and 7.
The movie was generated in Blender 3.4.1 with MolecularNodes 2.2.1 and the annotations and final movie export were performed in Adobe Premiere Pro 23.2.0.
Key resources table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| NEB® 5-alpha Competent Cells | New England Biolabs | Cat#C2987H |
| Rosetta 2(DE3) Competent Cells | Fisher Scientific Company | Cat#713974 |
| BL21-Gold (DE3) | Agilent | Cat#230132 |
| Chemicals, peptides, and recombinant proteins | ||
| 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) Free Acid | US Biological | Cat#H2010 |
| Dithiothreitol (DTT) | US Biological | Cat#D8070 |
| Glycerol | Fisher Scientific Company | Cat#BP229-4 |
| Tween 20, 100% Nonionic Detergent | Bio-RAD | Cat#1706531 |
| Ammonium Sulfate ACS Grade | Fisher Scientific Company | Cat#A702-10 |
| Sodium Chloride (NaCl) | Fisher Scientific Company | Cat#S6191-1KG |
| Potassium Acetate (KOAc) | Fisher Scientific Company | Cat#P171I-3 |
| 3-(decyldimethyl-ammonio)-propanesulfonate (3D-MAP) | Sigma | D4266-25G |
| Isopropyl β- d-1-thiogalactopyranoside (IPTG) | Fisher Scientific Company | Cat#BP162010 |
| UltraPure™ 1 M Tris-HCI Buffer, pH 7.5 | Fisher Scientific Company | Cat#15567027 |
| UltraPure™ 0.5M EDTA, pH 8.0 | Fisher Scientific Company | Cat#15575020 |
| 2-Mercaptoethanol, for molecular biology, 25 mL | Sigma Aldrich | M3148-25ML |
| Triton X-100 | Sigma Aldrich | Cat#11332481001 |
| Ultrapure GUANIDINE HYDROCHLORIDE -- 500 g | Invitrogen | Cat#15502016 |
| Imidazole | Acros Organics | Cat#AC301872500 |
| Urea | Sigma Aldrich | Cat#U5128-5KG |
| Pierce™ Trifluoroacetic Acid (TFA), LC-MS Grade | Thermo Fisher Scientific | Cat#85183 |
| Phenylmethanesulfonyl fluoride (PMSF) | Sigma Aldrich | Cat#P7626-100G |
| Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) | GoldBio | Cat#TCEP10 |
| Terrific Broth | RPI | Cat#T5000-1000.0 |
| Magnesium Acetate | Boston Bioproducts | Cat#MT-190 |
| 25% Glutaraldehyde Solution in H2O, EM grade | Sigma Aldrich | Cat#111-30-8 |
| Glycine | Sigma Aldrich | Cat#G8790-1KG |
| Trichloroacetic Acid Bioxtra | Sigma Aldrich | Cat#T9159-250g |
| triethylammonium bicarbonate | Sigma Aldrich | Cat#T7408-100ML |
| Ammonium bicarbonate - LC/MS Grade | Sigma Aldrich | Cat#09830-1KG |
| Sodium Dodecyl Sulfate, 10% (SDS) 500mL | Invitrogen | Cat#AM9822 |
| Disuccinimidyl Dibutyric Urea (DSBU) | Thermo Fisher Scientific | Cat#A35459 |
| Iodoacetamide (IAA) | Sigma Aldrich | Cat#I6125-5G |
| Trypsin | Thermo Scientific | Cat#90057 |
| Formic Acid, LC-MS Grade | Thermo Scientific | Cat#28905 |
| EZview™ Red ANTI-FLAG® M2 Affinity Gel | Sigma Aldrich | Cat#F2426-1ML |
| 3X FLAG Peptide | GLPBIO | Cat#GP10149-5 |
| Poly-L-glutamic acid sodium salt | Sigma Aldrich | Cat#P1818-25MG |
| 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide-hydrochloride (EDC-HCl) | Xantec Bioanalytics | Cat#EDCHCL-1G |
| N-Hydroxysuccinimide | Sigma Aldrich | Cat#130672 |
| Sodium borate | Sigma Aldrich | Cat#S9640 |
| Sodium acetate | Ameresco | Cat#0530-1KG |
| Magnesium chloride (MgCl2) | Fisher Scientific | Cat#M35-500 |
| Deposited data | ||
| Hir complex bound to Asf1/H3/H4 (Map 1) | This paper | EMD-40006 |
| C-terminal half of the Hir3 TPR repeats, the Hir2 WD40 domain, and Hpc2 (Map 2) | This paper | EMD-40029 |
| Hir1 WD40 domain and Asf1/H3/H4 (Map 3) | This paper | EMD-40030 |
| Composite Map | This paper | EMD-40037 |
| ctHir3 (Map 4) | EMD-40078 | |
| Hir complex bound to Asf1/H3/H4 | This paper | PDB: 8GHL |
| C-terminal half of the Hir3 TPR repeats, the Hir2 WD40 domain, and Hpc2 | This paper | PDB: 8GHA |
| Hir1 WD40 domain and Asf1/H3/H4 | This paper | PDB: 8GHM |
| Composite Model | This paper | PDB: 8GHN |
| ctHir3 | PDB: 8GIX | |
| Crosslinking Mass Spectrometry of Hir complex, Hir complex bound to Asf1/H3/H4 | This paper | PRIDE: PXD040705 |
| Raw pictures of SDS-PAGE gels, plates and representative micrographs | This papr | http://dx.doi.org/10.17632/7fbn428nsp.1 |
| Experimental models: Organisms/strains | ||
| S. cerevisiae: C-TAP Hir2 CB010 (MATa pep4::HIS3 prb1::LEU2 prc-HISG can1 ade2 trp1 ura3 his3 leu2-3,112 cir-o HIR2-TAP::KanMX) | This paper | N/A |
| S. cerevisiae: FY2713 (MATa kanMX-GAL1pr-flo8::HIS3 his3Δ200 leu2Δ1 lys2-128∂ trp1Δ63 ura3-52) | Cheung et. al.47 | N/A |
| S. cerevisiae: hir3Δ FY2713 (MATa kanMX-GAL1pr-flo8::HIS3 his3Δ200 leu2Δ1 lys2-128∂ trp1Δ63 ura3-52 hir3:hphNT1) | This paper | N/A |
| S. cerevisiae: hir3Δ 3XFlag Hir1 FY2713 (MATa kanMX-GAL1pr-flo8::HIS3 his3Δ200 leu2Δ1 lys2-128∂ trp1Δ63 ura3-52 hir3::hphNT1 HIR1-3XFLAG::NatMX) | This paper | N/A |
| S. cerevisiae: hir1Δ FY2713 (MATa kanMX-GAL1pr-flo8::HIS3 his3Δ200 leu2Δ1 lys2-128∂ trp1Δ63 ura3-52 hir1::hphNT1) | This paper | N/A |
| S. cerevisiae: CKY826 (kanMX-GAL1pr-flo8::HIS3 his3Δ200 leu2Δ1 lys2-128d trp1Δ63 ura3-52) | This paper | N/A |
| Oligonucleotides | ||
| ssRNA probe: a 5-FAM moiety at the 5’ end (5’AAAACAAAUAUGCAUAUUAUCGAGAGG3’) | This paper | N/A |
| dsDNA probe: a 5-FAM moiety on the 5’ end of the sense strand (5’AGCGGCCCCATGCCCGGGCGG3’) | This paper | N/A |
| ssDNA probe: a 5-FAM moiety at the 5’ end (5’TAGGGAGTAATCCCCTTGGCGG3’) | This paper | N/A |
| Primer: For Hir1(599-840) Forward: 5’-CGCGAATTCACGAGCAACAGTATTGAC-3’ | This paper | N/A |
| Primer: For Hir1(599-840) Reverse: 5’-GCGCTCGAGTTATTTATATAACGACCAACA-3’ | This paper | N/A |
| Primer: For Hir2(471-875) Forward: 5’-CGCGGATCCCAGAAAAAAGAGCTACAG-3’ | This paper | N/A |
| Primer: For Hir2(471-875) Reverse: 5’-GCGCTCGAGTTAAGATATTATATTCATTTC-3’ | This paper | N/A |
| Primer: For native Hir3 Forward: 5’-GTCTCTCGAGGACATGATAGAACTGTCATATATCTTAGAG-3’ | This paper | N/A |
| Primer: For native Hir3 Reverse: 5’- TCGGCGGCCGCCAATTAAATCATCCAAAGGCAACGTTTTAGT-3’ | This paper | N/A |
| Primer: For hir3 C-termΔ CRISPR-Cas9 generation Forward: 5’ gatcGTTGCCAAATATATTACTCAgttttagagctag 3’ | This paper | N/A |
| Primer: For hir3 C-termΔ CRISPR-Cas9 generation Reverse: 5’- ctagctctaaaacTGAGTAATATATTTGGCAAC-3’ | This paper | N/A |
| Primer: For hir3 C-termΔ amplification Forward: 5’-GAGGCATTGGATGACTTAGGATC-3’ | This paper | N/A |
| Primer: For hir3 C-termΔ amplification Reverse: 5’-caacaaattgtctatcgctaaccg-3’ | This paper | N/A |
| Primer: For hir3 PAM mutant and 7x mutant amplification Forward: 5’- agctttgttttagctggaaaagcagtgctgagaatacaatGAGATTGTCTGAAACCGGTG-3’ | This paper | N/A |
| Primer: For hir3 PAM mutant and 7x mutant amplification Reverse: 5’- taacgaggtctgtgctgccatttttttttatcaacagaTAGTAAATGGCGAAGTAGTCTT-3’ | This paper | N/A |
| Primer: For hir3 2x mutant CRISPR-Cas9 generation Forward: 5’ TTTTTACAATCTGGCCgcaTTCTTTTTCgcaACAGATGGTGGTAATAATTGTAAATTAGT 3’ | This paper | N/A |
| Primer: For hir3 2x mutant CRISPR-Cas9 generation Reverse: 5’-tgcGGCCAGATTGTAAAAACTAG-3’ | This paper | N/A |
| Competitor ssRNA for SPR: 5’- AGCAUUCAAAGAGGAGAGGU-3’ | This paper | N/A |
| Recombinant DNA | ||
| Plasmid: Nterm-GST-TEV-S.c. Hir1(599-840), Nterm-6xHis-TEV-S.c. Hir2(471-875) (pRSFDuet-1) | This paper | N/A |
| Plasmid: Nterm-6xHis-TEV-c.t. Hir3 Full-length (pET-Duet-1) | This paper | N/A |
| Plasmid: Nterm-6xHis-TEV-c.t. Hir3 Full-length (K1358A, K1362A) (pET-Duet-1) | This paper | N/A |
| Plasmid: N-term-6xHis-TEV-S.c. Asf1 Full-length (pET-Duet-1) | This paper | N/A |
| Plasmid: N-term-6xHis-TEV-S.c. H3 Full-length (pET-Duet-1) | This paper | N/A |
| Plasmid: N-term-6xHis-TEV-S.c. H4 Full-length (pET-Duet-1) | This paper | N/A |
| Plasmid: N-term-6xHis-TEV-human H3.3 Full-length (pET-Duet-1) | Ricketts, M.D. et. al.30 | N/A |
| Plasmid: N-term-6xHis-TEV-human H4 Full-length (pET-Duet-1) | Ricketts, M.D. et. al.30 | N/A |
| Plasmid: pRS315 Shuttle Vector | Chee M. K. et. al.81 | N/A |
| Plasmid: Hir3 wildtype (pRS315) | This paper | N/A |
| Plasmid: Hir3 knockout (pRS315) | This paper | N/A |
| Plasmid: Hir3 ssDNA/RNA-channel 2x mutant, K1288A, K1292A (pRS315) | This paper | N/A |
| Plasmid: Hir3 K1336A, R1340A (pRS315) | This paper | N/A |
| Plasmid: Hir3 K1288A, K1292A, K1336A, R1340A (pRS315) | This paper | N/A |
| Plasmid: Hir3 ssDNA/RNA-channel 7x mutant, W1280A, Y1284A, K1288A, K1292A, Y1328A, K1336A, R1340A (pRS315) | This paper | N/A |
| Plasmid: Hir3 DNA-patch 4x mutant, K1482A, K1483A, R1485A, R1486A (pRS315) | This paper | N/A |
| Plasmid: Hir3 DNA-patch 6x mutant, K1482A, K1483A, R1485A, R1486A, K1558A, K1565A (pRS315) | This paper | N/A |
| Plasmid: Hir3 DNA-patch point mutation W1436A (pRS315) | This paper | N/A |
| Plasmid: Hir3 DNA-patch 3x mutant K1558A, K1565A, R1486A (pRS315) | This paper | N/A |
| Plasmid: Hir3 DNA-patch 2x mutant K1558A, K1565A (pRS315) | This paper | N/A |
| Plasmid: Hir3 C-termD 1245-1648 (pRS315) | This paper | N/A |
| Plasmid: Hir1 wildtype (pRS315) | This paper | N/A |
| Plasmid: Hir1 knockout (pRS315) | This paper | N/A |
| Plasmid: Hir1 Δ579-615 (pRS315) | This paper | N/A |
| Plasmid: Hir1 Δ598-615 (pRS315) | This paper | N/A |
| Plasmid: Hir1 B-domain mutant 493KKR/AAA495 (pRS315) | This paper | N/A |
| Plasmid: Hir1 Δ501-540 (pRS315) | This paper | N/A |
| Plasmid: Hir1 Δ436-475 (pRS315) | This paper | N/A |
| Plasmid: Hir1 Δ511-542 (pRS315) | This paper | N/A |
| Plasmid: Hir1 Δ521-540 (pRS315) | This paper | N/A |
| Plasmid: Hir1 Δ493-504 (pRS315) | This paper | N/A |
| Plasmid: Hir1 Δ476-510 (pRS315) | This paper | N/A |
| Plasmid: pCK1099 TOPO 3xFLAG NATMX | This paper | N/A |
| Plasmid: CRISPR-Cas9 single-plasmid pML107 | Laughery et. al. 79 | N/A |
| Plasmid: Annealed guide RNA oligos cloned in pML107 | This paper | N/A |
| Software and algorithms | ||
| Relion 3.1.3 | Scheres, S.H.W63 | https://github.com/3dem/relion/releases/tag/3.1.3 RRID:SCR_016274 |
| cryoSPARC 3.3.2 (ScHir) / 3.1.1 (ctHir3) | Punjani et al.62 | https://cryosparc.com/ RRID:SCR_016501 |
| Prism | GraphPad | www.graphpad.com |
| UCSF Chimera 1.16 | Pettersen et al.64 | https://www.cgl.ucsf.edu/chimera/ RRID:SCR_004097 |
| UCSF Chimera X 1.5 | Pettersen et al.73 | https://www.rbvi.ucsf.edu/chimerax/ |
| XMAS 1.1.2 | Lagerwaard et al.74 | https://cxtoolshed.rbvi.ucsf.edu/ |
| XiNet | Combe et al.72 | http://github.com/colin-combe/crosslink-viewer |
| CX-Circos 2.0 alpha | Wang, J.71 | https://cx-circos.vercel.app/ |
| Seaborn | Waskom, M. L. | https://seaborn.pydata.org/ |
| Adobe Premier Pro | Adobe | https://www.adobe.com/products/premiere.html |
| Blender 3.4.1 | Blender Foundation | https://blender.org/ |
| MolecularNodes 2.2.1 | Johnston, B. | https://github.com/BradyAJohnston/MolecularNodes |
| Adobe Illustrator | Adobe | https://www.adobe.com/products/illustrator.html |
| Phenix | Afonine et al.67 | https://www.phenix-online.org/; RRID:SCR_014224 |
| Coot | Emsley et al.66 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/; RRID:SCR_014222 |
| MeroX 2.0.1.4 | Iacobucci et al.70 | http://www.stavrox.com/ |
| ThermoRawFileParser 1.2.3 | Hulstaert et al.69 | https://github.com/compomics/ThermoRawFileParser |
| SEDPHAT | Zhao et al.75 | https://sedfitsedphat.github.io/sedphat/default.htm |
| UltraScan III | Demeler, B. | https://ultrascan3.aucsolutions.com/ |
| AlphaFold v2 | Jumper et al.45 | https://alphafold.ebi.ac.uk/ |
| RoseTTAFold2NA | Baek et al. 82 | https://github.com/uw-ipd/RoseTTAFold2NA/tree/main |
| AcquireMP | Refeyn | https://www.refeyn.com/ |
| DiscoverMP (2022 R1) | Refeyn | https://www.refeyn.com/ |
| Biacore T200 Evaluation Software 3.2.1 | Cytiva | https://www.cytivalifesciences.com/en/us/support/software/biacore-downloads/biacore-t200-software |
Highlights:
Cryo-EM unveils S. cerevisiae Hir complex structure with Asf1/H3/H4.
Higher-order dimer formed by Hir1/Hir2/Hir3/Hpc2 with 2/4/2/4 stoichiometry.
Central cavity accommodates Hpc2-assisted histone tetramer assembly.
Hir3 “Tail” supports DNA positioning for histone tetrasome formation.
Acknowledgments
We thank Drs. Paul Kaufman at the University of Massachusetts and Jerry Workman at the Stowers Institute for yeast strains for purification of the Hir complex. We thank Dr. Stefan Steimle at the University of Pennsylvania for use of equipment and assistance with cryo-EM sample screening and data collection. We thank Dr. Nir Kalisman at the Hebrew University of Jerusalem for guidance and advice on XL-MS. We thank Dr. Joel Cassel at the Wistar Institute and Dr. Michael Murphy at Cytiva for assistance with SPR. We thank Dr. Fevzi Daldal, Dr. Nur Selamoglu, Dr. Xin Xu, Lauren Gardner, and Andy Nguyen for discussion and feedback on our manuscript. We thank Claire E. Kim for the complex color scheme development. This research was supported by the following funding sources: NIH NRSA F31AG069390 (HJK), NIH R01GM123233 (KM), NIH P01-NIA-AG031862 (RM), CA196539 and HD106051 (BAG), NIH NRSA F31AG067692 (MS), NIH R35GM144116 (CDK). Cryo-EM data collection was supported by the Beckman Center at the University of Pennsylvania (RRID: SCR_022375).
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Footnotes
Declaration of interests
Authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kornberg RD (1974). Chromatin structure: a repeating unit of histones and DNA. Science 184, 868–871. 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- 2.Das C, Tyler JK, and Churchill ME (2010). The histone shuffle: histone chaperones in an energetic dance. Trends Biochem Sci 35, 476–489, 2010. S0968–0004(10)00070–8 [pii] 10.1016/j.tibs.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talbert PB, and Henikoff S (2021). Histone variants at a glance. J Cell Sci 134. 10.1242/jcs.244749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrand J, Rondinelli B, and Polo SE (2020). Histone Variants: Guardians of Genome Integrity. Cells 9. 10.3390/cells9112424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurard-Levin ZA, Quivy JP, and Almouzni G (2014). Histone chaperones: assisting histone traffic and nucleosome dynamics. Annu Rev Biochem 83, 487–517. 10.1146/annurev-biochem-060713-035536. [DOI] [PubMed] [Google Scholar]
- 6.Pardal AJ, Fernandes-Duarte F, and Bowman AJ (2019). The histone chaperoning pathway: from ribosome to nucleosome. Essays Biochem 63, 29–43. 10.1042/EBC20180055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond CM, Stromme CB, Huang H, Patel DJ, and Groth A (2017). Histone chaperone networks shaping chromatin function. Nat Rev Mol Cell Biol 18, 141–158. 10.1038/nrm.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W, Zhu Y, Dong A, and Shen WH (2015). Histone H2A/H2B chaperones: from molecules to chromatin-based functions in plant growth and development. Plant J 83, 78–95. 10.1111/tpj.12830. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, and Vasudevan D (2020). Structure-function relationship of H2A-H2B specific plant histone chaperones. Cell Stress Chaperones 25, 1–17. 10.1007/s12192-019-01050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mousson F, Ochsenbein F, and Mann C (2007). The histone chaperone Asf1 at the crossroads of chromatin and DNA checkpoint pathways. Chromosoma 116, 79–93. 10.1007/s00412-006-0087-z. [DOI] [PubMed] [Google Scholar]
- 11.Loyola A, and Almouzni G (2004). Histone chaperones, a supporting role in the limelight. Biochimica et Biophysica Acta 1677, 3–11. [DOI] [PubMed] [Google Scholar]
- 12.McBryant SJ, Park YJ, Abernathy SM, Laybourn PJ, Nyborg JK, and Luger K (2003). Preferential binding of the histone (H3-H4)2 tetramer by NAP1 is mediated by the amino-terminal histone tails. J Biol Chem 278, 44574–44583. 10.1074/jbc.M305636200. [DOI] [PubMed] [Google Scholar]
- 13.Bowman A, Ward R, Wiechens N, Singh V, El-Mkami H, Norman DG, and Owen-Hughes T (2011). The histone chaperones Nap1 and Vps75 bind histones H3 and H4 in a tetrameric conformation. Mol Cell 41, 398–408. 10.1016/j.molcel.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilar-Gurrieri C, Larabi A, Vinayachandran V, Patel NA, Yen K, Reja R, Ebong IO, Schoehn G, Robinson CV, Pugh BF, and Panne D (2016). Structural evidence for Nap1-dependent H2A-H2B deposition and nucleosome assembly. EMBO J 35, 1465–1482. 10.15252/embj.201694105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamiche A, and Shuaib M (2013). Chaperoning the histone H3 family. Biochim Biophys Acta 1819, 230–237. 10.1016/j.bbagrm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Das C, and Tyler JK (2013). Histone exchange and histone modifications during transcription and aging. Biochim Biophys Acta 1819, 332–342. 10.1016/j.bbagrm.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bano D, Piazzesi A, Salomoni P, and Nicotera P (2017). The histone variant H3.3 claims its place in the crowded scene of epigenetics. Aging (Albany NY) 9, 602–614. 10.18632/aging.101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Y, Poustovoitov MV, Zhao K, Garfinkel M, Canutescu A, Dunbrack R, Adams PD, and Marmorstein R (2006). Structure of a human ASF1a-HIRA complex and insights into specificity of histone chaperone complex assembly. Nat Struct Mol Biol 13, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tagami H, Ray-Gallet D, Almouzni G, and Nakatani Y (2004). Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116, 51–61. 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, et al. (2005). Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell 8, 19–30. 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Mello JA, Sillje HH, Roche DM, Kirschner DB, Nigg EA, and Almouzni G (2002). Human Asf1 and CAF-1 interact and synergize in a repair-coupled nucleosome assembly pathway. EMBO Rep 3, 329–334. 10.1093/embo-reports/kvf068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu WH, Roemer SC, Zhou Y, Shen ZJ, Dennehey BK, Balsbaugh JL, Liddle JC, Nemkov T, Ahn NG, Hansen KC, et al. (2016). The Cac1 subunit of histone chaperone CAF-1 organizes CAF-1-H3/H4 architecture and tetramerizes histones. Elife 5. 10.7554/eLife.18023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkler DD, Zhou H, Dar MA, Zhang Z, and Luger K (2017). Yeast CAF-1 assembles histone (H3-H4) 2 tetramers prior to DNA deposition. Nucleic Acids Res 45, 9811–9812. 10.1093/nar/gkx657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamour V, Lecluse Y, Desmaze C, Spector M, Bodescot M, Aurias A, Osley MA, and Lipinski M (1995). A human homolog of the S. cerevisiae HIR1 and HIR2 transcriptional repressors cloned from the DiGeorge syndrome critical region. Hum Mol Genet 4, 791–799. [DOI] [PubMed] [Google Scholar]
- 25.Spector MS, and Osley MA (1993). The HIR4–1 mutation defines a new class of histone regulatory genes in Saccharomyces cerevisiae. Genetics 135, 25–34. 10.1093/genetics/135.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherwood PW, Tsang SV, and Osley MA (1993). Characterization of HIR1 and HIR2, two genes required for regulation of histone gene transcription in Saccharomyces cerevisiae. Mol Cell Biol 13, 28–38. 10.1128/mcb.13.1.28-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricketts MD, and Marmorstein R (2017). A Molecular Prospective for HIRA Complex Assembly and H3.3-Specific Histone Chaperone Function. J Mol Biol 429, 1924–1933. 10.1016/j.jmb.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricketts MD, Dasgupta N, Fan J, Han J, Gerace M, Tang Y, Black BE, Adams PD, and Marmorstein R (2019). The HIRA histone chaperone complex subunit UBN1 harbors H3/H4- and DNA-binding activity. J Biol Chem 294, 9239–9259. 10.1074/jbc.RA119.007480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray-Gallet D, Ricketts MD, Sato Y, Gupta K, Boyarchuk E, Senda T, Marmorstein R, and Almouzni G (2018). Functional activity of the H3.3 histone chaperone complex HIRA requires trimerization of the HIRA subunit. Nat Commun 9, 3103. 10.1038/s41467-018-05581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricketts MD, Frederick B, Hoff H, Tang Y, Schultz DC, Singh Rai T, Grazia Vizioli M, Adams PD, and Marmorstein R (2015). Ubinuclein-1 confers histone H3.3-specific-binding by the HIRA histone chaperone complex. Nat Commun 6, 7711. 10.1038/ncomms8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banumathy G, Somaiah N, Zhang R, Tang Y, Hoffmann J, Andrake M, Ceulemans H, Schultz D, Marmorstein R, and Adams PD (2009). Human UBN1 is an ortholog of yeast Hpc2p and has an essential role in the HIRA/ASF1a chromatin-remodeling pathway in senescent cells. Mol Cell Biol 29, 758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torne J, Ray-Gallet D, Boyarchuk E, Garnier M, Le Baccon P, Coulon A, Orsi GA, and Almouzni G (2020). Two HIRA-dependent pathways mediate H3.3 de novo deposition and recycling during transcription. Nat Struct Mol Biol 27, 1057–1068. 10.1038/s41594-020-0492-7. [DOI] [PubMed] [Google Scholar]
- 33.Ray-Gallet D, Woolfe A, Vassias I, Pellentz C, Lacoste N, Puri A, Schultz DC, Pchelintsev NA, Adams PD, Jansen LE, and Almouzni G (2011). Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol Cell 44, 928–941. 10.1016/j.molcel.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Ray-Gallet D, Quivy JP, Scamps C, Martini EM, Lipinski M, and Almouzni G (2002). HIRA is critical for a nucleosome assembly pathway independent of DNA synthesis. Mol Cell 9, 1091–1100. [DOI] [PubMed] [Google Scholar]
- 35.Delaney K, and Almouzni G (2023). Transcription-coupled H3.3 recycling: A link with chromatin states. Semin Cell Dev Biol 135, 13–23. 10.1016/j.semcdb.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Adam S, Polo SE, and Almouzni G (2013). Transcription recovery after DNA damage requires chromatin priming by the H3.3 histone chaperone HIRA. Cell 155, 94–106. 10.1016/j.cell.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 37.Tang Y, Puri A, Ricketts MD, Rai TS, Hoffmann J, Hoi E, Adams PD, Schultz DC, and Marmorstein R (2012). Identification of an Ubinuclein 1 Region Required for Stability and Function of the Human HIRA/UBN1/CABIN1/ASF1a Histone H3.3 Chaperone Complex. Biochemistry 51, 2366–2377, 2012. 10.1021/bi300050b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rai TS, Puri A, McBryan T, Hoffman J, Tang Y, Pchelintsev NA, van Tuyn J, Marmorstein R, Schultz DC, and Adams PD (2011). Human CABIN1 is a functional member of the human HIRA/UBN1/ASF1a histone H3.3 chaperone complex. Mol Cell Biol 31, 4107–4118. 10.1128/MCB.05546-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandt WF, Patterson K, and von Holt C (1980). The histones of yeast. The isolation and partial structure of the core histones. Eur J Biochem 110, 67–76. 10.1111/j.1432-1033.1980.tb04841.x. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman PD, Cohen JL, and Osley MA (1998). Hir proteins are required for position-dependent gene silencing in Saccharomyces cerevisiae in the absence of chromatin assembly factor I. Mol Cell Biol 18, 4793–4806. 10.1128/MCB.18.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green EM, Antczak AJ, Bailey AO, Franco AA, Wu KJ, Yates JR 3rd, and Kaufman PD (2005). Replication-independent histone deposition by the HIR complex and Asf1. Curr Biol 15, 2044–2049. 10.1016/j.cub.2005.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufman PD, Kobayashi R, and Stillman B (1997). Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev 11, 345–357. 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- 43.Vishnoi N, Flaherty K, Hancock LC, Ferreira ME, Amin AD, and Prochasson P (2011). Separation-of-function mutation in HPC2, a member of the HIR complex in S. cerevisiae, results in derepression of the histone genes but does not confer cryptic TATA phenotypes. Biochim Biophys Acta 1809, 557–566, 2011. S1874–9399(11)00109-X [pii] 10.1016/j.bbagrm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prochasson P, Florens L, Swanson SK, Washburn MP, and Workman JL (2005). The HIR corepressor complex binds to nucleosomes generating a distinct protein/DNA complex resistant to remodeling by SWI/SNF. Genes & Development 19, 2534–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang JH, Song TY, Jo C, Park J, Lee HY, Song I, Hong S, Jung KY, Kim J, Han JW, et al. (2016). Differential regulation of the histone chaperone HIRA during muscle cell differentiation by a phosphorylation switch. Exp Mol Med 48, e252. 10.1038/emm.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung V, Chua G, Batada NN, Landry CR, Michnick SW, Hughes TR, and Winston F (2008). Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol 6, e277. 10.1371/journal.pbio.0060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva AC, Xu X, Kim HS, Fillingham J, Kislinger T, Mennella TA, and Keogh MC (2012). The replication-independent histone H3-H4 chaperones HIR, ASF1, and RTT106 co-operate to maintain promoter fidelity. J Biol Chem 287, 1709–1718. 10.1074/jbc.M111.316489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zasadzińska E, Barnhart-Dailey MC, Kuich PHJL, and Foltz DR (2013). Dimerization of the CENP-A assembly factor HJURP is required for centromeric nucleosome deposition. The EMBO Journal 32, 2113–2124. 10.1038/emboj.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fazly A, Li Q, Hu Q, Mer G, Horazdovsky B, and Zhang Z (2012). Histone Chaperone Rtt106 Promotes Nucleosome Formation Using (H3-H4)2 Tetramers*. Journal of Biological Chemistry 287, 10753–10760. 10.1074/jbc.M112.347450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattiroli F, Gu Y, Yadav T, Balsbaugh JL, Harris MR, Findlay ES, Liu Y, Radebaugh CA, Stargell LA, Ahn NG, et al. (2017). DNA-mediated association of two histone-bound complexes of yeast Chromatin Assembly Factor-1 (CAF-1) drives tetrasome assembly in the wake of DNA replication. Elife 6. 10.7554/eLife.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He C, Sidoli S, Warneford-Thomson R, Tatomer DC, Wilusz JE, Garcia BA, and Bonasio R (2016). High-Resolution Mapping of RNA-Binding Regions in the Nuclear Proteome of Embryonic Stem Cells. Mol Cell 64, 416–430. 10.1016/j.molcel.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baek MM, R.; Anishchenko I.; Baker D.; DiMaio F. (2022). Accurate prediction of nucleic acid and protein-nucleic acid complexes using RoseTTAFoldNA. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sauer PV, Timm J, Liu D, Sitbon D, Boeri-Erba E, Velours C, Mucke N, Langowski J, Ochsenbein F, Almouzni G, and Panne D (2017). Insights into the molecular architecture and histone H3-H4 deposition mechanism of yeast Chromatin assembly factor 1. Elife 6. 10.7554/eLife.23474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clément Rouillon BVE, Kollenstart Leonie, Gruss Fabian, Verkennis Alexander E.E., Rondeel Inge, Krijger Peter H.L., Ricci Giulia, Biran Alva, Laar Theo van, Delvaux de Fenffe Charlotte M., Luppens Georgiana, Albanese Pascal, Scheltema Richard A., Laat Wouter de, Dekker Nynke H., Groth Anja, View ORCID ProfileFrancesca Mattiroli (2022). CAF-1 deposits newly synthesized histones during DNA replication using distinct mechanisms on the leading and lagging strands. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu CP, Yu Z, Xiong J, Hu J, Song A, Ding D, Yu C, Yang N, Wang M, Yu J, et al. (2023). Structural insights into histone binding and nucleosome assembly by chromatin assembly factor-1. Science 381, eadd8673. 10.1126/science.add8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dennehey BK, Noone S, Liu WH, Smith L, Churchill ME, and Tyler JK (2013). The C terminus of the histone chaperone Asf1 cross-links to histone H3 in yeast and promotes interaction with histones H3 and H4. Mol Cell Biol 33, 605–621. 10.1128/MCB.01053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karpenahalli MR, Lupas AN, and Soding J (2007). TPRpred: a tool for prediction of TPR-, PPR- and SEL1-like repeats from protein sequences. BMC Bioinformatics 8, 2. 10.1186/1471-2105-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dann GP, Liszczak GP, Bagert JD, Muller MM, Nguyen UTT, Wojcik F, Brown ZZ, Bos J, Panchenko T, Pihl R, et al. (2017). ISWI chromatin remodellers sense nucleosome modifications to determine substrate preference. Nature 548, 607–611. 10.1038/nature23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharp JA, Fouts ET, Krawitz DC, and Kaufman PD (2001). Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr. Biol. 11, 463–473. [DOI] [PubMed] [Google Scholar]
- 61.Cox J, and Mann M (2008). MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26, 1367–1372. 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 62.Punjani A, Rubinstein JL, Fleet DJ, and Brubaker MA (2017). cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nature Methods 14, 290–296. 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 63.Scheres SHW (2012). RELION: Implementation of a Bayesian approach to cryo-EM structure determination. Journal of Structural Biology 180, 519–530. 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 65.Pintilie G, and Chiu W (2012). Comparison of Segger and other methods for segmentation and rigid-body docking of molecular components in Cryo-EM density maps. Biopolymers 97, 742–760. 10.1002/bip.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta crystallographica. Section D, Biological crystallography 66, 486–501. 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afonine PV, Klaholz BP, Moriarty NW, Poon BK, Sobolev OV, Terwilliger TC, Adams PD, and Urzhumtsev A (2018). New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr D Struct Biol 74, 814–840. 10.1107/S2059798318009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slavin M, and Kalisman N (2018). Structural Analysis of Protein Complexes by Cross-Linking and Mass Spectrometry. In Protein Complex Assembly: Methods and Protocols, Marsh JA, ed. (Springer; New York: ), pp. 173–183. 10.1007/978-1-4939-7759-8_11. [DOI] [PubMed] [Google Scholar]
- 69.Hulstaert N, Shofstahl J, Sachsenberg T, Walzer M, Barsnes H, Martens L, and Perez-Riverol Y (2020). ThermoRawFileParser: Modular, Scalable, and Cross-Platform RAW File Conversion. J Proteome Res 19, 537–542. 10.1021/acs.jproteome.9b00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iacobucci C, Gotze M, Ihling CH, Piotrowski C, Arlt C, Schafer M, Hage C, Schmidt R, and Sinz A (2018). A cross-linking/mass spectrometry workflow based on MS-cleavable cross-linkers and the MeroX software for studying protein structures and protein-protein interactions. Nat Protoc 13, 2864–2889. 10.1038/s41596-018-0068-8. [DOI] [PubMed] [Google Scholar]
- 71.Wang J CX-Circos, In Preparation https://cx-circos.vercel.app/. [Google Scholar]
- 72.Combe CW, Fischer L, and Rappsilber J (2015). xiNET: cross-link network maps with residue resolution. Mol Cell Proteomics 14, 1137–1147. 10.1074/mcp.O114.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, and Ferrin TE (2021). UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci 30, 70–82. 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lagerwaard IM, Albanese P, Jankevics A, and Scheltema RA (2022). Xlink Mapping and AnalySis (XMAS) - Smooth Integrative Modeling in ChimeraX. bioRxiv, 2022.2004.2021.489026. 10.1101/2022.04.21.489026. [DOI] [Google Scholar]
- 75.Zhao H, Piszczek G, and Schuck P (2015). SEDPHAT – A platform for global ITC analysis and global multi-method analysis of molecular interactions. Methods 76, 137–148. 10.1016/j.ymeth.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cole JL (2004). Analysis of heterogeneous interactions. Methods Enzymol 384, 212–232. 10.1016/S0076-6879(04)84013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schuck P (2003). On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal Biochem 320, 104–124. 10.1016/s0003-2697(03)00289-6. [DOI] [PubMed] [Google Scholar]
- 78.Laughery MF, Hunter T, Brown A, Hoopes J, Ostbye T, Shumaker T, and Wyrick JJ (2015). New vectors for simple and streamlined CRISPR-Cas9 genome editing in Saccharomyces cerevisiae. Yeast 32, 711–720. 10.1002/yea.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laughery MF, and Wyrick JJ (2019). Simple CRISPR-Cas9 Genome Editing in Saccharomyces cerevisiae. Curr Protoc Mol Biol 129, e110. 10.1002/cpmb.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gietz RD, and Schiestl RH (2007). High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2, 31–34. 10.1038/nprot.2007.13. [DOI] [PubMed] [Google Scholar]
- 81.Chee MK, and Haase SB (2012). New and Redesigned pRS Plasmid Shuttle Vectors for Genetic Manipulation of Saccharomycescerevisiae. G3 (Bethesda) 2, 515–526. 10.1534/g3.111.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baek M, McHugh R, Anishchenko I, Jiang H, Baker D, and DiMaio F (2024). Accurate prediction of protein-nucleic acid complexes using RoseTTAFoldNA. Nat Methods 21, 117–121. 10.1038/s41592-023-02086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Crosslinking mass spectrometry search result of the Hir complex alone, related to Figure 2. (separate file)
MeroX search results were combined and curated to show only unique site-pairs in the table. All the columns after “unknown 2” column were generated during the postprocessing to generate unique site-pair entries, UCSF ChimeraX XMAS, and CX-Circos input files.
Data S2 Crosslinking mass spectrometry search result of the Hir complex bound to Asf1/H3/H4, related to Figure 2. (separate file)
Same as Data S1, but with Asf1/H3/H4.
Movie S1 Composite map of the Hir complex bound to Asf1/H3/H4, related to Figure 1 and 7.
The composite map was generated by combining Maps1–3. Models and color codes are as in Figure 1. The Movie was created by Chimera 64.
Movie S2 Slab views of composite map of the Hir complex bound to Asf1/H3/H4, related to Figure 1, 3, 4, 5, and 7.
Slab views to show the model fitting. Models and color codes are as in Figure 1. The Movie was created by Chimera 64.
Movie S3 Sequential ribbon display of the Hir complex, related to Figure 1, 3, 4, 5, and 7.
Models and color codes are as in Figure 1. The Movie was created by Chimera64.
Movie S4 Overall view of Hir complex and proposed model of recruitment of Asf1/H3/H4, related to Figure 1, 3, 4, 5, and 7.
The movie was generated in Blender 3.4.1 with MolecularNodes 2.2.1 and the annotations and final movie export were performed in Adobe Premiere Pro 23.2.0.
Data Availability Statement
The cryo-EM density maps of the Hir complex bound to Asf1/H3/H4 were deposited in the Electron Microscopy Data Bank (EMDB) (Map 1: EMD-40006, Map 2: EMD-40029, Map 3: EMD-40030, Composite Map: EMD-40037) and associated atomic coordinates were deposited in the Protein Data Bank (PDB IDs: 8GHL, 8GHA, 8GHM, and 8GHN respectively). The cryo-EM density map of ctHir3 was deposited in EMD-B (Map 4: EMD40078) and associated atomic coordinate was deposited in PDB (8GIX). Bottom-up proteomics and crosslinking mass spectrometry raw data, protein databases, and search results were deposited in the Proteomics Identifications Database (PRIDE) Archive (PXD040705). Raw pictures of SDS-PAGE gels, plates and representative micrographs were deposited in Mendeley Data: http://dx.doi.org/10.17632/7fbn428nsp.1
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


