Abstract
The yeast peroxisomal adenine nucleotide carrier, Ant1p, was shown to catalyse unidirectional transport in addition to exchange of substrates. In both transport modes, proton movement occurs. Nucleotide hetero-exchange is H+-compensated and electroneutral. Furthermore, microscopic fluorescence imaging of a pH-sensitive green fluorescent protein targeted to peroxisomes shows that Ant1p is involved in the formation of a ΔpH across the peroxisomal membrane, acidic inside.
Keywords: adenine nucleotide transport, bioenergetics, microscopic fluorescence imaging, mitochondrial carrier family, peroxisome, pH homoeostasis
Abbreviations: Ant1p, peroxisomal adenine nucleotide transporter from S. cerevisiae; GFP, green fluorescent protein; perox-pHluorin, a peroxisomally targeted version of pHluorin; PTS, peroxisomal targeting signal; R405/485, ratiomeric fluorescence value obtained at 405/485 nm excitation
INTRODUCTION
Evidence has accumulated over the last decade that peroxisomal membranes are impermeable to small solutes in vivo suggesting the existence of specific transporters for substrates and cofactors that maintain metabolic communication between the lumen of peroxisomes and the cytosol [1]. Recently, we identified the peroxisomal adenine nucleotide transporter from Saccharomyces cerevisiae, Ant1p, as the first functionally characterized peroxisomal transporter [2]. Upon reconstitution into liposomes, Ant1p was shown to catalyse a specific exchange reaction of ATP, ADP, and AMP [2]. Knock-out of Ant1p activity results in an inability of yeast cells to grow on medium-chain fatty acids as sole carbon source, while the utilization of long-chain fatty acids or peroxisomal protein import is not affected [2,3]. Thus a major physiological role of Ant1p is to import into the peroxisomal lumen cytosolic ATP in exchange for AMP that is generated from ATP in the activation of medium-chain fatty acids, a process occurring in yeast exclusively inside the peroxisomes [2].
Whether the peroxisomal membrane is also impermeable to protons is still a matter of debate [4–6]. Here we show that Ant1p catalyses not only the exchange but also the unidirectional transport of adenine nucleotides. In both modes, transport of adenine nucleotides involves proton movement. By fluorescence imaging of a specifically targeted pH-sensitive probe in living yeast cell, we provide evidence for the existence of a pH gradient (ΔpH) between the peroxisomal lumen and the cytosol. The ΔpH between peroxisomes and the cytosol is drastically reduced in the absence of Ant1p indicating that Ant1p-mediated transport is a major factor involved in ΔpH formation across the peroxisomal membrane.
EXPERIMENTAL
Transport assays
His6-tagged Ant1p was expressed at high-level in S. cerevisiae and, following purification by affinity chromatography, reconstituted into liposomes as reported previously [2]. External substrate was removed from proteoliposomes on a Sephadex G-75 column pre-equilibrated with 50 mM NaCl and 10 mM Pipes, pH 7.0 (unless otherwise indicated). Transport at 25 °C was started by adding labelled adenine nucleotides to substrate-loaded proteoliposomes (exchange) or to unloaded proteoliposomes (uniport), and terminated by addition of 30 mM pyridoxal 5′-phosphate and 10 mM bathophenanthroline (the ‘inhibitor-stop’ method [7]). K+-diffusion potentials were generated by adding valinomycin (1 μg/mg of phospholipid) to proteoliposomes in the presence of different KCl gradients. For the formation of an artificial pH gradient (ΔpH), nigericin (50 ng/mg of phospholipid) was added to proteoliposomes instead of valinomycin. In these experiments, proteoliposomes were eluted from Sepahadex G75 columns using 0.5 mM Pipes, pH 7, and different amounts of KCl were added, together with the labelled substrate. NaCl was also added as required to maintain the external osmolarity constantly at 100 mM. For efflux measurements, the proteoliposomes containing 5 mM ATP and 100 mM Mes/100 mM Hepes pH 6.0, were prelabelled by carrier-mediated exchange equilibration [7]. External substrate and buffer were removed from proteoliposomes on a Sephadex G-75 column in the presence of NaCl 50 mM and 1 mM Mes/1 mM Hepes, pH 6.0. Efflux was started by adding 20 mM Mes/20 mM Hepes at different pHs.
Plasmid construction and expression of ‘pHluorin’
pHluorin, a ratiometric pH-sensitive green fluorescent protein (GFP) ([8]; a gift from Dr James E. Rothman, Memorial Sloan–Kettering Cancer Center, New York, U.S.A.) was PCR amplified with primer pair RE801 (5′-TTAAGAATTCATGAGTAAAGGAGAAGAACTTTTC-3′) and RE802 (5′-TTAAGTCGACTTATTTGTATAGTTCATCCATGC-3′). The PCR product was cloned as an EcoRI–SalI fragment into the yeast expression vector pRSFOXpterm [9], harbouring the oleate-inducible FOX3 promoter (pHPR258). Plasmid pHPR282, designed to express a peroxisome-targeted pHluorin by appending the peroxisomal targeting signal-SKL to its C-terminus, was constructed in the same way but with the primer pair RE801 and RE803 (5′-TTAAGTCGACTTATAATTTGGATTTGTATAGTTCATCCATGC-3′). The constructs were transformed into the ant1Δ and pex13Δ yeast strains (EUROSCARF, Frankfurt, Germany) and the isogenic wild-type strain BY4741 (MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0). Transformants were selected for uracil prototrophy and precultured in synthetic minimal medium supplemented with 2% glucose. To induce the expression of cytosolic or peroxisomal pHluorin, yeasts were grown in Rytka medium [0.1% glucose, 0.1% yeast extract, 0.17% yeast nitrogen base (without amino acids), 0.5% ammonium sulphate and amino acids as required, pH 6] supplemented with 0.1% oleate and 0.25% Tween 80 for 12–14 h.
Image acquisition and analysis
For image acquisition, cells grown to mid-log phase were harvested by centrifugation at 500 g for 2 min at 22 °C, and washed twice with fresh medium. Aliquots of cell suspension (10 μl) were immobilized on to a coverslip in the presence of 0.5% low-melting-point agarose, and the cells were analysed at 25 °C using a Zeiss Axiovert 200 inverted epifluorescence microscope equipped with a 100×/1.30 Ph3 oil objective. Excitation of pHluorin was accomplished by alternating 405DF10 nm and 485BP20 nm excitation filters mounted in a Lambda 10-2 filter wheel controller (Sutter Instruments, Novato, CA, U.S.A.). Emission fluorescence from approx. 20–25 fluorescent yeast cells was selected through 540BP25 nm emission filter and acquired with a CoolSNAP HQ charge-coupled device camera (Roper Scientific, Trenton NJ, U.S.A.) using the MetaFluor 4.6 software (Universal Imaging Corporation, Downington, PA, U.S.A.). For each measurement, data from at least 5 independent images were acquired. The average emission intensities at the excitation wavelengths 405 nm and 485 nm were measured for 10 min subtracting the background. During this period of time, the 405/485 nm excitation ratio remained stable within ±5%. In the case of perox-pHluorin (a peroxisomally targeted version of pHluorin), data were obtained from the fluorescence of large regions within an individual cell that contained clusters of peroxisomes. Data acquisition was repeated 20–25 times using cells from independent cultures. All data are given as means±S.E.M.
RESULTS
Ant1p catalyses unidirectional transport of adenine nucleotides in addition to their exchange
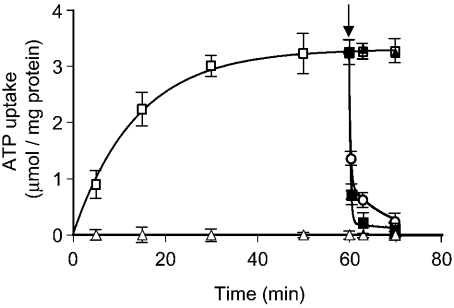
To investigate the possibility that Ant1p catalyses unidirectional transport of adenine nucleotides, uptake of [14C]ATP by ‘empty’ proteoliposomes (i.e. by proteoliposomes that had not been preloaded with any substrate) was studied in detail. Figure 1 shows the time-course of 0.5 mM [14C]ATP uptake in the absence of internal substrate. The data fitted a first-order rate equation. No activity was detected when inhibitors were added to proteoliposomes together with labelled substrate. Proteoliposomes also took up AMP and, with much lower efficiency, ADP (results not shown), whereas no uptake was detected with any of the other substrates tested (GTP, phosphate, malate, malonate, oxoglutarate, citrate, glutamate and ornithine). Addition of 10 mM unlabelled adenine nucleotides (ATP, ADP and AMP) to proteoliposomes after 60 min (i.e. when the radioactive uptake had approached equilibrium) caused an extensive efflux of radioactivity (Figure 1), indicating that the [14C]ATP taken up by uniport can be rapidly released by exchange for externally added substrates. No efflux was observed when GTP instead of ATP was added externally (Figure 1) or when Ant1p inhibitors were added together with ATP, ADP or AMP (results not shown). Altogether, these data indicate that Ant1p catalyses unidirectional transport of adenine nucleotides in addition to their exchange.
Figure 1. Time course of [14C]ATP uptake into proteoliposomes and its efflux after addition of unlabelled substrates.
[14C]ATP (0.5 mM) was added to unloaded proteoliposomes in the presence (▵) or the absence (□) of inhibitors (30 mM pyridoxal 5′-phosphate plus 10 mM bathophenantroline). Data are means±S.E.M. (error bars) for three independent experiments. The arrow indicates the addition of 10 mM unlabelled ATP (▪), ADP (○) or GTP (▴).
Ant1p-mediated uniport depends on the transmembrane pH gradient
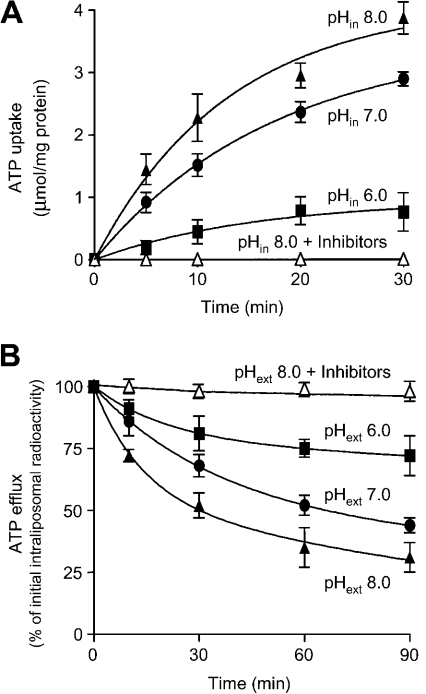
Several members of the mitochondrial carrier family have been shown to catalyse unidirectional transport of substrates in addition to their exchange. For charged substrates, the uniport is made electroneutral by simultaneous proton transport either in the same direction as substrate (for negatively charged substrates) or in the opposite direction (for positively charged substrates) [10–12]. In both cases the amount of substrate transported is dependent on the pH difference across the membrane [10–12]. We therefore analysed the influence of intraliposomal pH (at a fixed external pH of 6.0) on the unidirectional uptake of [14C]ATP by Ant1p. The amount of substrate taken up by uniport increased markedly with increasing internal pH from 6.0 to 8.0 (Figure 2A), suggesting that Ant1p catalyses the transport of protons in the same direction as nucleotides. The ΔpH dependence of adenine nucleotide uniport was confirmed by measuring the efflux of [14C]ATP from prelabelled active proteoliposomes (Figure 2B). Upon removal of external substrate we observed a significant efflux of intraliposomal radioactivity that was stimulated by increasing external pH from 6.0 to 8.0 and was prevented by the presence of Ant1p inhibitors (Figure 2B).
Figure 2. Dependence of ATP uniport on transmembrane pH gradient.
(A) Uptake of [14C]ATP by proteoliposomes reconstituted with Ant1p. Proteoliposomes were reconstituted in the presence of 100 mM Mes and 100 mM Hepes at pH 6.0 (▪), pH 7.0 (•) or pH 8.0 (▴) or pH 8.0 in the presence of inhibitors (30 mM pyridoxal 5′-phosphate and 10 mM bathophenantroline) (▵). External substrate and buffer were removed from proteoliposomes on a Sephadex G-75 column in the presence of 50 mM NaCl and 1 mM Mes/1 mM Hepes at the same pH as in the reconstitution mixture. Transport was started by adding 0.5 mM [14C]ATP and 20 mM Mes/20 mM Hepes at pH 6.0. (B) Efflux of [14C]ATP from proteoliposomes reconstituted with Ant1p. After carrier-mediated exchange equilibration and removal of external substrate and buffer by Sephadex G-75 chromatography, the efflux of [14C]ATP was started by adding 20 mM Mes/20 mM Hepes pH 6.0 (▪), pH 7.0 (•), pH 8.0 (▴), or pH 8.0 in the presence of inhibitors (30 mM pyridoxal 5′-phosphate and 10 mM bathophenantroline) (▵). Data are means±S.E.M. (error bars) for four independent experiments.
Ant1p-mediated hetero-exchange is electroneutral and H+-compensated
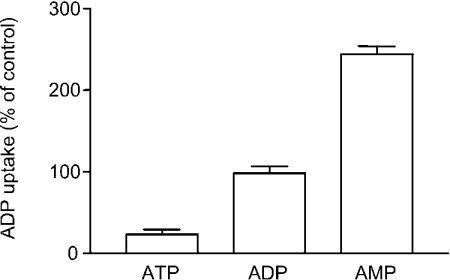
To investigate whether protons were also involved in the exchange transport mode, we first studied the influence of the membrane potential on nucleotide exchange catalysed by Ant1p. A K+-diffusion potential (calculated value approx. 100 mV, positive inside) was generated across the proteoliposomal membrane using valinomycin in the presence of an inwardly directed K+ gradient (1/50, mM/mM, in/out). In these experiments ADP exchange for other adenine nucleotides was measured as ADP is transported by uniport much less efficiently than ATP and AMP. The uptake of [14C]ADP by proteoliposomes reconstituted with Ant1p and containing 20 mM of either ATP, ADP or AMP was not influenced by the addition of valinomycin (results not shown). In contrast, addition of nigericin, a K+/H+ exchanger that generates a transmembrane ΔpH under these conditions (acidic outside), caused a 4-fold reduction of ADP exchange for ATP and a 2.5-fold increase of ADP exchange for AMP (Figure 3). The ADP/ADP homo-exchange was not significantly influenced by the addition of nigericin (Figure 3). Furthermore, neither valinomycin nor nigericin caused any effect when added in the absence of a K+ gradient (1/1 or 50/50, mM/mM, in/out) (results not shown).
Figure 3. Effect of pH gradient on the uptake of ADP by proteoliposomes reconstituted with Ant1p and containing different nucleotides.
The proteoliposomes contained 1 mM KCl and 20 mM of either ATP, ADP or AMP. The exchange reaction was started by adding 0.25 mM [14C]ADP and 50 mM KCl. Nigericin (50 ng/mg of phospholipid) was added in 10 μl of ethanol/ml of proteoliposomes immediately prior to start transport. In control samples ethanol alone was added. Data±S.E.M. (error bars) from four replicates are presented as the percentage of ADP uptake in controls.
In another set of experiments the uptake of ADP and AMP into liposomes containing 20 mM ATP were found to be inhibited (by 23% and by 67% respectively) in the presence of nigericin and a K+ gradient as described above (results not shown). No significant effect was observed when ATP/ATP homo-exchange was measured or when valinomycin was added instead of nigericin. Taken together, these results indicate that the exchange of adenine nucleotides catalysed by Ant1p is electroneutral and, as found for the uniport mode, nucleotide hetero-exchange in either direction is driven by ΔpH, indicating that the charge imbalance of the exchanged substrates is compensated by the movement of protons.
Peroxisomal pH is different from that of the cytosol
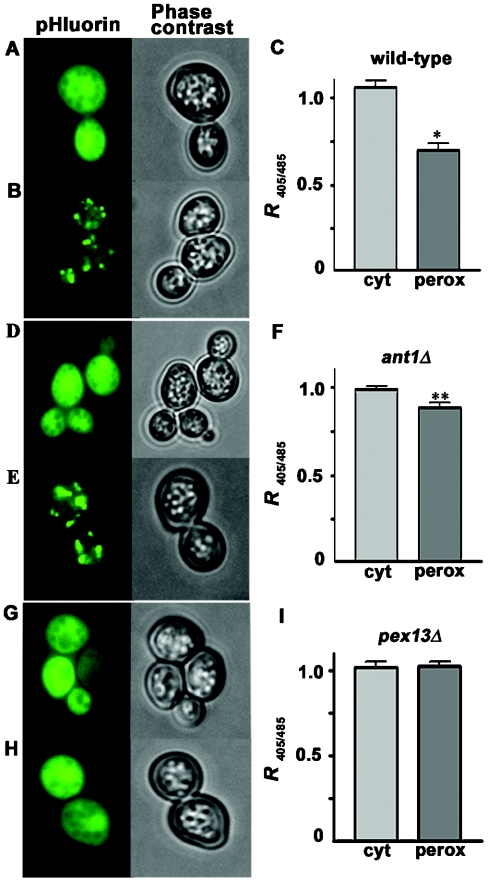
We then asked whether the electroneutral H+-compensated nucleotide transport catalysed by Ant1p would be physiologically influenced by ΔpH in its native habitat, and whether in turn this transport activity could affect peroxisomal pH homoeostasis. To tackle this issue, we used an engineered pH-sensitive GFP, referred to as ‘pHluorin’ [8]. Unlike wild-type GFP, pHluorin shows a striking pH-dependence of the relative emission intensities of the maxima of its bimodal excitation spectrum and therefore can be used as an accurate pH indicator that is particularly suited for small, mobile organelles like peroxisomes. Following expression of pHluorin in the cytosol of a wild-type strain, yeast cells displayed a diffuse and bright green fluorescence with equal intensities at 405 nm and 485 nm, the two pHluorin excitation maxima (Figures 4A and 4C). Expression of perox-pHluorin in the same strain resulted in a punctate staining pattern (Figures 4B and 4E) indicative of a peroxisomal localization. The peroxisomal identity of these structures was demonstrated by co-localization with the marker protein PTS2–DsRed [8] (results not shown). Furthermore, expression of perox-pHluorin in a pex13Δ mutant strain defective in peroxisomal matrix protein import [13], gave rise to a diffuse staining pattern (Figure 4H). Upon fluorescence imaging of perox-pHluorin, the ratiomeric fluorescence values obtained at 405/485 nm excitation (R405/485) were markedly lower than those measured with the same probe in the cytosol (Figures 4B and 4C). Differences between cytosolic and perox-pHluorin did not depend on the presence of the SKL tag, as demonstrated by the data obtained in a pex13Δ mutant where R405/485 was virtually the same for pHluorin and perox-pHluorin (Figure 4I). These results point to the existence of a pH gradient across the peroxisomal membrane.
Figure 4. Subcellular localization of pHluorin and determination of R405/485 in wild-type and ant1Δ cells.
pHluorin (A, D and G) and a peroxisomal version, perox-pHluorin (B, E and H), were expressed in wild-type strain BY4741 (A and B) or in the otherwise isogenic ant1Δ (D and E) and pex13Δ (G and H) mutant strains. Phase contrast images show the positions of the cells. (C, F and I), calculated R405/485 (means±S.E.M.), of the fluorescence emissions from the cytosol and peroxisomes from wild-type (C), ant1Δ (F) and pex13Δ (I) cells. Data are given as means±S.E.M. (error bars) for 20–25 separate cell cultures. For each culture, five replicate values were obtained. The statistical significance of differences between the cytosolic and the peroxisomal R405/485 values were calculated by the unpaired Student's t test with Bonferroni's correction. *P<0.0005, **P<0.05.
Ant1p is involved in pH gradient formation in peroxisomes
The R405/485 for pHluorin was virtually the same in ant1Δ mutant and wild-type cells (Figures 4C and 4F). By contrast, R405/485 for perox-pHluorin was significantly higher (P<0.001, unpaired Student's t test with Bonferroni's correction) in an ant1Δ knock-out strain (0.89±0.03, n=20; Figure 4F) than in wild-type cells (0.70±0.04, n=22; Figure 4C). These data indicate a marked reduction of the pH gradient across the peroxisomal membrane in the absence of the Ant1p carrier.
DISCUSSION
In this report we show that both uniport and exchange of adenine nucleotides catalysed by Ant1p involve proton movement, and that nucleotide hetero-exchange is H+-compensated and electroneutral. In this respect Ant1p is clearly different from the mitochondrial ADP/ATP carrier which is an electrogenic strict antiporter [14]. H+-compensated nucleotide exchange has also been demonstrated for the yeast mitochondrial GTP/GDP carrier [15]. Ant1p-mediated transport affects the peroxisomal pH, since in the absence of Ant1p the pH gradient across the peroxisomal membrane diminished. Our data demonstrate that (1) yeast peroxisomes are impermeable to protons and (2) Ant1p is a major factor involved in peroxisomal pH homoeostasis in unperturbed exponentially growing cells. Our finding of a reduced, but existing ΔpH in ant1Δ mutant cells indicates that a total break-down of the peroxisomal H+ homoeostasis is prevented in the absence of Ant1p by other factors. Indirect evidence was put forward suggesting the existence of a H+-ATPase in the peroxisomal membrane [16], but neither its molecular identity nor even its activity have been demonstrated.
In this work, subcellular pHs were monitored in vivo by utilizing ratiomeric pHluorin, a pH-sensitive GFP whose 405/485 nm excitation ratios were shown to decrease upon lowering the pH [8]. Although the absolute pH of peroxisomes was not determined in the present work, our data indicate a more acidic peroxisomal lumen relative to the cytosol. An acidic pH of the peroxisomal lumen was previously suggested based on 31P NMR measurements [17] and immunocytochemical staining of ‘DAMP’ [3-(2,4-dinitroanilino)-3′-amino-N-methyldipropylamine], a weak base that selectively accumulates in acidic compartments [18].
In a recent report the peroxisomal pH in human fibroblasts was inferred to be basic [5] based on fluorescence microscopic imaging of cells that had been preloaded with a PTS1-containing membrane-permeable peptide fused to a pH-sensitive fluorescent tag. By that approach, however, it was not possible to measure the pH of the cytosol in the same cells. When we applied the same experimental strategy in yeast, we found an accumulation of the conjugated peptide in non-peroxisomal structures (R. Erdmann and H. Rottensteiner, unpublished results). Thus we were unable to compare the two methodological approaches in our model system. More recently Jankowski et al. [6] used pHluorin to determine the peroxisomal pH in Chinese-hamster ovary cells. In spite of the clear differences between cytosolic and peroxisomal R405/485 measured under all conditions tested, which point to the acidity of peroxisomes, they interpreted their data to mean that a pH gradient between the cytosol and the peroxisomal lumen does not exist. This conclusion rests on the undemonstrated assumption that under the calibration conditions applied, the imposed extracellular pH is identical to both cytosolic and peroxisomal pHs. In fact, the observed parallel changes of peroxisomal pH upon manipulating the extracellular/cytosolic pH can be explained by the natural adaptation of a pH gradient to altered pH values on one side of the gradient and thus do not need to reflect the absence of a proton gradient.
Although we cannot rule out the occurrence of other mechanisms leading to acidification of the peroxisomal lumen, it should be noted that, in view of the different charges carried by ATP and AMP at physiological pH values, the generation of an acidic pH of peroxisomes is consistent with the main function of Ant1p, namely to import ATP in an H+-compensated electroneutral exchange for lumenal AMP. It may also be speculated that the Ant1p-mediated uniport is required to compensate for the constant decrease of the intraperoxisomal nucleotide concentration due to growth and division of the organelles. Uniport would thus provide the lumen of peroxisomes with ATP to initiate fatty acid activation before sufficient amount of AMP is generated to trigger further ATP import by an exchange mechanism, and to fuel other energy-requiring processes related to peroxisome proliferation [3,19], consistent with the very fast induction of Ant1p expression upon fatty acid sensing [20].
Acknowledgments
This work was supported by grants from MIUR-PRIN, MIUR-FIRB (Ministero dell'Istruzione, dell'Università e della Ricerca-Progetto di Ricerca di Rilevante Interesse Nazionale and -Fondo per gli Investimenti della Ricerca di Base), the Italian National Research Council (CNR), CNR-MIUR project ‘Functional genomics’, CEGBA (Centro di Eccellenza Geni in Campo Biosanitario e Agroalimentare), the European Social Fund and by the Deutsche Forschungsgemeinschaft, grants ER 178/2-4 and RO 2494/1-1.
References
- 1.Hettema E. H., Tabak H. F. Transport of fatty acids and metabolites across the peroxisomal membrane. Biochim. Biophys. Acta. 2000;1486:18–27. doi: 10.1016/s1388-1981(00)00045-7. [DOI] [PubMed] [Google Scholar]
- 2.Palmieri L., Rottensteiner H., Girzalsky W., Scarcia P., Palmieri F., Erdmann R. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 2001;20:5049–5059. doi: 10.1093/emboj/20.18.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Roermund C. W., Drissen R., van Den Berg M., Ijlst L., Hettema E. H., Tabak H. F., Waterham H. R., Wanders R. J. Identification of a peroxisomal ATP carrier required for medium-chain fatty acid β-oxidation and normal peroxisome proliferation in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:4321–4329. doi: 10.1128/MCB.21.13.4321-4329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulter G. J., Harder W., Veenhuis M. Structural and functional aspects of peroxisomal membranes in yeasts. FEMS Microbiol. Rev. 1993;11:285–296. doi: 10.1111/j.1574-6976.1993.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 5.Dansen T. B., Wirtz K. W., Wanders R. J., Pap E. H. Peroxisomes in human fibroblasts have a basic pH. Nat. Cell Biol. 2000;2:51–53. doi: 10.1038/71375. [DOI] [PubMed] [Google Scholar]
- 6.Jankowski A., Kim J. H., Collins R. F., Daneman R., Walton P., Grinstein S. In situ measurements of the pH of mammalian peroxisomes using the fluorescent protein pHluorin. J. Biol. Chem. 2001;276:48748–48753. doi: 10.1074/jbc.M109003200. [DOI] [PubMed] [Google Scholar]
- 7.Palmieri F., Indiveri C., Bisaccia F., Iacobazzi V. Mitochondrial metabolite carrier proteins: purification, reconstitution, and transport studies. Methods Enzymol. 1995;260:349–369. doi: 10.1016/0076-6879(95)60150-3. [DOI] [PubMed] [Google Scholar]
- 8.Miesenbock G., De Angelis D. A., Rothman J. E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature (London) 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 9.Stein K., Schell-Steven A., Erdmann R., Rottensteiner H. Interactions of Pex7p and Pex18p/Pex21p with the peroxisomal docking machinery: implications for the first steps in PTS2 protein import. Mol. Cell. Biol. 2002;22:6056–6069. doi: 10.1128/MCB.22.17.6056-6069.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stappen R., Kramer R. Kinetic mechanism of phosphate/phosphate and phosphate/OH-antiports catalysed by reconstituted phosphate carrier from beef heart mitochondria. J. Biol. Chem. 1994;269:11240–11246. [PubMed] [Google Scholar]
- 11.Indiveri C., Tonazzi A., Stipani I., Palmieri F. The purified and reconstituted ornithine/citrulline carrier from rat liver mitochondria catalyses a second transport mode: ornithine+/H+ exchange. Biochem. J. 1999;341:705–711. [PMC free article] [PubMed] [Google Scholar]
- 12.Fiermonte G., Palmieri L., Todisco S., Agrimi G., Palmieri F., Walker J. E. Identification of the mitochondrial glutamate transporter: bacterial expression, reconstitution, functional characterization, tissue distribution of two human isoforms. J. Biol. Chem. 2002;277:19289–19294. doi: 10.1074/jbc.M201572200. [DOI] [PubMed] [Google Scholar]
- 13.Erdmann R., Blobel G. Identification of Pex13p a peroxisomal membrane receptor for the PTS1 recognition factor. J. Cell Biol. 1996;135:111–121. doi: 10.1083/jcb.135.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klingenberg M. The ADP, ATP shuttle of the mitochondrion. Trends Biochem. Sci. 1979;4:249–252. [Google Scholar]
- 15.Vozza A., Blanco E., Palmieri L., Palmieri F. Identification of the mitochondrial GTP/GDP transporter in Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:20850–20857. doi: 10.1074/jbc.M313610200. [DOI] [PubMed] [Google Scholar]
- 16.Douma A. C., Veenhuis M., Sulter G. J., Harder W. A proton-translocating adenosine triphosphatase is associated with the peroxisomal membrane of yeasts. Arch. Microbiol. 1987;147:42–47. doi: 10.1007/BF00492903. [DOI] [PubMed] [Google Scholar]
- 17.Nicolay K., Veenhuis M., Douma A. C., Harder W. A 31P NMR study of the internal pH of yeast peroxisomes. Arch. Microbiol. 1987;147:37–41. doi: 10.1007/BF00492902. [DOI] [PubMed] [Google Scholar]
- 18.Waterham H. R., Keizer-Gunnink I., Goodman J. M., Harder W., Veenhuis M. Immunocytochemical evidence for the acidic nature of peroxisomes in methylotrophic yeasts. FEBS Lett. 1990;262:17–19. doi: 10.1016/0014-5793(90)80142-6. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa T., Imanaka T., Morita M., Ishiguro K., Yurimoto H., Yamashita A., Kato N., Sakai Y. Peroxisomal membrane protein Pmp47 is essential in the metabolism of middle-chain fatty acid in yeast peroxisomes and is associated with peroxisome proliferation. J. Biol. Chem. 2000;275:3455–3461. doi: 10.1074/jbc.275.5.3455. [DOI] [PubMed] [Google Scholar]
- 20.Rottensteiner H., Palmieri L., Hartig A., Hamilton B., Ruis H., Erdmann R., Gurvitz A. The peroxisomal transporter gene ANT1 is regulated by a novel ORE: characterization of the signal for fatty acid induction. Biochem. J. 2002;365:109–117. doi: 10.1042/BJ20011495. [DOI] [PMC free article] [PubMed] [Google Scholar]