Abstract
HS (heparan sulphate) has hitherto not been found on human red blood cells (RBCs, erythrocytes). However, malarial-parasite (Plasmodium falciparum)-infected RBCs adhere to uninfected RBCs via HS-like receptors. In the present paper we demonstrate that human RBCs carry epitopes for an anti-HS antibody. Glycans isolated from RBC membranes reacted to HS-specific degradations and adhered to an HS-binding malaria antigen. Additionally, an HS core protein was identified. This suggests that HS is present on human RBCs.
Keywords: glycans, heparan sulphate, malaria, Plasmodium falciparum, red blood cells (erythrocytes), sequestration of infected red blood cells
Abbreviations: DBL1α, Duffy-binding-like domain-1α; PfEMP1, P. falciparum erythrocyte membrane protein 1; GAG, glycosaminoglycan; GST, glutathione S-transferase; HS, heparan sulphate; HSPG, heparan sulphate proteoglycan; pRBC, Plasmodium falciparum-infected red blood cell; RBC, red blood cell; RT, room temperature
INTRODUCTION
HS (heparan sulphate) consists of carbohydrate chains covalently attached to core proteins. Through their carbohydrate chains, HS proteoglycans (HSPGs) have a variety of functions as receptors for endogenous mediators and micro-organisms such as parasites. HS chains are composed of a linear backbone of repeating disaccharides (GlcAα1-4GlcNAc). During biosynthesis, this backbone is modified by a number of enzymes to create a wealth of heterogeneous sequences. Hitherto, HS has not been reported on mature human RBCs (red blood cells, erythrocytes), although HSPGs have been found on erythroid precursor cell lines [1]. Indirect evidence of HS- and heparin lyase-sensitive adhesion of micro-organisms to RBCs has been reported for herpes simplex virus [2]. Similar observations have been reported for pRBCs (RBCs infected with the malarial parasite Plasmodium falciparum) [3].
The symptoms of severe malaria result, in part, from the occlusion of capillaries in vital organs by pRBCs and uninfected RBCs. The sequestration of pRBCs and RBCs is brought about by pRBC binding to endothelium (cytoadherence) and pRBCs binding RBCs (rosetting) [4]. PfEMP1 (P. falciparum erythrocyte membrane protein 1), a transmembrane protein containing several domains, has a key role in sequestration to diverse receptors [5]. Glycosaminoglycans (GAGs) such as HS, chondroitin sulphate A and hyaluronic acid have been suggested as important receptors for the binding of pRBCs to RBCs, endothelial cells and syncytiotrophoblasts [6–8], adhesive events in part mediated by PfEMP1 [3,9]. The N-terminal portion of PfEMP1, the Duffy-binding-like domain-1α (DBL1α), has been attributed binding properties to RBCs, CR1 (complement receptor 1), ABO-blood-group antigens and HS [3,9–12]. As RBCs treated with heparin lyase III can no longer form rosettes, HS-like structures on RBCs have been considered as receptors mediating rosetting [3,6]. Moreover, DBL1α by itself, HS and heparin may disrupt and block rosette formation both in cultured strains and in wild isolates of P. falciparum [3,6,13]. Correlations between the severity of the disease and the heparin- and rosetting-binding capacity of the pRBCs [14–16] has prompted us to investigate the nature of the GAG-like receptors on the RBCs as an important step in understanding the pathology of the disease and to potentially develop new treatment strategies. Here we describe the isolation of HS chains and their core protein from mature human RBCs and demonstrate the capacity of the RBC-derived HS chains to bind to the DBL1α of the malaria antigen PfEMP1.
MATERIALS AND METHODS
Isolation of RBCs and depletion of reticulocytes
Human RBC concentrates, collected in citrate-treated bags (Terumo, Gothenburg, Sweden), were purchased from the Blodcentralen, Karolinska University Hospital (KUS), Stockholm, Sweden. In order to obtain reticulocyte-free RBCs, samples of RBC concentrates were cultivated as previously described [17]. To determine the reticulocyte content, cell populations were stained with Cresyl Blue (Merck) and analysed by optical microscopy using an Optiphot-2 (Nikon, Tokyo, Japan) microscope.
Immunostaining with 3G10 antibody
RBCs were washed three times in PBS and treated with 0.2 i.u./ml neuraminidase (Sigma), and 5 munits/ml heparin lyase III or 5 munits/ml chondroitinase ABC (Seikagaku, Tokyo, Japan). After washing three times in PBS, cells were incubated with 3G10 antibody (IgG2b; 1:25, equal to 40 μg/ml) (Seikagaku), followed by three PBS washes and incubation with FITC-tagged anti-mouse antibody (1:25; Dako, Copenhagen, Denmark) and Alexa-Fluor-labelled anti-FITC antibody (1:25; Molecular Probes, Leiden, The Netherlands). All incubations were done at 37 °C for 1 h. Surface fluorescence was studied in an incident UV-light microscope (Optiphot-2).
In order to quantify the number of immunostained RBCs, samples were prepared as described above and analysed using a FACS instrument from Becton Dickinson (Mountain View, CA, U.S.A.). A minimum of 30000 cells per sample were collected.
RBC membrane preparation and isolation of O-glycans
RBCs were washed three times in PBS and lysed for 30 min on ice in hypo-osmotic buffer [10 mM Tris (pH 7.1)/0.1 mM EDTA] supplemented with Complete Mini protease inhibitor (Roche Diagnostics, Mannheim, Germany). Membranes were centrifuged at 9000 g for 30 min and washed in hypo-osmotic buffer until no haemoglobin was retained and finally washed in water. To remove most of the negatively charged sialic acid molecules, a membrane pellet (1 ml) was treated with 0.02 i.u./ml neuraminidase (Sigma) in 10 ml of 50 mM sodium acetate buffer, pH 6, and incubated for 24 h at 37 °C with shaking. The sample was subsequently washed three times in water. O-glycans were released from core proteins by incubation in 1 ml of 0.5 M NaOH for 16 h at 4 °C. The pH was adjusted to pH 9, followed by incubation with 2 mCi of NaB3H4 (55 Ci/mmol; Amersham Biosciences, Uppsala, Sweden) for 4 h at RT (room temperature, 21 °C). Remaining aldehydes were reduced by the addition of 200 μg of NaBH4 and incubation for 2 h. Excess NaBH4 was hydrolysed and the sample desalted on a Sephadex G-10 (Amersham Biosciences) column (1 cm×11 cm).
Purification of O-glycans
Separation of radiolabelled glycans (1×108 c.p.m.) according to size was performed on a column (1.5 cm×145 cm) of Sepharose G-50 (Amersham Biosciences) equilibrated in 0.5 M NH4HCO3. Aliquots of the fractions were analysed by liquid-scintillation counting. Fractions containing large glycans were pooled and desalted. Ion-exchange chromatography was performed on a column (1 cm×10 cm) of DEAE-Sephacel (Amersham Biosciences) equilibrated in 50 mM sodium acetate (pH 7)/0.1 M NaCl. Oligosaccharides were loaded in the same buffer and the column was washed with 10 ml of loading buffer and with 10 ml of 50 mM sodium acetate (pH 4)/0.1 M NaCl. Elution of the acidic glycans was achieved with a linear NaCl gradient in the same buffer. Fractions (1 ml each) were collected, aliquots were liquid-scintillation-counted and peak material was pooled and dialysed against water. All isolation steps were performed at least three times with different batches, and identical results were obtained.
Characterization of O-glycans
Aliquots of isolated O-glycans were either cleaved by heparin lyase III [10 munits/ml in 50 mM Hepes (pH 7)/1 mM CaCl2] or by deamination at pH 1.5 [18]. Untreated and treated samples were analysed by sizing chromatography on a column (3 cm×30 cm) of Superdex 12 (Amersham Biosciences) in 0.5 M NH4HCO3 and on DEAE-Sephacel as described above. Purified O-glycans were also analysed on GST (glutathione S-transferase) and DBL1α–GST fusion-protein [19] affinity columns [9]. The individual analyses were repeated at least twice with identical outcomes.
Analysis of RBC membrane proteins
RBC membranes were isolated as described above and treated with 5 munits/ml heparin lyase III, heat-inactivated heparin lyase III, chondroitinase ABC, or no enzyme, in PBS at 37 °C for 90 min. After incubation, samples were rinsed in PBS and dotted on to nitrocellulose membrane. The membrane was blocked in PBS with 5% (w/v) dried-milk powder (Semper AB, Stockholm, Sweden), incubated with 3G10 antibody (1:500) in PBS, washed three times in PBS and finally incubated with horseradish peroxidase-conjugated anti-mouse Ig antibody at 1:20,000 (Dako). All incubations were performed for 1 h at RT. The membrane was developed using enhanced chemiluminescence (ECL®; Amersham Biosciences). The dot-blot was performed three times with identical results.
Surface-restricted biotinylation was used to analyse RBC surface proteins. Approx. 5×109 RBCs were washed twice in 10 vol. of RPMI-1640 and twice in PBS and re-suspended in 500 μl of PBS. Membrane-impermeable NHS-PEO4-Biotin reagent (Pierce) in DMSO was diluted in 500 μl of PBS to a concentration of 2 mM and immediately added to the cells. Surface proteins were biotinylated for 10 min at RT, centrifuged, then washed in 100 mM glycine/PBS and PBS. Membranes were isolated and treated with active or heat-inactivated heparin lyase III, as described above. Material corresponding to 1.5×107 cells was separated by SDS/12%-(w/v)-PAGE and transferred to nitrocellulose membrane. The membrane was blocked in PBS with 5% milk powder and incubated with alkaline phosphatase-conjugated ExtrAvidin (1:10000) (Sigma). After three washes in PBS the membrane was developed with the alkaline phosphatase substrate 5-bromo-4-chloroindol-3-yl phosphate/Nitro Blue Tetrazolium (‘BCIP/NBT’; Sigma). All incubations were performed for 1 h at RT. Identical results were obtained from three individual experiments.
RESULTS AND DISCUSSION
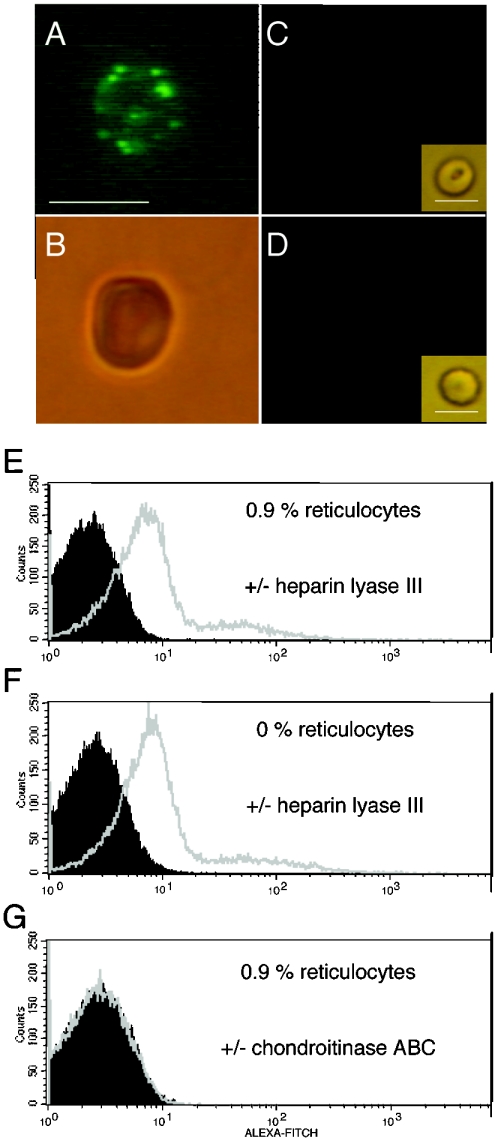
Since HS chains are heterogeneous molecules and differ in structure, there is a lack of antibodies recognizing all variant HS structures. Therefore, to identify HS chains on the intact RBC surface, we treated the RBCs with heparin lyase III and subsequently stained them with a monoclonal antibody, 3G10, which recognizes the conserved HS stub produced by cleavage of HS by the enzyme. An unsaturated hexuronate is created at the non-reducing end of the remaining HS stub that is essential for reactivity with the antibody [20]. Immunofluorescence staining of fresh RBCs with 3G10 indicated binding to 90% of all cells, although the staining was weak (Figures 1A and 1B). In a FACS scan a significant shift was detected between untreated and heparin lyase III-treated cell populations (Figure 1E). The 3G10 antibody did not react with stubs generated by chondroitinase ABC treatment of the RBCs (Figures 1C and 1G). No cross-reactivity was seen with any secondary antibody to enzyme-treated RBCs (results not shown) or with any of the antibodies to untreated RBCs (Figure 1D). Human RBC samples contain 0.5−2.5% reticulocytes, the immature RBC precursors still carrying mitochondria and ribosomes. Reticulocytes pass into the bloodstream, where they lose their organelles within a day or two to become mature RBCs. To ensure that the reactivity of the 3G10 antibody was not directed towards reticulocytes, a reticulocyte-free sample was produced by allowing the RBCs to mature in vitro. The content of organelles in cells decreases with time and, after 5 days of cultivation of RBCs, no mitochondria or ribosomes were present and the sample contained only mature RBCs. However, no difference was seen between the FACS analysis of RBC preparations containing or not containing reticulocytes (Figures 1E and 1F). Therefore RBC preparations containing reticulocytes were used in all further assays.
Figure 1. RBCs stained by an antibody that detects HS.
RBCs were treated with neuraminidase, and heparin lyase III or chondroitinase ABC, followed by staining with 3G10 antibody, anti-mouse FITC-labelled antibody and Alexa-Fluor-labelled anti-FITC antibody as described in the Materials and methods section. Cells were analysed by microscopy in visible light or incident UV light (A–D) and by FACS (E and G). RBCs treated with (A) neuraminidase and heparin lyase III under UV light, (B) under visible light, (C) RBCs treated with neuraminidase and chondroitinase ABC under UV light and under visible light (insert) and (D) untreated RBCs under UV light and under visible light (insert) (bar represents 6 μm) are shown. Also shown are FACS analyses (E) of RBC populations containing 0.9% reticulocytes after heparin lyase III treatment (grey) or in the absence of heparin lyase III treatment (black), (F) of a reticulocyte-free population after heparin lyase III treatment (grey) or in the absence of heparin lyase III treatment (black), and (G) of RBCs containing 0.9% reticulocytes after chondroitinase ABC treatment (grey) or in the absence of chondroitinase ABC treatment (black).
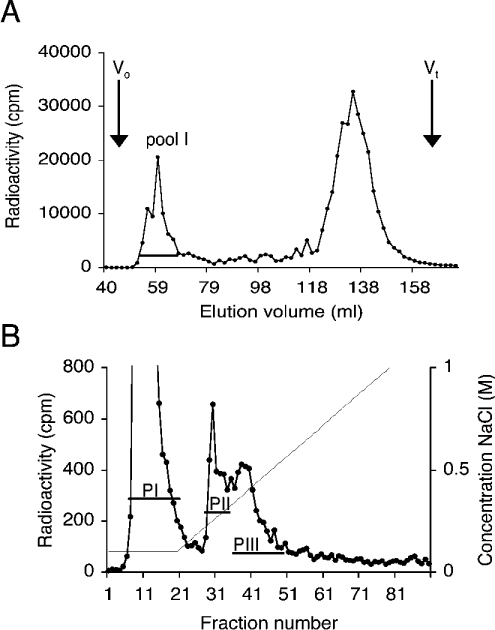
To isolate the potential PfEMP1 receptor of an HS nature from RBCs, large, acidic O-glycans were purified from neuraminidase-treated membrane preparations. The O-glycans were released from their core proteins and radiolabelled before separation by size chromatography (Figure 2A). Approximately one-fifth of the labelled molecules were eluted as large glycans close to the void volume, Vo (Figure 2A, pool I). The glycans in pool I were further subfractionated according to charge by anion-exchange chromatography. Most of the material was eluted as non-charged molecules. With a linear salt gradient a heterogeneous population of charged glycans was eluted, corresponding to approx. 20% of the loaded material. This material was pooled into two separate populations (Figure 2B, fractions PII and PIII).
Figure 2. Isolation of HS from RBCs.
(A) Size separation of O-glycans. 3H-labelled O-glycans isolated from RBCs were separated on a Sepharose G-50 column, and fractions containing large O-glycans in pool I were collected. The void volume (Vo) and the total included volume (Vt) are indicated. (B) Anion-exchange chromatography of large O-glycans. Pool I glycans (A) were separated on a DEAE-Sephacel column and eluted with an NaCl gradient. Fractions were collected, and two peaks (PII and PIII) were pooled and analysed for HS content. Both chromatograms represent typical results of at least three different preparations.
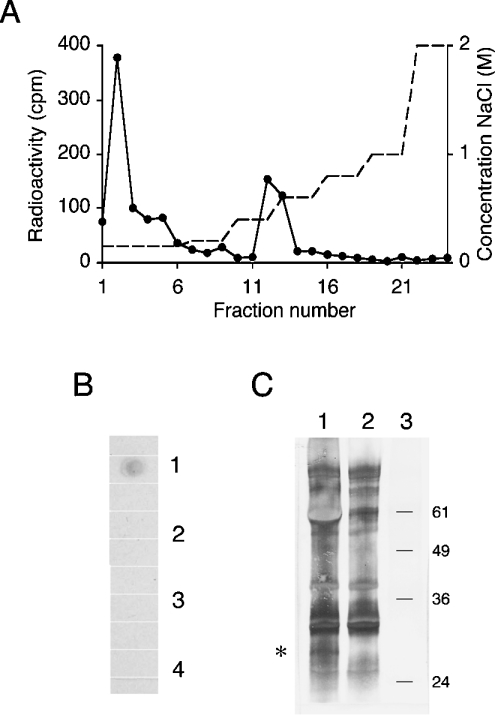
These negatively charged O-glycans (Figure 2B, fractions PII and PIII) were tested for their susceptibility towards selective degradation methods. Upon treatment with heparin lyase III and deamination at pH 1.5, no degradation was observed for the polysaccharides in PII (results not shown). This deamination procedure is a selective method to chemically cleave glycosidic linkages of N-sulphated glucosamine residues and is specific for HS, since no other O-linked GAG is known to contain this modification. The most charged O-glycans, PIII, however, were sensitive to heparin lyase III (35%) and to deamination at pH 1.5 (41%). Before treatment, the glycans from PIII were eluted with an apparent size of a 26-mer standard heparin oligosaccharide (≈7.5 kDa) on the Superose 12 sizing column (Figure 3A). After either cleavage the products were eluted as smaller species of ≈10−≈4-mers (3−1.2 kDa) on the sizing column (Figures 3B and 3C) and as non-retained oligosaccharides on the DEAE column (results not shown). This suggested the existence of HS chains in O-glycans isolated from human RBCs, although, as expected, the amount was very small. To test whether these glycans corresponded to the formerly suggested oligosaccharide receptor for malaria antigen, material from fraction PIII was tested for binding to DBL1α−GST fusion protein. About 40% of the O-glycans in PIII (Figure 2B) bound to the DBL1α−GST affinity column (Figure 4A), a similar amount as was sensitive to HS-specific degradation. No binding to GST was seen (results not shown). Using the specific radioactivity of NaB3H4, the activity of the radiolabelled starting material and the relative amounts of material that were sensitive to HS-specific reaction, a rough approximation of 2000 HS chains per single RBC could be estimated. The number of HS molecules is very low compared with many other RBC surface proteins, e.g. Band 3, which represents ≈106 molecules/cell, but not unknown in this range, as ≈102 Na/K-ATPase protein molecules have been estimated per RBC [21]. To prove that the HS is covalently bound to a membrane protein, we also attempted to demonstrate the existence of an HS core protein in RBCs. To detect potential HS core protein(s), a dot-blot assay was performed. Isolated RBC membranes were treated with either active or inactive heparin lyase III, chondroitinase ABC or no enzyme. Only the sample treated with active heparin lyase III reacted with the 3G10 antibody (Figure 4B), suggesting the existence of HS-core protein(s) in the RBC membrane. Therefore intact RBCs were biotinylated and membranes isolated. The material was treated with active or inactivated heparin lyase III. Samples were separated by SDS/PAGE, analysed by Western blot and biotinylated proteins were visualized with alkaline phosphatase-conjugated ExtrAvidin. In the sample treated with active heparin lyase III, a specific band of ≈30 kDa was detected in three separately isolated samples (Figure 4C). RBC proteoglycans analysed before heparin lyase III treatment still carrying glycans migrated as a polydisperse band and could not be studied. Notably, the biotinylation of RBCs was strictly directed to membrane proteins and did not occur in the cell, since, for example, haemoglobin was not labelled (results not shown). The 3G10 antibody was also used in direct immunoblotting, though no staining was seen, suggesting a low number of HS chains covalently attached to the RBCs.
Figure 3. HS-selective cleavage of RBC O-glycans.
Size separation of O-glycans (Figure 2B, PIII) on a Superose 12 column before (A) and after (B) heparin lyase III treatment, or after deamination at pH 1.5 (C). The void volume (Vo), the total included volume (Vt) and the elution positions of standard heparin oligosaccharides of the indicated sizes (26-, 16-, 10- and 2-mers) are marked. The analysis was repeated twice.
Figure 4. Affinity chromatography on DBL1α and identification of HS-core protein.
(A) O-glycans from RBCs (Figure 2B, PIII) were tested for binding to the DBL1α affinity column. Bound material was eluted with an NaCl gradient. (B) Dot-blot of RBC membrane extracts treated with (1) active heparin lyase III, (2) inactive heparin lyase III, (3) chondroitinase ABC or (4) buffer. Extracts were dotted on to a nitrocellulose membrane. The membrane was developed with 3G10 antibody and horseradish-peroxidase-conjugated anti-mouse antibody. (C) RBC membranes were isolated from biotinylated intact RBCs and treated with active heparin lyase III (lane 1) or inactive heparin lyase III (lane 2), separated by SDS/12%-(w/v)-PAGE and analysed by immunoblotting. Biotinylated proteins were detected with alkaline phosphatase-conjugated ExtrAvidin. An additional band of ≈30 kDa was detected after treatment with active heparin lyase III (asterisk). The mobility of molecular-mass standard proteins (kDa) is indicated to the right (lane 3).
HSPGs have been identified on a haematopoietic progenitor cell line induced to differentiate into erythroid cells [1], but whether these cells develop into reticulocytes is unknown. Yet, in the haematopoietic cell line studied, the quantity of HSPGs seemed to decrease during their differentiation into more mature erythroid cells. Considering that RBCs have a lifetime of about 120 days, it is tempting to speculate that, as for other cell compartments, the quantity and quality of an HSPG would change over time and that younger RBCs carry more HS than the older ones. This could have implications for the pathogenesis of severe malaria.
Several independent studies have proposed the involvement of HS on the RBC surface in the adhesion events occurring with pathogens such as herpes simplex virus and P. falciparum. The importance of GAGs on endothelial cells, syncytiotrophoblasts and RBCs in the development of severe malaria has indeed been appreciated, yet HS has so far not been found on mature human RBCs. One reason for this may be the small amounts of HS on the RBC surface, in combination with the lack of means to metabolically radiolabel carbohydrates in RBCs. Yet, we have here been able to isolate HS and identify its core protein, although the type and function of this HSPG are as yet unknown. The origin of the HS on the RBCs is probably erythroid, since HS has been found on erythroid precursors. Our findings suggest HS to be present on normal mature human RBCs.
A better knowledge of the structures of receptors involved in the causation of severe malaria is of importance for the design of receptor antagonists. The findings presented here will help us to further understand the development of pathology during severe P. falciparum malaria and hopefully aid in creating a drug that diminishes HS-dependent sequestration in vivo.
Acknowledgments
We thank Professor Ulf Lindahl (Department of Medical Biochemistry and Microbiology, The Biomedical Center, Uppsala University, Uppsala, Sweden) for helpful comments on the project. Technical support with FACS analysis by Ms Susanne Nylén and Ms Liv Eidsmo is very much appreciated. This project was supported by grants from the programme ‘Glycoconjugates in Biological Systems’ sponsored by the Swedish Foundation for Strategic Research (to M.W. and D.S.), the Swedish Research Council (to M.W.), the European Commission (QLRT-PL-1999-30109 to M.W.), the Swedish International Development Authority (Sida/SAREC; to M.W.) and Polysackaridforskning AB (to D.S.).
References
- 1.Drzeniek Z., Stöcker G., Siebertz B., Just U., Schroeder T., Ostertag W., Haubeck H.-D. Heparan sulfate proteoglycan expression is induced during early erythroid differentiation of multipotent hematopoietic stem cells. Blood. 1999;93:2884–2897. [PubMed] [Google Scholar]
- 2.Trybala E., Svennerholm B., Bergström T., Olofsson S., Jeansson S., Goodman J. L. Herpes simplex virus type 1-induced hemagglutination: glycoprotein C mediates virus binding to erythrocyte surface heparan sulphate. J. Virol. 1993;67:1278–1285. doi: 10.1128/jvi.67.3.1278-1285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q., Barragan A., Fernandez V., Sundström A., Schichtherle M., Sahlén A., Carlson J., Datta S., Wahlgren M. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J. Exp. Med. 1998;187:15–23. doi: 10.1084/jem.187.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller L. H., Good M. F., Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 5.Baruch D. I., Pasloske B. L., Singh H. B., Bi X., Ma X. C., Feldman M., Taraschi T. F., Howard R. J. Cloning the P. falciparum gene endcoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 6.Carlson J., Ekre H.-P., Helmby H., Gysin J., Greenwood B. M., Wahlgren M. Disruption of Plasmodium falciparum erythrocyte rosettes by standard heparin and heparin devoid of anticoagulant activity. Am. J. Trop. Med. Hyg. 1992;46:595–602. doi: 10.4269/ajtmh.1992.46.595. [DOI] [PubMed] [Google Scholar]
- 7.Fried M., Duffy P. E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 8.Beeson J., Rogerson S., Cooke B., Reeder J., Molyneux M., Brown G. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 2000;6:86–90. doi: 10.1038/71582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogt A. M., Barragan A., Chen Q., Kironde F., Spillmann D., Wahlgren M. Heparan sulfate on endothelial cells mediates the binding of Plasmodium falciparum-infected erythrocytes via the DBL1α domain of PfEMP1. Blood. 2003;101:2405–2411. doi: 10.1182/blood-2002-07-2016. [DOI] [PubMed] [Google Scholar]
- 10.Udomsangpetch R., Wåhlin B., Carlson J., Berzins K., Torii M., Aikawa M., Perlmann P., Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J. Exp. Med. 1989;169:1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe A. J., Moulds J. M., Newbold C. I., Miller L. H. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature (London) 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q., Heddini A., Barragan A., Fernandez V., Pearce S. F. A., Wahlgren M. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J. Exp. Med. 2000;192:1–9. doi: 10.1084/jem.192.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barragan A., Spillmann D., Wahlgren M., Carlson J. Plasmodium falciparum: molecular background of strain specific rosette disruption by glycosaminoglycans and sulfated glycoconjugates. Exp. Parasitol. 1999;99:133–143. doi: 10.1006/expr.1998.4349. [DOI] [PubMed] [Google Scholar]
- 14.Carlson J., Helmby H., Hill A. V. S., Brewster D., Greenwood B. M., Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 15.Rowe A., Berendt A. R., Marsh K., Newbold C. I. Plasmodium falciparum: a family of sulphated glycoconjugates disrupts erythrocytes rosettes. Exp. Parasitol. 1994;79:506–516. doi: 10.1006/expr.1994.1111. [DOI] [PubMed] [Google Scholar]
- 16.Heddini A., Pettersson F., Kai O., Shafi J., Obiero J., Chen Q., Barragan A., Wahlgren M., Marsh K. Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect. Immun. 2001;69:5849–5856. doi: 10.1128/IAI.69.9.5849-5856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noble N. A., Xu Q. P., Ward J. H. Reticulocytes. I. Isolation and in vitro maturation of synchronized populations. Blood. 1989;74:475–481. [PubMed] [Google Scholar]
- 18.Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976;15:3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- 19.Schlichtherle M., Wahlgren M., Perlmann H., Scherf A. Manassas: American Type Culture Collection; 2000. Methods in Malaria Research; pp. 1–88. [Google Scholar]
- 20.David G., Bai X. M., Van der Schueren B., Cassiman J. J., Van den Berghe H. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J. Cell Biol. 1992;119:961–975. doi: 10.1083/jcb.119.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J., Steck T. L. Isolation and characterization of band 3, the predominant polypeptide of the human erythrocyte membrane. J. Biol. Chem. 1975;250:9170–9175. [PubMed] [Google Scholar]