Abstract
Aims:
There is limited evidence to support the efficacy of sacral neuromodulation (SNM) for older adults with overactive bladder (OAB). This study aims to report outcomes following SNM among nursing home (NH) residents, a vulnerable population with high rates of frailty and comorbidity.
Methods:
This is a retrospective cohort study of long-stay NH residents who underwent a trial of percutaneous nerve evaluation (PNE) or Stage 1 permanent lead placement (Stage 1) between 2014–2016. Residents were identified using the Minimum Data Set linked to Medicare claims. The primary outcome of this study was successful progression from trial to implant. Rates of 1-year device explant/revisions were also investigated.
Results:
Trial of SNM was observed in 1089 residents (mean age: 77.9 years). PNE was performed in 66.9% of residents and 33.2% underwent Stage 1. Of Stage 1 procedures, 23.8% were performed with simultaneous device implant (single-stage). Overall, 53.1% of PNEs and 72.4% of Stage 1 progressed to device implant, which was associated with Stage 1 procedure versus PNE (adjusted relative risk [aRR] 1.34; 95% confidence interval [95% CI] 1.21–1.49) and female versus male sex (aRR 1.26; 95% CI: 1.09–1.46). One-year explant/revision was observed in 9.3% of residents (6.3% for PNE, 10.5% for Stage 1, 20.3% single-stage). Single-stage procedure versus PNE was significantly associated with device explant/revision (aRR 3.4; 95% CI: 1.9–6.2).
Conclusions:
In this large cohort of NH residents, outcomes following SNM were similar to previous reports of younger healthier cohorts. Surgeons managing older patients with OAB should use caution when selecting patients for single-stage SNM procedures.
Keywords: Frailty, neuromodulation, nursing home, older adults, overactive bladder, percutaneous nerve evaluation, third line therapy
INTRODUCTION:
Overactive bladder (OAB) affects nearly 60% of older adults 1 and has a significant detrimental impact on health-related quality of life 2. OAB in this population is also difficult to manage, as many medications are contraindicated. Anticholinergics, for example, are associated with dementia, and novel β-3 agonists with cardiotoxicity and hypertension3. According to American Urological Association (AUA) and Society of Urodynamics, Female Pelvic Medicine, and Urogenital Reconstruction (SUFU) guidelines on the diagnosis and treatment of OAB, those who are unable to tolerate medical therapy for OAB can be offered more invasive options 4. Sacral neuromodulation is an invasive OAB therapy with demonstrated efficacy in reducing OAB symptoms 5. Given the limiting potential side effects of medical therapy, SNM is often being offered earlier in the treatment pathway for older adults with OAB 6.
Despite the high burden of OAB in older adults, research on SNM is largely focused on younger and healthier subjects 7,8. Most studies conducted in older adults undergoing SNM are limited to single-institution series with small sample sizes of healthy subjects9,10. Little is known about the safety and efficacy of SNM in older adults, who tend to be comorbid, frail, and experience poorer outcomes following various urologic surgeries compared to younger individuals 11. Due to limited outcomes data and additional concerns related to anesthesia and complications, surgeons may be hesitant to offer sacral neuromodulation to older adults 12. Though well intentioned, these concerns may be overly cautious and result in unnecessary withholding of a treatment that has the potential to improve health-related quality of life in a population with otherwise limited options.
To address this knowledge gap, we designed a retrospective cohort study investigating the use of SNM in nursing home (NH) residents, one of the most vulnerable populations in the United States 13,14. Using data available in the Minimum Data Set (MDS) for nursing home residents linked to Medicare claims from 2014 to 2016, this study examines rates of device implantation and on subsequent rates of device explanation/revision in a population with high rates of frailty and comorbidity. Findings from this study will help guide clinicians to better counsel older and frail older adults considering for SNM.
MATERIALS & METHODS
Subjects and database
This study utilized a 100% sample of fee-for-service Medicare claims for beneficiaries undergoing SNM procedures from 2014 to 2016. It was deemed to be exempt by the institution’s review board.
This study specifically focused on long-stay NH residents, who were identified using Medicare claims data linked to the MDS 3.0 for NH residents. The MDS is a mandatory assessment for all NH residents who reside in facilities that receive Medicare payments in the United States and the data contain information related to cognitive, psychosocial, and functional status. The MDS is obtained by nursing staff quarterly, with admission or readmission to the NH, or with a change in resident clinical status 11. NH residents were defined as long-term if they had at least 2 or more consecutive MDS assessments more than 30 days apart in the year prior to their index SNM procedure.
The Index SNM procedure was identified using Current Procedural Terminology (CPT4) codes from the Medicare Carrier files. The index procedure was defined as any test procedure, either percutaneous nerve evaluation (PNE, CPT4 64561) or a Stage 1 procedure (Stage 1, CPT4 64581) identified during the study period. If a resident had both a PNE and a Stage 1 procedure, the test procedure that occurred first in the data was used in the analyses. Residents that progressed to Stage 2 (device implant) were identified using CPT4 code 64590. To identify the first-time index procedures, residents who underwent device explant/revision (CPT4 64585 or 64595, respectively) within 1 year before the test procedure were excluded from the analysis.
Covariates
Demographic data including age and gender was obtained from Medicare Master Beneficiary Files during the year before the index test procedures. Charlson Comorbidity Index (CCI) was calculated using comorbidities derived using International Classification of Diseases (ICD) 9 and 10 codes from Medicare Inpatient, Outpatient and Carrier files, according to prior literature 15. Socioeconomic status was evaluated using the Area Deprivation Index (ADI), which was linked to the Medicare data via beneficiary nine-digit ZIP code. The ADI is calculated using structural factors such as education, housing and poverty and has been shown to correlate with health outcomes and hospital readmission16. Higher ADI values correlate with increasing levels of social deprivation and a percentile of 50 represents the median national level.
Frailty was measured using the Claims-based Frailty Index (CFI) 17, which is calculated using a weighted deficit accumulation model of 93 clinical variables including 52 ICD-9 and 10 codes, 25 CPT-4 codes, and 15 Healthcare Common Procedure Coding System Level II Codes. The CFI is validated for use in Medicare data and is associated with poor outcomes following surgical procedures13. Residents were divided into three groups based on CFI measurement, consistent with prior studies: not frail to prefrail (CFI <0.25), mildly frail (0.25 ≤ CFI < 0.35) and moderately-to-severely frail (CFI ≥0.35), consistent with the literature12.
Outcome Measures
Primary outcome (device implantation)
NH residents who successfully progressed from a test procedure to device implantation (hereafter, Stage 2) were identified by the presence of CPT4 code 64590 within 90 days of PNE or Stage 1 procedures. More specifically, PNE success was defined as PNE followed by simultaneous claim for Stage 1 and Stage 2. If PNE was followed by Stage 2 alone (n = 26), this was considered a coding error and categorized as a PNE success. Stage 1 success was defined as claims for Stage 1 followed by Stage 2 on different dates. Single-stage procedures were identified by codes for Stage 1 and Stage 2 on the same day.
Residents who underwent test procedures without further codes for Stage 2 procedures were categorized as procedure failures, or cases that were not implanted. PNE followed by subsequent staged procedure (Stage 1 followed by Stage 2 on different dates, n = 28) were categorized as PNE failures. Residents who were reported to have undergone PNE and Stage 2 on the same visit (n < 11) were excluded, as this is not typical practice and was assumed to be related to coding error.
Secondary outcomes
Secondary outcomes included postoperative complications within 30 days of the index procedure and device explant/revision within 1 year of index procedure. Complications within 30 days were identified using ICD-9/10 diagnosis codes, consistent with existing literature 18. Residents who underwent device explant/revision in the year following their index procedure were identified using the CPT4 codes 64585 or 64595, respectively. ICD-9 and 10 codes associated with explant/revision, of which there may be multiple listed for each case, were categorized into unspecified, device complications, infection, and wound complications (including dehiscence). Residents that died within 12 months of test procedure (n = 108) were excluded from the models for device implant and explant.
Statistical analyses
Descriptive statistics including chi-squared test and analysis of variance were used for categorical and continuous variables, respectively. Generalized linear regression models with log link, Poisson distribution, and robust standard errors were used to determine the adjusted relative risk ratios for progression to Stage 2 and explant/revision. Independent variables in the model included age, race, gender, type of index test procedure group (PNE vs. Stage 1), CCI, CFI, ADI, and procedure year. Per best-practice guidelines for Medicare data, cell contents were masked if number of events was <1119.
Residents were included in the model for progression to Stage 2 according to the first test procedure. For residents who underwent single-stage procedures (n = 86), it was assumed that the decision to perform Stage 1 and Stage 2 simultaneously was made before the procedure date; therefore these cases were excluded from the progression to Stage 2 model.
For the device explant/revision model, residents were included based on the final test procedure they underwent. Residents who underwent PNE or Stage 1 followed by Stage 2 were included according to their index procedure. Residents who underwent PNE followed by subsequent staged procedure (Stage 1 and Stage 2 on different dates) were included in the device explant/revision model as having had Stage 1, as this is the ultimate procedure that led to implant. Residents who underwent single-stage procedures were included as a separate procedure group.
Because of variable length of follow-up time depending on when procedure was performed during the study period, Kaplan-Meier survival estimates were used to calculate cumulative risk of device explant/revision from the date of surgery.
For all analyses, a p value < 0.05 was considered statistically significant. Data management, statistical analyses, and figure development were completed using SAS version 9.4.
RESULTS:
Between 2014 and 2016, 1089 Medicare beneficiaries who were long-stay NH residents residing underwent a SNM test procedure. Table 1 shows the baseline characteristics of the study cohort. The average age of residents was 77.9 years, 52.8% were mildly frail (0.25 ≤ CFI ≤ 0.35) and 30.5% were moderately-to-severely frail (CFI > 0.35). Mean ± SD SD CFI was 3.2 ± 2.5. In total, 728 residents (66.9%) underwent PNE and 361 (33.2%) underwent Stage 1 procedures. Single-stage procedures were performed in 23.8% of Stage 1 procedures. There were no statistically significant differences between residents who underwent each type of test procedure. Women were more likely than men to undergo staged procedures compared to PNE procedures (adjusted relative risk [aRR] 1.23; 95% CI 1.00–1.52).
Table 1:
Baseline characteristics of long-stay NH residents who underwent PNE and Stage 1 SNM test procedure from 2014 to 2016.
| Variable name | Total N=1089 (100.0) | PNE n=728 (66.9) | Stage 1 n=361 (33.2) | p |
|---|---|---|---|---|
| Age in years | ||||
| Mean ± SD | 77.9 (7.1) | 78.0 ± 7.1 | 77.8 ± 7.2 | 0.666 |
| 65–74 | 405 (37.2) | 271 (37.2) | 134 (37.1) | 0.955 |
| 75–84 | 480 (44.1) | 319 (43.2) | 161 (44.6) | |
| ≥85 | 204 (18.7) | 138 (19.0) | 66 (18.3) | |
| Sex | ||||
| Male | 282 (25.9) | 201 (27.6) | 81 (22.4) | 0.067 |
| Female | 807 (74.1) | 527 (72.4) | 280 (77.6) | |
| Race | ||||
| White | 1008 (92.6) | 672 (92.3) | 336 (93.1) | 0.857 |
| Black | 53 (4.9) | <42 (<5.8) | ≥11 (≥3.0) | |
| Other | 28 (2.6) | ≥17 (≥2.3) | <11 (<3.0) | |
| CCI | ||||
| 0 | 128 (11.8) | 93 (12.8) | 35 (9.7) | 0.295 |
| 1 – 3 | 541 (49.7) | 361 (49.6) | 180 (49.9) | |
| ≥4 | 420 (38.6) | 274 (37.6) | 146 (40.4) | |
| Mean ± SD | 3.2 ± 2.5 | 3.2 ± 2.5 | 3.4 ± 2.5 | 0.172 |
| CFI | ||||
| Not Frail to Prefrail (CFI<0.25) | 182 (16.7) | 130 (17.9) | 52 (14.4) | 0.325 |
| Mildly Frail (0.25 ≤ CFI < 0.35) | 575 (52.8) | 382 (52.5) | 193 (53.5) | |
| Moderately to Severely Frail (CFI ≥ 0.35) | 332 (30.5) | 216 (29.7) | 116 (32.1) | |
| Mean ± SD | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.171 |
| ADI National Quartile | ||||
| Q1: 1 - < 32 | 226 (20.8) | 151 (20.8) | 75 (20.8) | 0.686 |
| Q2: 32 - < 51 | 252 (23.2) | 163 (22.4) | 89 (24.7) | |
| Q3: 51 - < 68 | 291 (26.8) | 192 (26.4) | 99 (27.4) | |
| Q4: ≥ 68 | 319 (29.3) | 221 (30.4) | 98 (27.2) | |
| Procedure Year | ||||
| 2014 | 341 (31.3) | 231 (31.7) | 110 (31.5) | 0.915 |
| 2015 | 375 (34.4) | 249 (34.2) | 126 (34.9) | |
| 2016 | 373 (34.3) | 248 (34.1) | 125 (34.6) | |
| Single-stage procedure | 86 (7.9) | -- | 74 (23.8) | -- |
Note: Observations ≤10 suppressed per Centers for Medicare & Medicaid Services (CMS) cell-suppression policy19.
Abbreviations: Area Deprivation Index; CCI, Charlson Comorbidity Index; CFI, Claims-based Frailty Index; CMS, Centers for Medicare & Medicaid Services; NH, nursing home; PNE, percutaneous nerve evaluation.
Table 2 demonstrates complications stratified by type of test procedure. Overall, 37.6% and 43.5% of residents undergoing PNE and Stage 1 procedures experienced at least one complication, respectively. Complications were similar between the two groups; however residents who underwent Stage 1 procedures were more likely to have a wound complication compared to those who underwent PNE, (2.5% versus 0.6%; p=0.005). The most common complications among both groups were urinary tract infection (UTI; 22.9%), cardiovascular complications (12.7%) and acute renal failure (3.7%). One-year mortality was 9.9% among all residents undergoing sacral SNM trial procedures.
Table 2:
Complications within 30-days and 1-year mortality following test procedure (PNE or Stage 1) among NH residents, by type of test procedure.
| Variable name | Total, N (%) 1089 (100.0) | PNE, n (%) 728 (66.9) | Stage 1, n (%) 361 (33.2) | P-value |
|---|---|---|---|---|
| Number complications | ||||
| ≥1 | 431 (39.6) | 274 (37.6) | 157 (43.5) | 0.063 |
| 0 | 658 (60.4) | 454 (62.4) | 204 (56.5) | 0.090 |
| 1–2 | 403 (37.0) | 259 (35.6) | 144 (39.9) | |
| ≥3 | 28 (2.6) | 15 (2.1) | 13 (3.6) | |
| Complication type | ||||
| UTI | 249 (22.9) | 164 (22.5) | 85 (23.6) | 0.706 |
| Cardiovascular | 138 (12.7) | 82 (11.3) | 56 (15.5) | 0.047 |
| Acute renal failure | 40 (3.7) | 21 (2.9) | 19 (5.3) | 0.050 |
| Pulmonary | 39 (3.6) | 26 (3.6) | 13 (3.6) | 0.980 |
| DVT/PE | 34 (3.1) | ≥23 (≥3.2) | <11 (<3.0) | 0.638 |
| Reoperation | 29 (2.7) | 15 (2.1) | 14 (3.9) | 0.080 |
| Other infection | <22 (<2.0) | <11 (<1.5) | <11 (<3.0) | 0.591 |
| Wound complication | <22 (<2.0) | <11 (<1.5) | <11 (<3.0) | 0.005 |
| Other complications | <11 (<1.0) | <11 (<1.5) | <11 (<3.0) | 0.584 |
| Postoperative shock | <11 (<1.0) | <11 (<1.5) | <11 (<3.0) | 0.080 |
| Delirium | <11 (<1.0) | <11 (<1.5) | <11 (<3.0) | 0.080 |
| Postoperative hemorrhage | <11 (<1.0) | <11 (<1.5) | <11 (<3.0) | 0.379 |
| Postoperative stroke | <11 (<1.0) | <11 (<1.5) | <11 (<3.0) | 0.473 |
| Anesthesia complications | <11 (<1.0) | <11 (<1.5) | 0 (0.0) | 0.481 |
| 1-Year mortality | 108 (9.9) | 71 (9.8) | 37 (10.3) | 0.796 |
Note: Observations ≤10 suppressed per Centers for Medicare & Medicaid Services (CMS) cell-suppression policy19.
Abbreviations: CMS, Centers for Medicare & Medicaid Services; DVT/PE, deep vein
thrombosis/pulmonary embolus; NH, nursing home; PNE, percutaneous nerve evaluation; UTI, urinary tract infection.
Results of the model for progression from test procedure to Stage 2 procedures are shown in Table 3. Of the 907 residents who underwent trial of SNM (single-stage procedures and deaths within 12-month excluded), 58.4% successfully proceeded to Stage 2 procedures. According to test procedure type, 53.1% residents who underwent PNE and 72.4% of residents who underwent Stage 1 procedures progressed to Stage 2 (p≤.0001). On multivariable analysis, Stage 1 procedures versus PNE (aRR 1.34; 95% CI: 1.21–1.49) and female versus male sex (aRR 1.26; 95% CI: 1.09–1.46) were significantly associated with successful progression to Stage 2 procedures. Age, CFI, and CCI were not significantly associated with the outcome of interest.
Table 3:
Relative risk associated with progression to device implant/Stage 2 procedure within 90 days of PNE or Stage 1, following exclusion of deaths within 1 year.
| Basic Statistics | Univariate Model RR | Multivariate Model RR | |||||
|---|---|---|---|---|---|---|---|
| Variable Name | Total, N (%) N=907 (100.0) | Event, n (%) 530 (58.4) | P value | Relative risk (RR, 95% CI) | P value | RR, 95% CI | P value |
| Index procedure | |||||||
| PNE | 657 (72.4) | 349 (65.8) | <0.001 | Ref. | <0.001 | Ref. | <0.001 |
| Stage 1 | 250 (27.6) | 181 (34.2) | 1.36 (1.23 – 1.51) | 1.34 (1.21 – 1.49) | |||
| Age in years | |||||||
| 65–74 | 340 (37.5) | 202 (38.1) | 0.448 | Ref. | 0.456 | Ref. | 0.612 |
| 75–84 | 397 (43.8) | 236 (44.5) | 1.00 (0.89 – 1.13) | 1.01 (0.90 – 1.14) | |||
| ≥85 | 170 (18.7) | 92 (17.4) | 0.91 (0.77 – 1.07) | 0.94 (0.80 – 1.10) | |||
| Sex | |||||||
| Male | 231 (25.5) | 111 (20.9) | <.001 | Ref. | <0.001 | Ref. | 0.001 |
| Female | 676 (74.5) | 419 (79.1) | 1.29 (1.11 – 1.49) | 1.26 (1.09 – 1.46) | |||
| Race | |||||||
| White | 836 (92.2) | 492 (92.8) | 0.382 | Ref. | 0.389 | Ref. | 0.501 |
| Non-white | 71 (7.8) | 38 (7.2) | 0.91 (0.73 – 1.14) | 0.93 (0.74 – 1.16) | |||
| Charlson Comorbidity Index | |||||||
| 0 | 114 (12.6) | 64 (10.2) | 0.794 | Ref. | 0.795 | Ref. | 0.864 |
| 1–3 | 461 (50.8) | 268 (50.6) | 1.04 (0.87 – 1.24) | 1.03 (0.85 – 1.23) | |||
| ≥4 | 332 (36.6) | 198 (37.4) | 1.06 (0.88 – 1.28) | 1.05 (0.86 – 1.28) | |||
| Claims-based Frailty Index | |||||||
| Not Frail or Prefrail (CFI<0.25) | 161 (17.8) | 92 (17.4) | 0.391 | Ref. | 0.387 | Ref. | 0.518 |
| Mildly Frail (0.25 ≤ CFI < 0.35) | 466 (51.4) | 265 (50.0) | 1.00 (0.85 – 1.16) | 0.96 (0.82 – 1.12) | |||
| Moderate To Severely Frail (CFI ≥ 0.35) | 280 (30.9) | 173 (32.6) | 1.08 (0.92 – 1.27) | 1.02 (0.86 – 1.21) | |||
| Area Deprivation Index National Quartile | |||||||
| Q1 (ADI 1 – 32) | 187 (20.6) | 99 (18.7) | 0.280 | Ref. | 0.286 | Ref. | 0.396 |
| Q2 (ADI 32 – 50) | 212 (23.4) | 130 (24.5) | 1.16 (0.97 – 1.38) | 1.14 (0.96 – 1.35) | |||
| Q3 (ADI 50 – 67) | 234 (25.8) | 143 (27.0) | 1.15 (0.97 – 1.37) | 1.12 (0.95 – 1.33) | |||
| Q4 (ADI ≥ 68) | 273 (30.1) | 157 (29.6) | 1.09 (0.92 – 1.29) | 1.06 (0.90 – 1.26) | |||
Note: Residents who underwent simultaneous Stage 1 and 2 procedures were excluded from this analysis. Model adjusted for procedure year.
Abbreviations: ADI, Area Deprivation Index; CCI, Charlson Comorbidity Index; CFI, Claims-based Frailty Index; PNE, percutaneous nerve evaluation.
Device explant/revision procedures were performed in 9.3% of residents at 1 year. Table 4 demonstrates relative risk of device explant/revision following Stage 2. Compared to PNE, explant/revision procedures were more likely for residents who underwent Stage 1 and Stage 2 on different dates (aRR 1.58; 95% CI: 0.90–2.76) and for residents who underwent single-stage procedures (aRR 3.37; 95% CI: 1.85–6.15). Resident age, CFI and CCI were not associated with device explant/revision. Race was excluded from the model for device explant/revision given limited number of events. Figure 1 shows a Kaplan-Meier curve illustrating device explant/revision procedures following Stage 2. Kaplan-Meier estimates for device explant at 3 years was 14.7%, based on the data available for residents with more than 1 year of follow-up. The most common reasons for explant/revision were unspecified (n≥22), device complications (n=17), infection (n=12) and wound complications (n≤11)19.
Table 4:
Relative risk associated with device explant/revision within 1 year of implant procedure for residents who successfully underwent device implant following PNE, Stage1, or simultaneous Stage 1 and Stage 2, following exclusion of deaths within 1 year.
| Basic Statistics | Univariate Model RR | Multivariate Model RR | |||||
|---|---|---|---|---|---|---|---|
| Variable Name | Total, N (%) N=632 (100.0) | Event, n (%) n=59 (9.3) | P value | Relative risk (HR, 95% CI) | P value | RR, 95% CI | P value |
| Procedure group | |||||||
| PNE followed by device implant | 349 (55.2) | 22 (37.3) | <0.001 | Ref. | 0.009 | Ref. | 0.008 |
| Stage 1 and Stage 2 on different dates | 209 (33.1) | 22 (37.3) | 1.67 (0.95 – 2.94) | 1.58 (0.90 – 2.76) | |||
| Single Stage procedure | 74 (11.7) | 15 (25.4) | 3.22 (1.75 – 5.90) | 3.37 (1.85 – 6.15) | |||
| Age | |||||||
| 65–74 | 235 (37.2) | 26 (44.1) | 0.484 | Ref. | 0.496 | Ref. | 0.381 |
| 75–84 | 288 (45.6) | >22 (>37.3) | 0.72 (0.42 – 1.23) | 0.69 (0.42 – 1.16) | |||
| ≥85 | 109 (17.3) | <11 (<18.6) | 0.83 (0.41 – 1.66) | 0.76 (0.38 – 1.52) | |||
| Sex | |||||||
| Male | 134 (21.2) | 13 (22.0) | 0.870 | Ref. | 0.871 | Ref. | 0.876 |
| Female | 498 (78.8) | 46 (78.0) | 0.95 (0.53 – 1.71) | 0.96 (0.55 – 1.66) | |||
| Charlson Comorbidity Index | |||||||
| 0 | 74 (11.7) | <11 (<18.6) | 0.318 | Ref. | 0.386 | Ref. | 0.349 |
| 1 – 3 | 325 (51.4) | >25 (>42.4) | 0.59 (0.30 – 1.17) | 0.57 (0.27 – 1.18) | |||
| ≥4 | 233 (36.9) | 23 (39.0) | 0.73 (0.36 – 1.46) | 0.71 (0.34 – 1.48) | |||
| Claims-based Frailty Index | |||||||
| Not Frail to Prefrail (CFI<0.25) | 113 (17.9) | 12 (20.3) | 0.807 | Ref. | 0.810 | Ref. | 0.874 |
| Mildly Frail (0.25 ≤ CFI < 0.35) | 317 (50.2) | 30 (50.8) | 0.89 (0.47 – 1.68) | 0.84 (0.45 – 1.59) | |||
| Moderately to Severely Frail (CFI ≥ 0.35) | 202 (32.0) | 17 (28.8) | 0.79 (0.39 – 1.60) | 0.89 (0.42 – 1.91) | |||
| Area Deprivation Index National Quartile | |||||||
| ADI <50% | 274 (43.4) | 31 (52.5) | 0.138 | Ref. | 0.146 | Ref. | 0.105 |
| ADI ≥50% | 357 (56.6) | 28 (57.5) | 0.69 (0.43 – 1.13) | 0.67 (0.41 – 1.09) | |||
Note: Model adjusted for procedure year. Observations ≤10 suppressed per CMS cell-suppression policy19.
Abbreviations: ADI, Area Deprivation Index; CCI, Charlson Comorbidity Index; CFI, Claims-based Frailty Index; CMS, Centers for Medicare & Medicaid Services; PNE, percutaneous nerve evaluation.
Figure 1:
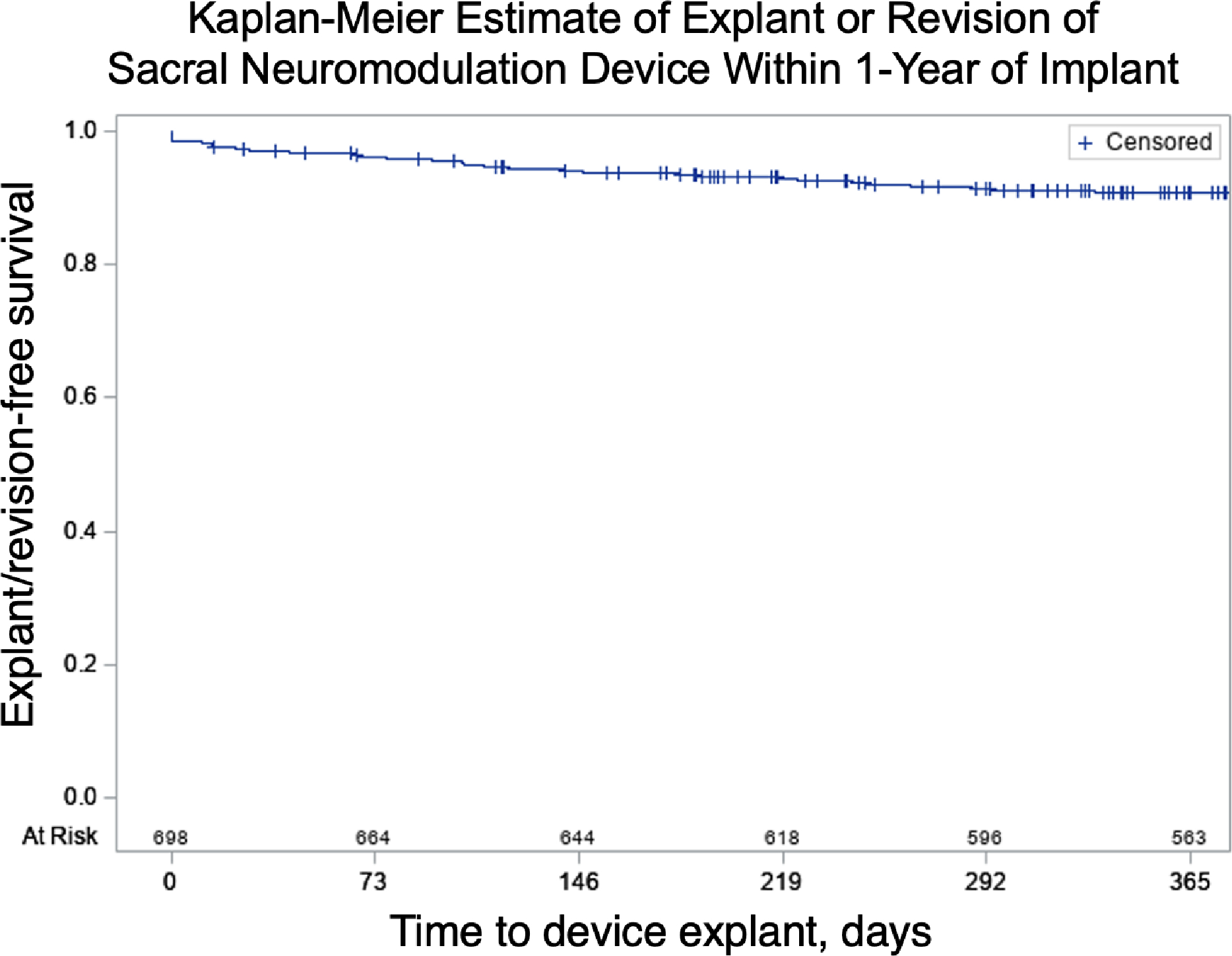
Kaplan-Meier estimate of device explant/revision (n=59), of all device implants (PNE and Stage 1, N=632) within 1 year of device implant. (PNE=Percutaneous Nerve Evaluation).
DISCUSSION:
NH residents are a particularly vulnerable population, with high rates of comorbidity and frailty that puts them at high risk for poor surgical outcomes. This is the largest cohort of NH residents who underwent a SNM. Mean age of residents was 77.9 years and over 80% were frail. Despite this, the majority (58.4%) of residents progressed from PNE or Stage 1 to implant and 9.3% underwent device explant/revision at 1 year. These findings were similar across age groups and no association was seen with CFI and comorbidity.
It is difficult to counsel older patients on the likelihood of treatment success following a trial of SNM, as reports in the literature are lacking. Prior work suggests that increasing age imparts a lower chance of success of progression to Stage 2, but sample sizes in these studies are limited. Two recent series offer insight into the safety and efficacy of SNM in an older population. Faris et al.20 retrospectively reviewed 356 subjects that underwent trial of SNM. Despite a relatively young age of subjects (mean 66.6 years), no difference in treatment success was seen according to comorbidity or age. Similarly, Zillioux et al.21 assessed the impact of cognitive impairment on treatment success among older adults with a mean age of 71.0 years. They found that overall rates of progression to Stage 2 were high in this population (76.4% for PNE, 88.3% Stage 1) and the authors concluded that there was no difference in rates of progression to Stage 2 for subjects with cognitive impairment compared to those without. Rates of progression to Stage 2 in the present study were similarly unaffected by age, CFI or comorbidity.
Overall rates of progression to Stage 2 in the present study are also high, at 58.4% for all subjects, which is comparable to existing literature with subjects that were younger and healthier 20,22, and higher than prior analyses of Medicare data23. Although high rates of device implant in the studies by Zillioux et al.21 and Faris et al.20 may be due to subjects receiving care at a high-volume center of excellence, the rate of progression to Stage 2 in the present analysis represents a nationwide aggregate across a mixture of practice settings. While beyond the scope of this study, it is possible that the mechanism of symptom improvement with SNM is distinct in older adults, as compared to younger individuals. It is also possible that older adults, with limited options in the treatment of OAB symptoms, are more likely to report subjective improvements following trial of SNM. Despite these reassuring rates of progression from test procedure to device implant, it is important to consider cost associated with neuromodulation procedures in patients with limited life expectancy; however this should not necessarily preclude its utilization.
In this study, 31% of NH residents who underwent neuromodulation experienced at least 1 complication within 30 days of trial procedure. It has been well-documented that increasing age, CFI and comorbidity is associated with complications following surgery 11,12. Although comparison to prior studies is difficult due to heterogeneity in reporting of timing and severity of adverse events, rates of complications appear similar to prior work3. The AUA guidelines on OAB state neuromodulation in older individuals can be offered in the context of a known rate of adverse events4.
Residents who underwent Stage 1 neuromodulation were more likely to progress to Stage 2 than those who underwent PNE. Although PNE can be performed in the office with just local anesthesia, the leads are prone to migration, dislodgement, and false negative responses, which can result in a lower rate of conversion from trial to Stage 224. Residents who underwent Stage 2 procedures following Stage 1 had explant/revision more than those who underwent PNE, but overall rates of explant/revision were still relatively low at 10.5% at 1 year. When counseling older adults on selection of neuromodulation procedure, it is important to keep these differences in mind, in addition to the higher rate of complications seen in Stage 1. For residents that underwent single-stage procedures, rates of explant/revision were nearly double that of staged procedures, suggesting that this strategy may not be ideal.
Despite high rates of progression to Stage 2 seen in the present study, rates of explant/revision were 9.3% in the first year. This was not impacted by age, comorbidity or CFI. Type of index procedure was the only identified factor predictive of device explant/revision. Single-stage procedures were strongly associated with device explant/revision at 12 months vs PNE. Although cost savings associated with this strategy support its use in select populations25, findings from this study suggest older adults may not be ideal candidates for single-stage procedures. However, single-stage may be considered to limit anesthesia exposure for patients at particularly high risk. The reasons for explant/revision are not offered by Medicare claims data; however, increasing age has been shown to result in lower risk of device revision 20, possibly due to unwillingness to undergo a second invasive procedure. Although these rates of device explant/revision may be related to symptom improvement and satisfaction among NH residents, it is possible that unmeasured factors represent risks for device explant/revision are unavailable Medicare claims data.
Results from this study must be taken in the context of its limitations. Although our large sample size and nationwide cohort allow for wider generalizability of these findings, this study is limited by its retrospective nature and claims data source and lack of patient reported outcomes. The CFI has been shown to predict poor surgical outcomes in Medicare beneficiaries12, but its role in NH residents – the majority of whom are frail – is less clearly understood. Complications were measured within 30 days of PNE or Stage 1 lead placement but it is unclear which complications are directly attributable to the index procedure versus incident medical events expected in a comorbid population. Multivariable models were created to account for confounding, but it is possible that unmeasured variables may influence findings. Importantly, measures relating to family and social support are not available in Medicare claims however this surely plays a role in outcomes following SNM. Finally, claims-based analyses are limited by potential errors in billing codes which can influence results.
Despite these limitations, this study presents important findings from the largest reported cohort of an under-studied and vulnerable population. Management of older adults with OAB can be challenging, and earlier use of invasive therapies such as SNM can improve symptoms without systemic toxicities associated with medical therapy. In NH residents undergoing trials of SNM, outcomes were similar to prior analyses of younger, healthier individuals, and not impacted by age, comorbidity or CFI. These findings are important to consider for surgeons who must balance the risks of any therapy with potential benefits in this medically complicated and vulnerable population.
CONCLUSION:
NH residing adults may be candidates for SNM and the majority of residents progress to device implant. Surgeons should exhibit caution when selecting older patients for single-stage procedures. Older adult candidates for SNM should be counseled on possible complications and perioperative risk, but reassured that chance of progression from trial to implant is similar to the general population.
Funding:
This work was funded through an NIH-NIA R01AG058616 grant.
Abbreviations:
- AUA
American Urological Association OAB: Overactive bladder
- PNE
Percutaneous Nerve Evaluation
- CFI
Claims-based Frailty Index
- CPT
Current Procedural Terminology
- ICD
International Classification of Diseases
- MDS
Minimum Data Set
Footnotes
Conflicts of interest: The authors have declared that no conflict of interest exist.
Ethics of approval: This study was deemed to be exempt by the University of California San Francisco review board.
Data availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES:
- 1.Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. Dec 2006;50(6):1306–14; discussion 1314–5. doi: 10.1016/j.eururo.2006.09.019 [DOI] [PubMed] [Google Scholar]
- 2.Angner E, Ray MN, Saag KG, Allison JJ. Health and happiness among older adults: a community-based study. J Health Psychol. May 2009;14(4):503–12. doi: 10.1177/1359105309103570 [DOI] [PubMed] [Google Scholar]
- 3.Zillioux J, Slopnick EA, Vasavada SP. Third-line therapy for overactive bladder in the elderly: Nuances and considerations. Neurourol Urodyn. Nov 2022;41(8):1967–1974. doi: 10.1002/nau.24965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lightner DJ, Gomelsky A, Souter L, Vasavada SP. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J Urol. Sep 2019;202(3):558–563. doi: 10.1097/JU.0000000000000309 [DOI] [PubMed] [Google Scholar]
- 5.Amundsen CL, Richter HE, Menefee S, et al. The Refractory Overactive Bladder: Sacral NEuromodulation vs. BoTulinum Toxin Assessment: ROSETTA trial. Contemp Clin Trials Mar 2014;37(2):272–83. doi: 10.1016/j.cct.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zillioux J, Welk B, Suskind AM, Gormley EA, Goldman HB. SUFU white paper on overactive bladder anticholinergic medications and dementia risk. Neurourol Urodyn. Nov 2022;41(8):1928–1933. doi: 10.1002/nau.25037 [DOI] [PubMed] [Google Scholar]
- 7.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol Nov 2007;178(5):2029–34. doi: 10.1016/j.juro.2007.07.032 [DOI] [PubMed] [Google Scholar]
- 8.Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs Sacral Neuromodulation on Refractory Urgency Urinary Incontinence in Women: A Randomized Clinical Trial. JAMA. Oct 04 2016;316(13):1366–1374. doi: 10.1001/jama.2016.14617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suskind AM, Kowalik C, Quanstrom K, et al. The impact of frailty on treatment for overactive bladder in older adults. Neurourol Urodyn. Sep 2019;38(7):1915–1923. doi: 10.1002/nau.24093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White WM, Mobley JD, Doggweiler R, Dobmeyer-Dittrich C, Klein FA. Sacral nerve stimulation for refractory overactive bladder in the elderly population. J Urol. Oct 2009;182(4):1449–52. doi: 10.1016/j.juro.2009.06.049 [DOI] [PubMed] [Google Scholar]
- 11.Suskind AM, Zhao S, Walter LC, Boscardin WJ, Finlayson E. Mortality and Functional Outcomes After Minor Urological Surgery in Nursing Home Residents: A National Study. J Am Geriatr Soc. May 2018;66(5):909–915. doi: 10.1111/jgs.15302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Kuiken ME, Zhao S, Covinsky K, Boscardin J, Finlayson E, Suskind AM. Frailty Is Associated with an Increased Risk of Complications and Need for Repeat Procedures after Sling Surgery in Older Adults. J Urol. Jun 2022;207(6):1276–1284. doi: 10.1097/JU.0000000000002441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suskind AM, Zhao S, Boscardin WJ, Covinsky K, Finlayson E. Comparative Outcomes for Pelvic Organ Prolapse Surgery among Nursing Home Residents and Matched Community Dwelling Older Adults. J Urol. Jan 2021;205(1):199–205. doi: 10.1097/JU.0000000000001331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finlayson E, Wang L, Landefeld CS, Dudley RA. Major abdominal surgery in nursing home residents: a national study. Ann Surg. Dec 2011;254(6):921–6. doi: 10.1097/SLA.0b013e3182383a78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. Dec 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0 [DOI] [PubMed] [Google Scholar]
- 16.Sheehy AM, Powell WR, Kaiksow FA, et al. Thirty-Day Re-observation, Chronic Re-observation, and Neighborhood Disadvantage. Mayo Clin Proc. Dec 2020;95(12):2644–2654. doi: 10.1016/j.mayocp.2020.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring Frailty in Administrative Claims Data: Comparative Performance of Four Claims-Based Frailty Measures in the U.S. Medicare Data. J Gerontol A Biol Sci Med Sci. May 22 2020;75(6):1120–1125. doi: 10.1093/gerona/glz224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massarweh NN, Legner VJ, Symons RG, McCormick WC, Flum DR. Impact of advancing age on abdominal surgical outcomes. Arch Surg. Dec 2009;144(12):1108–14. doi: 10.1001/archsurg.2009.204 [DOI] [PubMed] [Google Scholar]
- 19.(CMS) CfMMS. CMS Cell Suppression Policy https://www.hhs.gov/guidance/document/cms-cell-suppression-policy
- 20.Faris AER, Gill BC, Pizarro-Berdichevsky J, et al. Impact of Age and Comorbidities on Use of Sacral Neuromodulation. J Urol. Jul 2017;198(1):161–166. doi: 10.1016/j.juro.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 21.Zillioux J, Lewis KC, Hettel D, Goldman HB, Vasavada SP, Gill BC. Cognitive impairment does not impact sacral neuromodulation implant rates for overactive bladder. Neurourol Urodyn. Mar 2023;42(3):623–630. doi: 10.1002/nau.25138 [DOI] [PubMed] [Google Scholar]
- 22.Chughtai B, Clemens JQ, Thomas D, Sun T, Ghomrawi H, Sedrakyan A. Real World Performance of Sacral Neuromodulation and OnabotulinumtoxinA for Overactive Bladder: Focus on Safety and Cost. J Urol. Jan 2020;203(1):179–184. doi: 10.1097/JU.0000000000000462 [DOI] [PubMed] [Google Scholar]
- 23.Cameron AP, Anger JT, Madison R, Saigal CS, Clemens JQ, Project UDiA. National trends in the usage and success of sacral nerve test stimulation. J Urol. Mar 2011;185(3):970–5. doi: 10.1016/j.juro.2010.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leong RK, De Wachter SG, Nieman FH, de Bie RA, van Kerrebroeck PE. PNE versus 1st stage tined lead procedure: a direct comparison to select the most sensitive test method to identify patients suitable for sacral neuromodulation therapy. Neurourol Urodyn. Sep 2011;30(7):1249–52. doi: 10.1002/nau.20979 [DOI] [PubMed] [Google Scholar]
- 25.Lee W, Artenstein D, Tenggardjaja CF, et al. Single Institutional Experience with Single Stage Sacral Neuromodulation: Cost Savings and Outcomes in a Contemporary Case Series. J Urol. Mar 2020;203(3):604–610. doi: 10.1097/JU.0000000000000576 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


