Abstract
Many intracellular signalling events are accompanied by generation of reactive oxygen species in cells. Oxidation of protein thiol groups is an emerging theme in signal-transduction research. We have found that MEKK1 [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase kinase 1], an upstream activator of the SAPK/JNK (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, is directly inhibited by cysteine alkylation using NEM (N-ethylmaleimide). The related kinase, ASK1 (apoptosis signal-regulating kinase 1), was not inhibited, but was instead activated by NEM. Inhibition of MEKK1 requires a single unique cysteine residue (Cys1238) in the ATP-binding domain of MEKK1. Oxidative stress induced by menadione (2-methyl-1,4-naphthoquinone) also inhibited MEKK1, but activated ASK1, in cells. MEKK1 inhibition by menadione also required Cys1238. Oxidant-inhibited MEKK1 was re-activated by dithiothreitol and glutathione, supporting reversible cysteine oxidation as a mechanism. Using various chemical probes, we excluded modification by S-nitrosylation or oxidation of cysteine to sulphenic acid. Oxidant-inhibited MEKK1 migrated normally on non-reducing gels, excluding the possibility of intra- or inter-molecular disulphide bond formation. MEKK1 was inhibited by glutathionylation in vitro, and MEKK1 isolated from menadione-treated cells was shown by MS to be modified by glutathione on Cys1238. Our results support a model whereby the redox environment within the cell selectively regulates stress signalling through MEKK1 versus ASK1, and may thereby participate in the induction of apoptosis by oxidative stress.
Keywords: apoptosis signal-regulating kinase 1 (ASK1), glutathionylation, MEKK1, oxidative stress, redox, S-thiolation
Abbreviations: ASK1, apoptosis signal-regulating kinase 1; cAPK, cyclic AMP-dependent protein kinase; CBD, chitin-binding domain; C1238V (etc.), Cys1238→Val (etc.); DTT, dithiothreitol; EE epitope tag, MHEEEEYMPMEGPG (in the one-letter amino acid code); GST, glutathione S-transferase; HA, haemagglutinin; IL1, interleukin 1; JNK, c-Jun N-terminal kinase; LNCaP, lymph-node carcinoma of the prostate; MEKK1, MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase kinase 1; MKK4, mitogen-activated protein kinase kinase 4; MS/MS, tandem MS; NEM, N-ethylmaleimide; NF-κB, nuclear factor NF-κB; SNAP, S-nitroso-N-acetylpenicillamine; SAPK, stress-activated protein kinase; SEK, SAPK/ERK kinase; SEK-KR, a catalytically inactive mutant of SEK in which K (lysine) has been mutated to arginine (R); TNF, tumour necrosis factor
INTRODUCTION
Physiological stress events such as UV and γ-irradiation, as well as the inflammatory cytokines TNFα (tumour necrosis factor α) and IL1β (interleukin 1β), stimulate intracellular generation of reactive oxygen species, including H2O2 and superoxide [1–3]. This suggests that reactive oxygen species could be either mediators of stress signal transduction or might serve to control secondary events such as feedback regulation. These stress signals activate SAPK [stress-activated protein kinase; also known as JNK (c-Jun N-terminal kinase)] [4–6]. SAPK is a member of the MAPK (mitogen-activated protein kinase) family of protein kinases and is controlled by the serial activation of at least three upstream elements of a protein kinase cascade. The first characterized and best-known SAPK kinase kinase is MEKK1 [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase kinase 1] [7], which phosphorylates and activates the SAPK kinase SEK/MKK4 (SAPK/ERK kinase/mitogen-activated protein kinase kinase 4). However, several other related kinases such as ASK1 (apoptosis signal-regulating kinase 1) phosphorylate MKK4 on apparently identical sites [8]. ASK1 has a clear role in signalling to SAPK during apoptosis [8], whereas the activation of MEKK1 appears to transmit a survival signal [9–11]. It is an unsolved paradox how these two similar protein kinases, with apparently identical activities in phosphorylation of downstream target substrates, can have opposing functions in cell physiology.
MEKK1 was characterized by our group and others as an activator of the SAPK cascade [7,12]. Recent studies using embryonic stem-cell lines that lack MEKK1 have confirmed the crucial role of this kinase in activation of SAPK by a number of extracellular stimuli [13,14]. Several studies have also implicated MEKK1 in regulation of NF-κB (nuclear factor κB) signalling pathways [15–17], suggesting that NF-κB may represent a mechanism for the survival signalling associated with MEKK1. Although much is known about the downstream targets of MEKK1, the upstream signalling events leading to MEKK1 activation remain somewhat obscure. The full-length MEKK1 polypeptide has a large N-terminal regulatory domain containing a number of regions that could be important for MEKK1 regulation [18], including a provocative cysteine-rich region that could be a target for redox control. The role of this domain in regulation of MEKK1 activity, if any, remains to be elucidated.
We have been interested in mechanisms by which redox events regulate the function of the stress-activated SAPK/JNK kinase cascade. In general, oxidative stress is correlated with activation of SAPK/JNK as well as the p38 and ERK/MAPK kinases [19–22]. In preliminary experiments that led to the detailed work described in the present paper, we found that oxidative stress may provide clues to the contradictory signalling mediated by MEKK1 and ASK1. Specifically, some oxidants known to induce apoptosis and activate SAPK/JNK activation paradoxically inhibited MEKK1 activity. This led us to hypothesize that oxidative damage leading to stress-induced apoptosis was transmitted through an SAPK kinase kinase distinct from MEKK1 (perhaps ASK1, which is activated by oxidative events), whereas MEKK1 itself was subject to oxidant-mediated inhibition. The work described below confirms this hypothesis, and identifies a direct thiol oxidation event that serves to silence MEKK1 without inhibiting the activity of ASK1.
EXPERIMENTAL
Reagents
Unless otherwise specified, all chemicals were obtained from Sigma–Aldrich.
Plasmids
A full-length rat MEKK1 expression plasmid was obtained from Professor Melanie H. Cobb, Department of Pharmacology, University of Texas Southwestern Medical Center, Dallas, TX, U.S.A. The coding region was subcloned into plasmid pTM1 using a PCR strategy to engineer an EE (MHEEEEYMPMEGPG) epitope tag at the 5′ end and a chitin-binding domain (CBD) tag at the 3′ end. The truncated fragments were generated using PCR to amplify a portion of the coding region followed by cloning into the pTM1 vector. The C1238V (Cys1238→Val) mutant was generated using a PCR-based mutagenesis approach. The HA (haemagglutinin)–ASK1 expression clone was obtained from Professor Hidenori Ichijo (Department of Biomaterials Science, Tokyo Medical and Dental University, Tokyo, Japan). A CBD tag was added to the C-terminus of the coding region using PCR.
Protein expression and treatments
A 1 μg portion of DNA encoding full-length or truncated MEKK1 proteins or full-length ASK1 was expressed in CV-1 cells in six-well plates using a T7-polymerase-driven vaccinia-virus overexpression system as described in [23]. After incubation overnight, the cells were treated for 20–30 min at 37 °C with the compounds indicated in the Figure legends and then lysed in Mops lysis buffer [50 mM Mops (pH 7.0)/250 mM NaCl/5 mM EDTA/0.1% Nonidet P40] supplemented with 2.5 μg/ml aprotinin, 2.5 μg/ml leupeptin, 10 mM sodium fluoride, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate and 10 mM β-glycerol phosphate. Lysates were clarified by centrifugation at 13000 g at 4 °C for 5 min. Proteins were purified using chitin beads (New England Biolabs), washed twice with 30 vol. of Mops lysis buffer and once with 30 vol. of 50 mM Tris/HCl, pH 7.8. In vitro treatments were carried out at room temperature in 50 mM Tris/HCl, pH 7.8, at concentration and incubation times as indicated in the Figure legends. After incubation, the buffer containing the in vitro treatment was removed from the bead-bound kinase, which was washed once with 50 mM Tris/HCl, pH 7.4, and the activity measured using an in vitro kinase assay.
For experiments with endogenous MEKK1, LNCaP (lymph-node carcinoma of the prostate) cells were serum-starved for 16 h. Cells (2×107 per sample) were concentrated into 10 ml of serum-free medium, then left untreated or treated with 50 μM menadione (2-methyl-1,4-naphthoquinone) for 15 min. The cells were pelleted, lysed in 1 ml of Mops lysis buffer as described above, and MEKK1 was isolated by immunoprecipitation with anti-MEKK1 antibody (1-9C-2A; Santa Cruz Biotechnology) and Protein A–Sepharose (Repligen Corp., Waltham, MA, U.S.A.). Treatment with DTT (dithiothreitol) and assay for kinase activity were carried out as for the chitin-tagged recombinant proteins.
In vitro kinase assays
Purified MEKK1 or ASK1 on chitin beads (or immunoprecipitates on Protein A–Sepharose in the case of endogenous MEKK1) was assayed for activity by an in vitro kinase assay in a buffer containing 50 mM Tris/HCl, pH 7.4, 10 mM MgCl2 and 7.5 μM ATP in the presence of 10 μCi of [γ-32P]ATP. A 1 μg portion of bacterially expressed GST (glutathione S-transferase)-tagged catalytically inactive SEK (GST–SEK-KR) was included as substrate [SEK-KR is a catalytically inactive mutant of SEK in which K (lysine) has been mutated to arginine (R)]. Reaction mixtures were incubated for 30 min at room temperature, then the reaction stopped by the addition of an equal volume of 2×concentrated Laemmli sample buffer [2% (w/v) SDS/100 mM Tris/HCl (pH 7.0)/20% (v/v) glycerol/150 mM DTT/Bromophenol Blue]. The samples were boiled and subjected to SDS/10%-PAGE. Gels were transferred to PVDF membrane (Immobilon), followed by imaging and quantification using a Packard Instant Imager. Equivalent protein was confirmed by immunoblotting with anti-MEKK1 antibody (Santa Cruz Biotechnology) or anti-EE or anti-HA epitope antibodies as indicated.
MS analysis of MEKK1
An expression plasmid was engineered by PCR to encode the wild-type catalytic domain (Δ1172) of MEKK1 with a thrombin recognition sequence between the coding region and the CBD tag. This protein was expressed and isolated as described above from untreated cells or cells treated with 250 μM menadione. Soluble protein was released from the chitin beads by cleavage with thrombin and then submitted, for analysis under standard liquid chromatography–MS conditions, to W. M. Keck Biomedical Mass Spectrometry Laboratory, University of Virginia, Charlottesville, VA, U.S.A. Briefly, the protein was digested with modified trypsin (Promega, Madison, WI, U.S.A.) overnight at room temperature. A 100 ng portion of proteinase Asp-N was added and the material allowed to digest overnight at room temperature. The samples were acidified with formic acid, and the peptide extract was injected on to a self-packed Jupiter C18 (Phenomenex, Torrance, CA, U.S.A.) microcapillary column and eluted into a Finnigan LCQ (ThermoFinnigan, San Jose, CA, U.S.A.) ion-trap mass spectrometer using a 2–85% (v/v) acetonitrile/0.1 M acetic acid gradient. Full-scan (MS) and product-ion [MS/MS (tandem MS)] spectra were acquired using full data-dependent acquisition routines. MS/MS spectra were automatically batch-searched using Sequest (ThermoFinnigan) against the MEKK1 sequence. Peptides containing the modified cysteine residues were manually interpreted for confirmation.
RESULTS
MEKK1 activity is inhibited by cysteine alkylation
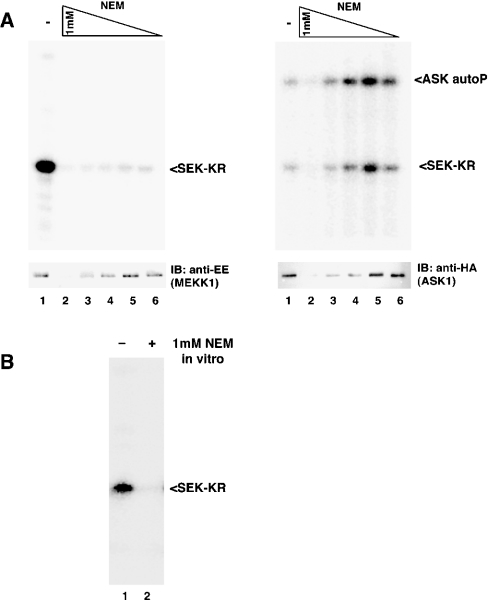
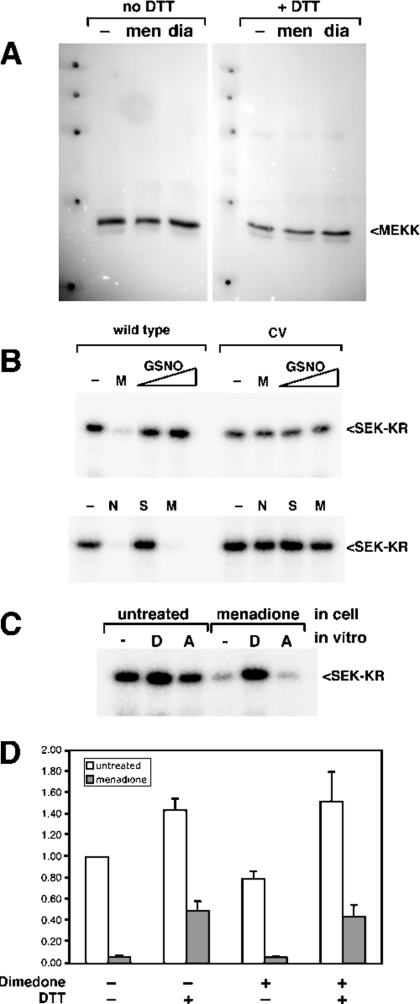
To test the hypothesis that cysteine residues play a role in the regulation of MEKK1 activity, we treated cells overexpressing full-length MEKK1 with increasing doses of NEM (N-ethylmaleimide), a reagent that covalently modifies cysteine residues. MEKK1 was then isolated by affinity purification on chitin beads and assayed for kinase activity. Treatment of cells with NEM completely inhibited the activity of MEKK1 (Figure 1A, left panel). At the highest doses of NEM (0.5–1.0 mM), the MEKK1 protein was undetectable (Figure 1A, left, lower panel), suggesting that these concentrations of NEM induced degradation of the full-length MEKK1 protein.
Figure 1. NEM specifically and directly inhibits MEKK1, but not ASK1.
(A) Cells overexpressing full-length MEKK1 (left) or ASK1 (right) fused to a CBD were treated with decreasing concentrations of NEM (1, 0.5, 0.25, 0.1 and 0.05 mM). Cells were lysed, the kinase purified and its activity was measured by an in vitro kinase assay using SEK-KR as substrate. MEKK1 was inhibited at all concentrations of NEM. The inhibition of MEKK1 is specific, since ASK1 is not inhibited (but instead activated) by NEM treatment. High concentrations of NEM cause degradation of both kinases. Abbreviations: autoP, autophosphorylation; IB, immunoblot. (B) Purified MEKK1 on chitin beads was treated with 1 mM NEM for 20 min. NEM was removed and the activity measured as described above. Purified MEKK1 is inhibited by NEM, suggesting that inhibition is due to direct modification of MEKK1.
The related MAP3K kinase, ASK1, was not inhibited by moderate doses of NEM, but was instead activated by NEM at the lower concentrations (Figure 1A, right panel, lanes 4–6). Similar to MEKK1, the higher doses of NEM caused a loss of ASK1 protein. Thus inhibition of MEKK1 by moderate concentrations of NEM occurs through a specific mechanism that is not common to all protein kinases. In contrast, the degradation induced by higher doses of NEM appears to operate through a mechanism that is common to both MEKK1 and ASK1.
Inhibition of MEKK1 by low concentrations of NEM might be due to a direct effect on MEKK1 itself, or could represent inhibition of events upstream of MEKK1. To determine whether MEKK1 is directly inhibited by NEM, we affinity-purified active MEKK1 and treated the purified protein with NEM in vitro. As shown in Figure 1(B), purified MEKK1 was completely inhibited by exposure to NEM in vitro, indicating direct modification and inhibition of MEKK1 by NEM.
NEM targets the catalytic domain of MEKK1
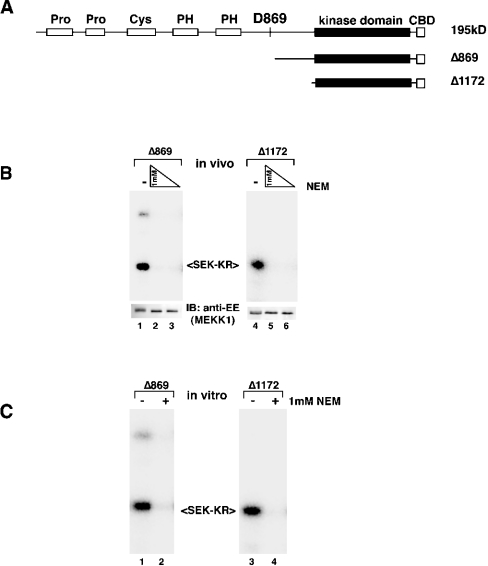
To determine which domain of MEKK1 mediates sensitivity to NEM, we compared the effect of NEM treatment on two C-terminal protein fragments of MEKK1 that lack the N-terminal regulatory domains (Figure 2A). The first truncation mutant, Δ869, mimics the fragment generated when MEKK1 undergoes caspase-mediated cleavage during apoptosis [9–11]. The second, Δ1172, is likely the minimal-catalytic-domain fragment of the kinase. We found that both the Δ869 fragment and the catalytic-domain fragment Δ1172 were inhibited by NEM after exposure in cells (Figure 2B) or in vitro (Figure 2C). Thus the NEM-targeted cysteine residue(s) that is relevant for kinase activity lies within the catalytic domain of the kinase. Importantly, the truncated kinases were not degraded in response to high doses of NEM (1 mM) (Figure 2B, lower panels), suggesting that the degradation of MEKK1 in response to NEM requires sequences upstream of the amino acid at position 869.
Figure 2. The catalytic domain of MEKK1 is the target of NEM inhibition.
(A) Schematic of full-length MEKK1 and the two truncated fragments (not drawn to scale). The site of caspase cleavage (D869) is indicated. N-terminally truncated fragments of MEKK1 representing the caspase cleavage product (Δ869) or the catalytic domain fragment (Δ1172) are shown. Abbreviation: PH, pleckstrin homology domain. The indicated CBD-tagged proteins were expressed and treated with 1 mM and 0.5 mM NEM on cells (B) or 1 mM NEM for purified protein in vitro (C). Abbreviation: IB, immunoblot. Kinase activity was measured by in vitro kinase assay. The catalytic fragment of the protein is inhibited by NEM, indicating that the target of modification lies within this domain. The truncated MEKK1 proteins are not degraded in response to NEM.
Cys1238 is the target of inhibitory NEM modification
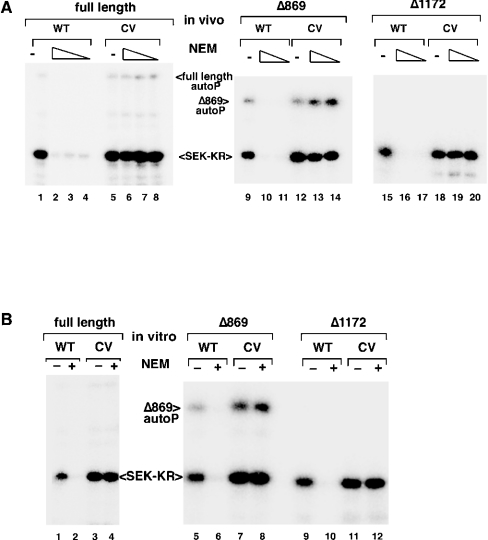
Comparison of the primary sequence of MEKK1 with those of other MEKK family members, including the NEM-insensitive kinase ASK1, revealed the presence of an unconserved cysteine residue in the ATP-binding domain of MEKK1. This position is occupied by a valine residue in most other kinases. To determine whether this cysteine residue could represent the target of NEM modification that is relevant for MEKK1 activity, we mutated the catalytic-domain fragment of MEKK1 to replace this cysteine residue with a valine residue. This mutation, at position 1238 (C1238V), resulted in the generation of a protein kinase that retained activity but was completely resistant to inhibition by NEM, either in treated cells (Figure 3A) or as purified protein treated with NEM in vitro (Figure 3B). In fact, mutation of this single cysteine residue to a valine residue was sufficient to completely abolish NEM sensitivity, even in the context of the full-length MEKK1 protein (Figures 3A and 3B, left panels). Thus, whereas NEM certainly alkylates numerous cysteine residues on full-length MEKK1, only Cys1238 alkylation is relevant for loss of catalytic activity.
Figure 3. MEKK1 C1238V substitution confers NEM resistance.
The cysteine codon at position 1238 was mutated to valine in the context of full-length or truncated MEKK1. The proteins were overexpressed and then treated with NEM either in cells (A) (full-length, 0.25, 0.1 and 0.05 mM; truncations, 1 and 0.5 mM) or following purification (B) (1 mM NEM for all). The C1238V mutant of MEKK1 is completely resistant to NEM. Abbreviations: autoP, autophosphorylation; WT, wild-type; CV, C1238V mutant.
Oxidative stress inhibits wild-type MEKK1 but not the C1238V mutant
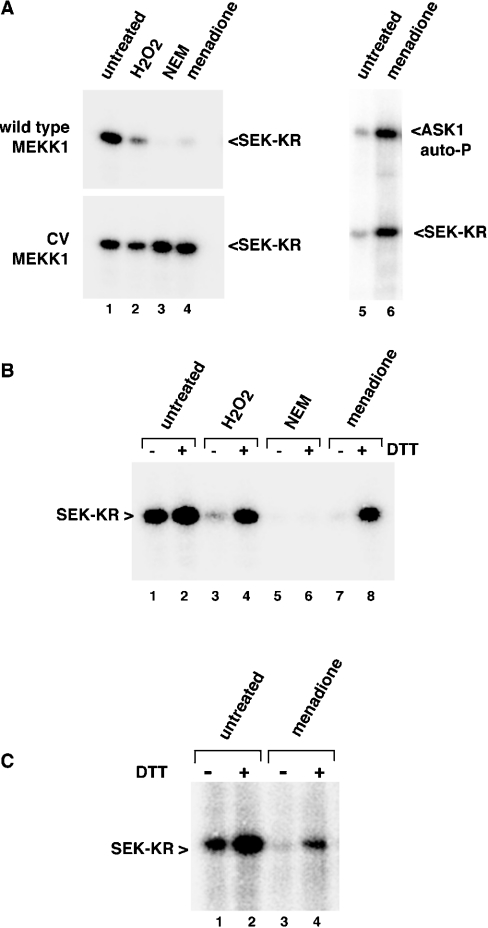
Treatment of MEKK1 with NEM indicates that modification of the cysteine at position 1238 is sufficient to completely abolish kinase activity. Therefore we set out to determine whether oxidative stress could induce cysteine modification(s) that impact MEKK1 activity. Treatment of cells overexpressing the catalytic domain of MEKK1 with 10 mM H2O2 inhibited MEKK1 activity (Figure 4, upper panel). This inhibition was unique to the wild-type protein, since the C1238V mutant was resistant to inhibition by H2O2 (Figure 4A, lower panel).
Figure 4. Oxidative stress inhibits MEKK1 by inducing a DTT-reversible modification.
(A) Wild-type or C1238V catalytic domain MEKK1 (Δ1172) (left) or full-length ASK1 (right) were expressed in cells that were then treated with hydrogen peroxide (H2O2, 10 mM), NEM (1 mM) or menadione (250 μM) as indicated. Kinase was purified and activity measured using an in vitro kinase assay. Stimuli that induce oxidative stress inhibited wild-type MEKK1, but not the C1238V (‘CV’) mutant. ASK1 is not inhibited, but instead activated, by menadione. (B) Wild-type MEKK1 was isolated from cells treated as in (A) and then incubated in vitro with 10 mM DTT. The activity of the kinase was measured by an in vitro kinase assay. DTT treatment restored the activity of H2O2- or menadione-inhibited MEKK1. (C) Endogenous MEKK1 was immunoprecipitated from either untreated or menadione-treated LNCaP cells. The protein was left untreated or treated with 10 mM DTT in vitro, and the activity measured by an in vitro kinase assay. Endogenous MEKK1 was inhibited by menadione treatment and was partially re-activated by reduction with DTT. Abbreviations: autoP, autophosphorylation; WT, wild-type; CV, C1238V mutant.
Menadione is a redox-cycling reactive quinone that has a number of effects on the cell, including the generation of reactive oxygen species and depletion of glutathione, resulting in intracellular oxidative damage [24–29]. We examined the effect of menadione treatment of cells on the activity of MEKK1. Similar to treatment with H2O2, exposure of cells to 250 μM menadione inhibited wild-type MEKK1 (Figure 4A, upper left panel). Although this concentration of menadione was used in most experiments in order to achieve near complete inhibition over the short time periods tested, MEKK1 was inhibited dose-responsively by menadione, with approx. 50% inhibition at 25 μM (results not shown). In contrast with wild-type MEKK1, the C1238V mutant of MEKK1 was completely resistant to inhibition (Figure 4A, lower left panel). Importantly, ASK1 was not only resistant to inhibition by menadione, but was instead activated in response to menadione (Figure 4A, right panel). Thus, like NEM, oxidants specifically inhibit MEKK1 activity through a single cysteine residue, amino acid position 1238.
Oxidant-mediated inhibition of MEKK1 is reversed by treatment with DTT in vitro
To verify that the inhibition of MEKK1 was due to cysteine oxidation and to begin characterization of the nature of the modification, we examined whether the inhibition could be reversed by reduction in vitro. Inhibited MEKK1 protein was purified from oxidant-treated cells and then incubated in vitro with the reducing agent DTT. DTT treatment completely restored the activity of MEKK1 protein isolated from menadione or H2O2-treated cells (Figure 4B). Since alkylation of cysteine residues by NEM is an irreversible reaction, MEKK1 protein isolated from NEM-treated cells could not be re-activated by DTT in vitro (Figure 4B). Importantly, DTT treatment of wild-type MEKK1 isolated from untreated cells consistently resulted in a small, but reproducible, increase in kinase activity (Figure 4B; see also Figures 5, 6C and 6D below), perhaps indicating that a fraction of the overexpressed protein is present in an oxidized and inhibited form that can be fully activated by reduction with DTT in vitro.
Figure 5. Oxidant-inhibited MEKK1 is protected from NEM exposure.
Wild-type or C1238V catalytic domain (Δ1172) MEKK1 was expressed in cells that were then left untreated or treated with 250 μM menadione. The cells were lysed in buffer containing 1 mM NEM. The MEKK1 protein was purified on chitin beads and then treated with 10 mM DTT as described above. The MEKK1 isolated from menadione-treated cells was protected from NEM modification during lysis, and could therefore be re-activated by subsequent DTT treatment in vitro.
Figure 6. MEKK1 inhibition by oxidants does not involve inter- or intra-molecular disulphide-bond formation, NO modification, or oxidation of cysteine to sulphenic acid.
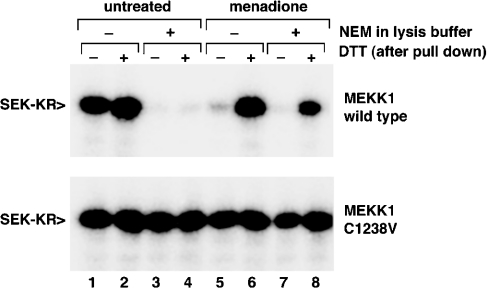
(A) Cells expressing the catalytic domain fragment of MEKK1 were left untreated (‘–’) or treated with menadione (‘men’; 250 μM) or diamide (‘dia’; 1 mM). The cells were lysed and samples prepared in Laemmli sample buffer without DTT supplemented with 10 mM NEM. Samples were divided into two fractions and one was adjusted to 75 mM DTT. The samples were subjected to SDS/10%-PAGE, transferred to PVDF membrane and blotted with anti-MEKK1 antibody. Oxidant-inhibited MEKK1 did not exhibit altered electrophoretic mobility under non-reducing conditions. (B) Cells expressing wild-type or C1238V (CV) mutant Δ1172 (upper panel) or N-terminally truncated MEKK1 Δ765 (lower pannel) were treated with menadione (M, 250 μM), GSNO (0.2 mM or 0.5 mM), NEM (N, 1 mM) or SNAP (S, 100 μM) as indicated. MEKK1 was purified and activity measured by in vitro kinase assay. MEKK1 activity was unaffected by either of the NO donors. (C) The catalytic domain of MEKK1 was isolated from either untreated cells or cells exposed to 250 μM menadione. Purified MEKK1 was treated with either 10 mM DTT (‘D’) or 10 mM ascorbate (‘A’) for 30 min, then MEKK1 activity was measured by in vitro kinase assay. Menadione-inhibited MEKK1 was not re-activated by exposure to ascorbate in vitro. (D) Wild-type catalytic domain MEKK1 was isolated from untreated cells (white bars) or cells treated with 250 μM menadione (grey bars). Purified MEKK1 was incubated with or without 20 mM dimedone for 1 h at room temperature. DTT at 10 mM was added to the indicated samples, and incubation continued for additional 30 min. The buffer containing the treatments was removed from the kinase immobilized on chitin beads, and activity was measured by using an in vitro kinase assay. A representative of three independent experiments is shown. Dimedone did not interfere with re-activation of menadione-inhibited MEKK by DTT.
Endogenous MEKK1 is inhibited by cysteine oxidation following oxidative stress
To confirm the role of redox events in regulation of MEKK1, we examined the effect of menadione treatment on endogenous MEKK1 protein activity. Endogenous MEKK1 protein was immunoprecipitated from either untreated cells or cells that were exposed to menadione for 20 min. The immunoprecipitated MEKK1 was either left untreated or incubated with 10 mM DTT for 30 min, and then assayed for kinase activity. Oxidative stress induced by menadione significantly inhibited the activity of the endogenous MEKK1 protein (Figure 4C; compare lanes 1 and 3). This loss of activity could be partially restored by exposure of the purified protein to DTT in vitro (Figure 4C, lane 4), suggesting that inhibition is due to a reversible, DTT-sensitive modification. Importantly, similar to the overexpressed catalytic-domain fragment, the activity of the endogenous protein isolated from untreated cells was also stimulated more than 2-fold by exposure to DTT in vitro (Figure 4C; compare lanes 1 and 2), suggesting that a fraction of the endogenous MEKK1 protein is inhibited by a DTT-sensitive modification, even in untreated cells.
Oxidized MEKK1 is resistant to inhibition by NEM
To further confirm that in vivo inhibition of MEKK1 by menadione is the result of a reversible cysteine-oxidation event, we asked whether MEKK1 oxidized by menadione treatment of cells would be protected from subsequent exposure to NEM. To this end, untreated or menadione-treated cells were lysed in a buffer containing 1 mM NEM to alkylate all non-oxidized cysteine residues. In the case of wild-type unoxidized protein, the presence of NEM in the lysis buffer completely inhibited the activity of MEKK1 (Figure 5; compare lane 3 with lane 1). This protein could not be re-activated by DTT in vitro (Figure 5, lane 4). Treatment of cells with menadione prior to lysis in NEM resulted in MEKK1 that was inhibited (Figure 5, lane 7). However, this protein could be re-activated by DTT (Figure 5, lane 8). This indicates that oxidative modification of the cysteine residue protects MEKK1 from irreversible alkylation by NEM. As expected, the C1238V mutant is unaffected by lysis in the presence of NEM, as well as by in vitro reduction with DTT.
Identification of the Cys1238 oxidation event on MEKK1
Cysteine residues within proteins can be modified in several ways following oxidative stress in cells. These include: (1) formation of inter- or intra-molecular disulphide bonds; (2) modification by reactive nitrogen species (i.e. S-nitrosylation); (3) direct oxidation of the cysteine thiol groups to sulphenic acid or to higher-order sulphinic or sulphonic acid; or (4) formation of mixed disulphides with a small intracellular thiol (e.g. glutathione).
To address the first possibility, we examined oxidant-inhibited MEKK1 by non-reducing gel electrophoresis (Figure 6A). The presence of an intramolecular disulphide bond would result in altered migration on non-reducing SDS/PAGE. Formation of a disulphide bond between two molecules of MEKK1 or between MEKK1 and another protein would reduce mobility on SDS/PAGE. Neither menadione nor diamide treatment of cells expressing MEKK1 resulted in altered electrophoretic mobility under non-reducing conditions, suggesting that the inhibition of MEKK1 is not due to either type of disulphide-bond formation.
To address the potential sensitivity of MEKK1 to S-nitrosylation, we used two different nitric oxide donors, S-nitrosoglutathione (GSNO) and S-nitroso-N-acetylpenicillamine (SNAP). Both failed to inhibit MEKK1 activity, suggesting that MEKK1 is not sensitive to modification by NO (Figure 6B). Even at concentrations as high as 10 mM (results not shown), SNAP had no effect on MEKK1 activity. In addition, ascorbate, which reverses S-nitrosylation, failed to re-activate menadione-inhibited MEKK1 (Figure 6C), suggesting that inhibition is not due to S-nitrosylation.
Since DTT reactivates oxidant-inhibited MEKK1 (shown in Figure 4B), higher-order oxidation of cysteine to sulphinic or sulphonic acid is unlikely, as these oxidized forms are insensitive to reduction by DTT. To address the possibility of oxidation of cysteine to sulphenic acid, we used a covalent probe for sulphenic acid, dimedone (5,5-dimethylcyclohexane-1,3-dione) [30]. Reaction of sulphenic acid with dimedone produces a DTT-irreversible modification [31]. If the inhibition of MEKK1 by menadione were due to sulphenic acid formation, in vitro exposure to dimedone would prevent subsequent re-activation by DTT. Figure 6(D) demonstrates that dimedone had no effect on the reactivation of MEKK1 by DTT, suggesting that inhibition is not due to sulphenic acid.
Taken together, these experiments leave formation of mixed disulphides with small thiol-containing molecules as the likely mechanism for inhibition of MEKK1 by oxidative stress. We examined whether menadione may inhibit MEKK1 by inducing glutathionylation of Cys1238.
Oxidized MEKK1 is re-activated by exposure to glutathione
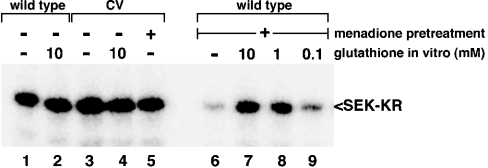
To further define the modification that occurs at Cys1238 in response to oxidant exposure, menadione-inhibited protein was exposed to various reducing agents in vitro. Like DTT, 2-mercaptoethanol was able to restore the activity of menadione-inhibited MEKK1 (results not shown). Interestingly, glutathione, a relatively weaker reducing agent, was also capable of re-activating menadione-inhibited MEKK1. The re-activation by glutathione was dose-responsive over three logarithmically decreasing concentrations (10 mM, 1.0 mM, and 0.1 mM; Figure 7, lanes 7, 8 and 9 respectively), with the re-activation nearly complete at 10 mM. The C1238V mutant was resistant to any effect of menadione or in vitro exposure to glutathione, even at the highest dose tested (Figure 7, lanes 3–5). These data support glutathionylation as the mechanism of inhibition of MEKK1 by menadione. It should be noted that these concentrations of glutathione are within the range predicted to exist in mammalian cells, suggesting that oxidant inhibition of MEKK1 would be reversible at physiologically relevant concentrations of glutathione.
Figure 7. Glutathione re-activates menadione-inhibited MEKK1.
The catalytic domain of MEKK1 was isolated from untreated cells or cells treated with 250 μM menadione. The purified protein was incubated with the indicated concentrations of glutathione for 30 min and activity measured using an in vitro kinase assay. Exposure to glutathione restored the activity of menadione-inhibited MEKK1.
MEKK1 is inhibited by in vitro oxidant-mediated glutathionylation
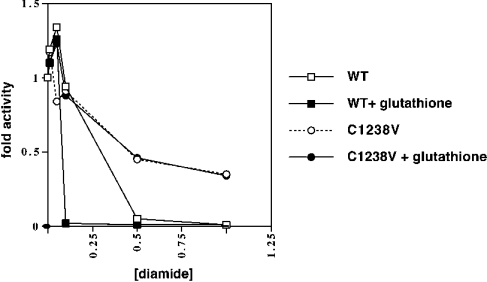
To directly examine the sensitivity of MEKK1 to glutathionylation, we applied an in vitro oxidant-induced glutathionylation assay [32,33]. Purified MEKK1 was exposed to an oxidant (diamide) in the presence or absence of glutathione (Figure 8). Wild-type MEKK1 was dose-responsively inhibited by diamide. This inhibition was markedly enhanced in the presence of glutathione. The C1238V mutant was partially resistant to diamide at the concentrations tested. Importantly, the C1238V protein was completely insensitive to the presence of glutathione in the reaction mixture. Thus MEKK1 is inhibited by glutathionylation in vitro, and inhibition requires Cys1238.
Figure 8. Oxidant-induced glutathionylation in vitro inhibits MEKK1.
Purified wild-type (‘WT’) or C1238V MEKK1 was treated with increasing concentrations of the oxidant diamide in the presence or absence of 100 μM glutathione for 30 min at room temperature. The treated proteins were washed, and the activity of the MEKK1 measured by in vitro kinase assay. A representative of three independent experiments is shown. Diamide inhibited MEKK1 dose-responsively over the concentration range tested. This inhibition was enhanced in the presence of glutathione. The C1238V mutant was less sensitive to diamide treatment and was unaffected by the inclusion of glutathione in the reaction mixture.
Glutathionylation of MEKK1 at Cys1238 in cells following oxidative stress
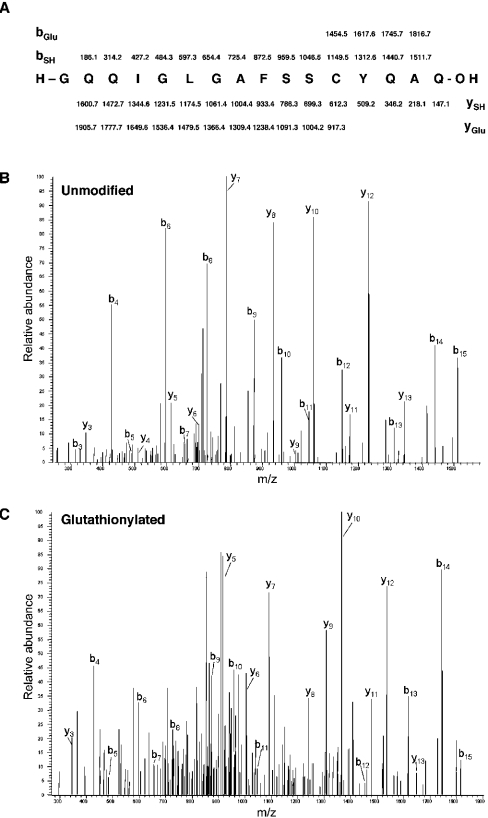
To confirm the results of the in vitro assay above and to determine whether MEKK1 is glutathionylated in intact cells, we examined MEKK1 from menadione-treated cells by MS (Figure 9). The peptide containing Cys1238 was observed in the spectra obtained from both the untreated MEKK1 protein and MEKK1 purified from menadione-treated cells. However, menadione-exposed MEKK1 contained an additional peak representing a modified form of the Cys1238-containing peptide with a 305 Da mass increase. This mass change is consistent with glutathionylation of the peptide. Sequencing of this peak by MS/MS localized the modification to the cysteine residue. This glutathionylated form of the peptide was absent from the untreated control, where the Cys1238-containing peptide existed exclusively in an unmodified reduced form. A second fraction of the Cys1238-containing peptide in the menadione-treated MEKK1 sample had a 171 Da addition. This mass difference is compatible with direct menadione adduct formation, a known consequence of menadione exposure [34]. Sequence analysis confirmed that this modification also occurred at Cys1238. These results demonstrate that oxidative stress induced by menadione leads to glutathionylation of MEKK1 at Cys1238 in intact cells.
Figure 9. MEKK1 is glutathionylated at Cys1238 in menadione-treated cells.
MEKK1 was purified from untreated cells or from cells treated with 250 μM menadione, digested with trypsin and proteinase Asp-N and analysed by MS. (A) Predicted production (MS/MS) values for unmodified and glutathionylated Cys1238-containing peptide. Representative MS/MS spectra for unmodified peptide (B) and glutathionylated peptide (C) are shown, with indicators identifying the predicted peaks.
DISCUSSION
Oxidation of specific cysteine residues on target proteins is an emerging theme in regulation of signal transduction. Like control by phosphorylation, redox control of signal transduction is likely to occur through several mechanisms that control protein localization and interactions, as well as the intrinsic activity of cell-signalling proteins. Here, we demonstrate that the MEKK1 protein kinase is sensitive to inhibition by direct cysteine alkylation as well as by exposure of cells to agents that induce oxidative stress. We identified a single cysteine residue that mediates this sensitivity. By examining various chemical and physical properties of oxidized MEKK1, we characterized the nature of the redox modification at this cysteine residue. Finally, using an in vitro assay as well as direct analysis of MEKK1 purified from oxidant-exposed cells by MS we demonstrated that inhibition of MEKK1 involves glutathionylation at Cys1238.
Several examples of oxidation events that impact protein activity have been described previously. Formation of an intramolecular disulphide bond in the bacterial OxyR transcription factor following exposure to H2O2 results in activation of the protein [35]. In mammalian systems, the serotonin (5-hydroxytryptamine) N-acetyltransferase (arylalkylamine N-acetyltransferase, ‘AA-NAT’) forms an intramolecular disulphide between cysteine residues at positions 61 and 177 following exposure to H2O2 in vitro, resulting in inhibition of enzymic activity [36]. Formation of sulphenic acid has been proposed to be involved in the regulation of a variety of proteins (e.g. N-acetyltransferases, peroxiredoxins, p50 NF-κB and human serum albumin [37–39]). However, the oxidation to sulphenic acid is generally considered unstable and is often modelled as an intermediate in an oxidation pathway leading to formation of a more stable oxidized form.
Oxidative control has been shown in several signalling molecules, largely through in vitro studies. Some protein kinases are regulated through interaction with oxidation-sensitive partner molecules. For example, the ASK1 protein kinase is inhibited by non-covalent binding to reduced thioredoxin [40]. Upon oxidation the thioredoxin dissociates, resulting in activation of ASK1. A similar mechanism has been proposed for inhibition of SAPK by GST Pi [41].
Other signalling proteins are regulated by direct oxidation events. The small G protein Ras can be modified by both glutathionylation and S-nitrosylation. These modifications can prevent the required farnyslation of the Ras protein [42], as well as stimulate its guanine-nucleotide exchange function [43]. The mammalian cell-cycle phosphatase cdc25B is susceptible to inhibition in the presence of H2O2 in vitro through disulphide-bond formation involving a sulphenic acid intermediate [44,45]. The active-site cysteine residue of protein phosphatases has been examined as a target for oxidative inhibition [32,46]. The best-characterized example of this is the inhibition of protein tyrosine phosphatase 1B by H2O2 in vitro. This is caused by a stable sulphenyl–amide intermediate formed between a sulphenic acid at the catalytic cysteine and the main peptide chain amine of the adjacent serine residue [47,48]. Finally, cAPK (cyclic AMP-dependent protein kinase) is inhibited by the oxidant diamide in vitro, resulting in disulphide-bond formation between cysteine residues at position 199 and 343 [49]. However, in the presence of glutathione, either in vitro or in the context of an intact cell, diamide inhibits cAPK by glutathionylation at Cys199 [49]. This cysteine residue lies within the activation loop of the protein kinase and is predicted to be important for interaction with substrates.
The modification we have characterized on MEKK1 is unique in these examples. The Cys1238 residue of MEKK1 lies within the ATP-binding pocket of the kinase. Modification of this residue by either experimental alkylation with NEM or by inducible glutathionylation in oxidant-exposed cells would therefore be predicted to inhibit the catalytic activity by interfering with the ability of the kinase to bind ATP. The presence of a cysteine codon at this location is rare. Only a few other protein kinases share this feature, including the polo family kinases [PLK (polo-like kinase), polo, cdc5] and the src-family tyrosine kinase Lck (lymphocyte kinase). This shared cysteine residue suggests that these kinases may also be sensitive to oxidative stress via modification at this site.
Inhibition of MEKK1 by oxidation appears to be dominant over activation of the kinase by phosphorylation. This is based on the observation that oxidative inhibition does not remove the activating autophosphorylation [50], as judged by an unchanged electrophoretic mobility. In addition, re-activation of oxidant-inhibited MEKK1 by DTT does not require ATP. Cys1238 appears unique in its contribution to redox control of MEKK1, as individual mutation of each of four cysteine residues in the catalytic domain (Cys1295, Cys1411, Cys1425 and Cys1469) does not affect the oxidant sensitivity of the protein (results not shown). Mutation of the fifth cysteine (Cys1418), which is conserved in ASK1, results in a protein that is catalytically inactive. The conservation of this cysteine residue in other MEKK family members suggests a potential structural role.
MEKK1 and the related kinase ASK1 both phosphorylate MKK4, leading to activation of SAPK/JNK by both kinases. Paradoxically, ASK1 promotes apoptosis [8], whereas MEKK1 provides a cell-survival signal [9–11], probably through coincident activation of other pathways. Our results provide a possible explanation for this contradiction, in that oxidative stress can activate ASK1 while inhibiting MEKK1. Thus, while many stress signals (e.g. inflammatory cytokines) may stimulate both kinases and result in a mixed death and survival signal, the addition of oxidative damage serves to shut down one kinase while stimulating the other. Therefore, glutathionylation of MEKK1 might participate in shifting the balance from cell survival to cell death.
In addition to a role in the regulation of MEKK1 protein following oxidative stress, inhibition of basal MEKK1 activity by oxidation of Cys1238 may be a part of normal cellular physiology. The activity of endogenous MEKK1 is enhanced more than 2-fold by DTT in vitro, and the wild-type catalytic domain fragment (but not the C1238V mutant) is also moderately activated by DTT. This suggests that MEKK1 is inhibited by a DTT-reversible modification, even in unstressed cells. In addition, cysteine oxidation may play a role in feedback inhibition of MEKK1 activity following stimulatory signalling. Many extracellular stimuli, including inflammatory cytokines and UV exposure, are associated with generation of reactive oxygen species such as H2O2. Glutathionylation of MEKK1 may represent a means of feedback inhibition following responses to these stimuli.
The contribution of oxidative control in the regulation of signal transduction is emerging through the examples cited above and the work described here. Experimental evaluation of redox regulation of protein function is severely limited by the lack of appropriate reagents and methods. We are interested in further developing techniques to allow examination of these events. New approaches for these studies could reveal a substantial role for protein-oxidation events in the control of signal transduction, the complexity and magnitude of which probably complements the significant role of phosphorylation in the exquisite control of cell signalling.
Acknowledgments
This work was funded by grants from the National Cancer Institute (NCI; CA66134) and the Burroughs Wellcome Fund. We thank Dr Kristina T. Nelson and Dr Nicholas E. Sherman in the W.M. Keck Biomedical Mass Spectrometry Laboratory at the University of Virginia for their assistance with the MS analysis. We are grateful to Dr Chao Zhang and Dr Kevan Shokat (Department of Cellular and Molecular Pharmacology, University of California San Francisco, San Francisco, CA, U.S.A.) for focusing our attention on the unique cysteine residue in the ATP-binding pocket of MEKK1. We also thank Dr John Mieyal and Mr David Starke (Department of Pharmacology, Case Western Reserve University, Cleveland, OH, U.S.A.) for helpful discussions, Professor Melanie H. Cobb for providing the full-length MEKK1 expression plasmid, and Professor Hidenori Ichijo for the ASK1 expression plasmid.
References
- 1.Pentland A. P. Active oxygen mechanisms of UV inflammation. Adv. Exp. Med. Biol. 1994;366:87–97. doi: 10.1007/978-1-4615-1833-4_7. [DOI] [PubMed] [Google Scholar]
- 2.Riley P. A. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 3.Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem. J. 1989;263:539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hibi M., Lin A., Smeal T., Minden A., Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 5.Sluss H. K., Barrett T., Derijard B., Davis R. J. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol. Cell. Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y. R., Meyer C. F., Tan T. H. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J. Biol. Chem. 1996;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- 7.Yan M., Dai T., Deak J. C., Kyriakis J. M., Zon L. I., Woodgett J. R., Templeton D. J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature (London) 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 8.Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signalling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 9.Cardone M. H., Salvesen G. S., Widmann C., Johnson G., Frisch S. M. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 10.Widmann C., Gerwins P., Johnson N. L., Jarpe M. B., Johnson G. L. MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol. Cell. Biol. 1998;18:2416–2429. doi: 10.1128/mcb.18.4.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deak J. C., Cross J. V., Lewis M., Qian Y., Parrott L. A., Distelhorst C. W., Templeton D. J. Fas-induced proteolytic activation and intracellular redistribution of the stress-signalling kinase MEKK1. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5595–5600. doi: 10.1073/pnas.95.10.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minden A., Lin A., McMahon M., Lange-Carter C., Derijard B., Davis R. J., Johnson G. L., Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y., Makris C., Su B., Li E., Yang J., Nemerow G. R., Karin M. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5243–5248. doi: 10.1073/pnas.97.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yujiri T., Ware M., Widmann C., Oyer R., Russell D., Chan E., Zaitsu Y., Clarke P., Tyler K., Oka Y., Fanger G. R., Henson P., Johnson G. L. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-κB activation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7272–7277. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer C. F., Wang X., Chang C., Templeton D., Tan T. H. Interaction between c-Rel and the mitogen-activated protein kinase kinase kinase 1 signalling cascade in mediating κB enhancer activation. J. Biol. Chem. 1996;271:8971–8976. doi: 10.1074/jbc.271.15.8971. [DOI] [PubMed] [Google Scholar]
- 16.Nemoto S., DiDonato J. A., Lin A. Coordinate regulation of IκB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB-inducing kinase. Mol. Cell. Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano H., Shindo M., Sakon S., Nishinaka S., Mihara M., Yagita H., Okumura K. Differential regulation of IκB kinase alpha and beta by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu S., Robbins D. J., Christerson L. B., English J. M., Vanderbilt C. A., Cobb M. H. Cloning of rat MEK kinase 1 cDNA reveals an endogenous membrane-associated 195-kDa protein with a large regulatory domain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5291–5295. doi: 10.1073/pnas.93.11.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clerk A., Fuller S. J., Michael A., Sugden P. H. Stimulation of “stress-regulated” mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J. Biol. Chem. 1998;273:7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- 20.Clerk A., Michael A., Sugden P. H. Stimulation of multiple mitogen-activated protein kinase sub-families by oxidative stress and phosphorylation of the small heat shock protein, HSP25/27, in neonatal ventricular myocytes. Biochem. J. 1998;333:581–589. doi: 10.1042/bj3330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendelson K. G., Contois L. R., Tevosian S. G., Davis R. J., Paulson K. E. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L., Jope R. S. Oxidative stress differentially modulates phosphorylation of ERK, p38 and CREB induced by NGF or EGF in PC12 cells. Neurobiol. Aging. 1999;20:271–278. doi: 10.1016/s0197-4580(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 23.Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J. Biol. Chem. 1982;257:12419–12425. [PubMed] [Google Scholar]
- 25.Di Monte D., Bellomo G., Thor H., Nicotera P., Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch. Biochem. Biophys. 1984;235:343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- 26.Di Monte D., Ross D., Bellomo G., Eklow L., Orrenius S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch. Biochem. Biophys. 1984;235:334–342. doi: 10.1016/0003-9861(84)90206-6. [DOI] [PubMed] [Google Scholar]
- 27.Ross D., Thor H., Threadgill M. D., Sandy M. S., Smith M. T., Moldeus P., Orrenius S. The role of oxidative processes in the cytotoxicity of substituted 1,4-naphthoquinones in isolated hepatocytes. Arch. Biochem. Biophys. 1986;248:460–466. doi: 10.1016/0003-9861(86)90499-6. [DOI] [PubMed] [Google Scholar]
- 28.Bellomo G., Mirabelli F., DiMonte D., Richelmi P., Thor H., Orrenius C., Orrenius S. Formation and reduction of glutathione-protein mixed disulfides during oxidative stress. A study with isolated hepatocytes and menadione (2-methyl-1,4-naphthoquinone) Biochem. Pharmacol. 1987;36:1313–1320. doi: 10.1016/0006-2952(87)90087-6. [DOI] [PubMed] [Google Scholar]
- 29.Gant T. W., Rao D. N., Mason R. P., Cohen G. M. Redox cycling and sulphydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem.–Biol. Interact. 1988;65:157–173. doi: 10.1016/0009-2797(88)90052-x. [DOI] [PubMed] [Google Scholar]
- 30.Benitez L. V., Allison W. S. The inactivation of the acyl phosphatase activity catalyzed by the sulfenic acid form of glyceraldehyde 3-phosphate dehydrogenase by dimedone and olefins. J. Biol. Chem. 1974;249:6234–6243. [PubMed] [Google Scholar]
- 31.Ellis H. R., Poole L. B. Novel application of 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole to identify cysteine sulfenic acid in the AhpC component of alkyl hydroperoxide reductase. Biochemistry. 1997;36:15013–15018. doi: 10.1021/bi972191x. [DOI] [PubMed] [Google Scholar]
- 32.Barrett W. C., DeGnore J. P., Konig S., Fales H. M., Keng Y. F., Zhang Z. Y., Yim M. B., Chock P. B. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 33.Ward N. E., Stewart J. R., Ioannides C. G., O'Brian C. A. Oxidant-induced S-glutathiolation inactivates protein kinase C-α (PKC-α): a potential mechanism of PKC isozyme regulation. Biochemistry. 2000;39:10319–10329. doi: 10.1021/bi000781g. [DOI] [PubMed] [Google Scholar]
- 34.Bhatnagar A., Liu S. Q., Petrash J. M., Srivastava S. K. Mechanism of inhibition of aldose reductase by menadione (vitamin K3) Mol. Pharmacol. 1992;42:917–921. [PubMed] [Google Scholar]
- 35.Zheng M., Aslund F., Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 36.Tsuboi S., Kotani Y., Ogawa K., Hatanaka T., Yatsushiro S., Otsuka M., Moriyama Y. An intramolecular disulfide bridge as a catalytic switch for serotonin N-acetyltransferase. J. Biol. Chem. 2002;277:44229–44235. doi: 10.1074/jbc.M203305200. [DOI] [PubMed] [Google Scholar]
- 37.Peshenko I. V., Shichi H. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radicals Biol. Med. 2001;31:292–303. doi: 10.1016/s0891-5849(01)00579-2. [DOI] [PubMed] [Google Scholar]
- 38.Pineda-Molina E., Klatt P., Vazquez J., Marina A., Garcia de Lacoba M., Perez-Sala D., Lamas S. Glutathionylation of the p50 subunit of NF-κB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 39.Carballal S., Radi R., Kirk M. C., Barnes S., Freeman B. A., Alvarez B. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry. 2003;42:9906–9914. doi: 10.1021/bi027434m. [DOI] [PubMed] [Google Scholar]
- 40.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adler V., Yin Z., Fuchs S. Y., Benezra M., Rosario L., Tew K. D., Pincus M. R., Sardana M., Henderson C. J., Wolf C. R., Davis R. J., Ronai Z. Regulation of JNK signalling by GSTπ. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallis R. J., Buss J. E., Thomas J. A. Oxidative modification of H-ras: S-thiolation and S-nitrosylation of reactive cysteines. Biochem. J. 2001;355:145–153. doi: 10.1042/0264-6021:3550145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lander H. M., Ogiste J. S., Pearce S. F., Levi R., Novogrodsky A. Nitric oxide-stimulated guanine nucleotide exchange on p21ras. J. Biol. Chem. 1995;270:7017–7020. doi: 10.1074/jbc.270.13.7017. [DOI] [PubMed] [Google Scholar]
- 44.Savitsky P. A., Finkel T. Redox regulation of Cdc25C. J. Biol. Chem. 2002;277:20535–20540. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- 45.Sohn J., Rudolph J. Catalytic and chemical competence of regulation of cdc25 phosphatase by oxidation/reduction. Biochemistry. 2003;42:10060–10070. doi: 10.1021/bi0345081. [DOI] [PubMed] [Google Scholar]
- 46.Lee S. R., Kwon K. S., Kim S. R., Rhee S. G. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 47.Salmeen A., Andersen J. N., Myers M. P., Meng T. C., Hinks J. A., Tonks N. K., Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature (London) 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 48.van Montfort R. L., Congreve M., Tisi D., Carr R., Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature (London) 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 49.Humphries K. M., Juliano C., Taylor S. S. Regulation of cAMP-dependent protein kinase activity by glutathionylation. J. Biol. Chem. 2002;277:43505–43511. doi: 10.1074/jbc.M207088200. [DOI] [PubMed] [Google Scholar]
- 50.Deak J. C., Templeton D. J. Regulation of the activity of MEK kinase 1 (MEKK1) by autophosphorylation within the kinase activation domain. Biochem. J. 1997;322:185–192. doi: 10.1042/bj3220185. [DOI] [PMC free article] [PubMed] [Google Scholar]