Abstract
The binding of enoyl-ACP (acyl-carrier protein) reductase from Plasmodium falciparum (PfENR) with its substrates and inhibitors has been analysed by SPR (surface plasmon resonance). The binding of the substrate analogue crotonoyl-CoA and coenzyme NADH to PfENR was monitored in real time by observing changes in response units. The binding constants determined for crotonoyl-CoA and NADH were 1.6×104 M−1 and 1.9×104 M−1 respectively. Triclosan, which has recently been demonstrated as a potent antimalarial agent, bound to the enzyme with a binding constant of 1.08×105 M−1. However, there was a 300-fold increase in the binding constant in the presence of NAD+. The increase in the binding constant was due to a 17 times increase in the association rate constant (k1) from 741 M−1·s−1 to 1.3×104 M−1 ·s−1 and a 16 times decrease in the dissociation rate constant (k−1) from 6.84×10−3 s−1 to 4.2×10−4 s−1. These values are in agreement with those determined by steady-state kinetic analysis of the inhibition reaction [Kapoor, Reddy, Krishnasastry, N. Surolia and A. Surolia (2004) Biochem. J. 381, 719–724]. In SPR experiments, the binding of NAD+ to PfENR was not detected. However, a binding constant of 6.5×104 M−1 was obtained in the presence of triclosan. Further support for these observations was provided by the crystal structures of the binary and ternary complexes of PfENR. Thus the dramatic enhancement in the binding affinity of both triclosan and NAD+ in the ternary complex can be explained by increased van der Waals contacts in the ternary complex, facilitated by the movement of residues 318–324 of the substrate-binding loop and the nicotinamide ring of NAD+. Interestingly, the results of the present study also provide a rationale for the increased affinity of NAD+ for the enzyme in the ternary complex.
Keywords: antimalarial drug, enoyl-ACP (acyl-carrier protein) reductase, fatty acid biosynthesis, malaria, surface plasmon resonance, triclosan
Abbreviations: ACP, acyl-carrier protein; ENR, enoyl-ACP reductase; FAS, fatty acid synthase; PDB, Protein Data Bank; PfENR, Plasmodium falciparum; RU, resonance unit; SPR, surface plasmon resonance
INTRODUCTION
It is estimated that malaria causes 1–3 million deaths annually [1], thus attesting to the need of better antimalarials. The human malaria parasite, Plasmodium falciparum, harbours the enzymes of type II FAS (fatty acid synthase) and is capable of synthesizing fatty acids [2,3]. In contrast, humans utilize type I FAS [4]. Due to this difference in the enzymes of fatty acid synthesis between Plasmodium and man, it is an attractive target for the ongoing research for the development of new antimalarials [5,6]. The final reduction step in the elongation phase of fatty acid synthesis is catalysed by enoyl-ACP (acyl-carrier protein) reductase (ENR; also known as Fab1) to form acyl-ACP, which in turn serves as a substrate for another round of elongation. The enzyme catalyses the NADH/NADPH-dependent reduction of 2-trans-enoyl-ACP by the direct transfer of the 4S hydrogen atom of NADH to the C3 position of the α,β-unsaturated thioester via an intermediate enolate anion [2,7–11].
The antimicrobial activity of triclosan and other 2-hydroxydiphenyl ethers and diazaborine derivatives has been attributed to their inhibitory action on the reaction catalysed by ENR [2,12–14]. Due to the importance of ENR in type II dissociative FAS, its inhibitors appear attractive agents against broad spectrum of organisms. ENR from P. falciparum has been studied previously [10] and its structure solved both in binary complex with NADH, as well as ternary complex with NAD+ and triclosan [15]. However, no attempt was made to answer the question regarding the high-affinity binding of the enzyme to triclosan in relation to cofactor binding and the structural transitions involved therein.
In the present work we have studied the interaction of Plasmodium falciparum ENR (PfENR) with its substrates and inhibitors in real time. Real-time BIA (biomolecular interaction analysis) by SPR (surface plasmon resonance) relies exclusively on the mass change during a reaction, without the need for labelling any of the interactants, which can sometimes alter the nature of the reaction [16–18]. Also, it provides data for both the association and dissociation phases of a reaction and the affinities involved therein in a single experimental run. The crystal structure of PfENR was reported previously; however, the co-ordinates had not been deposited until recently [15]. Also, the positions of the water molecules were not deposited. In the meantime, we had independently determined the crystal structures of PfENR in binary complex with NADH andin ternary complex with triclosan and NAD+ at 2.5 and 2.2 Å resolution respectively [Protein Data Bank (PDB) numbers, 1UH5 (ternary complex) and 1V35 (binary complex)]. In the present paper we provide a kinetic basis for the observed increase in inhibition of ENR activity by triclosan in the presence of NAD+. These results are supported by structural studies, which indicate tighter binding in the ternary complex due to the movement of the substrate-binding loop in PfENR.
EXPERIMENTAL
Materials
Media components were obtained from Hi-media (Delhi, India). β-NADH, β-NAD+, crotonoyl-CoA, imidazole and SDS/PAGE reagents were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Triclosan was obtained from Kumar Organic Products (Bangalore, India). His-bind resin and anti-His tag antibody were obtained from Novagen (Madison, WI, U.S.A.). All other chemicals used were of analytical grade.
Expression and purification of PfENR
PfENR was expressed and purified as described previously [10]. Briefly, the plasmid containing pfENR was transformed into BL21(DE3) cells. Cultures were grown at 37 °C for 12 h, followed by subsequent purification of the His-tagged ENR on Ni-NTA (Ni2+-nitrilotriacetate)–agarose column using an imidazole gradient. PfENR eluted at 400 mM imidazole concentration. The purity of the protein was confirmed by SDS/PAGE.
SPR analysis
Biospecific-interaction analysis was performed using a BIAcore 2000 biosensor system (Amersham Pharmacia Biotech, Uppsala, Sweden). The immobilization of PfENR on one of the flow cells of a CM5 (carboxymethylated)-certified grade sensor chip involved activation of the sensor surface with a 7 min flow of an equal mixture of 0.4 M N-hydroxysuccinimide and N-ethyl-N′-(dimethylaminopropyl)carbodiimide at a rate of 10 μl/min on the sensor surface. This was followed by injecting PfENR (0.1 mg/ml) in 10 mM sodium acetate buffer (pH 6.2) at 1 μl/min for 5 min. Nearly 2000 resonance units (RUs) were coupled, where 1 RU corresponds to immobilized protein concentration of approx. 1 pg/mm2. The unreacted N-hydroxysuccinimide esters of the surface were blocked by flowing ethanolamine at a rate of 5 μl/min for 7 min. PBS was used as the running buffer during the immobilization. Another flow cell was kept as the reference surface. The reference surface was treated in the same way as the ligand surface, except that PfENR was not passed over this surface. This is important to normalize the chemistries between the two flow cells.
All measurements were carried out in 10 mM Hepes, pH 7.4, 150 mM NaCl and 3.4 mM EDTA. In order to determine the association rate constant for the binding of the various compounds to the immobilized PfENR, NADH, crotonoyl-CoA and NAD+ were passed over the surfaces at various concentrations at 20 °C in Hepes buffer at a flow rate of 5 μl/min. For the determination of dissociation rate constant, the same buffer was passed at a flow rate of 5 μl/min. Triclosan stock solutions were initially made in methanol and diluted in the running buffer with the final concentration of methanol being 0.5%. After each triclosan injection, the surfaces were regenerated with a 10 μl pulse of 5 mM sodium acetate, pH 5.2, flowing at 50 μl/min. To study the interaction of triclosan with PfENR in the presence of NAD+, 50 μM NAD+ in Hepes buffer was continuously passed over the sensor chip surface for 3 h at a flow rate of 5 μl/min, followed by the injection of various concentrations of triclosan made in the NAD+-containing buffer. To observe the effect of triclosan on the binding of NAD+ to PfENR, 50 μM triclosan in Hepes buffer was run over the sensor chip surface for 3 h at a flow rate of 5 μl/min followed by injections of various concentrations of NAD+ made in the triclosan buffer.
Evaluation of kinetic parameters
The association (k1) and dissociation (k−1) rate constants are obtained by non-linear fitting of the primary sensorgram data using the BIA evaluation software version 3.0. The sensorgrams were fit globally to the 1:1 Langmuir dissociation model (equation 1) to obtain the k−1 values.
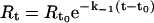 |
(1) |
where, Rt is the response at time t, Rt0 is the amplitude of the initial response. The measured k−1 values were used to determine the k1 values using 1:1 Langmuir association model (equation 2).
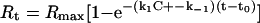 |
(2) |
Where, Rmax is the maximum response, C is the concentration of the analyte in the solution. The ratio of k1 and k−1 yields the value of association constant Ka (k1/k−1). χ2 and residual values were used to evaluate the quality of fit between the experimental data and binding model. The residuals obtained upon fitting the curves are provided as part of the supplemental data (http://www.BiochemJ.org/bj/381/bj3810725add.htm).
Crystallization and structure solution
Purified PfENR was crystallized both as a binary complex with NADH, as well as a ternary complex with NAD+ and triclosan, using the hanging-drop vapour diffusion methods, using the conditions reported previously [15]. In order to obtain crystals of the ternary complex, triclosan and NAD+ were added to a dilute solution of purified PfENR. The mixture was concentrated in a 10 kDa Amicon concentrator in order to obtain a protein concentration of 20 mg/ml. The final solution obtained was then used to set up crystallization experiments.
Diffraction data werecollected at room temperature to 2.5 Å (binary complex) and 2.2 Å (ternary complex) resolution using a MAR imaging plate detector coupled to a Rigaku X-ray generator with a copper rotating anode (λ=1.5418Å). The space group and the cell parameters for the binary and ternary complexes were respectively: P43212, a=b=133.32, c=83.65 Å; P43212, a=b=133.02, c=83.66 Å (Table 1). The structure was solved by the molecular replacement method with the program AmoRe [19] using the coordinates of the structure of ENR from Brassica napus (PDB code, 1ENO) as the search model. Electron density maps showed clear density for NADH, NAD+ and triclosan. A final Rcryst of 18% and Rfree of 21.1% were obtained. The structures of the binary and ternary complexes have been deposited in the PDB (PDB code of ternary complex, 1UH5; PDB code of binary complex, 1V35).
Table 1. Data collection and refinement statistics.
| Binary complex | Ternary complex | |
|---|---|---|
| Data collection | ||
| Resolution (Å) | 2.5 | 2.2 |
| Space group | P43212 | P43212 |
| Cell parameters (Å) | a=b=133.32, c=83.65 | a=b=133.02, c=83.66 |
| Unique reflections | 26266 | 37772 |
| Rmerge (%) | 10.1 | 10.1 |
| Completeness (%) | 98.2 | 97.8 |
| I/σ | 14 | 20.2 |
| Refinement | ||
| Resolution range (Å) | 20-2.5 | 20-2.2 |
| Number of reflections | 25266 | 34648 |
| Number of atoms | ||
| Protein | 4342 | 4321 |
| Water | 178 | 164 |
| Ligand(s) | 88 | 122 |
| Rcryst (%) | 16.5 | 18 |
| Rfree (%) | 20.2 | 21.1 |
| Average B-factor | 33.6 | 35.9 |
The binary and the ternary complexes were superposed using ALIGN [20]. The hydrogen bonds and van der Waals contacts were calculated using CCP4 (Collaborative Computational Project No. 4, 1994) program suite CONTACT [21]. Molecules were displayed using the program O [22] in order to compare the structures.
RESULTS
ENR plays a determinant role in completing cycles of fatty acid elongation [23]. In vivo, ACP thioesters are utilized by the enzymes of fatty acid biosynthesis, although other thio moieties, such as CoA and N-acetylcysteamine, can also be exploited in vitro for their study. Reduction of crotonoyl-CoA into butyryl-CoA, wherein NADH acts as the coenzyme, has most often been used in studies with ENR, because of the ready availability of the former [24]:
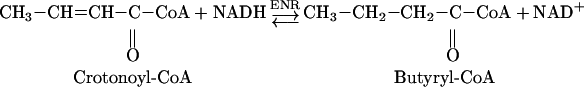 |
(3) |
Immobilization of PfENR on CM5 surfaces for SPR studies
The first requirement for the analyses of binding reaction by SPR is the immobilization of one of the reactants on the surface of the sensor chip. The immobilization of proteins on CM5 surfaces requires the proteins to be at least one pH unit below their pI values, which ensures effective electrostatic interaction between the protein molecules and the negatively charged dextran surface. PfENR has a calculated pI of 8.86. Hence, the stock solution of protein was diluted in 10 mM sodium acetate buffer pH 6.2 and injected over the activated CM5 chip surface. Approx. 2000 RUs of PfENR were immobilized over the chip surface. All the binding studies were conducted at 20 °C. After the immobilization of the ligand, buffer was flowed over the surface until a stable baseline was attained. The binding studies were carried out in the presence of 10 mM Hepes containing 5 mM EDTA and 150 mM NaCl.
SPR studies for binding of crotonoyl-CoA and NADH to PfENR
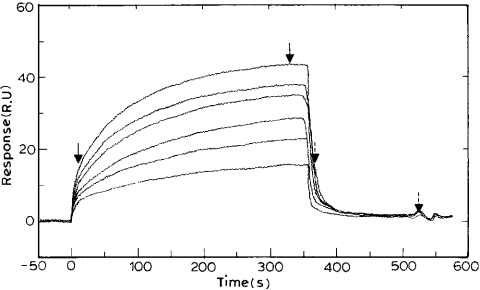
Various concentrations of crotonoyl-CoA were injected over the surface of the chip. A complete cycle of binding was observed, which had the first phase of increase in RU, the second phase in which the plateau was reached and the third phase during which the RU signal returned to baseline (Figure 1). Thus the ENR immobilized surface devoid of crotonoyl-CoA from a previous reaction could be regenerated by passing buffer alone.
Figure 1. Sensorgram depicting the binding of crotonoyl-CoA to PfENR.
Varying concentrations of crotonoyl-CoA (5, 10, 15, 20, 25 and 30 μM) dissolved in 10 mM Hepes buffer, containing 150 mM NaCl/3.4 mM EDTA, pH 7.4, were passed over the immobilized PfENR at a flow rate of 10 μl/min. The dissociation was studied subsequently by passing the same buffer at a flow rate of 10 μl/min. The fit of the sensorgram yielded association and dissociation values of 1.28×103 M−1·s−1 and 0.08 s−1 respectively, and an Rmax of 45. The first two arrows mark the regions selected to obtain the association rate constant. The other two arrows (with broken line) mark the regions selected to obtain the dissociation rate constant.
A typical sensorgram for the binding of varying concentrations of crotonoyl-CoA to immobilized PfENR is shown in Figure 1. The occurrence of an enhancement in the RUs is indicative of the increase in mass on the chip surface, which indicates a binding reaction. The fit of the sensorgram gave the association and dissociation rates for the interaction of crotonoyl-CoA with PfENR as 1.28×103 M−1·s−1 and 0.08 s−1 respectively, giving an overall binding constant of 1.6×104 M−1 (Table 2). The value of Kb thus determined is close to that obtained (i.e. Km=165 μM) by non-linear least-squares fit of the enzyme kinetic data [9]. The distribution of residuals indicated that the time dependence of the RUs for both the association and dissociation reactions fitted satisfactorily to a mono-exponential reaction (see supplemental data, http://www.BiochemJ.org/bj/381/bj3810725add.htm).
Table 2. Rate constants for the interaction of PfENR with crotonoyl-CoA, NAD+ and triclosan at 25 °C.
| Rate constant | ||||
|---|---|---|---|---|
| Analyte | k1 (M−1·s−1) | k−1 (s−1) | Ka (M−1) | Kd (M) |
| Crotonoyl-CoA | 1.28×103 | 0.08 | 1.6×104 | 6.25×10−5 |
| NADH | 1.54×103 | 0.079 | 1.9×104 | 5.16×10−5 |
| NAD+ | ND* | ND | ND | ND |
| NAD+ (presence of triclosan)† | 1.9×103 | 0.029 | 6.5×104 | 1.5×10−5 |
| Triclosan | 741 | 6.84×10−3 | 1.08×105 | 3.71×10−6 |
| Triclosan (presence of NAD+) | 1.3×104 | 4.2×10−4 | 0.3×108 | 3.2 ×10−8 |
* ND no binding observed for NAD+.
† To determine the parameters for binding of NAD+ to ENR in the presence of triclosan, buffer containing 250 μM triclosan was passed over the surface for 1h before the passage of NAD+ and vice versa.
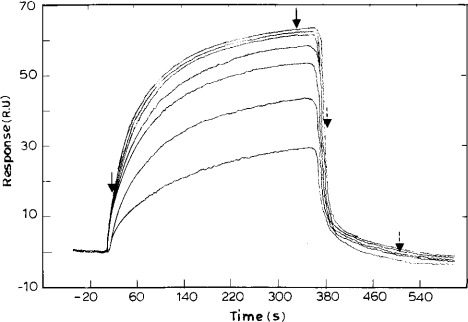
The binding of NADH to PfENR also fitted to a simple 1:1 interaction model. The association rate for the binding of NADH to PfENR was obtained as 1.54×103 M−1·s−1. The dissociation rate was obtained as 0.0793 s−1, giving an overall binding constant of 1.9×104 M−1 (Figure 2). No binding was observed for the product of the reaction, namely, NAD+. This is not surprising as the Km for NAD+ for PfENR is 2 mM, which gives a Kb of approx. 5×102 M−1, a value too low for detection of binding in SPR measurements [25].
Figure 2. Sensorgram depicting the binding of NADH to PfENR.
Varying concentrations of NADH (5, 10, 20, 25, 30, 35 and 40 μM) were passed over the immobilized ENR. The fit of the sensorgram yielded association and dissociation rates of 1.54×103 M−1·s−1 and 0.079 s−1 respectively, and an Rmax of 65.
Binding of triclosan and NAD+ to PfENR
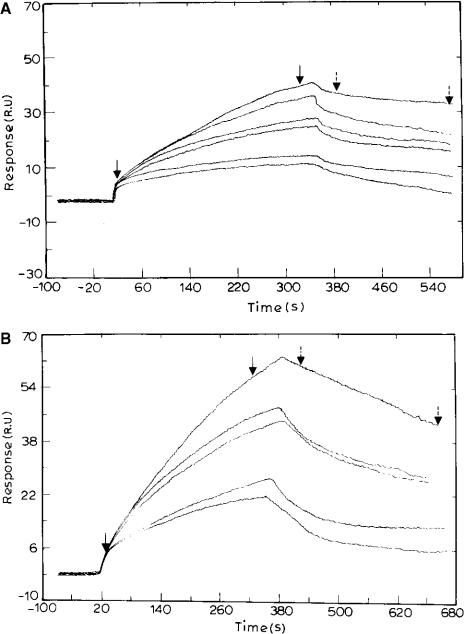
To study the interaction of PfENR and triclosan, stocks of triclosan were made in methanol keeping a final concentration of methanol during each injection as 0.5%. Methanol was chosen over DMSO or ethanol as it led to the least change in refractive index and hence RUs. Due to the tight binding of triclosan to the surface, after each injection the surface was regenerated with a 10 μl pulse of 5 mM sodium acetate, pH 5.2, flowing at 50 μl/min. An ‘on’ rate (k1) and an ‘off’ rate (k−1) of 741 M−1·s−1 and 6.84×10−3 s−1 respectively were obtained for triclosan binding, giving a binding constant (Kb) of 1.08×105 M−1 (Figure 3A).
Figure 3. Sensorgram depicting the binding of triclosan to ENR in the absence (A) and presence (B) of NAD+.
(A) Various concentrations of triclosan (0.4, 0.8, 1, 1.5, 3 and 4 μM) were passed over the immobilized PfENR. The surfaces were regenerated by a 10-s pulse of 10 mM glycine/HCl (pH 4.2) flowing at 50 μl/min. The fit of the sensorgram yielded association and dissociation values of 741 M−1·s−1 and 6.84×10−3 s−1 respectively, and an Rmax of 150. (B) NAD+ was passed over immobilized PfENR for 1 h followed by varying concentrations of triclosan (0.4, 0.8, 1, 1.5 and 3 μM). The fit of the curve yielded forward and reverse rate constants of 1.3×104 M−1·s−1and 4.2×10−4 s−1 respectively, and an Rmax of 175.
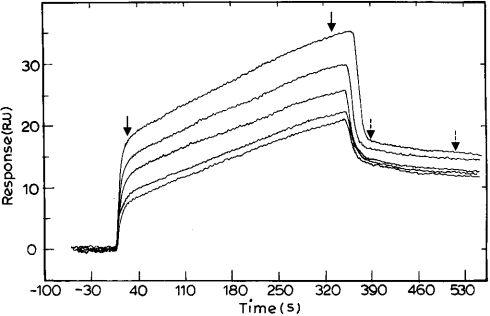
To study the effect of the presence of NAD+ on triclosan binding to PfENR, NAD+ was passed over immobilized PfENR continuously for 1 h prior to passing over a solution of triclosan in buffer containing NAD+. This led to an increase in the binding constant of triclosan for PfENR by 300-fold (Figure 3B). The association and dissociation rate constants for the binding of triclosan to PfENR in the presence of NAD+ were obtained as 1.3×104 M−1·s−1 and 4.2×10−4 s−1, leading to an overall binding constant of 0.3×108 M−1. In order to ascertain the effect of the presence of triclosan on NAD+ binding to PfENR, 50 μM triclosan was passed over immobilized PfENR for 1 h, followed by injection of NAD+ into the buffer solution containing triclosan. The on and the off rate constants were obtained as 1.9×103 M−1 ·s−1 and 0.029 s−1 respectively, leading to an overall binding constant of 6.5×104 M−1 (Figure 4). Thus, in SPR experiments, binding of NAD+ is detected only to the complex of ENR with triclosan. This in turn indicates that there is an increase in the affinity for NAD+, perhaps due to a change in the conformation of the enzyme upon binding to triclosan.
Figure 4. Sensorgram depicting the binding of NAD+ to ENR in the presence of triclosan.
Triclosan (50 μM) was passed over immobilized PfENR for 1 h followed by varying concentrations of NAD+ (5, 10, 15, 20 and 25 μM). The surfaces were regenerated by a 10-s pulse of 10 mM glycine/HCl (pH 4.2) flowing at 50 μl/min. The fit of the sensorgram yielded association and dissociation values of 1.9×103 M−1·s−1 and 0.029 s−1 respectively, and an Rmax of 60.
Analysis of the PfENR crystal structure
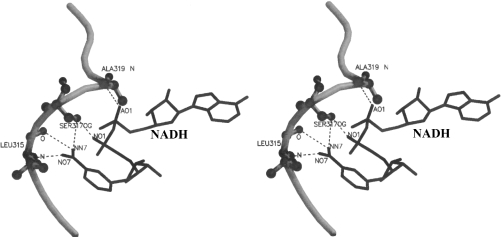
The structure of PfENR has the Rossmann fold. Each subunit comprises seven β-strands forming a parallel β-sheet and connected to nine α-helices by loops. The nicotinamide ring of NADH, as observed in the binary complex of the enzyme, interacts via five specific hydrogen bonds formed by the oxygen and nitrogen of the carboxamide moiety and the pyrophosphate moiety with the substrate binding loop (315–324), as shown in Figure 5. We have compared the binary and ternary complexes of PfENR to explain the increase in affinity of NAD+ for PfENR in the presence of triclosan and vice versa. Two striking observations can be made that explain this: first, the movement in the substrate-binding loop, specifically residues 318–324 of PfENR, present in a helical (310) conformation, and, second, the movement of the nicotinamide ring of NAD+.
Figure 5. Stereo image showing the hydrogen bonds present in the binary complex of PfENR with NADH.
The nicotinamide ring of NADH interacts via five hydrogen bonds formed between oxygen and nitrogen of the carboxamide moiety and the pyrophosphate moiety with the substrate-binding loop (315–324). The figure was generated using MOLSCRIPT [38] and rendered in Raster3D [39].
Movement of the Ala318–Asn324 loop
When compared with ENR from other organisms, PfENR has a 43-amino-acid insertion (325–366) localized near the catalytic centre of the protein. In the crystal structure of both binary and ternary complexes, there is no clear density for this loop. The PfENR structure is similar to that of ENR from B. napus, except for a flexible loop, Lys315–Asn324, involved in a reverse turn, which is present before the 43-amino-acid insertion. This substrate-binding loop fixes the nicotinamide ring of the coenzyme NADH by forming hydrogen bonds. The structure of Perozzo et al. [15] reported it to exist in an α-helical conformation in PfENR. However, we note that this region has characteristics of a 310 helix. Also, this region is flanked by residues in a loop conformation. Hence, in the following section the region 315–324 is being referred to as the loop region.
It would have been desirable to have the structure of the binary complex between PfENR and triclosan in addition to those of the binary complex involving NADH and the ternary complex with NAD+ and triclosan. However, despite repeated attempts, a binary complex involving triclosan could not be crystallized. Therefore, the effect of triclosan on the enzyme–coenzyme association has to be examined on the basis of the other two complexes, one binary (E–NADH) and the other ternary (E–NAD+–triclosan).
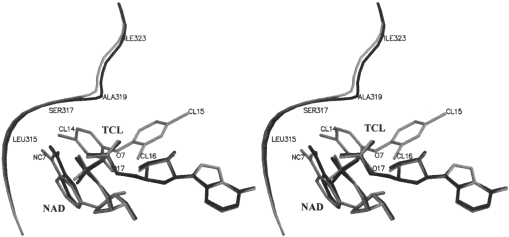
The most significant difference between the two complexes, presumably caused by the binding of triclosan, is the movement of the 315–324 loop. As illustrated in Figure 6, a substantial part of this loop moves away from the body of the molecule in the ternary complex with respect to its position in the binary complex. As can be seen from Table 3, this movement appears to have been caused by the steric effects resulting from the binding of triclosan. Clearly, triclosan has many unacceptable short contacts with the loop at its position in the binary complex. Many of them have distances less than the limits prescribed by Ramachandran and Sasisekharan [26]. They are relieved when the loop moves to its position in the ternary complex.
Figure 6. Stereo image showing the position of the substrate-binding loop and NAD+ in the two complexes.
Binary (black) and ternary (grey). The figures were generated using MOLSCRIPT [38] and rendered in Raster3D [39]. TCL, triclosan.
Table 3. van der Waals contact distances present in the binary and ternary positions of the loop and triclosan.
Distances (Å) of the atoms in the 318–324 loop, as observed in the binary and ternary complexes from the atoms in triclosan, as observed in the ternary complex. Distances less than 4 Å in one of the cases are listed. Distances less than the limits prescribed by Ramachandran et al. [40] are shown in bold. Please see Figures 5 and 6 for further information regarding positions of the atoms.
| van der Waals contact distance (Å) | ||||
|---|---|---|---|---|
| ENR atom | Triclosan atom | Complex… | Binary | Ternary |
| Ala319CB | C10 | 3.10 | 3.91 | |
| C9 | 2.55 | 3.61 | ||
| CL16 | 2.28 | 3.46 | ||
| Ala320CA | C4 | 3.14 | 3.74 | |
| C3 | 3.64 | 4.07 | ||
| Ala320CB | C4 | 3.22 | 3.85 | |
| C3 | 3.35 | 3.83 | ||
Movement of the nicotinamide ring of NAD+
As can be seen from contact distances listed in Table 4, the binding of triclosan also leads to a movement of the position of the coenzyme in the ternary complex compared with that in the binary complex. The nicotinamide ring of the coenzyme is firmly anchored to the protein through hydrogen bonds involving the carboxamide moiety. Yet, the ring moves, at least partly to facilitate good stacking interactions between the ring and the phenolic ring of triclosan. Thecarboxamide moiety itself moves, with a maximum movement of 0.5 Å seen at NC7 (Figure 6).
Table 4. van der Waals contact distances present between the binary and ternary positions of NAD+ and triclosan.
Distances (Å) of the atoms of NAD+, as observed in the binary and ternary complexes from the atoms in triclosan, as observed in the ternary complex. Distances less than 4 Å in one of the cases are listed. Please see Figures 5 and 6 for further information regarding positions of the atoms.
| van der Waals contact distance (Å) | ||||
|---|---|---|---|---|
| Triclosan | NAD+ | Complex… | Binary | Ternary |
| C1 | NC3 | 3.31 | 3.75 | |
| NC4 | 2.84 | 3.34 | ||
| NC5 | 3.65 | 3.71 | ||
| C2 | NC7 | 3.18 | 3.77 | |
| NO7 | 2.51 | 3.43 | ||
| NC3 | 3.58 | 3.82 | ||
| NC4 | 3.34 | 3.56 | ||
| C6 | NO2* | 3.75 | 3.48 | |
| NC3 | 3.22 | 3.79 | ||
| NC4 | 3.22 | 3.82 | ||
| NC2 | 3.73 | 3.97 | ||
| NC5 | 3.79 | 3.99 | ||
| C5 | NC3 | 3.44 | 3.92 | |
| C4 | NC7 | 3.03 | 3.69 | |
| NO7 | 3.07 | 3.91 | ||
| NN7 | 3.26 | 3.90 | ||
| C3 | NC7 | 2.94 | 3.49 | |
| NO7 | 2.40 | 3.25 | ||
| NC3 | 3.81 | 3.98 | ||
| O7 | NC2* | 3.52 | 3.35 | |
| CL14 | NO7 | 3.12 | 3.74 | |
| CL16 | O3 | 3.57 | 3.72 | |
| AC5* | 3.96 | 3.85 | ||
| NO5* | 3.52 | 3.60 | ||
| NC5* | 3.96 | 3.99 | ||
| NC3* | 3.74 | 3.69 | ||
| NC2* | 3.83 | 3.81 | ||
| O17 | NC2* | 3.55 | 3.42 | |
* The atoms correspond to those present in the ribose sugar.
The movement of the coenzyme molecule consequent to the binding of triclosan is such as to alter the strength of the protein–coenzyme interactions. The number of van der Waals contacts increases from 15 between NADH and ENR in the binary complex to 21 between NAD+ and ENR in the ternary complex. From Table 5, it is clear that six of the contacts are strengthened as the distance between them decreases. On the whole the contact distances decrease, but not to the point where repulsive forces could set in. Hence, the interactions between the enzyme and the coenzyme become tighter, thus providing a structural rationale for the results of the SPR study.
Table 5. van der Waals contact distances present in the binary and ternary positions of NAD+ and the loop of PfENR.
Distances (Å) of the atoms in NAD+ as observed in the binary and ternary complexes from the atoms in 318–324 loop of PfENR. Six of the contacts that are strengthened, as the distance between them decreases, are shown in bold. Please see Figures 5 and 6 for further information regarding positions of the atoms.
| van der Waals contact distance (Å) | ||||
|---|---|---|---|---|
| ENR | NAD+ | Complex… | Binary | Ternary |
| Leu315N | NC7 | 3.77 | 3.70 | |
| NN7 | 3.97 | 4.05 | ||
| Leu315CA | NO7 | 4.12 | 3.74 | |
| Leu315CB | NO7 | 4.36 | 3.87 | |
| NC7 | 4.22 | 3.97 | ||
| NN7 | 3.78 | 3.67 | ||
| Leu315C | NO7 | 4.18 | 3.91 | |
| Leu315C | NN7 | 3.96 | 3.99 | |
| Leu315O | NC7 | 3.67 | 3.62 | |
| NO7 | 3.40 | 3.23 | ||
| Ser317CB | AO2 | 3.37 | 3.50 | |
| O3 | 4.05 | 3.96 | ||
| NP | 3.93 | 3.72 | ||
| NO1 | 3.06 | 3.01 | ||
| NO2 | 4.39 | 3.92 | ||
| NN7 | 4.35 | 3.99 | ||
| Ser317OG | AO2 | 3.73 | 3.91 | |
| O3 | 3.64 | 3.63 | ||
| NP | 3.48 | 3.44 | ||
| Ala319CA | AO1 | 3.52 | 3.77 | |
| Ala319CB | O3 | 3.51 | 3.87 | |
| AO1 | 2.90 | 2.91 | ||
DISCUSSION
SPR has been routinely used to determine the binding constants of macromolecular interactions [27]. Recently, it has been demonstrated that it can also be used to study the direct binding of small molecules to larger targets, which has been possible due to the advancements in instrumentation and data handling [28]. In earlier experiments, it has been demonstrated that the constants determined by SPR match well with those determined using solution techniques, such as isothermal titration calorimetry or stopped-flow spectrofluorimetry [29–31].
Although there have been various studies on PfENR, there has been no direct determination of the binding constants of the substrates and inhibitor for this enzyme [2,10,15]. In the present study we have used SPR to directly determine these parameters for its substrate crotonoyl-CoA, the coenzyme NADH and the inhibitor triclosan. We analysed the interaction of PfENR and its substrates and inhibitors utilizing the chemistry of the CM5 chip for the immobilization of PfENR. The individual rates for the binding of the substrate crotonoyl-CoA and the coenzyme NADH can be determined by SPR as the catalytic reaction requires the presence of both the components of the reaction at the same time.
Studies on ENR from B. napus have shown that the reaction catalysed by this enzyme must proceed via an ordered ternary complex, with NADH binding first [24]. However, on the basis of kinetic isotope studies, ENR from Haemophilus influenzae has been shown to have a rapid equilibrium random kinetic mechanism [32]. We studied the binding of NADH and crotonoyl-CoA to PfENR in the absence of the other. Both bound to PfENR with overall binding constants of 1.9×104 M−1 and 1.6×104 M−1 respectively. Thus confirming that, indeed, both the substrate and the coenzyme are capable of binding the enzyme in the absence of the other. Hence, PfENR, like H. influenzae enzyme but unlike its counterpart from B. napus, accepts random order of the addition of the substrate. These studies provide for the first time information on the kinetic parameters for the interaction of the enzyme with NADH, crotonoyl-CoA and triclosan. Also, it is of note that the values of the binding constants determined from SPR studies correlate well with the enzyme kinetic data, where a Km of 33 μM and 165 μM were obtained for NADH and crotonoyl-CoA respectively.
Triclosan dramatically increases the affinity of E. coli ENR for NAD+ [33]. However, the extent to which an enhancement in the affinity for NAD+ occurs was not known. Also, the onset of inhibition of E. coli ENR by triclosan was more rapid when NAD+ was added [34]. Indeed, triclosan became a better inhibitor of PfENR in the presence of NAD+ [10]. The onset of inhibition is faster when NAD+ is included along with triclosan and the degree of inhibition increases still further upon pre-incubation of triclosan with NAD+. This is due to the formation of a stable complex between E. coli ENR and triclosan in the presence of NAD+, as shown qualitatively in an [3H]NAD+ binding assay [33]. Such a trend has been noted for PfENR as well ([8] and M. Kapoor, J. Gopalakrishnan, N. Surolia and A. Surolia, unpublished work). However, in the previous studies, neither the kinetics of the recognition of all the interacting molecules have been determined nor any structural basis for such enhancements in the affinities has been provided for ENR from any of the sources mentioned. To further characterize this interaction, we analysed the binding of triclosan in the absence and presence of NAD+ and vice versa. Triclosan bound to PfENR with a binding constant of 1.08×105 M−1. However, it increased by 300-fold in the presence of NAD+.
When NAD+ alone was passed over immobilized PfENR, no binding was observed. However, a binding constant of 6.5×104 M−1 was obtained in the presence of triclosan. An approximate value for the Kb of 5×102 M−1 can be calculated from the Km value for its binding to PfENR [9]. Triclosan binding therefore increases the affinity of NAD+ by 13-fold. These studies show for the first time that not only NAD+ binding, but also triclosan binding, induces a conformational change in the binary complex of the enzyme, potentiating the association of NAD+–PfENR, which in turn facilitates the formation of the ternary complex.
The crystal structure of ENR has been solved from E. coli [35,36], B. napus [8] and M. tuberculosis [9]. A comparison of the structures of binary complex (InhA and NADH) with that of the ternary complex (InhA, NAD+ and C16 fatty acyl substrate) identified a major conformational change in the protein upon fatty acyl substrate binding. The substrate-binding loop (196–219) was shifted by 4 Å. This along with a rotation in the side chain of Tyr158 causes the substrate-binding cavity to widen and therefore accommodate the bound C16 fatty acyl substrate [9], thus demonstrating the flexibility of this loop in ENRs to accommodate substrates of various chain lengths.
Diazaborine acts as an inhibitor of ENR from B. napus in the presence of NAD+ and not NADH. An analysis of the B. napus ENR(Ala138→Gly)–NAD+–diazaborine and the ENR(Ala138→Gly)–NADH–diazaborine complexes surprisingly revealed no structural differences [8]. It was proposed that the charge on the nicotinamide ring, the presence of aromaticity and the loss of the hydride could influence the affinity of the site for diazaborine. However, no structural basis for the higher affinity of the inhibitor for ENR–NAD+ as compared with ENR–NADH could be obtained.
The crystal structure of P. falciparum ENR revealed a similar binding mode for triclosan as found in B. napus and E. coli complexes [15]. Superimposition of the binary and ternary complex structures revealed subtle conformational changes in PfENR upon inhibitor binding, with a slight shift of helix α7 by 0.5 Å towards the solvent. However, the significance of this movement in the context of triclosan and the coenzyme binding was not explained. To investigate the nature of the conformational change upon binding of the inhibitor to the P. falciparum enzyme, the structure of the binary complex of the enzyme with the coenzyme (NADH) and the ternary complex (NAD+ and triclosan) were determined by X-ray crystallography. A comparison of these structures provides information on the subtle features that help explain the tight binding of the coenzyme and the inhibitor in the ternary complex and the higher affinity of the inhibitor for ENR–NAD+ as compared with PfENR.
Upon analysis of the crystal structure of the binary and ternary complexes of PfENR, a concerted backward movement in the latter part of the loop (315–324) and in the nicotinamide ring of NAD+ is observed. This leads to better stacking interactions between the nicotinamide ring of NAD+ and the phenolic ring of triclosan. Occurrence of this stacking interaction in the ternary complex also constitutes an important reason as to why NAD+ and triclosan show striking enhancements in their affinities for interaction with PfENR–triclosan and PfENR–NAD+ binary complexes respectively. The movement of the nicotinamide ring also leads to an increase in the interactions between NAD+ and protein, as seen from the increase in the number of van der Waals contacts between NAD+ and protein (Table 5).
The binding affinity of a ligand is, to a significant extent, proportional to the solvent-accessible surface area that becomes hidden from the solvent upon binding [37]. The buried hydrophobic surface area for the binary complex was calculated by subtracting the accessible hydrophobic surface area (ENR and NAD+) from that of the complex. Similar calculations were made for the ternary complex. Upon formation of the binary complex, an area of 637.7 Å2 becomes buried. There is still greater burial of hydrophobic surface area upon formation of the ternary complex (1188.6 Å2). Of the accessible hydrophobic surface area, 424.4 Å2 of triclosan, only 27.6 Å2 remains accessible in the ternary complex. Indeed, triclosan buries 92% of its accessible surface area upon binding to the enzyme in the ternary complex. Also, 328.7 Å2 and 346.7 Å2 of the hydrophobic surface area of NAD+ is buried in the binary and the ternary complexes respectively. These results provide further support for the formation of a high-affinity ternary complex between the enzyme, NAD+ and triclosan.
Molecular interactions involving conformational changes in the interacting molecules are more versatile. Conformational changes in the proteins can occur due to minimum expenditure of energy and might occur due to changes in the main torsional angles or side chain orientations. The observed shift in the position of the loop in the ternary complex as compared with its binary position, hints towards it being caused by the presence of triclosan. Further support to the loop movement is provided by the differences in the accessible surface areas of the residues of the loop between the binary and ternary complexes. Specifically residues Lys316, Ala319, Thr321 and Ile-323 become accessible to the solvent by 8.92, 10.43, 15.51 and 7.3 Å2 respectively. The other residues remain at the same position in the ternary complex as their positions in the binary complex. Thus, on the whole, the crystal structure analysis describing the movement of the loop residues and the nicotinamide ring of NAD+, supports the SPR observation, i.e. higher affinity for NAD+ in the presence of triclosan. Thus these studies add new dimensions to the knowledge of the kinetics and mechanism of the interaction of Pf ENR with its cofactor, substrate and triclosan, hitherto inaccessible to the steady-state kinetic analyses of the reaction reported in the preceding paper [41].
In conclusion, PfENR unlike its B. napus counterpart interacts with its substrate and cofactor NADH independently and in a random manner. Importantly, however, binding of NAD+, the oxidized cofactor is facilitated by triclosan in a dramatic fashion. Indeed, the ternary complex between NAD+, triclosan and the enzyme is facilitated by a conformational transition. Consequently, several new contacts between PfENR, NAD+ and triclosan are formed in addition to potentiation of many existing ones. These studies thus help explain the high potency of triclosan towards PfENR. ENR holds an important place in the fatty acid biosynthesis pathway. The inhibition of the enzymes of fatty acid biosynthesis pathway and their use as targets of potent antimalarials/antibacterials has been validated previously. These studies, taken together with the steady-state kinetic analysis [41] and mutational studies [42] reported in this issue, should help in the design and choice of future antimalarials targeted to P. falciparum ENR.
Online data
Acknowledgments
This work was supported by a grant from the Department of Biotechnology, Government of India, to N. S. and A. S. The BIAcore™ facility is funded by the Department of Biotechnology, Government of India, for the program support to Indian Institute of Science, Bangalore, in the area of Drug and Molecular Design. X-ray diffraction data were collected at the X-ray facility for structural biology at the Indian Institute of Science, supported by the Department of Science and Technology and the Department of Biotechnology, India.
References
- 1.World Health Organization. Fact Sheet No. 94, Revised October 1998.
- 2.Surolia N., Surolia A. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 2001;7:167–173. doi: 10.1038/84612. [DOI] [PubMed] [Google Scholar]
- 3.Waller R. F., Keeling P. J., Donald R. G. K., Striepen B., Handman E., Lang-Unnasch N., Cowman A. F., Besra G. S., Roos D. S., McFadden G. I. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12352–12357. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. Faseb J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- 5.Surolia N., Satish P. R., Surolia A. Paradigm shifts in malaria parasite biochemistry and anti-malarial chemotherapy. Bioessays. 2002;24:192–196. doi: 10.1002/bies.10042. [DOI] [PubMed] [Google Scholar]
- 6.Gornicki P. Apicoplast fatty acid biosynthesis as a target for medical intervention in apicomplexan parasites. Int. J. Parasitol. 2003;3:885–896. doi: 10.1016/s0020-7519(03)00133-4. [DOI] [PubMed] [Google Scholar]
- 7.Bergler H., Wallner P., Ebeling A., Leitinger B., Fuchsbichler S., Aschauer H., Kollenz G., Högenauer G., Turnowsky F. Protein EnvM is the NADH-dependent enoyl-ACP reductase (FabI) of Escherichia coli. J. Biol. Chem. 1994;269:5493–5496. [PubMed] [Google Scholar]
- 8.Rafferty J. B., Simon J. W., Baldock C., Artymiuk P. J., Baker P. J., Stuitje A. R., Slabas A. R., Rice D. W. Common themes in redox chemistry emerge from the X-ray structure of oilseed rape (Brassica napus) enoyl-acyl carrier protein reductase. Structure. 1995;3:927–938. doi: 10.1016/S0969-2126(01)00227-1. [DOI] [PubMed] [Google Scholar]
- 9.Rozwarski D. A., Grant G. A., Barton D. H. R., Jacobs W. R., Jr, Sacchettini J. C. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science. 1998;279:98–102. doi: 10.1126/science.279.5347.98. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor M., Dar M. J., Surolia A., Surolia N. Kinetic determinants of the interaction of enoyl-ACP reductase from Plasmodium falciparum with its substrates and inhibitors. Biochem. Biophys. Res. Commun. 2001;289:832–837. doi: 10.1006/bbrc.2001.6061. [DOI] [PubMed] [Google Scholar]
- 11.Bergler H., Fuchsbichler S., Högenauer G., Turnowsky F. The enoyl-(acyl-carrier-protein) reductase (FabI) of Escherichia coli, which catalyzes a key regulatory step in fatty acid biosynthesis, accepts NADH and NADPH as cofactors and is inhibited by palmitoyl-CoA. Eur. J. Biochem. 1996;242:689–694. doi: 10.1111/j.1432-1033.1996.0689r.x. [DOI] [PubMed] [Google Scholar]
- 12.Heath R. J., Yu Y. T., Shapiro M. A., Olson E., Rock C. O. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 1998;273:30316–30320. doi: 10.1074/jbc.273.46.30316. [DOI] [PubMed] [Google Scholar]
- 13.McMurray L. M., Oethinger M., Levy S. B. Triclosan targets lipid synthesis. Nature (London) 1998;394:531–532. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- 14.Webster J. Handwashing in a neonatal intensive care nursery: product acceptability and effectiveness of chlorhexidine gluconate 4% and triclosan 1% J. Hosp. Infect. 1992;21:137–141. doi: 10.1016/0195-6701(92)90033-i. [DOI] [PubMed] [Google Scholar]
- 15.Perozzo R., Kuo M., Sidhu A. S., Valiyaveettil J. T., Bittman R., Jacobs W. R., Jr, Fidock D. A., Sacchettini J. C. Structural elucidation of the specificity of the antibacterial agent triclosan for malarial enoyl acyl carrier protein reductase. J. Biol. Chem. 2002;277:13106–13114. doi: 10.1074/jbc.M112000200. [DOI] [PubMed] [Google Scholar]
- 16.Vadgama P., Crump P. W. Biosensors: Recent trends, a review. Analyst. 1992;117:1657–1670. [Google Scholar]
- 17.Jonsson U., Fagerstam L., Ivarsson B., Johnsson B., Karlsson R., Lundh K., Lofas S., Persson B., Roos H., Ronnberg I. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. Biotechniques. 1991;11:620–627. [PubMed] [Google Scholar]
- 18.Szabo A., Stolz L., Granzow R. Surface plasmon resonance and its use in biomolecular interaction analysis (BIA) Curr. Opin. Struct. Biol. 1995;5:699–705. doi: 10.1016/0959-440x(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 19.Navaza J. AMoRe: an automated package for molecular replacement. Acta Cryst. 1994;A50:157–163. [Google Scholar]
- 20.Cohen G. H. ALIGN: a program to superimpose protein coordinates, accounting for insertions and deletions. J. Appl. Crystal. 1997;30:1160–1161. [Google Scholar]
- 21.Collaborative Computational Project No. 4. The CCP4 suite: programs for protein crystallography. Acta Cryst. D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 22.Jones T. A., Kjeldgaard M. Electron-density map interpretation. Methods Enzymol. 1997;277:173–208. doi: 10.1016/s0076-6879(97)77012-5. [DOI] [PubMed] [Google Scholar]
- 23.Heath R. J., Rock C. O. Enoyl-acyl carrier protein reductase (FabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J. Biol. Chem. 1995;44:26538–26542. doi: 10.1074/jbc.270.44.26538. [DOI] [PubMed] [Google Scholar]
- 24.Fawcett T., Copse C. L., Simon J. W., Slabas A. R. Kinetic mechanism of NADH-enoyl-ACP reductase from Brassica napus. FEBS Lett. 2000;484:65–68. doi: 10.1016/s0014-5793(00)02128-1. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor M., Thomas C. J., Bachhawat-Sikder K., Surolia A. Exploring kinetics and mechanism of protein-sugar recognition by surface plasmon resonance. Methods Enzymol. 2003;362:312–329. doi: 10.1016/S0076-6879(03)01022-X. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran G. N., Sasisekharan V. Conformation of polypeptides and proteins. Adv. Prot. Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- 27.Myszka D. G. Kinetic analysis of macromolecular interactions using surface plasmon resonance biosensors. Curr. Opin. Biotechnol. 1997;8:50–57. doi: 10.1016/s0958-1669(97)80157-7. [DOI] [PubMed] [Google Scholar]
- 28.Day Y. S. N., Baird C. L., Rich R. L., Myszka D. G. Direct comparison of binding equilibrium, thermodynamic, and rate constants determined by surface- and solution-based biophysical methods. Prot. Sci. 2002;11:1017–1025. doi: 10.1110/ps.4330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachhawat K., Thomas C. J., Amutha B., Krishnasastry M. V., Khan M. I., Surolia A. On the stringent requirement of mannosyl substitution in mannooligosaccharides for the recognition by garlic (Allium sativum) lectin: A surface plasmon resonance study. J. Biol. Chem. 2001;276:5541–5546. doi: 10.1074/jbc.M009533200. [DOI] [PubMed] [Google Scholar]
- 30.Thomas C. J., Gangadhar B. P., Surolia N., Surolia A. Kinetics and mechanism of the recognition of endotoxin by polymyxin B. J. Am. Chem. Soc. 1998;120:12428–12434. [Google Scholar]
- 31.Thomas C. J., Surolia N., Surolia A. Surface plasmon resonance studies resolve the enigmatic endotoxin neutralizing activity of polymyxin B. J. Biol. Chem. 1999;274:29624–29627. doi: 10.1074/jbc.274.42.29624. [DOI] [PubMed] [Google Scholar]
- 32.Marcinkeviciene J., Jiang W., Kopcho L. M., Locke G., Luo Y., Copeland R. A. Enoyl-ACP reductase (FabI) of Haemophilus influenzae: steady-state kinetic mechanism and inhibition by triclosan and hexachlorophene. Arch. Biochem. Biophys. 2001;390:101–108. doi: 10.1006/abbi.2001.2349. [DOI] [PubMed] [Google Scholar]
- 33.Heath R. J., Rubin J. R., Holland D. R., Zhang E., Snow M. E., Rock C. O. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 1999;274:11110–11114. doi: 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- 34.Ward W. H. J., Holdgate G. A., Rowsell S., McLean E. G., Pauptit R. A., Clayton E., Nichols W. W., Colls J. G., Minshull C. A., Jude D. A., et al. Kinetic and structural characteristics of the inhibition of enoyl (acyl carrier protein) reductase by triclosan. Biochemistry. 1999;38:12514–12525. doi: 10.1021/bi9907779. [DOI] [PubMed] [Google Scholar]
- 35.Stewart M. J., Parikh S., Xiao G., Tonge P. J., Kisker C. Structural basis and mechanism of enoyl reductase inhibition by triclosan. J. Mol. Biol. 1999;290:859–865. doi: 10.1006/jmbi.1999.2907. [DOI] [PubMed] [Google Scholar]
- 36.Roujeinikova A., Levy C. W., Rowsell S., Sedelnikova S., Baker P. J., Minshull C. A., Mistry A., Colls J. G., Camble R., Stuitje A. R., et al. Crystallographic analysis of triclosan bound to enoyl reductase. J. Mol. Biol. 1999;294:527–535. doi: 10.1006/jmbi.1999.3240. [DOI] [PubMed] [Google Scholar]
- 37.Luque I., Freire E. Structural stability of binding sites: consequences for binding affinity and allosteric effects. Proteins Suppl. 2000;4:63–71. doi: 10.1002/1097-0134(2000)41:4+<63::aid-prot60>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 38.Kraulis P. J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystal. 1991;24:946–950. [Google Scholar]
- 39.Bacon D. J., Anderson W. F. A fast algorithm for rendering space-filling molecule pictures. J. Mol. Graph. 1988;6:219–220. [Google Scholar]
- 40.Ramachandran G. N., Ramakrishnan C., Sasisekharan V. Stereochemistry of polypeptide chain configuration. J. Mol. Biol. 1963;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- 41.Kapoor M., Reddy C. C., Krishnasastry M. V., Surolia N., Surolia A. Slow-tight-binding inhibition of enoyl-acyl carrier protein reductase from Plasmodium falciparum. Biochem. J. 2004;381:719–724. doi: 10.1042/BJ20031821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapoor K., Gopalakrishnapai J., Surolia N., Surolia A. Mutational analysis of the triclosan-binding region of enoyl-ACP (acyl-carrier protein) reductase from Plasmodium falciparum. Biochem. J. 2004;381:735–741. doi: 10.1042/BJ20040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.